Structural Features and In Vitro Antiviral Activities of Fungal Metabolites Sphaeropsidins A and B Against Bovine Coronavirus
Abstract
1. Introduction
2. Results
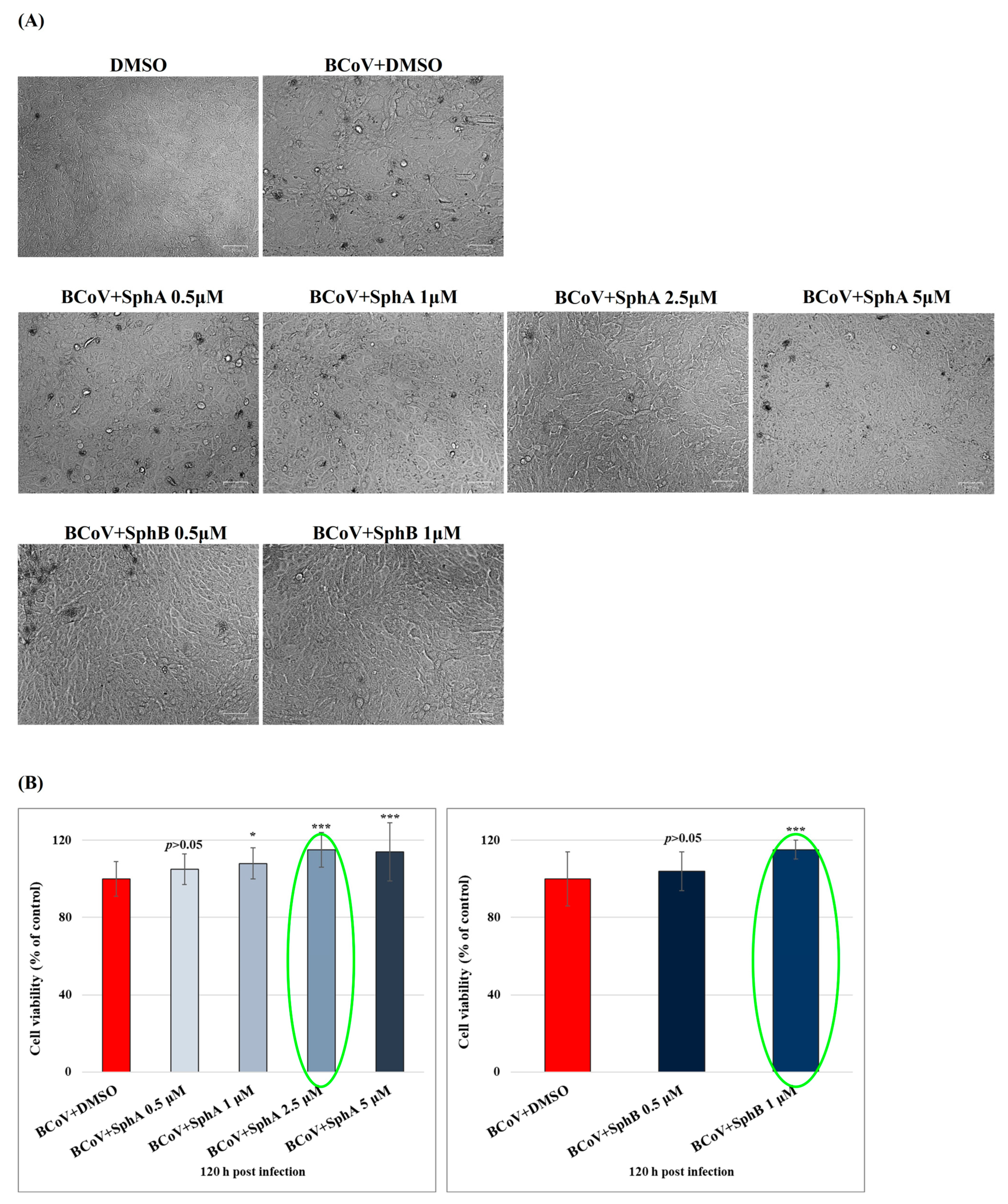
2.1. SphA and SphB Increased Cell Viability During BCoV Infection
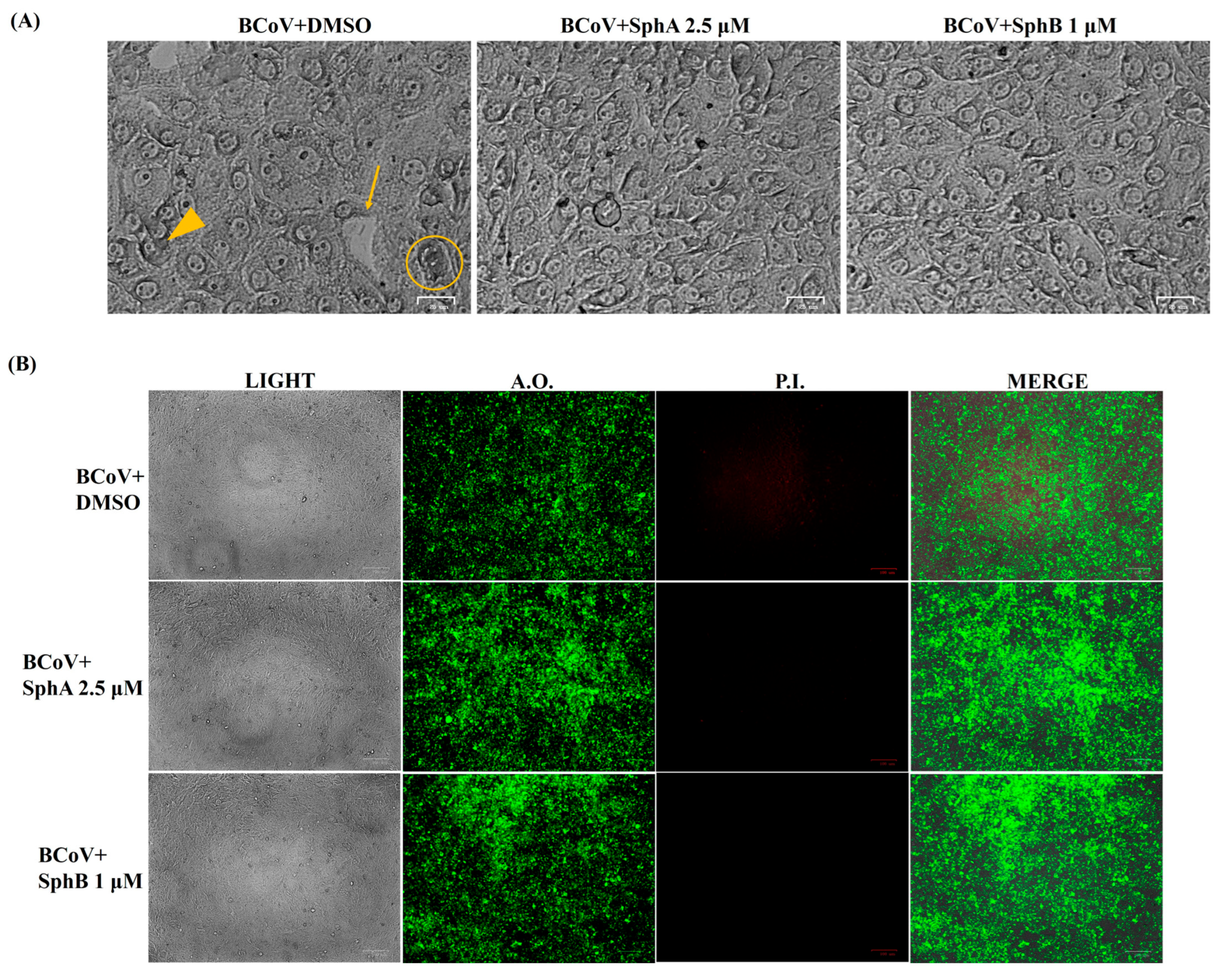
2.2. SphA and SphB Reduced Signs of the Cell Death Morphology During BCoV Infection in MDBK Cells
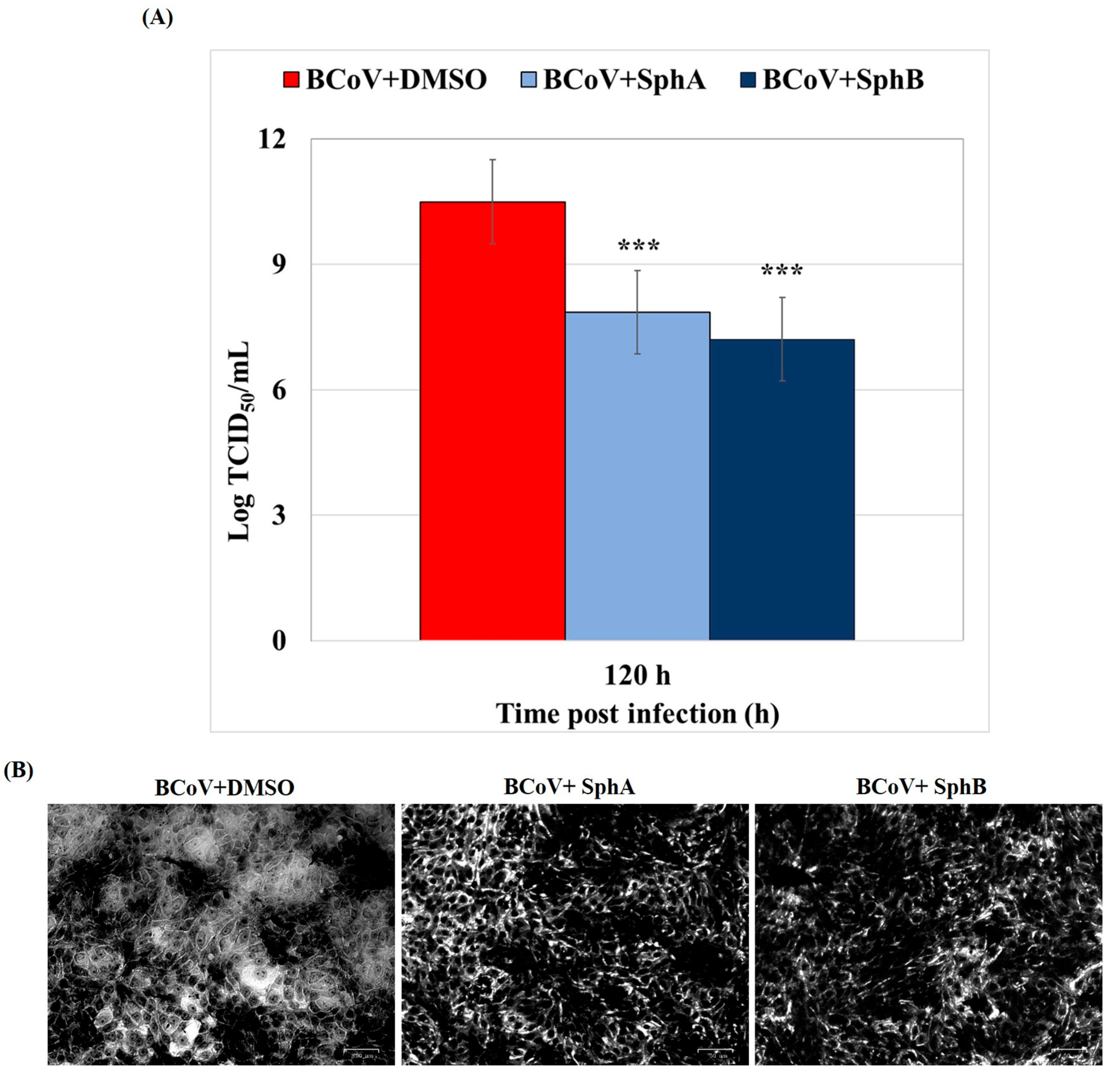
2.3. SphA and SphB Induced Reductions in the Virus Yield During BCoV Infection
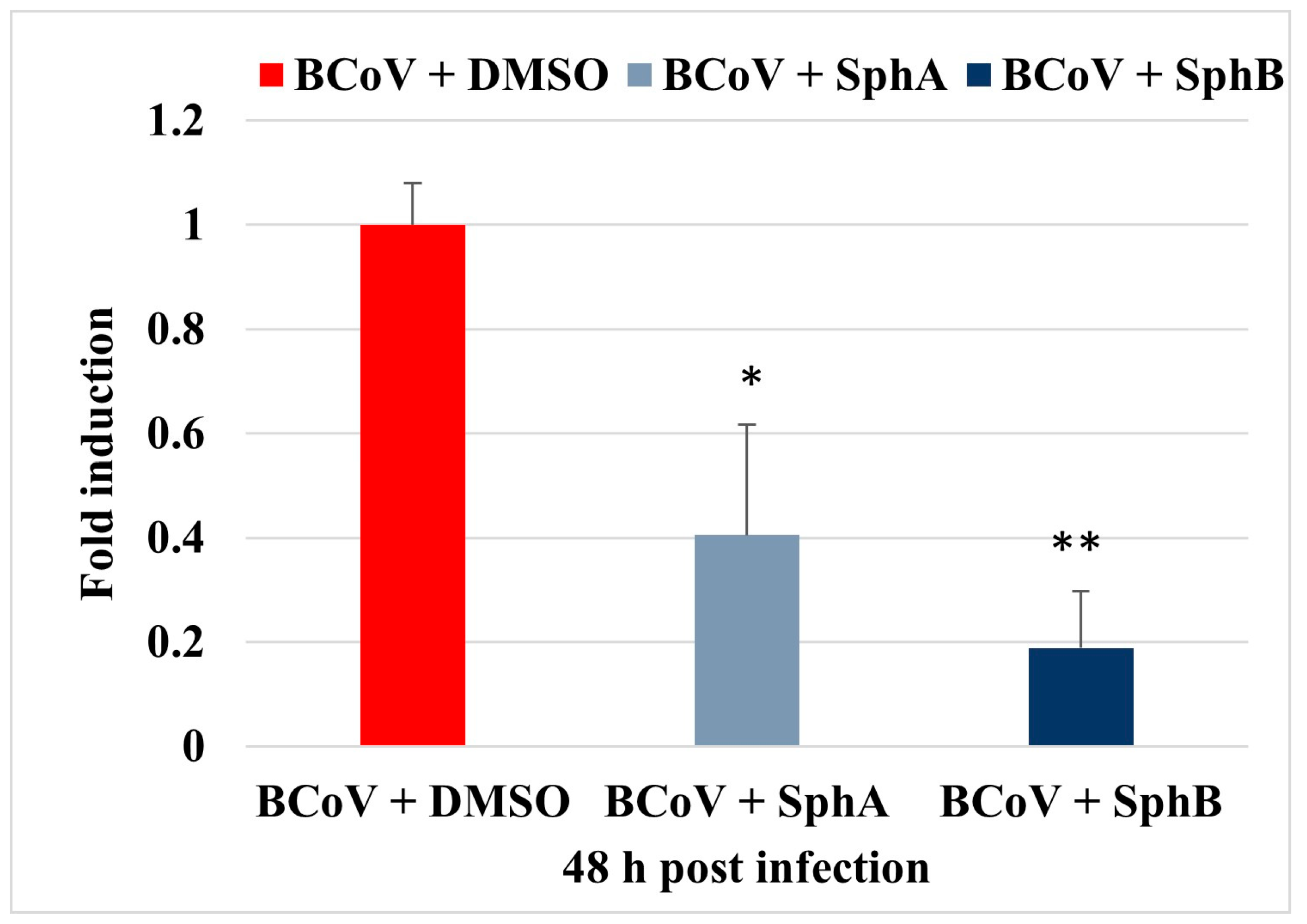
2.4. SphA and SphB Reduced NP Gene Expression During BCoV Infection in MDBK Cells
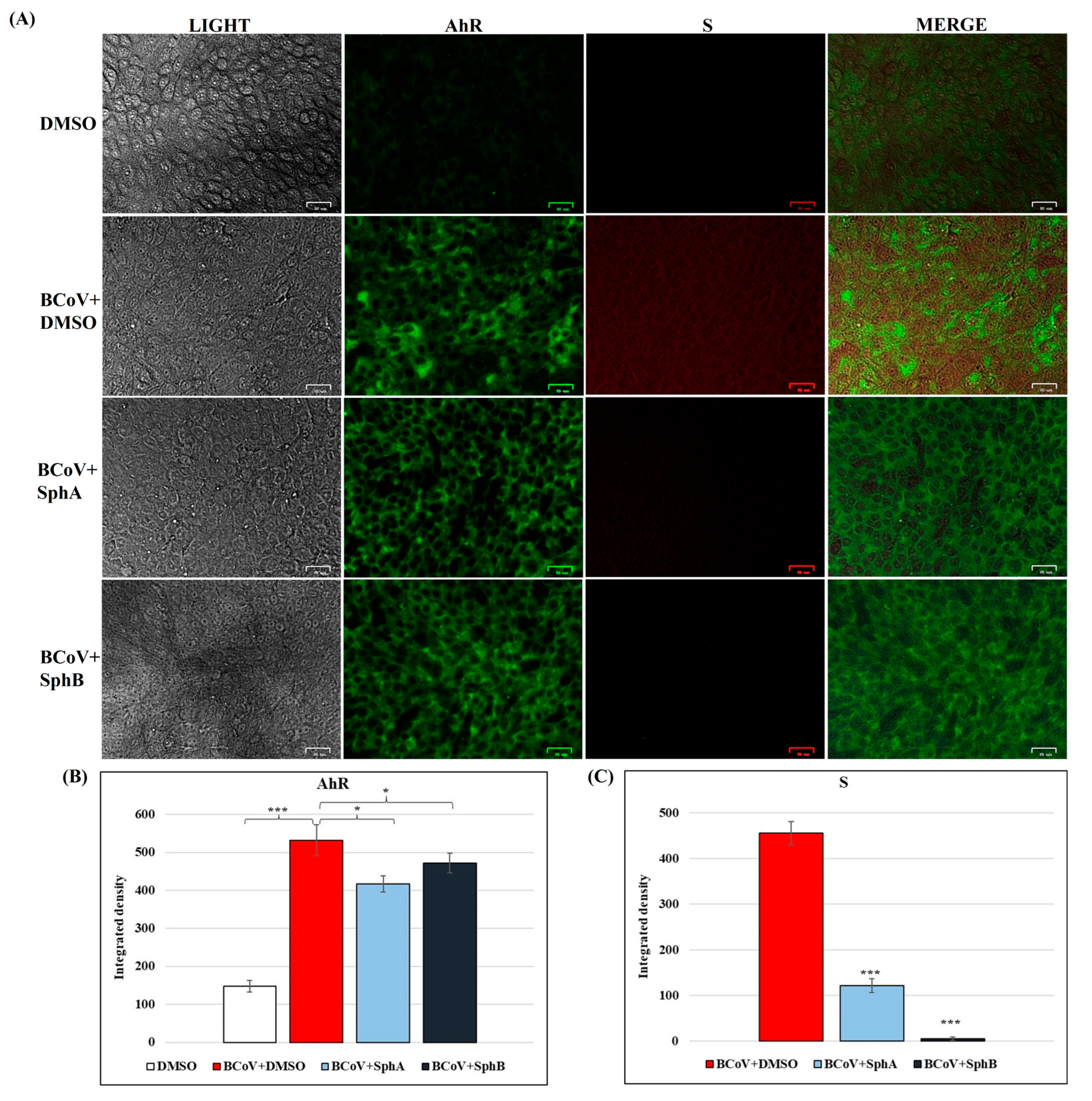
2.5. SphA and SphB Caused Reductions in Cellular AhR and Viral S Protein Expressions During BCoV Infection
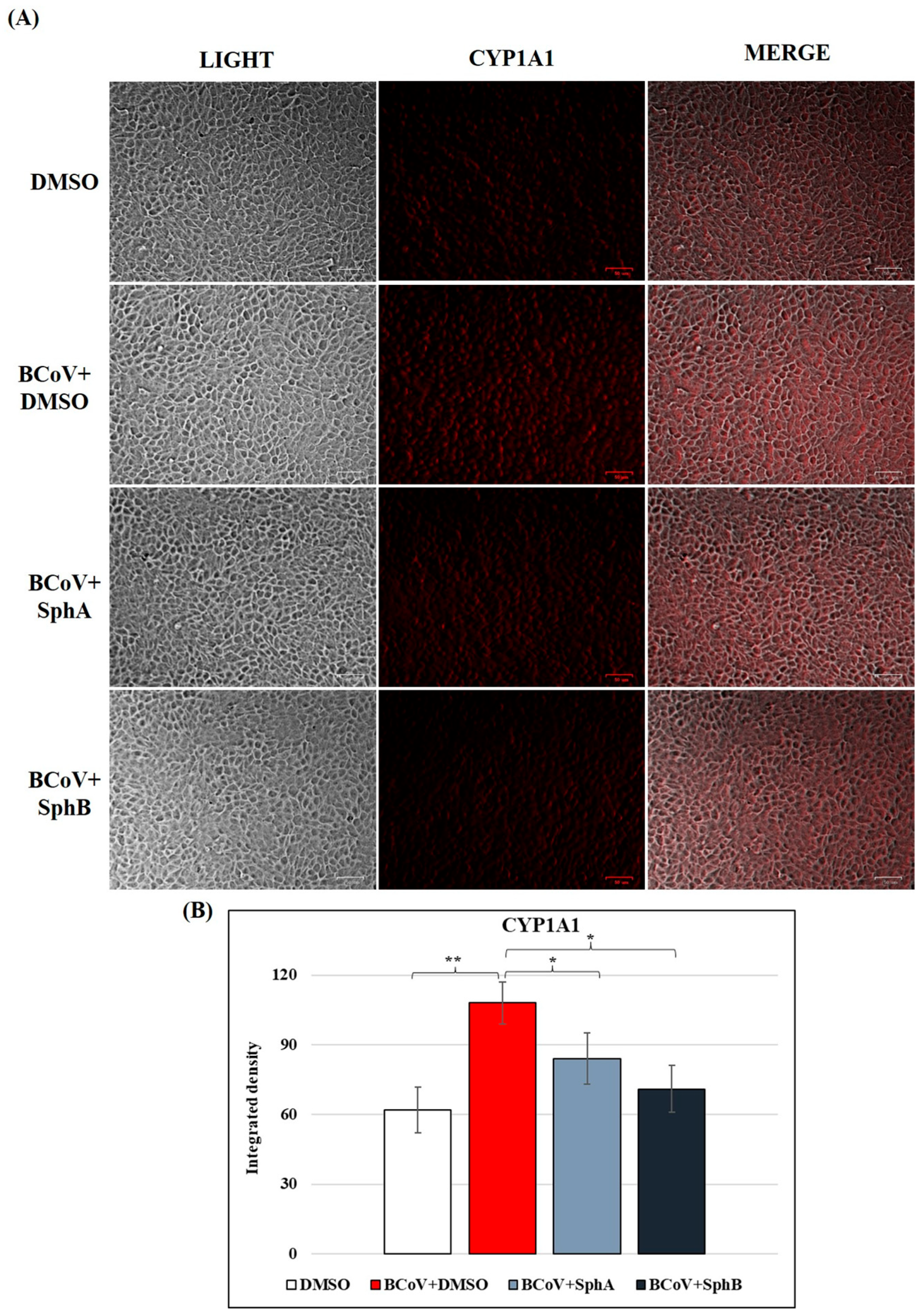
2.6. SphA and SphB Caused Reductions in CYP1A1 Protein Levels During BCoV Infection
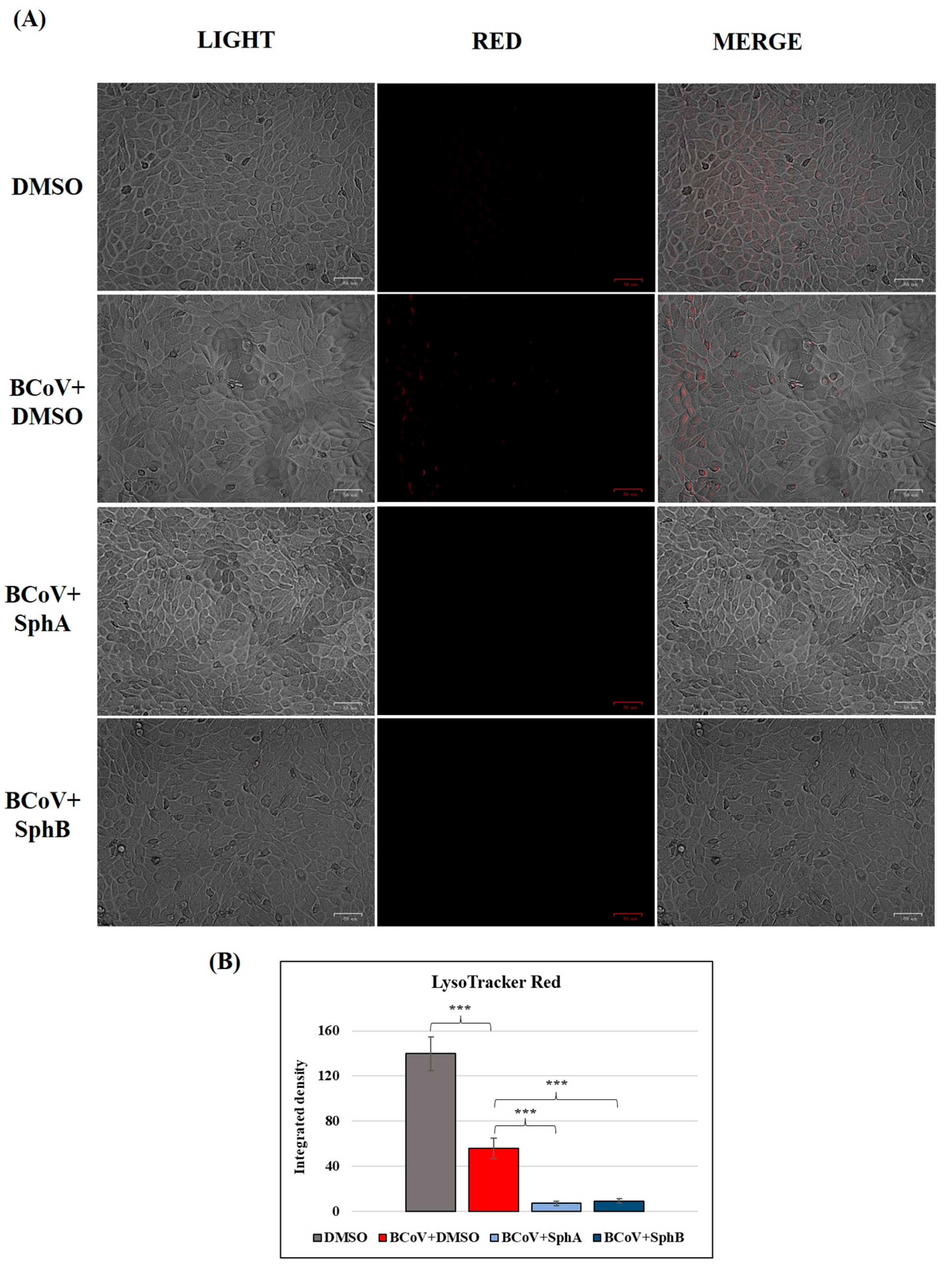
2.7. SphA and SphB Deacidified Lysosomes During BCoV Infection in MDBK Cells
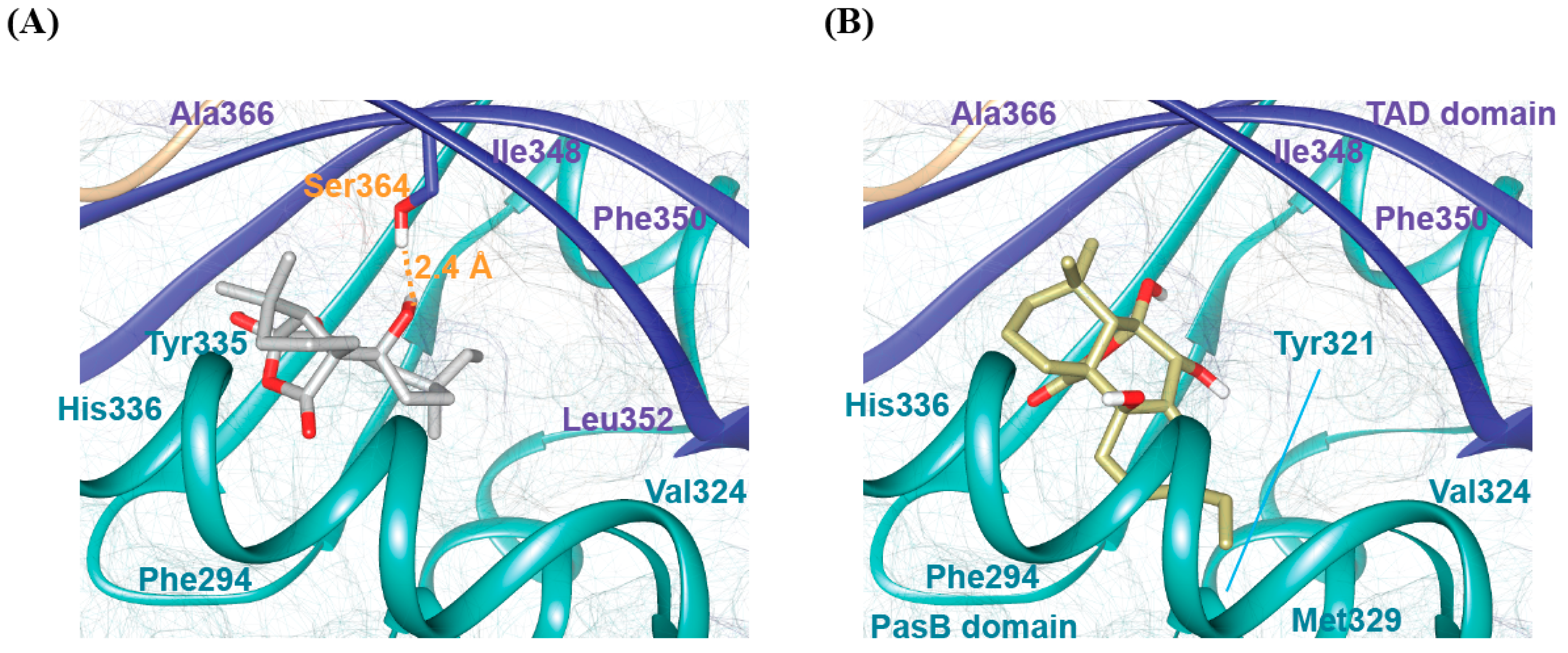
2.8. Molecular Docking Analysis of bAhR in Complexes with SphA and SphB
3. Discussion
4. Materials and Methods
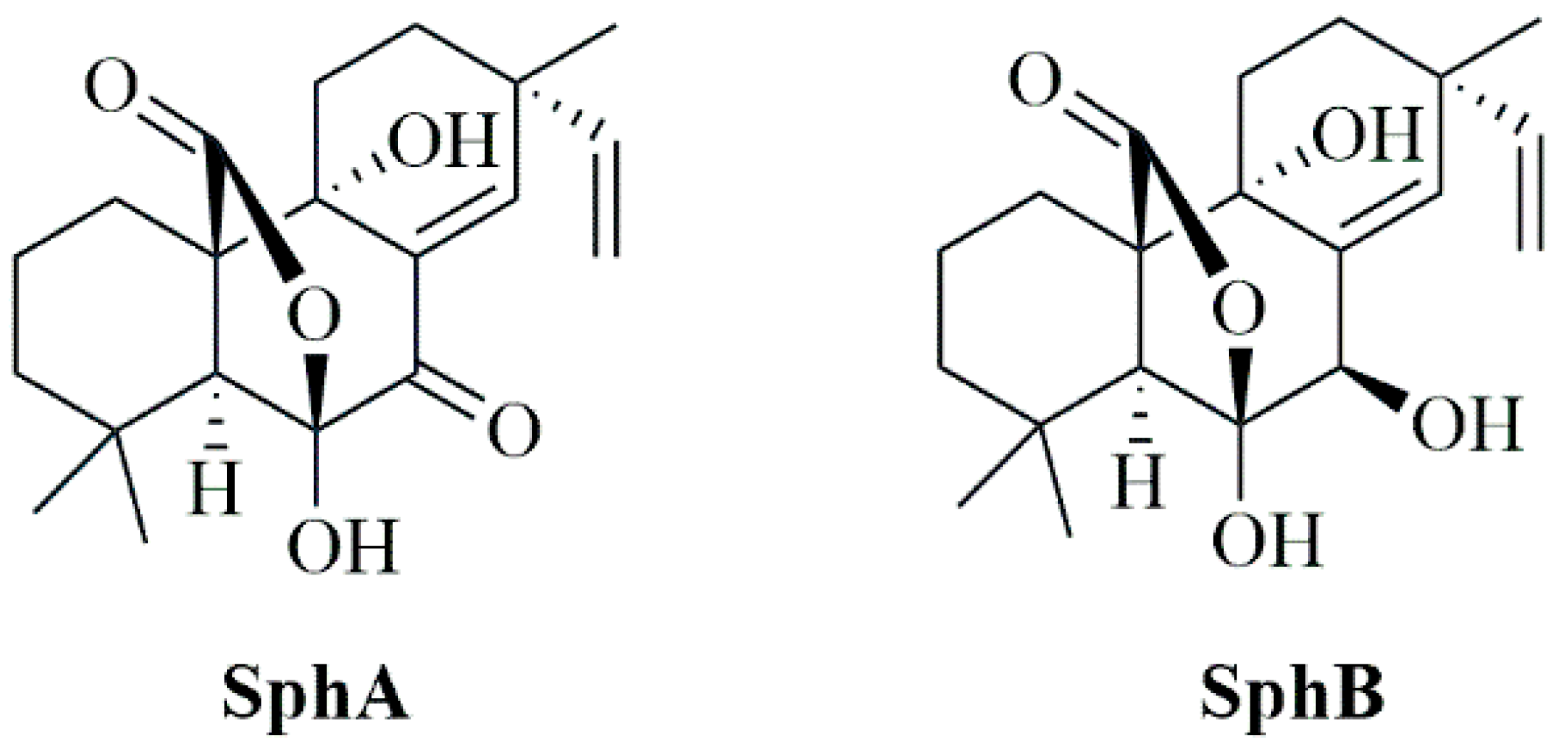
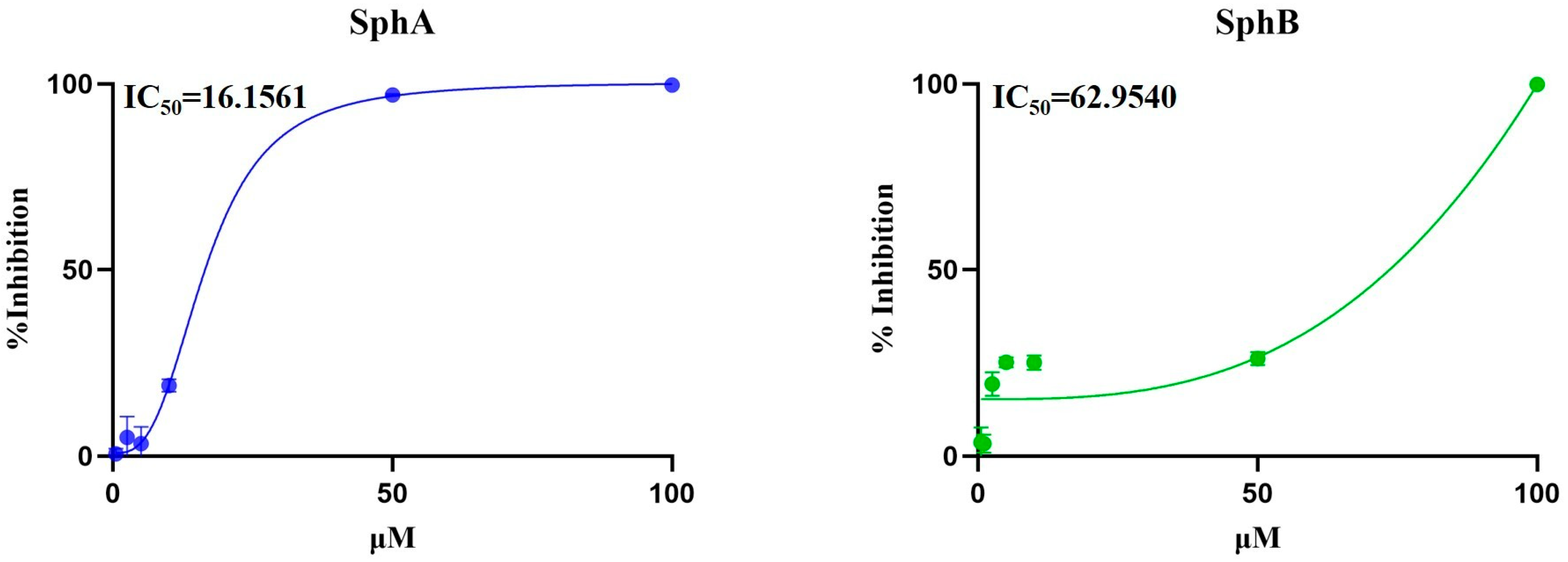
4.1. Production, Isolation, and Identification of Sphaeropsidin A and Sphaeropsidin B
4.2. Cell Cultures and Virus Infection
4.3. Cell Viability
4.4. Examination of the Cell Morphology
4.5. Immunofluorescence Staining
4.6. Virus Production
4.7. Gene Expression
4.8. LysoRed Staining
4.9. Statistical Analysis
4.10. Computational Studies of bAhR Interactions with SphA and SphB
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pusztahelyi, T.; Holb, I.J.; Pócsi, I. Secondary metabolites in fungus-plant interactions. Front. Plant Sci. 2015, 6, 573. [Google Scholar] [CrossRef] [PubMed]
- Macheleidt, J.; Mattern, D.J.; Fischer, J.; Netzker, T.; Weber, J.; Schroeckh, V.; Valiante, V.; Brakhage, A.A. Regulation and role of fungal secondary metabolites. Annu. Rev. Genet. 2016, 50, 371–392. [Google Scholar] [CrossRef] [PubMed]
- Keller, N.P. Fungal secondary metabolism: Regulation, function and drug discovery. Nat. Rev. Microbiol. 2019, 17, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Bills, G.F.; Gloer, J.B. Biologically active secondary metabolites from the fungi. Fungal Kingd. 2017, 4, 1087–1119. [Google Scholar]
- Aly, A.H.; Debbab, A.; Proksch, P. Fifty years of drug discovery from fungi. Fungal Divers. 2011, 50, 3–19. [Google Scholar] [CrossRef]
- Conrado, R.; Gomes, T.C.; Roque, G.S.C.; De Souza, A.O. Overview of bioactive fungal secondary metabolites: Cytotoxic and antimicrobial compounds. Antibiotics 2022, 11, 1604. [Google Scholar] [CrossRef] [PubMed]
- Barreca, D.; Gattuso, G.; Bellocco, E.; Calderaro, A.; Trombetta, D.; Smeriglio, A.; Laganà, G.; Daglia, M.; Meneghini, S.; Nabavi, S.M. Flavanones: Citrus phytochemical with health-promoting properties. BioFactors 2017, 43, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Galgano, M.; Capozza, P.; Pellegrini, F.; Cordisco, M.; Sposato, A.; Sblano, S.; Camero, M.; Lanave, G.; Fracchiolla, G.; Corrente, M.; et al. Antimicrobial activity of essential oils evaluated in vitro against Escherichia coli and Staphylococcus aureus. Antibiotics 2022, 11, 979. [Google Scholar] [CrossRef] [PubMed]
- Galgano, M.; Pellegrini, F.; Mrenoshki, D.; Capozza, P.; Omar, A.H.; Salvaggiulo, A.; Camero, M.; Lanave, G.; Tempesta, M.; Pratelli, A.; et al. Assessing contact time and concentration of Thymus vulgaris essential oil on antibacterial efficacy in vitro. Antibiotics 2023, 12, 1129. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, R.; Dev, K.; Sourirajan, A. Antiviral activity of bioactive phytocompounds against coronavirus: An update. J. Virol. Methods 2021, 290, 114070. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, S.K.; Agrawal, S.; Gupta, M.K.; Patidar, R.K.; Ranjan, N. Recent advances in the discovery of antiviral metabolites from fungi. Curr. Pharm. Biotechnol. 2022, 23, 495–537. [Google Scholar] [CrossRef] [PubMed]
- Lacerda, Í.C.D.S.; Polonio, J.C.; Golias, H.C. Endophytic fungi as a source of antiviral compounds—A review. Chem. Biodivers. 2022, 19, e202100971. [Google Scholar] [CrossRef] [PubMed]
- Raihan, T.; Rabbee, M.F.; Roy, P.; Choudhury, S.; Baek, K.H.; Azad, A.K. Microbial metabolites: The emerging hotspot of antiviral compounds as potential candidates to avert viral pandemic alike COVID-19. Front. Mol. Biosci. 2021, 8, 732256. [Google Scholar] [CrossRef] [PubMed]
- Carabelli, A.M.; Peacock, T.P.; Thorne, L.G.; Harvey, W.T.; Hughes, J.; de Silva, T.I.; Peacock, S.J.; Barclay, W.S.; de Silva, T.I.; Towers, G.J.; et al. SARS-CoV-2 variant biology: Immune escape, transmission and fitness. Nat. Rev. Microbiol. 2023, 21, 162–177. [Google Scholar] [CrossRef] [PubMed]
- Steiner, S.; Kratzel, A.; Barut, G.T.; Lang, R.M.; Aguiar Moreira, E.; Thomann, L.; Kelly, J.N.; Thiel, V. SARS-CoV-2 biology and host interactions. Nat. Rev. Microbiol. 2024, 22, 206–225. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.R. Intra-host variability of SARS-CoV-2: Patterns, causes and impact on COVID-19. Virology 2025, 603, 110366. [Google Scholar] [CrossRef] [PubMed]
- Fiorito, F.; Cerracchio, C.; Salvatore, M.M.; Serra, F.; Pucciarelli, A.; Amoroso, M.G.; Nicoletti, R.; Andolfi, A. Antiviral property of the fungal metabolite 3-O-methylfunicone in bovine Herpesvirus 1 infection. Microorganisms 2022, 10, 188. [Google Scholar] [CrossRef] [PubMed]
- Cerracchio, C.; Iovane, V.; Salvatore, M.M.; Amoroso, M.G.; Dakroub, H.; DellaGreca, M.; Nicoletti, R.; Andolfi, A.; Fiorito, F. Effectiveness of the fungal metabolite 3-O-methylfunicone towards canine coronavirus in a canine fibrosarcoma cell line (A72). Antibiotics 2022, 11, 1594. [Google Scholar] [CrossRef] [PubMed]
- Cerracchio, C.; Salvatore, M.M.; Del Sorbo, L.; Serra, F.; Amoroso, M.G.; Dellagreca, M.; Nicoletti, R.; Andolfi, A.; Fiorito, F. In vitro evaluation of antiviral activities of funicone-like compounds vermistatin and penisimplicissin against canine coronavirus infection. Antibiotics 2023, 12, 1319. [Google Scholar] [CrossRef] [PubMed]
- Cerracchio, C.; Del Sorbo, L.; Serra, F.; Staropoli, A.; Amoroso, M.G.; Vinale, F.; Fiorito, F. Fungal metabolite 6-pentyl-α-pyrone reduces canine coronavirus infection. Heliyon 2024, 10, e28351. [Google Scholar] [CrossRef] [PubMed]
- Rahmah, L.; Abarikwu, S.O.; Arero, A.G.; Essouma, M.; Jibril, A.T.; Fal, A.; Flisiak, R.; Makuku, R.; Marquez, L.; Mohamed, K.; et al. Oral antiviral treatments for COVID-19: Opportunities and challenges. Pharmacol. Rep. 2022, 74, 1255–1278. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yang, Q.; Ran, T.; Zhang, G.; Li, W.; Zhou, P.; Tang, J.; Dai, M.; Zhong, J.; Chen, H.; et al. Discovery of orally bioavailable SARS-CoV-2 papain-like protease inhibitor as a potential treatment for COVID-19. Nat. Commun. 2024, 15, 10169. [Google Scholar] [CrossRef] [PubMed]
- Barghash, R.F.; Gemmati, D.; Awad, A.M.; Elbakry, M.M.M.; Tisato, V.; Awad, K.; Singh, A.V. Navigating the COVID-19 therapeutic landscape: Unveiling novel perspectives on FDA-approved medications, vaccination targets, and emerging novel strategies. Molecules 2024, 29, 5564. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, A.; Ludwig, S. Host-targeted antivirals against SARS-CoV-2 in clinical development—Prospect or disappointment? Antiviral Res. 2025, 235, 106101. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Zheng, M.; Gao, Y.; Lin, S.; Zhang, X.; Chen, C.; Zhu, H.; Sun, W.; Zhang, Y. Overview of host-directed antiviral targets for future research and drug development. Acta Pharm. Sin. B 2025, 15, 1723–1751. [Google Scholar] [CrossRef] [PubMed]
- Barreira-Silva, P.; Lian, Y.; Kaufmann, S.H.E.; Moura-Alves, P. The role of the AHR in host–pathogen interactions. Nat. Rev. Immunol. 2024, 25, 178–194. [Google Scholar] [CrossRef] [PubMed]
- Grycová, A.; Vyhlídalová, B.; Dvořák, Z. The role of aryl hydrocarbon receptor in antiviral immunity: A focus on RNA viruses. Crit. Rev. Microbiol. 2025, 29, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Grunewald, M.E.; Shaban, M.G.; Mackin, S.R.; Fehr, A.R.; Perlman, S. Murine coronavirus infection activates the aryl hydrocarbon receptor in an indoleamine 2,3-dioxygenase-independent manner, contributing to cytokine modulation and proviral TCDD-inducible-PARP expression. J. Virol. 2020, 94, e01743-19. [Google Scholar] [CrossRef] [PubMed]
- Giovannoni, F.; Li, Z.; Remes-Lenicov, F.; Dávola, M.E.; Elizalde, M.; Paletta, A.; Ashkar, A.A.; Mossman, K.L.; Dugour, A.V.; Figueroa, J.M.; et al. AHR signaling is induced by infection with coronaviruses. Nat. Commun. 2021, 12, 5148. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Du, T.; Wang, J.; Tang, C.; Lei, M.; Yu, W.; Yang, Y.; Ma, Y.; Huang, P.; Chen, H.; et al. Aryl hydrocarbon receptor is a proviral host factor and a candidate pan-SARS-CoV-2 therapeutic target. Sci. Adv. 2023, 9, eadf0211. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, M.; Lee, W.S.; Chan, W.O.Y.; He, W.; Mah, M.G.; Yong, C.L.; Deerain, J.M.; Wang, L.; Arcinas, C.; Yan, B.; et al. Betacoronaviruses SARS-CoV-2 and HCoV-OC43 infections in IGROV-1 cell line require aryl hydrocarbon receptor. Emerg. Microbes Infect. 2023, 12, 2256416. [Google Scholar] [CrossRef] [PubMed]
- Cerracchio, C.; Serra, F.; Amoroso, M.G. Canine coronavirus activates aryl hydrocarbon receptor during in vitro infection. Viruses 2022, 14, 2437. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, Y.; Cui, T.; Bao, H.; Gao, M.; Cheng, M.; Sun, Y.; Lu, Y. AhR ligands from LGG metabolites promote piglet intestinal ILC3 activation activation and IL-22 secretion to inhibit PEDV infection. J. Virol. 2024, 98, e01039-24. [Google Scholar] [CrossRef] [PubMed]
- Healey, A.M.; Fenner, K.N.; O’Dell, C.T.; Lawrence, B.P. Aryl hydrocarbon receptor activation alters immune cell populations in the lung and bone marrow during coronavirus infection. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2024, 326, L313–L329. [Google Scholar] [CrossRef] [PubMed]
- Del Sorbo, L.; Giugliano, R.; Cerracchio, C.; Iovane, V.; Salvatore, M.M.; Serra, F.; Amoroso, M.G.; Pellegrini, F.; Levante, M.; Capozza, P.; et al. In Vitro evaluation of aryl hydrocarbon receptor involvement in feline coronavirus infection. Viruses 2025, 17, 227. [Google Scholar] [CrossRef] [PubMed]
- Del Sorbo, L.; Acconcia, C.; Salvatore, M.M.; Fusco, G.; Vasinioti, V.; Lucente, M.S.; Zhu, L.; Pratelli, A.; Russo, L.; Andolfi, A.; et al. Insight into the role of the aryl hydrocarbon receptor in bovine coronavirus infection by an integrated approach combining in vitro and in silico methods. Microorganisms 2025, 13, 579. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, M.M.; Masi, M.; Andolfi, A. The current status on secondary metabolites produced by fungi of the genus Diplodia associated with plants. Phytochem. Rev. 2024, 24, 1565–1589. [Google Scholar] [CrossRef]
- Masi, M.; Evidente, A. Sphaeropsidin A: A pimarane diterpene with interesting biological activities and promising practical applications. ChemBioChem 2021, 22, 3263–3269. [Google Scholar] [CrossRef] [PubMed]
- Roscetto, E.; Masi, M.; Esposito, M.; Di Lecce, R.; Delicato, A.; Maddau, L.; Calabro, V.; Evidente, A.; Catania, M.R. Anti-biofilm activity of the fungal phytotoxin sphaeropsidin A against clinical isolates of antibiotic-resistant bacteria. Toxins 2020, 12, 444. [Google Scholar] [CrossRef] [PubMed]
- Buonanno, A.; Salvatore, M.M.; Feola, A.; Siciliano, A.; Bellavita, R.; Imbò, L.E.; Guida, M.; Andolfi, A.; Nicoletti, R.; Maione, A.; et al. Sphaeropsidin A loaded in liposomes to reduce its cytotoxicity and preserve antifungal activity against Candida auris. Molecules 2024, 29, 5949. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, B.D.A.P.; Medeiros, J.; Rubens, M.; Lobato, C. Globally approved vaccines for COVID-19: A systematic review. Braz. J. Microbiol. 2025, 56, 511–527. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Chen, J.; Liu, Q.; Wu, Q.; Chen, S.; Wang, J.; Li, J.; Liu, L.; Gao, Z. Cyclohexenone derivative and drimane sesquiterpenes from the seagrass-derived fungus Aspergillus insuetus. Chem. Biodivers. 2023, 20, e202300424. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Dellibovi-Ragheb, T.A.; Kerviel, A.; Pak, E.; Qiu, Q.; Fisher, M.; Takvorian, P.M.; Bleck, C.; Hsu, V.W.; Fehr, A.R.; et al. β-Coronaviruses use lysosomes for egress instead of the biosynthetic secretory pathway. Cell 2020, 183, 1520–1535. [Google Scholar] [CrossRef] [PubMed]
- Contreras, P.S.; Tapia, P.J.; Jeong, E.; Ghosh, S.; Altan-Bonnet, N.; Puertollano, R. Beta-coronaviruses exploit cellular stress responses by modulating TFEB and TFE3 activity. iScience 2023, 26, 106169. [Google Scholar] [CrossRef] [PubMed]
- Blaess, M.; Kaiser, L.; Sommerfeld, O.; Csuk, R.; Deigner, H.P. Drugs, metabolites, and lung accumulating small lysosomotropic molecules: Multiple targeting impedes SARS-CoV-2 infection and progress to COVID-19. Int. J. Mol. Sci. 2021, 22, 1797. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Qin, P.; Huang, Y.W. Lysosomal ion channels involved in cellular entry and uncoating of enveloped viruses: Implications for therapeutic strategies against SARS-CoV-2. Cell Calcium 2021, 94, 102360. [Google Scholar] [CrossRef] [PubMed]
- Carrière, F.; Longhi, S.; Record, M. The endosomal lipid bis(monoacylglycero) phosphate as a potential key player in the mechanism of action of chloroquine against SARS-CoV-2 and other enveloped viruses hijacking the endocytic pathway. Biochimie 2020, 179, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Gorshkov, K.; Chen, C.Z.; Bostwick, R.; Rasmussen, L.; Tran, B.N.; Cheng, Y.S.; Xu, M.; Pradhan, M.; Henderson, M.; Zhu, W.; et al. The SARS-CoV-2 cytopathic effect is blocked by lysosome alkalizing small molecules. ACS Infect. Dis. 2021, 7, 1389–1408. [Google Scholar] [CrossRef] [PubMed]
- Payne, H.R.; Storz, J.; Henk, W.G. Initial events in bovine coronavirus infection: Analysis through immunogold probes and lysosomotropic inhibitors. Arch. Virol. 1990, 114, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.A.; Kar, M.; Panwar, A.; Wangchuk, J.; Kumar, S.; Das, A.; Pandey, A.K.; Lodha, R.; Medigeshi, G.R. Oxidative stress specifically inhibits replication of dengue virus. J. Gen. Virol. 2021, 102, 001596. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, M.M.; Russo, M.T.; Nicoletti, R.; Mahamedi, A.E.; Berraf-tebbal, A.; Cimmino, A.; Masi, M.; Andolfi, A. Comparative analysis of secondary metabolites in Diplodia corticola strains with different virulence degrees associated with canker and dieback of Quercus spp. Molecules 2023, 28, 6302. [Google Scholar] [CrossRef] [PubMed]
- Ramón, A.C.; Pérez, G.V.; Caballero, E.; Rosales, M.; Aguilar, D.; Vázquez-Blomquist, D.; Ramos, Y.; Rodríguez-Ulloa, A.; Falcón, V.; Rodríguez-Moltó, M.P.; et al. Targeting of protein kinase CK2 elicits antiviral activity on bovine coronavirus infection. Viruses 2022, 14, 552. [Google Scholar] [CrossRef] [PubMed]
- Strober, W. Tryptan blue exclusion of cell viability. Curr. Protoc. Immunol. 2017, 176, 139–148. [Google Scholar]
- Bank, H.L. Assessment of islet cell viability using fluorescent dyes. Diabetologia 1987, 30, 812–816. [Google Scholar] [CrossRef] [PubMed]
- Fiorito, F.; Marfè, G.; Granato, G.E.; Ciarcia, R.; De Blasio, E.; Tafani, M.; Florio, S.; De Martino, L.; Muzi, G.; Pagnini, U.; et al. 2,3,7,8-Tetrachlorodibenzo-p-dioxin modifies expression and nuclear/cytosolic localization of bovine herpesvirus 1 immediate-early protein (bICP0) during infection. J. Cell. Biochem. 2010, 111, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Leite, M.; Quinta-Costa, M.; Leite, P.S.; Guimarães, J.E. Critical evaluation of techniques to detect and measure cell death—Study in a model of UV radiation of the leukaemic cell line HL60. Anal. Cell. Pathol. 1999, 19, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Levine, B. Autophagic cell death: The story of a misnomer. Nat. Rev. Mol. Cell Biol. 2008, 9, 1004–1010. [Google Scholar] [CrossRef] [PubMed]
- Zakeri, Z.; Lockshin, R.A. Cell death: History and future. Adv. Exp. Med. Biol. 2008, 615, 1–11. [Google Scholar] [PubMed]
- Altamura, G.; Power, K.; Martano, M.; degli Uberti, B.; Galiero, G.; De Luca, G.; Maiolino, P.; Borzacchiello, G. Felis catus papillomavirus type-2 E6 binds to E6AP, promotes E6AP/p53 binding and enhances p53 proteasomal degradation. Sci. Rep. 2018, 8, 17529. [Google Scholar] [CrossRef] [PubMed]
- Fiorito, F.; Irace, C.; Nocera, F.P.; Piccolo, M.; Ferraro, M.G.; Ciampaglia, R.; Tenore, G.C.; Santamaria, R.; De Martino, L. MG-132 interferes with iron cellular homeostasis and alters virulence of bovine herpesvirus 1. Res. Vet. Sci. 2021, 137, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Brandolini, M.; Taddei, F.; Marino, M.M.; Grumiro, L.; Scalcione, A.; Turba, M.E.; Gentilini, F.; Fantini, M.; Zannoli, S.; Dirani, G.; et al. Correlating qrt-pcr, dpcr and viral titration for the identification and quantification of SARS-CoV-2: A new approach for infection management. Viruses 2021, 13, 1022. [Google Scholar] [CrossRef] [PubMed]
- Reed, L.J.; Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Hyg. 1938, 27, 493–497. [Google Scholar]
- Pratelli, A.; Cirone, F.; Capozza, P.; Trotta, A.; Corrente, M.; Balestrieri, A.; Buonavoglia, C. Bovine respiratory disease in beef calves supported long transport stress: An epidemiological study and strategies for control and prevention. Res. Vet. Sci. 2021, 135, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Alkharashi, N.A.O.; Periasamy, V.S.; Athinarayanan, J.; Alshatwi, A.A. Sulforaphane alleviates cadmium-induced toxicity in human mesenchymal stem cells through POR and TNFSF10 genes expression. Biomed. Pharmacother. 2019, 115, 108896. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, R.A.; Rullmann, J.A.C.; MacArthur, M.W.; Kaptein, R.; Thornton, J.M. AQUA and PROCHECK-NMR: Programs for checking the quality of protein structures solved by NMR. J. Biomol. NMR 1996, 8, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.C.; Rodrigues, J.P.; Kastritis, P.L.; Bonvin, A.M.; Vangone, A. PRODIGY: A web server for predicting the binding affinity of protein-protein complexes. Bioinformatics 2016, 32, 3676–3678. [Google Scholar] [CrossRef] [PubMed]
- Vangone, A.; Bonvin, A.M.J.J. Contacts-based prediction of binding affinity in protein–protein complexes. eLife 2015, 4, e07454. [Google Scholar] [CrossRef] [PubMed]
- DeLano, W.L. The PyMOL Molecular Graphics System. DeLano Scientific: San Carlos, CA, USA, 2002. [Google Scholar]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Sorbo, L.; Salvatore, M.M.; Acconcia, C.; Giugliano, R.; Fusco, G.; Galdiero, M.; Vasinioti, V.I.; Lucente, M.S.; Capozza, P.; Pratelli, A.; et al. Structural Features and In Vitro Antiviral Activities of Fungal Metabolites Sphaeropsidins A and B Against Bovine Coronavirus. Int. J. Mol. Sci. 2025, 26, 7045. https://doi.org/10.3390/ijms26157045
Del Sorbo L, Salvatore MM, Acconcia C, Giugliano R, Fusco G, Galdiero M, Vasinioti VI, Lucente MS, Capozza P, Pratelli A, et al. Structural Features and In Vitro Antiviral Activities of Fungal Metabolites Sphaeropsidins A and B Against Bovine Coronavirus. International Journal of Molecular Sciences. 2025; 26(15):7045. https://doi.org/10.3390/ijms26157045
Chicago/Turabian StyleDel Sorbo, Luca, Maria Michela Salvatore, Clementina Acconcia, Rosa Giugliano, Giovanna Fusco, Massimiliano Galdiero, Violetta Iris Vasinioti, Maria Stella Lucente, Paolo Capozza, Annamaria Pratelli, and et al. 2025. "Structural Features and In Vitro Antiviral Activities of Fungal Metabolites Sphaeropsidins A and B Against Bovine Coronavirus" International Journal of Molecular Sciences 26, no. 15: 7045. https://doi.org/10.3390/ijms26157045
APA StyleDel Sorbo, L., Salvatore, M. M., Acconcia, C., Giugliano, R., Fusco, G., Galdiero, M., Vasinioti, V. I., Lucente, M. S., Capozza, P., Pratelli, A., Russo, L., Iacovino, R., Andolfi, A., & Fiorito, F. (2025). Structural Features and In Vitro Antiviral Activities of Fungal Metabolites Sphaeropsidins A and B Against Bovine Coronavirus. International Journal of Molecular Sciences, 26(15), 7045. https://doi.org/10.3390/ijms26157045













