Salivary Proteome Profile of Xerostomic Patients Reveals Pathway Dysregulation Related to Neurodegenerative Diseases: A Pilot Study
Abstract
1. Introduction
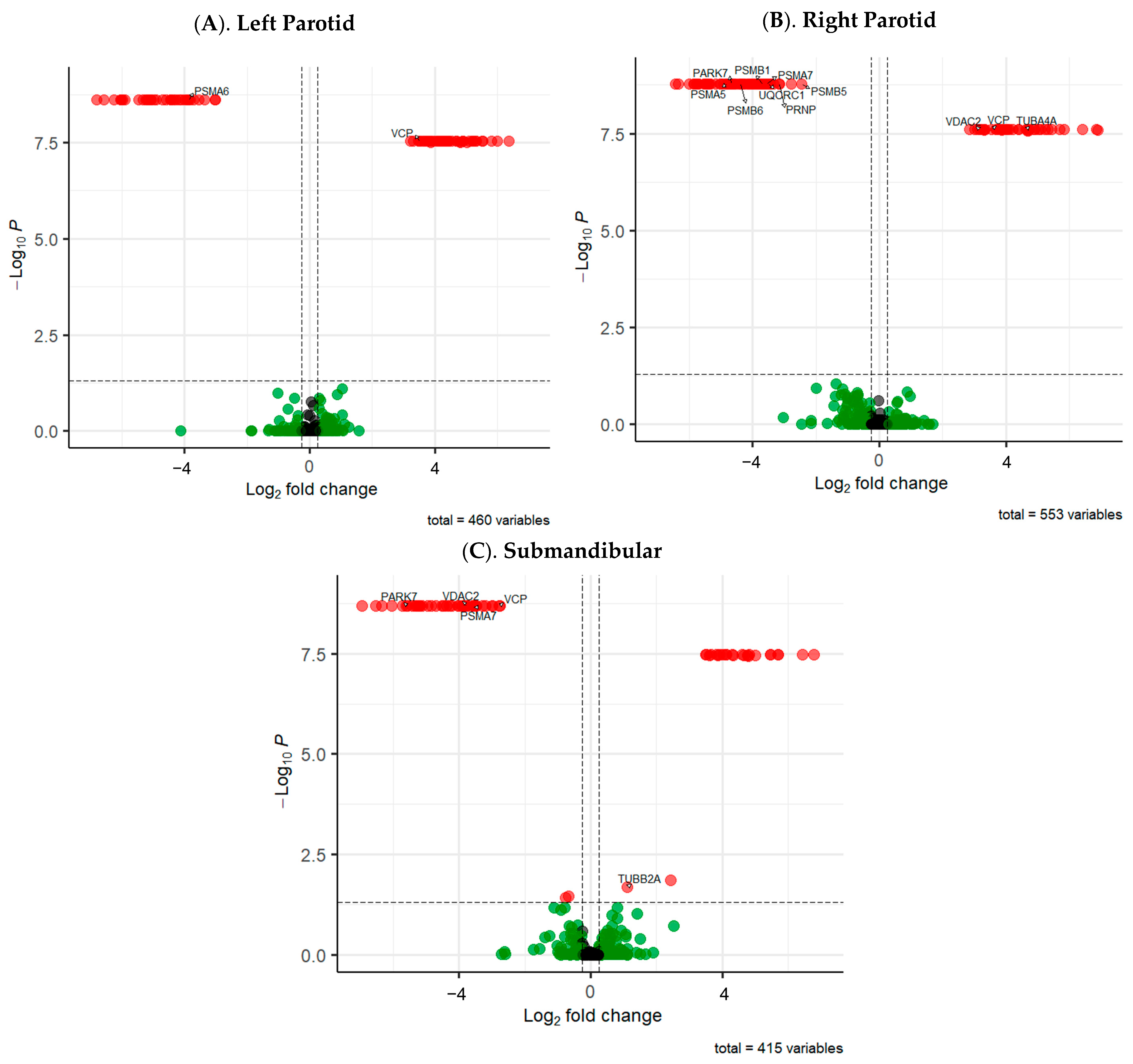
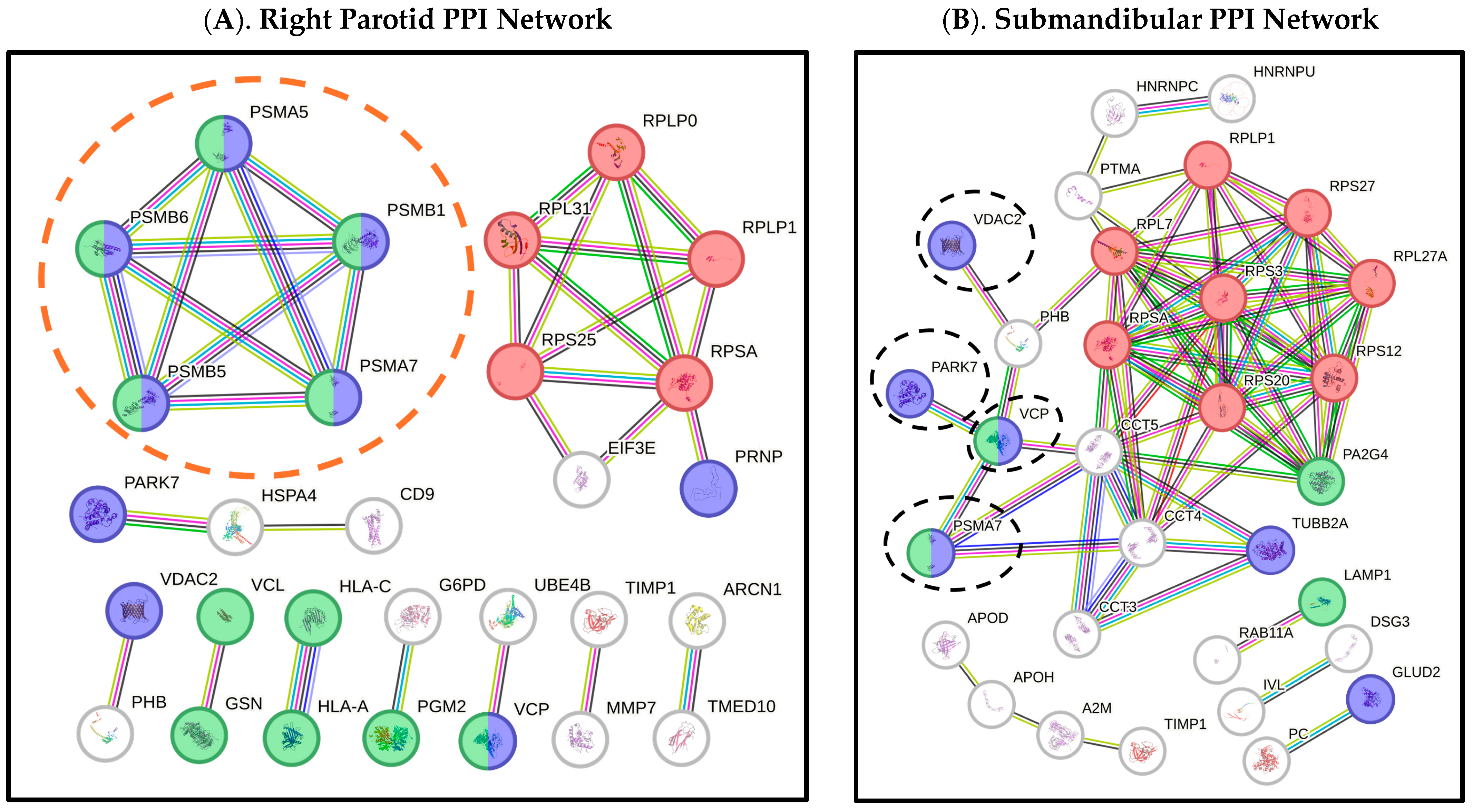
2. Results
3. Discussion
4. Methods
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jacob, L.E.; Krishnan, M.; Mathew, A.; Mathew, A.L.; Baby, T.K.; Krishnan, A. Xerostomia—A Comprehensive Review with a Focus on Mid-Life Health. J. Mid-Life Health 2022, 13, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.S.; Bhayat, A.; Zafar, M.S.; Al-Samadani, K.H. The Impact of Hyposalivation on Quality of Life (QoL) and Oral Health in the Aging Population of Al Madinah Al Munawarrah. Int. J. Environ. Res. Public Health 2017, 14, 445. [Google Scholar] [CrossRef] [PubMed]
- Ship, J.A.; Pillemer, S.R.; Baum, B.J. Xerostomia and the geriatric patient. J. Am. Geriatr. Soc. 2002, 50, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.D.; Ship, J.A. Dry mouth and its effects on the oral health of elderly people. J. Am. Dent. Assoc. 2007, 138 (Suppl. S1), 15S–20S. [Google Scholar] [CrossRef] [PubMed]
- Elting, L.S.; Chang, Y.-C. Costs of Oral Complications of Cancer Therapies: Estimates and a Blueprint for Future Study. JNCI Monogr. 2019, 2019, lgz010. [Google Scholar] [CrossRef] [PubMed]
- Leimola-Virtanen, R.; Salo, T.; Toikkanen, S.; Pulkkinen, J.; Syrjänen, S. Expression of estrogen receptor (ER) in oral mucosa and salivary glands. Maturitas 2000, 36, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Mathews, S.A.; Kurien, B.T.; Scofield, R.H. Oral manifestations of Sjögren’s syndrome. J. Dent. Res. 2008, 87, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Kapourani, A.; Kontogiannopoulos, K.N.; Manioudaki, A.E.; Poulopoulos, A.K.; Tsalikis, L.; Assimopoulou, A.N.; Barmpalexis, P. A Review on Xerostomia and Its Various Management Strategies: The Role of Advanced Polymeric Materials in the Treatment Approaches. Polymers 2022, 14, 850. [Google Scholar] [CrossRef] [PubMed]
- Eliasson, L.; Carlén, A.; Laine, M.; Birkhed, D. Minor gland and whole saliva in postmenopausal women using a low potency oestrogen (oestriol). Arch. Oral Biol. 2003, 48, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Momm, F.; Volegova-Neher, N.J.; Schulte-Mönting, J.; Guttenberger, R. Different saliva substitutes for treatment of xerostomia following radiotherapy. A prospective crossover study. Strahlenther. Und Onkol. 2005, 181, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.S.; Akula, R.; Satyanarayana, T.S.V.; Indugu, V. Recent Advances of Pacemakers in Treatment of Xerostomia: A Systematic Review. J. Int. Soc. Prev. Community Dent. 2019, 9, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Khosravani, N.; Birkhed, D.; Ekström, J. The cholinesterase inhibitor physostigmine for the local treatment of dry mouth: A randomized study. Eur. J. Oral Sci. 2009, 117, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Moreno, G.; Guardia, J.; Aguilar-Salvatierra, A.; Cabrera-Ayala, M.; Maté-Sánchez de-Val, J.-E.; Calvo-Guirado, J.-L. Effectiveness of malic acid 1% in patients with xerostomia induced by antihypertensive drugs. Med. Oral Patol. Oral Y Cir. Bucal 2013, 18, e49–e55. [Google Scholar] [CrossRef] [PubMed]
- Hamada, T.; Nakane, T.; Kimura, T.; Arisawa, K.; Yoneda, K.; Yamamoto, T.; Osaki, T. Treatment of Xerostomia with the Bile Secretion-Stimulating Drug Anethole Trithione: A Clinical Trial. Am. J. Med. Sci. 1999, 318, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Sardellitti, L.; Bortone, A.; Filigheddu, E.; Serralutzu, F.; Milia, E.P. Xerostomia: From Pharmacological Treatments to Traditional Medicine-An Overview on the Possible Clinical Management and Prevention Using Systemic Approaches. Curr. Oncol. 2023, 30, 4412–4426. [Google Scholar] [CrossRef] [PubMed]
- Ryo, K.; Ito, A.; Takatori, R.; Tai, Y.; Arikawa, K.; Seido, T.; Yamada, T.; Shinpo, K.; Tamaki, Y.; Fujii, K.; et al. Effects of coenzyme Q10 on salivary secretion. Clin. Biochem. 2011, 44, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Alevizos, I.; Zheng, C.; Cotrim, A.P.; Liu, S.; McCullagh, L.; Billings, M.E.; Goldsmith, C.M.; Tandon, M.; Helmerhorst, E.J.; Catalán, M.A.; et al. Late responses to adenoviral-mediated transfer of the aquaporin-1 gene for radiation-induced salivary hypofunction. Gene Ther. 2017, 24, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Lai, Z.; Yin, H.; Cabrera-Pérez, J.; Guimaro, M.C.; Afione, S.; Michael, D.G.; Glenton, P.; Patel, A.; Swaim, W.D.; Zheng, C.; et al. Aquaporin gene therapy corrects Sjögren’s syndrome phenotype in mice. Proc. Natl. Acad. Sci. USA 2016, 113, 5694–5699. [Google Scholar] [CrossRef] [PubMed]
- Katsani, K.R.; Sakellari, D. Saliva proteomics updates in biomedicine. J. Biol. Res. Thessalon. 2019, 26, 17. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, W.L.; Dawes, C. The salivary proteome: Challenges and perspectives. Proteomics Clin. Appl. 2011, 5, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Loo, J.A.; Yan, W.; Ramachandran, P.; Wong, D.T. Comparative human salivary and plasma proteomes. J. Dent. Res. 2010, 89, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Jasim, H.; Olausson, P.; Hedenberg-Magnusson, B.; Ernberg, M.; Ghafouri, B. The proteomic profile of whole and glandular saliva in healthy pain-free subjects. Sci. Rep. 2016, 6, 39073. [Google Scholar] [CrossRef] [PubMed]
- Saitou, M.; Gaylord, E.A.; Xu, E.; May, A.J.; Neznanova, L.; Nathan, S.; Grawe, A.; Chang, J.; Ryan, W.; Ruhl, S.; et al. Functional Specialization of Human Salivary Glands and Origins of Proteins Intrinsic to Human Saliva. Cell Rep. 2020, 33, 108402. [Google Scholar] [CrossRef] [PubMed]
- Davidson, K.; Pickering, A.M. The proteasome: A key modulator of nervous system function, brain aging, and neurodegenerative disease. Front. Cell Dev. Biol. 2023, 11, 1124907. [Google Scholar] [CrossRef] [PubMed]
- Moscovitz, O.; Ben-Nissan, G.; Fainer, I.; Pollack, D.; Mizrachi, L.; Sharon, M. The Parkinson’s-associated protein DJ-1 regulates the 20S proteasome. Nat. Commun. 2015, 6, 6609. [Google Scholar] [CrossRef] [PubMed]
- Coukos, J.S.; Lee, C.W.; Pillai, K.S.; Shah, H.; Moellering, R.E. PARK7 Catalyzes Stereospecific Detoxification of Methylglyoxal Consistent with Glyoxalase and Not Deglycase Function. Biochemistry 2023, 62, 3126–3133. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.K.; Park, S.B.; Yu, H.Y.; Kim, J. Improvement effect of gemigliptin on salivary gland dysfunction in exogenous methylglyoxal-injected rats. Heliyon 2024, 10, e29362. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Jousilahti, P.; Bidel, S.; Antikainen, R.; Tuomilehto, J. Type 2 diabetes and the risk of Parkinson’s disease. Diabetes Care 2007, 30, 842–847. [Google Scholar] [CrossRef] [PubMed]
- López-Pintor, R.M.; Casañas, E.; González-Serrano, J.; Serrano, J.; Ramírez, L.; de Arriba, L.; Hernández, G. Xerostomia, Hyposalivation, and Salivary Flow in Diabetes Patients. J. Diabetes Res. 2016, 2016, 4372852. [Google Scholar] [CrossRef] [PubMed]
- Kawedia, J.D.; Yang, F.; Sartor, M.A.; Gozal, D.; Czyzyk-Krzeska, M.; Menon, A.G. Hypoxia and hypoxia mimetics decrease aquaporin 5 (AQP5) expression through both hypoxia inducible factor-1α and proteasome-mediated pathways. PLoS ONE 2013, 8, e57541. [Google Scholar] [CrossRef] [PubMed]
- Bae, T.; Hallis, S.P.; Kwak, M.K. Hypoxia, oxidative stress, and the interplay of HIFs and NRF2 signaling in cancer. Exp. Mol. Med. 2024, 56, 501–514. [Google Scholar] [CrossRef] [PubMed]
- Ben-Nissan, G.; Katzir, N.; Füzesi-Levi, M.G.; Sharon, M. Biology of the Extracellular Proteasome. Biomolecules 2022, 12, 619. [Google Scholar] [CrossRef] [PubMed]
- Forbes, Z.; Zeldin, R.K.; Brennan, M. Gene Therapy for the Treatment of Radiation-Induced Xerostomia: AAV2-hAQP1 Program Update [PowerPoint Slides]; MeiraGTx: New York, NY, USA, 2022. [Google Scholar]
- Plaitakis, A.; Latsoudis, H.; Kanavouras, K.; Ritz, B.; Bronstein, J.M.; Skoula, I.; Mastorodemos, V.; Papapetropoulos, S.; Borompokas, N.; Zaganas, I.; et al. Gain-of-function variant in GLUD2 glutamate dehydrogenase modifies Parkinson’s disease onset. Eur. J. Hum. Genet. EJHG 2010, 18, 336–341. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, W.; Yang, T.; Thomas, E.R.; Dai, R.; Li, X. The Potential Role of Voltage-Dependent Anion Channel in the Treatment of Parkinson’s Disease. Oxidative Med. Cell. Longev. 2022, 2022, 4665530. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Tsai, P.I.; Lin, H.Y.; Hattori, N.; Funayama, M.; Jeon, B.; Sato, K.; Abe, K.; Mukai, Y.; Takahashi, Y.; et al. Mitochondrial UQCRC1 mutations cause autosomal dominant parkinsonism with polyneuropathy. Brain J. Neurol. 2020, 143, 3352–3373. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, B.L.; Sun, F.R.; Li, J.Q.; Cao, X.P.; Tan, L. The role of UNC5C in Alzheimer’s disease. Ann. Transl. Med. 2018, 6, 178. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Ahn, E.H.; Kang, S.S.; Xia, Y.; Liu, X.; Zhang, Z.; Ye, K. UNC5C Receptor Proteolytic Cleavage by Active AEP Promotes Dopaminergic Neuronal Degeneration in Parkinson’s Disease. Adv. Sci. 2022, 9, e2103396. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Kang, S.S.; Liu, X.; Ahn, E.H.; Zhang, Z.; He, L.; Iuvone, P.M.; Duong, D.M.; Seyfried, N.T.; Benskey, M.J.; et al. Asparagine endopeptidase cleaves α-synuclein and mediates pathologic activities in Parkinson’s disease. Nat. Struct. Mol. Biol. 2017, 24, 632–642. [Google Scholar] [CrossRef] [PubMed]
- Cyske, Z.; Gaffke, L.; Pierzynowska, K.; Węgrzyn, G. Tubulin Cytoskeleton in Neurodegenerative Diseases-not Only Primary Tubulinopathies. Cell. Mol. Neurobiol. 2023, 43, 1867–1884. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.H.; Hou, N.N.; Zhang, D.L.; Liu, D.N.; Tang, R.T.; Luo, H.T.; Song, Y.D.; Cui, L.; Zhang, X.; Zhu, J.H. Substantia nigra and blood gene signatures and biomarkers for Parkinson’s disease from integrated multicenter microarray-based transcriptomic analyses. Front. Aging Neurosci. 2025, 17, 1540830. [Google Scholar] [CrossRef] [PubMed]
- Schulte, F.; Hasturk, H.; Hardt, M. Mapping Relative Differences in Human Salivary Gland Secretions by Dried Saliva Spot Sampling and nanoLC-MS/MS. Proteomics 2019, 19, e1900023. [Google Scholar] [CrossRef] [PubMed]
- Mollan, K.; Trumble, I.; Reifeis, S.; Ferrer, O.; Bay, C.; Baldoni, P.; Hudgens, M. Precise and accurate power of the rank-sum test for a continuous outcome. J. Biopharm. Stat. 2020, 30, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Blighe, K.; Rana, S.; Lewis, M. EnhancedVolcano: Publication-Ready Volcano Plots with Enhanced Colouring and Labeling. Available online: https://github.com/kevinblighe/EnhancedVolcano (accessed on 4 June 2025).
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
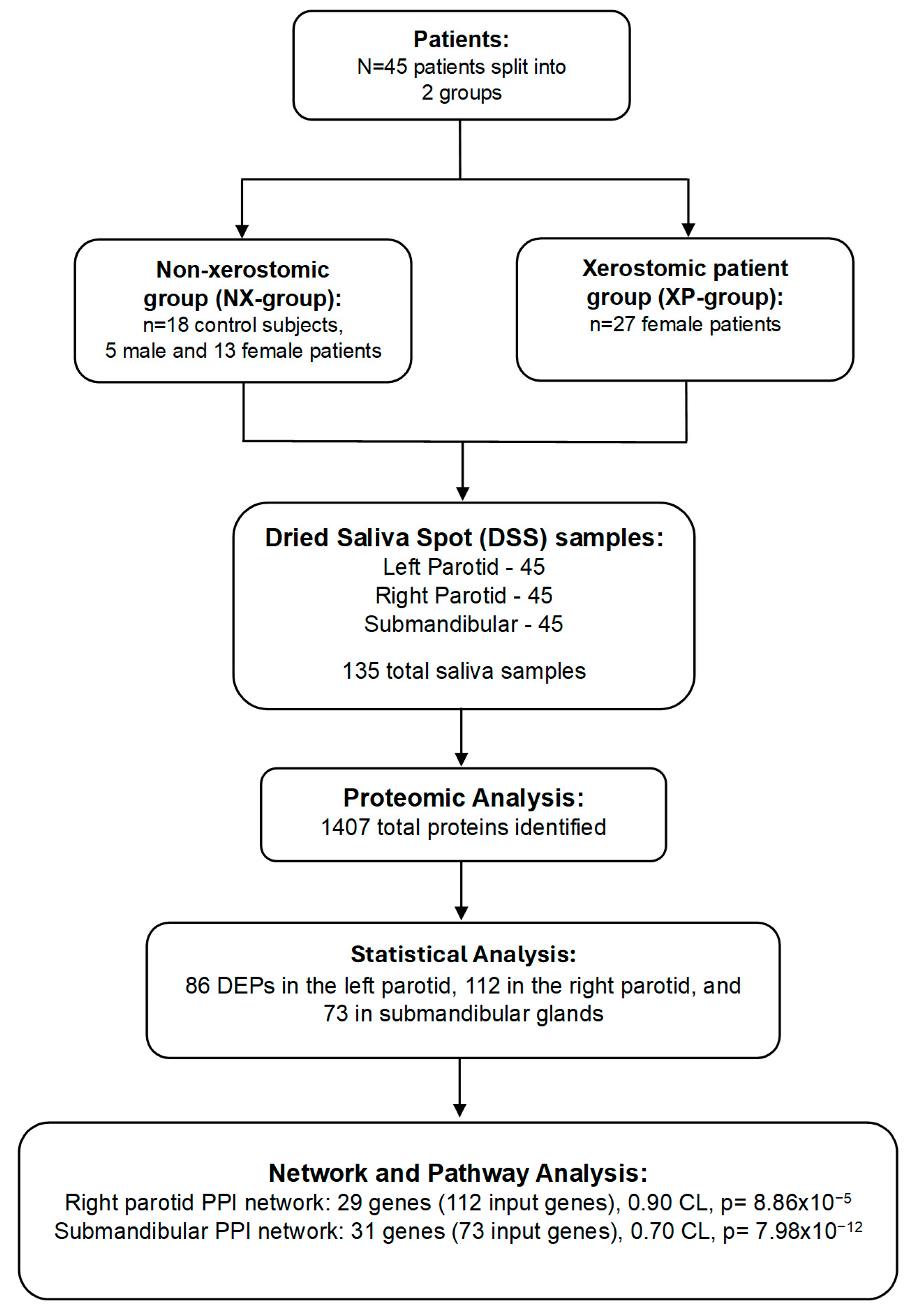

| Group a | # Patients b | M/F c | Age (Min./Max./SD) d | Age (avg) e | Ethnicity (AA/Caucasian/Other) f |
|---|---|---|---|---|---|
| NX-group g | 18 | 5/13 | 31/82/12.74 | 57.89 | 3/10/5 |
| XP-group h | 27 | 0/27 | 48/79/6.82 | 64.41 | 1/26/0 |
| Gene Name a | Protein Name b | Direct Saliva Secretion c | Exosome (Indirect Secretion) d | Oral Involvement e | Salivary Gland/s f | Up/Down-Regulated g | Fold Change h |
|---|---|---|---|---|---|---|---|
| DSG3 | Desmoglein 3 | No | Yes | Yes | LP; RP; SMSL | Up; Down; Down | 35.90; 32.33; 37.23 |
| GLO1 | Glyoxalase I | No | Yes | Yes | RP | Down | 14.06 |
| GLOD4 | Glyoxalase Domain Containing 4 | No | Yes | Yes | LP | Up | 26.63 |
| GLUD2 * | Glutamate Dehydrogenase 2 | Unknown | No | Yes | SMSL | Down | 43.00 |
| MUC1 | Mucin 1 | No | Yes | Yes | LP | Down | 18.10 |
| MUC7 | Mucin 7 | Yes | No | Yes | LP | Up | 132.36 |
| PARK7 * | Parkinson Disease Protein 7 | Yes | No | Yes | RP; SMSL | Down; Down | 25.58; 47.43 |
| PRNP * | Prion Protein | No | Yes | Yes | RP | Down | 8.92 |
| PSMA5 * | Proteasome 20S Subunit Alpha 5 | No | Yes | Yes | RP | Down | 28.26 |
| PSMA6 * | Proteasome 20S Subunit Alpha 6 | No | Yes | Yes | LP | Down | 14.64 |
| PSMA7 * | Proteasome 20S Subunit Alpha 7 | No | Yes | Yes | RP; SMSL | Down; Down | 11.79; 11.36 |
| PSMB1 * | Proteasome 20S Subunit Beta 1 | No | Yes | Yes | RP | Down | 12.80 |
| PSMB5 * | Proteasome 20S Subunit Beta 5 | No | Yes | Yes | RP | Down | 5.46 |
| PSMB6 * | Proteasome 20S Subunit Beta 6 | No | Yes | Yes | RP | Down | 20.96 |
| TUBA4A * | Tubulin Alpha 4a | No | Yes | Yes | RP | Up | 23.67 |
| TUBB2A * | Tubulin Beta 2a | No | Yes | Yes | SMSL | Up | 1.16 |
| UNC5C * | Unc-5 Netrin Receptor C | Unknown | Unknown | Unknown | RP | Down | 28.03 |
| UQCRC1 * | Ubiquinol-Cytochrome C Reductase Core Protein 1 | Unknown | Unknown | Yes | RP | Down | 11.27 |
| VCP * | Valosin Containing Protein | No | Yes | Yes | LP; RP; SMSL | Up; Up; Down | 10.49; 10.75; 6.79 |
| VDAC2 * | Voltage Dependent Anion Channel 2 | No | Yes | Yes | RP; SMSL | Up; Down | 8.04; 13.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henry, A.A.; Beckman, M.F.; Fry, T.S.; Brennan, M.T.; Bahrani Mougeot, F.; Mougeot, J.-L.C. Salivary Proteome Profile of Xerostomic Patients Reveals Pathway Dysregulation Related to Neurodegenerative Diseases: A Pilot Study. Int. J. Mol. Sci. 2025, 26, 7037. https://doi.org/10.3390/ijms26157037
Henry AA, Beckman MF, Fry TS, Brennan MT, Bahrani Mougeot F, Mougeot J-LC. Salivary Proteome Profile of Xerostomic Patients Reveals Pathway Dysregulation Related to Neurodegenerative Diseases: A Pilot Study. International Journal of Molecular Sciences. 2025; 26(15):7037. https://doi.org/10.3390/ijms26157037
Chicago/Turabian StyleHenry, Abhijeet A., Micaela F. Beckman, Thomas S. Fry, Michael T. Brennan, Farah Bahrani Mougeot, and Jean-Luc C. Mougeot. 2025. "Salivary Proteome Profile of Xerostomic Patients Reveals Pathway Dysregulation Related to Neurodegenerative Diseases: A Pilot Study" International Journal of Molecular Sciences 26, no. 15: 7037. https://doi.org/10.3390/ijms26157037
APA StyleHenry, A. A., Beckman, M. F., Fry, T. S., Brennan, M. T., Bahrani Mougeot, F., & Mougeot, J.-L. C. (2025). Salivary Proteome Profile of Xerostomic Patients Reveals Pathway Dysregulation Related to Neurodegenerative Diseases: A Pilot Study. International Journal of Molecular Sciences, 26(15), 7037. https://doi.org/10.3390/ijms26157037







