Insights into the Molecular Basis of Pollen Coat Development and Its Role in Male Sterility
Abstract
1. Introduction
2. Pollen Coat Formation and Morphology of Pollen in Arabidopsis and Rice
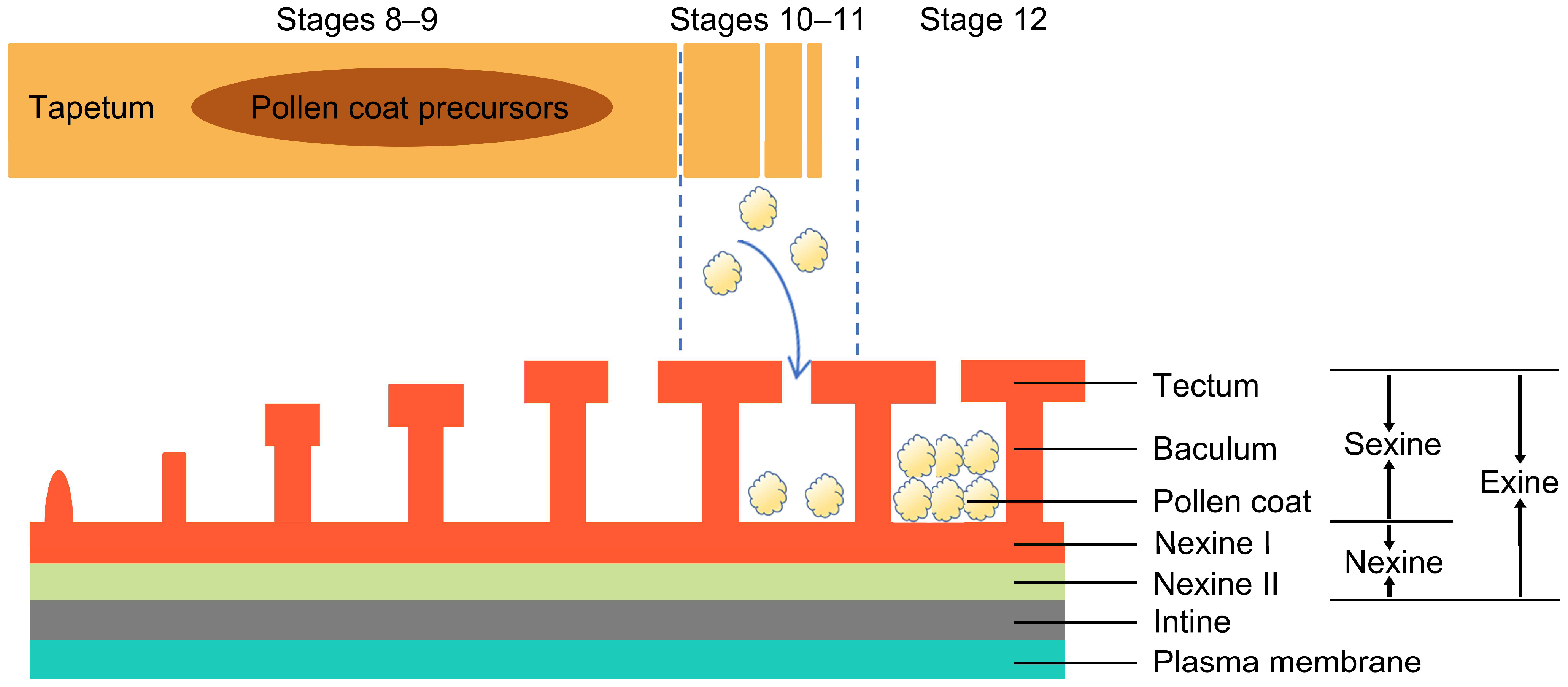
2.1. Pollen Coat Development and Formation
2.2. Pollen Morphology
3. The Main Components of Plant Pollen Coats
3.1. Phenolic Biosynthesis
3.2. Sterol and Triterpenoid Biosynthesis
3.3. Fatty Acid Biosynthesis
4. Transport Mechanism and Related Genes of Pollen Coat Precursors
5. Function of Pollen Coat
5.1. Pollen Adhesion to Pollinators and Pollen Protection
5.2. Communication and Recognition Between Pollen and Stigma
5.3. Water Retention and Hydration Response
5.4. Pollen Coat and Humidity-Sensitive Genic Male Sterility
6. Diversity and Evolutionary Significance of Pollen Coat in Plants
| Species | Methodology | Author List |
|---|---|---|
| Arabidopsis thaliana | Molecular genetic analysis, proteomic analysis, cell biology observation, biochemical analysis | Shuaijie Wei, et al. [19], Ludi Wang, et al. [152], D. J. Murphy, [156] |
| Arabidopsis lyrata | Proteomic analysis | Ludi Wang, et al. [152] |
| Caulokaempferia coenobialis | Cytological analysis, RNA-seq, positive selection analysis | Guo Hui Lu, et al. [185] |
| Hornstedtia hainanensis | ||
| Pyrgophyllum yunnanense | ||
| Zingiber nudicarpum | ||
| Zea mays | Multi-omics integration | Yanhua Li, Giampiero Cai, et al. [184] |
| Oryza sativa L. | Lipid analysis, molecular biology analysis, physiological analysis | Huiqiong Chen, et al. [17] |
| Helianthus annuus L. cv. Morden | Lipid and enzyme activity analysis | Shakya Rashmi, et al. [186], Sharma Basudha, et al. [199] |
| B. oleracea | Molecular genetic analysis, proteomic analysis, cell biology observation, biochemical analysis | Iwano Megumi et al. [183] |
| Brassica napus | Proteomic analysis, cell biology observation | Jiabao Huang, et al. [167] |
| Brassica rapa | Multi-omics integration, molecular genetic analysis | |
| Brassica campestris | Biochemical identification, proteomic analysis, molecular genetic analysis, cell biology observation | Takayama Seiji, et al. [159], Xueqing Liu, et al. [200] |
| Gossypium hirsutum L. | Cytological/morphological observation, transcriptome analysis, phytohormone analysis, bioinformatics analysis | Ji Liu, Chaoyou Pang, Shuxun Yu, et al. [201] |
| Sorghum halepense | Proteomic analysis, biochemical analysis, cell biology observation, molecular genetic analysis | Bashir, et al. [202] |
| Phleum pratense | ||
| Cynodon dactylon | ||
| Secale cereale | Proteomic analysis, cytological analysis | Kalinowski, et al. [203] |
| Festuca pratensis | ||
| Hordeum vulgare | Proteomic analysis, molecular genetic analysis, biochemical analysis, cytological analysis | Suen, et al. [204] |
| Raphanus sativus | Molecular genetic analysis, gene expression analysis | Yung Geun Yoo, et al. [205] |
| Picea pungens | Biochemical identification, cell biology observation | Maria Breygina, Alexander Voronkov, Tatiana Ivanova, Ksenia Babushkina [206] |
| Nicotiana tabacum | ||
| Lilium longiflorum | ||
| Solanum lycopersicum | Cytological analysis, gene expression analysis, biochemical analysis | Anna Smirnova, Jana Leide, Markus Riederer [101] |
| Lolium multiflorum | Proteomic analysis, biochemical identification, cytological analysis | Andrzej Kalinowski, Marek Radlowski, Aleksandra Bocian [191] |
| Citrullus lanatus | Cytological observation, proteomic analysis, bioinformatic analysis | Chunhua Wei, et al. [207] |
7. Conclusions and Future Prospects
7.1. Conclusions
7.2. Future Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chichiriccò, G.; Pacini, E.; Lanza, B. Pollenkitt of some monocotyledons: Lipid composition and implications for pollen germination. Plant Biol. 2019, 21, 920–926. [Google Scholar] [CrossRef]
- Yan, C.L.; Guan, K.X.; Lin, H.; Feng, T.; Meng, J.G. Peptides in plant reproduction-small yet powerful. Front. Plant Sci. 2025, 16, 1506617. [Google Scholar] [CrossRef]
- Qiao, Y.; Hou, B.; Qi, X. Biosynthesis and transport of pollen coat precursors in angiosperms. Nat. Plants 2023, 9, 864–876. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Cui, M.; Yang, L.; Kim, Y.J.; Zhang, D. Genetic and Biochemical Mechanisms of Pollen Wall Development. Trends Plant Sci. 2015, 20, 741–753. [Google Scholar] [CrossRef] [PubMed]
- Stokes, M.; Geitmann, A. Screening methods for thermotolerance in pollen. Ann. Bot. 2025, 135, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Radja, A. Pollen wall patterns as a model for biological self-assembly. J. Exp. Zool. B Mol. Dev. Evol. 2021, 336, 629–641. [Google Scholar] [CrossRef]
- Shi, Q.S.; Lou, Y.; Shen, S.Y.; Wang, S.H.; Zhou, L.; Wang, J.J.; Liu, X.L.; Xiong, S.X.; Han, Y.; Zhou, H.S.; et al. A cellular mechanism underlying the restoration of thermo/photoperiod-sensitive genic male sterility. Mol. Plant 2021, 14, 2104–2114. [Google Scholar] [CrossRef]
- Xu, D.; Shi, J.; Rautengarten, C.; Yang, L.; Qian, X.; Uzair, M.; Zhu, L.; Luo, Q.; An, G.; Waßmann, F.; et al. Defective Pollen Wall 2 (DPW2) Encodes an Acyl Transferase Required for Rice Pollen Development. Plant Physiol. 2017, 173, 240–255. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, F.; Li, X.; Yang, Z. Arabidopsis ECERIFERUM3 (CER3) Plays a Critical Role in Maintaining Hydration for Pollen-Stigma Recognition during Fertilization. bioRxiv 2019. [Google Scholar] [CrossRef]
- Preuss, D.; Lemieux, B.; Yen, G.; Davis, R.W. A conditional sterile mutation eliminates surface components from Arabidopsis pollen and disrupts cell signaling during fertilization. Genes. Dev. 1993, 7, 974–985. [Google Scholar] [CrossRef]
- Cheung, A.Y.; Duan, Q.; Li, C.; James Liu, M.C.; Wu, H.M. Pollen-pistil interactions: It takes two to tangle but a molecular cast of many to deliver. Curr. Opin. Plant Biol. 2022, 69, 102279. [Google Scholar] [CrossRef] [PubMed]
- Abhinandan, K.; Sankaranarayanan, S.; Macgregor, S.; Goring, D.R.; Samuel, M.A. Cell-cell signaling during the Brassicaceae self-incompatibility response. Trends Plant Sci. 2022, 27, 472–487. [Google Scholar] [CrossRef] [PubMed]
- Fiebig, A.; Mayfield, J.A.; Miley, N.L.; Chau, S.; Fischer, R.L.; Preuss, D. Alterations in CER6, a gene identical to CUT1, differentially affect long-chain lipid content on the surface of pollen and stems. Plant Cell 2000, 12, 2001–2008. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, H.; Ankita, K.; Abhinandan, K.; Sharma, T.; Sankaranarayanan, S. From lock and key to molecular diplomacy: Understanding pollen recognition and discrimination in brassicaceae. Plant Reprod. 2024, 38, 2. [Google Scholar] [CrossRef]
- Raut, V.K.; Yadav, A.; Kaur, V.; Rao, M.; Pathania, P.; Wankhede, D.; Singh, M.; Singh, G.P. Pollen-pistil interactions in divergent wide crosses lead to spatial and temporal pre-fertilization reproductive barrier in flax (Linum usitatissimum L.). Sci. Rep. 2025, 15, 6806. [Google Scholar] [CrossRef]
- Ni, E.; Deng, L.; Chen, H.; Lin, J.; Ruan, J.; Liu, Z.; Zhuang, C.; Zhou, H. OsCER1 regulates humidity-sensitive genic male sterility through very-long-chain (VLC) alkane metabolism of tryphine in rice. Funct. Plant Biol. 2021, 48, 461–468. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Z.; Ni, E.; Lin, J.; Peng, G.; Huang, J.; Zhu, L.; Deng, L.; Yang, F.; Luo, Q.; et al. HMS1 interacts with HMS1I to regulate very-long-chain fatty acid biosynthesis and the humidity-sensitive genic male sterility in rice (Oryza sativa). New Phytol. 2020, 225, 2077–2093. [Google Scholar] [CrossRef]
- Badiger, M.; Yadav, R.K.; Sharma, B.B.; Bhat, K.V.; Tomar, B.S.; Lata, S.; Vinay, N.D.; Das, A. Pollen germination, pollen–pistil interaction and crossability studies in interspecific and induced colchiploid population of Abelmoschus species. Genet. Resour. Crop Evol. 2024, 71, 107–127. [Google Scholar] [CrossRef]
- Wei, S.; Ma, L. Comprehensive Insight into Tapetum-Mediated Pollen Development in Arabidopsis thaliana. Cells 2023, 12, 247. [Google Scholar] [CrossRef]
- Moreira, G.L.L.S.; Ferreira, M.E.P.; Linhares, F.S. Identity Transitions of Tapetum Phases: Insights into Vesicular Dynamics and in Mortem Support During Pollen Maturation. Plants 2025, 14, 749. [Google Scholar] [CrossRef]
- Wang, R.; Owen, H.A.; Dobritsa, A.A. Dynamic changes in primexine during the tetrad stage of pollen development. Plant Physiol. 2021, 187, 2393–2404. [Google Scholar] [CrossRef]
- Foubert-Mendes, S.; Silva, J.; Ferreira, M.J.; Pereira, L.G.; Coimbra, S. A review on the function of arabinogalactan-proteins during pollen grain development. Plant Reprod. 2025, 38, 8. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.Y.; Wu, C.C.; Lin, C.C.; Jane, W.N.; Suen, D.F. 3D Imaging of Tapetal Mitochondria Suggests the Importance of Mitochondrial Fission in Pollen Growth. Plant Physiol. 2019, 180, 813–826. [Google Scholar] [CrossRef] [PubMed]
- Rejón, J.D.; Delalande, F.; Schaeffer-Reiss, C.; Alché, J.D.; Rodríguez-García, M.I.; Van Dorsselaer, A.; Castro, A.J. The Pollen Coat Proteome: At the Cutting Edge of Plant Reproduction. Proteomes 2016, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Zhang, B.; Lu, S.; Sun, X.; Liu, X.; Xi, R.; Deng, X. Delayed degradation of tapetal cells causes the male sterility in Lagerstroemia speciosa ‘Zichan’. Sci. Hortic. 2025, 349, 114261. [Google Scholar] [CrossRef]
- Ye, Q.; Jiang, W.; Wang, X.; Hu, X.; Zhang, Z.; Wu, Z.; Wang, H.; Li, S.; Guo, D.; He, H.; et al. Identification of the new allele ptc1-2 and analysis of the regulatory role of PTC1 gene in rice anther development. BMC Plant Biol. 2024, 24, 1062. [Google Scholar] [CrossRef]
- Wiese, A.J.; Torutaeva, E.; Honys, D. The transcription factors and pathways underpinning male reproductive development in Arabidopsis. Front. Plant Sci. 2024, 15, 1354418. [Google Scholar] [CrossRef]
- Yin, G.-M.; Fang, Y.-R.; Wang, J.-G.; Liu, Y.; Xiang, X.; Li, S.; Zhang, Y. Arabidopsis HAPLESS13/AP-1µ is critical for pollen sac formation and tapetal function. Plant Sci. 2024, 341, 111998. [Google Scholar] [CrossRef]
- Gasperini, D.; Howe, G.A. Phytohormones in a universe of regulatory metabolites: Lessons from jasmonate. Plant Physiol. 2024, 195, 135–154. [Google Scholar] [CrossRef]
- Blackmore, S.; Wortley, A.H.; Skvarla, J.J.; Rowley, J.R. Pollen wall development in flowering plants. New Phytol. 2007, 174, 483–498. [Google Scholar] [CrossRef]
- Gómez-Mena, C.; Honys, D.; Datla, R.; Testillano, P.S. Editorial: Advances in Pollen Research: Biology, Biotechnology, and Plant Breeding Applications. Front. Plant Sci. 2022, 13, 876502. [Google Scholar] [CrossRef]
- Althiab-Almasaud, R.; Teyssier, E.; Chervin, C.; Johnson, M.A.; Mollet, J.C. Pollen viability, longevity, and function in angiosperms: Key drivers and prospects for improvement. Plant Reprod. 2024, 37, 273–293. [Google Scholar] [CrossRef]
- Wang, X.; Yu, H.; Gao, R.; Liu, M.; Xie, W. A comprehensive review of the family of very-long-chain fatty acid elongases: Structure, function, and implications in physiology and pathology. Eur. J. Med. Res. 2023, 28, 532. [Google Scholar] [CrossRef]
- Scott, R.J.; Spielman, M.; Dickinson, H.G. Stamen structure and function. Plant Cell 2004, 16 (Suppl. S1), S46–S60. [Google Scholar] [CrossRef]
- Albano, L.; Bento, A.; Correia, V.G.; Silva Pereira, C. The Chemistry of Sporopollenin Ektexine and Endexine Layers Isolated from Sunflower Pollen through an Ionic Liquid-Mediated Process. ACS Omega 2025, 10, 411–421. [Google Scholar] [CrossRef]
- Ariizumi, T.; Hatakeyama, K.; Hinata, K.; Inatsugi, R.; Nishida, I.; Sato, S.; Kato, T.; Tabata, S.; Toriyama, K. Disruption of the novel plant protein NEF1 affects lipid accumulation in the plastids of the tapetum and exine formation of pollen, resulting in male sterility in Arabidopsis thaliana. Plant J. 2004, 39, 170–181. [Google Scholar] [CrossRef]
- Ma, X.; Wu, Y.; Zhang, G. Formation pattern and regulatory mechanisms of pollen wall in Arabidopsis. J. Plant Physiol. 2021, 260, 153388. [Google Scholar] [CrossRef]
- Wang, K.; Zhao, X.; Pang, C.; Zhou, S.; Qian, X.; Tang, N.; Yang, N.; Xu, P.; Xu, X.; Gao, J. IMPERFECTIVE EXINE FORMATION (IEF) is required for exine formation and male fertility in Arabidopsis. Plant Mol. Biol. 2021, 105, 625–635. [Google Scholar] [CrossRef]
- Li, H.; Zhang, D. Biosynthesis of anther cuticle and pollen exine in rice. Plant Signal Behav. 2010, 5, 1121–1123. [Google Scholar] [CrossRef]
- Park, J.H.; Seo, J.; Jackman, J.A.; Cho, N.J. Inflated Sporopollenin Exine Capsules Obtained from Thin-Walled Pollen. Sci. Rep. 2016, 6, 28017. [Google Scholar] [CrossRef]
- Liu, F.; Yang, H.; Tang, R.; Wang, W.; Shen, H.; Xu, M.; Hao, T.; Hu, Y.; Zhang, Y.; Bao, Y. OsTKPR1 proteins with a single amino acid substitution fail the synthesis of a specific sporopollenin precursor and cause abnormal exine and pollen development in rice. Plant Sci. 2023, 335, 111792. [Google Scholar] [CrossRef]
- Gotelli, M.M.; Lattar, E.C.; Zini, L.M.; Galati, B.G. Style morphology and pollen tube pathway. Plant Reprod. 2017, 30, 155–170. [Google Scholar] [CrossRef]
- Zhang, D.; Shi, J.; Yang, X. Role of Lipid Metabolism in Plant Pollen Exine Development. Subcell. Biochem. 2016, 86, 315–337. [Google Scholar] [CrossRef]
- Xu, W.; Peng, X.; Li, Y.; Zeng, X.; Yan, W.; Wang, C.; Wang, C.R.; Chen, S.; Xu, C.; Tang, X. OsSNDP4, a Sec14-nodulin Domain Protein, is Required for Pollen Development in Rice. Rice 2024, 17, 54. [Google Scholar] [CrossRef]
- Zhang, L.; Cao, Z.; Hong, Y.; He, H.; Chen, L.; Yu, Z.; Gao, Y. Squalene Epoxidase: Its Regulations and Links with Cancers. Int. J. Mol. Sci. 2024, 25, 3874. [Google Scholar] [CrossRef]
- Oh, J.-W. The Formation of Pollen. In Pollen Allergy in a Changing World; Springer Nature: Singapore, 2018; pp. 9–19. [Google Scholar] [CrossRef]
- Volkova, O.A.; Severova, E.E.; Polevova, S.V. Structural basis of harmomegathy: Evidence from Boraginaceae pollen. Plant Syst. Evol. 2013, 299, 1769–1779. [Google Scholar] [CrossRef]
- Dobritsa, A.A.; Lei, Z.; Nishikawa, S.; Urbanczyk-Wochniak, E.; Huhman, D.V.; Preuss, D.; Sumner, L.W. LAP5 and LAP6 encode anther-specific proteins with similarity to chalcone synthase essential for pollen exine development in Arabidopsis. Plant Physiol. 2010, 153, 937–955. [Google Scholar] [CrossRef]
- Fellenberg, C.; Vogt, T. Evolutionarily conserved phenylpropanoid pattern on angiosperm pollen. Trends Plant Sci. 2015, 20, 212–218. [Google Scholar] [CrossRef]
- Ortiz, A.; Sansinenea, E. Phenylpropanoid Derivatives and Their Role in Plants’ Health and as antimicrobials. Curr. Microbiol. 2023, 80, 380. [Google Scholar] [CrossRef]
- Pozdniakova, T.A.; Cruz, J.P.; Silva, P.C.; Azevedo, F.; Parpot, P.; Domingues, M.R.; Carlquist, M.; Johansson, B. Optimization of a hybrid bacterial/Arabidopsis thaliana fatty acid synthase system II in Saccharomyces cerevisiae. Metab. Eng. Commun. 2023, 17, e00224. [Google Scholar] [CrossRef]
- de Azevedo Souza, C.; Kim, S.S.; Koch, S.; Kienow, L.; Schneider, K.; McKim, S.M.; Haughn, G.W.; Kombrink, E.; Douglas, C.J. A novel fatty Acyl-CoA Synthetase is required for pollen development and sporopollenin biosynthesis in Arabidopsis. Plant Cell 2009, 21, 507–525. [Google Scholar] [CrossRef]
- Troncoso-Ponce, M.A.; Nikovics, K.; Marchive, C.; Lepiniec, L.; Baud, S. New insights on the organization and regulation of the fatty acid biosynthetic network in the model higher plant Arabidopsis thaliana. Biochimie 2016, 120, 3–8. [Google Scholar] [CrossRef]
- Lallemand, B.; Erhardt, M.; Heitz, T.; Legrand, M. Sporopollenin biosynthetic enzymes interact and constitute a metabolon localized to the endoplasmic reticulum of tapetum cells. Plant Physiol. 2013, 162, 616–625. [Google Scholar] [CrossRef]
- Shang, H.Q.; Bo Yang, Q.; Qiang, S.; Zheng, R.; Zhang, C.Q.; Hu, C.Y.; Chen, Q.H.; Meng, Y.H. Engineering Caffeic Acid O-Methyltransferase for Efficient De Novo Ferulic Acid Synthesis. Eng. Life Sci. 2025, 25, e70018. [Google Scholar] [CrossRef]
- He, B.T.; Li, B.Z. Engineering yeast to produce fraxetin from ferulic acid and lignin. Appl. Microbiol. Biotechnol. 2025, 109, 26. [Google Scholar] [CrossRef]
- Grienenberger, E.; Kim, S.S.; Lallemand, B.; Geoffroy, P.; Heintz, D.; Souza Cde, A.; Heitz, T.; Douglas, C.J.; Legrand, M. Analysis of TETRAKETIDE α-PYRONE REDUCTASE function in Arabidopsis thaliana reveals a previously unknown, but conserved, biochemical pathway in sporopollenin monomer biosynthesis. Plant Cell 2010, 22, 4067–4083. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Ma, M.; Lin, Z.; Hu, W.; Lin, W.; Zhang, P. Structural and Biochemical Insights Into Two BAHD Acyltransferases (AtSHT and AtSDT) Involved in Phenolamide Biosynthesis. Front. Plant Sci. 2020, 11, 610118. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, S.; Tan, H.; Sun, Z.; Li, C.; Zhang, G. Tissue-specific transcriptome analyses unveils candidate genes for flavonoid biosynthesis, regulation and transport in the medicinal plant Ilex asprella. Sci. Rep. 2024, 14, 29999. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Z.; Zhou, S.; Li, S.M.; Ran, H.; Song, Z.; Yu, T.; Yin, W.B. A fungal NRPS-PKS enzyme catalyses the formation of the flavonoid naringenin. Nat. Commun. 2022, 13, 6361. [Google Scholar] [CrossRef]
- Knoch, E.; Sugawara, S.; Mori, T.; Nakabayashi, R.; Saito, K.; Yonekura-Sakakibara, K. UGT79B31 is responsible for the final modification step of pollen-specific flavonoid biosynthesis in Petunia hybrida. Planta 2018, 247, 779–790. [Google Scholar] [CrossRef]
- Yonekura-Sakakibara, K.; Nakabayashi, R.; Sugawara, S.; Tohge, T.; Ito, T.; Koyanagi, M.; Kitajima, M.; Takayama, H.; Saito, K. A flavonoid 3-O-glucoside:2″-O-glucosyltransferase responsible for terminal modification of pollen-specific flavonols in Arabidopsis thaliana. Plant J. 2014, 79, 769–782. [Google Scholar] [CrossRef]
- Faulkner, R.; Jo, Y. Synthesis, function, and regulation of sterol and nonsterol isoprenoids. Front. Mol. Biosci. 2022, 9, 1006822. [Google Scholar] [CrossRef]
- Noushahi, H.A.; Khan, A.H.; Noushahi, U.F.; Hussain, M.; Javed, T.; Zafar, M.; Batool, M.; Ahmed, U.; Liu, K.; Harrison, M.T.; et al. Biosynthetic pathways of triterpenoids and strategies to improve their Biosynthetic Efficiency. Plant Growth Regul. 2022, 97, 439–454. [Google Scholar] [CrossRef]
- Thimmappa, R.; Geisler, K.; Louveau, T.; O’Maille, P.; Osbourn, A. Triterpene biosynthesis in plants. Annu. Rev. Plant Biol. 2014, 65, 225–257. [Google Scholar] [CrossRef]
- Wangeline, M.A.; Vashistha, N.; Hampton, R.Y. Proteostatic Tactics in the Strategy of Sterol Regulation. Annu. Rev. Cell Dev. Biol. 2017, 33, 467–489. [Google Scholar] [CrossRef]
- Gao, M.; Sun, J.; Xiao, Q.; Zhai, Y.; Tian, Y.; Zhang, Z.; Xu, F.; Zhang, P. Sensitive quantification of mevalonate pathway intermediates and prediction of relative novel analogs by chemical derivatization-based LC-MS/MS. J. Chromatogr. A 2024, 1731, 465163. [Google Scholar] [CrossRef]
- Movahedi, A.; Wei, H.; Pucker, B.; Ghaderi-Zefrehei, M.; Rasouli, F.; Kiani-Pouya, A.; Jiang, T.; Zhuge, Q.; Yang, L.; Zhou, X. Isoprenoid biosynthesis regulation in poplars by methylerythritol phosphate and mevalonic acid pathways. Front. Plant Sci. 2022, 13, 968780. [Google Scholar] [CrossRef]
- Vranová, E.; Coman, D.; Gruissem, W. Network analysis of the MVA and MEP pathways for isoprenoid synthesis. Annu. Rev. Plant Biol. 2013, 64, 665–700. [Google Scholar] [CrossRef]
- Yu, H.; Chen, B.; Li, J.; Dong, N.; Chang, X.; Wang, J.; Peng, H.; Zha, L.; Gui, S. Identification and functional characterization of two trans-isopentenyl diphosphate synthases and one squalene synthase involved in triterpenoid biosynthesis in Platycodon grandiflorus. Planta 2023, 258, 115. [Google Scholar] [CrossRef]
- Nagegowda, D.A.; Gupta, P. Advances in biosynthesis, regulation, and metabolic engineering of plant specialized terpenoids. Plant Sci. 2020, 294, 110457. [Google Scholar] [CrossRef]
- Tholl, D. Biosynthesis and Biological Functions of Terpenoids in Plants. Adv. Biochem. Eng./Biotechnol. 2015, 148, 63–106. [Google Scholar]
- Câmara, J.S.; Perestrelo, R.; Ferreira, R.; Berenguer, C.V.; Pereira, J.A.M.; Castilho, P.C. Plant-Derived Terpenoids: A Plethora of Bioactive Compounds with Several Health Functions and Industrial Applications—A Comprehensive Overview. Molecules 2024, 29, 3861. [Google Scholar] [CrossRef]
- Gallo, C.; Landi, S.; d’Ippolito, G.; Nuzzo, G.; Manzo, E.; Sardo, A.; Fontana, A. Diatoms synthesize sterols by inclusion of animal and fungal genes in the plant pathway. Sci. Rep. 2020, 10, 4204. [Google Scholar] [CrossRef] [PubMed]
- Takemura, M.; Tanaka, R.; Misawa, N. Pathway engineering for the production of β-amyrin and cycloartenol in Escherichia coli-a method to biosynthesize plant-derived triterpene skeletons in E. coli. Appl. Microbiol. Biotechnol. 2017, 101, 6615–6625. [Google Scholar] [CrossRef]
- Du, Y.; Fu, X.; Chu, Y.; Wu, P.; Liu, Y.; Ma, L.; Tian, H.; Zhu, B. Biosynthesis and the Roles of Plant Sterols in Development and Stress Responses. Int. J. Mol. Sci. 2022, 23, 2332. [Google Scholar] [CrossRef]
- Liao, P.; Lung, S.C.; Chan, W.L.; Bach, T.J.; Lo, C.; Chye, M.L. Overexpression of HMG-CoA synthase promotes Arabidopsis root growth and adversely affects glucosinolate biosynthesis. J. Exp. Bot. 2020, 71, 272–289. [Google Scholar] [CrossRef]
- Suzuki, M.; Nakagawa, S.; Kamide, Y.; Kobayashi, K.; Ohyama, K.; Hashinokuchi, H.; Kiuchi, R.; Saito, K.; Muranaka, T.; Nagata, N. Complete blockage of the mevalonate pathway results in male gametophyte lethality. J. Exp. Bot. 2009, 60, 2055–2064. [Google Scholar] [CrossRef]
- Kobayashi, K.; Suzuki, M.; Muranaka, T.; Nagata, N. The mevalonate pathway but not the methylerythritol phosphate pathway is critical for elaioplast and pollen coat development in Arabidopsis. Plant Biotechnol 2018, 35, 381–385. [Google Scholar] [CrossRef]
- Xue, Z.; Xu, X.; Zhou, Y.; Wang, X.; Zhang, Y.; Liu, D.; Zhao, B.; Duan, L.; Qi, X. Deficiency of a triterpene pathway results in humidity-sensitive genic male sterility in rice. Nat. Commun. 2018, 9, 604. [Google Scholar] [CrossRef]
- Batsale, M.; Bahammou, D.; Fouillen, L.; Mongrand, S.; Joubès, J.; Domergue, F. Biosynthesis and Functions of Very-Long-Chain Fatty Acids in the Responses of Plants to Abiotic and Biotic Stresses. Cells 2021, 10, 1284. [Google Scholar] [CrossRef]
- Shang, B.; Xu, C.; Zhang, X.; Cao, H.; Xin, W.; Hu, Y. Very-long-chain fatty acids restrict regeneration capacity by confining pericycle competence for callus formation in Arabidopsis. Proc. Natl. Acad. Sci. USA 2016, 113, 5101–5106. [Google Scholar] [CrossRef]
- Zheng, H.; Liang, Y.; Hong, B.; Xu, Y.; Ren, M.; Wang, Y.; Huang, L.; Yang, L.; Tao, J. Genome-Scale Analysis of the Grapevine KCS Genes Reveals Its Potential Role in Male Sterility. Int. J. Mol. Sci. 2023, 24, 6510. [Google Scholar] [CrossRef]
- Hegebarth, D.; Buschhaus, C.; Joubès, J.; Thoraval, D.; Bird, D.; Jetter, R. Arabidopsis ketoacyl-CoA synthase 16 (KCS16) forms C(36)/C(38) acyl precursors for leaf trichome and pavement surface wax. Plant Cell Environ. 2017, 40, 1761–1776. [Google Scholar] [CrossRef]
- Jessen, D.; Olbrich, A.; Knüfer, J.; Krüger, A.; Hoppert, M.; Polle, A.; Fulda, M. Combined activity of LACS1 and LACS4 is required for proper pollen coat formation in Arabidopsis. Plant J. 2011, 68, 715–726. [Google Scholar] [CrossRef]
- Xie, L.J.; Tan, W.J.; Yang, Y.C.; Tan, Y.F.; Zhou, Y.; Zhou, D.M.; Xiao, S.; Chen, Q.F. Long-Chain acyl-CoA Synthetase LACS2 Contributes to Submergence Tolerance by Modulating Cuticle Permeability in Arabidopsis. Plants 2020, 9, 262. [Google Scholar] [CrossRef]
- Zhao, H.; Kosma, D.K.; Lü, S. Functional Role of Long-Chain Acyl-CoA Synthetases in Plant Development and Stress Responses. Front. Plant Sci. 2021, 12, 640996. [Google Scholar] [CrossRef]
- Pascal, S.; Bernard, A.; Deslous, P.; Gronnier, J.; Fournier-Goss, A.; Domergue, F.; Rowland, O.; Joubès, J. Arabidopsis CER1-LIKE1 Functions in a Cuticular Very-Long-Chain Alkane-Forming Complex. Plant Physiol. 2019, 179, 415–432. [Google Scholar] [CrossRef]
- Wu, H.; Shi, S.; Lu, X.; Li, T.; Wang, J.; Liu, T.; Zhang, Q.; Sun, W.; Li, C.; Wang, Z.; et al. Expression Analysis and Functional Characterization of CER1 Family Genes Involved in Very-Long-Chain Alkanes Biosynthesis in Brachypodium distachyon. Front. Plant Sci. 2019, 10, 1389. [Google Scholar] [CrossRef]
- Haslam, T.M.; Gerelle, W.K.; Graham, S.W.; Kunst, L. The Unique Role of the ECERIFERUM2-LIKE Clade of the BAHD Acyltransferase Superfamily in Cuticular Wax Metabolism. Plants 2017, 6, 23. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, H.; Kosma, D.K.; Tomasi, P.; Dyer, J.M.; Li, R.; Liu, X.; Wang, Z.; Parsons, E.P.; Jenks, M.A.; et al. The Acyl Desaturase CER17 Is Involved in Producing Wax Unsaturated Primary Alcohols and Cutin Monomers. Plant Physiol. 2017, 173, 1109–1124. [Google Scholar] [CrossRef]
- Zhan, H.; Xiong, H.; Wang, S.; Yang, Z.N. Anther Endothecium-Derived Very-Long-Chain Fatty Acids Facilitate Pollen Hydration in Arabidopsis. Mol. Plant 2018, 11, 1101–1104. [Google Scholar] [CrossRef]
- Haslam, T.M.; Kunst, L. Arabidopsis ECERIFERUM2-LIKEs Are Mediators of Condensing Enzyme Function. Plant Cell Physiol. 2021, 61, 2126–2138. [Google Scholar] [CrossRef]
- Gonzales-Vigil, E.; vonLoessl, M.E.; Chen, J.Y.; Li, S.; Haslam, T.M.; Kunst, L.; Mansfield, S.D. Understanding the Role of Populus ECERIFERUM2-Likes in the Biosynthesis of Very-Long-Chain Fatty Acids for Cuticular Waxes. Plant Cell Physiol. 2021, 62, 827–838. [Google Scholar] [CrossRef]
- Haslam, T.M.; Haslam, R.; Thoraval, D.; Pascal, S.; Delude, C.; Domergue, F.; Fernández, A.M.; Beaudoin, F.; Napier, J.A.; Kunst, L.; et al. ECERIFERUM2-LIKE proteins have unique biochemical and physiological functions in very-long-chain fatty acid elongation. Plant Physiol. 2015, 167, 682–692. [Google Scholar] [CrossRef]
- Pascal, S.; Bernard, A.; Sorel, M.; Pervent, M.; Vile, D.; Haslam, R.P.; Napier, J.A.; Lessire, R.; Domergue, F.; Joubès, J. The Arabidopsis cer26 mutant, like the cer2 mutant, is specifically affected in the very long chain fatty acid elongation process. Plant J. 2013, 73, 733–746. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Ayaz, A.; Zheng, M.; Yang, X.; Zaman, W.; Zhao, H.; Lü, S. ArabidopsisKCS5 and KCS6 Play Redundant Roles in Wax Synthesis. Int. J. Mol. Sci. 2022, 23, 4450. [Google Scholar] [CrossRef]
- Bernard, A.; Domergue, F.; Pascal, S.; Jetter, R.; Renne, C.; Faure, J.D.; Haslam, R.P.; Napier, J.A.; Lessire, R.; Joubès, J. Reconstitution of plant alkane biosynthesis in yeast demonstrates that Arabidopsis ECERIFERUM1 and ECERIFERUM3 are core components of a very-long-chain alkane synthesis complex. Plant Cell 2012, 24, 3106–3118. [Google Scholar] [CrossRef]
- Xu, F.; Zheng, L.; Yang, Z.; Zhang, S. Arabidopsis ECERIFERUM3 (CER3) Functions to Maintain Hydration for Pollen–Stigma Recognition During Fertilization. J. Plant Biol. 2020, 63, 347–359. [Google Scholar] [CrossRef]
- Wang, X.; Guan, Y.; Zhang, D.; Dong, X.; Tian, L.; Qu, L.Q. A β-Ketoacyl-CoA Synthase Is Involved in Rice Leaf Cuticular Wax Synthesis and Requires a CER2-LIKE Protein as a Cofactor. Plant Physiol. 2017, 173, 944–955. [Google Scholar] [CrossRef]
- Smirnova, A.; Leide, J.; Riederer, M. Deficiency in a very-long-chain fatty acid β-ketoacyl-coenzyme a synthase of tomato impairs microgametogenesis and causes floral organ fusion. Plant Physiol. 2013, 161, 196–209. [Google Scholar] [CrossRef]
- Xiong, T.; Ye, F.; Chen, J.; Chen, Y.; Zhang, Z. Peptide signaling in anther development and pollen-stigma interactions. Gene 2023, 865, 147328. [Google Scholar] [CrossRef]
- Yu, B.; Liu, L.; Wang, T. Deficiency of very long chain alkanes biosynthesis causes humidity-sensitive male sterility via affecting pollen adhesion and hydration in rice. Plant Cell Environ. 2019, 42, 3340–3354. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.Q.; Zhang, X.Y.; Xue, F.; Zhu, S.H.; Li, Y.J.; Zhu, Q.H.; Liu, F.; Sun, J. Characterization and transcriptome analysis of a dominant genic male sterile cotton mutant. BMC Plant Biol. 2020, 20, 312. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Yuan, C.; Niu, Y.; Tang, Q.; Wei, D.; Wang, Z. Regulation of plant MYB transcription factors in anther development. Sheng Wu Gong. Cheng Xue Bao 2020, 36, 2277–2286. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; Zhou, H.S.; Han, Y.; Zeng, Q.Y.; Zhu, J.; Yang, Z.N. Positive regulation of AMS by TDF1 and the formation of a TDF1-AMS complex are required for anther development in Arabidopsis thaliana. New Phytol. 2018, 217, 378–391. [Google Scholar] [CrossRef]
- Gu, J.N.; Zhu, J.; Yu, Y.; Teng, X.D.; Lou, Y.; Xu, X.F.; Liu, J.L.; Yang, Z.N. DYT1 directly regulates the expression of TDF1 for tapetum development and pollen wall formation in Arabidopsis. Plant J. 2014, 80, 1005–1013. [Google Scholar] [CrossRef]
- Tidy, A.C.; Ferjentsikova, I.; Vizcay-Barrena, G.; Liu, B.; Yin, W.; Higgins, J.D.; Xu, J.; Zhang, D.; Geelen, D.; Wilson, Z.A. Sporophytic control of pollen meiotic progression is mediated by tapetum expression of ABORTED MICROSPORES. J. Exp. Bot. 2022, 73, 5543–5558. [Google Scholar] [CrossRef]
- Xu, J.; Ding, Z.; Vizcay-Barrena, G.; Shi, J.; Liang, W.; Yuan, Z.; Werck-Reichhart, D.; Schreiber, L.; Wilson, Z.A.; Zhang, D. ABORTED MICROSPORES Acts as a Master Regulator of Pollen Wall Formation in Arabidopsis. Plant Cell 2014, 26, 1544–1556. [Google Scholar] [CrossRef]
- Ito, T.; Nagata, N.; Yoshiba, Y.; Ohme-Takagi, M.; Ma, H.; Shinozaki, K. Arabidopsis MALE STERILITY1 encodes a PHD-type transcription factor and regulates pollen and tapetum development. Plant Cell 2007, 19, 3549–3562. [Google Scholar] [CrossRef]
- Lu, J.Y.; Xiong, S.X.; Yin, W.; Teng, X.D.; Lou, Y.; Zhu, J.; Zhang, C.; Gu, J.N.; Wilson, Z.A.; Yang, Z.N. MS1, a direct target of MS188, regulates the expression of key sporophytic pollen coat protein genes in Arabidopsis. J. Exp. Bot. 2020, 71, 4877–4889. [Google Scholar] [CrossRef]
- Yang, C.; Vizcay-Barrena, G.; Conner, K.; Wilson, Z.A. MALE STERILITY1 is required for tapetal development and pollen wall biosynthesis. Plant Cell 2007, 19, 3530–3548. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhan, H.; Lu, J.; Xiong, S.; Yang, N.; Yuan, H.; Yang, Z.N. Tapetal 3-Ketoacyl-Coenzyme A Synthases Are Involved in Pollen Coat Lipid Accumulation for Pollen-Stigma Interaction in Arabidopsis. Front. Plant Sci. 2021, 12, 770311. [Google Scholar] [CrossRef] [PubMed]
- Goodman, K.; Paez-Valencia, J.; Pennington, J.; Sonntag, A.; Ding, X.; Lee, H.N.; Ahlquist, P.G.; Molina, I.; Otegui, M.S. ESCRT components ISTL1 andLIP5 are required for tapetal function and pollen viability. Plant Cell 2021, 33, 2850–2868. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Shi, H.; Wang, W.; Liu, X.; Gao, H.; Wang, X.; Zhang, Y.; Yang, M.; Li, R.; Guo, Y. Secretory COPII Protein SEC31B Is Required for Pollen Wall Development. Plant Physiol. 2016, 172, 1625–1642. [Google Scholar] [CrossRef]
- Liu, X.; Tong, M.; Zhang, A.; Liu, M.; Zhao, B.; Liu, Z.; Li, Z.; Zhu, X.; Guo, Y.; Li, R. COPII genes SEC31A/B are essential for gametogenesis and interchangeable in pollen development in Arabidopsis. Plant J. 2021, 105, 1600–1614. [Google Scholar] [CrossRef]
- Li, N.; Gügel, I.L.; Giavalisco, P.; Zeisler, V.; Schreiber, L.; Soll, J.; Philippar, K. FAX1, a novel membrane protein mediating plastid fatty acid export. PLoS Biol. 2015, 13, e1002053. [Google Scholar] [CrossRef]
- Choi, H.; Ohyama, K.; Kim, Y.Y.; Jin, J.Y.; Lee, S.B.; Yamaoka, Y.; Muranaka, T.; Suh, M.C.; Fujioka, S.; Lee, Y. The role of Arabidopsis ABCG9 and ABCG31 ATP binding cassette transporters in pollen fitness and the deposition of steryl glycosides on the pollen coat. Plant Cell 2014, 26, 310–324. [Google Scholar] [CrossRef]
- Chang, Z.; Chen, Z.; Yan, W.; Xie, G.; Lu, J.; Wang, N.; Lu, Q.; Yao, N.; Yang, G.; Xia, J.; et al. An ABC transporter, OsABCG26, is required for anther cuticle and pollen exine formation and pollen-pistil interactions in rice. Plant Sci. 2016, 253, 21–30. [Google Scholar] [CrossRef]
- Hsieh, K.; Huang, A.H. Tapetosomes in Brassica tapetum accumulate endoplasmic reticulum-derived flavonoids and alkanes for delivery to the pollen surface. Plant Cell 2007, 19, 582–596. [Google Scholar] [CrossRef]
- Grunewald, S.; Marillonnet, S.; Hause, G.; Haferkamp, I.; Neuhaus, H.E.; Veß, A.; Hollemann, T.; Vogt, T. The Tapetal Major Facilitator NPF2.8 Is Required for Accumulation of Flavonol Glycosides on the Pollen Surface in Arabidopsis thaliana. Plant Cell 2020, 32, 1727–1748. [Google Scholar] [CrossRef]
- Chen, L.; Ji, C.; Zhou, D.; Gou, X.; Tang, J.; Jiang, Y.; Han, J.; Liu, Y.G.; Chen, L.; Xie, Y. OsLTP47 may function in a lipid transfer relay essential for pollen wall development in rice. J. Genet. Genom. 2022, 49, 481–491. [Google Scholar] [CrossRef]
- Zhang, D.; Liang, W.; Yin, C.; Zong, J.; Gu, F.; Zhang, D. OsC6, encoding a lipid transfer protein, is required for postmeiotic anther development in rice. Plant Physiol. 2010, 154, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.; Gao, H.; Yang, Y.; Wu, H. Changes in the content of pollen total lipid and TAG in Arabidopsis thaliana DGAT1 mutant as11. AoB Plants 2023, 15, plad012. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, J.; Wong, C.K.; Lim, B.L. Movement of Lipid Droplets in the Arabidopsis Pollen Tube Is Dependent on the Actomyosin System. Plants 2023, 12, 2489. [Google Scholar] [CrossRef] [PubMed]
- Colin, L.A.; Jaillais, Y. Phospholipids across scales: Lipid patterns and plant development. Curr. Opin. Plant Biol. 2020, 53, 1–9. [Google Scholar] [CrossRef]
- Kim, H.U.; Wu, S.S.; Ratnayake, C.; Huang, A.H. Brassica rapa has three genes that encode proteins associated with different neutral lipids in plastids of specific tissues. Plant Physiol. 2001, 126, 330–341. [Google Scholar] [CrossRef]
- Sierra, J.; Escobar-Tovar, L.; Leon, P. Plastids: Diving into their diversity, their functions, and their role in plant development. J. Exp. Bot. 2023, 74, 2508–2526. [Google Scholar] [CrossRef]
- Suzuki, T.; Tsunekawa, S.; Koizuka, C.; Yamamoto, K.; Imamura, J.; Nakamura, K.; Ishiguro, S. Development and disintegration of tapetum-specific lipid-accumulating organelles, elaioplasts and tapetosomes, in Arabidopsis thaliana and Brassica napus. Plant Sci. 2013, 207, 25–36. [Google Scholar] [CrossRef]
- Aboulela, M.; Nakagawa, T.; Oshima, A.; Nishimura, K.; Tanaka, Y. The Arabidopsis COPII components, AtSEC23A and AtSEC23D, are essential for pollen wall development and exine patterning. J. Exp. Bot. 2018, 69, 1615–1633. [Google Scholar] [CrossRef]
- Kobayashi, K.; Akita, K.; Suzuki, M.; Ohta, D.; Nagata, N. Fertile Arabidopsis cyp704b1 mutant, defective in sporopollenin biosynthesis, has a normal pollen coat and lipidic organelles in the tapetum. Plant Biotechnol. 2021, 38, 109–116. [Google Scholar] [CrossRef]
- Ma, H.; Wu, Y.; Lv, R.; Chi, H.; Zhao, Y.; Li, Y.; Liu, H.; Ma, Y.; Zhu, L.; Guo, X.; et al. Cytochrome P450 mono-oxygenase CYP703A2 plays a central role in sporopollenin formation and ms5ms6 fertility in cotton. J. Integr. Plant Biol. 2022, 64, 2009–2025. [Google Scholar] [CrossRef]
- Shi, J.; Tan, H.; Yu, X.H.; Liu, Y.; Liang, W.; Ranathunge, K.; Franke, R.B.; Schreiber, L.; Wang, Y.; Kai, G.; et al. Defective pollen wall is required for anther and microspore development in rice and encodes a fatty acyl carrier protein reductase. Plant Cell 2011, 23, 2225–2246. [Google Scholar] [CrossRef]
- Yang, X.; Wu, D.; Shi, J.; He, Y.; Pinot, F.; Grausem, B.; Yin, C.; Zhu, L.; Chen, M.; Luo, Z.; et al. Rice CYP703A3, a cytochrome P450 hydroxylase, is essential for development of anther cuticle and pollen exine. J. Integr. Plant Biol. 2014, 56, 979–994. [Google Scholar] [CrossRef]
- Chen, W.; Yu, X.H.; Zhang, K.; Shi, J.; De Oliveira, S.; Schreiber, L.; Shanklin, J.; Zhang, D. Male Sterile2 encodes a plastid-localized fatty acyl carrier protein reductase required for pollen exine development in Arabidopsis. Plant Physiol. 2011, 157, 842–853. [Google Scholar] [CrossRef]
- Dobritsa, A.A.; Shrestha, J.; Morant, M.; Pinot, F.; Matsuno, M.; Swanson, R.; Møller, B.L.; Preuss, D. CYP704B1 is a long-chain fatty acid omega-hydroxylase essential for sporopollenin synthesis in pollen of Arabidopsis. Plant Physiol. 2009, 151, 574–589. [Google Scholar] [CrossRef]
- Morant, M.; Jørgensen, K.; Schaller, H.; Pinot, F.; Møller, B.L.; Werck-Reichhart, D.; Bak, S. CYP703 is an ancient cytochrome P450 in land plants catalyzing in-chain hydroxylation of lauric acid to provide building blocks for sporopollenin synthesis in pollen. Plant Cell 2007, 19, 1473–1487. [Google Scholar] [CrossRef] [PubMed]
- Qian, D.; Li, T.; Zheng, C.; Wang, M.; Chen, S.; Li, C.; An, J.; Yang, Y.; Niu, Y.; An, L.; et al. Heat-stable protein PGSL1 enhances pollen germination and tube growth at high temperature. Nat. Commun. 2025, 16, 3642. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Yao, D.; Duan, H.; Zhang, J.; Cai, Y.; Lan, C.; Zhao, B.; Mei, Y.; Zheng, Y.; Yang, E.; et al. VAMP726 from maize and Arabidopsis confers pollen resistance to heat and UV radiation by influencing lignin content of sporopollenin. Plant Commun. 2023, 4, 100682. [Google Scholar] [CrossRef] [PubMed]
- Nottebrock, H.; Schmid, B.; Mayer, K.; Devaux, C.; Esler, K.J.; Böhning-Gaese, K.; Schleuning, M.; Pagel, J.R.; Schurr, F.M. Sugar landscapes and pollinator-mediated interactions in plant communities. Ecography 2017, 40, 1129–1138. [Google Scholar] [CrossRef]
- Seitz, J.; Reimann, T.M.; Fritz, C.; Schröder, C.; Knab, J.; Weber, W.; Stadler, R. How pollen tubes fight for food: The impact of sucrose carriers and invertases of Arabidopsis thaliana on pollen development and pollen tube growth. Front. Plant Sci. 2023, 14, 1063765. [Google Scholar] [CrossRef]
- Lin, H.; Gomez, I.; Meredith, J.C. Pollenkitt wetting mechanism enables species-specific tunable pollen adhesion. Langmuir 2013, 29, 3012–3023. [Google Scholar] [CrossRef] [PubMed]
- Denisow, B.; Denisow-Pietrzyk, M. Biological and therapeutic properties of bee pollen: A review. J. Sci. Food Agric. 2016, 96, 4303–4309. [Google Scholar] [CrossRef] [PubMed]
- Kiyono, H.; Katano, K.; Suzuki, N. Links between Regulatory Systems of ROS and Carbohydrates in Reproductive Development. Plants 2021, 10, 1652. [Google Scholar] [CrossRef] [PubMed]
- Nepi, M.; Franchi, G.G.; Pacini, E. Pollen hydration status at dispersal: Cytophysiological features and strategies. Protoplasma 2001, 216, 171–180. [Google Scholar] [CrossRef]
- Kuljarusnont, S.; Iwakami, S.; Iwashina, T.; Tungmunnithum, D. Flavonoids and Other Phenolic Compounds for Physiological Roles, Plant Species Delimitation, and Medical Benefits: A Promising View. Molecules 2024, 29, 5351. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef]
- El Ghouizi, A.; Bakour, M.; Laaroussi, H.; Ousaaid, D.; El Menyiy, N.; Hano, C.; Lyoussi, B. Bee Pollen as Functional Food: Insights into Its Composition and Therapeutic Properties. Antioxidants 2023, 12, 557. [Google Scholar] [CrossRef]
- Agati, G.; Brunetti, C.; Di Ferdinando, M.; Ferrini, F.; Pollastri, S.; Tattini, M. Functional roles of flavonoids in photoprotection: New evidence, lessons from the past. Plant Physiol. Biochem. 2013, 72, 35–45. [Google Scholar] [CrossRef]
- Battat, M.; Eitan, A.; Rogachev, I.; Hanhineva, K.; Fernie, A.; Tohge, T.; Beekwilder, J.; Aharoni, A. A MYB Triad Controls Primary and Phenylpropanoid Metabolites for Pollen Coat Patterning. Plant Physiol. 2019, 180, 87–108. [Google Scholar] [CrossRef]
- Walters, D.; Meurer-Grimes, B.; Rovira, I. Antifungal activity of three spermidine conjugates. FEMS Microbiol. Lett. 2001, 201, 255–258. [Google Scholar] [CrossRef]
- Wang, L.; Lau, Y.L.; Fan, L.; Bosch, M.; Doughty, J. Pollen Coat Proteomes of Arabidopsis thaliana, Arabidopsis lyrata, and Brassica oleracea Reveal Remarkable Diversity of Small Cysteine-Rich Proteins at the Pollen-Stigma Interface. Biomolecules 2023, 13, 157. [Google Scholar] [CrossRef]
- Doucet, J.; Lee, H.K.; Goring, D.R. Pollen Acceptance or Rejection: A Tale of Two Pathways. Trends Plant Sci. 2016, 21, 1058–1067. [Google Scholar] [CrossRef]
- Hiscock, S.J.; McInnis, S.M. Pollen recognition and rejection during the sporophytic self-incompatibility response: Brassica and beyond. Trends Plant Sci. 2003, 8, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Fobis-Loisy, I.; Ivanov, R.; Gaude, T. The S-LOCUS CYSTEINE-RICH PROTEIN (SCR): A Small Peptide with A High Impact on the Evolution of Flowering Plants. In Plant Signaling Peptides; Springer: Berlin/Heidelberg, Germany, 2012; pp. 77–92. [Google Scholar]
- Murphy, D.J. The extracellular pollen coat in members of the Brassicaceae: Composition, biosynthesis, and functions in pollination. Protoplasma 2006, 228, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Du, Q.; Yang, F.; Chen, L.Y. The emerging role of cysteine-rich peptides in pollen-pistil interactions. J. Exp. Bot. 2024, 75, 6228–6243. [Google Scholar] [CrossRef] [PubMed]
- Doughty, J.; Dixon, S.; Hiscock, S.J.; Willis, A.C.; Parkin, I.A.; Dickinson, H.G. PCP-A1, a defensin-like Brassica pollen coat protein that binds the S locus glycoprotein, is the product of gametophytic gene expression. Plant Cell 1998, 10, 1333–1347. [Google Scholar] [CrossRef]
- Takayama, S.; Shiba, H.; Iwano, M.; Asano, K.; Hara, M.; Che, F.S.; Watanabe, M.; Hinata, K.; Isogai, A. Isolation and characterization of pollen coat proteins of Brassica campestris that interact with S locus-related glycoprotein 1 involved in pollen-stigma adhesion. Proc. Natl. Acad. Sci. USA 2000, 97, 3765–3770. [Google Scholar] [CrossRef]
- Liu, C.; Shen, L.; Xiao, Y.; Vyshedsky, D.; Peng, C.; Sun, X.; Liu, Z.; Cheng, L.; Zhang, H.; Han, Z.; et al. Pollen PCP-B peptides unlock a stigma peptide-receptor kinase gating mechanism for pollination. Science 2021, 372, 171–175. [Google Scholar] [CrossRef]
- Zhou, L.Z.; Qu, L.J.; Dresselhaus, T. Stigmatic ROS: Regulator of compatible pollen tube perception? Trends Plant Sci. 2021, 26, 993–995. [Google Scholar] [CrossRef]
- Lan, Z.; Song, Z.; Wang, Z.; Li, L.; Liu, Y.; Zhi, S.; Wang, R.; Wang, J.; Li, Q.; Bleckmann, A.; et al. Antagonistic RALF peptides control an intergeneric hybridization barrier on Brassicaceae stigmas. Cell 2023, 186, 4773–4787.e12. [Google Scholar] [CrossRef]
- Lan, Z.; Zhong, S.; Qu, L.J. Insights into pollen-stigma recognition: Self-incompatibility mechanisms serve as interspecies barriers in Brassicaceae? aBIOTECH 2023, 4, 176–179. [Google Scholar] [CrossRef]
- Nasrallah, J.B. Self-incompatibility in the Brassicaceae: Regulation and mechanism of self-recognition. Curr. Top. Dev. Biol. 2019, 131, 435–452. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Akram, A.; Farman, K.; Abbas, T.; Awan, M.M.W. Molecular Markers and Marker Assisted Plant Breeding: Current Status and their Applications in Agricultural Development. J. Environ. Agric. Sci. 2017, 11, 35–50. [Google Scholar]
- Lobaton, J.; Andrew, R.; Duitama, J.; Kirkland, L.; Macfadyen, S.; Rader, R. Using RNA-seq to characterize pollen-stigma interactions for pollination studies. Sci. Rep. 2021, 11, 6635. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Yang, L.; Yang, L.; Wu, X.; Cui, X.; Zhang, L.; Hui, J.; Zhao, Y.; Yang, H.; Liu, S.; et al. Stigma receptors control intraspecies and interspecies barriers in Brassicaceae. Nature 2023, 614, 303–308. [Google Scholar] [CrossRef]
- Ruiter, R.K.; Eldik, G.J.V.; Herpen, M.M.A.V.; Schrauwen, J.A.M.; Wullems, G.J. Hydration-dependent gene expression in Brassica oleracea anthers. Sex. Plant Reprod. 1999, 12, 135–143. [Google Scholar] [CrossRef]
- Williams, J.H.; Brown, C.D.; Williams, J.H.; Brown, C.D. Pollen has higher water content when dispersed in a tricellular state than in a bicellular state. Acta Bot. Bras. 2018, 32, 454–461. [Google Scholar] [CrossRef]
- Gong, F.; Wu, X.; Wang, W. Diversity and function of maize pollen coat proteins: From biochemistry to proteomics. Front. Plant Sci. 2015, 6, 199. [Google Scholar] [CrossRef]
- Jia, X.L.; Xue, J.S.; Zhang, F.; Yao, C.; Shen, S.Y.; Sui, C.X.; Peng, Y.J.; Xu, Q.L.; Feng, Y.F.; Hu, W.J.; et al. A dye combination for the staining of pollen coat and pollen wall. Plant Reprod. 2021, 34, 91–101. [Google Scholar] [CrossRef]
- Hiscock, S.J.; Allen, A.M. Diverse cell signalling pathways regulate pollen-stigma interactions: The search for consensus. New Phytol. 2008, 179, 286–317. [Google Scholar] [CrossRef]
- Mayfield, J.A.; Fiebig, A.; Johnstone, S.E.; Preuss, D. Gene families from the Arabidopsis thaliana pollen coat proteome. Science 2001, 292, 2482–2485. [Google Scholar] [CrossRef]
- Nasrallah, J.B. Stop and go signals at the stigma-pollen interface of the Brassicaceae. Plant Physiol. 2023, 193, 927–948. [Google Scholar] [CrossRef]
- Chaudhary, K.; Geeta, R.; Panjabi, P. Origin and diversification of ECERIFERUM1 (CER1) and ECERIFERUM3 (CER3) genes in land plants and phylogenetic evidence that the ancestral CER1/3 gene resulted from the fusion of pre-existing domains. Mol. Phylogenet Evol. 2021, 159, 107101. [Google Scholar] [CrossRef] [PubMed]
- Updegraff, E.P.; Zhao, F.; Preuss, D. The extracellular lipase EXL4 is required for efficient hydration of Arabidopsis pollen. Sex. Plant Reprod. 2009, 22, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Hu, W.; Yang, Z.N. The contributions of sporophytic tapetum to pollen formation. Seed Biol. 2022, 1, 13. [Google Scholar] [CrossRef]
- Aarts, M.G.; Keijzer, C.J.; Stiekema, W.J.; Pereira, A. Molecular characterization of the CER1 gene of arabidopsis involved in epicuticular wax biosynthesis and pollen fertility. Plant Cell 1995, 7, 2115–2127. [Google Scholar] [CrossRef]
- Ni, E.; Zhou, L.; Li, J.; Jiang, D.; Wang, Z.; Zheng, S.; Qi, H.; Zhou, Y.; Wang, C.; Xiao, S.; et al. OsCER1 Plays a Pivotal Role in Very-Long-Chain Alkane Biosynthesis and Affects Plastid Development and Programmed Cell Death of Tapetum in Rice (Oryza sativa L.). Front. Plant Sci. 2018, 9, 1217. [Google Scholar] [CrossRef]
- Chen, X.; Goodwin, S.M.; Boroff, V.L.; Liu, X.; Jenks, M.A. Cloning and characterization of the WAX2 gene of Arabidopsis involved in cuticle membrane and wax production. Plant Cell 2003, 15, 1170–1185. [Google Scholar] [CrossRef]
- Ariizumi, T.; Hatakeyama, K.; Hinata, K.; Sato, S.; Kato, T.; Tabata, S.; Toriyama, K. A novel male-sterile mutant of Arabidopsis thaliana, faceless pollen-1, produces pollen with a smooth surface and an acetolysis-sensitive exine. Plant Mol. Biol. 2003, 53, 107–116. [Google Scholar] [CrossRef]
- Wang, W.; Liu, X.; Gai, X.; Ren, J.; Liu, X.; Cai, Y.; Wang, Q.; Ren, H. Cucumis sativus L. WAX2 Plays a Pivotal Role in Wax Biosynthesis, Influencing Pollen Fertility and Plant Biotic and Abiotic Stress Responses. Plant Cell Physiol. 2015, 56, 1339–1354. [Google Scholar] [CrossRef]
- Iwano, M.; Igarashi, M.; Tarutani, Y.; Kaothien-Nakayama, P.; Nakayama, H.; Moriyama, H.; Yakabe, R.; Entani, T.; Shimosato-Asano, H.; Ueki, M.; et al. A pollen coat-inducible autoinhibited Ca2+-ATPase expressed in stigmatic papilla cells is required for compatible pollination in the Brassicaceae. Plant Cell 2014, 26, 636–649. [Google Scholar] [CrossRef]
- Li, Y.; Niu, L.; Wu, X.; Faleri, C.; Tai, F.; Zhang, M.; Liu, H.; Wang, W.; Cai, G. Genome-Wide Identification and Comparison of Cysteine Proteases in the Pollen Coat and Other Tissues in Maize. Front. Plant Sci. 2021, 12, 709534. [Google Scholar] [CrossRef]
- Lu, G.H.; Xu, J.L.; Zhong, M.X.; Li, D.L.; Chen, M.; Li, K.T.; Wang, Y.Q. Cytochemical and comparative transcriptome analyses elucidate the formation and ecological adaptation of three types of pollen coat in Zingiberaceae. BMC Plant Biol. 2022, 22, 407. [Google Scholar] [CrossRef] [PubMed]
- Shakya, R.; Bhatla, S.C. A comparative analysis of the distribution and composition of lipidic constituents and associated enzymes in pollen and stigma of sunflower. Sex. Plant Reprod. 2010, 23, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, J.; Ma, Q.; Qin, Y.; Wang, H.; Chen, P.; Ma, L.; Fu, X.; Zhu, L.; Wei, H.; et al. Deficiencies in the formation and regulation of anther cuticle and tryphine contribute to male sterility in cotton PGMS line. BMC Genom. 2020, 21, 825. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, Y.Y.; Zhao, X.; Zhang, C.; Liu, D.K.; Lan, S.; Yin, W.; Liu, Z.J. Molecular insights into self-incompatibility systems: From evolution to breeding. Plant Commun. 2024, 5, 100719. [Google Scholar] [CrossRef]
- Cabrillac, D.; Cock, J.M.; Dumas, C.; Gaude, T. The S-locus receptor kinase is inhibited by thioredoxins and activated by pollen coat proteins. Nature 2001, 410, 220–223. [Google Scholar] [CrossRef]
- Vanoosthuyse, V.; Miege, C.; Dumas, C.; Cock, J.M. Two large Arabidopsis thaliana gene families are homologous to the Brassica gene superfamily that encodes pollen coat proteins and the male component of the self-incompatibility response. Plant Mol. Biol. 2001, 46, 17–34. [Google Scholar] [CrossRef]
- Kalinowski, A.; Radłowski, M.; Bocian, A. Effects of interaction between pollen coat eluates and pistil at the molecular level in self-compatible and self-incompatible plants of Lolium multiflorum Lam. J. Appl. Genet. 2006, 47, 319–329. [Google Scholar] [CrossRef]
- Shiba, H.; Takayama, S.; Iwano, M.; Shimosato, H.; Funato, M.; Nakagawa, T.; Che, F.S.; Suzuki, G.; Watanabe, M.; Hinata, K.; et al. A pollen coat protein, SP11/SCR, determines the pollen S-specificity in the self-incompatibility of Brassica species. Plant Physiol. 2001, 125, 2095–2103. [Google Scholar] [CrossRef]
- Shimosato, H.; Yokota, N.; Shiba, H.; Iwano, M.; Entani, T.; Che, F.-S.; Watanabe, M.; Isogai, A.; Takayama, S. Characterization of the SP11/SCR High-Affinity Binding Site Involved in Self/Nonself Recognition in Brassica Self-Incompatibility. Plant Cell 2007, 19, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Sawa, T.; Moriwaki, Y.; Jiang, H.; Murase, K.; Takayama, S.; Shimizu, K.; Terada, T. Comprehensive computational analysis of the SRK–SP11 molecular interaction underlying self-incompatibility in Brassicaceae using improved structure prediction for cysteine-rich proteins. Comput. Struct. Biotechnol. J. 2023, 21, 5228–5239. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Zhong, S.; Qu, L.J. FERONIA and reactive oxygen species: Regulators in the self-incompatibility response and in interspecific pollination. Mol. Hortic. 2023, 3, 10. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Zhao, T.; Yang, Z.; Liang, L.; Ma, W.; Wang, G.; Ma, Q. Stigmatic Transcriptome Analysis of Self-Incompatible and Compatible Pollination in Corylus heterophylla Fisch × Corylus avellana L. Front. Plant Sci. 2022, 13, 800768. [Google Scholar] [CrossRef]
- Boggs, N.A.; Nasrallah, J.B.; Nasrallah, M.E. Independent S-locus mutations caused self-fertility in Arabidopsis thaliana. PLoS Genet. 2009, 5, e1000426. [Google Scholar] [CrossRef]
- Naithani, S.; Ripoll, D.; Nasrallah, J. The S-locus cysteine-rich peptide SCR/SP11. In Handbook of Biologically Active Peptides; Academic Press: New York, NY, USA, 2006; pp. 41–48. [Google Scholar]
- Sharma, B.; Bhatla, S.C. Structural analysis of stigma development in relation with pollen–stigma interaction in sunflower. Flora Morphol. Distrib. Funct. Ecol. Plants 2013, 208, 420–429. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Tang, X.; Wang, W.; Khan, A.; Pang, X.; Wang, Y.; Wang, C.; Yuan, L.; Hou, J.; et al. Genome-wide identification, transcript profiling and functional analyses of PCP gene family in Wucai (Brassica campestris). Sci. Rep. (Nat. Publ. Group) 2024, 14, 28236. [Google Scholar] [CrossRef]
- Liu, J.; Pang, C.; Wei, H.; Song, M.; Meng, Y.; Ma, J.; Fan, S.; Yu, S. iTRAQ-facilitated proteomic profiling of anthers from a photosensitive male sterile mutant and wild-type cotton (Gossypium hirsutum L.). J. Proteom. 2015, 126, 68–81. [Google Scholar] [CrossRef]
- Bashir, M.E.H.; Ward, J.M.; Cummings, M.; Karrar, E.E.; Root, M.; Abu Bekr, A.M.; Naclerio, R.M.; Preuss, D. Dual Function of Novel Pollen Coat (Surface) Proteins: IgE-binding Capacity and Proteolytic Activity Disrupting the Airway Epithelial Barrier. PLoS ONE 2013, 8, e53337. [Google Scholar] [CrossRef]
- Kalinowski, A.; Winiarczyk, K.; Radlowski, M. Pollen coat proteins after two-dimensional gel electrophoresis and pollen wall ultrastructure of Secale cereale and Festuca pratensis. Sex. Plant Reprod. 2002, 15, 75–83. [Google Scholar] [CrossRef]
- Suen, D.-F. Tapetum and Pollen Interaction in the Anthers of Maize and Barley. Ph.D. Thesis, University of California, Riverside, CA, USA, 2005. [Google Scholar]
- Yoo, Y.G.; Lee, S.C.; Kim, S.R. Identification of a flower-specific cDNA, RsPCP1, encoding putative pollen coat protein from radish. J. Plant Biol. 2003, 46, 130–133. [Google Scholar] [CrossRef]
- Breygina, M.; Voronkov, A.; Ivanova, T.; Babushkina, K. Fatty Acid Composition of Dry and Germinating Pollen of Gymnosperm and Angiosperm Plants. Int. J. Mol. Sci. 2023, 24, 9717. [Google Scholar] [CrossRef]
- Wei, C.; Zhang, R.; Yue, Z.; Yan, X.; Cheng, D.; Li, J.; Li, H.; Zhang, Y.; Ma, J.; Yang, J.; et al. The impaired biosynthetic networks in defective tapetum lead to male sterility in watermelon. J. Proteom. 2021, 243, 104241. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Guo, L.; Qin, Y.; Gao, Q. PCP-bε is a novel positive regulator of pollen germination in Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 2024, 733, 150698. [Google Scholar] [CrossRef] [PubMed]
- Li, D.D.; Xue, J.S.; Zhu, J.; Yang, Z.N. Gene Regulatory Network for Tapetum Development in Arabidopsis thaliana. Front. Plant Sci. 2017, 8, 1559. [Google Scholar] [CrossRef] [PubMed]
- Haslam, T.M.; Kunst, L. Extending the story of very-long-chain fatty acid elongation. Plant Sci. 2013, 210, 93–107. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyu, B.; Liang, C. Insights into the Molecular Basis of Pollen Coat Development and Its Role in Male Sterility. Int. J. Mol. Sci. 2025, 26, 7036. https://doi.org/10.3390/ijms26157036
Lyu B, Liang C. Insights into the Molecular Basis of Pollen Coat Development and Its Role in Male Sterility. International Journal of Molecular Sciences. 2025; 26(15):7036. https://doi.org/10.3390/ijms26157036
Chicago/Turabian StyleLyu, Binyang, and Cuiyue Liang. 2025. "Insights into the Molecular Basis of Pollen Coat Development and Its Role in Male Sterility" International Journal of Molecular Sciences 26, no. 15: 7036. https://doi.org/10.3390/ijms26157036
APA StyleLyu, B., & Liang, C. (2025). Insights into the Molecular Basis of Pollen Coat Development and Its Role in Male Sterility. International Journal of Molecular Sciences, 26(15), 7036. https://doi.org/10.3390/ijms26157036





