Cytochalasins Suppress 3D Migration of ECM-Embedded Tumoroids at Non-Toxic Concentrations
Abstract
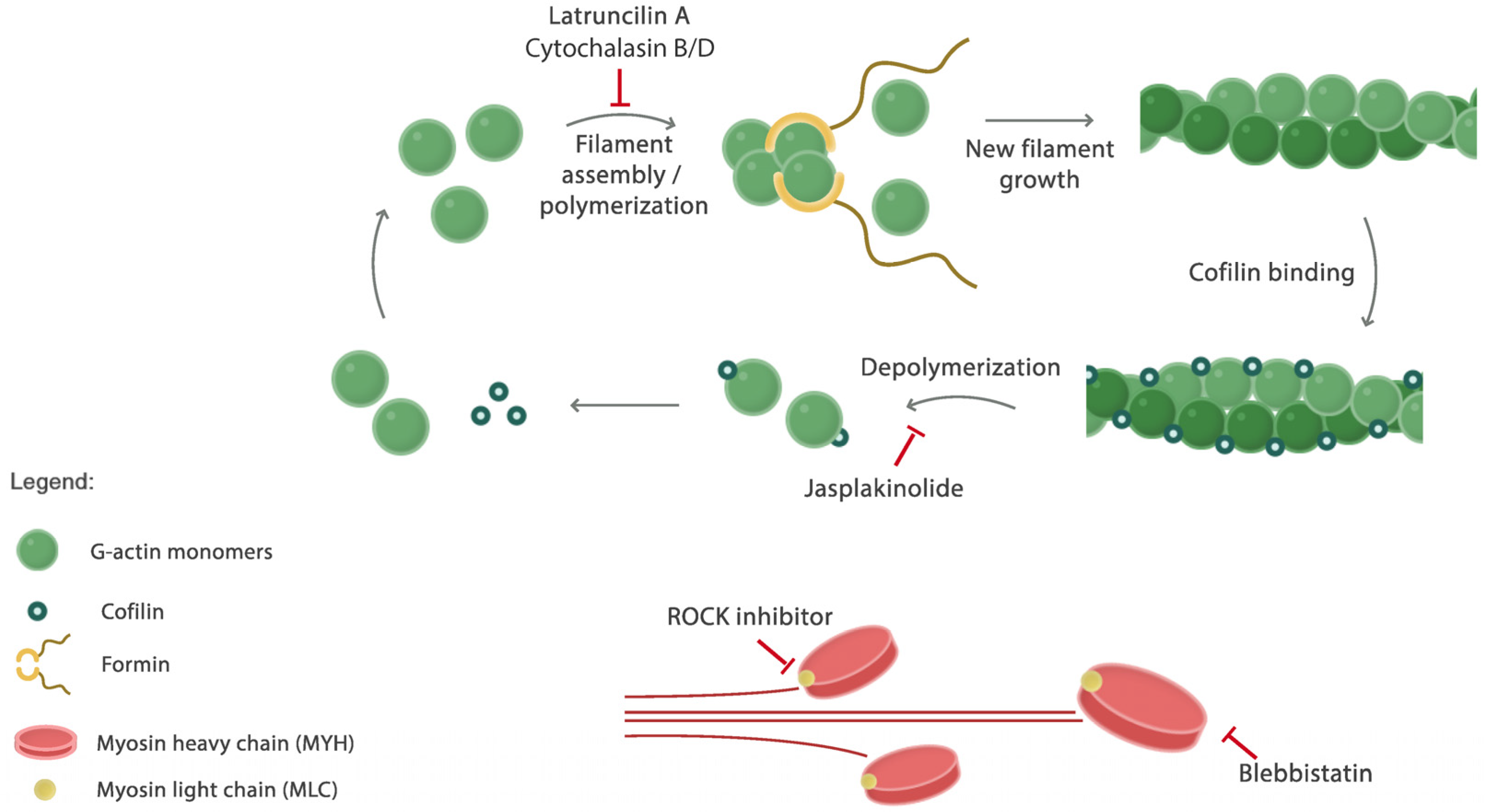
1. Introduction
2. Results
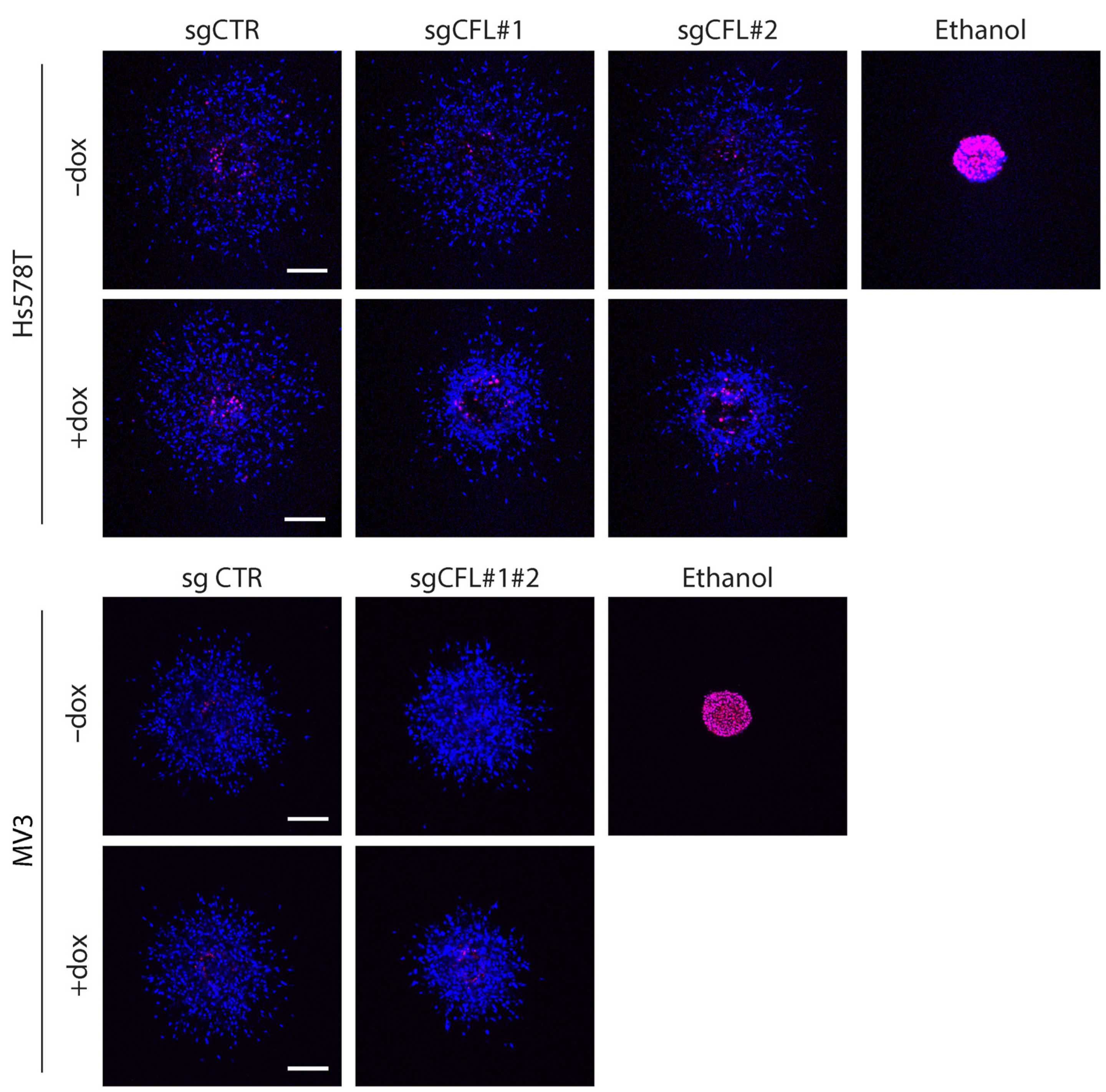
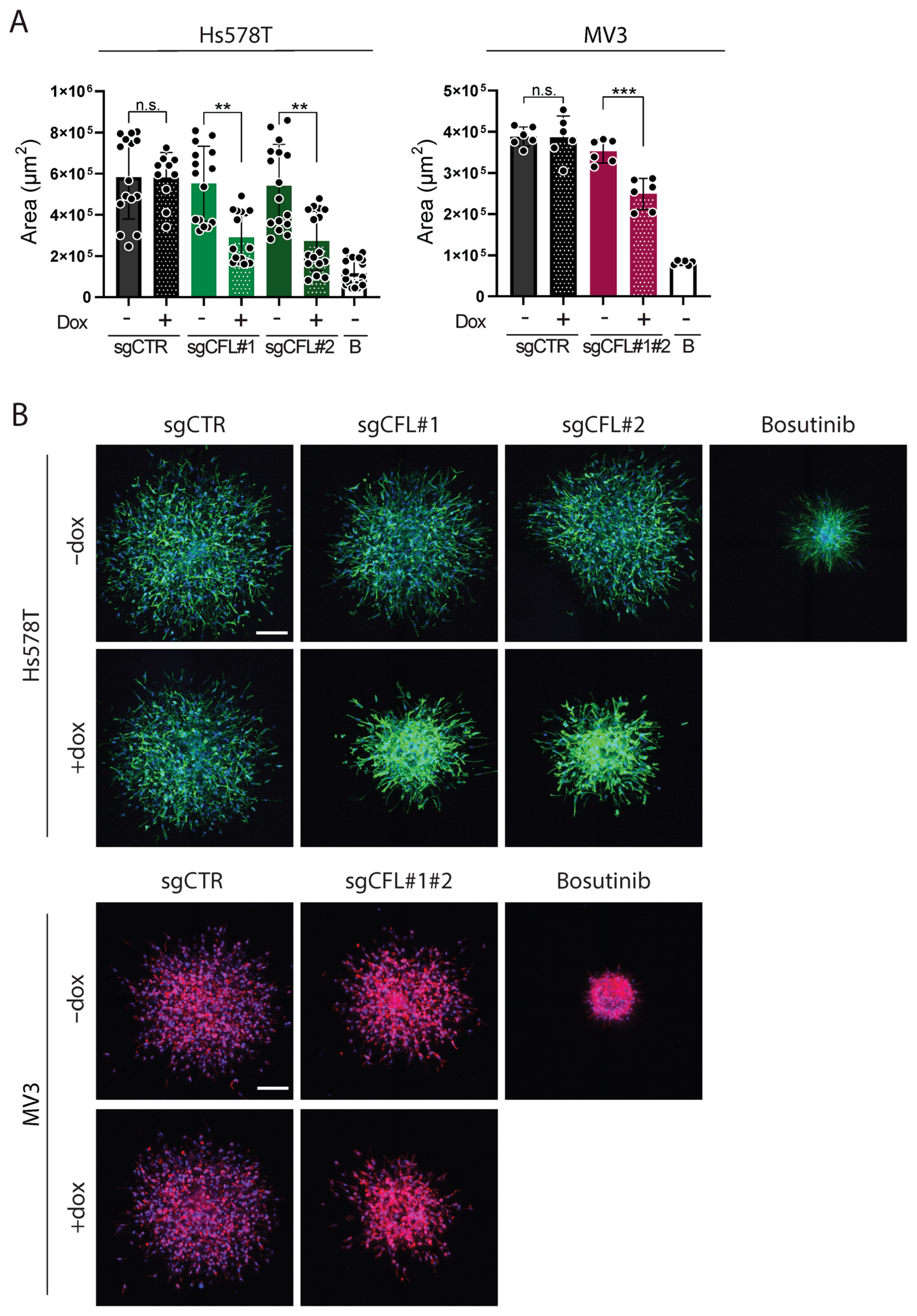
2.1. Deletion of CFL Attenuates Migration of Cancer Cells from ECM-Embedded Tumoroids
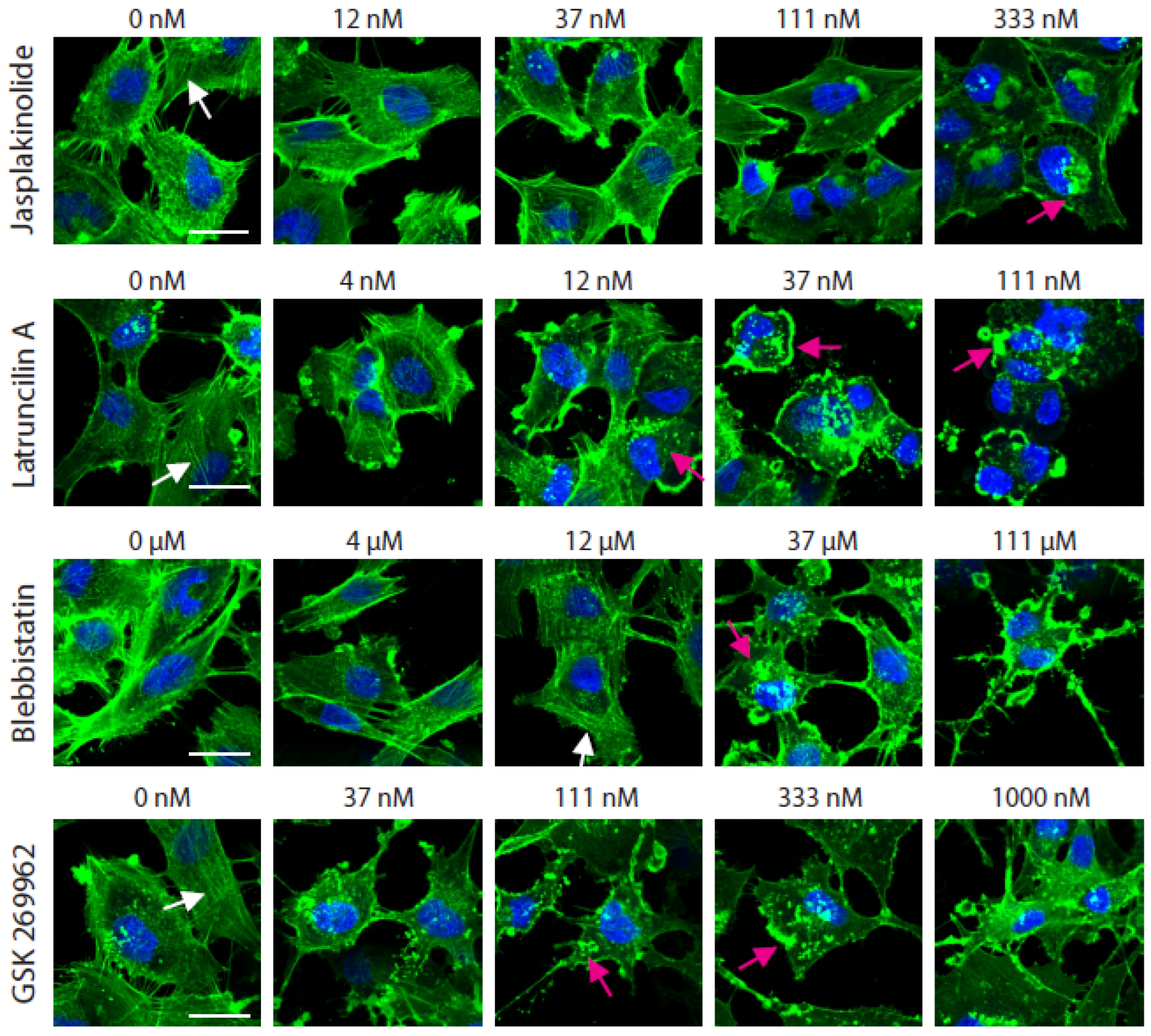
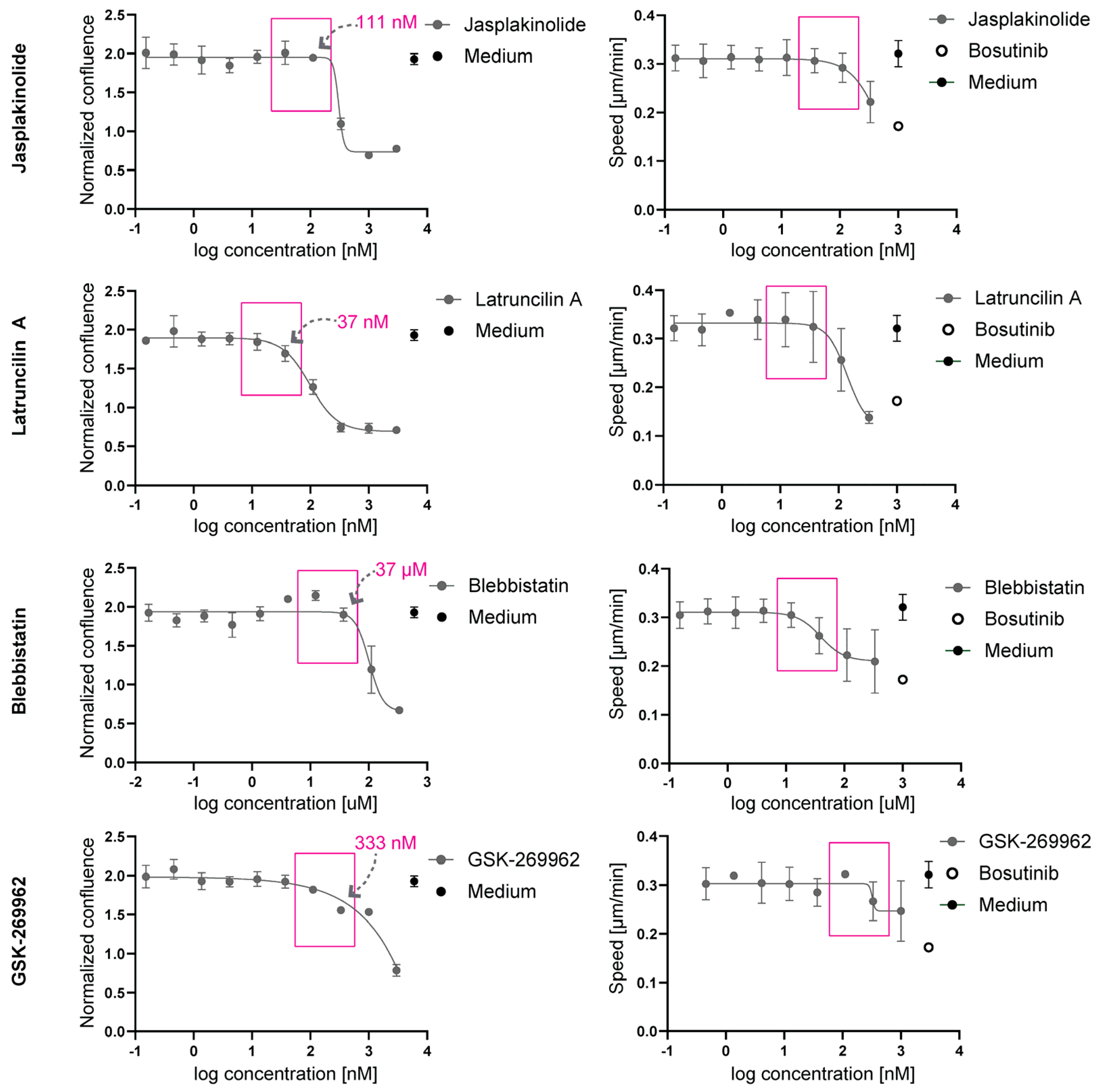
2.2. At Non-Toxic Concentrations, Jasplakinolide, Latruncilin A, Blebbistatin, and GSK-269962 Do Not Affect 2D Migration Speed
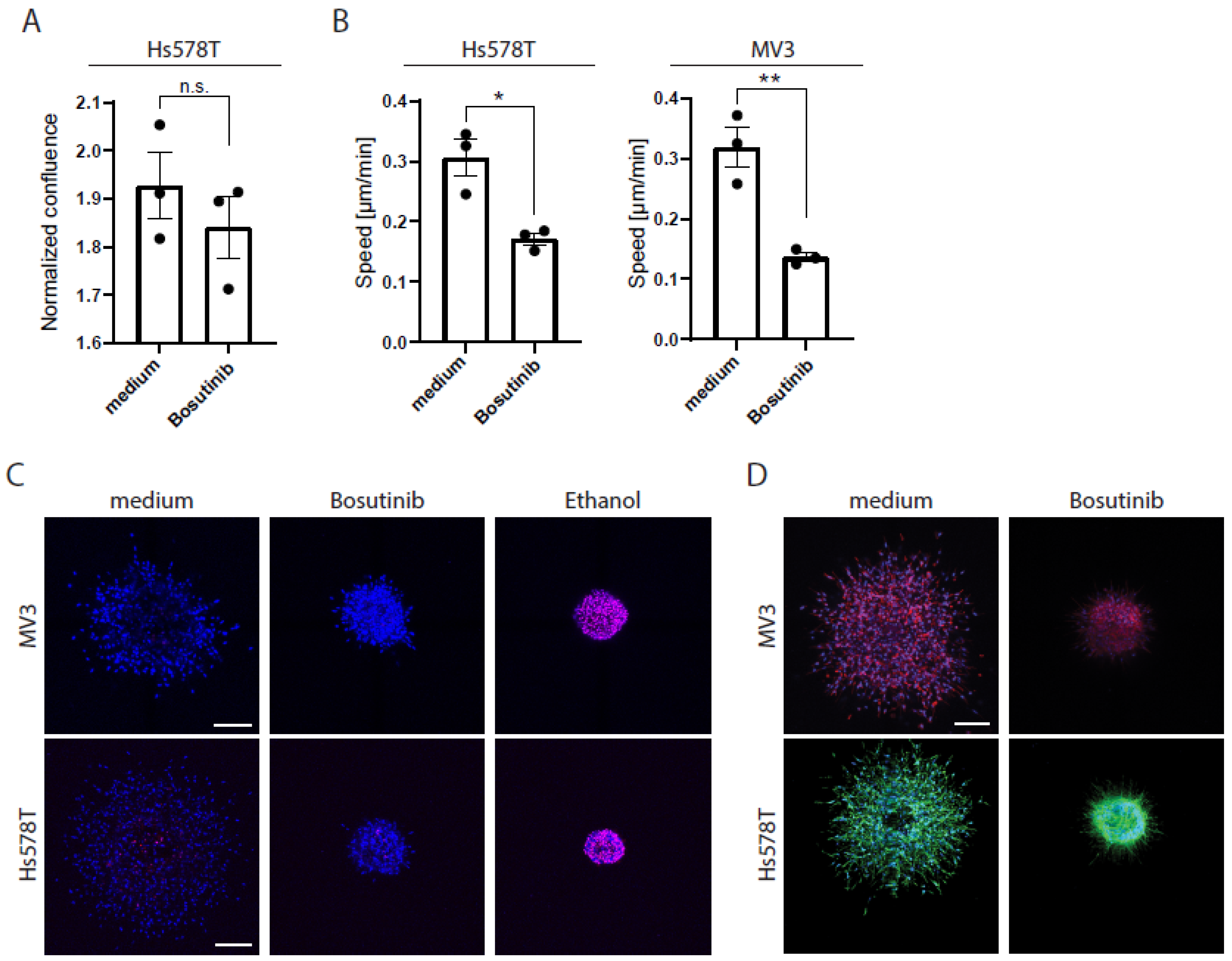
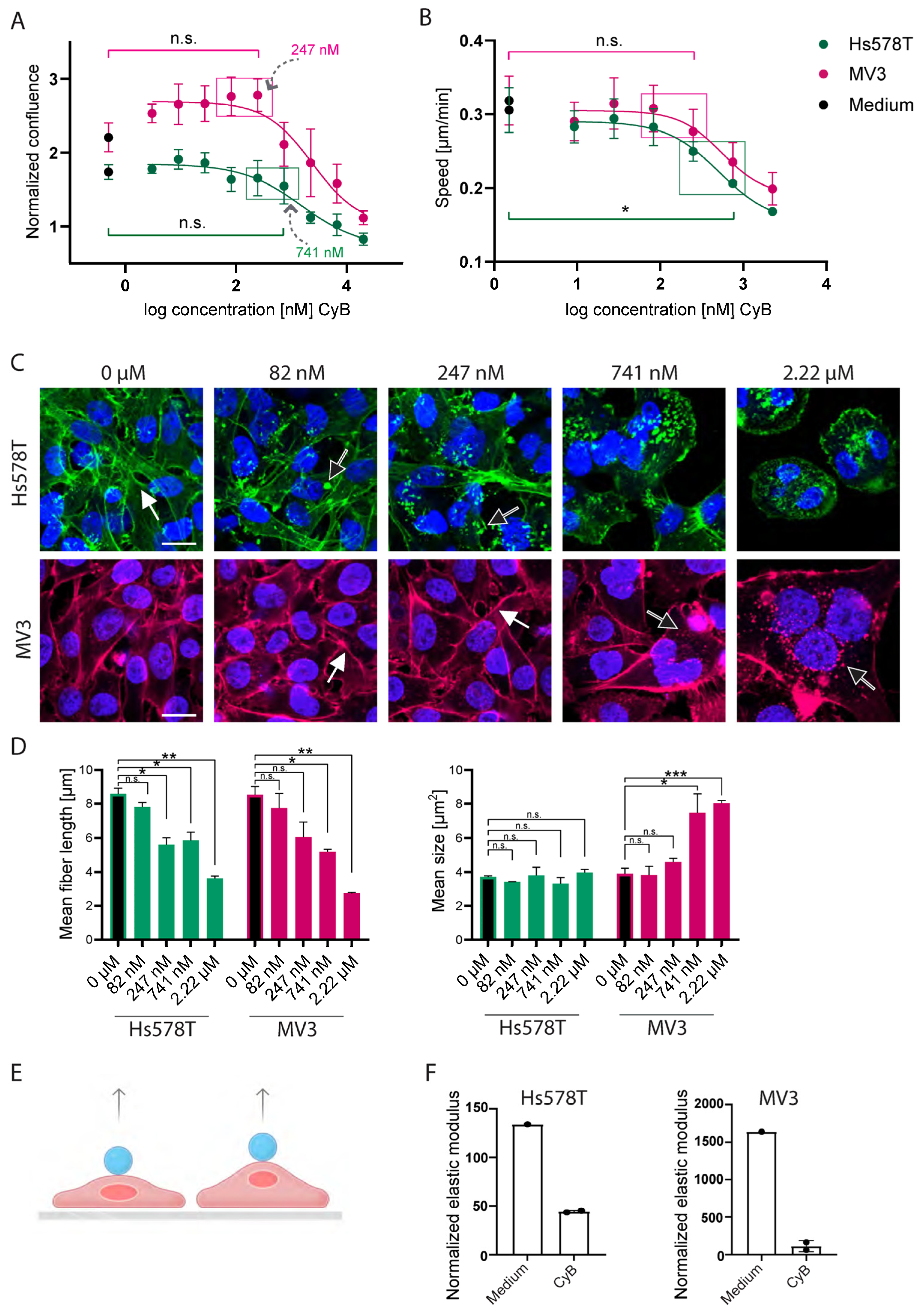
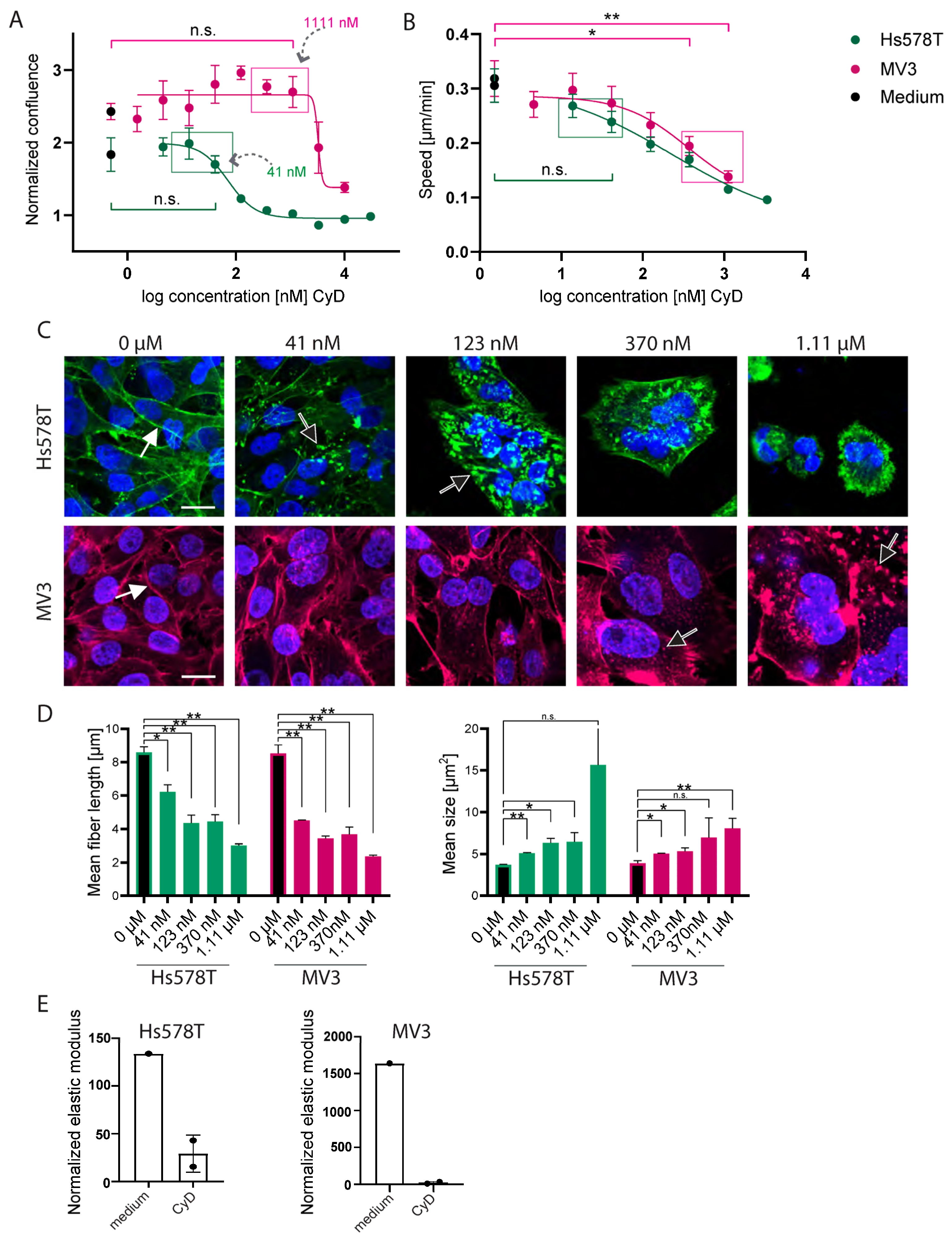
2.3. CyB and cyD Suppress 2D Migration Speed and Cell Stiffness at Non-Toxic Concentrations
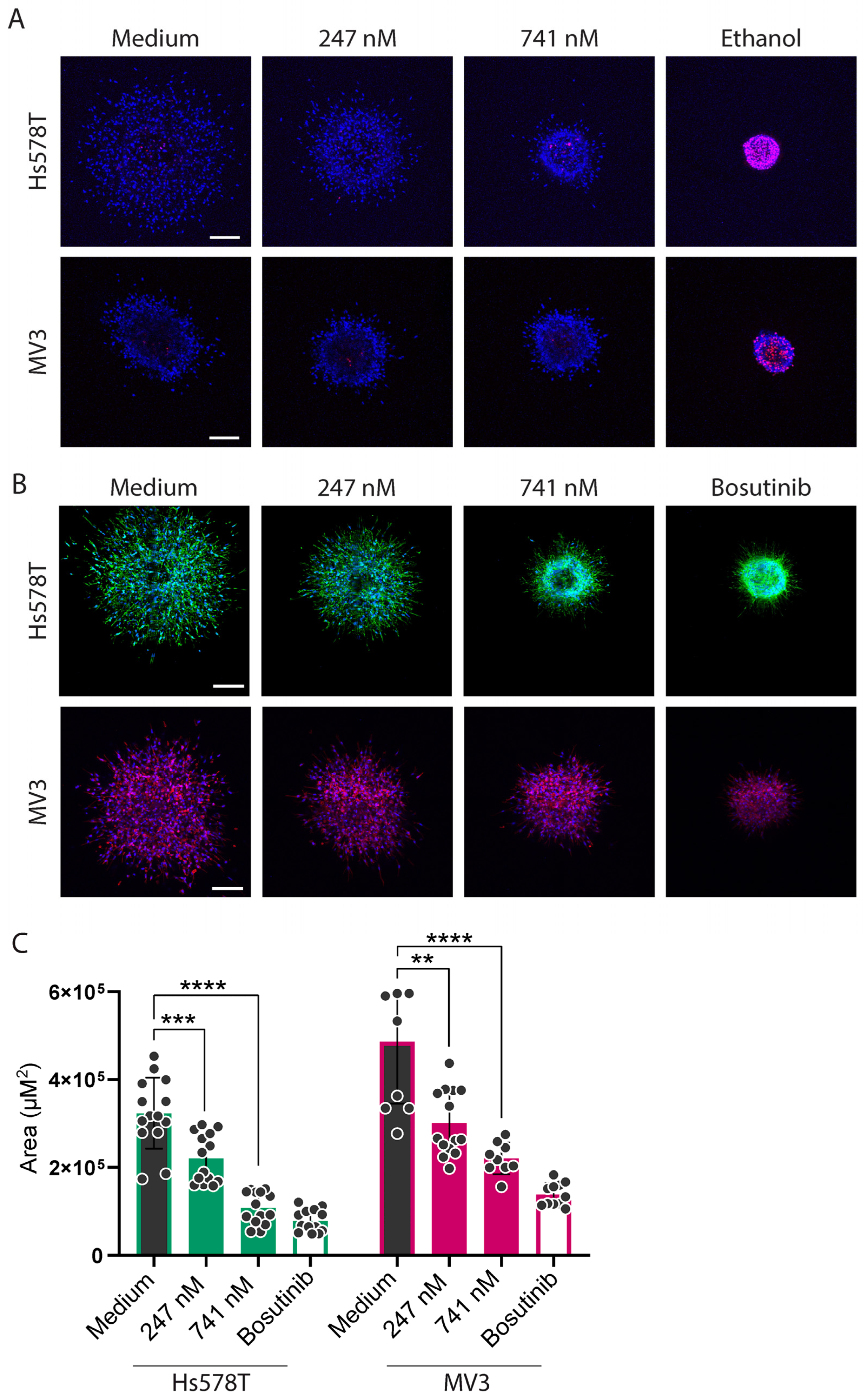
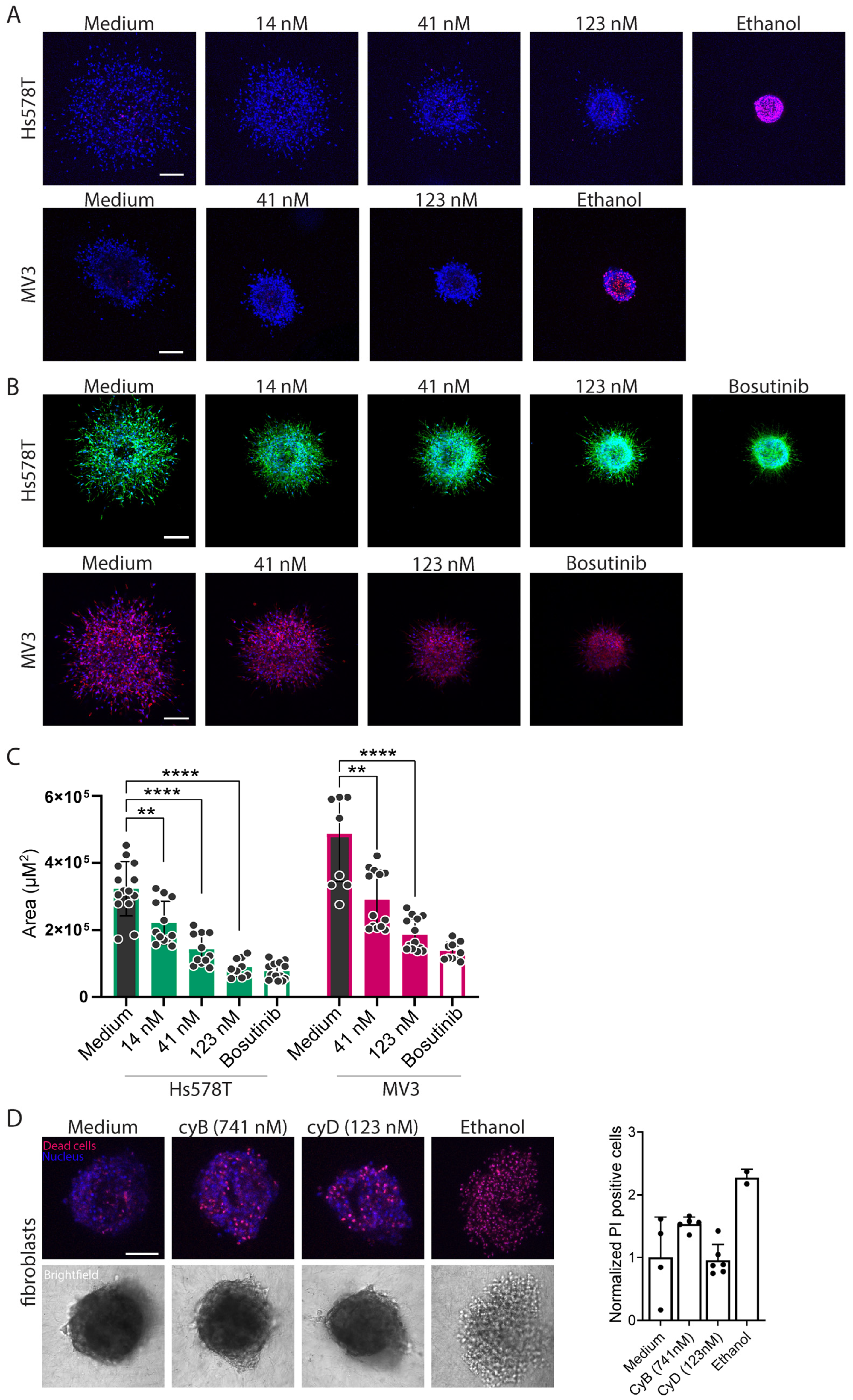
2.4. Cytochalasins Are Migrastatic in the Context of 3D ECM-Embedded Tumoroids
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Conditional Knockout Procedure
4.3. Pharmacological Inhibitors
4.4. Proliferation Assay
4.5. F-Actin Morphology
4.6. Two-Dimensional Random Cell Migration
4.7. Acoustic Force Spectroscopy (AFS)
4.8. Three-Dimensional ECM-Embedded Tumoroids
4.9. Scanning Confocal Microscopy
4.10. Data Visualization and Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ECM | Extracellular matrix |
| CFL | Cofilin-1 |
| CyB | Cytochalasin B |
| CyD | Cytochalasin D |
Appendix A

References
- Lekka, M.; Laidler, P.; Gil, D.; Lekki, J.; Stachura, Z.; Hrynkiewicz, A.Z. Elasticity of normal and cancerous human bladder cells studied by scanning force microscopy. Eur. Biophys. J. 1999, 28, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Mezencev, R.; Kim, B.; Wang, L.; McDonald, J.; Sulchek, T. Cell stiffness is a biomarker of the metastatic potential of ovarian cancer cells. PLoS ONE 2012, 7, e46609. [Google Scholar] [CrossRef] [PubMed]
- Galie, P.A.; Georges, P.C.; Janmey, P.A. How do cells stiffen? Biochem. J. 2022, 479, 1825–1842. [Google Scholar] [CrossRef] [PubMed]
- Pollard, T.D. Regulation of actin filament assembly by arp2/3 complex and formins. Annu. Rev. Biophys. Biomol. Struct. 2007, 36, 451–477. [Google Scholar] [CrossRef] [PubMed]
- Evers, T.M.J.; Holt, L.J.; Alberti, S.; Mashaghi, A. Publisher correction: Reciprocal regulation of cellular mechanics and metabolism. Nat. Metab. 2021, 3, 876–877. [Google Scholar] [CrossRef] [PubMed]
- Bamburg, J.R. Proteins of the adf/cofilin family: Essential regulators of actin dynamics. Annu. Rev. Cell Dev. Biol. 1999, 15, 185–230. [Google Scholar] [CrossRef] [PubMed]
- Power, G.; Lateef, O.M.; Ramirez-Perez, F.I.; Lazo-Fernandez, Y.; Augenreich, M.A.; Ferreira-Santos, L.; Soares, R.N.; Gonzalez-Vallejo, J.D.; Morales-Quinones, M.; Norton, C.E.; et al. Reduced cofilin activity as a mechanism contributing to endothelial cell stiffening in type 2 diabetes. Am. J. Physiol. Heart Circ. Physiol. 2025, 328, H84–H92. [Google Scholar] [CrossRef] [PubMed]
- Ullo, M.F.; Logue, J.S. Adf and cofilin-1 collaborate to promote cortical actin flow and the leader bleb-based migration of confined cells. eLife 2021, 10, e67856. [Google Scholar] [CrossRef] [PubMed]
- Goode, B.L.; Eskin, J.; Shekhar, S. Mechanisms of actin disassembly and turnover. J. Cell Biol. 2023, 222, e202309021. [Google Scholar] [CrossRef] [PubMed]
- Morton, W.M.; Ayscough, K.; Mclaughlin, P. Latrunculin alters the actin-monomer subunit interface to prevent polymerization. Nat. Cell Biol. 2000, 2, 376–378. [Google Scholar] [CrossRef] [PubMed]
- MacLean-Fletcher, S.; Pollard, T. Mechanism of action of cytochalasin b on actin. Cell 1980, 20, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Casella, J.F.; Flanagan, M.D.; Lin, S. Cytochalasin d inhibits actin polymerization and induces depolymerization of actin filaments formed during platelet shape change. Nature 1981, 293, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Bubb, M.; Senderowicz, A.; Sausville, E.; Duncan, K.; Korn, E. Jasplakinolide, a cytotoxic natural product, induces actin polymerization and competitively inhibits the binding of phalloidin to f-actin. J. Biol. Chem. 1994, 269, 14869–14871. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.A. Effects of cytochalasin and phalloidin on actin. J. Cell Biol. 1987, 105, 1473–1478. [Google Scholar] [CrossRef] [PubMed]
- Bonder, E.M.; Mooseker, M.S. Cytochalasin b slows but does not prevent monomer addition at the barbed end of the actin filament. J. Cell Biol. 1986, 102, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Goddette, D.W.; Frieden, C. Actin polymerization. The mechanism of action of cytochalasin d. J. Biol. Chem. 1986, 261, 15974–15980. [Google Scholar] [CrossRef] [PubMed]
- Shoji, K.; Ohashi, K.; Sampei, K.; Oikawa, M.; Mizuno, K. Cytochalasin d acts as an inhibitor of the actin–cofilin interaction. Biochem. Biophys. Res. Commun. 2012, 424, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Song, K.; Jin, E.-J.; Sonn, J. Staurosporine and cytochalasin d induce chondrogenesis by regulation of actin dynamics in different way. Exp. Mol. Med. 2012, 44, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Lambert, C.; Schmidt, K.; Karger, M.; Stadler, M.; Stradal, T.E.B.; Rottner, K. Cytochalasans and their impact on actin filament remodeling. Biomolecules 2023, 13, 1247. [Google Scholar] [CrossRef] [PubMed]
- Vicar, T.; Chmelik, J.; Navratil, J.; Kolar, R.; Chmelikova, L.; Cmiel, V.; Jagos, J.; Provaznik, I.; Masarik, M.; Gumulec, J. Cancer cell viscoelasticity measurement by quantitative phase and flow stress induction. Biophys. J. 2022, 121, 1632–1642. [Google Scholar] [CrossRef] [PubMed]
- Connolly, S.; McGourty, K.; Newport, D. The influence of cell elastic modulus on inertial positions in poiseuille microflows. Biophys. J. 2021, 120, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Zhang, L.; Kang, H.; Zhang, B.; Bao, G.; Wang, J. Mechanical, nanomorphological and biological reconstruction of early-stage apoptosis in hela cells induced by cytochalasin b. Oncol. Rep. 2019, 41, 928–938. [Google Scholar] [CrossRef] [PubMed]
- Hayot, C.; Debeir, O.; Van Ham, P.; Van Damme, M.; Kiss, R.; Decaestecker, C. Characterization of the activities of actin-affecting drugs on tumor cell migration. Toxicol. Appl. Pharmacol. 2006, 211, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Keurhorst, D.; Liashkovich, I.; Frontzek, F.; Nitzlaff, S.; Hofschröer, V.; Dreier, R.; Stock, C. Mmp3 activity rather than cortical stiffness determines nhe1-dependent invasiveness of melanoma cells. Cancer Cell Int. 2019, 19, 285. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, F.; Sommariva, M.; Cornaghi, L.B.; Denti, L.; Nava, A.; Arnaboldi, F.; Moscheni, C.; Gagliano, N. Mechanical cues, ecadherin expression and cell “sociality” are crucial crossroads in determining pancreatic ductal adenocarcinoma cells behavior. Cells 2022, 11, 1318. [Google Scholar] [CrossRef] [PubMed]
- Hitsuda, A.; Dan, R.; Urakawa, A.; Hiraoka, Y.; Murakami, C.; Yamamoto, H.; Tanaka, A.R. 25-hydroxycholesterol–induced cell death via activation of rock/limk/cofilin axis in colorectal cancer cell spheroids. J. Steroid Biochem. Mol. Biol. 2022, 216, 106037. [Google Scholar] [CrossRef] [PubMed]
- Remsing Rix, L.L.; Rix, U.; Colinge, J.; Hantschel, O.; Bennett, K.L.; Stranzl, T.; Müller, A.; Baumgartner, C.; Valent, P.; Augustin, M.; et al. Global target profile of the kinase inhibitor bosutinib in primary chronic myeloid leukemia cells. Leukemia 2009, 23, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Doe, C.; Bentley, R.; Behm, D.J.; Lafferty, R.; Stavenger, R.; Jung, D.; Bamford, M.; Panchal, T.; Grygielko, E.; Wright, L.L.; et al. Novel rho kinase inhibitors with anti-inflammatory and vasodilatory activities. J. Pharmacol. Exp. Ther. 2007, 320, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, R.; Baradaran, B.; Khordadmehr, M.; Safaei, S.; Baghbanzadeh, A.; Jigari, F.; Ezzati, H. Targeting rock signaling in health, malignant and non-malignant diseases. Immunol. Lett. 2020, 219, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Amano, M.; Ito, M.A.; Kimura, K.; Fukata, Y.; Chihara, K.; Nakano, T.; Matsuura, Y.; Kaibuchi, K. Phosphorylation and activation of myosin by rho-associated kinase (rhokinase). J. Biol. Chem. 1996, 271, 20246–20249. [Google Scholar] [CrossRef] [PubMed]
- Kovács, M.; Tóth, J.; Hetényi, C.; Málnási-Csizmadia, A.; Sellers, J. Mechanism of blebbistatin inhibition of myosin ii. J. Biol. Chem. 2004, 279, 35557–35563. [Google Scholar] [CrossRef] [PubMed]
- Rosel, D.; Fernandes, M.; Sanz-Moreno, V.; Brábek, J. Migrastatics: Redirecting r&d in solid cancer towards metastasis? Trends Cancer 2019, 5, 755–756. [Google Scholar] [CrossRef] [PubMed]
- Kanellos, G.; Zhou, J.; Patel, H.; Ridgway, R.; Huels, D.; Gurniak, C.; Sandilands, E.; Carragher, N.; Sansom, O.; Witke, W.; et al. Adf and cofilin1 control actin stress fibers, nuclear integrity, and cell survival. Cell Rep. 2015, 13, 1949–1964. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Cordero, J.J.; Magalhaes, M.A.O.; Eddy, R.J.; Hodgson, L.; Condeelis, J. Functions of cofilin in cell locomotion and invasion. Nat. Rev. Mol. Cell Biol. 2013, 14, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-H.; Kundu, J.K.; Chae, J.-I.; Shim, J.-H. Targeting rock/limk/cofilin signaling pathway in cancer. Arch. Pharmacal Res. 2019, 42, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Antmen, E.; Demirci, U.; Hasirci, V. Polymeric micropatterned substrates: A tool for imaging and study of breast cancer cell morphology. Adv. Biol. 2021, 5, e2000048. [Google Scholar]
- Shankar, J.; Nabi, I.R. Correction: Actin cytoskeleton regulation of epithelial mesenchymal transition in metastatic cancer cells. PLoS ONE 2015, 10, e0132759. [Google Scholar] [CrossRef] [PubMed]
- Garcia, K.Y.M.; Quimque, M.T.J.; Lambert, C.; Schmidt, K.; Primahana, G.; Stradal, T.E.B.; Ratzenböck, A.; Dahse, H.-M.; Phukhamsakda, C.; Stadler, M.; et al. Antiproliferative and cytotoxic cytochalasins from sparticola triseptata inhibit actin polymerization and aggregation. J. Fungi 2022, 8, 560. [Google Scholar] [CrossRef] [PubMed]
- Hamann, I.; Krys, D.; Glubrecht, D.; Bouvet, V.; Marshall, A.; Vos, L.; Mackey, J.R.; Wuest, M.; Wuest, F. Expression and function of hexose transporters glut1, glut2, and glut5 in breast cancer—Effects of hypoxia. FASEB J. 2018, 32, 5104–5118. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, L.; Wu, X.; Wen, B.; Lin, W.; Cao, Y.; Xie, L.; Zhang, H.; Dong, G.; Li, E.; et al. Anti-tumour effects of a macrolide analog f806 in oesophageal squamous cell carcinoma cells by targeting and promoting glut1 autolysosomal degradation. FEBS J. 2022, 289, 6782–6798. [Google Scholar] [CrossRef] [PubMed]
- Akopian, A.; Szikra, T.; Cristofanilli, M.; Krizaj, D. Glutamate-induced Ca2+ influx in thirdorder neurons of salamander retina is regulated by the actin cytoskeleton. Neuroscience 2006, 138, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.K.; Song, W. The actin cytoskeleton is required for the trafficking of the b cell antigen receptor to the late endosomes. Traffic 2001, 2, 414–427. [Google Scholar] [CrossRef] [PubMed]
- Rotsch, C.; Radmacher, M. Drug-induced changes of cytoskeletal structure and mechanics in fibroblasts: An atomic force microscopy study. Biophys. J. 2000, 78, 520–535. [Google Scholar] [CrossRef] [PubMed]
- Kunschmann, T.; Puder, S.; Fischer, T.; Steffen, A.; Rottner, K.; Mierke, C. The small gtpase rac1 increases cell surface stiffness and enhances 3d migration into extracellular matrices. Sci. Rep. 2019, 9, 7675. [Google Scholar] [CrossRef] [PubMed]
- Cross, S.; Jin, Y.; Rao, J.; Gimzewski, J. Nanomechanical analysis of cells from cancer patients. Nat. Nanotechnol. 2007, 2, 780–783. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Pegoraro, A.; Li, H.; Li, K.; Yuan, Y.; Xu, G.; Gu, Z.; Sun, J.; Hao, Y.; Gupta, S.; et al. Cell swelling, softening and invasion in a three-dimensional breast cancer model. Nat. Phys. 2019, 16, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, V.; Mythreye, K.; O’Brien, E.T.; Berchuck, A.; Blobe, G.C.; Superfine, R. Mechanical stiffness grades metastatic potential in patient tumor cells and in cancer cell lines. Cancer Res. 2011, 71, 5075–5080. [Google Scholar] [CrossRef] [PubMed]
- Guck, J.; Schinkinger, S.; Lincoln, B.; Wottawah, F.; Ebert, S.; Romeyke, M.; Lenz, D.; Erickson, H.M.; Ananthakrishnan, R.; Mitchell, D.; et al. Optical deformability as an inherent cell marker for testing malignant transformation and metastatic competence. Biophys. J. 2005, 88, 3689–3698. [Google Scholar] [CrossRef] [PubMed]
- Kashani, A.; Packirisamy, M. Cancer cells optimize elasticity for efficient migration. R. Soc. Open Sci. 2020, 7, 200747. [Google Scholar] [CrossRef] [PubMed]
- van Muijen, G.N.P.; Jansen, K.F.J.; Cornelissen, I.M.H.A.; Smeets, D.F.C.M.; Beck, J.L.M.; Ruiter, D.J. Establishment and characterization of a human melanoma cell line (mv3) which is highly metastatic in nude mice. Int. J. Cancer 1991, 48, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.S.W.; Haaland, B.; Gan, J.M.; Tham, S.C.; Sinha, I.; Tan, E.H.; Lim, K.H.; Takano, A.; Krisna, S.S.; Thu, M.M.M.; et al. Bosutinib inhibits migration and invasion via ack1 in kras mutant non-small cell lung cancer. Mol. Cancer 2014, 13, 13. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Verbeek, F.J. Segmentation for high-throughput image analysis: Watershed masked clustering. In Leveraging Applications of Formal Methods, Verification and Validation. Applications and Case Studies: 5th International Symposium, ISoLA 2012, Heraklion, Crete, Greece, October 15–18, 2012, Proceedings, Part II 5; Springer: Berlin/Heidelberg, Germany, 2012; pp. 25–41. [Google Scholar]
- Burger, G. CPTrackRApp. Available online: https://github.com/burgerga/CPTrackRApp/ (accessed on 1 February 2023).
- Evers, T.M.J.; Babaei, M.; Mashaghi, A. Probing human immune cell mechanics using acoustic force spectroscopy. STAR Protoc. 2024, 5, 102861. [Google Scholar] [CrossRef] [PubMed]
- Rajan, N.; Habermehl, J.; Coté, M.-F.; Doillon, C.J.; Mantovani, D. Preparation of ready-to-use, storable and reconstituted type i collagen from rat tail tendon for tissue engineering applications. Nat. Protoc. 2006, 1, 2753–2758. [Google Scholar] [CrossRef] [PubMed]
- Truong, H.H.; Xiong, J.; Ghotra, V.P.S.; Nirmala, E.; Haazen, L.; Le Dévédec, S.E.; Balcioğlu, H.E.; He, S.; Snaar-Jagalska, B.E.; Vreugdenhil, E.; et al. β1 integrin inhibition elicits a prometastatic switch through the tgfβ–mir200–zeb network in e-cadherin–positive triple-negative breast cancer. Sci. Signal. 2014, 7, ra15. [Google Scholar] [CrossRef] [PubMed]
- Truong, H.H.; de Sonneville, J.; Ghotra, V.P.S.; Xiong, J.; Price, L.; Hogendoorn, P.C.W.; Spaink, H.H.; van de Water, B.; Danen, E.H.J. Automated microinjection of cell-polymer suspensions in 3d ecm scaffolds for high-throughput quantitative cancer invasion screens. Biomaterials 2012, 33, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Beslmüller, K.; de Mercado, R.R.; Koenderink, G.H.; Danen, E.H.J.; Schmidt, T. Matrix stiffness affects spheroid invasion, collagen remodeling, and effective reach of stress into ecm. Organoids 2025, 4, 11. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beslmüller, K.; van Megen, L.J.A.; Struik, T.; Batenburg, D.; Neubert, E.; Evers, T.M.J.; Mashaghi, A.; Danen, E.H.J. Cytochalasins Suppress 3D Migration of ECM-Embedded Tumoroids at Non-Toxic Concentrations. Int. J. Mol. Sci. 2025, 26, 7021. https://doi.org/10.3390/ijms26147021
Beslmüller K, van Megen LJA, Struik T, Batenburg D, Neubert E, Evers TMJ, Mashaghi A, Danen EHJ. Cytochalasins Suppress 3D Migration of ECM-Embedded Tumoroids at Non-Toxic Concentrations. International Journal of Molecular Sciences. 2025; 26(14):7021. https://doi.org/10.3390/ijms26147021
Chicago/Turabian StyleBeslmüller, Klara, Lieke J. A. van Megen, Timo Struik, Daisy Batenburg, Elsa Neubert, Tom M. J. Evers, Alireza Mashaghi, and Erik H. J. Danen. 2025. "Cytochalasins Suppress 3D Migration of ECM-Embedded Tumoroids at Non-Toxic Concentrations" International Journal of Molecular Sciences 26, no. 14: 7021. https://doi.org/10.3390/ijms26147021
APA StyleBeslmüller, K., van Megen, L. J. A., Struik, T., Batenburg, D., Neubert, E., Evers, T. M. J., Mashaghi, A., & Danen, E. H. J. (2025). Cytochalasins Suppress 3D Migration of ECM-Embedded Tumoroids at Non-Toxic Concentrations. International Journal of Molecular Sciences, 26(14), 7021. https://doi.org/10.3390/ijms26147021





