Unraveling the Complexity of Plant Trichomes: Models, Mechanisms, and Bioengineering Strategies
Abstract
1. Introduction
2. Epidermal Appendage
2.1. Root Hairs: Plant Growth and Intercellular Signaling
2.2. Prickles: Morphological Adaptations and Functional Diversity
2.3. Trichomes: Non-Glandular and Glandular Variants
3. Non-Glandular Trichomes: A Model System for Studying Cell Differentiation
3.1. Structure and Morphology Characteristics of NGTs
3.2. Biological Functions of NGTs
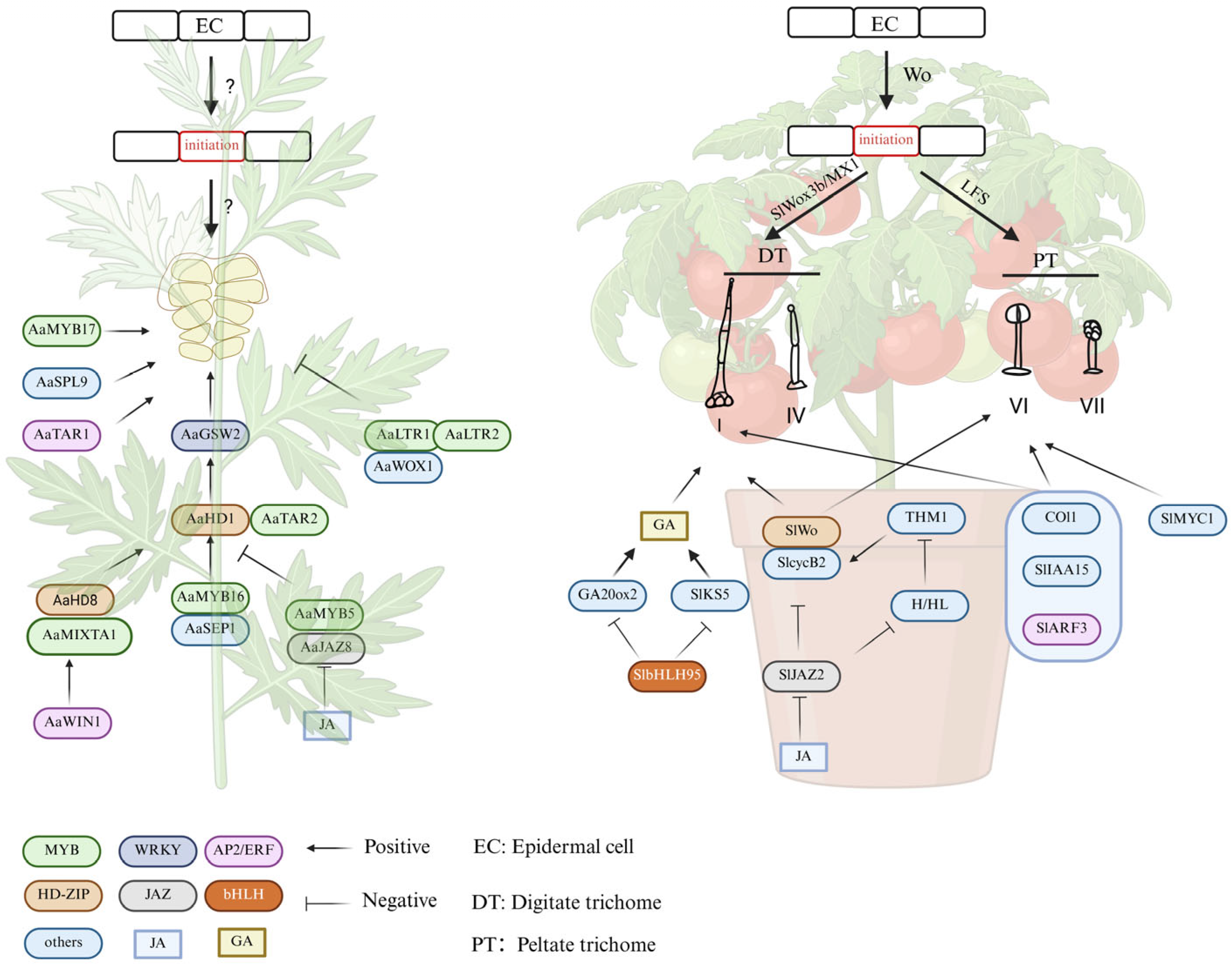
3.3. Regulatory Mechanism of NGTs Development
4. Glandular Secretory Trichomes (GSTs): Natural Biofactories
4.1. Structure and Morphology Characteristics of GSTs

4.2. Secondary Metabolite Synthesis in GSTs
4.3. Regulatory Mechanism of GSTs Development
5. Applications and Future Perspectives
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Chalvin, C.; Drevensek, S.; Dron, M.; Bendahmane, A.; Boualem, A. Genetic Control of Glandular Trichome Development. Trends Plant Sci. 2020, 25, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Boehm, C.R.; Bock, R. Recent Advances and Current Challenges in Synthetic Biology of the Plastid Genetic System and Metabolism. Plant Physiol. 2019, 179, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Grierson, C.; Nielsen, E.; Ketelaarc, T.; Schiefelbein, J. Root hairs. Arab. Book 2014, 12, e0172. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Simonneau, F.; Thouroude, T.; Oyant, L.H.; Foucher, F. Morphological Studies of Rose Prickles Provide New Insights. Hortic. Res. 2021, 8, 221. [Google Scholar] [CrossRef]
- Han, G.; Li, Y.; Yang, Z.; Wang, C.; Zhang, Y.; Wang, B. Molecular Mechanisms of Plant Trichome Development. Front. Plant Sci. 2022, 13, 910228. [Google Scholar] [CrossRef]
- Kim, Y.; Chung, Y.S.; Lee, E.; Tripathi, P.; Heo, S.; Kim, K.H. Root response to drought stress in rice (Oryza sativa L.). Int. J. Mol. Sci. 2020, 21, 1513. [Google Scholar] [CrossRef]
- de Jesus Vieira Teixeira, C.; Bellande, K.; van der Schuren, A.; O’Connor, D.; Hardtke, C.S.; Vermeer, J.E.M. An atlas of Brachypodium distachyon lateral root development. Biol. Open. 2024, 13, bio060531. [Google Scholar] [CrossRef]
- Yasin, M.U.; Liu, Y.; Wu, M.; Chen, N.; Gan, Y. Regulatory mechanisms of trichome and root hair development in Arabidopsis. Plant Mol. Biol. 2025, 115, 14. [Google Scholar] [CrossRef]
- Kellogg, A.A.; Branaman, T.J.; Jones, N.M.; Little, C.Z.; Swanson, J.D. Morphological studies of developing Rubus prickles suggest that they are modified glandular trichomes. Botany 2011, 89, 217–226. [Google Scholar] [CrossRef]
- Khadgi, A.; Weber, C.A. Morphological Characterization of Prickled and Prickle-Free Rubus Using Scanning Electron Microscopy. Hort Sci. 2020, 55, 676–683. [Google Scholar] [CrossRef]
- Zhou, N.N.; Tang, K.X.; Jeauffre, J.; Thouroude, T.; Arias, D.L.; Foucher, F.; Oyant, L.H.S. Genetic determinism of prickles in rose. Theor. Appl. Genet. 2020, 133, 3017–3035. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhu, Y.; Jia, H.; Han, Y.; Zheng, X.; Wang, M.; Feng, W. From Plant to Yeast—Advances in Biosynthesis of Artemisinin. Molecules 2022, 27, 6888. [Google Scholar] [CrossRef] [PubMed]
- Livingston, S.J.; Quilichini, T.D.; Booth, J.K.; Wong, D.C.J.; Rensing, K.H.; Laflamme-Yonkman, J.; Castellarin, S.D.; Bohlmann, J.; Page, J.E.; Samuels, A.L. Cannabis Glandular Trichomes Alter Morphology and Metabolite Content during Flower Maturation. Plant J. 2020, 101, 37–56. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, J.; Stiller, W.N.; Wilson, L.J. Identification of Host Plant Resistance to Silverleaf Whitefly in Cotton: Implications for Breeding. Field Crops Res. 2013, 154, 145–152. [Google Scholar] [CrossRef]
- Huchelmann, A.; Boutry, M.; Hachez, C. Plant Glandular Trichomes: Natural Cell Factories of High Biotechnological Interest. Plant Physiol. 2017, 175, 6–22. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Y.; Li, L.; Tang, K.; Hao, X.; Kai, G. Advanced Metabolic Engineering Strategies for Increasing Artemisinin Yield in Artemisia annua L. Hortic. Res. 2024, 11, uhad292. [Google Scholar] [CrossRef]
- Gao, S.; Wang, B.; Xie, S.; Xu, X.; Zhang, J.; Pei, L.; Yu, Y.; Yang, W.; Zhang, Y. A High-Quality Reference Genome of Wild Cannabis Sativa. Hortic. Res. 2020, 7, 73. [Google Scholar] [CrossRef]
- Zhou, Y.; Tang, N.; Huang, L.; Zhao, Y.; Tang, X.; Wang, K. Effects of Salt Stress on Plant Growth, Antioxidant Capacity, Glandular Trichome Density, and Volatile Exudates of Schizonepeta Tenuifolia Briq. Int. J. Mol. Sci. 2018, 19, 252. [Google Scholar] [CrossRef]
- Blanco-Sánchez, L.; Planelló, R.; Llorente, L.; Díaz-Pendón, J.A.; Ferrero, V.; Fernández-Muñoz, R.; Herrero, Ó.; De La Peña, E. Characterization of the Detrimental Effects of Type IV Glandular Trichomes on the Aphid Macrosiphum euphorbiae in Tomato. Pest Manag. Sci. 2021, 77, 4117–4127. [Google Scholar] [CrossRef]
- Hülskamp, M. Plant Trichomes: A Model for Cell Differentiation. Nat. Rev. Mol. Cell Biol. 2004, 5, 471–480. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Q.; Meng, Z.; Abid, M.A.; Wang, Y.; Wei, Y.; Guo, S.; Zhang, R.; Liang, C. Multi-dimensional molecular regulation of trichome development in Arabidopsis and cotton. Front. Plant Sci. 2022, 13, 892381. [Google Scholar] [CrossRef]
- Mathur, V.; Tytgat, T.O.; de Graaf, R.M.; Kalia, V.; Sankara Reddy, A.; Vet, L.E.; van Dam, N.M. Dealing with double trouble: Consequences of single and double herbivory in Brassica juncea. Chemoecology 2013, 23, 71–82. [Google Scholar] [CrossRef]
- Qin, Y.; Sun, M.; Li, W.; Xu, M.; Shao, L.; Liu, Y.; Zhao, G.; Liu, Z.; Xu, Z.; You, J.; et al. Single-cell RNA-seq reveals fate determination control of an individual fibre cell initiation in cotton (Gossypium hirsutum). Plant Biotechnol. J. 2022, 20, 2372–2388. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Fan, L.; Liu, Z.; Yang, X.; Zhang, Z.; Duan, Z.; Liang, Q.; Imran, M.; Zhang, M.; Tian, Z. A Pd1-Ps-P1 Feedback Loop Controls Pubescence Density in Soybean. Mol. Plant 2020, 13, 1768–1783. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Fang, C.; Yuan, Z.; Hu, Q.; Huang, W.; Li, H.; Ma, R.; Wang, L.; Su, T.; Li, S.; et al. A Retrotransposon Insertion in the Mao1 Promoter Results in Erect Pubescence and Higher Yield in Soybean. Proc. Natl. Acad. Sci. USA 2023, 120, e2210791120. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Lyu, X.; Liu, J.; Gao, W.; Ma, Y.; Liao, N.; Li, Z.; Bo, Y.; Hu, Z.; Yang, J.; et al. Structural variation of GL1 gene deter-mines the trichome formation in Brassica juncea. Theor. Appl. Genet. 2023, 136, 75. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, M.; Liu, Y.; Li, Z.; Hu, Z.; Zhang, M.; Yang, J. An HD-Zip III transcription factor, BjPHVa, negatively regulates non-glandular trichome formation in Brassica juncea. Physiol. Plant. 2024, 176, e14553. [Google Scholar] [CrossRef]
- Happyana, N.; Agnolet, S.; Muntendam, R.; Van Dam, A.; Schneider, B.; Kayser, O. Analysis of Cannabinoids in Laser-Microdissected Trichomes of Medicinal Cannabis Sativa Using LCMS and Cryogenic NMR. Phytochemistry 2013, 87, 51–59. [Google Scholar] [CrossRef]
- Xie, Z.; Mi, Y.; Kong, L.; Gao, M.; Chen, S.; Chen, W.; Meng, X.; Sun, W.; Chen, S.; Xu, Z. Cannabis Sativa: Origin and History, Glandular Trichome Development, and Cannabinoid Biosynthesis. Hortic. Res. 2023, 10, uhad150. [Google Scholar] [CrossRef]
- Xie, L.; Yan, T.; Li, L.; Chen, M.; Hassani, D.; Li, Y.; Qin, W.; Liu, H.; Chen, T.; Fu, X.; et al. An HD-ZIP-MYB Complex Regulates Glandular Secretory Trichome Initiation in Artemisia annua. New Phytol. 2021, 231, 2050–2064. [Google Scholar] [CrossRef]
- Yan, T.; Li, L.; Xie, L.; Chen, M.; Shen, Q.; Pan, Q.; Fu, X.; Shi, P.; Tang, Y.; Huang, H.; et al. A Novel HD-ZIP IV/MIXTA Complex Promotes Glandular Trichome Initiation and Cuticle Development in Artemisia annua. New Phytol. 2018, 218, 567–578. [Google Scholar] [CrossRef]
- Tattini, M.; Guidi, L.; Morassi-Bonzi, L.; Pinelli, P.; Remorini, D.; Degl’Innocenti, E.; Giordano, C.; Massai, R.; Agati, G. On the role of flavonoids in the integrated mechanisms of response of Ligustrum vulgare and Phillyrea latifolia to high solar radiation. New Phytol. 2025, 167, 457–470. [Google Scholar] [CrossRef]
- Yang, S.; Xue, S.; Shan, L.; Fan, S.; Sun, L.; Dong, Y.; Li, S.; Gao, Y.; Qi, Y.; Yang, L.; et al. The CsTM alters multicellular trichome morphology and enhances resistance against aphid by interacting with CsTIP1; 1 in cucumber. J. Adv. Res. 2025, 69, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.; Dong, M.; Liu, X.; Xu, S.; Pang, J.; Zhang, W.; Weng, Y.; Ren, H. Classification of Fruit Trichomes in Cucumber and Effects of Plant Hormones on Type II Fruit Trichome Development. Planta 2019, 249, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, J.; Miao, J.; Sun, G.; Bai, H.; Xiao, J.; Sun, M.; Shi, L. Micromorphology and Molecular Insights Into Glandular Trichomes in Two Different Thymes: Glandular Trichomes Formation Process and the Function of the Main Regulator TqHD1. Plant Cell Environ. 2025, 48, 6269–6284. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Wang, Q.; Tong, B.; Liu, D.; Liu, H.; Yan, Q.; Xin, H. Morphological studies of developing glandular trichomes and a novel HD-ZIP gene LjROC3 increasing glandular trichome density on leaves in honeysuckle (Lonicera japonica). Ind. Crops Prod. 2023, 198, 116696. [Google Scholar] [CrossRef]
- Huang, X.; Chen, W.; Zhao, Y.; Chen, J.; Ouyang, Y.; Li, M.; Gu, Y.; Wu, Q.; Cai, S.; Guo, F.; et al. Deep learning-based quantification and transcriptomic profiling reveal a methyl jasmonate-mediated glandular trichome formation pathway in Cannabis sativa. Plant J. 2024, 118, 1155–1173. [Google Scholar] [CrossRef]
- Luo, X.; Reiter, M.A.; d’Espaux, L.; Wong, J.; Denby, C.M.; Lechner, A.; Zhang, Y.; Grzybowski, A.T.; Harth, S.; Lin, W.; et al. Complete Biosynthesis of Cannabinoids and Their Unnatural Analogues in Yeast. Nature 2019, 567, 123–126. [Google Scholar] [CrossRef]
- Talman, A.M.; Clain, J.; Duval, R.; Ménard, R.; Ariey, F. Artemisinin Bioactivity and Resistance in Malaria Parasites. Trends Parasitol. 2019, 35, 953–963. [Google Scholar] [CrossRef]
- Wang, J.; Xu, C.; Wong, Y.K.; Li, Y.; Liao, F.; Jiang, T.; Tu, Y. Artemisinin, the Magic Drug Discovered from Traditional Chinese Medicine. Engineering 2019, 5, 32–39. [Google Scholar] [CrossRef]
- Efferth, T. From Ancient Herb to Modern Drug: Artemisia annua and Artemisinin for Cancer Therapy. Semin. Cancer Biol. 2017, 46, 65–83. [Google Scholar] [CrossRef]
- Majid, I.; Kumar, A.; Abbas, N. A Basic Helix Loop Helix Transcription Factor, AaMYC2-Like Positively Regulates Artemisinin Biosynthesis in Artemisia annua L. Ind. Crops Prod. 2019, 128, 115–125. [Google Scholar] [CrossRef]
- Yan, T.; Chen, M.; Shen, Q.; Li, L.; Fu, X.; Pan, Q.; Tang, Y.; Shi, P.; Lv, Z.; Jiang, W.; et al. HOMEODOMAIN PROTEIN 1 Is Required for Jasmonate-mediated Glandular Trichome Initiation in Artemisia annua. New Phytol. 2017, 213, 1145–1155. [Google Scholar] [CrossRef]
- He, Y.; Fu, X.; Li, L.; Sun, X.; Tang, K.; Zhao, J. AaSPL9 Affects Glandular Trichomes Initiation by Positively Regulating Expression of AaHD1 in Artemisia Annu. L. Plant Sci. 2022, 317, 111172. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Xie, L.; Li, Y.; Liu, H.; Fu, X.; Chen, T.; Hassani, D.; Li, L.; Sun, X.; Tang, K. An R2R3-MYB Transcription Factor Positively Regulates the Glandular Secretory Trichome Initiation in Artemisia annua L. Front. Plant Sci. 2021, 12, 657156. [Google Scholar] [CrossRef]
- Shi, P.; Fu, X.; Shen, Q.; Liu, M.; Pan, Q.; Tang, Y.; Jiang, W.; Lv, Z.; Yan, T.; Ma, Y.; et al. The Roles of AaMIXTA1 in Regulating the Initiation of Glandular Trichomes and Cuticle Biosynthesis in Artemisia annua. New Phytol. 2018, 217, 261–276. [Google Scholar] [CrossRef]
- Xie, L.; Yan, T.; Li, L.; Chen, M.; Ma, Y.; Hao, X.; Fu, X.; Shen, Q.; Huang, Y.; Qin, W.; et al. The WRKY Transcription Factor AaGSW2 Promotes Glandular Trichome Initiation in Artemisia annua. J. Exp. Bot. 2021, 72, 1691–1701. [Google Scholar] [CrossRef]
- Wang, C.; Chen, T.; Li, Y.; Liu, H.; Qin, W.; Wu, Z.; Peng, B.; Wang, X.; Yan, X.; Fu, X.; et al. AaWIN1, an AP2/ERF Protein, Positively Regulates Glandular Secretory Trichome Initiation in Artemisia annua. Plant Sci. 2023, 329, 111602. [Google Scholar] [CrossRef]
- Chen, T.; Liu, H.; Li, Y.; Yao, X.; Qin, W.; Yan, X.; Wang, X.; Peng, B.; Zhang, Y.; Shao, J.; et al. AaSEPALLATA1 Integrates Jasmonate and Light-Regulated Glandular Secretory Trichome Initiation in Artemisia annua. Plant Physiol. 2023, 192, 1483–1497. [Google Scholar] [CrossRef]
- Zhou, Z.; Tan, H.; Li, Q.; Li, Q.; Wang, Y.; Bu, Q.; Li, Y.; Wu, Y.; Chen, W.; Zhang, L. Trichome and Artemisinin Regulator 2 Positively Regulates Trichome Development and Artemisinin Biosynthesis in Artemisia annua. New Phytol. 2020, 228, 932–945. [Google Scholar] [CrossRef]
- Matías-Hernández, L.; Jiang, W.; Yang, K.; Tang, K.; Brodelius, P.E.; Pelaz, S. AaMYB1 and Its Orthologue AtMYB61 Affect Terpene Metabolism and Trichome Development in Artemisia annua and Arabidopsis thaliana. Plant J. 2017, 90, 520–534. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Li, J.; Qiu, S.; Qi, F.; Su, H.; Bu, Q.; Jiang, R.; Tang, K.; Zhang, L.; Chen, W. The Transcription Factors TLR1 and TLR2 Negatively Regulate Trichome Density and Artemisinin Levels in Artemisia annua. JIPB 2022, 64, 1212–1228. [Google Scholar] [CrossRef]
- Tan, H.; Xiao, L.; Gao, S.; Li, Q.; Chen, J.; Xiao, Y.; Ji, Q.; Chen, R.; Chen, W.; Zhang, L. TRICHOME AND ARTEMISININ REGULATOR 1 Is Required for Trichome Development and Artemisinin Biosynthesis in Artemisia annua. Mol. Plant 2015, 8, 1396–1411. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Li, H.; Zhang, J.; Luo, Z.; Gong, P.; Zhang, C.; Li, J.; Wang, T.; Zhang, Y.; Lu, Y.; et al. A Regulatory Gene Induces Trichome Formation and Embryo Lethality in Tomato. Proc. Natl. Acad. Sci. USA 2011, 108, 11836–11841. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Gao, Y.; Xiong, C.; Yu, G.; Chang, J.; Yang, Q.; Yang, C.; Ye, Z. The Tomato B-Type Cyclin Gene, SlCycB2, Plays Key Roles in Reproductive Organ Development, Trichome Initiation, Terpenoids Biosynthesis and Prodenia litura Defense. Plant Sci. 2017, 262, 103–114. [Google Scholar] [CrossRef]
- Chen, Y.; Su, D.; Li, J.; Ying, S.; Deng, H.; He, X.; Zhu, Y.; Li, Y.; Chen, Y.; Pirrello, J.; et al. Overexpression of bHLH95, a Basic Helix–Loop–Helix Transcription Factor Family Member, Impacts Trichome Formation via Regulating Gibberellin Biosynthesis in Tomato. J. Exp. Bot. 2020, 71, 3450–3462. [Google Scholar] [CrossRef]
- Hua, B.; Chang, J.; Wu, M.; Xu, Z.; Zhang, F.; Yang, M.; Xu, H.; Wang, L.; Chen, X.; Wu, S. Mediation of JA Signalling in Glandular Trichomes by the Woolly/SlMYC1 Regulatory Module Improves Pest Resistance in Tomato. Plant Biotechnol. J. 2021, 19, 375–393. [Google Scholar] [CrossRef]
- Hua, B.; Chang, J.; Han, X.; Xu, Z.; Hu, S.; Li, S.; Wang, R.; Yang, L.; Yang, M.; Wu, S.; et al. H and HL Synergistically Regulate Jasmonate-Triggered Trichome Formation in Tomato. Hortic. Res. 2022, 9, uhab080. [Google Scholar] [CrossRef]
- Chen, G.; Klinkhamer, P.G.L.; Escobar-Bravo, R.; Leiss, K.A. Type VI Glandular Trichome Density and Their Derived Volatiles Are Differently Induced by Jasmonic Acid in Developing and Fully Developed Tomato Leaves: Implications for Thrips Resistance. Plant Sci. 2018, 276, 87–98. [Google Scholar] [CrossRef]
- Yu, X.; Chen, G.; Tang, B.; Zhang, J.; Zhou, S.; Hu, Z. The Jasmonate ZIM-Domain Protein Gene SlJAZ2 Regulates Plant Morphology and Accelerates Flower Initiation in Solanum lycopersicum Plants. Plant Sci. 2018, 267, 65–73. [Google Scholar] [CrossRef]
- Li, L.; Zhao, Y.; McCaig, B.C.; Wingerd, B.A.; Wang, J.; Whalon, M.E.; Pichersky, E.; Howe, G.A. The Tomato Homolog of CORONATINE-INSENSITIVE1 Is Required for the Maternal Control of Seed Maturation, Jasmonate-Signaled Defense Responses, and Glandular Trichome Development. Plant Cell 2004, 16, 126–143. [Google Scholar] [CrossRef] [PubMed]
- Hua, B.; Chang, J.; Xu, Z.; Han, X.; Xu, M.; Yang, M.; Yang, C.; Ye, Z.; Wu, S. HOMEODOMAIN PROTEIN8 Mediates Jasmonate-triggered Trichome Elongation in Tomato. New Phytol. 2021, 230, 1063–1077. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yan, F.; Tang, Y.; Yuan, Y.; Deng, W.; Li, Z. Auxin Response Gene SlARF3 Plays Multiple Roles in Tomato Development and Is Involved in the Formation of Epidermal Cells and Trichomes. Plant Cell Physiol. 2015, 56, 2210–2224. [Google Scholar] [CrossRef]
- Deng, W.; Yang, Y.; Ren, Z.; Audran-Delalande, C.; Mila, I.; Wang, X.; Song, H.; Hu, Y.; Bouzayen, M.; Li, Z. The Tomato SlIAA15 Is Involved in Trichome Formation and Axillary Shoot Development. New Phytol. 2012, 194, 379–390. [Google Scholar] [CrossRef]
- Wu, M.; Chang, J.; Han, X.; Shen, J.; Yang, L.; Hu, S.; Huang, B.-B.; Xu, H.; Xu, M.; Wu, S.; et al. A HD-ZIP Transcription Factor Specifies Fates of Multicellular Trichomes via Dosage-Dependent Mechanisms in Tomato. Dev. Cell 2023, 58, 278–288.e5. [Google Scholar] [CrossRef]
- Wu, M.; Bian, X.; Huang, B.; Du, Y.; Hu, S.; Wang, Y.; Shen, J.; Wu, S. HD-Zip Proteins Modify Floral Structures for Self-Pollination in Tomato. Science 2024, 384, 124–130. [Google Scholar] [CrossRef]
- Molina-Hidalgo, F.J.; Vazquez-Vilar, M.; D’Andrea, L.; Demurtas, O.C.; Fraser, P.; Giuliano, G.; Bock, R.; Orzáez, D.; Goossens, A. Engineering Metabolism in Nicotiana Species: A Promising Future. Trends Biotechnol. 2021, 39, 901–913. [Google Scholar] [CrossRef]
- Paddon, C.J.; Westfall, P.J.; Pitera, D.J.; Benjamin, K.; Fisher, K.; McPhee, D.; Leavell, M.D.; Tai, A.; Main, A.; Eng, D.; et al. High-Level Semi-Synthetic Production of the Potent Antimalarial Artemisinin. Nature 2013, 496, 528–532. [Google Scholar] [CrossRef]
- Fesenko, E.; Edwards, R. Plant Synthetic Biology: A New Platform for Industrial Biotechnology. J. Exp. Bot. 2014, 65, 1927–1937. [Google Scholar] [CrossRef]
- Liu, W.; Stewart, C.N. Plant Synthetic Biology. Trends Plant Sci. 2015, 20, 309–317. [Google Scholar] [CrossRef]
- Khairul Ikram, N.K.B.; Beyraghdar Kashkooli, A.; Peramuna, A.V.; Van Der Krol, A.R.; Bouwmeester, H.; Simonsen, H.T. Stable Production of the Antimalarial Drug Artemisinin in the Moss Physcomitrella patens. Front. Bioeng. Biotechnol. 2017, 5, 47. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, P. Recent advances and challenges in trichome research and essential oil biosynthesis in Mentha arvensis L. Ind. Crops Prod. 2016, 82, 141–148. [Google Scholar] [CrossRef]
- Li, C.; Wood, J.C.; Vu, A.H.; Hamilton, J.P.; Rodriguez Lopez, C.E.; Payne, R.M.; Serna Guerrero, D.A.; Gase, K.; Yamamoto, K.; Vaillancourt, B.; et al. Single-cell multi-omics in the medicinal plant Catharanthus roseus. Nat. Chem. Biol. 2023, 19, 1031–1041. [Google Scholar] [CrossRef]
- Sun, S.; Shen, X.; Li, Y.; Li, Y.; Wang, S.; Li, R.; Zhang, H.; Shen, G.; Guo, B.; Wei, J.; et al. Single-Cell RNA Sequencing Provides a High-Resolution Roadmap for Understanding the Multicellular Compartmentation of Specialized Metabolism. Nat. Plants 2023, 9, 179–190. [Google Scholar] [CrossRef]
- Zhao, B.; Gao, Y.; Ma, Q.; Wang, X.; Zhu, J.-K.; Li, W.; Wang, B.; Yuan, F. Global Dynamics and Cytokinin Participation of Salt Gland Development Trajectory in Recretohalophyte Limonium bicolor. Plant Physiol. 2024, 195, 2094–2110. [Google Scholar] [CrossRef] [PubMed]

| Plant Species | Trichome Types | Trichome Morphology | Major Biological Function(s) |
|---|---|---|---|
| Arabidopsis thaliana | NGT | unicellular branched | Physical barrier defense |
| Gossypium spp. | NGT | unicellular unbranched | Cellulose storage (commercial fiber) |
| Glycine max | NGT | unicellular unbranched | Enhanced photosynthetic efficiency |
| Brassica juncea | NGT | unicellular unbranched | Defense-related responses |
| Cannabis sativa | GST | Multicellular: capitate, bulbous | Secretion of cannabinoids (THC/CBD) |
| Solanum lycopersicum | GST | Multicellular: digitate, peltate | Secretion of acyl sugars, insect repellence |
| Artemisia annua | GST | Multicellular: peltate | Artemisinin secretion (antimalarial) |
| Schizonepeta tenuifolia | GST | Multicellular: peltate, capitate | Secretion of menthol-rich essential oils (antibacterial, insecticidal) |
| Lonicera japonica | GST | Multicellular: peltate, capitate | Secretion of chlorogenic acid (antioxidant, medicinal) |
| Ocimum basilicum | GST | Multicellular: bulbous, capitate | Secretion of eugenol/linalool (antibacterial, spice) |
| Phillyrea latifolia | GST | Multicellular: peltate | Secretion of triterpenoids (stress responses) |
| Cucumis sativus | GST | Multicellular: conical | Secretion of cucurbitacins (anti-herbivory) |
| Thymus vulgaris | GST | Multicellular: peltate, capitate | Secretion of thymol (antimicrobial, medicinal) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.; Ma, Y.; Qi, J. Unraveling the Complexity of Plant Trichomes: Models, Mechanisms, and Bioengineering Strategies. Int. J. Mol. Sci. 2025, 26, 7008. https://doi.org/10.3390/ijms26147008
Chen T, Ma Y, Qi J. Unraveling the Complexity of Plant Trichomes: Models, Mechanisms, and Bioengineering Strategies. International Journal of Molecular Sciences. 2025; 26(14):7008. https://doi.org/10.3390/ijms26147008
Chicago/Turabian StyleChen, Tiantian, Yanfei Ma, and Jiyan Qi. 2025. "Unraveling the Complexity of Plant Trichomes: Models, Mechanisms, and Bioengineering Strategies" International Journal of Molecular Sciences 26, no. 14: 7008. https://doi.org/10.3390/ijms26147008
APA StyleChen, T., Ma, Y., & Qi, J. (2025). Unraveling the Complexity of Plant Trichomes: Models, Mechanisms, and Bioengineering Strategies. International Journal of Molecular Sciences, 26(14), 7008. https://doi.org/10.3390/ijms26147008





