Abstract
Human leukocyte extract (HLE), a non-immunogenic dialyzable leukocyte preparation (<10 kDa), may serve as a safe adjuvant in immunotherapy. We investigated the effects of albendazole (ABZ), HLE, and their combination in Mesocestoides vogae infected mice, focusing on lymphoid cells in the peritoneal cavity, the site of larval proliferation and parasite-induced immunosuppression. Peritoneal lymphoid cells were analysed by flow cytometry and qPCR. Cells proliferative responses to ConA, LPS, and parasite excretory/secretory (E/S) antigens, cytokine production (ELISA), IgM and IgG isotypes in exudates and parasite antigen recognition (Western blot) were assessed. Efficacy was measured by larval burden and 14-3-3 gene expression in larvae. HLE combined with ABZ enhanced larval clearance and suppressed 14-3-3 gene expression in larvae. HLE and combination therapy increased CD3+ T cell frequencies, especially CD3+high, reduced regulatory CD3+/IL-10 Tregs and expression of Foxp3+. All treatments diminished CD19+/IL-10+ Bregs, correlating with lower CD9 and Atf3 mRNA levels compared to infected mice. Transcription factors T-bet expression was strongly upregulated, while GATA3 was moderately elevated. IFN-γ production and T/B cell proliferation were restored after HLE and combination therapy, partially, even in the presence of E/S antigens. IgM and total IgG levels against parasite antigens declined, while Th1-associated IgG2a increased in ABZ+HLE and HLE-treated groups. Albendazole failed to reverse the immunosuppressive Treg-type immunity but was more effective in reducing Breg populations and their functions. HLE enhanced ABZ efficacy by restoring Th1 responsiveness, reducing Treg/Breg activity, and modulating antibody profiles. It represents a promising immunomodulatory adjuvant in the treatment of the infections associated with Th2/Treg-driven immunosuppression.
1. Introduction
The immune system plays a pivotal role in defending against infectious and parasitic diseases in both humans and animals. Traditional therapy typically relies on chemotherapeutic interventions involving chemically synthesised drugs. In recent decades, however, immunotherapy has garnered considerable attention, and research is increasingly focused on the effects of bioactive molecules derived from natural sources. Mixtures of low molecular weight molecules (up to 10 kDa), obtained from disintegrated white blood cells of humans or animals through dialysis and ultrafiltration, have demonstrated beneficial immunomodulatory properties without inducing immunogenicity. These preparations have shown therapeutic potential in the treatment of various diseases [].
Commercially available human dialysable leukocyte extracts (hDLEs) in countries such as Mexico, Cuba, the Czech Republic and China have exhibited promise at diseases, characterised by impaired cell-mediated immunity, including viral [,], bacterial [,], and parasitic infections [,,]. In the murine breast cancer model, the hDLE preparation Immodin alleviated paclitaxel-induced toxicity, reduced tumour growth, and increased lymphocyte counts, decreased myeloid-derived suppressor cells in both the blood and spleen []. Similarly, hDLE preparation, Transferon™ (Instituto Politecnico Nacional, Mexico City, Mexico) exhibited anti-neoplastic effects and significantly reduced brain metastases in a murine model of prostate cancer []. Furthermore, the administration of hDLE in a study involving 143 patients with sepsis was associated with an increased survival rate []. Positive outcomes have also been reported in patients with multiple sclerosis [], chronic fatigue syndrome [], and allergic rhinitis [].
Infectious diseases caused by parasitic protozoa and helminths remain significant health and economic burdens for humans, livestock, and companion animals worldwide. These conditions are classified among the so-called neglected tropical diseases (NTDs), as they predominantly occur in tropical regions and disproportionately affect socioeconomically disadvantaged populations and domestic animals. Among the NTDs, an important subgroup comprises zoonotic larval cestodiases induced by flatworms []. The larval stages of flatworms Echinococcus multilocularis, Echinococcus granulosus, and Taenia solium causing cysticercosis are of particular concern. Although Mesocestoides vogae (syn. Mesocestoides corti) rarely infects humans [], its adult stage commonly parasitises carnivores such as dogs and cats. Rodents serve as intermediate hosts for the metacestode stage, known as the tetrathyridium, which exhibits an exceptional capacity for asexual proliferation. This characteristic is also observed in the metacestodes of Echinococcus and Taenia species, reviewed by [,]. Rapid progression of M. vogae larvae in the liver and peritoneal cavity of laboratory rodents [] makes it a valuable experimental model in pharmacological studies.
For the treatment of echinococcosis in humans, benzimidazole-based anthelmintics such as albendazole (ABZ) or mebendazole are employed, either as monotherapy or in combination with other agents [,,]. However, treatment extending over several years is frequently associated with adverse effects including bone marrow suppression, gastrointestinal disturbances, proteinuria, and hepatotoxicity []. Beyond their antiparasitic properties, these benzimidazoles have also demonstrated anticancer activities []. Given that long-term treatment of these chronic infections is typically parasitostatic rather than parasiticidal [,], and may negatively impact the immune system, combining anthelmintic therapy with an appropriate immunotherapeutic agent represents a rational and potentially more effective approach.
A notable feature of tissue-dwelling flatworm larvae is their ability to persist in the host for extended periods despite an active immune response. This reflects their sophisticated capacity to modulate host defence mechanisms. In mice, infection with M. vogae larvae initially elicits a transient Th1-type response in the spleen, liver, and peritoneal cavity. Over time, this response shifts towards Th2 and T regulatory (Treg) profiles, both at the cellular and humoral levels, thereby promoting chronic infection likely due to parasite-derived excretory/secretory (E/S) products playing central role in immune modulation [,]. The proliferative larval stages of M. vogae in the peritoneal cavity of rodents constitutes a distinct inflammatory model, wherein larvae directly interact with various immune cell populations. This specific immunological milieu presents a valuable model for investigating antiparasitic efficacy and immunomodulation following the administration of pharmacological agents and biomolecules, both in vivo and ex vivo.
Our study, based on a flatworm-induced model of peritoneal inflammation, was designed to explore the effects of human dialysable leukocyte extract (HLE) and albendazole (ABZ), administered either individually or in combination. The investigation focused on the differential immunomodulatory effects of each therapy on the phenotypic and functional changes in lymphocyte T and B subsets related to Th1, Th2, and Treg-type immunity, as well as to the production of peritoneal IgM and IgG subclass antibodies against immunogenic somatic and E/S larval antigens. These immunological parameters were then correlated with the observed reduction in larval burden.
2. Results
2.1. Phenotypic Analysis of Lymphoid Sub-Populations in the Peritoneal Cavities of Mice
In this study, we investigated whether ABZ, HLE, and their combination modulate the proportions and functions of T and B lymphocytes, as well as plasma cells and plasmablasts, representing up to ~40% of total peritoneal exudate cells (PECs). Non-adherent cells recovered after 18 h of cultivation contained a small proportion of granulocytes, mainly eosinophils, as confirmed by Giemsa-stained smears, reflecting their limited adherence to plastic. Flow cytometric analysis of non-adherent cells revealed that within the lymphocyte gate, the frequency of mature CD3+/CD19−/CD138− T cells exceeded that of CD3−/CD19+/CD138− B1 cells, the main B cell population in the murine peritoneal cavity. Compared to infected controls, HLE and combination therapies led to an increase in total CD3+ T cells (Figure 1A), particularly those expressing high levels of CD3 (Figure 1B). Since the CD3 complex acts as a co-receptor of the TCR [], this suggests enhanced functional activity. Treatment did not affect the proportions of CD4+ helper or CD8+ cytotoxic T cells (Figure 1C).
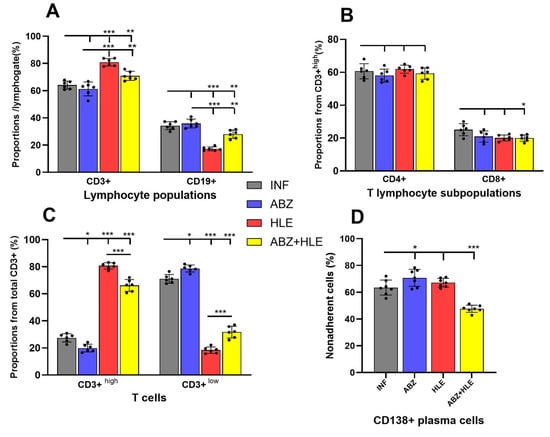
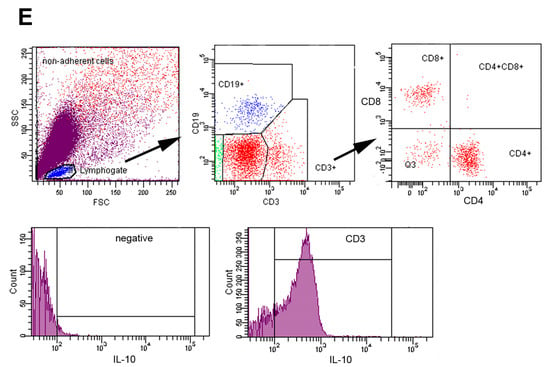
Figure 1.
Phenotypic characterisation of lymphoid subpopulations in the peritoneal cavity of mice infected with M. vogae and treated with albendazole (ABZ), human leukocyte extract (HLE), or their combination. (A) Proportion of total CD3+ T lymphocytes in the peritoneal exudate. (B) Frequency of CD3+high T cells, indicating enhanced TCR/CD3 complex expression. (C) Relative proportions of CD4+ helper and CD8+ cytotoxic T lymphocyte subsets. (D) Proportions of antibody-secreting CD19+/CD138+ plasmablasts and CD19−/CD138+ plasma cells within the non-adherent lymphoid population. (E) Gating strategy. Data represent mean ± SD (n = 7); * p < 0.05 ** p < 0.01, *** p < 0.001 vs. infected group.
Antigen-driven activation of B cells yielded large, short-lived CD19+/CD138+ plasmablasts and terminally differentiated CD19−/CD138+ plasma cells, which dominated the non-adherent fraction (Figure 1D). A significant reduction in these populations was observed only after ABZ+HLE treatment (p < 0.001).
2.2. HLE and Combination Therapy Reduce the Frequency of IL-10-Producing T and B Lymphocytes
Regulatory B cells (Bregs) and effector B cell subsets differentiation pathways are based on the priming signals received []. Breg-derived plasmablasts and plasma cells, as well as helminth-derived E/S products—particularly those rich in saccharides—can stimulate IL-10 production, promoting both Breg and Treg regulatory activities [,]. Flow cytometric analysis revealed that over 60% of CD3+ T (Figure 2A). In contrast, HLE alone or in combination with ABZ significantly cells in infected mice expressed IL-10, a pattern similarly observed following ABZ treatment (reduced the proportions of IL-10+ CD3+ T cells (p < 0.001), indicating attenuation of Treg-mediated suppression. Similarly, IL-10–producing CD19+ B1 cells were significantly diminished in all treated groups compared with infected controls (p < 0.001) (Figure 2B), suggesting suppression of infection-induced Breg responses.
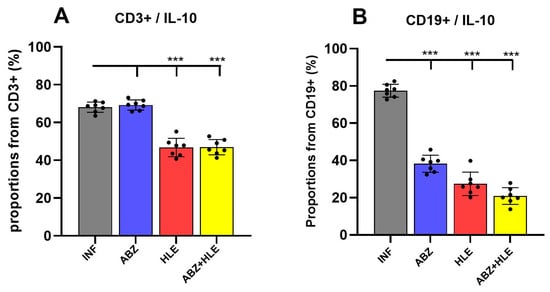
Figure 2.
IL-10–producing regulatory T and B cells in the peritoneal cavity of Mesocestoides vogae infected mice following therapy. (A) Proportions of CD3+IL-10+ regulatory T cells and (B) Frequencies of IL-10–producing CD19+ B1 cells (Bregs). Data represent mean ± SD from flow cytometric analysis (n = 7); statistical comparison is indicated by connecting lines and significant difference is at *** p < 0.001.
2.3. Gene Expression of Transcription Factors Regulating Th1/Th2/Treg Immunity
To investigate T cell polarisation, we examined the mRNA expression of lineage-defining transcription factors: Tbet (Th1), GATA3 (Th2), and Foxp3 (Tregs). T-bet, a master regulator of Th1 differentiation, governs IFN-γ expression [], whereas GATA3 promotes Th2 responses and suppresses Th1 lineage factors [] and Foxp3 is necessary for Treg development []. Infected mice exhibited low T-bet expression, which was significantly upregulated in all treated groups, most notably after ABZ and ABZ+HLE therapy (p < 0.001) (Figure 3A). In contrast, both GATA3 and Foxp3 were markedly elevated in infected and ABZ-only groups, while their expression levels significantly declined following HLE and combination therapy (p < 0.001) (Figure 3B,C), indicating a rebalancing of Th1/Th2/Treg responses.
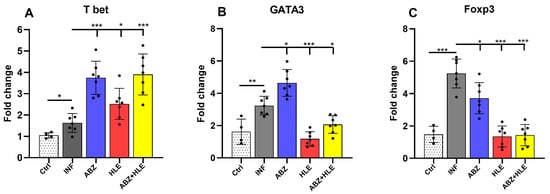
Figure 3.
Modulation of transcription factors defining Th1, Th2 and Treg responses in peritoneal lymphoid cells. mRNA expression levels of (A) T-bet, (B) GATA3, and (C) Foxp3 were analysed in peritoneal lymphocytes by qPCR following termination of therapy corresponding to day 26 p.i. Data are shown as mean ± SEM (means of triplicate analysis) (n = 7); * p < 0.05 ** p < 0.01, *** p < 0.001.
2.4. Gene Expression of Breg-Associated Markers
We analysed mRNA expression levels of Breg-associated markers Cd9 and Atf3 in peritoneal lymphocytes []. The expression of Cd9, a surface marker characteristic of Bregs, was significantly upregulated in infected mice but markedly reduced in all treated groups (p < 0.001), indicating effective suppression of the Breg phenotype by the therapies (Figure 4A). Conversely, infection with M. vogae led to a downregulation of Atf3, a transcription factor involved in cellular stress responses, suggesting stress-induced modulation of the lymphoid microenvironment. Combination therapy induced a modest increase in Atf3 expression, although this did not correspond with the changes observed in Cd9 expression.
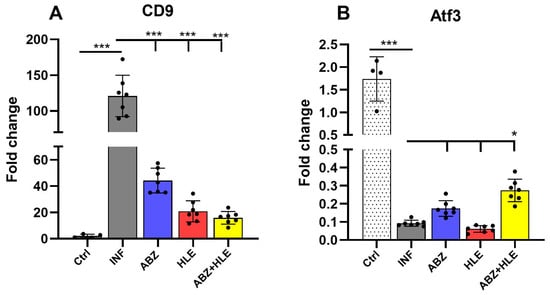
Figure 4.
Gene expression of Breg-associated markers Cd9 and Atf3 in peritoneal lymphocytes. (A) Relative mRNA expression levels of Cd9 and (B) Atf3 were assessed by qPCR in lymphoid cell populations isolated from the peritoneal cavity of infected and treated mice. Data are shown as mean ± SEM (means of triplicate analysis) (n = 7); * p < 0.05, *** p < 0.001.
2.5. Proliferation of ConA- and LPS-Stimulated Lymphocytes and Response to E/S Antigens
Helminth-derived excretory/secretory (E/S) products are known to modulate host immunity to promote parasite survival []. To assess lymphocyte functionality, we evaluated the proliferative capacity of non-adherent peritoneal lymphocytes ex vivo using BrdU incorporation following 72-h stimulation with ConA (T cell mitogen), LPS (B cell mitogen), or re-stimulation with larval E/S antigens. Cells from infected and ABZ-treated mice exhibited significantly reduced proliferative responses to ConA, indicative of impaired T cell function. In contrast, proliferation was partially restored in the HLE and ABZ+HLE groups (p < 0.001) (Figure 5A). Similarly, LPS-induced B cell proliferation was low in infected and ABZ-treated groups but showed moderate recovery following HLE and combination therapies (Figure 5B). Re-stimulation with E/S antigens led to further suppression of T and B cell proliferation across all groups, with the most profound inhibition observed in the infected and ABZ-only groups. However, cells from the HLE and ABZ+HLE groups displayed a partial restoration of responsiveness despite E/S exposure (Figure 5C,D), suggesting that HLE may mitigate E/S-induced immune hyporesponsiveness through its immunomodulatory components.
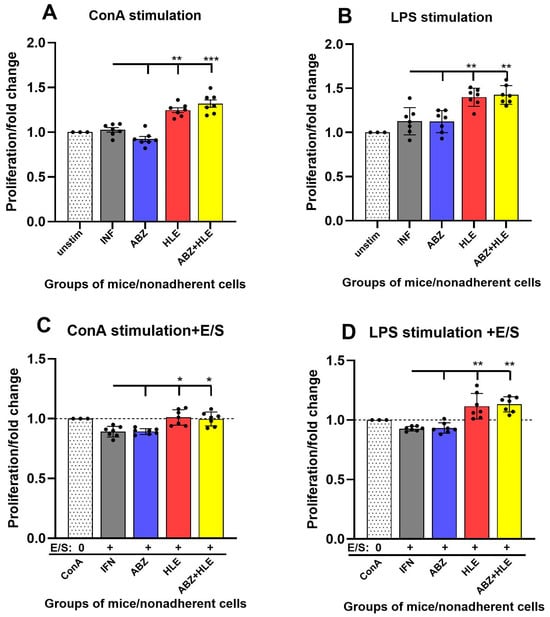
Figure 5.
Proliferative responses of peritoneal lymphocytes to mitogenic stimulation and larval E/S antigens. BrdU incorporation assay was used to assess proliferation of non-adherent peritoneal lymphocytes after 72 h culture. (A) Fold change in proliferation after ConA stimulation (T cells). (B) Fold change after LPS stimulation (B cells). (C,D) Re-stimulation of mitogen-activated T and B cells with larval E/S products. Data are shown as mean ± SEM (means of triplicate analysis) (n = 7); * p < 0.05 ** p < 0.01, *** p < 0.001 vs. infected group.
2.6. Production of IFN-γ, IL-4, and IL-10 by Stimulated Peritoneal Lymphocytes
The polarising effects of the treatments on T and B lymphocytes were evaluated by quantifying the canonical Th1/Th2/Treg cytokines IFN-γ, IL-4, and IL-10 in culture supernatants following stimulation with ConA, LPS or re-stimulation with larval E/S antigens. IFN-γ production by ConA-stimulated T cells was low in infected and ABZ-treated mice and significantly increased in the HLE and ABZ+HLE treated groups (p < 0.001), consistent with elevated T-bet expression and proliferative activity (Figure 6A). LPS-stimulated B cells from infected and ABZ-treated mice produced only low levels of IFN-γ, whereas B cells from HLE and ABZ+HLE groups secreted significantly higher levels, though E/S antigens reduced this secretion in all groups. (Figure 6B). Compared to the infected group, IL-4 levels were significantly increased in cultures of ConA-stimulated T cells from ABZ-treated mice (p < 0.001) and were markedly reduced in the HLE and ABZ+HLE groups (Figure 6C). In contrast, LPS-stimulated B cells from infected mice secreted high levels of IL-4, which declined in all treated groups, suggesting differential regulatory effects of ABZ on T and B1 cells (Figure 6D). Notably, IL-4 production was also suppressed in all groups following stimulation with E/S antigens.
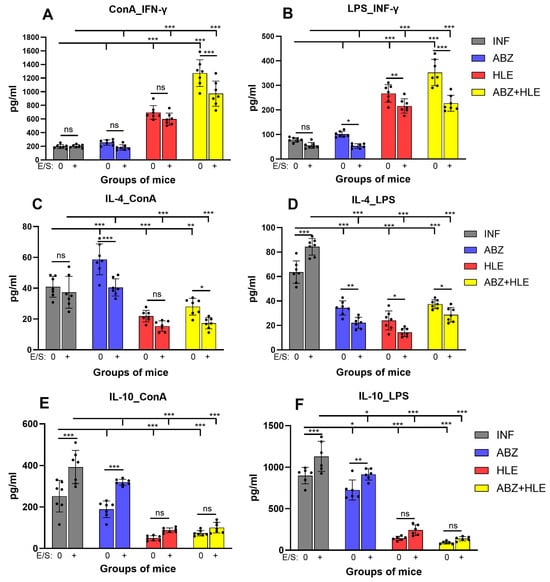
Figure 6.
Cytokine production by stimulated peritoneal lymphocytes. Levels of IFN-γ, IL-4, and IL-10 were measured in culture supernatants of non-adherent peritoneal cells after 72 h of stimulation with ConA (T cell mitogen), LPS (B cell mitogen), or re-stimulation with Mesocestoides vogae E/S antigens. (A,C,E) Cytokine levels from ConA-stimulated cultures representing T cell responses. (B,D,F) Cytokine levels from LPS-stimulated cell cultures representing B cell responses. Data represent mean ± SEM (means of triplicate analysis) (n = 7); * p < 0.05 ** p < 0.01, *** p < 0.001 vs. infected group. Differences between untreated and E/S treated cells are indicated by connecting line; ns = not significant.
Production of the regulatory cytokine IL-10, is feature of regulatory Tregs and Bregs to control over-activated inflammation and maintain suppression by helminth parasites. IL-10 was elevated in cultures of stimulated T and B cells from infected mice and only partially reduced following ABZ therapy. Significant decline was detected in the cell supernatants from HLE and ABZ+HLE treated groups (Figure 6E,F). Co-culture with E/S antigens significantly elevated IL-10 levels in the infected and ABZ-treated groups, confirming the immunosuppressive role of larval products, whereas the addition of HLE partially attenuated this effect.
2.7. Analysis of Antibody Isotypes and Immunoreactive E/S and MvH Antigens in Peritoneal Exudates and Detection of Antibody Secreting Peritoneal Cells
Flow cytometric analysis of lymphoid subpopulations revealed elevated proportions of antibody-secreting plasma cells and plasmablasts. Therefore, we investigated the effects of the treatments on IgM and IgG antibody subclass levels in peritoneal exudates, with specificity to larval somatic (MvH) and excretory/secretory (E/S) antigens. In addition, the expression profiles of immunogenic molecules were examined by Western blotting, and the presence of isotype-specific antibody-secreting cells was demonstrated by immunocytochemical staining of lymphoid populations.
Monitoring the kinetics of IgM levels in infected untreated mice revealed peak concentrations between days 35 and 40 post-infection (p.i.), followed by a gradual decline (Figure 7A). These optical density (OD) values were used to calculate arbitrary units for antibody levels across experimental groups (Figure 7B). Compared with infected controls, IgM levels specific to MvH antigens were elevated in all treated groups, with the highest increase observed following HLE therapy (p < 0.001). In contrast, IgM levels specific to E/S antigens were significantly reduced in the ABZ and ABZ+HLE treatment groups. Compared with infected controls, treated groups displayed altered antigen recognition profiles for MvH and E/S, with some bands absent and others newly appearing in (Figure 7C). Immunocytochemical staining of non-adherent lymphoid cells using HRP-labelled monoclonal anti-IgM antibodies revealed IgM-specific plasma cells. In addition, large, strongly stained cells, likely representing short-lived plasmablasts with irregular plasma membranes, were frequently observed (Figure 7D,E).
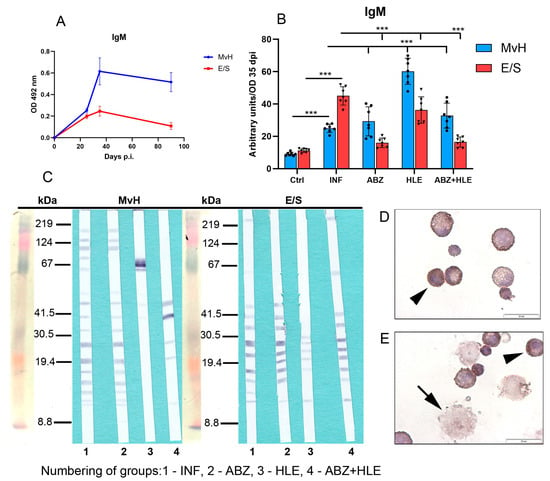
Figure 7.
IgM antibody response and immunoreactivity to MvH and E/S antigens in peritoneal exudates of M. vogae infected mice. (A) Kinetics of IgM levels in infected untreated mice showed a peak between days 35 and 40 p.i. (B) IgM antibody levels specific to MvH and E/S antigens in treated groups. Data represent mean ± SEM (means of triplicate analysis) (n = 7); *** p < 0.001. (C) Representative images from Western blot analysis showing revealed distinct immunoreactive profiles for MvH and E/S antigens across treatment groups (1-INF, 2-ABZ, 3-HLE, 4-ABZ+HLE). (D,E) Immunocytochemical staining of non-adherent lymphoid cells with HRP-conjugated anti-IgM antibodies identified IgM-producing plasma cells (arrowhead) and large plasmablast-like cells (arrow) (scale bar = 20 µM).
Evaluation of total IgG antibodies revealed activation of B cell differentiation into plasma cells, as IgG titres against both antigens increased with the progression of infection. Optical density (OD) values corresponding to IgG titres on day 90 p.i., representing the chronic stage of infection, were used to calculate arbitrary units (Figure 8A). IgG antibodies specific to MvH antigens were elevated in both the infected group and HLE-treated group and significantly declined following ABZ treatment alone and in combination with HLE (p < 0.001), likely due to the decreased parasite burden. Similarly, IgG levels specific to E/S antigens were markedly elevated in the infected group but significantly reduced in all treated groups, most notably following combination therapy (Figure 8B), suggesting that HLE may interfere with larval secretory activity on molecular level. Western blot analysis revealed a differential expression profile of MvH-immunoreactive antigens in peritoneal exudates among all experimental groups (Figure 8C), which differed substantially from the profiles observed after probing E/S-specific membranes. In all groups, IgG antibodies prominently recognised a dominant ~27 kDa antigen, while exudates from treated mice also reacted with additional antigens in the 35–67 kDa molecular weight range. Immunocytochemical staining confirmed IgG immunoreactivity in enlarged plasma cells and, to a lesser extent, in plasmablasts (Figure 8D,E).
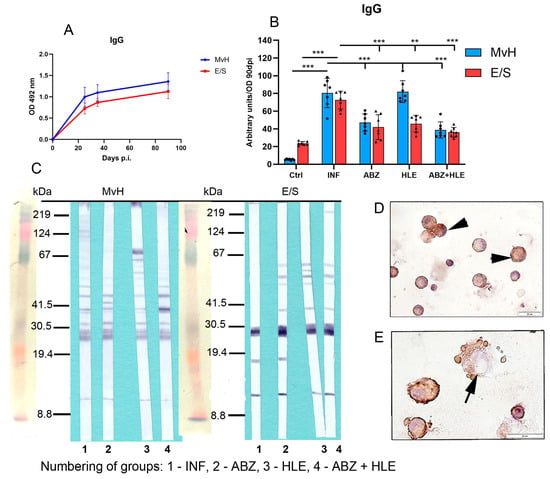
Figure 8.
IgG antibody levels and immunoreactivity of MvH and E/S antigens in peritoneal exudates. (A,B) Total IgG antibody levels specific to MvH (A) and E/S. (B) Antigens were measured in peritoneal exudates by ELISA. Arbitrary units were calculated from OD values on day 90 p.i. Data represent mean ± SEM (means of triplicate analysis) (n = 7); ** p < 0.01, *** p < 0.001. (C) Representative images from Western blot analysis showing differential profiles of immunoreactive MvH and E/S antigens for individual groups (1-INF, 2-ABZ, 3-HLE, 4-ABZ+HLE). (D,E) Immunocytochemical detection of IgG-producing peritoneal cells showing positive staining in enlarged plasma cells (arrowhead) and a smaller number of plasmablasts (arrows); (scale bar = 20 µM).
The proportions of antibody subclasses vary depending on the prevailing cytokine environment []. IgG1 production is typically induced by IL-4 []. OD values for IgG1 specific to MvH and E/S antigens measured at day 90 p.i. were used to calculate arbitrary units representing IgG1 levels in peritoneal exudates (Figure 9A). Interestingly, IgG1 responses showed a different pattern from total IgG. MvH antigens elicited a weaker IgG1 response than E/S antigens, with no significant differences in IgG1 levels to MvH between infected and treated groups (Figure 9B). However, IgG1 levels to E/S antigens increased following therapy, particularly in the HLE group (p < 0.05). Western blotting revealed weak reactivity with MvH antigens (30–67 kDa) in all groups, with altered band patterns in treated animals. In contrast, IgG1 antibodies strongly recognised three dominant E/S antigens across all groups, with additional bands emerging after HLE or combined treatment (Figure 9C). Immunocytochemical staining confirmed IgG1 secretion by plasma cells and plasmablasts (Figure 9D,E).
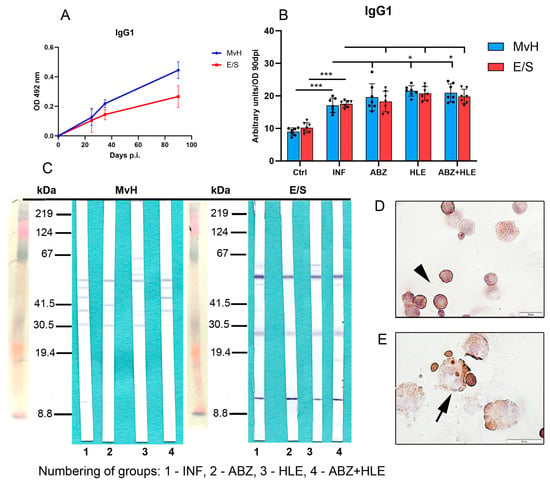
Figure 9.
IgG1 antibody levels and antigen recognition patterns in peritoneal exudates. (A) IgG1 levels specific to MvH and E/S antigens on day 90 p.i., expressed in arbitrary units derived from OD values. (B) IgG1 levels to MvH remained unchanged across groups, while E/S-specific IgG1 increased post-treatment. Data represent mean ± SEM (means of triplicate analysis) (n = 7); * p < 0.05, *** p < 0.001. (C) Western blot profiles showing differential IgG1 reactivity to MvH and E/S antigen for individual groups (1-INF, 2-ABZ, 3-HLE, 4-ABZ+HLE). (D,E) IgG1-producing plasma cells (arrowhad) and plasmablasts (arrow) detected by immunocytochemistry (scale bar = 20 µM).
Previous studies have shown that Th1 cytokines, especially IFN-γ, promote IgG2a and IgG3 production, while TGF-β induces class-switching to IgG2b []. We assessed IgG2a and IgG2b levels specific to MvH and E/S antigens, using OD values from day 90 p.i. to calculate arbitrary units (Figure 10A). Overall, IgG2a concentrations were lower than IgG1 and IgM. While ABZ therapy did not enhance IgG2a levels, significantly higher concentrations were observed in mice treated with HLE and combination therapy (Figure 10B), consistent with observed increased IFN-γ secretion ex vivo. Western blotting revealed weak IgG2a reactivity with MvH in infected mice, with different antigen profiles in treated groups. For E/S antigens, only faint bands were visible, with low-MW antigens (~12 kDa) detected in exudates obtained after HLE and combination therapy (Figure 10C). IgG2a-secreting cells were localised to smaller plasma cells (Figure 10D,E).
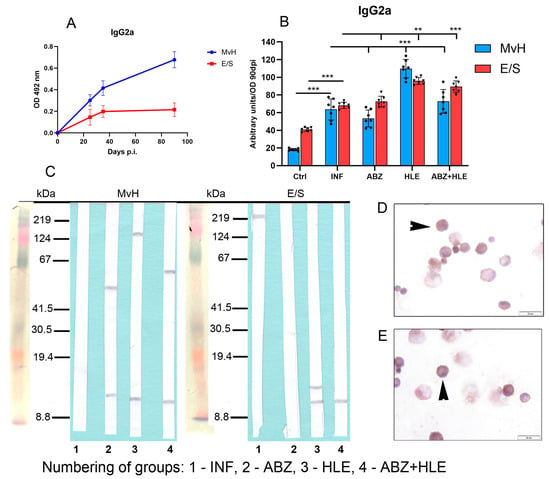
Figure 10.
IgG2a antibody response and antigen recognition in peritoneal exudates and cell staining. (A) ELISA quantification of IgG2a levels specific to MvH and E/S antigens. (B) IgG2a levels in exudates from infected and treated groups of mice. Data represent mean ± SEM (means of triplicate analysis) (n = 7); ** p < 0.01, *** p < 0.001. (C) Western blotting analysis for individual groups (1-INF, 2-ABZ, 3-HLE, 4-ABZ+HLE) revealed weak IgG2a reactivity; low-MW bands (~12 kDa) were visible in treated groups. (D,E) Immunocytochemical localisation of IgG2a-secreting plasma cells (arrowhead) (scale bar = 20 µM).
Infection induced higher IgG2b responses to MvH than to E/S antigens. Surprisingly, a significant increase in MvH-specific IgG2b was detected in the HLE group (p < 0.001), while E/S-specific IgG2b levels were not significantly altered by any treatment (Figure 11B). Correspondingly, more immunogenic MvH antigens were detected in exudates from infected mice, whereas responses were markedly diminished following ABZ+HLE therapy. Similarly, E/S-specific IgG2b reactivity was weak across all groups, with fewer bands observed in HLE and combination-treated mice than in infected controls (Figure 11C). Smaller IgG2b-expressing plasma cells were identified by a weak immunocytochemical staining (Figure 11D,E).
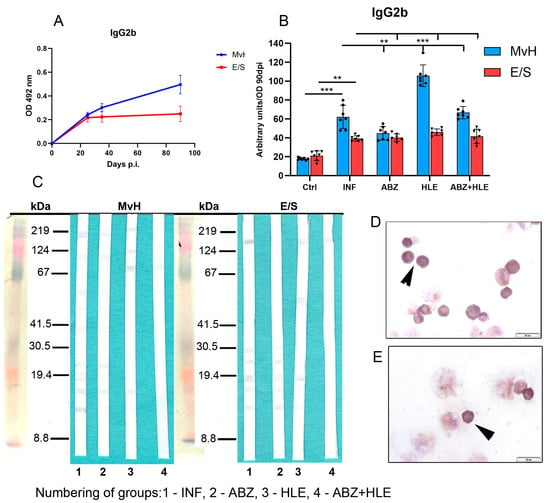
Figure 11.
IgG2b antibody response, antigen recognition in peritoneal exudates and antibody secreting cells. (A) IgG2b levels expressed as arbitrary units at day 90 p.i. (B) Effect of infection and treatments on MvH and E/S-specific IgG2b levels. Data represent mean ± SEM (means of triplicate analysis) (n = 7); ** p < 0.01, *** p < 0.001. (C) Western blot detection of MvH and E/S antigens recognised by IgG2b in individual groups (1-INF, 2-ABZ, 3-HLE, 4-ABZ+HLE). (D,E) Representative images of IgG2b-secreting plasma cells (arrowhead), (scale bar = 20 µM).
2.8. Efficacy of Treatments on Larval Burden and mRNA Levels of the Regulatory 14-3-3 Gene
The larvicidal effects of ABZ, HLE, and their combination are shown in Figure 12A. In comparison with infected mice, the greatest reduction in larval burden in comparison with counts in infected mice (%) was observed in mice treated with the combination of ABZ and HLE, and less effective was ABZ monotherapy (p < 0.001). Intraperitoneal administration of HLE alone led to a modest reduction of larval counts and efficacy, indicating a synergistic effect. To determine whether the reduction in larval burden was associated with interference in larval proliferation or development, we examined the expression of the 14-3-3 gene. This gene plays a critical role in cell cycle regulation and general physiology in a wide range of eukaryotic organisms, including helminths []. We quantified 14-3-3 mRNA expression in larvae isolated from treated and untreated mice. Compared to larvae from the infected control group, expression of 14-3-3 was significantly downregulated in all treated groups, with the most pronounced suppression observed in ABZ+HLE treated group, followed by HLE alone and then ABZ monotherapy (Figure 12B). Results suggest that both HLE and the combination treatment impair larval proliferation, likely through disruption of regulatory pathways essential for parasite development.
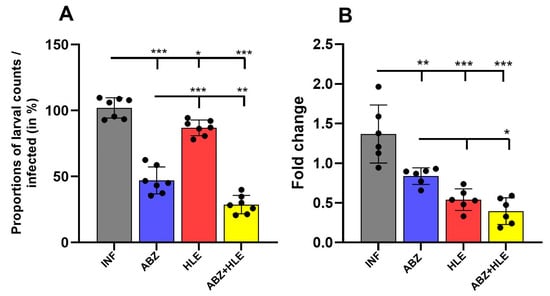
Figure 12.
Larvicidal efficacy of ABZ, HLE, and combination therapy against Mesocestoides vogae infection. (A) Total larval burden in the peritoneal cavity of mice following treatment with albendazole (ABZ), human leukocyte extract (HLE), or their combination (ABZ+HLE). (B) Relative mRNA expression of the larval 14-3-3 gene, a marker of cellular proliferation and development showing significant downregulation in all treated groups to the infected control group. Data are expressed as means ± SEM (means of triplicate analysis) (n = 7); * p < 0.05 ** p < 0.01, *** p < 0.001.
3. Discussion
In the infected peritoneal cavity, M. vogae induces a marked increase in myeloid-derived suppressor cells (MDSCs), comprising predominantly M2 macrophages and eosinophils, at the expense of lymphoid populations [,,], along with elevated concentrations of the Th2/Treg-associated cytokines IL-4, IL-10 and transforming growth factor-β (TGF-β). This permissive immune environment, typically elicited by helminths, facilitates parasite survival and proliferation while limiting host tissue pathology. Ideally, an effective treatment would not only eliminate the parasite but also induce host-protective immune responses that enhance the efficacy of chemotherapy and reduce the adverse effects of drugs.
In the present study, we examined the effects of ABZ, the immunomodulatory product HLE, and their combination on non-adherent lymphoid cell populations in the peritoneal cavity of BALB/c mice, using experimental conditions identical to our previous investigation focused on myeloid populations []. The greatest reduction in larval burden was observed following combined ABZ+HLE therapy, while HLE monotherapy resulted in a modest yet significant decrease, supporting its role as a beneficial adjuvant.
In our study, flow cytometric analysis of non-adherent cells revealed that CD3+ T lymphocytes and CD19+ B lymphocytes were present at lower frequencies compared with antibody-secreting CD138+ plasmablasts and various plasma cell stages. Immune modulation towards a Treg phenotype, coupled with suppression of Th1 responses, is a common feature of helminth infections, documented, for E. multilocularis [], Taenia crassiceps [], and M. vogae [,]. In our study, a significant increase in CD3+ T cells and a decrease in CD19+ B cells were observed following HLE and combined ABZ+HLE therapy compared with untreated infected controls. CD3+high cells predominated after HLE and combined therapy, whereas their frequency slightly decreased following ABZ given alone. As CD3 is a multimeric protein complex that associates with the T cell receptor (TCR) and is essential for T cell lineage commitment [], these results suggest that components of HLE may modulate this process.
The peritoneal cavity resides a distinct subset of B cells, known as CD19+ B1 cells, which differ from conventional B2 cells in phenotype, function, and self-renewal capacity [,]. Upon antigen encounter, parasite-induced B cells can differentiate into short-lived plasmablasts or undergo clonal expansion into high-affinity antibody-producing plasma cells and memory B cells. In our study, a high frequency of plasmablasts/plasma cells was noted following infection and ABZ therapy, whereas a significant decline was recorded after combined treatment, correlating with the greatest reduction in parasite burden. To further investigate the impact of therapy on regulatory lymphocyte subtypes, we examined IL-10-secreting CD3+ Treg and CD19+/IL-10 Breg cells using intracellular staining. Regulatory B cells (Bregs) are known to suppress various immune cells, including T cells, dendritic cells and monocytes, partly by promoting regulatory T cells (Tregs) []. IL-10-producing T cell frequencies were reduced following HLE and combined treatment, but not after ABZ administered alone. In contrast, IL-10-producing B1 cells were significantly reduced in all treated groups. Collectively, these data indicate that HLE, particularly in combination with ABZ, effectively reversed the infection-driven dominance of Treg/Breg populations. This finding aligns with our previous study, which showed that HLE and combination therapy shifted peritoneal macrophage polarisation from M2 to M1, mediated via STAT-1/IFN-γ signalling pathway activation []. M1 versus M2 polarisation is driven by IFN-γ and IL-4, respectively, with M2 macrophages being prominent producers of IL-10 [].
To date, limited information is available on the effects of HLE on lymphocyte phenotypes and cytokine profiles during flatworm infections. In a related murine model of E. multilocularis infection, combination therapy with ABZ and a swine-derived dialysable leukocyte extract (Imunor, ImunomedicA, Ltd., Ústí nad Labem, Czech Republic) resulted in an expanded splenic CD4+ T cell population with elevated IFN-γ production []. In the same model, HLE therapy (Immodin, SevaPharma Ltd., Prague, Czech Republic) downregulated IL-4 and TGF-β production by ConA-stimulated lymphocytes ex vivo, alongside suppression of GATA3 and Foxp3 gene expression []. A human leukocyte extract (Transferon®, Instituto Politecnico Nacional, Mexico City, Mexico) promoted the differentiation of human haematopoietic stem/progenitor cells into CD11c+ NK-like cells and stimulated the proliferation of bone marrow CD34+ cells [], The immunomodulatory effects of ABZ on the immune system have also been consistently monitored. For example, in the liver biopsies from patients infected with E. multilocularis and treated with high doses of ABZ, lesions surrounding the parasitic cysts exhibited significantly increased infiltration of CD4+ T cells, CD20+ B cells, and CD38+ plasma cells, indicative of an activated adaptive immune response [].
We further explored the association between treatment-induced changes in lymphocyte phenotypes and the expression of key transcription factors orchestrating T cell polarisation. Compared to infected mice, ABZ treatment significantly upregulated T-bet and GATA3, and partially downregulated Foxp3, demonstrating a mixed polarisation profile. However, HLE and especially ABZ+HLE therapy induced a more balanced Th1/Th2 response, characterised by strong T-bet activation, moderate GATA3 downregulation, and more pronounced Foxp3 suppression. This was consistent with the lower proportions of IL-10-producing CD3+ cells observed in these groups. Regulatory T cells (Tregs; CD4+CD25+Foxp3+) mediate their suppressive effects on adaptive immunity by inhibiting T cell proliferation, downregulating pro-inflammatory cytokines such as IFN-γ and TNF-α, and promoting the secretion of TGF-β and IL-10, which also induce M2 macrophage polarisation []. Increased CD4+CD25+Foxp3+ Tregs have also been documented in the peritoneal cavity of mice intraperitoneally infected with E. multilocularis, and their depletion improved infection control []. Our results suggest that ABZ+HLE therapy effectively restricted Treg expansion while supporting Th1-polarised, anti-parasitic immunity.
B cells, including the B1 subset, serve as an important source of cytokines and as precursors of plasma cells, playing pivotal roles in coordinating interactions among macrophages, dendritic cells, and T cells []. In another flatworm infection, schistosomiasis, CD9 has recently been identified as a key surface marker for murine IL-10+ CD19+ Bregs involved in infection progression []. In our study, expression of Cd9 was strongly upregulated in B1 cells from infected mice and significantly downregulated in all treated groups, including the ABZ treated mice. This was not entirely consistent with the pattern of Foxp3 expression, suggesting that ABZ may have exerted a more pronounced molecular effect on B cells than on peritoneal T cells. We also found that in comparison with control larval infection strongly downregulated Atf3 gene expression, another transcription factor associated with Breg function, immune regulation, and homeostasis [,] and therapies only moderately modulated mRNA levels.
Attenuated T cell proliferation is a hallmark of helminth-induced immunosuppression, often directed by E/S antigens. To assess whether changes in lymphocyte phenotype were translated into altered functional capacity, we stimulated peritoneal T and B1 cells with ConA or LPS and measured proliferation. T cells from infected and ABZ-treated mice exhibited poor proliferative responses to ConA, which were partially restored following HLE treatment and markedly improved by ABZ+HLE therapy. ConA, lectin, which binds mannose residues on T cell membranes and is internalised to mitochondria [], induces proliferation and cytokine release. Thus, the enhanced proliferation suggests a shift towards a more functional T cell pool. The T cell proliferation after re-stimulation with parasite products was hardly detectable in all groups, indicating a state of functional anergy, a known helminth-induced immune evasion mechanism that preserves lymphocyte viability while impairing effector function []. Helminth-derived molecules are also known to inhibit TLR4 signalling in myeloid cells; for instance, glycomolecules from E. granulosus were shown to impair TLR4-mediated activation in dendritic cells []. In our study, LPS-stimulated B1 cell proliferation was poor in infected mice but was partially restored by HLE and combination therapy. This suggests that HLE may counteract the inhibitory effects of E/S products on TLR4 signaling in B1 cells.
Cytokine production in response to mitogen stimulation reflected these changes in functional responsiveness. In our study, IFN-γ secretion increased significantly in HLE and combination-treated groups after ConA or LPS stimulation, while IL-4 and IL-10 levels declined. This shift away from Th2/Treg cytokines further confirms reversal of helminth-induced immunosuppression. Notably, ABZ alone was less effective in restoring IFN-γ production and dampening IL-10 secretion. There is little known regarding the effects of oral ABZ therapy on peritoneal lymphoid population during cestode infections in mice. Clinical data on the effects of ABZ in echinococcosis patients revealed significant reductions in IL-10 and partial reductions in IL-4 in serum that was associated with the successful therapy [,]. In M. vogae infected mice, the role of IL-4 appears complex. C57BL/6 mice are more resistant to this infection than BALB/c and IL-4-deficient (IL4−/−) C57BL/6 mice exhibited increased parasite burdens, heightened IFN-γ and TNF-α production, and a shift from IgG1 to IgG2a antibodies [,]. These findings suggest that while Th1 responses are important for parasite control, as demonstrated by IFN-γ’s larvicidal effects [], a balanced Th1/Th2 profile may be more effective. Our data support this conclusion, with ABZ+HLE therapy producing the most balanced and protective immune profile.
Antibodies are essential effectors of adaptive immunity, with isotype switching governed by the cytokine milieu. In mice, elevated secretion of IL-4 promotes IgG1 and IgE, while IFN-γ induces IgG2a and IgG3 and inhibits Th2-associated isotypes []. In mouse M. corti infection, larval antigens selectively induced IgM and IgG1 in serum [], and similar subclass patterns were found across four different helminth infections [], including M. corti. We observed relatively strong peritoneal antibody responses dominated by IgM and IgG and antigen-specific variations in isotype reactivity. Treatments significantly modulated these responses. ABZ+HLE therapy resulted in the greatest reduction of total IgG and IgM, which corresponded with the lowest CD138+ plasma cell counts and larval burden. Interestingly, IgG1, the isotype dominant in systemic responses to M. corti, did not prevail in the peritoneal cavity, what may reflect reduced IL-4 levels in this microenvironment. Notably, elevated IFN-γ correlated with increased IgG2a levels after HLE and combination therapy, indicating a Th1-driven class switch. These differential antibody profiles highlight the complex interplay between host immunity and parasite antigens, and the ability of immunomodulatory therapy to redirect these responses. Proteomic analysis of an identical HLE product, formerly marketed as Immodin, revealed the presence of 48 unique proteins/polypeptides, classified into three functional groups: molecules regulating immune responses, proteins involved in inflammatory processes and tissue repair, and proteins regulating cell growth [].
Finally, we found a correlation of drug efficacy on larval biology by measuring expression of the 14-3-3 gene, a cell cycle regulator in eukaryotic organisms, including helminths. Expression was significantly downregulated following therapy, especially in the ABZ+HLE group. Surprisingly, HLE alone also reduced gene expression, potentially explaining its modest larvicidal activity. Similar correlations between 14-3-3 downregulation and parasite mass reduction were observed in E. multilocularis infected mice []. We suppose that suppressed 14-3-3 mRNA expression may not fully account for the inhibition of all larval pro-survival adaptation mechanisms. This could explain the relatively modest reduction in larval burden compared to the effect of ABZ, which acts directly through inhibition of tubulin polymerisation. A deeper investigation of this phenomenon, along with studies on the safety and risks of this adjuvant, could support future research and the development of innovative therapeutic strategies against parasitic diseases.
In summary, our study showed that co-administration of HLE with ABZ significantly enhanced the drug’s larvicidal efficacy, likely through synergistic immunomodulatory effects on T and B lymphocyte subsets and by suppressing expression of the parasite’s 14-3-3 gene. While ABZ alone showed limited capacity to counteract larval E/S-induced T cell suppression, it partially reduced IL-10-producing CD9+ Bregs and inhibited their secretion of IL-10 following LPS stimulation. However, upregulation of Th1/Th2/Treg transcription factors under ABZ monotherapy was not mirrored by reduced IL-10 production in T cells, indicating ongoing functional hyporesponsiveness. In contrast, HLE markedly improved T cell responsiveness, as evidenced by enhanced Th1 proliferation, increased T-bet expression, and restoration of IFN-γ production. These effects were accompanied by augmented IgG2a antibody responses and suppression of Treg- and Breg-associated markers. Downregulation of GATA3 and reduced IL-4 production further suggested that HLE rebalanced the local Th1/Th2 response in favour of parasite clearance.
4. Materials and Methods
4.1. Albendazole and HLE
Albendazole (ABZ), used as the standard anthelmintic, was purchased from Sigma-Aldrich (St. Louis, MO, USA). For oral administration, ABZ was suspended in 1.0% cremophor oil in 0.9% sterile NaCl and stored at 4 °C. Human dialysable leukocyte extract (HLE) used in our study was manufactured by Aumed, Ltd. (Prague, Czech Republic), a GMP-certified pharmaceutical company, and supplied exclusively for research purposes. The preparation is registered in the European Union under International Publication No. WO 2021/249581 A1 and patented in the Czech Republic by the Industrial Property Office (Patent No. 34517). According to the manufacturer’s protocol, HLE is a soluble extract of peripheral blood mononuclear cells (PBMCs) obtained from over 150 healthy donors, which were screened at certified transfusion centres and tested negative for HIV 1/2, HCV, HBsAg, and syphilis. The extract contains a complex mixture of proteins and other molecules with a molecular weight below 10 kDa. Each batch was prepared by pooling leukocytes from donors, followed by disintegration using six cycles of freezing at a temperature below −50 °C and thawing at a temperature above +30 °C. The resulting homogenate was concentrated by dialysis to a defined protein concentration, and the low molecular weight fraction was isolated using 10 kDa ultrafiltration membranes and further concentrated by ultrafiltration. The final product was pasteurised and quality-controlled for sterility, endotoxins, and biological activity using proliferation assays on the Jurkat T cell line.
4.2. Infection and Experimental Design
Infections with Mesocestoides vogae (syn. M. corti) larvae were maintained through serial passage in ICR mice housed under pathogen-free conditions at the animal facility of the Institute of Parasitology. In the experiment, we used 7-week-old male BALB/c mice, bred at the accredited animal facilities of the Institute of Parasitology of the Slovak Academy of Sciences under pathogen-free conditions. Mice strain was originally purchased from Velaz (Prague, Czech Republic). Mice were bred under standard laboratory conditions (12 h light/dark cycle, 22 ± 2 °C, standard chow diet). Four healthy mice served as uninfected controls (Ctrl), and 28 mice were orally infected with 60–65 tetrathyridia isolated from the peritoneal cavity of ICR mouse. Infected mice were randomly allocated into four groups (n = 7 per group): INF—infected, untreated control; ABZ—infected, treated with albendazole; HLE—infected, treated with human leukocyte extract (HLE); and ABZ+HLE—infected, treated with both ABZ and HLE. Treatments were administered daily for 11 consecutive days, from day 15 to day 25 post-infection (p.i.). ABZ was administered orally by gavage at a dose of 10 mg/kg body weight, and HLE was delivered intraperitoneally at a dose of 0.2 mL (equivalent to an extract derived from 1 × 109 leukocytes). On day 26 p.i., after termination of therapy, mice were subjected to mild anesthesia in a CO2 atmosphere and were then euthanised by cervical dislocation. Peritoneal exudates, peritoneal exudate cells (PECs), and larvae were subsequently collected for further analyses.
4.3. Isolation of Peritoneal Non-Adherent Cell Populations and Exudates
Peritoneal exudates were aseptically collected from mice by injecting 1 mL of sterile PBS. Following isolation of larvae and peritoneal cells, exudates were stored at −80 °C. Peritoneal cells were obtained via a second lavage with 5 mL of RPMI medium (Biochrom, Berlin, Germany) supplemented with 2 mM stable glutamine, 10% heat-inactivated foetal bovine serum (Biochrom, Berlin, Germany), 100 U/mL penicillin, 100 μg/mL streptomycin, 10 μg/mL gentamicin, and 2.5 μg/mL amphotericin B (complete medium, CM; all from Sigma-Aldrich, St. Louis, MO, USA). Cells from both lavages were pooled. Peritoneal exudate cells (PECs) were resuspended in CM, seeded into culture flasks, and incubated for 18 h to separate non-adherent lymphoid cells from adherent macrophages and eosinophils. Non-adherent cells were washed and counted using the trypan blue exclusion method (Sigma-Aldrich, St. Luis, MO, USA). Cell aliquots (2 × 106 cells/mouse) were preserved in RiboZol reagent (VWR Chemicals, Radnor, PA, USA) and stored at −80 °C for RNA extraction. Remaining cells were subjected to flow cytometry, qPCR, proliferation assays, and immunocytochemistry. Exudates were used for antibody detection and immunoblotting.
4.4. Flow Cytometric Analysis
Non-adherent peritoneal cells (0.3 × 106 cells/50 μL) were stained with monoclonal anti-mouse antibodies: CD45.2-PE-Cy7 (clone C.104, eBioscience, San Diego, CA, USA), CD3e-FITC (clone 145-2C11, Invitrogen, Waltham, MA, USA), CD19-PE (clone 1D3, eBioscience, San Diego, CA, USA), and CD138-APC (clone 300506, Invitrogen, Waltham, MA, USA). Other cell samples were stained with anti-mouse antibodies: CD3-PE-Cy7 (clone 17A2), CD4-FITC (clone GK 1.5), and CD8-PE (clone C:53-6.7); all from eBioscience, San Diego, CA, USA). Cells were incubated for 30 min at room temperature in the dark, washed, and analysed using a FACS Canto analyser (BD Biosciences, Franklin Lakes, NJ, USA). For intracellular cytokine detection, cells were permeabilised using the BD Cytofix/Cytoperm Kit (BD Life Sciences, Franklin Lakes, NJ, USA), then stained with anti-IL-10-PE (clone J4S5-16E3, SONY Biotechnology, San Jose, CA, USA) in combination with anti-CD3e-FITC or CD19-APC-Cy7 (clone MB 19-1), both from Invitrogen (Waltham, MA, USA). Data were analysed using FACS Diva software version 9.0 and represent mean ± standard deviation, (SD), (n = 7).
4.5. Cell Proliferation Assay
Cell proliferation was assessed using the BrdU Cell Proliferation ELISA Kit (Roche, Basel, Switzerland). Non-adherent cells (1 × 106/mL, 200 μL/well) were cultured in triplicates in 96-well flat-bottom plates type Falcon, (Corning, New York, NY, USA), either unstimulated or stimulated with concanavalin A (ConA, 3 μg/mL), ConA + E/S antigens (10 μg/well), lipopolysaccharide (LPS, 1 μg/mL), or LPS + E/S for 72 h at 37 °C in 5% CO2. BrdU (5 μM) was added for the final 22 h. Proliferation was expressed as the fold change of absorbance in stimulated vs. unstimulated cells or as the ratio of absorbance of mitogen-stimulated cells to OD values for mitogen+E/S. Tests were performed in triplicate for each cell sample and data shown in graphs are mean ± standard error of the mean (SEM) (n = 7).
4.6. RNA Isolation and Real-Time qPCR
Total RNA was extracted from 3 × 106 non-adherent cells using TRIzol reagent (Invitrogen, Waltham, MA, USA). RNA quality and quantity were determined using a NanoSpectrophotometer (AstraGene, Cambridge, UK). cDNA was synthesised from 3 μg of RNA using ReverseAid H Minus M-MuLV Reverse Transcriptase and oligo dT primers (Thermo Fisher Scientific, Waltham, MA, USA). The cDNA from each sample was used as a template for quantitative RT-PCR (qPCR) analysis using SYBR Green Mix (Bio-Rad, Hercules, CA, USA) in 20 μL reactions with 2 μL cDNA gene-specific primers (10 pM each) run for 39 cycles. Transcriptional profiles of following genes with published sequences were analysed: T-bet [], GATA3 [], Foxp3 [], Atf3 [], CD9 [] and housekeeping gene GAPDH []. Primer sequences are listed in Table S1 in Supplementary Materials. Relative gene expression was calculated using the 2−∆∆Ct method [], using normalised Ct values from healthy mice as the calibrator. RNA from isolated larvae was processed similarly, with expression of 14-3-3 gene normalised to α-smooth actin reference gene. Each reaction was run in triplicates and data shown in graphs are mean ± standard error of the mean (SEM) (n = 7).
4.7. Preparation of Excretory/Secretory (E/S) and Larval Homogenate Antigens
Tetrathyridia of M. vogae were harvested from ICR mice under aseptic conditions and washed extensively with LPS-free DPBS (Sigma-Aldrich, St. Luis, MO, USA). Surface host cells were removed by 48 h incubation in RPMI, without FCS, supplemented with antibiotics (culture medium) under standard culture conditions. Larvae were then intensively washed, transferred to fresh medium, and incubated for 7 days in culture medium. The culture supernatant after the first 24 h of culture was discarded. From day 2, supernatants were collected daily, filtered in 0.22 μm filters (Sarstedt, Numbrecht, Germany), concentrated, and buffer-exchanged into endotoxin free-DPBS using 3 kDa columns (Merck Millipore, Merck KGaA, Darmstadt, Germany). Larval viability was confirmed by motility and morphology. A portion of viable larvae was used for preparation of mixture of somatic antigens (Mesocestoides vogae homogenate, MvH). Larvae were sonicated in DPBS (30 s, 25% watt on ice), centrifuged at 10,000× g, and the supernatant (MvH) was collected. Protein content was quantified using the Bradford assay (Bio-Rad) and BSA as standard. The same batch of E/S and MvH was used throughout the study.
4.8. Detection of Antibodies to E/S and MvH Antigens
Enzyme-linked immunosorbent assay (ELISA) was used to quantify levels of total IgG, IgM, IgG1, IgG2a, and IgG2b antibodies against E/S and MvH antigens in peritoneal exudates. Plates (Nunc Maxisorp, Roskilde, Denmark) were coated with 2.5 μg/mL E/S or 1.5 μg/mL MvH antigens in carbonate–bicarbonate buffer (pH 9.6) overnight at 4 °C. After blocking with 10% FBS, 0.05% Tween-20 in PBS for 1 h, plates were incubated with diluted exudates (1:50) for 1 h at 37 °C. After washing, the secondary HRP-conjugated goat anti-mouse antibodies were used at the following dilutions: anti-IgG1 (1:2000), anti-mouse IgG (1:4000), anti-IgM (1:2000), anti-IgG2a (1:2000), and anti-IgG2b (1:2000), all from Abcam (Cambridge, United Kingdom). Signals were developed using o-phenylenediamine substrate in citrate buffer (pH 4.5) after addition of H2O2, and the reaction was stopped with 2 M H2SO4. Absorbance was measured at 492 nm using Multiskan FC reader (Thermo Fisher Scientific, Waltham, MA, USA). Results were expressed in arbitrary units (AU) relative to OD for calibrator, representing exudates from chronically infected mice (n = 3). Each sample was examined in triplicates and data shown in graphs are mean ± standard error of the mean (SEM) (n = 7).
4.9. Immunoblot Analysis
Expression of immunogenic larval MvH and E/S antigens present in the peritoneal exudates was performed by Western blotting to examine immunoreactivity with antibodies IgG, IgM, IgG1, IgG2a and IgG2b. Proteins (10 μg/lane) were denatured at 95 °C for 5 min, resolved by 12% SDS-PAGE, and transferred onto PVDF membranes (0.45 μm, Merck Millipore, Merck KGaA, Darmstadt, Germany) using a Mini Trans-Blot Electrophoretic Transfer Cell (Bio-Rad, Hercules, CA, USA) including protein Kaleidoscope prestained standard, cat. No.161-0324). Nonspecific binding was blocked upon incubation of strips in 5% fat-free milk in PBS for 2 h. Membranes with blotted E/S or MvH antigens were then incubated with pooled exudates (n = /group) at 1:20 dilution (IgG, IgG1, IgM) or 1:10 (IgG2a, IgG2b) in 3% fat-free milk overnight at 10 °C using IKA Roller 6 (Biosan, Riga, Latvia). After washing, HRP-conjugated goat anti-mouse antibodies were applied at the dilutions of 1:500 for IgG, IgG1, IgM and 1:200 for IgG2a, IgG2b (Abcam). Finally, immunoreactive bands were visualised using 4-chloronaphthol with H2O2. Figures show representative immunoblots.
4.10. Immunocytochemical Staining
Non-adherent cells were fixed in 4% paraformaldehyde and processed for intracellular immunodetection of antibody subclasses. Following permeabilisation with 0.5% Triton-X100/PBS, slides were treated with 0.3% H2O2 for 20 min to block endogenous peroxidase and nonspecific bindings was blocked with 5% goat serum/PBS. Slides were then incubated in HRP-conjugated antibodies to IgG, IgM, IgG1, IgG2a, and IgG2b (Abcam, 1:100) overnight at room temperature. Following washing, and incubation with substrate (0.4% DAB in Tris-NaCl) for 10 min, slides were counterstained with Gill’s haematoxylin, differentiated in Scott’s tap water (pH 8.2), mounted, and analysed microscopically using Olympus BX51 (Hachioji, Tokyo, Japan).
4.11. Larval Burden and Therapeutic Efficacy
Larvae were recovered from the peritoneal cavity, washed, and resuspended in an appropriate volume of 0.1% agar to prevent sedimentation. The suspension was thoroughly mixed, and 0.5 mL was randomly taken, placed on a glass slide, and the larvae were counted under a light microscope. For each sample, counts were performed in triplicate. The number of larvae per ml was calculated and then extrapolated to the total volume of the larval suspension in agar. Approximately 50 mg of larvae per mouse were preserved in TRIzol (Invitrogen) at −80 °C for RNA extraction. Therapeutic effect was expressed as the proportions of larval numbers/mouse in treated groups calculated as percentage from infected untreated group, where the mean number (n = 7) represented 100%. Data represent mean ± stand error of the mean (SEM).
4.12. Statistical Analysis
Depending on the assay used, analyses were performed in duplicates or triplicates and the mean was calculated and was used to calculate the final mean and standard error of mean (SEM). Data from flow cytometric analysis of cell phenotypes, results of which are shown in graphs, represent the mean and standard deviation (SD). Statistically significant differences between the infected and treated groups were calculated using one-way ANOVA followed by Tukey’s post hoc test. In the case of group analysis, two-way ANOVA was applied, followed by Bonferroni’s or Sidak’s multiple comparison analyses. Data were evaluated with GraphPad Prism (version 10.4.2.) (GraphPad Software, Inc., San Diego, CA, USA) and statistical differences in Figures are denoted as * p < 0.05, ** p < 0.01 and *** p < 0.001.
5. Conclusions
In conclusion, this study demonstrates that co-administration of ABZ+HLE significantly improves larvicidal efficacy in a murine model of Mesocestoides vogae infection. The combination therapy not only boosted the larval-killing effect of ABZ but also rebalanced the immune environment by enhancing Th1 responses and suppressing regulatory T and B cell subsets. Therefore, HLE represents a promising immunomodulatory supplement to chemotherapy in helminth infections. Additional studies are needed in order to explore the broader applicability of HLE as an adjuvant treatment in other helminthiases revealing the biological mechanism of action, to improve therapeutic strategies for parasite control and host protection.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms26146994/s1.
Author Contributions
Conceptualization, G.H. and D.M.; methodology, G.H., D.M., S.C. and K.R.; validation, G.H.; formal analysis, S.C. and I.B.; investigation, G.H., K.R. and D.M.; resources, G.H.; data curation, G.H., D.M. and S.C.; writing—original draft preparation, G.H.; writing—review and editing, G.H., D.M., S.C. and I.B.; project administration, G.H.; funding acquisition, G.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Scientific Grant Agency of the Ministry of Education of the Slovak Republic and the Slovak Academy of Sciences, grant number APVV—17–0410 and partially by international EU project “COST Action no. CA21111—One Health drugs against parasitic vector borne diseases in Europe and beyond (OneHealthdrugs)/MVTS” (Slovak Republic). Open access funding was provided by grant number APVV—17–0410.
Institutional Review Board Statement
The animal study was conducted in accordance with guidelines laid down by Government Regulation § 35, no. 377/2012 Coll., establishing requirements for the protection of animals used for scientific purposes or educational purposes as amended by SR Government Regulation No. 199/2019 Coll. All methods are reported per ARRIVE guidelines. The study protocol was approved by the Ethics Committee of the State Veterinary and Food Administration of the Slovak Republic under protocol No. 3871/15-221d in 2021.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analysed during this study are included in this article.
Acknowledgments
The authors thank AUMED Company Ltd. (Czech Republic) and Ľuboš Jakub, for the donation of the certified batch of HLE preparation for research purposes. The authors would like to acknowledge Zuzana Jučacková, for technical help and assistance with the preparation of images from immunoblot analyses, and Silvia Spišáková, for help with animal handling and drug application, both from Parasitological Institute of SAS, Košice.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Arnaudov, A.; Kostova, Z. Dialysable leukocyte extracts in immunotherapy. Biotechnol. Biotechnol. Equip. 2015, 29, 1017–1023. [Google Scholar] [CrossRef]
- Estrada-Parra, S.; Nagaya, A.; Serrano, E.; Rodriguez, O.; Santamaria, V.; Ondarza, R.; Chavez, R.; Correa, B.; Monges, A.; Cabezas, R.; et al. Comparative study of transfer factor and acyclovir in the treatment of herpes zoster. Int. J. Immunopharmacol. 1998, 20, 521–535. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Ortega, C.; Dubed, M.; Ramos, Y.; Navea, L.; Alvarez, G.; Lobaina, L.; Lopez, L.; Casillas, D.; Rodriguez, L. Non-induced leukocyte extract reduces HIV replication and TNF secretion. Biochem. Biophys. Res. Commun. 2004, 325, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- Fabre, R.A.; Pérez, T.M.; Aguilar, L.D.; Rangel, M.J.; Estrada-Garcìa, I.; Hernández-Pando, R.; Parra, S.E. Transfer factors as immunotherapy and supplement of chemotherapy in experimental pulmonary tuberculosis. Clin. Exp. Immunol. 2004, 136, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Espinosa, O.; Moreno-García, S.; Arce-Paredes, P.; Becerril-Villanueva, E.; Juárez-Ortega, M. Effect of dialyzable leukocyte extract, sodium butyrate, and valproic acid in the development of anergy in murine leprosy. Int. J. Mycobacteriol. 2020, 9, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Ciglanová, D.; Jurčacková, Z.; Mudroňová, D.; Dvorožňaková, E.; Hrčková, G. Differential Activity of Human Leukocyte Extract on Systemic Immune Response and Cyst Growth in Mice with Echinococcus Multilocularis Infection After Oral, Subcutaneous and Intraperitoneal Routes of Administration. Helminthologia 2022, 59, 341–356. [Google Scholar] [CrossRef] [PubMed]
- Kubašková, T.M.; Mudroňová, D.; Velebný, S.; Hrčková, G. The utilisation of human dialyzable leukocyte extract (IMMODIN) as adjuvant in albendazole therapy on mouse model of larval cestode infection: Immunomodulatory and hepatoprotective effects. Int. Immunopharmacol. 2018, 65, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Viza, D.; Fudenberg, H.H.; Palareti, A.; Ablashi, D.; De Vinci, C.; Pizza, G. Transfer Factor: An Overlooked Potential for the Prevention and Treatment of Infectious Diseases. Folia Biol. (Prague) 2013, 59, 53–67. [Google Scholar] [CrossRef]
- Demečková, V.; Solar, P.; Hrčková, G.; Mudroňová, D.; Bojková, B.; Kassayová, M.; Gancarčiková, S. Immodin and its immune system supportive role in paclitaxel therapy of 4T1 mouse breast cancer. Biomed. Pharmacother. 2017, 89, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Esquivel, M.A.; Perez-Torres, A.; Romero-Romero, L.; Reyes-Matute, A.; Loaiza, B.; Mellado-Sanchez, G.; Pavon, L.; Medina-Rivero, E.; Pestell, R.G.; Perez-Tapia, S.M.; et al. The dialyzable leukocyte extract Transferon(TM) inhibits tumor growth and brain metastasis in a murine model of prostate cancer. Biomed. Pharmacother. 2018, 101, 938–944. [Google Scholar] [CrossRef] [PubMed]
- Castrejon Vazquez, M.I.; Resendiz-Albor, A.A.; Ynga-Durand, M.A.; Martinez, I.M.A.; Orellana-Villazon, V.I.; Lopez, C.A.G.; Noguera, M.L.L.; Camano, M.E.V. Dialyzable Leukocyte Extract (Transferon) Administration in Sepsis: Experience from a Single Referral Pediatric Intensive Care Unit. BioMed Res. Int. 2019, 2019, 8980506. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Gonzalez, L.G.; Panameno-Cruz, V.; Luna-Arias, J.P.; Reyna-Beltran, E. Implementation of Dialyzable Leukocyte Extract to Treat Multiple Sclerosis: A Clinical Case. Clin. Case Rep. 2024, 12, e70016. [Google Scholar] [CrossRef] [PubMed]
- Hana, I.; Vrubel, J.; Pekarek, J.; Cech, K. The influence of age on transfer factor treatment of cellular immunodeficiency, chronic fatigue syndrome and/or chronic viral infections. Biotherapy 1996, 9, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Homberg, T.A.; Lara, I.; Andaluz, C.; Cervantes-Trujano, E.; Hernandez-Martinez, P.M.; Perez-Tapia, S.M.; Jimenez-Martinez, M.C. Quality of life in adult patients using dialyzable leukocyte extract for allergic rhinitis. Medicine (Baltimore) 2023, 102, e34186. [Google Scholar] [CrossRef] [PubMed]
- Budke, C.M.; White, A.C., Jr.; Garcia, H.H. Zoonotic larval cestode infections: Neglected, neglected tropical diseases? PLoS Neglected Trop. Dis. 2009, 3, e319. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, M.V.; Galán-Puchades, M.T.; Malone, J.B. Short report: A new case report of human Mesocestoides infection in the United States. Am. J. Trop. Med. Hyg. 2003, 68, 566–567. [Google Scholar] [CrossRef] [PubMed]
- Eckert, J.; Deplazes, P. Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin. Microbiol. Rev. 2004, 17, 107–135. [Google Scholar] [CrossRef] [PubMed]
- Filho, P.T.H.; Rodriguez-Rivas, R.; Fleury, A. Neurocysticercosis: A Review into Treatment Options, Indications, and Their Efficacy. Res. Rep. Trop. Med. 2022, 13, 67–79. [Google Scholar] [CrossRef] [PubMed]
- White, T.R.; Thompson, R.C.; Penhale, W.J. A comparative study of the susceptibility of inbred strains of mice to infection with Mesocestoides corti. Int. J. Parasitol. 1982, 12, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Hemphill, A.; Stadelmann, B.; Rufener, R.; Spiliotis, M.; Boubaker, G.; Müller, J.; Müller, N.; Gorgas, D.; Gottstein, B. Treatment of echinococcosis: Albendazole and mebendazole—What else? Parasite 2014, 21, 70. [Google Scholar] [CrossRef] [PubMed]
- Popova, G.; Vuchev, D.; Anichina, K. Treatment of hepatic and pulmonary hydatidosis with albendazole and praziquantel. Helminthologia 2023, 60, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Vuitton, D.A. Benzimidazoles for the treatment of cystic and alveolar echinococcosis: What is the consensus? Expert Rev. Anti-Infect. Ther. 2009, 7, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Kern, P. Medical treatment of echinococcosis under the guidance of Good Clinical Practice (GCP/ICH). Parasitol. Int. 2006, 55, S273–S282. [Google Scholar] [CrossRef] [PubMed]
- Son, D.S.; Lee, E.S.; Adunyah, S.E. The Antitumor Potentials of Benzimidazole Anthelmintics as Repurposing Drugs. Immune Netw. 2020, 20, e29. [Google Scholar] [CrossRef] [PubMed]
- Kubašková, T.M.; Mudroňová, D.; Vargová, M.; Reiterová, K.; Hrčková, G. Cellular and humoral peritoneal immunity to Mesocestoides vogae metacestode infection in mice. Parasites Vectors 2021, 14, 54. [Google Scholar] [CrossRef] [PubMed]
- Vendelova, E.; Lutz, M.B.; Hrčková, G. Immunity and immune modulation elicited by the larval cestode Mesocestoides vogae and its products. Parasite Immunol. 2015, 37, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Smith-Garvin, J.E.; Koretzky, G.A.; Jordan, M.S. T cell activation. Annu. Rev. Immunol. 2009, 27, 591–619. [Google Scholar] [CrossRef] [PubMed]
- Lund, F.E. Cytokine-producing B lymphocytes-key regulators of immunity. Curr. Opin. Immunol. 2008, 20, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Fillatreau, S. Regulatory functions of B cells and regulatory plasma cells. Biomed. J. 2019, 42, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Nono, J.K.; Lutz, M.B.; Brehm, K. Expansion of Host Regulatory T Cells by Secreted Products of the Tapeworm Echinococcus multilocularis. Front. Immunol. 2020, 11, 798. [Google Scholar] [CrossRef] [PubMed]
- Kallies, A.; Good-Jacobson, K.L. Transcription Factor T-bet Orchestrates Lineage Development and Function in the Immune System. Trends Immunol. 2017, 38, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.Y. GATA3: A master of many trades in immune regulation. Trends Immunol. 2014, 35, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Hori, S.; Nomura, T.; Sakaguchi, S. Control of regulatory T cell development by the transcription factor Foxp3. Science 2003, 299, 1057–1061. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.Y.; Long, J.; Huang, M.X.; Luo, P.Y.; Bian, Z.H.; Xu, Y.F.; Wang, C.B.; Yang, S.H.; Li, L.; Selmi, C.; et al. Characterization of Organ-Specific Regulatory B Cells Using Single-Cell RNA Sequencing. Front. Immunol. 2021, 12, 711980. [Google Scholar] [CrossRef] [PubMed]
- Maizels, R.M.; Smits, H.H.; McSorley, H.J. Modulation of Host Immunity by Helminths: The Expanding Repertoire of Parasite Effector Molecules. Immunity 2018, 49, 801–818. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.M. IgG subclass co-expression brings harmony to the quartet model of murine IgG function. Immunol. Cell Biol. 2016, 94, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Snapper, C.M.; Mond, J.J. Towards a comprehensive view of immunoglobulin class switching. Immunol. Today 1993, 14, 15–17. [Google Scholar] [CrossRef] [PubMed]
- Siles-Lucas Mdel, M.; Gottstein, B. The 14-3-3 protein: A key molecule in parasites as in other organisms. Trends Parasitol. 2003, 19, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Hrčková, G.; Kubašková, T.M.; Mudroňová, D.; Jurčacková, Z.; Ciglanová, D. Co-Treatment with Human Leukocyte Extract and Albendazole Stimulates Drug’s Efficacy and Th1 Biased Immune Response in Mesocestoides vogae (Cestoda) Infection via Modulation of Transcription Factors, Macrophage Polarization, and Cytokine Profiles. Pharmaceutics 2023, 15, 541. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, A.E.; Kerepesi, L.A.; Vandergrift, G.L.; Herbert, D.R.; Van Winkle, T.J.; Hooper, D.C.; Pearce, E.J.; Abraham, D. IL-4-/- mice with lethal Mesocestoides corti infections—reduced Th2 cytokines and alternatively activated macrophages. Parasite Immunol. 2009, 31, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Peon, A.N.; Espinoza-Jimenez, A.; Terrazas, L.I. Immunoregulation by Taenia crassiceps and its antigens. BioMed Res. Int. 2013, 2013, 498583. [Google Scholar] [CrossRef] [PubMed]
- Vendelova, E.; de Lima, J.C.; Lorenzatto, K.R.; Monteiro, K.M.; Mueller, T.; Veepaschit, J.; Grimm, C.; Brehm, K.; Hrčková, G.; Lutz, M.B.; et al. Proteomic Analysis of Excretory-Secretory Products of Mesocestoides corti Metacestodes Reveals Potential Suppressors of Dendritic Cell Functions. PLoS Neglected Trop. Dis. 2016, 10, e0005061. [Google Scholar] [CrossRef] [PubMed]
- Dorshkind, K.; Montecino-Rodriguez, E. Fetal B-cell lymphopoiesis and the emergence of B-1-cell potential. Nat. Rev. Immunol. 2007, 7, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, M.I.; Catalan-Dibene, J.; Zlotnik, A. B cells responses and cytokine production are regulated by their immune microenvironment. Cytokine 2015, 74, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Rosser, E.C.; Mauri, C. Regulatory B Cells: Origin, Phenotype, and Function. Immunity 2015, 42, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Oishi, S.; Takano, R.; Tamura, S.; Tani, S.; Iwaizumi, M.; Hamaya, Y.; Takagaki, K.; Nagata, T.; Seto, S.; Horii, T.; et al. M2 polarization of murine peritoneal macrophages induces regulatory cytokine production and suppresses T-cell proliferation. Immunology 2016, 149, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Dvorožnáková, E.; Porubcová, J.; Ševcíková, Z. Immune response of mice with alveolar echinococcosis to therapy with transfer factor, alone and in combination with albendazole. Parasitol. Res. 2009, 105, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Ramirez, D.; Vadillo, E.; Arriaga-Pizano, L.A.; Mayani, H.; Estrada-Parra, S.; Velasco-Velazquez, M.A.; Perez-Tapia, S.M.; Pelayo, R. Early Differentiation of Human CD11c(+)NK Cells with gammadelta T Cell Activation Properties Is Promoted by Dialyzable Leukocyte Extracts. J. Immunol. Res. 2016, 2016, 4097642. [Google Scholar] [CrossRef] [PubMed]
- Ricken, F.J.; Nell, J.; Grüner, B.; Schmidberger, J.; Kaltenbach, T.; Kratzer, W.; Hillenbrand, A.; Henne-Bruns, D.; Deplazes, P.; Moller, P.; et al. Albendazole increases the inflammatory response and the amount of Em2-positive small particles of Echinococcus multilocularis (spems) in human hepatic alveolar echinococcosis lesions. PLoS Neglected Trop. Dis. 2017, 11, e0005636. [Google Scholar] [CrossRef] [PubMed]
- Tiemessen, M.M.; Jagger, A.L.; Evans, H.G.; van Herwijnen, M.J.C.; John, S.; Taams, L.S. CD4-CD25-Foxp3 regulatory T cells induce alternative activation of human monocytes/macrophages. Proc. Natl. Acad. Sci. USA 2007, 104, 19446–19451. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Müller, S.; Lin, R.Y.; Siffert, M.; Vuitton, D.A.; Wen, H.; Gottstein, B. Depletion of FoxP3+Tregs improves control of larval Echinococcus multilocularis infection by promoting co-stimulation and Th1/17 immunity. Immun. Inflamm. Dis. 2017, 5, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Ludwig-Portugall, I.; Layland, L.E. TLRs, Treg, and B cells, an interplay of regulation during helminth infection. Front. Immunol. 2012, 3, 8. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, H.; Ni, Y.; Li, C.; Xu, X.; Chang, H.; Xu, Z.; Hou, M.; Ji, M. Helminth-induced CD9(+) B-cell subset alleviates obesity-associated inflammation via IL-10 production. Int. J. Parasitol. 2022, 52, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Ku, H.C.; Cheng, C.F. Master Regulator Activating Transcription Factor 3 (ATF3) in Metabolic Homeostasis and Cancer. Front. Endocrinol. 2020, 11, 556. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.Y.; Chang, C.P. Lectin of Concanavalin A as an anti-hepatoma therapeutic agent. J. Biomed. Sci. 2009, 16, 10. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, R.H. T cell anergy. Annu. Rev. Immunol. 2003, 21, 305–334. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Li, L.; Dong, D.; Wang, L.; Wang, X.; Yang, K.; Xu, X.; Chen, C.; Wu, X.; Chen, X. Glycomolecules in Echinococcus granulosus cyst fluid inhibit TLR4-mediated inflammatory responses via c-Raf. Cell Mol. Immunol. 2020, 17, 423–425. [Google Scholar] [CrossRef] [PubMed]
- Gruner, B.; Peters, L.; Hillenbrand, A.; Vossberg, P.; Schweiker, J.; Rollmann, E.G.; Rodriguez, L.H.; Blumhardt, J.; Burkert, S.; Kern, P.; et al. Echinococcus multilocularis specific antibody, systemic cytokine, and chemokine levels, as well as antigen-specific cellular responses in patients with progressive, stable, and cured alveolar echinococcosis: A 10-year follow-up. PLoS Neglected Trop. Dis. 2022, 16, e0010099. [Google Scholar] [CrossRef] [PubMed]
- Rigáno, R.; Profumo, E.; Ioppolo, S.; Notargiacomo, S.; Teggi, A.; Siracusano, A. Serum cytokine detection in the clinical follow up of patients with cystic echinococcosis. Clin. Exp. Immunol. 1999, 115, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Rawat, J.; Dixon, J.B.; Macintyre, A.R.; McGarry, H.F.; Taylor, M.J. IL-4 dependent resistance to the tapeworm Mesocestoides corti (Cestoda) in mice. Parasite Immunol. 2003, 25, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, P.; Dixon, J.B.; Haywood, S.; Rakha, N.K.; Carter, S.D. Differential Regulation of Murine Mesocestoides corti Infection by Bacterial Lipopolysaccharide and Interferon-Gamma. Parasitology 1991, 102, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Abraham, K.M.; Teale, J.M. Isotype Restriction during Infection of Mice with the Cestode Mesocestoides corti—Role of Immune Suppression. J. Immunol. 1987, 138, 1699–1704. [Google Scholar] [CrossRef] [PubMed]
- Zakroff, S.G.H.; Beck, L.; Platzer, E.G.; Spiegelberg, H.L. The Ige and Igg Subclass Responses of Mice to 4 Helminth-Parasites. Cell Immunol. 1989, 119, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Zuniga-Navarrete, F.; Zavala-Meneses, S.G.; Zelnik, V.; Kopacek, J.; Skultety, L. Initial proteomic characterization of IMMODIN, commercially available dialysable leukocytes extract. Chem. Pap. 2021, 75, 1959–1968. [Google Scholar] [CrossRef]
- Matsumoto, J.; Muller, N.; Hemphill, A.; Oku, Y.; Kamiya, M.; Gottstein, B. 14-3-3- and II/3-10-gene expression as molecular markers to address viability and growth activity of Echinococcus multilocularis metacestodes. Parasitology 2006, 132 Pt 1, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Lighvani, A.A.; Frucht, D.M.; Jankovic, D.; Yamane, H.; Aliberti, J.; Hissong, B.D.; Nguyen, B.V.; Gadina, M.; Sher, A.; Paul, W.E.; et al. T-bet is rapidly induced by interferon-γ in lymphoid and myeloid cells. Proc. Natl. Acad. Sci. USA 2001, 98, 15137–15142. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.P.; Flavell, R.A. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell 1997, 89, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Ichiyama, K.; Yoshida, H.; Wakabayashi, Y.; Chinen, T.; Saeki, K.; Nakaya, M.; Takaesu, G.; Hori, S.; Yoshimura, A.; Kobayashi, T. Foxp3 inhibits RORγt-mediated IL-17A mRNA transcription through direct interaction with RORγt. J. Biol. Chem. 2008, 283, 17003–17008. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Chen, J.; Hai, T. The regulation of ATF3 gene expression by mitogen-activated protein kinases. Biochem. J. 2007, 401, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Won, W.J.; Kearney, J.F. CD9 is a unique marker for marginal zone B cells, B1 cells, and plasma cells in mice. J. Immunol. 2002, 168, 5605–5611. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Geng, J.; Gao, J.H.; Zhao, H.; Li, J.H.; Shi, Y.R.; Yang, B.Y.; Xiao, C.; Linghu, Y.Y.; Sun, X.F.; et al. Macrophage achieves self-protection against oxidative stress-induced ageing through the Mst-Nrf2 axis. Nat. Commun. 2019, 10, 755. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).