Effect of Environmental Exposure to Zearalenone on the Metabolic Profile of Patients with Sigmoid Colorectal Cancer or Colorectal Cancer on the Day of Hospital Admission
Abstract
1. Introduction
2. Results
2.1. Concentrations of ZEN and Its Metabolites
2.2. Metabolic Reprogramming During SCC and CRC [2]
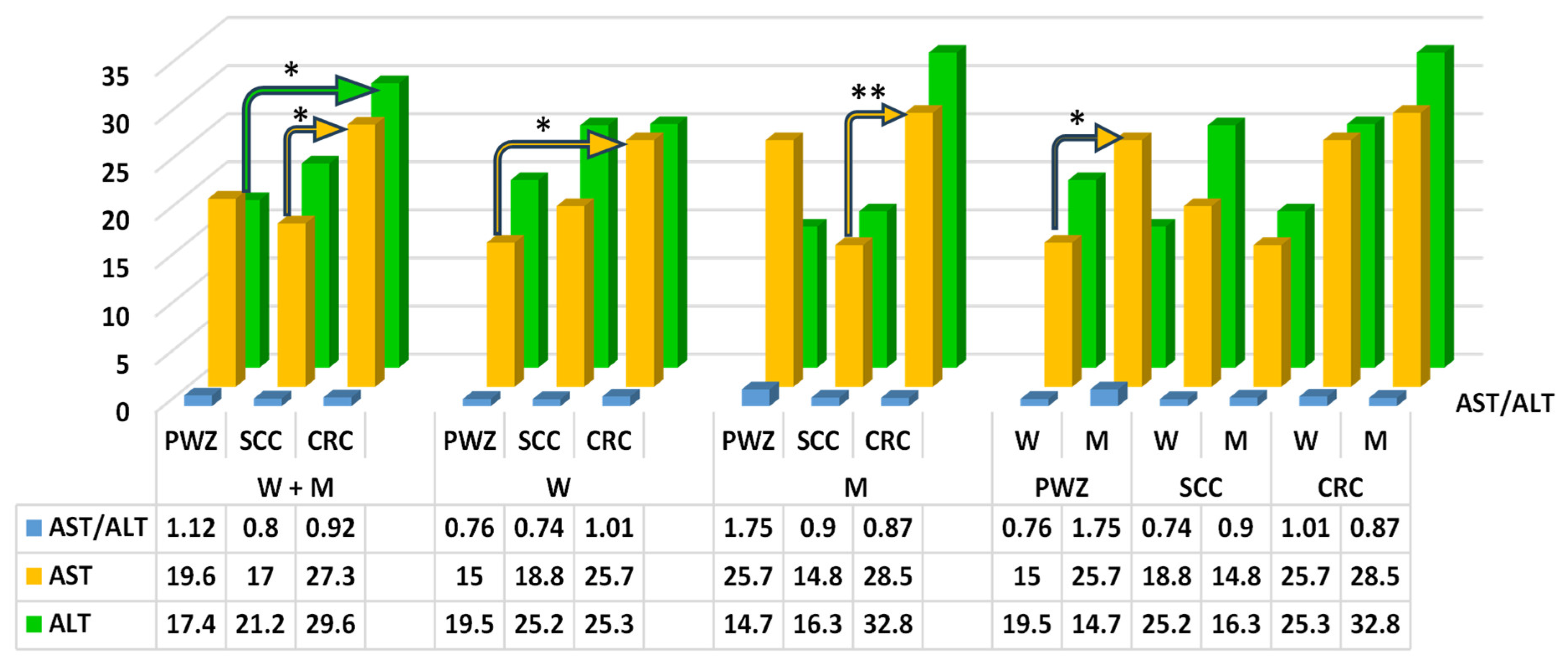
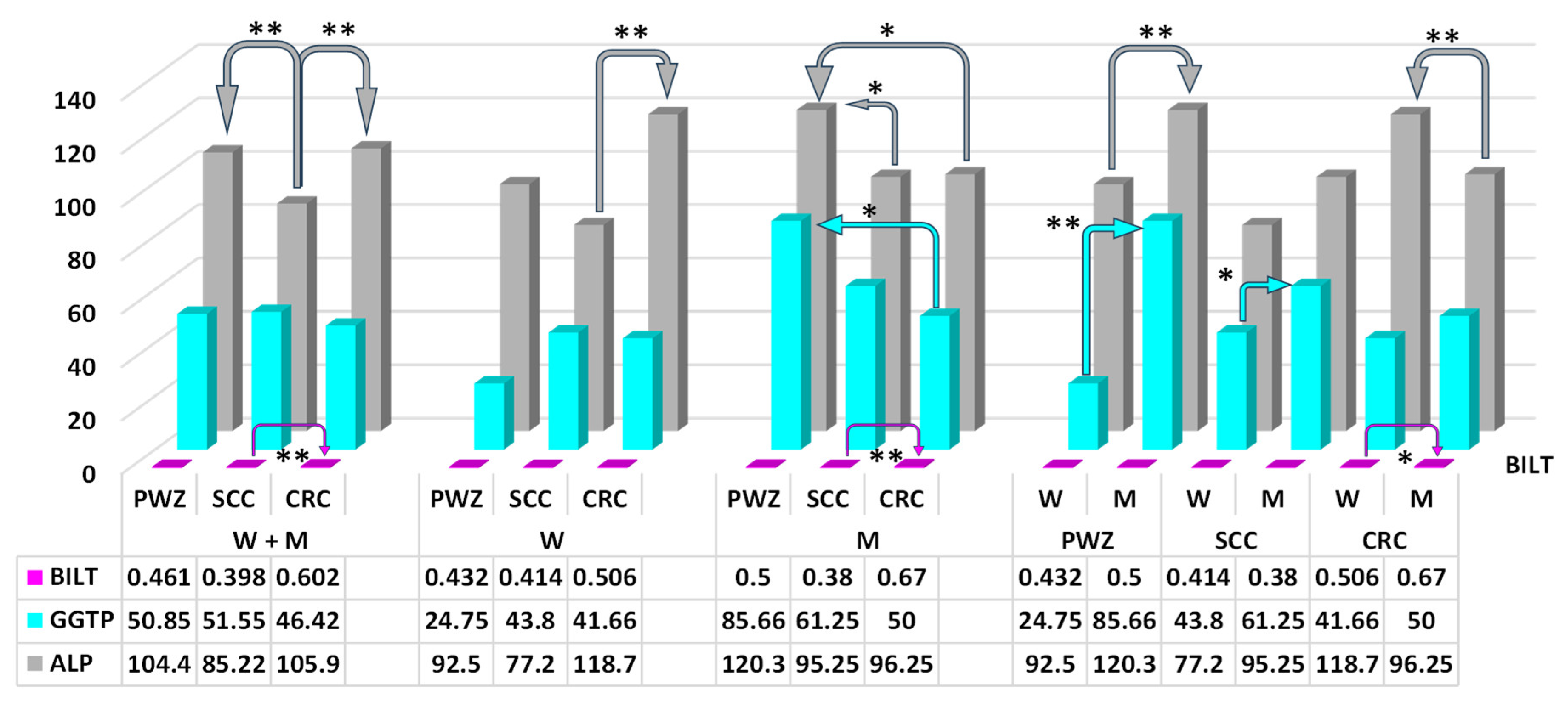
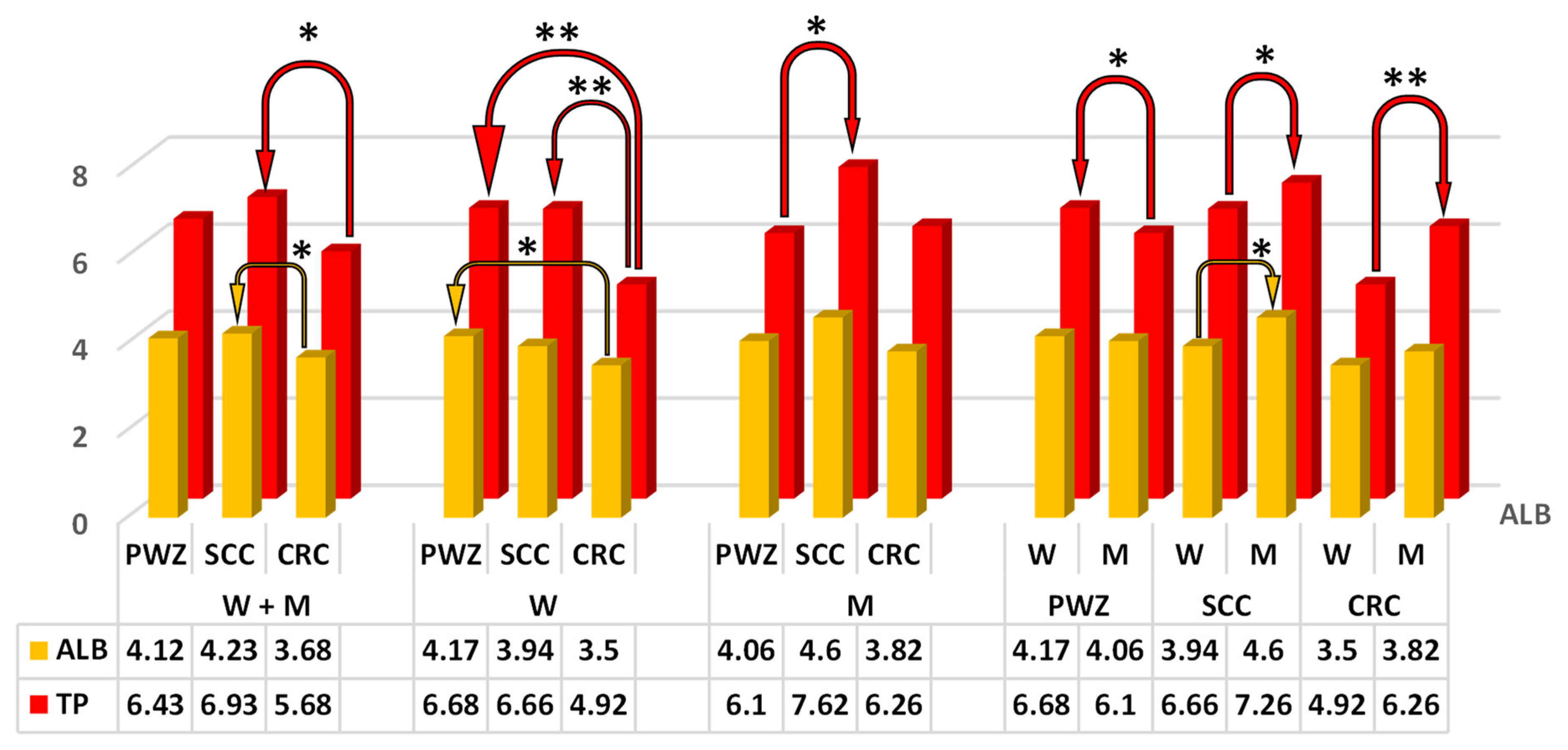
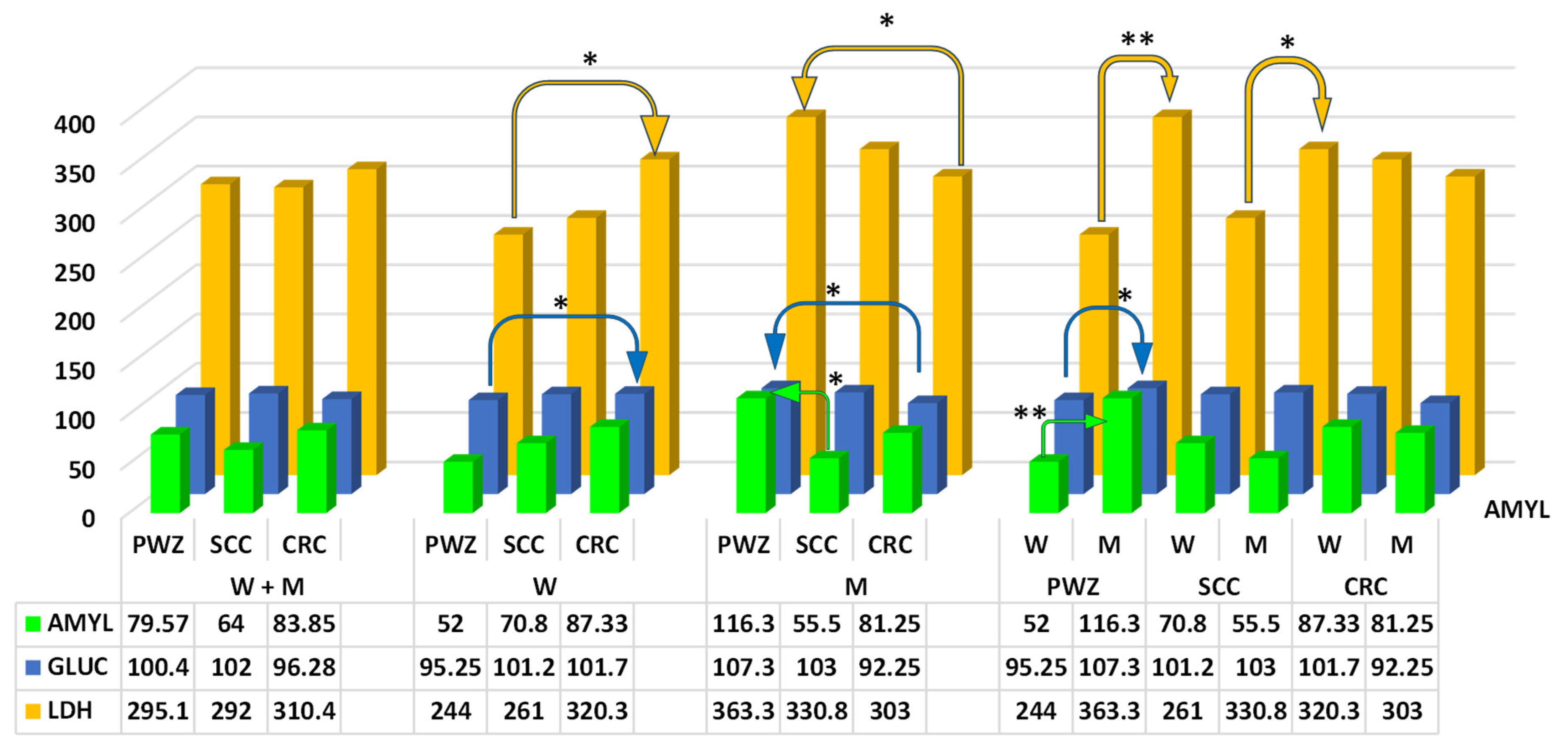
2.2.1. Liver Function Test
2.2.2. Comprehensive Metabolic Panel
3. Discussion
3.1. Zearalenone
3.2. Metabolic Reprogramming Effect in Patients with SCC and CRC
4. Materials and Methods
4.1. Patients
Inclusion Criteria
4.2. Study Design
4.3. Blood Sampling
4.4. Extraction Procedure and Quantification of ZEN, α-ZEL, and β-ZEL
4.5. Quantification of Blood Biochemical Parameters
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zou, S.; Fang, L. Metabolic reprogramming in colorectal cancer: Regulatory networks and therapy. Cell Biosci. 2023, 13, 25. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Liu, X.; Wang, L.; Hang, D. The Potential of Metabolomics in Colorectal Cancer Prognosis. Metabolites 2024, 14, 708. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Siegel, R.L.; Laversanne, M.; Jiang, C.; Morgan, E.; Zahwe, M.; Cao, Y.; Bray, F.; Jema, A. Colorectal cancer incidence trends in younger versus older adults: An analysis of population-based cancer registry data. Lancet Oncol. 2025, 26, 51–63. [Google Scholar] [CrossRef] [PubMed]
- do Rêgo, A.C.M.; Araújo-Filho, I. The Role of Gut Microbiota in Tumor Recurrence and Therapy Resistance in Colorectal Cancer: Molecular Mechanisms and Clinical Implications. Jpn. J. Res. 2025, 6, 105. [Google Scholar] [CrossRef]
- Wang, D.; Zhu, L.; Liu, H.; Feng, X.; Zhang, C.; Liu, B.; Li, T.; Liu, L.; Chang, H.; Sun, J.; et al. Altered gut metabolites and metabolic reprogramming involved in the pathogenesis of colitis-associated colorectal cancer and the transition of colon “inflammation to cancer”. J. Pharm. Biomed. 2025, 253, 116553. [Google Scholar] [CrossRef] [PubMed]
- Maresca, M.; Fantini, J. Some food-associated mycotoxins as potential risk factors in humans predisposed to chronic intestinal inflammatory diseases. Toxicon 2010, 56, 282–294. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Huang, Y.; Jiang, P.; Yuan, X.; Long, Q.; Yan, X.; Huang, Y.; Wang, Z.; Li, Z. Research progress on anti-inflammatory drugs for preventing colitis-associated colorectal cancer. Int. Immunopharmacol. 2025, 144, 113583. [Google Scholar] [CrossRef] [PubMed]
- Przybylska-Gornowicz, B.; Lewczuk, B.; Prusik, M.; Hanuszewska, M.; Petrusewicz-Kosińska, M.; Gajęcka, M.; Zielonka, Ł.; Gajęcki, M. The Effects of Deoxynivalenol and Zearalenone on the Pig Large Intestine. A Light and Electron Microscopic study. Toxins 2018, 10, 148. [Google Scholar] [CrossRef] [PubMed]
- Fousekis, F.S.; Mpakogiannis, K.; Filis, P.; Skamnelos, A.; Christodoulou, D.K.; Mauri, D.; Katsanos, K.H. Exploring Chemoprevention in Colorectal Cancer for Patients with Inflammatory Bowel Disease: Mechanisms of Action and Clinical Aspects. Cancers 2025, 17, 229. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska-Kempisty, H.; Klupczyńska, A.; Trzybulska, D.; Kulcenty, K.; Sulej-Suchomska, A.M.; Kucińska, M.; Mikstacka, R.; Wierzchowski, M.; Murias, M.; Baer-Dubowska, W.; et al. Role of CYP1A1 in the biological activity of methylated resveratrol analogue, 3,4,5,40-tetramethoxystilbene (DMU-212) in ovarian cancer A-2780 and non-cancerous HOSE cells. Toxicol. Lett. 2017, 267, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Aoyama, T.; Kimura, S.; Gonzalez, F.J. Targeting nuclear receptors for the treatment of fatty liver disease. Pharmacol. Ther. 2017, 179, 142–157. [Google Scholar] [CrossRef] [PubMed]
- Lisieska-Żołnierczyk, S.; Gajęcka, M.; Zielonka, Ł.; Dąbrowski, M.; Gajęcki, M.T. Blood levels of zearalenone, thyroid-stimulating hormone, and thyroid hormones in patients with colorectal cancer. Toxicon 2024, 251, 108125. [Google Scholar] [CrossRef] [PubMed]
- Lisieska-Żołnierczyk, S.; Gajęcka, M.; Dąbrowski, M.; Zielonka, Ł.; Gajęcki, M.T. A Cohort Study Investigating Zearalenone Concentrations and Selected Steroid Levels in Patients with Sigmoid Colorectal Cancer or Colorectal Cancer. Toxins 2024, 16, 15. [Google Scholar] [CrossRef] [PubMed]
- Gajęcka, M.; Otrocka-Domagała, I.; Brzuzan, P.; Zielonka, Ł.; Dąbrowski, M.; Gajęcki, M.T. Influence of deoxynivalenol and zearalenone on the immunohistochemical expression of oestrogen receptors and liver enzyme genes in vivo in prepubertal gilts. Arch. Toxicol. 2023, 97, 2155–2168. [Google Scholar] [CrossRef] [PubMed]
- Gajęcka, M.; Otrocka-Domagała, I.; Brzuzan, P.; Dąbrowski, M.; Lisieska-Żołnierczyk, S.; Zielonka, Ł.; Gajęcki, M.T. Immunohistochemical expression (IE) of oestrogen receptors in the intestines of prepubertal gilts exposed to zearalenone. Toxins 2023, 15, 122. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Huangfu, B.; Xu, T.; Huang, K.; He, X. Zearalenone exacerbates lipid metabolism disorders by promoting liver lipid droplet formation and disrupting gut microbiota. Ecotoxicol. Environ. Saf. 2025, 289, 117664. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Huangfu, B.; Xu, T.; Xu, W.; Asakiya, C.; Huang, K.; He, X. Research Progress of Safety of Zearalenone: A Review. Toxins 2022, 14, 386. [Google Scholar] [CrossRef] [PubMed]
- Cieplińska, K.; Gajęcka, M.; Nowak, A.; Dąbrowski, M.; Zielonka, Ł.; Gajęcki, M.T. The Genotoxicity of Caecal Water in Gilts Exposed to Low Doses of Zearalenone. Toxins 2018, 10, 350. [Google Scholar] [CrossRef] [PubMed]
- Alassane-Kpembi, I.; Pinton, P.; Oswald, I.P. Effects of Mycotoxins on the Intestine. Toxins 2019, 11, 159. [Google Scholar] [CrossRef] [PubMed]
- Cieplińska, K.; Gajęcka, M.; Dąbrowski, M.; Rykaczewska, A.; Zielonka, Ł.; Lisieska-Żołnierczyk, S.; Bulińska, M.; Gajęcki, M.T. Time-dependent changes in the intestinal microbiome of gilts exposed to low zearalenone doses. Toxins 2019, 11, 296. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef] [PubMed]
- Enkobahry, A.; Sime, T.; Kene, K.; Mateos, T.; Dilnesa, S.; Zawdie, B. Blood biomarkers as potential malnutrition screening alternatives among adult patients with cancer on treatment in oncology unit of jimma tertiary hospital: A cross-sectional analysis. BMC Nutr. 2023, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Menyhart, O.; Fekete, J.T.; Győrffy, B. Inflammation and Colorectal Cancer: A Meta-Analysis of the Prognostic Significance of the Systemic Immune–Inflammation Index (SII) and the Systemic Inflammation Response Index (SIRI). Int. J. Mol. Sci. 2024, 25, 8441. [Google Scholar] [CrossRef] [PubMed]
- Mróz, M.; Gajęcka, M.; Brzuzan, P.; Lisieska-Żołnierczyk, S.; Leski, D.; Zielonka, Ł.; Gajecki, M.T. Carry-Over of Zearalenone and Its Metabolites to Intestinal Tissues and the Expression of CYP1A1 and GSTπ1 in the Colon of Gilts before Puberty. Toxins 2022, 14, 354. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ma, Z.; Yi, Z.; Wu, E.; Shang, Z.; Tuo, B.; Li, T.; Liu, X. The effects of metabolism on the immune microenvironment in colorectal cancer. Cell Death Discov. 2024, 10, 118. [Google Scholar] [CrossRef] [PubMed]
- Ashrafian, H.; Sounderajah, V.; Glen, R.; Ebbels, T.; Blaise, B.J.; Kalra, D.; Kultima, K.; Spjuth, O.; Tenori, L.; Salek, R.M.; et al. Metabolomics: The Stethoscope for the Twenty-First Century. Med. Princ. Pract. 2021, 30, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Salmerón, A.M.; Tristán, A.I.; Abreu, A.C.; Fernández, I. Serum Colorectal Cancer Biomarkers Unraveled by NMR Metabolomics: Past, Present, and Future. Anal. Chem. 2022, 94, 417–430. [Google Scholar] [CrossRef] [PubMed]
- Rykaczewska, A.; Gajęcka, M.; Dąbrowski, M.; Wiśniewska, A.; Szcześniewska, J.; Gajęcki, M.T.; Zielonka, Ł. Growth performance, selected blood biochemical parameters and body weight of pre-pubertal gilts fed diets supplemented with different doses of zearalenone (ZEN). Toxicon 2018, 152, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Panteghini, M.; Forest, J.C. Standardization in laboratory medicine: New challenges. Clin. Chim. Acta 2005, 355, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Etim, N.N.; Williams, M.E.; Akpabio, U.; Offiong, E.E. Haematological parameters and factors affecting their values. Agric. Sci. 2014, 2, 37–47. [Google Scholar] [CrossRef]
- Pei, J.P.; Zhang, C.D.; Fu, X.; Ba, Y.; Yue, S.; Zhao, Z.M.; Dai, D.Q. A Modified Tumor-Node-Metastasis Classification for Stage III Colorectal Cancers Based on Treating Tumor Deposits as Positive Lymph Nodes. Front. Med. 2020, 7, 571154. [Google Scholar] [CrossRef] [PubMed]
- Nie, T.; Li, J.; You, L.; Wu, Q. Environmental Mycotoxins: A Potential Etiological Factor for Neurodegenerative Diseases? Toxicology 2025, 511, 154056. [Google Scholar] [CrossRef] [PubMed]
- Brook, N.; Gill, J.; Dharmarajan, A.; Chan, A.; Dass, C.R. NFκB-Mediated Mechanisms Drive PEDF Expression and Function in Pre- and Post-Menopausal Oestrogen Levels in Breast Cancer. Int. J. Mol. Sci. 2022, 23, 15641. [Google Scholar] [CrossRef] [PubMed]
- Balló, A.; Busznyákné Székvári, K.; Czétány, P.; Márk, L.; Török, A.; Szántó, Á.; Máté, G. Estrogenic and Non-Estrogenic Disruptor Effect of Zearalenone on Male Reproduction: A Review. Int. J. Mol. Sci. 2023, 24, 1578. [Google Scholar] [CrossRef] [PubMed]
- Kinkade, C.W.; Rivera-Núñez, Z.; Gorcyzca, L.; Aleksunes, L.M.; Barrett, E.S. Impact of Fusarium-Derived Mycoestrogens on Female Reproduction: A Systematic Review. Toxins 2021, 13, 373. [Google Scholar] [CrossRef] [PubMed]
- Ruan, H.; Zhang, J.; Wang, Y.; Huang, Y.; Wu, J.; He, C.; Ke, T.; Luo, J.; Yang, M. 7-Hydroxycholesterol/liver X receptor/apolipoprotein E mediates zearalenone-induced intestinal immunosuppression: A key target potentially linking zearalenone and cancer. J. Pharm. Anal. 2024, 14, 371–388. [Google Scholar] [CrossRef] [PubMed]
- Kościelecka, K.; Kuć, A.; Kubik-Machura, D.; Męcik-Kronenberg, T.; Włodarek, J.; Radko, L. Endocrine Effect of Some Mycotoxins on Humans: A Clinical Review of the Ways to Mitigate the Action of Mycotoxins. Toxins 2023, 15, 515. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Wang, F.; Tang, Z.; Yang, X.; Liu, Y.; Zhao, M.; Liu, S.; Han, S.; Zhang, Z.; Chen, B. Quercetagetin alleviates zearalenone-induced liver injury in rabbits through Keap1/Nrf2/ARE signaling pathway. Front. Pharmacol. 2023, 14, 1271384. [Google Scholar] [CrossRef] [PubMed]
- Llorens, P.; Herrera, M.; Juan-García, A.; Payá, J.J.; Moltó, J.C.; Ariño, A.; Juan, C. Biomarkers of Exposure to Zearalenone in In Vivo and In Vitro Studies. Toxins 2022, 14, 291. [Google Scholar] [CrossRef] [PubMed]
- Mavrommatis, A.; Giamouri, E.; Tavrizelou, S.; Zacharioudaki, M.; Danezis, G.; Simitzis, P.E.; Zoidis, E.; Tsiplakou, E.; Pappas, A.C.; Georgiou, C.A.; et al. Impact of Mycotoxins on Animals’ Oxidative Status. Antioxidants 2021, 10, 214. [Google Scholar] [CrossRef] [PubMed]
- Rykaczewska, A.; Gajęcka, M.; Onyszek, E.; Cieplińska, K.; Dąbrowski, M.; Lisieska-Żołnierczyk, S.; Bulińska, M.; Babuchowski, A.; Gajęcki, M.T.; Zielonka, Ł. Imbalance in the Blood Concentrations of Selected Steroids in Prepubertal Gilts Depending on the Time of Exposure to Low Doses of Zearalenone. Toxins 2019, 11, 561. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.Y.; Li, S.; Ogamune, K.J.; Ahmed, A.A.; Kim, I.H.; Zhang, Y.; Cai, D. Fungi in the Gut Microbiota: Interactions, Homeostasis, and Host Physiology. Microorganisms 2025, 13, 70. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, F.; Baldini, E.; Lori, E.; Cardarelli, S.; Pironi, D.; Lauro, A.; Tripodi, D.; Palumbo, P.; D’Armiento, E.; Cavallaro, G.; et al. Insights on the association between thyroid diseases and colorectal cancer. J. Clin. Med. 2023, 12, 2234. [Google Scholar] [CrossRef] [PubMed]
- Vagvala, S.H.; O’Connor, S.D. Imaging of abnormal liver function tests. Clin. Liver Dis. 2018, 11, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Abe, Y.; Nishise, S.; Yagi, M.; Mizumoto, N.; Kon, T.; Onozato, Y.; Sakai, T.; Umehara, M.; Ito, M.; et al. Low serum pancreatic amylase levels as a novel latent risk factor for colorectal adenoma in non-alcohol drinkers. J. Gastroen. Hepatol. 2022, 37, 660–668. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Chen, Z.; Qin, S.; Zhou, J.; Huang, Y.; Peng, S.; Huang, P.; Lin, Y.; Alenzi, M.; Huang, J.; et al. Gamma-glutamyl transferase to aspartateaminotransferase ratio (GSR) predicts prognoses in patients with colorectal cancer with liver metastasis after microwave ablation. BMC Gastroenterol. 2024, 24, 327. [Google Scholar] [CrossRef] [PubMed]
- Scheipner, L.; Smolle, M.A.; Barth, D.; Posch, F.; Stotz, M.; Pichler, M.; Stöger, H.; Gerger, A.; Riedl, J.M. The AST/ALT Ratio Is an Independent Prognostic Marker for Disease-free Survival in Stage II and III Colorectal Carcinoma. Anticancer Res. 2021, 41, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S.M.; Varma, A.; Kumar, S.; Acharya, S.; Patil, R. Navigating Disease Management: A Comprehensive Review of the De Ritis Ratio in Clinical Medicine. Cureus 2024, 16, e64447. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Du, F.; Tian, T.; Huang, H.; Zhang, L.; Li, D.; Liu, Y.; Zhang, D.; Gao, L.; Zheng, T.; et al. Development and validation of prognostic nomograms based on De Ritis ratio and clinicopathological features for patients with stage II/III colorectal cancer. BMC Cancer 2023, 23, 620. [Google Scholar] [CrossRef] [PubMed]
- De Ritis, F.; Coltorti, M.; Giusti, G. Transaminase activity in liver disease. Lancet 1958, 272, 214–215. [Google Scholar] [CrossRef]
- Wang, H.; Fang, K.; Zhang, J.; Jiang, Y.; Wang, G.; Zhang, H.; Chen, T.; Shi, X.; Li, Y.; Duan, F.; et al. The significance of De Ritis (aspartate transaminase/alanine transaminase) ratio in predicting pathological outcomes and prognosis in localized prostate cancer patients. Int. Urol. Nephrol. 2017, 49, 1391–1398. [Google Scholar] [CrossRef] [PubMed]
- Bezan, A.; Mrsic, E.; Krieger, D.; Stojakovic, T.; Pummer, K.; Zigeuner, R.; Hutterer, G.C.; Pichler, M. The preoperative AST/ALT (De Ritis) ratio represents a poor prognostic factor in a cohort of patients with nonmetastatic renal cell carcinoma. J. Urol. 2015, 194, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Seyed Khoei, N.; Jenab, M.; Murphy, N.; Banbury, B.L.; Carreras-Torres, R.; Viallon, V.; Kühn, T.; Bueno-de-Mesquita, B.; Aleksandrova, K.; Cross, A.J.; et al. Circulating bilirubin levels and risk of colorectal cancer: Serological and Mendelian randomization analyses. BMC Med. 2020, 18, 229. [Google Scholar] [CrossRef] [PubMed]
- Iaciu, C.I.; Emilescu, R.A.; Cotan, H.T.; Orlov, C.; Popa, A.C.; Nitipir, C. Role of Systemic Inflammation Markers in Colorectal Cancer Prognosis–A Narrative Review. Clin. Case Rep. Int. 2023, 7, 1551. [Google Scholar]
- Creeden, J.F.; Gordon, D.M.; Stec, D.E.; Hinds, T.D., Jr. Bilirubin as a metabolic hormone: The physiological relevance of low levels. Am. J. Physiol. Endocrinol. Metab. 2021, 320, E191–E207. [Google Scholar] [CrossRef] [PubMed]
- Wagner, K.H.; Wallner, M.; Molzer, C.; Gazzin, S.; Bulmer, A.C.; Tiribelli, C.; Vitek, L. Looking to the horizon: The role of bilirubin in the development and prevention of age-related chronic diseases. Clin. Sci. 2015, 129, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Xing, P.; Ji, Z.N. Correlation between pre-treatment serum total blood bilirubin and unconjugated bilirubin and prognosis in patients with colorectal cancer. World J. Gastrointest. Surg. 2023, 15, 2456–2462. [Google Scholar] [CrossRef] [PubMed]
- Damiano, S.; Longobardi, C.; Ferrara, G.; Piscopo, N.; Riccio, L.; Russo, V.; Meucci, V.; De Marchi, L.; Esposito, L.; Florio, S.; et al. Oxidative Status and Histological Evaluation of Wild Boars’ Tissues Positive for Zearalenone Contamination in the Campania Region, Southern Italy. Antioxidants 2023, 12, 1748. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Zhao, Q. Clinical significance of preoperative albumin and alkaline phosphatase in colorectal cancer: A systematic review and meta-analysis. Am. J. Transl. Res. 2024, 16, 3449–3461. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Cai, Y.; Ding, Y.; Kong, X.; Li, Z. The association between serum albumin and alkaline phosphatase in cancer patients. Medicine 2024, 103, e37526. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Bian, J.; Yuan, J.; Zhao, S.; Huang, S.; Wu, R.; Fei, F. Study on the correlation between controlling nutritional status score and clinical biochemical indicators in patients with colorectal cancer. Heliyon 2024, 10, e27202. [Google Scholar] [CrossRef] [PubMed]
- Nannini, G.; Meoni, G.; Amedei, A.; Tenori, L. Metabolomics profile in gastrointestinal cancers: Update and future perspectives. World J. Gastroenterol. 2020, 26, 2514–2532. [Google Scholar] [CrossRef] [PubMed]
- Pakiet, A.; Kobiela, J.; Stepnowski, P.; Śledziński, T.; Mika, A. Changes in lipids composition and metabolism in colorectal cancer: A review. Lipids Health Dis. 2019, 18, 29. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.H.; Rampal, S.; Sung, J.; Choi, Y.-H.; Son, H.J.; Lee, J.H.; Ho, K.Y.; Kyung, C.D.; Poong-Lyul, R.; Jae, K.; et al. Association of Serum Lipids with Colorectal Adenomas. Am. J. Gastroenterol. 2013, 108, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Zaytseva, Y.Y.; Harris, J.W.; Mitov, M.I.; Kim, J.T.; Butterfield, D.A.; Lee, E.Y.; Weiss, H.L.; Gao, T.; Evers, B.M. Increased expression of fatty acid synthase provides a survival advantage to colorectal cancer cells via upregulation of cellular respiration. Oncotarget 2015, 6, 18891–18904. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lu, J.; Fu, J.; Wang, Q.; Wang, T.; Huo, Y.; Xu, Y.; Xu, M.; Zhao, Z.; Chen, Y.; et al. The association and joint effect of serum cholesterol, glycemic status with the risk of incident cancer among middle-aged and elderly population in china cardiometabolic disease and cancer cohort (4C)-study. Am. J. Cancer Res. 2020, 10, 975–986. [Google Scholar] [PubMed]
- Fang, Z.; He, M.; Song, M. Serum lipid profiles and risk of colorectal cancer: A prospective cohort study in the UK Biobank. Br. J. Cancer 2021, 124, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Sever, Ö.N.; Başoğlu, T.; Yıldırım, S. Relationship of Tumor Localization and Lipid Parameters with Survival in Patients with Colorectal Cancer. J. Clin. Med. 2025, 14, 1302. [Google Scholar] [CrossRef] [PubMed]
- Munir, R.; Lisec, J.; Swinnen, J.V.; Zaidi, N. Lipid metabolism in cancer cells under metabolic stress. Br. J. Cancer 2019, 120, 1090–1098. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Lan, H.; Jin, K.; Liu, F. Cholesterol in colorectal cancer: An essential but tumorigenic precursor? Front. Oncol. 2023, 13, 1276654. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Li, W.; Mo, L.; Deng, S.; Lin, W.; Ma, C.; Luo, Z.; Luo, C.; Hon, H. Adipose triglyceride lipase promotes the proliferation of colorectal cancer cells via enhancing the lipolytic pathway. J. Cell Mol. Med. 2021, 25, 3963–3975. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Luo, J.; Zhang, Y.; Lu, B.; Li, N.; Zhou, Y.; Chen, S.; Wu, S.; Zhang, Q.; Dai, M.; et al. Associations between blood glucose and early- and late-onset colorectal cancer: Evidence from two prospective cohorts and Mendelian randomization analyses. J. Natl. Cancer Cent. 2024, 4, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wu, K. Effect of hyperglycemia on the occurrence and prognosis of colorectal cancer. Am. J. Transl. Res. 2024, 16, 2070–2081. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wan, X.; Li, Z.; Zhou, L. High glucose inhibits autophagy and promotes the proliferation and metastasis of colorectal cancer through the PI3K/AKT/mTOR pathway. Cancer Med. 2024, 13, e7382. [Google Scholar] [CrossRef] [PubMed]
- Kasprzak, A. Insulin-like growth factor 1 (IGF-1) signaling in glucose metabolism in colorectal cancer. Int. J. Mol. Sci. 2021, 22, 6434. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.C.; Yang, W.V. Hyperglycemia, tumorigenesis, and chronic inflammation. Crit. Rev. Oncol. Hematol. 2016, 108, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Cruz, E.; Mendez, A.; Ting, A.; Spiegel, D.; Tsai, T.C.; Carver, C.S.; Kim, Y. The associations of spirituality and Hispanic ethnicity with neuroendocrine biomarkers among patients with colorectal cancer. J. Psychosom. Res. 2024, 185, 111865. [Google Scholar] [CrossRef] [PubMed]
- Gajęcki, M.; Gajęcka, M.; Zielonka, Ł.; Jakimiuk, E.; Obremski, K. Zearalenone as a potential allergen in the alimentary tract–a review. Pol. J. Food Nutr. Sci. 2006, 56, 263–268. [Google Scholar]
- Cai, S.; Xia, Q.; Duan, D.; Fu, J.; Wu, Z.; Yang, Z.; Yu, C. Creatine kinase mitochondrial 2 promotes the growth and progression of colorectal cancer via enhancing Warburg effect through lactate dehydrogenase B. Peer J. 2024, 28, e17672. [Google Scholar] [CrossRef] [PubMed]
- Mowat, A.N.; Al-Abady, Z.N. Study the Effect of Caspase-3, Lactate Dehydrogenase, and Oxidative Stress Levels as Promising Biomarkers to Mediate Colon Cancer Therapy. J. Biomed. Biochem. 2024, 3, 23–30. [Google Scholar] [CrossRef]
- Ding, H.; Yuan, M.; Yang, Y.; Gupta, M.; Xu, X.S. Evaluating Prognostic Value of Dynamics of Circulating Lactate Dehydrogenase in Colorectal Cancer Using Modeling and Machine Learning. Clin. Pharm. Ther. 2024, 115, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Skiepko, N.; Przybylska-Gornowicz, B.; Gajęcka, M.; Gajęcki, M.; Lewczuk, B. Effects of Deoxynivalenol and Zearalenone on the Histology and Ultrastructure of Pig Liver. Toxins 2020, 12, 463. [Google Scholar] [CrossRef] [PubMed]
- Mróz, M.; Gajęcka, M.; Przybyłowicz, K.E.; Sawicki, T.; Lisieska-Żołnierczyk, S.; Zielonka, Ł.; Gajęcki, M.T. The Effect of Low Doses of Zearalenone (ZEN) on the Bone Marrow Microenvironment and Haematological Parameters of Blood Plasma in Pre-Pubertal Gilts. Toxins 2022, 14, 105. [Google Scholar] [CrossRef] [PubMed]
- Ropejko, K.; Twarużek, M. Zearalenone and Its Metabolites—General Overview, Occurrence, and Toxicity. Toxins 2021, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- Guder, W.G.; Narayanan, S.; Wisser, H.; Zawta, B. Diagnostic Samples: From the Patient to the Laboratory: The Impact of Preanalytical Variables on the Quality of Laboratory Results, 4th ed.; Wiley-Blackwell: Oxford, UK, 2014; ISBN 978-3-527-69108-1. [Google Scholar]

| Groups | ZEN | α-ZEL | β-ZEL | |||
|---|---|---|---|---|---|---|
| Patients | W | M | W | M | W | M |
| PWZ | 0 | 0 | 0 | 0 | 0 | 0 |
| SCC | 224.19 ± 98.96 | 229.12 ± 84.02 | 0 | 0 | 0 | 0 |
| CRC | 289.14 * ± 38.72 | 214.61 ± 144.75 | 0 | 0 | 0 | 0 |
| Analyte | Precursor (m/z) | Production (m/z) | Fragmentor Voltage (V) | Collision Energy (eV) | LOD (ng mL−1) | LOQ (ng mL−1) | Linearity (%R2) |
|---|---|---|---|---|---|---|---|
| ZEN | 317.1 | 273.3 187.1 | 160 | 25 33 | 0.03 | 0.1 | 0.999 |
| α-ZEL | 319.2 | 275.2 160.1 | 144 | 21 33 | 0.3 | 0.9 | 0.997 |
| β-ZEL | 319.2 | 275.2 160.1 | 144 | 21 33 | 0.3 | 1 | 0.993 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lisieska-Żołnierczyk, S.; Gajęcka, M.; Zielonka, Ł.; Przybyłowicz, K.E.; Gajęcki, M.T. Effect of Environmental Exposure to Zearalenone on the Metabolic Profile of Patients with Sigmoid Colorectal Cancer or Colorectal Cancer on the Day of Hospital Admission. Int. J. Mol. Sci. 2025, 26, 6967. https://doi.org/10.3390/ijms26146967
Lisieska-Żołnierczyk S, Gajęcka M, Zielonka Ł, Przybyłowicz KE, Gajęcki MT. Effect of Environmental Exposure to Zearalenone on the Metabolic Profile of Patients with Sigmoid Colorectal Cancer or Colorectal Cancer on the Day of Hospital Admission. International Journal of Molecular Sciences. 2025; 26(14):6967. https://doi.org/10.3390/ijms26146967
Chicago/Turabian StyleLisieska-Żołnierczyk, Sylwia, Magdalena Gajęcka, Łukasz Zielonka, Katarzyna E. Przybyłowicz, and Maciej T. Gajęcki. 2025. "Effect of Environmental Exposure to Zearalenone on the Metabolic Profile of Patients with Sigmoid Colorectal Cancer or Colorectal Cancer on the Day of Hospital Admission" International Journal of Molecular Sciences 26, no. 14: 6967. https://doi.org/10.3390/ijms26146967
APA StyleLisieska-Żołnierczyk, S., Gajęcka, M., Zielonka, Ł., Przybyłowicz, K. E., & Gajęcki, M. T. (2025). Effect of Environmental Exposure to Zearalenone on the Metabolic Profile of Patients with Sigmoid Colorectal Cancer or Colorectal Cancer on the Day of Hospital Admission. International Journal of Molecular Sciences, 26(14), 6967. https://doi.org/10.3390/ijms26146967







