Precision Recovery After Spinal Cord Injury: Integrating CRISPR Technologies, AI-Driven Therapeutics, Single-Cell Omics, and System Neuroregeneration
Abstract
1. Introduction
1.1. Overview of Spinal Cord Injury (SCI)
1.2. Importance of Molecular Understanding in SCI
- Neuroinflammation: The Paradox of Destruction and Repair
- M1 Microglia: The cellular stormtrooper, a tissue-level synergy of chemical defense, predominantly from the TNF-α and IL-1β that are produced, with their main contribution being to the ongoing death of neurons, which includes bystander neuronal death [27];
- M2 Microglia: The cellular repairers, which contribute to recycling debris and initiate reparative changes by way of a suite of neurotrophic compounds, including IL-10 and TGF-β, to commence regeneration [28].
- Oxidative Stress: The Silent Destroyer
- Emerging Pathways: Ferroptosis and Systems Biology Insights
2. Molecular Pathophysiology of SCI
2.1. Primary Injury: The Mechanical Trigger and Immediate Molecular Response
- Biomechanical Complexity and Axonal Vulnerability
- Vascular Dysfunction and Hypoxic Stress
- Molecular Triggers: Calcium Dysregulation and DAMP Release
- Necroptosis and Autophagy: Divergent Cellular Responses
2.2. Secondary Injury: The Amplifying Cascade
2.2.1. Neuroinflammation: The Janus-Faced Immune Response
- Microglial Senescence and Plasticity
- Extracellular Vesicles in Inflammatory Propagation
2.2.2. Oxidative Stress: The Molecular Wildfire
- Proteostasis and Oxidative Stress Interplay
2.2.3. Excitotoxicity and Programmed Cell Death
- Emerging Concepts and Translational Perspectives
- 1.
- Adaptive Gene Editing: The future ability to adapt gene editing, such as CRISPR, to the future adaptive technological improvements of using base editing and prime editing will help adjust unnecessary inhibitory genes like CSPGs and reduce unwanted off-target effects [91];
- 2.
- Systems Biology with SCI: The use of AI-based models allowing for changing omics data (including transcriptomic, metabolomics, and lipidomics) imaging as well as clinical outcomes through diagnosis and personalization of treatment, is pivotal in knowledge translation of SCI, better and more targeted subjects for future SCI recovery technologies [92];
- 3.
- Therapies Embedded with Nanotechnology: The emerging field combining the application of lectins to understand different cell targeting and the multi-functional nanoparticles combining antioxidant and anti-inflammatory properties, as well as delivering growth factors, is poised to be the new precision/aligned therapeutics [93];
- 4.
- Holistic approaches: Not just focusing on the body but on the entire body system’s rehabilitation, e.g., gut–brain axis, healing, and metabolic reprogramming, these are a new set of paradigms, although still undefined, utilizing a rehabilitation approach to recovery mechanisms [94].
3. Multimolecular Interactions in SCI
3.1. Glial Cell Responses: Commanders of Environmental Remodeling
3.1.1. Astrocyte Reactivity and Glial Scar Dynamics
- Knowledge Gap: Astrocyte-Vascular Crosstalk
3.1.2. Microglial Dynamics and Immunometabolism
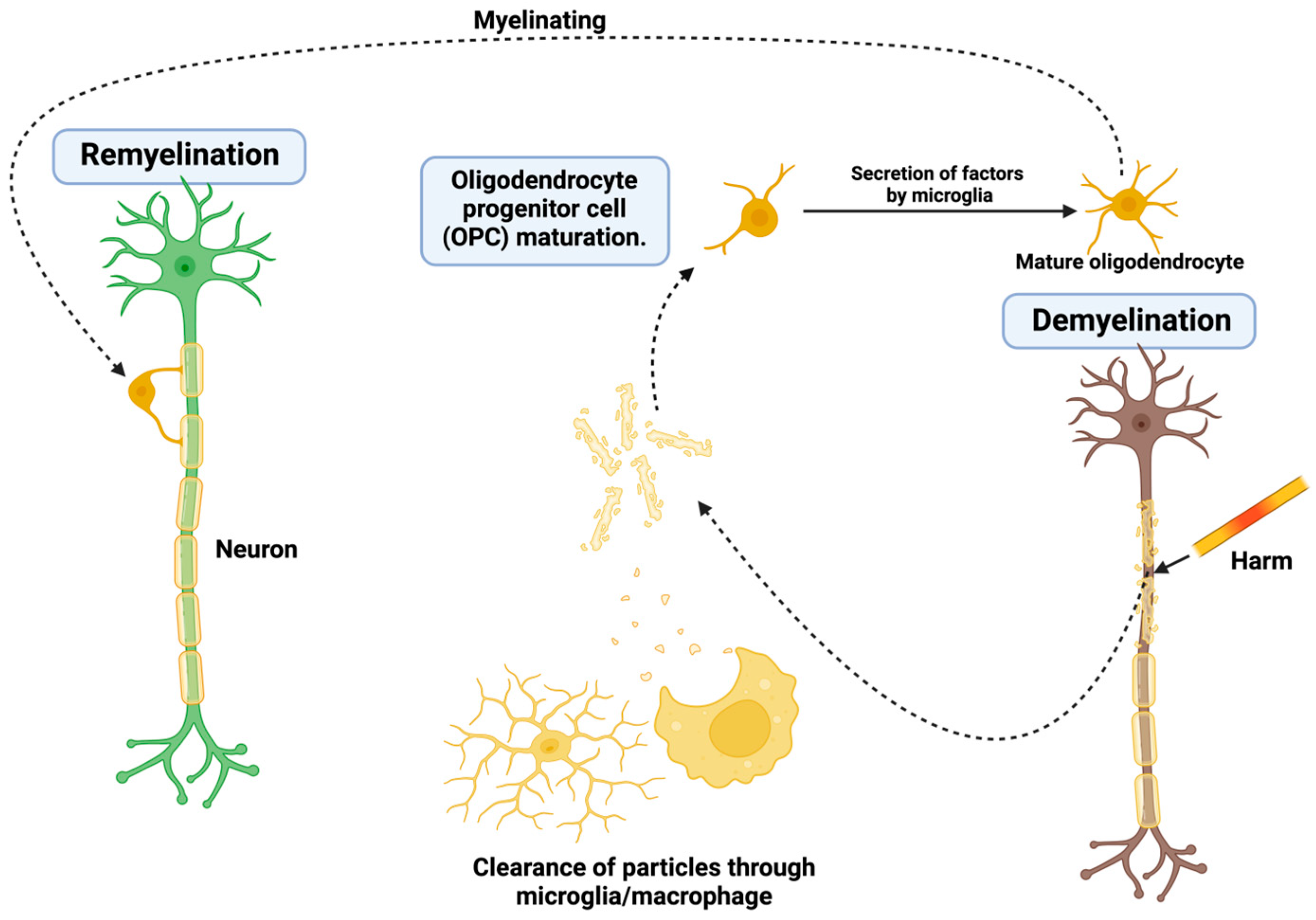
3.1.3. Oligodendrocyte Death and Challenges in Remyelination
- Knowledge Gap: Axon-OPC Interactions
3.2. Inhibitory Molecules: Molecular Barriers to Axonal Growth
3.2.1. Myelin-Associated Inhibitors
3.2.2. Chondroitin Sulfate Proteoglycans
3.3. Intracellular Signaling Pathways: Translating Inhibition into Regeneration
3.4. Future Perspectives
4. Potential Recovery Mechanisms and Therapies
4.1. Modulating the Immune Response: Turning Adversaries into Allies
4.2. Overcoming Axonal Growth Inhibition: A Path Through Barriers
4.3. Neuroprotection: Preserving What Remains
4.4. Stem Cell Therapies: Rebuilding from Within
4.5. Gene Therapy: Precision Healing
4.6. Artificial Intelligence in SCI Therapy: Precision, Prediction, and Personalization
5. Challenges and Future Directions
5.1. Translational Barriers: From Models to Clinical Realities
5.2. Novel Non-Coding RNA Targets
5.3. Advanced Stem Cell Engineering
5.4. Bioelectronic Interfaces
5.5. Neuroprotective Innovations
5.6. Ethical and Regulatory Considerations
5.7. Future Technologies
6. Conclusions and Future Directions
6.1. Summary of Key Molecular Mechanisms
- Unraveling the Complexity of Injury
6.2. The Path Ahead
- Interdisciplinary Collaboration for Complex Challenges
- Accelerating Clinical Translation
- Enhancing Quality of Life
6.3. Emerging Trends and Opportunities
6.4. Unresolved Challenges
6.5. A Vision for the Future
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lorach, H.; Galvez, A.; Spagnolo, V.; Martel, F.; Karakas, S.; Intering, N.; Vat, M.; Faivre, O.; Harte, C.; Komi, S.; et al. Walking naturally after spinal cord injury using a brain–spine interface. Nature 2023, 618, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Guest, J.D.; Kelly-Hedrick, M.; Williamson, T.; Park, C.; Ali, D.M.; Sivaganesan, A.; Neal, C.J.; Tator, C.H.; Fehlings, M.G. Development of a Systems Medicine Approach to Spinal Cord Injury. J. Neurotrauma 2023, 40, 1849–1877. [Google Scholar] [CrossRef] [PubMed]
- Oude Alink, M.; Stassen, H.; Spoor, J.; Renkens, J.; Moors, X.; Dremmen, M.; Stolker, R.J.; van der Marel, C. Traumatic Spinal Injury in Children; Time to Revise Pre-Hospital and Diagnostic Protocols? J. Clin. Med. 2024, 13, 2372. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Shang, Z.; Zhang, W.; Pang, M.; Hu, X.; Dai, Y.; Shen, R.; Wu, Y.; Liu, C.; Luo, T.; et al. Global incidence and characteristics of spinal cord injury since 2000-2021: A systematic review and meta-analysis. BMC Med. 2024, 22, 285. [Google Scholar] [CrossRef] [PubMed]
- Yokota, K.; Sakai, H.; Kawano, O.; Morishita, Y.; Masuda, M.; Hayashi, T.; Kubota, K.; Ideta, R.; Ariji, Y.; Koga, R.; et al. Changing trends in traumatic spinal cord injury in an aging society: Epidemiology of 1152 cases over 15 years from a single center in Japan. PLoS ONE 2024, 19, e0298836. [Google Scholar] [CrossRef] [PubMed]
- Jazayeri, S.B.; Maroufi, S.F.; Mohammadi, E.; Dabbagh Ohadi, M.A.; Hagen, E.-M.; Chalangari, M.; Jazayeri, S.B.; Safdarian, M.; Zadegan, S.A.; Ghodsi, Z.; et al. Incidence of traumatic spinal cord injury worldwide: A systematic review, data integration, and update. World Neurosurg. X 2023, 18, 100171. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, Y.; Chu, H.; Xu, Z.; Sun, X.; Fang, H. Association between Health-Related Quality of Life and Access to Chronic Disease Management by Primary Care Facilities in Mainland China: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2023, 20, 4288. [Google Scholar] [CrossRef] [PubMed]
- Diop, M.; Epstein, D. A Systematic Review of the Impact of Spinal Cord Injury on Costs and Health-Related Quality of Life. PharmacoEconomics–Open 2024, 8, 793–808. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, K.; Kempe, S.; Carwana, M.; Racine, N. Global and inclusive considerations for the future of ACEs research. Child Prot. Pract. 2024, 3, 100054. [Google Scholar] [CrossRef]
- Cardile, D.; Calderone, A.; De Luca, R.; Corallo, F.; Quartarone, A.; Calabrò, R.S. The Quality of Life in Patients with Spinal Cord Injury: Assessment and Rehabilitation. J. Clin. Med. 2024, 13, 1820. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, M.; Deng, Z.; Yuan, L.; Mai, Z.; Zhong, M.; Ye, J.-M. Association of systemic inflammatory response index and clinical outcome in acute traumatic spinal cord injury patients. Sci. Rep. 2024, 14, 19085. [Google Scholar] [CrossRef] [PubMed]
- Toader, C.; Tataru, C.P.; Munteanu, O.; Serban, M.; Covache-Busuioc, R.-A.; Ciurea, A.V.; Enyedi, M. Decoding Neurodegeneration: A Review of Molecular Mechanisms and Therapeutic Advances in Alzheimer’s, Parkinson’s, and ALS. Int. J. Mol. Sci. 2024, 25, 12613. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.; Swift-LaPointe, T.; Yung, A.; Prevost, V.; George, S.; Bauman, A.; Kozlowski, P.; Samadi-Bahrami, Z.; Fournier, C.; Mattu, P.S.; et al. Advanced Magnetic Resonance Imaging Biomarkers of the Injured Spinal Cord: A Comparative Study of Imaging and Histology in Human Traumatic Spinal Cord Injury. J. Neurotrauma 2024, 41, 1223–1239. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Sun, L.; Zhong, W.; Zhang, N.; Zhao, Z.; Tian, W. Artificial intelligence-assisted repair of peripheral nerve injury: A new research hotspot and associated challenges. Neural Regen. Res. 2024, 19, 663. [Google Scholar] [CrossRef] [PubMed]
- Tao, G.; Yang, S.; Xu, J.; Wang, L.; Yang, B. Global research trends and hotspots of artificial intelligence research in spinal cord neural injury and restoration—A bibliometrics and visualization analysis. Front. Neurol. 2024, 15, 1361235. [Google Scholar] [CrossRef] [PubMed]
- Javdani-Mallak, A.; Salahshoori, I. Environmental pollutants and exosomes: A new paradigm in environmental health and disease. Sci. Total Environ. 2024, 925, 171774. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Song, Y.; He, T.; Wen, R.; Li, Y.; Chen, T.; Huang, S.; Wang, Y.; Tang, Y.; Shen, F.; et al. M2 microglial small extracellular vesicles reduce glial scar formation via the miR-124/STAT3 pathway after ischemic stroke in mice. Theranostics 2021, 11, 1232–1248. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Nash, M.S.; Farraj, A.K. Metabolomic profiling reveals systemic metabolic disruptions induced by combined exposure to particulate matter and ozone. Curr. Res. Toxicol. 2025, 8, 100216. [Google Scholar] [CrossRef] [PubMed]
- Toader, C.; Brehar, F.-M.; Radoi, M.P.; Covache-Busuioc, R.-A.; Glavan, L.-A.; Grama, M.; Corlatescu, A.-D.; Costin, H.P.; Bratu, B.-G.; Popa, A.A.; et al. Machine Learning-Based Prediction of Clinical Outcomes in Microsurgical Clipping Treatments of Cerebral Aneurysms. Diagnostics 2024, 14, 2156. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Waisberg, E.; Ong, J.; Paladugu, P.; Amiri, D.; Saintyl, J.; Yelamanchi, J.; Nahouraii, R.; Jagadeesan, R.; Tavakkoli, A. Artificial Intelligence-Based Methodologies for Early Diagnostic Precision and Personalized Therapeutic Strategies in Neuro-Ophthalmic and Neurodegenerative Pathologies. Brain Sci. 2024, 14, 1266. [Google Scholar] [CrossRef] [PubMed]
- Onciul, R.; Tataru, C.-I.; Dumitru, A.V.; Crivoi, C.; Serban, M.; Covache-Busuioc, R.-A.; Radoi, M.P.; Toader, C. Artificial Intelligence and Neuroscience: Transformative Synergies in Brain Research and Clinical Applications. J. Clin. Med. 2025, 14, 550. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Zhang, D.; Guo, H.; Ma, W. Beyond blood: Advancing the frontiers of liquid biopsy in oncology and personalized medicine. Cancer Sci. 2024, 115, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Lima, R.; Monteiro, A.; Salgado, A.J.; Monteiro, S.; Silva, N.A. Pathophysiology and Therapeutic Approaches for Spinal Cord Injury. Int. J. Mol. Sci. 2022, 23, 13833. [Google Scholar] [CrossRef] [PubMed]
- Guha, L.; Singh, N.; Kumar, H. Different Ways to Die: Cell Death Pathways and Their Association With Spinal Cord Injury. Neurospine 2023, 20, 430–448. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimy, N.; Gasterich, N.; Behrens, V.; Amini, J.; Fragoulis, A.; Beyer, C.; Zhao, W.; Sanadgol, N.; Zendedel, A. Neuroprotective effect of the Nrf2/ARE/miRNA145-5p signaling pathway in the early phase of spinal cord injury. Life Sci. 2022, 304, 120726. [Google Scholar] [CrossRef] [PubMed]
- Maurya, S.K.; Borgonovo, J.E.; Biswal, S.; Martínez-Cerdeño, V.; Mishra, R.; Muñoz, E.M. Editorial: Trends in neuroimmunology: Cross-talk between brain-resident and peripheral immune cells in both health and disease. Front. Immunol. 2024, 15, 1442322. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Du, M.; Yang, Y.; Lin, X.; Wang, Y.; Li, H.; Ren, J.; Xu, W.; Yan, J.; Wang, N. Sp1 Regulates the M1 Polarization of Microglia Through the HuR/NF-κB Axis after Spinal Cord Injury. Neuroscience 2024, 544, 50–63. [Google Scholar] [CrossRef] [PubMed]
- Kuboyama, T.; Kominato, S.; Nagumo, M.; Tohda, C. Recovery from spinal cord injury via M2 microglial polarization induced by Polygalae Radix. Phytomedicine 2021, 82, 153452. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, Y.; Sun, H.; Lei, D.; Liu, J.; Fei, Y.; Wang, C.; Han, C. Resveratrol ameliorates postoperative cognitive dysfunction in aged mice by regulating microglial polarization through CX3CL1/CX3CR1 signaling axis. Neurosci. Lett. 2024, 847, 138089. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Liu, Z.; Liu, Q.; Xu, Q.; Ding, C.; Chen, Z.; Li, J.; Wu, Z. Enhanced neural recovery and reduction of secondary damage in spinal cord injury through modulation of oxidative stress and neural response. Sci. Rep. 2024, 14, 19042. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Han, X.; Zhang, M.; Kou, H.; Liu, H.; Cheng, T. Melatonin exerts neuroprotective effects in mice with spinal cord injury by activating the Nrf2/Keap1 signaling pathway via the MT2 receptor. Exp. Ther. Med. 2024, 27, 37. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Tian, H.; Li, X.; Mao, L.; Zhao, X.; Lin, J.; Lin, S.; Xu, C.; Liu, Y.; Guo, Y.; et al. Zinc promotes functional recovery after spinal cord injury by activating Nrf2/HO-1 defense pathway and inhibiting inflammation of NLRP3 in nerve cells. Life Sci. 2020, 245, 117351. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Lu, L.; Yao, X.; Wu, Z.; Sun, P.; Wen, X.; Li, X.; Wang, K.; Yin, X. The NFATC2/Nrf2 cascade regulates spinal cord ischemia-reperfusion injury by controlling inflammation, apoptosis and oxidative stress. Regen. Ther. 2025, 28, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Ding, Y.; Shi, C.; Yuan, F.; Sheng, X.; Liu, Y.; Xie, Y.; Lu, H.; Duan, C.; Hu, J.; et al. Identification of Cathepsin B as a Therapeutic Target for Ferroptosis of Macrophage after Spinal Cord Injury. Aging Dis. 2024, 15, 421–443. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Chen, Z.; Wu, Y.; Shi, Q.; Yang, E.; Zhang, B.; Qian, Y.; Lian, X.; Xu, J. ADSC-Exos enhance functional recovery after spinal cord injury by inhibiting ferroptosis and promoting the survival and function of endothelial cells through the NRF2/SLC7A11/GPX4 pathway. Biomed. Pharmacother. 2024, 172, 116225. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Li, Z.; Jing, S.; Wang, B.; Ye, Z.; Xiong, W.; Liu, Y.; Liu, Y.; Xu, C.; Kumeria, T.; et al. Repair spinal cord injury with a versatile anti-oxidant and neural regenerative nanoplatform. J. Nanobiotechnol. 2024, 22, 351. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Li, Z.; Shi, L.; Jian, H.; Yang, F.; Qiu, J.; Li, C.; Xiao, P.; Ruan, W.; Li, H.; et al. Machine learning identifies key cells and therapeutic targets during ferroptosis after spinal cord injury. Neural Regen. Res. 2024, 20, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hua, R.; Zhao, C.; Xu, Z.; Liu, D.; Shen, W.; Yuan, W.; Li, Y.; Ma, J.; Wang, Z.; Feng, S. ROS-responsive nanoparticle delivery of ferroptosis inhibitor prodrug to facilitate mesenchymal stem cell-mediated spinal cord injury repair. Bioact. Mater. 2024, 38, 438–454. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Ma, S.; Li, L.; Xia, Y. Tanshinone IIA attenuates fluoride-induced spinal cord injury by inhibiting ferroptosis and inflammation. Heliyon 2024, 10, e40549. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Peng, Z.; Guo, M.; Wang, Y.; Liu, J.; Liu, Y.; Li, M.; Wei, T.; Li, P.; Zhao, Y.; et al. TET3-facilitated differentiation of human umbilical cord mesenchymal stem cells into oligodendrocyte precursor cells for spinal cord injury recovery. J. Transl. Med. 2024, 22, 1118. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Li, Y.; Wang, Z.; Chen, P.; Xie, Y.; Qu, W.; Wang, M.; Yu, Z.; Luo, X. Regulatory T cells promote functional recovery after spinal cord injury by alleviating microglia inflammation via STAT3 inhibition. CNS Neurosci. Ther. 2023, 29, 2129–2144. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Cai, Z.; Liang, J.; Miao, P.; Ruan, Y.; Li, P.; Lin, S.; Tian, H.; Yu, Q.; He, X. Heme Oxygenase-1 Overexpression Activates the IRF1/DRP1 Signaling Pathway to Promote M2-Type Polarization of Spinal Cord Microglia. Drug Dev. Res. 2024, 85, e70033. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zheng, M.; Ouyang, F.; Ye, J.; Huang, J.; Zhao, Y.; Wang, J.; Shan, F.; Li, Z.; Yu, S.; et al. Interleukin-3 Modulates Macrophage Phagocytic Activity and Promotes Spinal Cord Injury Repair. CNS Neurosci. Ther. 2024, 30, e70181. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Zhou, J.; Zhu, H.; Xu, L.; Yang, J.; Mu, X.; Fan, X. Tetramethylpyrazine inhibits ferroptosis in spinal cord injury by regulating iron metabolism through the NRF2/ARE pathway. Front. Pharmacol. 2024, 15, 1503064. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Lin, B.; Jin, W.; Tang, L.; Hu, S.; Cai, R. NRF2, a Superstar of Ferroptosis. Antioxidants 2023, 12, 1739. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Wu, X.; Zhu, Y.; Yang, S.; Lu, K.; Zhang, X.; Zhang, D.; Wang, X. Screening of orthopedic medicines identifies raloxifene hydrochloride as a novel ferroptosis inhibitor for spinal cord injury therapy. Int. Immunopharmacol. 2024, 143, 113542. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yu, S.; Jiao, B.; Zhang, C.; Zhang, K.; Liu, B.; Zhang, X. Vitamin D Attenuates Neuropathic Pain via Suppression of Mitochondria-Associated Ferroptosis by Inhibiting PKCα/NOX4 Signaling Pathway. CNS Neurosci. Ther. 2024, 30, e70067. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Kang, J.; Wang, Y.; Meng, X.; Huang, Y.; Bai, Y.; Feng, Z. Transcranial direct current stimulation promotes angiogenesis and improves neurological function via the OXA-TF-AKT/ERK signaling pathway in traumatic brain injury. Aging 2024, 16, 6566–6587. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.J.; Mun, H.; Oh, J.K.; Choi, G.-M.; Yoo, D.Y.; Hwang, I.K.; Kim, D.W.; Moon, S.M. Neuroprotective Effects of Chaperonin Containing TCP1 Subunit 2 (CCT2) on Motor Neurons Following Oxidative or Ischemic Stress. Neurochem. Res. 2024, 50, 42. [Google Scholar] [CrossRef] [PubMed]
- Walling, I.; Baumgartner, S.; Patel, M.; Crone, S.A. Electrical Stimulation of the Sciatic Nerve Restores Inspiratory Diaphragm Function in Mice after Spinal Cord Injury. Front. Neural Circuits 2024, 18, 1480291. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Srinivasan, P.R.; Tajiknia, V.; Sanchez Sevilla Uruchurtu, A.F.; Seyhan, A.A.; Carneiro, B.A.; De La Cruz, A.; Pinho-Schwermann, M.; George, A.; Zhao, S.; et al. Targeting apoptotic pathways for cancer therapy. J. Clin. Investig. 2024, 134, e179570. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, M.; Guo, K. Exploring the mechanisms and current status of acupuncture in alleviating tumor metabolism and associated diseases: Insights from the central nervous system and immune microenvironment. SLAS Technol. 2024, 29, 100208. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Guha, L.; Kumar, H. From hope to healing: Exploring the therapeutic potential of exosomes in spinal cord injury. Extracell. Vesicle 2024, 3, 100044. [Google Scholar] [CrossRef]
- Xie, A.; Zhang, X.; Ju, F.; Zhou, Y.; Wu, D.; Han, J. Sevoflurane impedes neuropathic pain by maintaining endoplasmic reticulum stress and oxidative stress homeostasis through inhibiting the activation of the PLCγ/CaMKII/IP3R signaling pathway. Aging 2024, 16, 11062–11071. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Tan, H.; Zhang, Y.; Qi, T.; Li, Y.; Li, N.; Zhou, Z.; Wang, Y.; Wang, H.; Zhang, H.; et al. Irisflorentin improves functional recovery after spinal cord injury by protecting the blood-spinal cord barrier and promoting axonal growth. Exp. Neurol. 2024, 379, 114886. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, K.; Zhang, Y.; Jiang, K.; Sun, Z. Developmental endothelial locus-1 enhances ER calmodulin expression, mitigates ER stress, suppresses inflammation, and attenuates neuronal apoptosis. J. Chin. Pharm. Sci. 2024, 33, 795. [Google Scholar] [CrossRef]
- Talabis, K. IRE1 Activation in Spinal Cord Development and Repair in the Zebrafish Model. 2024. Available online: https://mspace.lib.umanitoba.ca/items/d81ed71a-3391-4c65-85fa-b4fa2514308b (accessed on 25 December 2024).
- Kong, L.; Gao, X.; Qian, Y.; Sun, W.; You, Z.; Fan, C. Biomechanical microenvironment in peripheral nerve regeneration: From pathophysiological understanding to tissue engineering development. Theranostics 2022, 12, 4993–5014. [Google Scholar] [CrossRef] [PubMed]
- Sáez, P.; Borau, C.; Antonovaite, N.; Franze, K. Brain tissue mechanics is governed by microscale relations of the tissue constituents. Biomaterials 2023, 301, 122273. [Google Scholar] [CrossRef] [PubMed]
- Bradfield, C.; Voo, L.; Bhaduri, A.; Ramesh, K.T. Validation of a computational biomechanical mouse brain model for rotational head acceleration. Biomech. Model. Mechanobiol. 2024, 23, 1347–1367. [Google Scholar] [CrossRef] [PubMed]
- Falconieri, A.; Folino, P.; Da Palmata, L.; Raffa, V. Nano-pulling stimulates axon regeneration in dorsal root ganglia by inducing stabilization of axonal microtubules and activation of local translation. Front. Mol. Neurosci. 2024, 17, 1340958. [Google Scholar] [CrossRef] [PubMed]
- Al-Jipouri, A.; Eritja, À.; Bozic, M. Unraveling the Multifaceted Roles of Extracellular Vesicles: Insights into Biology, Pharmacology, and Pharmaceutical Applications for Drug Delivery. Int. J. Mol. Sci. 2024, 25, 485. [Google Scholar] [CrossRef] [PubMed]
- Keri, D.; Walker, M.; Singh, I.; Nishikawa, K.; Garces, F. Next generation of multispecific antibody engineering. Antib. Ther. 2024, 7, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Dull, R.O.; Hahn, R.G. The glycocalyx as a permeability barrier: Basic science and clinical evidence. Crit. Care 2022, 26, 273. [Google Scholar] [CrossRef] [PubMed]
- Kunz, S.; Durandy, M.; Seguin, L.; Feral, C.C. NANOBODY® Molecule, a Giga Medical Tool in Nanodimensions. Int. J. Mol. Sci. 2023, 24, 13229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Han, B.; Zhang, H.; Fu, R.; Lu, Y.; Zhang, G. Integrated transcriptomic and metabolomic analysis of cortical neurons reveals dysregulated lipid metabolism, enhanced glycolysis and activated HIF-1 signaling pathways in acute hypoxia. Heliyon 2023, 9, e14949. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xu, X.; Xiong, S.; Zhang, P.; Yuan, J.; Gao, X.; Guan, W.; Wang, F.; Li, X.; Leng, T.; et al. Calcium-chelated nanosystem reversing cancer chemoresistance via replenishing intracellular calcium ions. Chem. Eng. J. 2022, 448, 137500. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, X.; Shi, L.; Cai, Y.; Hu, B. Wolfram syndrome 1b mutation suppresses Mauthner-cell axon regeneration via ER stress signal pathway. Acta Neuropathol. Commun. 2022, 10, 184. [Google Scholar] [CrossRef] [PubMed]
- Gulen, M.F.; Samson, N.; Keller, A.; Schwabenland, M.; Liu, C.; Glück, S.; Thacker, V.V.; Favre, L.; Mangeat, B.; Kroese, L.J.; et al. cGAS–STING drives ageing-related inflammation and neurodegeneration. Nature 2023, 620, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, Y.; Liu, Y.; Wang, X.; Yang, Y.; Shi, L. The cGAS/STING signaling pathway is involved in sevoflurane induced neuronal necroptosis via regulating microglia M1 polarization. Cell. Signal. 2024, 119, 111195. [Google Scholar] [CrossRef] [PubMed]
- Bastioli, G.; Piccirillo, S.; Graciotti, L.; Carone, M.; Sprega, G.; Taoussi, O.; Preziuso, A.; Castaldo, P. Calcium Deregulation in Neurodegeneration and Neuroinflammation in Parkinson’s Disease: Role of Calcium-Storing Organelles and Sodium–Calcium Exchanger. Cells 2024, 13, 1301. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Chen, A.; Chen, W.; Cheng, S.; Lin, S.; Mei, R.; Mei, X. Knockout of TNF-α in microglia decreases ferroptosis and convert microglia phenotype after spinal cord injury. Heliyon 2024, 10, e36488. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Luo, Y.; Liu, Z.; Chen, Y.; Wei, L.; Luo, X.; Zhou, G.; Lai, J.; Ji, J.; Lin, Y.; et al. Ferrostatin-1 ameliorates Bupivacaine-Induced spinal neurotoxicity in rats by inhibiting ferroptosis. Neurosci. Lett. 2023, 809, 137308. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Deng, B.; Sun, C.; McComb, D.W.; Gu, C. The Mechanical Microenvironment Regulates Axon Diameters Visualized by Cryo-Electron Tomography. Cells 2022, 11, 2533. [Google Scholar] [CrossRef] [PubMed]
- Syková, E.; Voříšek, I.; Starčuk, Z.; Kratochvíla, J.; Pavlova, I.; Ichikawa, Y.; Kwok, J.C.F.; Kmoníčková, E.; Myronchenko, S.; Hromádka, T.; et al. Disruption of Extracellular Matrix and Perineuronal Nets Modulates Extracellular Space Volume and Geometry. J. Neurosci. Off. J. Soc. Neurosci. 2025, 45, e0517242024. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xing, F.; Yu, P.; Lu, R.; Ma, S.; Shakya, S.; Zhou, X.; Peng, K.; Zhang, D.; Liu, M. Biomimetic fabrication bioprinting strategies based on decellularized extracellular matrix for musculoskeletal tissue regeneration: Current status and future perspectives. Mater. Des. 2024, 243, 113072. [Google Scholar] [CrossRef]
- Alves, N.G.; Breslin, J.W. Microvascular Endothelial Glycocalyx Surface Layer Visualization and Quantification. In Vascular Hyperpermeability: Methods and Protocols; Methods in Molecular Biology; Humana: New York, NY, USA, 2024; Volume 2711, pp. 163–175. [Google Scholar] [CrossRef]
- Chehelgerdi, M.; Chehelgerdi, M.; Allela, O.Q.B.; Pecho, R.D.C.; Jayasankar, N.; Rao, D.P.; Thamaraikani, T.; Vasanthan, M.; Viktor, P.; Lakshmaiya, N.; et al. Progressing nanotechnology to improve targeted cancer treatment: Overcoming hurdles in its clinical implementation. Mol. Cancer 2023, 22, 169. [Google Scholar] [CrossRef] [PubMed]
- Steinecker-Frohnwieser, B.; Lohberger, B.; Toegel, S.; Windhager, R.; Glanz, V.; Kratschmann, C.; Leithner, A.; Weigl, L. Activation of the Mechanosensitive Ion Channels Piezo1 and TRPV4 in Primary Human Healthy and Osteoarthritic Chondrocytes Exhibits Ion Channel Crosstalk and Modulates Gene Expression. Int. J. Mol. Sci. 2023, 24, 7868. [Google Scholar] [CrossRef] [PubMed]
- Guerini, D. STING Agonists/Antagonists: Their Potential as Therapeutics and Future Developments. Cells 2022, 11, 1159. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.-J.; Park, W.-T.; Lee, G.W. Extracellular vesicles for neural regeneration after spinal cord injury. Neural Regen. Res. 2023, 19, 491–492. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Tong, K.; Li, S.; Huang, Z.; Liu, S.; Zhu, H.; Zhong, Y.; Zhou, Z.; Jiao, G.; Wei, F.; et al. Extracellular vesicles released by transforming growth factor-beta 1-preconditional mesenchymal stem cells promote recovery in mice with spinal cord injury. Bioact. Mater. 2024, 35, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Wu, J.; Wang, C.; Xu, Z.; Jin, Z.; Yan, D.; Chen, S. BMSCs-derived exosomes inhibit macrophage/microglia pyroptosis by increasing autophagy through the miR-21a-5p/PELI1 axis in spinal cord injury. Aging 2024, 16, 5184–5206. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Song, P.; Wu, Z.; Liu, Y.; Ying, W.; Shen, C. Inflammation Modifies miR-21 Expression Within Neuronal Extracellular Vesicles to Regulate Remyelination Following Spinal Cord Injury. Stem Cell Rev. Rep. 2023, 19, 2024–2037. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Sun, R.; Sun, K.; Yan, C.; Jiang, J.; Kong, F.; Shi, J. The deubiquitinase USP11 ameliorates intervertebral disc degeneration by regulating oxidative stress-induced ferroptosis via deubiquitinating and stabilizing Sirt3. Redox Biol. 2023, 62, 102707. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Fan, Y.; Feng, J.; Zhu, Z.; Luo, Z.; Hu, H.; Li, W.; Yang, H.; Ding, G. ALCAT1-mediated abnormal cardiolipin remodelling promotes mitochondrial injury in podocytes in diabetic kidney disease. Cell Commun. Signal. 2024, 22, 26. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ren, J.; Li, Y.; Wu, Q.; Wei, J. Oxidative stress: The nexus of obesity and cognitive dysfunction in diabetes. Front. Endocrinol. 2023, 14, 1134025. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Sun, T. Impact of TRPV1 on Pathogenesis and Therapy of Neurodegenerative Diseases. Molecules 2024, 29, 181. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Liu, S.; Li, S.; Chen, Y.; Xie, B.; Zhou, J. Induction Mechanism of Ferroptosis, Necroptosis, and Pyroptosis: A Novel Therapeutic Target in Nervous System Diseases. Int. J. Mol. Sci. 2023, 24, 10127. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Liu, W.-C.; Chen, X.-Y.; Wang, X.; Li, J.-L.; Zhang, X. Gasdermin D-mediated pyroptosis: Mechanisms, diseases, and inhibitors. Front. Immunol. 2023, 14, 1178662. [Google Scholar] [CrossRef] [PubMed]
- Kraus, C.; Sontheimer, E.J. Applications of Anti-CRISPR Proteins in Genome Editing and Biotechnology. J. Mol. Biol. 2023, 435, 168120. [Google Scholar] [CrossRef] [PubMed]
- Betz, U.A.K.; Arora, L.; Assal, R.A.; Azevedo, H.; Baldwin, J.; Becker, M.S.; Bostock, S.; Cheng, V.; Egle, T.; Ferrari, N.; et al. Game changers in science and technology—Now and beyond. Technol. Forecast. Soc. Change 2023, 193, 122588. [Google Scholar] [CrossRef]
- Jia, Z.; Li, W. Nanosystems-enabled regenerative strategies for spinal cord Injury: Recent advances and future prospects. Mater. Des. 2024, 237, 112617. [Google Scholar] [CrossRef]
- Babu, M.; Snyder, M. Multi-Omics Profiling for Health. Mol. Cell. Proteomics MCP 2023, 22, 100561. [Google Scholar] [CrossRef] [PubMed]
- Alhazmi, A.M.; Hakami, M.A.; Aldosari, O.M.O.; Al-Kharif, S.A.; Alduhaish, A.A.; Alrshood, A.S.S.; Bader, K.A.; Alshamrani, K.A.A.; Asiri, S.A.S. Rehabilitation Programs for Patients with Spinal Cord Injuries-An Updated Review. Egypt. J. Chem. 2024, 67, 1603–1611. [Google Scholar] [CrossRef]
- Li, J.; Peng, C.; Huang, C.; Wan, L.; Wang, K.; Wu, P.; Chen, T.; Sun, G.; Guo, R.; Lin, H.; et al. Metal Ruthenium Complexes Treat Spinal Cord Injury By Alleviating Oxidative Stress Through Interaction With Antioxidant 1 Copper Chaperone Protein. Adv. Sci. 2024, 11, 2407225. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Tu, W.-J. Editorial: Brain-computer interfaces in neurological disorders: Expanding horizons for diagnosis, treatment, and rehabilitation. Front. Neurosci. 2024, 18, 1526723. [Google Scholar] [CrossRef] [PubMed]
- Brommeland, T.; Strøm, M.; Mirzamohammadi, J.; Glott, T.; Linnerud, H.; Rønning, P.A.; Rizvi, S.A.M.; Holla, T.M.; Høydal, B.J.; Biernat, D.; et al. Traumatic cervical spinal cord injury in southeastern Norway: Acute treatment, specialized rehabilitation referral and mortality. Front. Neurol. 2024, 15, 1452194. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, A.; Zandieh, A.; Behroozi, Z.; Hamblin, M.R.; Mayahi, S.; Yousefifard, M.; Ramezani, F. Sustained delivery of chABC improves functional recovery after a spine injury. BMC Neurosci. 2022, 23, 60. [Google Scholar] [CrossRef] [PubMed]
- Rau, C.-S.; Wu, S.-C.; Kuo, P.-J.; Lin, C.-W.; Lu, T.-H.; Wu, Y.-C.; Tsai, C.-W.; Hsieh, C.-H. Tracking adipose-derived stem cell exosomes applied in a mouse crush injury model: Insights from fluorescent labeling and spatial transcriptomics—an experimental study. Int. J. Surg. 2025, 111, 1860–1873. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.M.; Borys, B.; Karimi-Abdolrezaee, S. Neural stem cell therapies for spinal cord injury repair: An update on recent preclinical and clinical advances. Brain 2024, 147, 766–793. [Google Scholar] [CrossRef] [PubMed]
- Menta, A.K.; Fuleihan, A.A.; Li, M.; Azad, T.D.; Witham, T.F. Enabling Technologies in the Management of Cervical Spine Trauma. Clin. Spine Surg. 2024, 37, 459. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, W.; Yang, H.; Wang, Y.; Shi, Y.; Chen, Y.; Luo, D.; Guo, D.; Lin, D.; Yue, K.; et al. Microenvironment-Responsive Injectable Conductive Hydrogel for Spinal Cord Injury Repair. Adv. Funct. Mater. 2024, 34, 2406376. [Google Scholar] [CrossRef]
- Xu, J.; Shi, C.; Yuan, F.; Ding, Y.; Xie, Y.; Liu, Y.; Zhu, F.; Lu, H.; Duan, C.; Hu, J.; et al. Targeted transplantation of engineered mitochondrial compound promotes functional recovery after spinal cord injury by enhancing macrophage phagocytosis. Bioact. Mater. 2024, 32, 427–444. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, H.; Li, C.; Guan, Y.; Xiong, X.; He, R.; Jia, Z.; Liang, L.; Zhao, J.; Miao, X.; et al. Exogenous Mitochondrial Transplantation Facilitates the Recovery of Autologous Nerve Grafting in Repairing Nerve Defects. Cell Transplant. 2024, 33, 09636897241291278. [Google Scholar] [CrossRef] [PubMed]
- Riou, A.; Broeglin, A.; Grimm, A. Mitochondrial transplantation in brain disorders: Achievements, methods, and challenges. Neurosci. Biobehav. Rev. 2025, 169, 105971. [Google Scholar] [CrossRef] [PubMed]
- Cavanagh, R.J.; Monteiro, P.F.; Moloney, C.; Travanut, A.; Mehradnia, F.; Taresco, V.; Rahman, R.; Martin, S.G.; Grabowska, A.M.; Ashford, M.B.; et al. Free drug and ROS-responsive nanoparticle delivery of synergistic doxorubicin and olaparib combinations to triple negative breast cancer models. Biomater. Sci. 2024, 12, 1822–1840. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Shen, H.; Chen, F.; Liu, W.; Zhao, Y.; Xiao, Z.; Wu, X.; Chen, B.; Lu, J.; Shao, D.; et al. Inflammatory Microenvironment-Responsive Nanomaterials Promote Spinal Cord Injury Repair by Targeting IRF5. Adv. Healthc. Mater. 2022, 11, 2201319. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wan, L.; Xiao, Y.; Wang, K.; Wu, P.; Li, C.; Huang, C.; Liu, X.; Xue, W.; Sun, G.; et al. Curcumin/pEGCG-encapsulated nanoparticles enhance spinal cord injury recovery by regulating CD74 to alleviate oxidative stress and inflammation. J. Nanobiotechnol. 2024, 22, 653. [Google Scholar] [CrossRef] [PubMed]
- Quel de Oliveira, C.; Bundy, A.; Middleton, J.W.; Refshauge, K.; Rogers, K.; Davis, G.M. Activity-Based Therapy for Mobility, Function and Quality of Life after Spinal Cord Injuries—A Mixed-Methods Case Series. J. Clin. Med. 2023, 12, 7588. [Google Scholar] [CrossRef] [PubMed]
- Mu, Z.; Qin, J.; Zhou, X.; Wang, K. Synergistic effects of human umbilical cord mesenchymal stem cells/neural stem cells and epidural electrical stimulation on spinal cord injury rehabilitation. Sci. Rep. 2024, 14, 26090. [Google Scholar] [CrossRef] [PubMed]
- Scheffler, M.S.; Martin, C.A.; Dietz, V.; Faraji, A.H.; Sayenko, D.G. Synergistic implications of combinatorial rehabilitation approaches using spinal stimulation on therapeutic outcomes in spinal cord injury. Clin. Neurophysiol. 2024, 165, 166–179. [Google Scholar] [CrossRef] [PubMed]
- Novakovic, M.M.; Korshunov, K.S.; Grant, R.A.; Martin, M.E.; Valencia, H.A.; Budinger, G.R.S.; Radulovic, J.; Prakriya, M. Astrocyte reactivity and inflammation-induced depression-like behaviors are regulated by Orai1 calcium channels. Nat. Commun. 2023, 14, 5500. [Google Scholar] [CrossRef] [PubMed]
- Schiera, G.; Di Liegro, C.M.; Schirò, G.; Sorbello, G.; Di Liegro, I. Involvement of Astrocytes in the Formation, Maintenance, and Function of the Blood–Brain Barrier. Cells 2024, 13, 150. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, Y.; Guo, Y.; Wang, W. Bioinformatics analysis reveals multiple functional changes in astrocytes in temporal lobe epilepsy. Brain Res. 2024, 1831, 148820. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Wu, H.; Wang, X.; Zhang, G.; Lu, J.; Xu, W.; Xu, S.; Fang, Y.; Zhang, A.; Shao, A.; et al. Temporal dynamics of microglia-astrocyte interaction in neuroprotective glial scar formation after intracerebral hemorrhage. J. Pharm. Anal. 2023, 13, 862–879. [Google Scholar] [CrossRef] [PubMed]
- Qian, D.; Dong, Y.; Liu, X.; Yu, H.; Song, Z.; Jia, C.; Zhang, Z.; Cao, S.; Hu, F.; Zhang, X. Salidroside promotes the repair of spinal cord injury by inhibiting astrocyte polarization, promoting neural stem cell proliferation and neuronal differentiation. Cell Death Discov. 2024, 10, 224. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.M.; Nemati, S.; Karimi-Abdolrezaee, S. Astrocytes originated from neural stem cells drive the regenerative remodeling of pathologic CSPGs in spinal cord injury. Stem Cell Rep. 2024, 19, 1451–1473. [Google Scholar] [CrossRef] [PubMed]
- Quan, L.; Uyeda, A.; Manabe, I.; Muramatsu, R. Astrocytic heterogeneous nuclear ribonucleoprotein U is involved in scar formation after spinal cord injury. J. Neuroinflamm. 2025, 22, 28. [Google Scholar] [CrossRef] [PubMed]
- Koltai, T.; Fliegel, L. Exploring monocarboxylate transporter inhibition for cancer treatment. Explor. Target. Anti-Tumor Ther. 2024, 5, 135–169. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhou, R.; Chen, Z.; Gao, S.; Zhou, F. The Glial Cells Respond to Spinal Cord Injury. Front. Neurol. 2022, 13, 844497. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, A.R.; Barbosa, D.J.; Remião, F.; Silva, R. Co-Culture Models: Key Players in In Vitro Neurotoxicity, Neurodegeneration and BBB Modeling Studies. Biomedicines 2024, 12, 626. [Google Scholar] [CrossRef] [PubMed]
- Gomes, C.; Huang, K.-C.; Harkin, J.; Baker, A.; Hughes, J.M.; Pan, Y.; Tutrow, K.; VanderWall, K.B.; Lavekar, S.S.; Hernandez, M.; et al. Induction of astrocyte reactivity promotes neurodegeneration in human pluripotent stem cell models. Stem Cell Rep. 2024, 19, 1122–1136. [Google Scholar] [CrossRef] [PubMed]
- Lilienberg, J.; Hegyi, Z.; Szabó, E.; Hathy, E.; Málnási-Csizmadia, A.; Réthelyi, J.M.; Apáti, Á.; Homolya, L. Pharmacological Modulation of Neurite Outgrowth in Human Neural Progenitor Cells by Inhibiting Non-muscle Myosin II. Front. Cell Dev. Biol. 2021, 9, 719636. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yang, L.; Wu, Z.; Li, C.; Wang, S.; Xiao, Z.; Ma, B.; Zhu, R.; Cheng, L. Ferroptosis-related genes participate in the microglia-induced neuroinflammation of spinal cord injury via NF-κB signaling: Evidence from integrated single-cell and spatial transcriptomic analysis. J. Transl. Med. 2025, 23, 43. [Google Scholar] [CrossRef] [PubMed]
- Mirarchi, A.; Albi, E.; Arcuri, C. Microglia Signatures: A Cause or Consequence of Microglia-Related Brain Disorders? Int. J. Mol. Sci. 2024, 25, 10951. [Google Scholar] [CrossRef] [PubMed]
- Adamu, A.; Li, S.; Gao, F.; Xue, G. The role of neuroinflammation in neurodegenerative diseases: Current understanding and future therapeutic targets. Front. Aging Neurosci. 2024, 16, 1347987. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, L.; Lin, W.; Yao, Y.; Li, H.; Shen, G.; Cao, X.; He, N.; Chen, J.; Hu, J.; et al. Single-cell transcriptome analysis reveals the immune heterogeneity and the repopulation of microglia by Hif1α in mice after spinal cord injury. Cell Death Dis. 2022, 13, 432. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Hu, X.; Chen, Z.; Ouyang, F.; Li, J.; Hu, Y.; Zhao, Y.; Wang, J.; Yao, F.; Jing, J.; et al. Fascin-1 limits myosin activity in microglia to control mechanical characterization of the injured spinal cord. J. Neuroinflamm. 2024, 21, 88. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Yang, L.; Chen, J.; Zhou, C.; Zong, N.; Geng, Y.; Xia, S.; Yang, H.; Bao, X.; Chen, Y.; et al. SRGN amplifies microglia-mediated neuroinflammation and exacerbates ischemic brain injury. J. Neuroinflamm. 2024, 21, 35. [Google Scholar] [CrossRef] [PubMed]
- Woods, I.; O’Connor, C.; Frugoli, L.; Kerr, S.; Gutierrez Gonzalez, J.; Stasiewicz, M.; McGuire, T.; Cavanagh, B.; Hibbitts, A.; Dervan, A.; et al. Biomimetic Scaffolds for Spinal Cord Applications Exhibit Stiffness-Dependent Immunomodulatory and Neurotrophic Characteristics. Adv. Healthc. Mater. 2022, 11, e2101663. [Google Scholar] [CrossRef] [PubMed]
- Valiukas, Z.; Tangalakis, K.; Apostolopoulos, V.; Feehan, J. Microglial activation states and their implications for Alzheimer’s Disease. J. Prev. Alzheimers Dis. 2025, 12, 100013. [Google Scholar] [CrossRef] [PubMed]
- Martens, N.; Zhan, N.; Voortman, G.; Leijten, F.P.J.; van Rheenen, C.; van Leerdam, S.; Geng, X.; Huybrechts, M.; Liu, H.; Jonker, J.W.; et al. Activation of Liver X Receptors and Peroxisome Proliferator-Activated Receptors by Lipid Extracts of Brown Seaweeds: A Potential Application in Alzheimer’s Disease? Nutrients 2023, 15, 3004. [Google Scholar] [CrossRef] [PubMed]
- Boccazzi, M.; Macchiarulo, G.; Lebon, S.; Janowska, J.; Le Charpentier, T.; Faivre, V.; Hua, J.; Marangon, D.; Lecca, D.; Fumagalli, M.; et al. G protein-coupled receptor 17 is regulated by WNT pathway during oligodendrocyte precursor cell differentiation. Neurobiol. Dis. 2023, 187, 106315. [Google Scholar] [CrossRef] [PubMed]
- Wei, C. The role of glutathione peroxidase 4 in neuronal ferroptosis and its therapeutic potential in ischemic and hemorrhagic stroke. Brain Res. Bull. 2024, 217, 111065. [Google Scholar] [CrossRef] [PubMed]
- Del Giovane, A.; Russo, M.; Tirou, L.; Faure, H.; Ruat, M.; Balestri, S.; Sposato, C.; Basoli, F.; Rainer, A.; Kassoussi, A.; et al. Smoothened/AMP-Activated Protein Kinase Signaling in Oligodendroglial Cell Maturation. Front. Cell. Neurosci. 2022, 15, 801704. [Google Scholar] [CrossRef] [PubMed]
- Pukos, N.; Marion, C.M.; Arnold, W.D.; Noble, B.T.; Popovich, P.G.; McTigue, D.M. Chronic demyelination and myelin repair after spinal cord injury in mice: A potential link for glutamatergic axon activity. Glia 2023, 71, 2096–2116. [Google Scholar] [CrossRef] [PubMed]
- Al Mamun, A.; Quan, Z.; Geng, P.; Wang, S.; Shao, C.; Xiao, J. Targeting Remyelination in Spinal Cord Injury: Insights and Emerging Therapeutic Strategies. CNS Neurosci. Ther. 2024, 30, 1–15. [Google Scholar] [CrossRef] [PubMed]
- López-Muguruza, E.; Matute, C. Alterations of Oligodendrocyte and Myelin Energy Metabolism in Multiple Sclerosis. Int. J. Mol. Sci. 2023, 24, 12912. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Leung, G.K.K. Oligodendrocyte Precursor Cells in Spinal Cord Injury: A Review and Update. BioMed Res. Int. 2015, 2015, 235195. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, Y.; Meng, H.; Meng, F. SREBPs as the potential target for solving the polypharmacy dilemma. Front. Physiol. 2024, 14, 1272540. [Google Scholar] [CrossRef] [PubMed]
- Cherchi, F.; Bulli, I.; Venturini, M.; Pugliese, A.M.; Coppi, E. Ion Channels as New Attractive Targets to Improve Re-Myelination Processes in the Brain. Int. J. Mol. Sci. 2021, 22, 7277. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Shen, Q.; Liu, Y.; Zhang, Y.; Sun, L.; Ma, X.; Song, N.; Xie, J. Homeostasis and metabolism of iron and other metal ions in neurodegenerative diseases. Signal Transduct. Target. Ther. 2025, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Sandoz, G.; Levitz, J. Optogenetic techniques for the study of native potassium channels. Front. Mol. Neurosci. 2013, 6, 6. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tian, T.; Zhang, S.; Yang, M. Recent progress and challenges in the treatment of spinal cord injury. Protein Cell 2023, 14, 635–652. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, Y.; Zhuo, Y. Synaptic or Non-synaptic? Different Intercellular Interactions with Retinal Ganglion Cells in Optic Nerve Regeneration. Mol. Neurobiol. 2022, 59, 3052–3072. [Google Scholar] [CrossRef] [PubMed]
- Hirt, J.; Khanteymoori, A.; Hohenhaus, M.; Kopp, M.A.; Howells, D.W.; Schwab, J.M.; Watzlawick, R. Inhibition of the Nogo-pathway in experimental spinal cord injury: A meta-analysis of 76 experimental treatments. Sci. Rep. 2023, 13, 22898. [Google Scholar] [CrossRef] [PubMed]
- Maynard, G.; Kannan, R.; Liu, J.; Wang, W.; Lam, T.K.T.; Wang, X.; Adamson, C.; Hackett, C.; Schwab, J.M.; Liu, C.; et al. Soluble Nogo-Receptor-Fc decoy (AXER-204) in patients with chronic cervical spinal cord injury in the USA: A first-in-human and randomised clinical trial. Lancet Neurol. 2023, 22, 672–684. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Luo, W.; Xiao, C.; Zhao, J.; Xiang, C.; Liu, W.; Gu, R. Recent advances in endogenous neural stem/progenitor cell manipulation for spinal cord injury repair. Theranostics 2023, 13, 3966–3987. [Google Scholar] [CrossRef] [PubMed]
- Punjani, N.; Deska-Gauthier, D.; Hachem, L.D.; Abramian, M.; Fehlings, M.G. Neuroplasticity and regeneration after spinal cord injury. N. Am. Spine Soc. J. (NASSJ) 2023, 15, 100235. [Google Scholar] [CrossRef] [PubMed]
- Blake, M.R.; Parrish, D.C.; Staffenson, M.A.; Sueda, S.; Woodward, W.R.; Habecker, B.A. Chondroitin sulfate proteoglycan 4,6 sulfation regulates sympathetic nerve regeneration after myocardial infarction. eLife 2022, 11, e78387. [Google Scholar] [CrossRef] [PubMed]
- Göritzer, K.; Grandits, M.; Grünwald-Gruber, C.; Figl, R.; Mercx, S.; Navarre, C.; Ma, J.K.-C.; Teh, A.Y.-H. Engineering the N-glycosylation pathway of Nicotiana tabacum for molecular pharming using CRISPR/Cas9. Front. Plant Sci. 2022, 13, 1003065. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, S.M.; He, Y. Exploring Extracellular Matrix Crosslinking as a Therapeutic Approach to Fibrosis. Cells 2024, 13, 438. [Google Scholar] [CrossRef] [PubMed]
- Ghuman, H.; Mauney, C.; Donnelly, J.; Massensini, A.R.; Badylak, S.F.; Modo, M. Biodegradation of ECM hydrogel promotes endogenous brain tissue restoration in a rat model of stroke. Acta Biomater. 2018, 80, 66–84. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.; Zheng, M.; Zhang, Y.; Jiao, Y.; Wang, J.; Zhang, S. RhoA-ROCK2 signaling possesses complex pathophysiological functions in cancer progression and shows promising therapeutic potential. Cancer Cell Int. 2024, 24, 339. [Google Scholar] [CrossRef] [PubMed]
- Slater, P.G.; Domínguez-Romero, M.E.; Villarreal, M.; Eisner, V.; Larraín, J. Mitochondrial function in spinal cord injury and regeneration. Cell. Mol. Life Sci. (CMLS) 2022, 79, 239. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zhang, J.; Zhang, X.; Luo, W.; Liu, L.; Zhu, Y.; Liu, Q.; Zhang, X. Emerging role and function of Hippo-YAP/TAZ signaling pathway in musculoskeletal disorders. Stem Cell Res. Ther. 2024, 15, 386. [Google Scholar] [CrossRef] [PubMed]
- Pankratova, M.D.; Riabinin, A.A.; Butova, E.A.; Selivanovskiy, A.V.; Morgun, E.I.; Ulianov, S.V.; Vorotelyak, E.A.; Kalabusheva, E.P. YAP/TAZ Signalling Controls Epidermal Keratinocyte Fate. Int. J. Mol. Sci. 2024, 25, 12903. [Google Scholar] [CrossRef] [PubMed]
- Grass, T.; Dokuzluoglu, Z.; Rodríguez-Muela, N. Neuromuscular Organoids to Study Spinal Cord Development and Disease. In Methods in Molecular Biology; Springer: New York, NY, USA, 2024; pp. 1–23. [Google Scholar] [CrossRef]
- Yuan, T.-Y.; Zhang, J.; Yu, T.; Wu, J.-P.; Liu, Q.-Y. 3D Bioprinting for Spinal Cord Injury Repair. Front. Bioeng. Biotechnol. 2022, 10, 847344. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yuan, Y.; Liu, J.; Li, C.; Jiang, X. The role of mitochondria in aging, cell death, and tumor immunity. Front. Immunol. 2024, 15, 1520072. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Zhang, S.J.; Meng, H.F.; Xu, H.Q.; Guo, Z.X.; Yan, J.F.; Gao, J.L.; Niu, L.N.; Wang, S.L.; Jiao, K. DPSCs regulate epithelial-T cell interactions in oral submucous fibrosis. Stem Cell Res. Ther. 2024, 15, 113. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Peng, H.; Zhao, Y.; Yu, Y.; Yang, J.; Liu, C.; Ren, S.; Miao, L. Remodeling periodontal osteoimmune microenvironment through MAPK/NFκB phosphorylation pathway of macrophage via intelligent ROS scavenging. Hum. Cell 2023, 36, 1991–2005. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.-P.; Qin, Z.-H.; Zhang, Y.; Ning, B. Implications of microglial heterogeneity in spinal cord injury progression and therapy. Exp. Neurol. 2023, 359, 114239. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, Y.; Huang, R.; Chen, Z.; Wang, X.; Chen, F.; Huang, Y. Interleukin-10 gene intervention ameliorates liver fibrosis by enhancing the immune function of natural killer cells in liver tissue. Int. Immunopharmacol. 2024, 127, 111341. [Google Scholar] [CrossRef] [PubMed]
- Toader, C.; Tataru, C.P.; Munteanu, O.; Covache-Busuioc, R.-A.; Serban, M.; Ciurea, A.V.; Enyedi, M. Revolutionizing Neuroimmunology: Unraveling Immune Dynamics and Therapeutic Innovations in CNS Disorders. Int. J. Mol. Sci. 2024, 25, 13614. [Google Scholar] [CrossRef] [PubMed]
- Rezzani, R.; Favero, G.; Cominelli, G.; Pinto, D.; Rinaldi, F. Skin Aging and the Upcoming Role of Ferroptosis in Geroscience. Int. J. Mol. Sci. 2024, 25, 8238. [Google Scholar] [CrossRef] [PubMed]
- Kvistad, C.E.; Kråkenes, T.; Gavasso, S.; Bø, L. Neural regeneration in the human central nervous system—From understanding the underlying mechanisms to developing treatments. Where do we stand today? Front. Neurol. 2024, 15, 1398089. [Google Scholar] [CrossRef] [PubMed]
- Yari, D.; Saberi, A.; Salmasi, Z.; Ghoreishi, S.A.; Etemad, L.; Movaffagh, J.; Ganjeifar, B. Recent Advances in the Treatment of Spinal Cord Injury. Arch. Bone Jt. Surg. 2024, 12, 380–399. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.D.; Pournoori, N.; Saksala, E.; Oommen, O.P. Glycosaminoglycans’ for brain health: Harnessing glycosaminoglycan based biomaterials for treating central nervous system diseases and in-vitro modeling. Biomaterials 2024, 309, 122629. [Google Scholar] [CrossRef] [PubMed]
- Ikiz, E.D.; Hascup, E.R.; Bae, C.; Hascup, K.N. Microglial Piezo1 mechanosensitive channel as a therapeutic target in Alzheimer’s disease. Front. Cell. Neurosci. 2024, 18, 1423410. [Google Scholar] [CrossRef] [PubMed]
- Zong, B.; Yu, F.; Zhang, X.; Pang, Y.; Zhao, W.; Sun, P.; Li, L. Mechanosensitive Piezo1 channel in physiology and pathophysiology of the central nervous system. Ageing Res. Rev. 2023, 90, 102026. [Google Scholar] [CrossRef] [PubMed]
- Ning, S.; Chen, Y.; Shao, J.; Zhu, H.; Zhang, Z.; Miao, J. The effects of acteoside on locomotor recovery after spinal cord injury—The role of autophagy and apoptosis signaling pathway. Biomed. Pharmacother. 2024, 175, 116607. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Shen, J.; Bao, B.; Hu, W.; Sun, Y.; Zhu, T.; Lin, J.; Gao, T.; Li, X.; Zheng, X. Mitochondrial-targeting antioxidant MitoQ modulates angiogenesis and promotes functional recovery after spinal cord injury. Brain Res. 2022, 1786, 147902. [Google Scholar] [CrossRef] [PubMed]
- Márquez, B.T.; Leung, T.C.S.; Hui, J.; Charron, F.; McKinney, R.A.; Watt, A.J. A mitochondrial-targeted antioxidant (MitoQ) improves motor coordination and reduces Purkinje cell death in a mouse model of ARSACS. Neurobiol. Dis. 2023, 183, 106157. [Google Scholar] [CrossRef] [PubMed]
- Lizama, B.N.; Kahle, J.; Catalano, S.M.; Caggiano, A.O.; Grundman, M.; Hamby, M.E. Sigma-2 Receptors—From Basic Biology to Therapeutic Target: A Focus on Age-Related Degenerative Diseases. Int. J. Mol. Sci. 2023, 24, 6251. [Google Scholar] [CrossRef] [PubMed]
- Mohammad; Khan, U.A.; Warsi, M.H.; Alkreathy, H.M.; Karim, S.; Jain, G.K.; Ali, A. Intranasal cerium oxide nanoparticles improves locomotor activity and reduces oxidative stress and neuroinflammation in haloperidol-induced parkinsonism in rats. Front. Pharmacol. 2023, 14, 1188470. [Google Scholar] [CrossRef]
- Toader, C.; Dumitru, A.V.; Eva, L.; Serban, M.; Covache-Busuioc, R.-A.; Ciurea, A.V. Nanoparticle Strategies for Treating CNS Disorders: A Comprehensive Review of Drug Delivery and Theranostic Applications. Int. J. Mol. Sci. 2024, 25, 13302. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Y.; Ji, M.; Wu, C.; Zhang, Y.; Ji, S. Targeting ferroptosis in neuroimmune and neurodegenerative disorders for the development of novel therapeutics. Biomed. Pharmacother. 2024, 176, 116777. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.; Xue, X.; Xian, J.; Yuan, L.; Wang, L.; Zou, Y.; Zhong, J.; Jiang, Z.; Shi, J.; Chen, T.; et al. Ferrostatin-1 Alleviates White Matter Injury Via Decreasing Ferroptosis Following Spinal Cord Injury. Mol. Neurobiol. 2022, 59, 161–176. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Yu, W.; Sun, M.; Zhou, D.; Sun, J.; Jiang, T.; Zhang, W.; Wang, M. Research trends and hotspots of ferroptosis in neurodegenerative diseases from 2013 to 2023: A bibliometrics study. Heliyon 2024, 10, e29418. [Google Scholar] [CrossRef] [PubMed]
- Miquel-Rio, L.; Sarriés-Serrano, U.; Sancho-Alonso, M.; Florensa-Zanuy, E.; Paz, V.; Ruiz-Bronchal, E.; Manashirov, S.; Campa, L.; Pilar-Cuéllar, F.; Bortolozzi, A. ER stress in mouse serotonin neurons triggers a depressive phenotype alleviated by ketamine targeting eIF2α signaling. iScience 2024, 27, 109787. [Google Scholar] [CrossRef] [PubMed]
- Nazerian, Y.; Nazerian, A.; Mohamadi-Jahani, F.; Sodeifi, P.; Jafarian, M.; Javadi, S.A.H. Hydrogel-encapsulated extracellular vesicles for the regeneration of spinal cord injury. Front. Neurosci. 2023, 17, 1309172. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhao, T.; Ding, J.; Gu, H.; Wang, Q.; Wang, Y.; Zhang, D.; Gao, C. A reactive oxygen species-responsive hydrogel encapsulated with bone marrow derived stem cells promotes repair and regeneration of spinal cord injury. Bioact. Mater. 2023, 19, 550–568. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, B.; Mutlu, E.C.; Ahmed, Z.; Ben-Nissan, B.; Stamboulis, A. Applications of Stem Cell-Derived Extracellular Vesicles in Nerve Regeneration. Int. J. Mol. Sci. 2024, 25, 5863. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.-Y.; Sun, X.; Pan, H.-X.; Wang, L.; He, C.-Q.; Wei, Q. Cell transplantation therapies for spinal cord injury focusing on bone marrow mesenchymal stem cells: Advances and challenges. World J. Stem Cells 2023, 15, 385–399. [Google Scholar] [CrossRef] [PubMed]
- Putthanbut, N.; Lee, J.Y.; Borlongan, C.V. Extracellular vesicle therapy in neurological disorders. J. Biomed. Sci. 2024, 31, 85. [Google Scholar] [CrossRef] [PubMed]
- Santa Cruz-Pavlovich, F.J.; Bolaños-Chang, A.J.; Del Rio-Murillo, X.I.; Aranda-Preciado, G.A.; Razura-Ruiz, E.M.; Santos, A.; Navarro-Partida, J. Beyond Vision: An Overview of Regenerative Medicine and Its Current Applications in Ophthalmological Care. Cells 2024, 13, 179. [Google Scholar] [CrossRef] [PubMed]
- Ajgaonkar, B.S.; Kumaran, A.; Kumar, S.; Jain, R.D.; Dandekar, P.P. Cell-based Therapies for Corneal and Retinal Disorders. Stem Cell Rev. Rep. 2023, 19, 2650–2682. [Google Scholar] [CrossRef] [PubMed]
- Vo, Q.D.; Saito, Y.; Ida, T.; Nakamura, K.; Yuasa, S. The use of artificial intelligence in induced pluripotent stem cell-based technology over 10-year period: A systematic scoping review. PLoS ONE 2024, 19, e0302537. [Google Scholar] [CrossRef] [PubMed]
- Chehelgerdi, M.; Behdarvand Dehkordi, F.; Chehelgerdi, M.; Kabiri, H.; Salehian-Dehkordi, H.; Abdolvand, M.; Salmanizadeh, S.; Rashidi, M.; Niazmand, A.; Ahmadi, S.; et al. Exploring the promising potential of induced pluripotent stem cells in cancer research and therapy. Mol. Cancer 2023, 22, 189. [Google Scholar] [CrossRef] [PubMed]
- Mirgayazova, R.; Khadiullina, R.; Chasov, V.; Mingaleeva, R.; Miftakhova, R.; Rizvanov, A.; Bulatov, E. Therapeutic Editing of the TP53 Gene: Is CRISPR/Cas9 an Option? Genes 2020, 11, 704. [Google Scholar] [CrossRef] [PubMed]
- Kalter, N.; Gulati, S.; Rosenberg, M.; Ayaz, Q.; Nguyen, J.; Wang, S.; Schroeder, B.; Li, C.-Y.; Hendel, A. Precise measurement of CRISPR genome editing outcomes through single-cell DNA sequencing. Mol. Ther. Methods Clin. Dev. 2025, 33, 101449. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z. Assessing Tumorigenicity in Stem Cell-Derived Therapeutic Products: A Critical Step in Safeguarding Regenerative Medicine. Bioeng. 2023, 10, 857. [Google Scholar] [CrossRef] [PubMed]
- Leahy, L.M.; Woods, I.; Gutierrez-Gonzalez, J.; Maughan, J.; O’Connor, C.; Stasiewicz, M.; Kaur, K.; Monaghan, M.G.; Dervan, A.; O’Brien, F.J. Electrostimulation via a 3D-printed, biomimetic, neurotrophic, electroconductive scaffold for the promotion of axonal regrowth after spinal cord injury. Mater. Today 2024, 79, 60–72. [Google Scholar] [CrossRef]
- Huang, L.-C.; McKeown, C.R.; He, H.-Y.; Ta, A.C.; Cline, H.T. BRCA1 and ELK-1 regulate neural progenitor cell fate in the optic tectum in response to visual experience in Xenopus laevis tadpoles. Proc. Natl. Acad. Sci. USA 2024, 121, e2316542121. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xie, L. AI-driven multi-omics integration for multi-scale predictive modeling of genotype-environment-phenotype relationships. Comput. Struct. Biotechnol. J. 2025, 27, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Wu, Z.; Hsieh, C.-Y.; Chen, G.; Liao, B.; Wang, Z.; Shen, C.; Cao, D.; Wu, J.; Hou, T. Could graph neural networks learn better molecular representation for drug discovery? A comparison study of descriptor-based and graph-based models. J. Cheminf. 2021, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhang, T.; Liu, Q.; Sutcharitchan, C.; Zhou, Z.; Zhang, D.; Li, S. Elucidating the role of artificial intelligence in drug development from the perspective of drug-target interactions. J. Pharm. Anal. 2025, 15, 101144. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.-Q.; Zeng, L.-H.; Li, C.-T.; He, D.-H.; Zhao, H.-D.; Xu, Y.-N.; Jin, Z.-T.; Gao, C. Brain organoids are new tool for drug screening of neurological diseases. Neural Regen. Res. 2023, 18, 1884–1889. [Google Scholar] [CrossRef] [PubMed]
- Gharibshahian, M.; Torkashvand, M.; Bavisi, M.; Aldaghi, N.; Alizadeh, A. Recent advances in artificial intelligent strategies for tissue engineering and regenerative medicine. Skin Res. Technol. 2024, 30, e70016. [Google Scholar] [CrossRef] [PubMed]
- Mikołajewska, E.; Masiak, J.; Mikołajewski, D. Applications of Artificial Intelligence-Based Patient Digital Twins in Decision Support in Rehabilitation and Physical Therapy. Electronics 2024, 13, 4994. [Google Scholar] [CrossRef]
- Li, Y.; Nie, Y.; Quan, Z.; Zhang, H.; Song, R.; Feng, H.; Cheng, X.; Liu, W.; Geng, X.; Sun, X.; et al. Brain-machine interactive neuromodulation research tool with edge AI computing. Heliyon 2024, 10, e32609. [Google Scholar] [CrossRef] [PubMed]
- Jagt, M.; Ganis, F.; Serafin, S. Enhanced neural phase locking through audio-tactile stimulation. Front. Neurosci. 2024, 18, 1425398. [Google Scholar] [CrossRef] [PubMed]
- Mienye, I.D.; Obaido, G.; Jere, N.; Mienye, E.; Aruleba, K.; Emmanuel, I.D.; Ogbuokiri, B. A survey of explainable artificial intelligence in healthcare: Concepts, applications, and challenges. Inform. Med. Unlocked 2024, 51, 101587. [Google Scholar] [CrossRef]
- Valero-Cuevas, F.J.; Finley, J.; Orsborn, A.; Fung, N.; Hicks, J.L.; Huang, H.; Reinkensmeyer, D.; Schweighofer, N.; Weber, D.; Steele, K.M. NSF DARE—Transforming modeling in neurorehabilitation: Four threads for catalyzing progress. J. Neuroeng. Rehabil. 2024, 21, 46. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Pang, S.; Li, Y.; Gao, J. Progress in the generation of spinal cord organoids over the past decade and future perspectives. Neural Regen. Res. 2024, 19, 1013. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ning, X.; Zhao, F.; Zhao, H.; Li, D. Human organoids-on-chips for biomedical research and applications. Theranostics 2024, 14, 788–818. [Google Scholar] [CrossRef] [PubMed]
- Dufva, M. A quantitative meta-analysis comparing cell models in perfused organ on a chip with static cell cultures. Sci. Rep. 2023, 13, 8233. [Google Scholar] [CrossRef] [PubMed]
- Memon, B.; Abdelalim, E.M. Toward Precision Medicine with Human Pluripotent Stem Cells for Diabetes. Stem Cells Transl. Med. 2022, 11, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, B.; Bin, X.; Xie, C.; Li, B.; Liu, O.; Tang, Z. CircHIPK3: Key Player in Pathophysiology and Potential Diagnostic and Therapeutic Tool. Front. Med. 2021, 8, 615417. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Li, Y.; Ho, W.; Chen, C.; Chen, Q.; Li, F.; Tang, M.; Fan, Q.; Wan, J.; Yu, W.; et al. Targeted mRNA Nanoparticles Ameliorate Blood–Brain Barrier Disruption Postischemic Stroke by Modulating Microglia Polarization. ACS Nano 2024, 18, 3260–3275. [Google Scholar] [CrossRef] [PubMed]
- Ruetz, T.J.; Pogson, A.N.; Kashiwagi, C.M.; Gagnon, S.D.; Morton, B.; Sun, E.D.; Na, J.; Yeo, R.W.; Leeman, D.S.; Morgens, D.W.; et al. CRISPR–Cas9 screens reveal regulators of ageing in neural stem cells. Nature 2024, 634, 1150–1159. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hao, M.; Chen, Z.; Zhang, T.; Huang, J.; Dai, J.; Zhang, Z. 3D bioprinted neural tissue constructs for spinal cord injury repair. Biomaterials 2021, 272, 120771. [Google Scholar] [CrossRef] [PubMed]
- Islamov, R.; Bashirov, F.; Fadeev, F.; Shevchenko, R.; Izmailov, A.; Markosyan, V.; Sokolov, M.; Kuznetsov, M.; Davleeva, M.; Garifulin, R.; et al. Epidural Stimulation Combined with Triple Gene Therapy for Spinal Cord Injury Treatment. Int. J. Mol. Sci. 2020, 21, 8896. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Zhu, Q.; Huang, Q.; Gu, Q.; Zhu, Y.; Tang, M.; Tian, S.; Wang, L.; Yan, F.; Ge, J.; et al. FGF21 prevents neuronal cell ferroptosis after spinal cord injury by activating the FGFR1/β-Klotho pathway. Brain Res. Bull. 2023, 202, 110753. [Google Scholar] [CrossRef] [PubMed]
- Musleh-Vega, S.; Ojeda, J.; Vidal, P.M. Gut Microbiota–Brain Axis as a Potential Modulator of Psychological Stress after Spinal Cord Injury. Biomedicines 2022, 10, 847. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.S.; Srikruthi, K.S.; Goudanavar, P.; Naveen, N.R. Navigating the frontier: Comprehensive insights into CRISPR technology advancements, delivery strategies, and ethical considerations in cancer research. Oral Oncol. Rep. 2024, 9, 100224. [Google Scholar] [CrossRef]
- Li, W.; Yin, Z.; Li, X.; Ma, D.; Yi, S.; Zhang, Z.; Zou, C.; Bu, K.; Dai, M.; Yue, J.; et al. A hybrid quantum computing pipeline for real world drug discovery. Sci. Rep. 2024, 14, 16942. [Google Scholar] [CrossRef] [PubMed]
- De Fazio, R.; Mastronardi, V.M.; De Vittorio, M.; Visconti, P. Wearable Sensors and Smart Devices to Monitor Rehabilitation Parameters and Sports Performance: An Overview. Sensors 2023, 23, 1856. [Google Scholar] [CrossRef] [PubMed]
| Reference | Molecular Pathway | Key Findings | Mechanism of Action | Therapeutic Target/Agent | Implications for SCI Recovery | Limitations |
|---|---|---|---|---|---|---|
| [38,39,40] | Ferroptosis | Lipid peroxidation drives oligodendrocyte death; ferroptosis inhibitors reduce cell death by 50% | ROS-mediated lipid damage; ACSL4 overexpression in SCI | Ferroptosis inhibitors (e.g., liproxstatin-1) | Preserves white matter; supports remyelination | Off-target effects and systemic toxicity concerns |
| [41,42,43] | Neuroinflammation | Reprogramming microglia from M1 to M2 reduces inflammation by 40% | CX3CR1 agonists shift microglial phenotype | Nanocarriers delivering IL-10; CX3CR1 agonists | Harnesses immune response for repair; promotes axonal regrowth | Microglial plasticity timing is critical for therapy |
| [44,45,46,47] | Nrf2 pathway activation | Reduced ROS-induced damage; enhanced mitochondrial resilience | Activation of Nrf2-dependent antioxidant pathways | Antioxidants (MitoQ, SS-31) | Protects against oxidative stress; improves recovery timelines | Bioavailability of agents in CNS remains a challenge |
| [48,49,50] | Excitotoxicity | Inhibiting glutamate-mediated excitotoxicity preserves neuronal viability | Blockade of NMDA receptors | NMDA receptor antagonists (memantine) | Reduces neuronal apoptosis; neuroprotective | Potential cognitive side effects |
| [51,52,53] | Apoptosis pathways | Reduced neuronal apoptosis by 30% with caspase inhibitors | Inhibition of caspase-3 and -9 | Caspase inhibitors | Prevents secondary injury-induced neuronal death | Target specificity and systemic side effects |
| [54,55,56,57] | Endoplasmic reticulum (ER) stress | Reduces protein misfolding; promotes neuronal survival | Modulation of UPR signaling | Salubrinal, ISRIB | Enhances neuronal survival | Long-term safety of ER-targeted agents is unknown |
| Therapeutic Approach | Mechanism/Methodology | Key Findings | Model/Evidence | Therapeutic Implications | Limitations | Reference |
|---|---|---|---|---|---|---|
| CRISPR-Cas9 editing targeting Nogo-A | Gene editing via CRISPR-Cas9 combined with activity-based therapy | 40% axonal sprouting; improved motor recovery | Preclinical: Rodent (SCI + treadmill) | Restores neural connectivity; potential for chronic SCI | Off-target effects and long-term gene stability | [95,96,97] |
| Nanoparticle delivery of ChABC | Local nanocarrier-mediated delivery of chondroitinase ABC (ChABC) | CSPGs; glial scar; 60% axonal growth | Preclinical: Chronic SCI rat model | Combines precision delivery with matrix remodeling | Nanoparticle stability in systemic delivery | [98,99,100] |
| MSC transplantation with biomaterial scaffolds | 3D-printed scaffolds seeded with MSCs + rehab protocols | 70% motor function in large animals | Preclinical: Porcine SCI model | Enhances integration and axonal regrowth | Immune rejection risk and scalability issues | [101,102,103] |
| Mitochondrial transplantation | Injection of isolated healthy mitochondria into lesion | oxidative stress; preserved white matter | Preclinical: Rodent model | Novel acute-phase neuroprotective approach | Targeting and distribution challenges | [104,105,106] |
| Antioxidant-loaded nanoparticles | Nanoparticles encapsulating SOD mimetics or MitoQ | ROS levels; axonal survival | Preclinical: Murine SCI | Shields tissue from oxidative damage | Requires precise targeting | [107,108,109] |
| Combined gene therapy and exercise | Gene therapy suppressing CSPGs + treadmill training | 45% functional recovery | Preclinical: Rodent model | Shows synergistic potential of genetic + physical therapy | High cost and logistics | [110,111,112] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Covache-Busuioc, R.-A.; Toader, C.; Rădoi, M.P.; Șerban, M. Precision Recovery After Spinal Cord Injury: Integrating CRISPR Technologies, AI-Driven Therapeutics, Single-Cell Omics, and System Neuroregeneration. Int. J. Mol. Sci. 2025, 26, 6966. https://doi.org/10.3390/ijms26146966
Covache-Busuioc R-A, Toader C, Rădoi MP, Șerban M. Precision Recovery After Spinal Cord Injury: Integrating CRISPR Technologies, AI-Driven Therapeutics, Single-Cell Omics, and System Neuroregeneration. International Journal of Molecular Sciences. 2025; 26(14):6966. https://doi.org/10.3390/ijms26146966
Chicago/Turabian StyleCovache-Busuioc, Răzvan-Adrian, Corneliu Toader, Mugurel Petrinel Rădoi, and Matei Șerban. 2025. "Precision Recovery After Spinal Cord Injury: Integrating CRISPR Technologies, AI-Driven Therapeutics, Single-Cell Omics, and System Neuroregeneration" International Journal of Molecular Sciences 26, no. 14: 6966. https://doi.org/10.3390/ijms26146966
APA StyleCovache-Busuioc, R.-A., Toader, C., Rădoi, M. P., & Șerban, M. (2025). Precision Recovery After Spinal Cord Injury: Integrating CRISPR Technologies, AI-Driven Therapeutics, Single-Cell Omics, and System Neuroregeneration. International Journal of Molecular Sciences, 26(14), 6966. https://doi.org/10.3390/ijms26146966





