Nitric Oxide and Photosynthesis Interplay in Plant Interactions with Pathogens
Abstract
1. Introduction
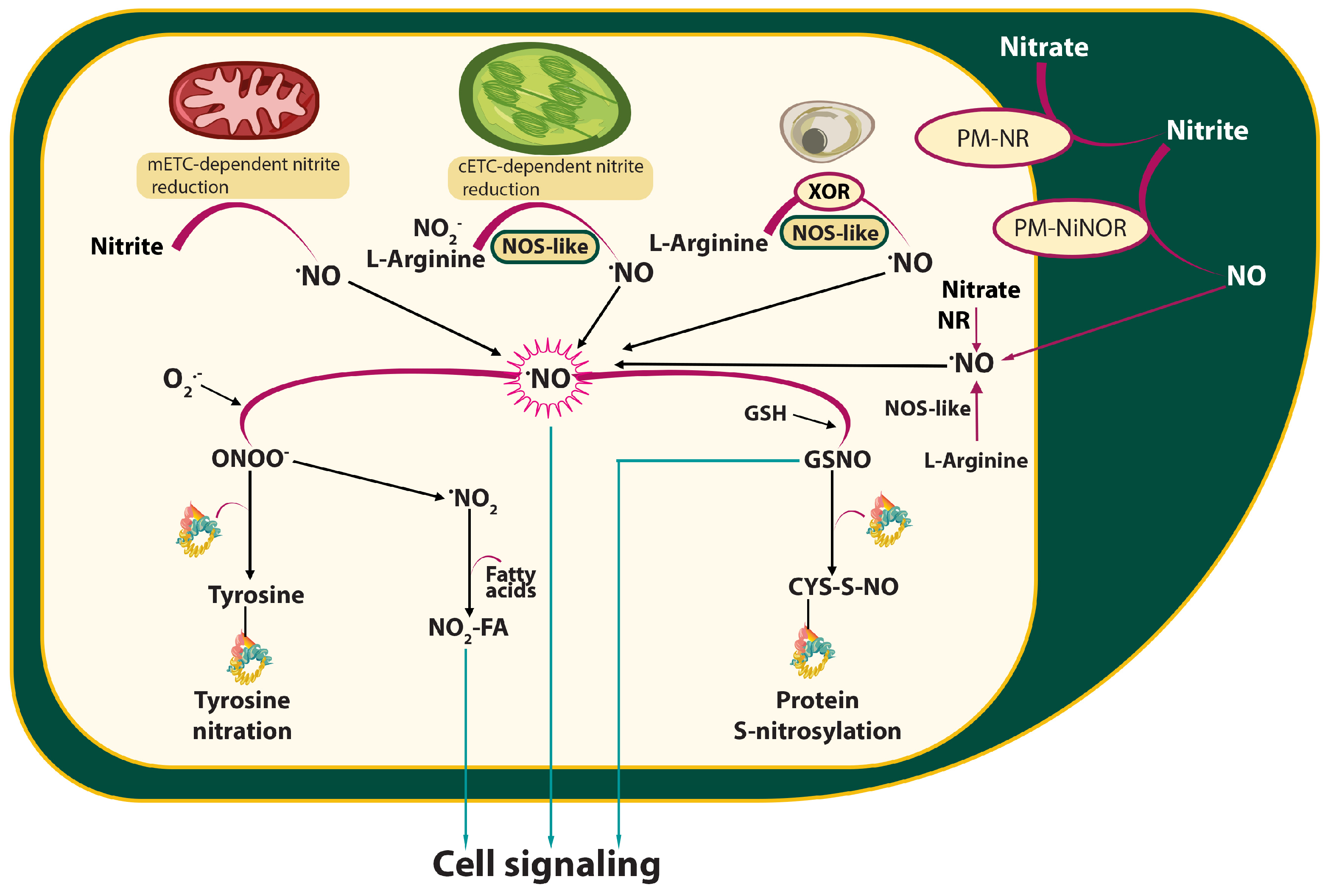
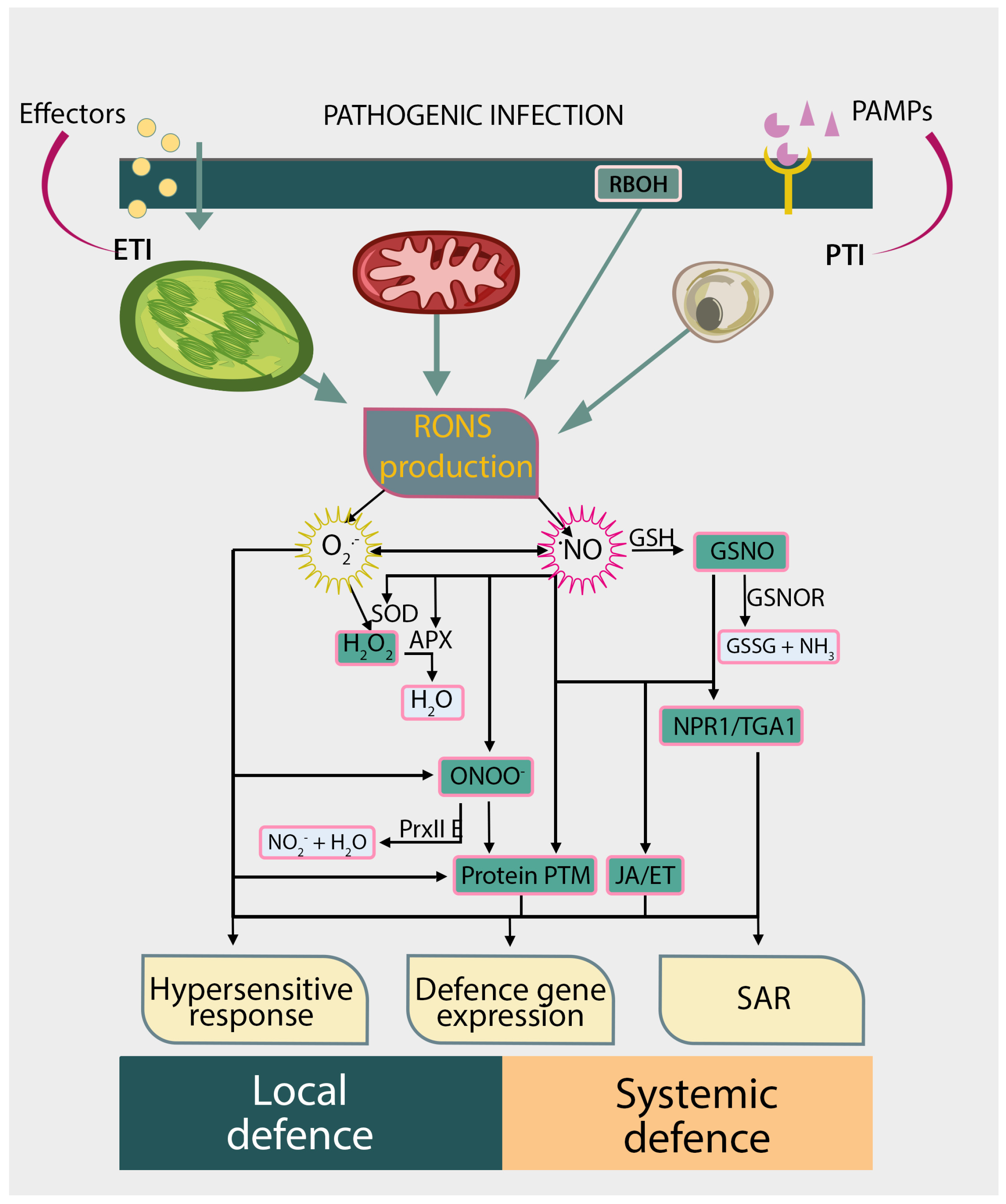
2. RONS Production and Catabolism During Plant-Pathogen Interactions
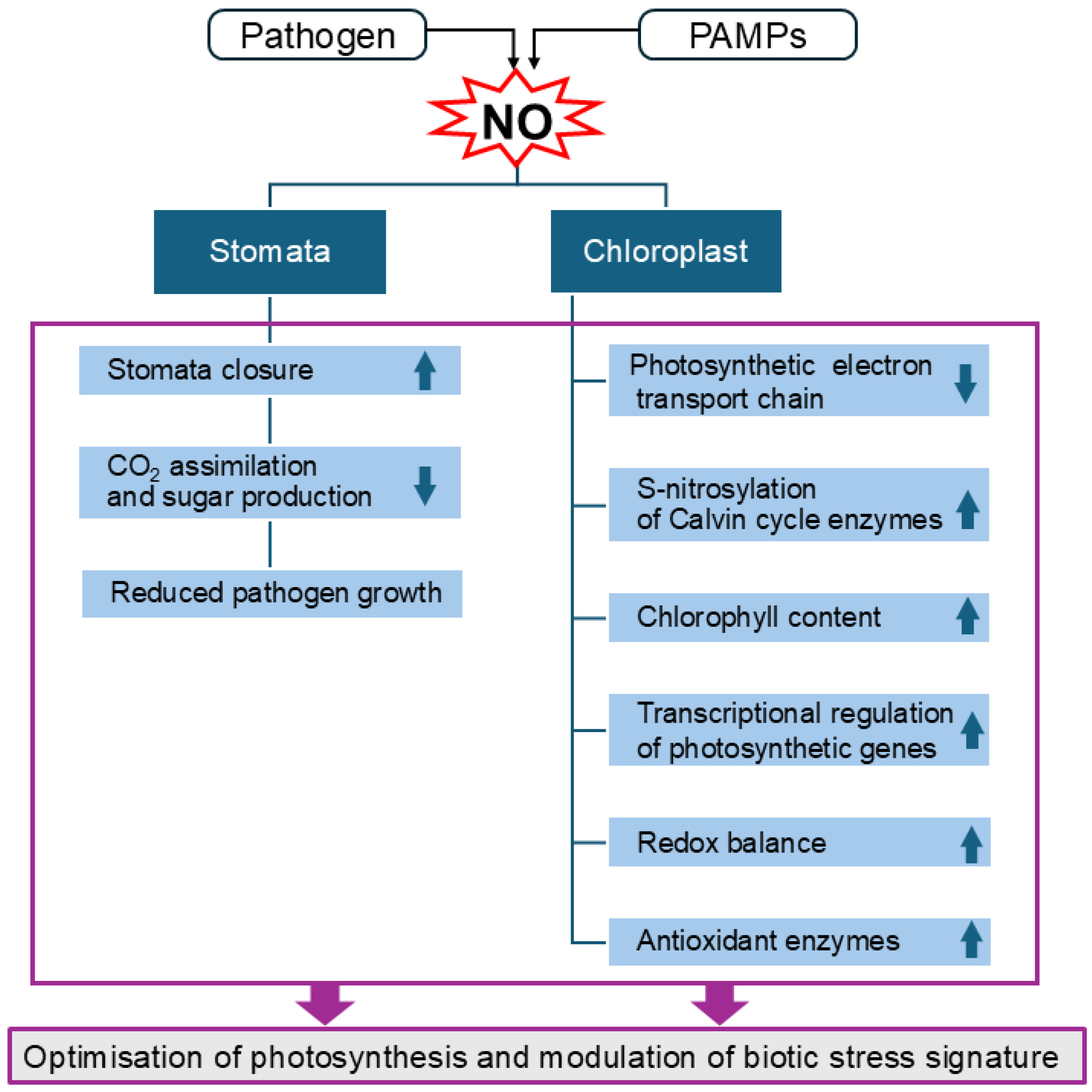
3. The Role of NO in Regulating Stomata Movement
4. The Role of NO in the Regulation of Photosynthesis
5. Protein S-Nitrosylation in Regulating Plant Immune Responses
| Nitrosylated Protein | Function in Plant Cells | Plant–Pathogen Interaction | S-Nitrosylation Effects | References |
|---|---|---|---|---|
| NPR1 (Non-Pathogenesis Related 1) | Non-expressor of Pathogen Related genes 1 | Arabidopsis thaliana–Pseudomonas syringae pv. maculicola | S-nitrosylation of cysteine 156 in NPR1 facilitates oligomerization/retention in cytosol (thioredoxin-h 5, TRXh5-capacity to denitrosylate Cys156 of NPR1) | [169] |
| S-nitrosylation of the NADPH oxidase, AtRBOHD, at Cys 890 | NADPH oxidases generate ROS after pathogen recognition | A. thaliana–P. syringae pv. tomato | AtRBOHD S-nitrosylation at Cys890 reduces ROS production during pathogen attacks, thus weakening the development of HR | [63] |
| SABP3-salicylic acid binding protein 3 | Role during the establishment of plant disease resistance; possesses carbonic anhydrase activity | A. thaliana–P. syringae | AtSABP3 S-nitrosylated at Cys280 inhibition of SA-binding activities; negative regulation of SA-dependent defence response at the later stage of infection | [171] |
| SCE1-small ubiquitin-like modifier (SUMO)-conjugating enzyme 1 | Inhibition of SCE1 enzyme activity facilitates SA-dependent immune responses | A. thaliana–P. syringae pv. tomato | S-nitrosylation of SCE1 at Cys139 stimulates PR1 expression and enhances plant immunity | [174] |
| SRG1-zinc finger transcription factor (ZF-TF) | NO accumulation promotes SRG1 expression | A. thaliana–P. syringae pv. tomato | S-nitrosylation of SRG1 at Cys87 positively regulates plant growth and immunity | [175] |
| HopAI1, bacterial effector HopAI1 | HopAI1 targets and suppresses mitogen-activated protein kinases (MAPK) | A. thaliana–P. syringae pv. tomato | S-nitrosylation of HopAI1 restores MAPK signalling and is required during the HR for activation of the HR-associated cell death | [166] |
| CDC48, chaperon-like protein | Chaperon-like protein Cdc48, cryptogein-induced immune response | Nicotiana tabacum–Phytophthora cryptogea | S-nitrosylation of NtCDC48 at Cys526 compromises immune responses in plant cells | [80,176] |
| COMT2– caffeic acid O-methyltransfe-rase 2 | NO and JA enhanced COMT-mediated infection-induced melatonin biosynthesis; melatonin inhibited cell death by scavenging ROS | Solanum lycopersicum–Botrytis cinerea | S-nitrosylation of SlCOMT2 at Cys344, enhances COMT2 stability and prevents its degradation via the 26S proteasome | [177] |
| GDC glycine decarboxylase complex (EC 2.1.2.10) | Mitochondria-localised GDC is a key enzyme of photorespiration in C3 plants | A. thaliana–harpin proteins (bacterial elicitors of Erwinia and Pseudomonas) | S-nitrosylation and S-glutathionylation inhibit GDC activity | [178] |
| Pyruvate kinase (EC 2.7.1.40) | Cytoplasm-localised, glycolysis enzyme | A. thaliana–P. syringae pv. tomato | S-nitrosylation of pyruvate kinase probably regulates enzyme activity | [179] |
| PrxII E peroxiredoxin | Chloroplast-localized peroxiredoxin, efficiently removes H2O2 | A. thaliana–P. syringae pv. tomato | S-nitrosylation of PrxII E at Cys121 leads to the inhibition of both the peroxidase and ONOO− reductase activities | [180] |
| Fructose-1,6-bisphosphatase (FBPase, EC 3.1.3.11) | Calvin–Benson cycle enzyme converting Fru-1,6-BP to Fru-6-P and Pi | Pisum sativum | S-nitrosylation at Cys153 of cFBP1 (FBPase isoform) leads to the formation of a regulatory disulfide bridge | [181] |
| Ribulose 1,5-bisphosphate carboxylase/oxygenase (Rubisco, EC 4.1.1.39) | Chloroplast-stroma localized key enzyme of the Calvin–Benson cycle | A. thaliana–P. syringae pv. tomato | S-nitrosylation of large/small Rubisco subunits likely regulates its activity and turnover | [146,164,179,182] |
| Rubisco activase | Calvin–Benson cycle enzyme activation | A. thaliana–P. syringae pv. tomato | S-nitrosylation of Rubisco activase detected | [182] |
| Rubisco large subunit-binding protein (subunit alpha) | Chloroplastic chaperone | A. thaliana–P. syringae pv. tomato | S-nitrosylation influences chloroplast organization-protein folding/chaperone | [179] |
| PSBP-1 PSBO2 | Photosystem II subunits | A. thaliana–P. syringae pv. tomato | S-nitrosylation of PS II subunits detected | [182] |
| 23 kDa subunit of oxygen evolving system of PSII | Photosystem II protein | A. thaliana–P. syringae pv. tomato | S-nitrosylation of 23 kDa subunit of PSII detected | [179] |
| Glyceraldehyde-3-phosphate dehydrogenase (GAPDH, EC 1.2.1.13) | Calvin–Benson cycle enzyme | A. thaliana–P. syringae pv. tomato | S-nitrosylation of B subunit of GAPDH inhibits its activity | [179] |
| CPCK2-α subunit of casein kinase II (CK2, Ser/Thr kinase) | Chloroplast-localised protein kinase CK2 subunit, CPCK2 regulates various pathways in plants | A. thalina–Golovinomyces cichoracearum A. thaliana–P. syringae pv. tomato | CPCK2 negatively regulates plant immunity by promoting S-nitrosylation of SABP3; cpck2 mutants accumulate SA and show resistance against the fungal pathogen powdery mildew | [183] |
| MPK6 protein kinase (a part of MAPK cascade) | MPK6 controls stomatal development by phosphorylating transcription factor SPCH | A. thaliana mutants treated with ABA | S-nitrosylation of MPK6 at Cys201 promotes stomatal development and controls stress responses | [184] |
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pathak, P.K.; Yadav, N.; Kaladhar, V.C.; Jaiswal, R.; Kumari, A.; Igamberdiev, A.U.; Loake, G.J.; Gupta, K.J. The Emerging Roles of Nitric Oxide and Its Associated Scavengers-Phytoglobins-in Plant Symbiotic Interactions. J. Exp. Bot. 2024, 75, 563–577. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Singh, R.D.; Pandey, G.K.; Mukherjee, P.K.; Foyer, C.H. Unravelling the Molecular Dialogue of Beneficial Microbe−Plant Interactions. Plant Cell Environ. 2025, 48, 2534–2548. [Google Scholar] [CrossRef]
- Mandal, M.; Sarkar, M.; Khan, A.; Biswas, M.; Masi, A.; Rakwal, R.; Agrawal, G.K.; Srivastava, A.; Sarkar, A. Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS) in Plants–Maintenance of Structural Individuality and Functional Blend. Adv. Redox Res. 2022, 5, 100039. [Google Scholar] [CrossRef]
- Talaat, N.B. Role of Reactive Oxygen Species Signaling in Plant Growth and Development. In Reactive Oxygen, Nitrogen and Sulfur Species in Plants: Production, Metabolism, Signaling and Defense Mechanisms; Hasanuzzaman, M., Fotopoulos, V., Nahar, K., Fujita, M., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2019; Volume 1, pp. 225–266. ISBN 9781119468677. [Google Scholar]
- Nawrocka, J.; Szymczak, K.; Maćkowiak, A.; Skwarek-Fadecka, M.; Małolepsza, U. Determination of Reactive Oxygen or Nitrogen Species and Novel Volatile Organic Compounds in the Defense Responses of Tomato Plants Against Botrytis cinerea Induced by Trichoderma virens TRS 106. Cells 2022, 11, 3051. [Google Scholar] [CrossRef]
- Ciacka, K.; Staszek, P.; Sobczynska, K.; Krasuska, U.; Gniazdowska, A. Nitric Oxide in Seed Biology. Int. J. Mol. Sci. 2022, 23, 14951. [Google Scholar] [CrossRef] [PubMed]
- Bykova, N.V.; Igamberdiev, A.U. Redox Control of Seed Germination Is Mediated by the Crosstalk of Nitric Oxide and Reactive Oxygen Species. Antioxid. Redox Signal. 2024, 42, 442–461. [Google Scholar] [CrossRef] [PubMed]
- Thiruvengadam, R.; Venkidasamy, B.; Easwaran, M.; Chi, H.Y.; Thiruvengadam, M.; Kim, S.H. Dynamic Interplay of Reactive Oxygen and Nitrogen Species (ROS and RNS) in Plant Resilience: Unveiling the Signaling Pathways and Metabolic Responses to Biotic and Abiotic Stresses. Plant Cell Rep. 2024, 43, 198. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Joshi, S.; Patil, S.; Khare, T.; Kumar, V. Reactive Oxygen, Nitrogen, Carbonyl and Sulfur Species and Their Roles in Plant Abiotic Stress Responses and Tolerance. J. Plant Growth Regul. 2022, 41, 119–142. [Google Scholar] [CrossRef]
- Christou, A.; Manganaris, G.A.; Fotopoulos, V. Systemic Mitigation of Salt Stress by Hydrogen Peroxide and Sodium Nitroprusside in Strawberry Plants via Transcriptional Regulation of Enzymatic and Non-Enzymatic Antioxidants. Environ. Exp. Bot. 2014, 107, 46–54. [Google Scholar] [CrossRef]
- Antoniou, C.; Xenofontos, R.; Chatzimichail, G.; Christou, A.; Kashfi, K.; Fotopoulos, V. Exploring the Potential of Nitric Oxide and Hydrogen Sulfide (NOSH)-Releasing Synthetic Compounds as Novel Priming Agents Against Drought Stress in Medicago Sativa Plants. Biomolecules 2020, 10, 120. [Google Scholar] [CrossRef]
- Medrano-Macías, J.; Flores-Gallegos, A.C.; Nava-Reyna, E.; Morales, I.; Tortella, G.; Solís-Gaona, S.; Benavides-Mendoza, A. Reactive Oxygen, Nitrogen, and Sulfur Species (RONSS) as a Metabolic Cluster for Signaling and Biostimulation of Plants: An Overview. Plants 2022, 11, 3203. [Google Scholar] [CrossRef]
- Gogoi, K.; Gogoi, H.; Borgohain, M.; Saikia, R.; Chikkaputtaiah, C.; Hiremath, S.; Basu, U. The Molecular Dynamics between Reactive Oxygen Species (ROS), Reactive Nitrogen Species (RNS) and Phytohormones in Plant’s Response to Biotic Stress. Plant Cell Rep. 2024, 43, 263. [Google Scholar] [CrossRef]
- Corpas, F.J.; Barroso, J.B. Nitro-Oxidative Stress vs Oxidative or Nitrosative Stress in Higher Plants. New Phytol. 2013, 199, 633–635. [Google Scholar] [CrossRef] [PubMed]
- Berger, S.; Papadopoulos, M.; Schreiber, U.; Kaiser, W.; Roitsch, T. Complex Regulation of Gene Expression, Photosynthesis and Sugar Levels by Pathogen Infection in Tomato. Physiol. Plant. 2004, 122, 419–428. [Google Scholar] [CrossRef]
- Gorshkov, V.; Tsers, I. Plant Susceptible Responses: The Underestimated Side of Plant–Pathogen Interactions. Biol. Rev. 2022, 97, 45–66. [Google Scholar] [CrossRef] [PubMed]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The Global Burden of Pathogens and Pests on Major Food Crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef]
- Cheaib, A.; Killiny, N. Photosynthesis Responses to the Infection with Plant Pathogens. Mol. Plant-Microbe Interact. 2024, 38, 9–29. [Google Scholar] [CrossRef]
- Yang, H.; Luo, P. Changes in Photosynthesis Could Provide Important Insight into the Interaction Between Wheat and Fungal Pathogens. Int. J. Mol. Sci. 2021, 22, 8865. [Google Scholar] [CrossRef]
- Croce, R.; Carmo-Silva, E.; Cho, Y.B.; Ermakova, M.; Harbinson, J.; Lawson, T.; McCormick, A.J.; Niyogi, K.K.; Ort, D.R.; Patel-Tupper, D.; et al. Perspectives on Improving Photosynthesis to Increase Crop Yield. Plant Cell 2024, 36, 3944–3973. [Google Scholar] [CrossRef]
- Jian, Y.; Gong, D.; Wang, Z.; Liu, L.; He, J.; Han, X.; Tsuda, K. How Plants Manage Pathogen Infection. EMBO Rep. 2024, 25, 31–44. [Google Scholar] [CrossRef]
- Piasecka, A.; Jedrzejczak-Rey, N.; Bednarek, P. Secondary Metabolites in Plant Innate Immunity: Conserved Function of Divergent Chemicals. New Phytol. 2015, 206, 948–964. [Google Scholar] [CrossRef] [PubMed]
- Malinovsky, F.G.; Fangel, J.U.; Willats, W.G.T. The Role of the Cell Wall in Plant Immunity. Front. Plant Sci. 2014, 5, 178. [Google Scholar] [CrossRef] [PubMed]
- Serrano, M.; Coluccia, F.; Torres, M.; L’Haridon, F.; Métraux, J.P. The Cuticle and Plant Defense to Pathogens. Front. Plant Sci. 2014, 5, 274. [Google Scholar] [CrossRef]
- Jones, J.D.G.; Dangl, J.L. The Plant Immune System. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef]
- Katagiri, F.; Tsuda, K. Understanding the Plant Immune System. Mol. Plant-Microbe Interact. 2010, 23, 1531–1536. [Google Scholar] [CrossRef]
- Cai, J.; Jiang, Y.; Ritchie, E.S.; Macho, A.P.; Yu, F.; Wu, D. Manipulation of Plant Metabolism by Pathogen Effectors: More than Just Food. FEMS Microbiol. Rev. 2023, 47, 1–16. [Google Scholar] [CrossRef]
- Zhou, J.M.; Zhang, Y. Plant Immunity: Danger Perception and Signaling. Cell 2020, 181, 978–989. [Google Scholar] [CrossRef]
- Daudi, A.; Cheng, Z.; O’Brien, J.A.; Mammarella, N.; Khan, S.; Ausubel, F.M.; Paul Bolwell, G. The Apoplastic Oxidative Burst Peroxidase in Arabidopsis Is a Major Component of Pattern-Triggered Immunity. Plant Cell 2012, 24, 275–287. [Google Scholar] [CrossRef]
- Torres, M.A.; Dangl, J.L.; Jones, J.D.G. Arabidopsis Gp91phox Homologues Atrbohd and Atrbohf Are Required for Accumulation of Reactive Oxygen Intermediates in the Plant Defense Response. Proc. Natl. Acad. Sci. USA 2002, 99, 517–522. [Google Scholar] [CrossRef]
- Littlejohn, G.R.; Breen, S.; Smirnoff, N.; Grant, M. Chloroplast Immunity Illuminated. New Phytol. 2021, 229, 3088–3107. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, G.; Ning, Y.; Wang, X.; Wang, G.L. Mitochondrial Functions in Plant Immunity. Trends Plant Sci. 2022, 27, 1063–1076. [Google Scholar] [CrossRef] [PubMed]
- Camejo, D.; Guzmán-Cedeño, Á.; Moreno, A. Reactive Oxygen Species, Essential Molecules, during Plant-Pathogen Interactions. Plant Physiol. Biochem. 2016, 103, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Ciereszko, I.; Kuźniak, E. Photorespiratory Metabolism and Its Regulatory Links to Plant Defence Against Pathogens. Int. J. Mol. Sci. 2024, 25, 12134. [Google Scholar] [CrossRef] [PubMed]
- Chmielowska-Bąk, J.; Sobieszczuk-Nowicka, E.; Arasimowicz-Jelonek, M. Nitro-Oxidative Nucleotide Modifications in Plants and Associated Microorganisms–Signaling Sensors or Stress Symptoms? J. Exp. Bot. 2025, eraf188. [Google Scholar] [CrossRef]
- Bleau, J.R.; Spoel, S.H. Selective Redox Signaling Shapes Plant-Pathogen Interactions. Plant Physiol. 2021, 186, 53–65. [Google Scholar] [CrossRef]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive Oxygen Species Signalling in Plant Stress Responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef]
- Li, M.; Kim, C. Chloroplast ROS and Stress Signaling. Plant Commun. 2022, 3, 100264. [Google Scholar] [CrossRef]
- Kuźniak, E.; Skłodowska, M. Compartment-Specific Role of the Ascorbate-Glutathione Cycle in the Response of Tomato Leaf Cells to Botrytis cinerea Infection. J. Exp. Bot. 2005, 56, 921–933. [Google Scholar] [CrossRef]
- Khan, M.; Ali, S.; Al Azzawi, T.N.I.; Saqib, S.; Ullah, F.; Ayaz, A.; Zaman, W. The Key Roles of ROS and RNS as a Signaling Molecule in Plant–Microbe Interactions. Antioxidants 2023, 12, 268. [Google Scholar] [CrossRef]
- Kuźniak, E.; Kopczewski, T. The Chloroplast Reactive Oxygen Species-Redox System in Plant Immunity and Disease. Front. Plant Sci. 2020, 11, 572686. [Google Scholar] [CrossRef]
- Rai, K.K. Revisiting the Critical Role of ROS and RNS in Plant Defense. J. Plant Growth Regul. 2023, 42, 6202–6227. [Google Scholar] [CrossRef]
- Sedlářová, M.; Jedelská, T.; Lebeda, A.; Petřivalský, M. Progress in Plant Nitric Oxide Studies: Implications for Phytopathology and Plant Protection. Int. J. Mol. Sci. 2025, 26, 2087. [Google Scholar] [CrossRef]
- Astier, J.; Gross, I.; Durner, J. Nitric Oxide Production in Plants: An Update. J. Exp. Bot. 2018, 69, 3401–3411. [Google Scholar] [CrossRef] [PubMed]
- León, J.; Costa-Broseta, Á. Present Knowledge and Controversies, Deficiencies, Andmisconceptions on Nitric Oxide Synthesis, Sensing, and Signalingin Plants. Plant Cell Environ. 2020, 43, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Allagulova, C.R.; Lubyanova, A.R.; Avalbaev, A.M. Multiple Ways of Nitric Oxide Production in Plants and Its Functional Activity Under Abiotic Stress Conditions. Int. J. Mol. Sci. 2023, 24, 11637. [Google Scholar] [CrossRef] [PubMed]
- Rockel, P.; Strube, F.; Rockel, A.; Wildt, J.; Kaiser, W.M. Regulation of Nitric Oxide (NO) Production by Plant Nitrate Reductase In Vivo and In Vitro. J. Exp. Bot. 2002, 53, 103–110. [Google Scholar] [CrossRef]
- Tejada-Jimenez, M.; Llamas, A.; Galván, A.; Fernández, E. Role of Nitrate Reductase in NO Production in Photosynthetic Eukaryotes. Plants 2019, 8, 56. [Google Scholar] [CrossRef]
- Corpas, F.J.; González-Gordo, S.; Palma, J.M. NO Source in Higher Plants: Present and Future of an Unresolved Question. Trends Plant Sci. 2022, 27, 116–119. [Google Scholar] [CrossRef]
- Corpas, F.J.; Taboada, J.; Sánchez-Romera, B.; López-Jaramillo, J.; Palma, J.M. Peroxisomal Sulfite Oxidase (SOX), an Alternative Source of NO in Higher Plants Which Is Upregulated by H2S. Plant Physiol. Biochem. 2025, 225, 110000. [Google Scholar] [CrossRef]
- Modolo, L.V.; Augusto, O.; Almeida, I.M.G.; Magalhaes, J.R.; Salgado, I. Nitrite as the Major Source of Nitric Oxide Production by Arabidopsis thaliana in Response to Pseudomonas syringae. FEBS Lett. 2005, 579, 3814–3820. [Google Scholar] [CrossRef]
- Perchepied, L.; Balagué, C.; Riou, C.; Claudel-renard, C.; Rivière, N.; Grezes-besset, B.; Roby, D.; Lipm, I.P.; Cnrs-inra, U.M.R.; Genomics, U.; et al. Nitric Oxide Participates in the Complex Interplay of Defense-Related Signaling Pathways Controlling Disease Resistance to Sclerotinia sclerotiorum in Arabidopsis thaliana. Mol. Plant-Microbe Interact. 2010, 23, 846–860. [Google Scholar] [CrossRef]
- Modolo, L.V.; Augusto, O.; Almeida, I.M.G.; Pinto-Maglio, C.A.F.; Oliveira, H.C.; Seligman, K.; Salgado, I. Decreased Arginine and Nitrite Levels in Nitrate Reductase-Deficient Arabidopsis thaliana Plants Impair Nitric Oxide Synthesis and the Hypersensitive Response to Pseudomonas syringae. Plant Sci. 2006, 171, 34–40. [Google Scholar] [CrossRef]
- Zeidler, D.; Zähringer, U.; Gerber, I.; Dubery, I.; Hartung, T.; Bors, W.; Hutzler, P.; Durner, J. Innate Immunity in Arabidopsis thaliana: Lipopolysaccharides Activate Nitric Oxide Synthase (NOS) and Induce Defense Genes. Proc. Natl. Acad. Sci. USA 2004, 101, 15811–15816. [Google Scholar] [CrossRef]
- Moreau, M.; Gyu, I.L.; Wang, Y.; Crane, B.R.; Klessig, D.F. AtNOS/AtNOA1 Is a Functional Arabidopsis thaliana CGTPase and Not a Nitric-Oxide Synthase. J. Biol. Chem. 2008, 283, 32957–32967. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.-Q.; Okamoto, M.; Crawford, N.M. Identification of Plant Nitric Oxide Synthase Gene Involved in Hormonal Signaling. Science 2003, 302, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Monjil, M.S.; Shibata, Y.; Takemoto, D.; Kawakita, K. Bis-Aryl Methanone Compound Is a Candidate of Nitric Oxide Producing Elicitor and Induces Resistance in Nicotiana benthamiana against Phytophthora infestans. Nitric Oxide-Biol. Chem. 2013, 29, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Jedelská, T.; Luhová, L.; Petřivalský, M. Nitric Oxide Signalling in Plant Interactions with Pathogenic Fungi and Oomycetes. J. Exp. Bot. 2021, 72, 848–863. [Google Scholar] [CrossRef]
- Rasul, S.; Dubreuil-Maurizi, C.; Lamotte, O.; Koen, E.; Poinssot, B.; Alcaraz, G.; Wendehenne, D.; Jeandroz, S. Nitric Oxide Production Mediates Oligogalacturonide-Triggered Immunity and Resistance to Botrytis cinerea in Arabidopsis thaliana. Plant Cell Environ. 2012, 35, 1483–1499. [Google Scholar] [CrossRef]
- Lamotte, O.; Gould, K.; Lecourieux, D.; Sequeira-Legrand, A.; Lebrun-Garcia, A.; Durner, J.; Pugin, A.; Wendehenne, D. Analysis of Nitric Oxide Signaling Functions in Tobacco Cells Challenged by the Elicitor Cryptogein. Plant Physiol. 2004, 135, 516–529. [Google Scholar] [CrossRef]
- Govrin, E.M.; Rachmilevitch, S.; Tiwari, B.S.; Solomon, M.; Levine, A. An Elicitor from Botrytis cinerea Induces the Hypersensitive Response in Arabidopsis thaliana and Other Plants and Promotes the Gray Mold Disease. Phytopathology 2006, 96, 299–307. [Google Scholar] [CrossRef]
- Govrin, E.M.; Levine, A. The Hypersensitive Response Facilitates Plant Infection by the Necrotrophic Pathogen Botrytis cinerea. Curr. Biol. 2000, 10, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Yun, B.W.; Feechan, A.; Yin, M.; Saidi, N.B.B.; Le Bihan, T.; Yu, M.; Moore, J.W.; Kang, J.G.; Kwon, E.; Spoel, S.H.; et al. S-Nitrosylation of NADPH Oxidase Regulates Cell Death in Plant Immunity. Nature 2011, 478, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, Q.; Mou, Z. Redox Signaling and Oxidative Stress in Systemic Acquired Resistance. J. Exp. Bot. 2024, 75, 4535–4548. [Google Scholar] [CrossRef] [PubMed]
- Borrowman, S.; Kapuganti, J.G.; Loake, G.J. Expanding Roles for S-Nitrosylation in the Regulation of Plant Immunity. Free Radic. Biol. Med. 2023, 194, 357–368. [Google Scholar] [CrossRef]
- Kolbert, Z.; Barroso, J.B.; Boscari, A.; Corpas, F.J.; Gupta, K.J.; Hancock, J.T.; Lindermayr, C.; Palma, J.M.; Petřivalský, M.; Wendehenne, D.; et al. Interorgan, Intraorgan and Interplant Communication Mediated by Nitric Oxide and Related Species. New Phytol. 2024, 244, 786–797. [Google Scholar] [CrossRef]
- Mur, L.A.J.; Mandon, J.; Persijn, S.; Cristescu, S.M.; Moshkov, I.E.; Novikova, G.V.; Hall, M.A.; Harren, F.J.M.; Hebelstrup, K.H.; Gupta, K.J. Nitric Oxide in Plants: An Assessment of the Current State of Knowledge. AoB Plants 2013, 5, pls052. [Google Scholar] [CrossRef]
- Turrion-Gomez, J.L.; Benito, E.P. Flux of Nitric Oxide Between the Necrotrophic Pathogen Botrytis cinerea and the Host Plant. Mol. Plant Pathol. 2011, 12, 606–616. [Google Scholar] [CrossRef]
- Sedlářová, M.; Kubienová, L.; Drábková Trojanová, Z.; Luhová, L.; Lebeda, A.; Petřivalský, M. The Role of Nitric Oxide in Development and Pathogenesis of Biotrophic Phytopathogens—Downy and Powdery Mildews. Adv. Bot. Res. 2016, 77, 263–283. [Google Scholar] [CrossRef]
- Truchon, A.N.; Hendrich, C.G.; Bigott, A.F.; Dalsing, B.L.; Allen, C. NorA, HmpX, and NorB Cooperate to Reduce NO Toxicity during Denitrification and Plant Pathogenesis in Ralstonia solanacearum. Microbiol. Spectr. 2022, 10, e0026422. [Google Scholar] [CrossRef]
- Gajewska, J.; Floryszak-Wieczorek, J.; Kosmala, A.; Perlikowski, D.; Żywicki, M.; Sobieszczuk-Nowicka, E.; Judelson, H.S.; Arasimowicz-Jelonek, M. Insight into Metabolic Sensors of Nitrosative Stress Protection in Phytophthora infestans. Front. Plant Sci. 2023, 14, 1148222. [Google Scholar] [CrossRef]
- Vandelle, E.; Delledonne, M. Peroxynitrite Formation and Function in Plants. Plant Sci. 2011, 181, 534–539. [Google Scholar] [CrossRef]
- Ergashev, U.; Yu, M.; Luo, L.; Tang, J.; Han, Y. The Key Targets of NO-Mediated Post-Translation Modification (PTM) Highlighting the Dynamic Metabolism of ROS and RNS in Peroxisomes. Int. J. Mol. Sci. 2024, 25, 8873. [Google Scholar] [CrossRef] [PubMed]
- León, J. Protein Tyrosine Nitration in Plant Nitric Oxide Signaling. Front. Plant Sci. 2022, 13, 859374. [Google Scholar] [CrossRef] [PubMed]
- Arasimowicz-Jelonek, M.; Floryszak-Wieczorek, J. A Physiological Perspective on Targets of Nitration in NO-Based Signaling Networks in Plants. J. Exp. Bot. 2019, 70, 4379–4389. [Google Scholar] [CrossRef] [PubMed]
- Rustérucci, C.; Espunya, M.C.; Díaz, M.; Chabannes, M.; Martínez, M.C. S-Nitrosoglutathione Reductase Affords Protection Against Pathogens in Arabidopsis, Both Locally and Systemically. Plant Physiol. 2007, 143, 1282–1292. [Google Scholar] [CrossRef]
- Yu, Z.; Cao, J.; Zhu, S.; Zhang, L.; Peng, Y.; Shi, J. Exogenous Nitric Oxide Enhances Disease Resistance by Nitrosylation and Inhibition of S-Nitrosoglutathione Reductase in Peach Fruit. Front. Plant Sci. 2020, 11, 543. [Google Scholar] [CrossRef]
- Hussain, A.; Yun, B.W.; Kim, J.H.; Gupta, K.J.; Hyung, N.I.; Loake, G.J. Novel and Conserved Functions of S-Nitrosoglutathione Reductase in Tomato. J. Exp. Bot. 2019, 70, 4877–4886. [Google Scholar] [CrossRef]
- Feechan, A.; Kwon, E.; Yun, B.W.; Wang, Y.; Pallas, J.A.; Loake, G.J. A Central Role for S-Nitrosothiols in Plant Disease Resistance. Proc. Natl. Acad. Sci. USA 2005, 102, 8054–8059. [Google Scholar] [CrossRef]
- Lubega, J.; Umbreen, S.; Loake, G.J. Recent Advances in the Regulation of Plant Immunity by S-Nitrosylation. J. Exp. Bot. 2021, 72, 864–872. [Google Scholar] [CrossRef]
- Feng, J.; Chen, L.; Zuo, J. Protein S-Nitrosylation in Plants: Current Progresses and Challenges. J. Integr. Plant Biol. 2019, 61, 1206–1223. [Google Scholar] [CrossRef]
- Wei, L.; Zhao, J.; Zhong, Y.; Wu, X.; Wei, S.; Liu, Y. The Roles of Protein S-Nitrosylation in Regulating the Growth and Development of Plants: A Review. Int. J. Biol. Macromol. 2025, 307, 142204. [Google Scholar] [CrossRef]
- Noritake, T.; Kawakita, K.; Doke, N. Nitric Oxide Induces Phytoalexin Accumulation in Potato Tuber Tissues. Plant Cell Physiol. 1996, 37, 113–116. [Google Scholar] [CrossRef]
- Imran, Q.M.; Hussain, A.; Mun, B.G.; Lee, S.U.; Asaf, S.; Ali, M.A.; Lee, I.J.; Yun, B.W. Transcriptome Wide Identification and Characterization of NO-Responsive WRKY Transcription Factors in Arabidopsis thaliana L. Environ. Exp. Bot. 2018, 148, 128–143. [Google Scholar] [CrossRef]
- Al Azzawi, T.N.; Khan, M.; Mun, B.G.; Lee, S.U.; Imran, M.; Hussain, A.; Rolly, N.K.; Lee, D.S.; Ali, S.; Lee, I.J.; et al. Enhanced Resistance of Atnigr1 Against Pseudomonas syringae pv. Tomato Suggests Negative Regulation of Plant Basal Defense and Systemic Acquired Resistance by AtNIGR1 Encoding NAD(P)-Binding Rossmann-Fold in Arabidopsis thaliana. Antioxidants 2023, 12, 989. [Google Scholar] [CrossRef]
- Lawson, T.; Vialet-Chabrand, S. Speedy Stomata, Photosynthesis and Plant Water Use Efficiency. New Phytol. 2019, 221, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, D.B.; da Luz, L.M.; de Oliveira, H.O.; Araújo, W.L.; Daloso, D.M.; Fernie, A.R. Metabolomics for Understanding Stomatal Movements. Theor. Exp. Plant Physiol. 2019, 31, 91–102. [Google Scholar] [CrossRef]
- Melotto, M.; Underwood, W.; Koczan, J.; Nomura, K.; He, S.Y. Plant Stomata Function in Innate Immunity Against Bacterial Invasion. Cell 2006, 126, 969–980. [Google Scholar] [CrossRef]
- Raffeiner, M.; Üstün, S.; Guerra, T.; Spinti, D.; Fitzner, M.; Sonnewald, S.; Baldermann, S.; Börnke, F. The Xanthomonas Type-III Effector XopS Stabilizes CaWRKY40a to Regulate Defense Responses and Stomatal Immunity in Pepper (Capsicum annuum). Plant Cell 2022, 34, 1684–1708. [Google Scholar] [CrossRef]
- Gahir, S.; Bharath, P.; Raghavendra, A.S. Stomatal Closure Sets in Motion Long-Term Strategies of Plant Defense Against Microbial Pathogens. Front. Plant Sci. 2021, 12, 761952. [Google Scholar] [CrossRef]
- Wu, J.; Liu, Y. Stomata–Pathogen Interactions: Over a Century of Research. Trends Plant Sci. 2022, 27, 964–967. [Google Scholar] [CrossRef]
- Gayatri, G.; Agurla, S.; Raghavendra, A.S. Nitric Oxide in Guard Cells as an Important Secondary Messenger During Stomatal Closure. Front. Plant Sci. 2013, 4, 425. [Google Scholar] [CrossRef]
- Rodrigues, O.; Shan, L. Stomata in a State of Emergency: H2O2 Is the Target Locked. Trends Plant Sci. 2022, 27, 274–286. [Google Scholar] [CrossRef]
- Wang, P.; Du, Y.; Hou, Y.J.; Zhao, Y.; Hsu, C.C.; Yuan, F.; Zhu, X.; Tao, W.A.; Song, C.P.; Zhu, J.K. Nitric Oxide Negatively Regulates Abscisic Acid Signaling in Guard Cells by S-Nitrosylation of OST1. Proc. Natl. Acad. Sci. USA 2015, 112, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.M.; Mori, I.C.; Pei, Z.M.; Leonhard, N.; Angel Torres, M.; Dangl, J.L.; Bloom, R.E.; Bodde, S.; Jones, J.D.G.; Schroeder, J.I. NADPH Oxidase AtrbohD and AtrbohF Genes Function in ROS-Dependent ABA Signaling in Arabidopsis. EMBO J. 2003, 22, 2623–2633. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.R.; Yue, C.M.; Hao, F.S. Update on Roles of Nitric Oxide in Regulating Stomatal Closure. Plant Signal. Behav. 2019, 14, e1649569. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Huang, L.; Sun, C.; Wang, L.; Jin, C.; Lin, X. Cross-Talk between Hydrogen Peroxide and Nitric Oxide During Plant Development and Responses to Stress. J. Agric. Food Chem. 2021, 69, 9485–9497. [Google Scholar] [CrossRef]
- Yang, H.; Mu, J.; Chen, L.; Feng, J.; Hu, J.; Li, L.; Zhou, J.M.; Zuo, J. S-Nitrosylation Positively Regulates Ascorbate Peroxidase Activity During Plant Stress Responses. Plant Physiol. 2015, 167, 1604–1615. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, H. Reactive Oxygen Species and Nitric Oxide as Mediators in Plant Hypersensitive Response and Stomatal Closure. Plant Signal. Behav. 2021, 16, 1985860. [Google Scholar] [CrossRef]
- Wei, Y.S.; Javed, T.; Liu, T.T.; Ali, A.; Gao, S.J. Mechanisms of Abscisic Acid (ABA)-Mediated Plant Defense Responses: An Updated Review. Plant Stress 2025, 15, 100724. [Google Scholar] [CrossRef]
- Sun, Q.; Liu, X.; Kitagawa, Y.; Calamita, G.; Ding, X. Plant Aquaporins: Their Roles Beyond Water Transport. Crop J. 2024, 12, 641–655. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, L.; Dong, H. Plant Aquaporins in Infection by and Immunity Against Pathogens—A Critical Review. Front. Plant Sci. 2019, 10, 632. [Google Scholar] [CrossRef]
- Mukherjee, S.; Roy, S.; Corpas, F.J. Aquaporins: A Vital Nexus in H2O2-Gasotransmitter Signaling. Trends Plant Sci. 2024, 29, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Mur, L.A.J.; Simpson, C.; Kumari, A.; Gupta, A.K.; Gupta, K.J. Moving Nitrogen to the Centre of Plant Defence Against Pathogens. Ann. Bot. 2017, 119, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Molina-Moya, E.; Terrón-Camero, L.C.; Pescador-Azofra, L.; Sandalio, L.M.; Romero-Puertas, M.C. Reactive Oxygen Species and Nitric Oxide Production, Regulation and Function during Defense Response. In Reactive Oxygen, Nitrogen and Sulfur Species in Plants: Production, Metabolism, Signaling and Defense Mechanisms; Hasanuzzaman, M., Fotopoulos, V., Nahar, K., Fujita, M., Eds.; John Wiley and Sons Ltd.: Hoboken, NJ, USA, 2019; Volume 1, pp. 573–590. ISBN 9781119468677. [Google Scholar]
- Chang, Q.; Liu, J.; Lin, X.; Hu, S.; Yang, Y.; Li, D.; Chen, L.; Huai, B.; Huang, L.; Voegele, R.T.; et al. A Unique Invertase Is Important for Sugar Absorption of an Obligate Biotrophic Pathogen During Infection. New Phytol. 2017, 215, 1548–1561. [Google Scholar] [CrossRef] [PubMed]
- Scharte, J.; Schön, H.; Weis, E. Photosynthesis and Carbohydrate Metabolism in Tobacco Leaves during an Incompatible Interaction with Phytophthora nicotianae. Plant Cell Environ. 2005, 28, 1421–1435. [Google Scholar] [CrossRef]
- Liu, Y.-H.; Song, Y.-H.; Ruan, Y.-L. Sugar Conundrum in Plant–Pathogen Interactions: Roles of Invertase and Sugar Transporters Depend on Pathosystems. J. Exp. Bot. 2022, 73, 1910–1925. [Google Scholar] [CrossRef]
- Qian, Y.; Tan, D.X.; Reiter, R.J.; Shi, H. Comparative Metabolomic Analysis Highlights the Involvement of Sugars and Glycerol in Melatonin-Mediated Innate Immunity Against Bacterial Pathogen in Arabidopsis. Sci. Rep. 2015, 5, 15815. [Google Scholar] [CrossRef]
- Bilgin, D.D.; Zavala, J.; Zhu, J.; Clough, S.J.; Ort, D.R.; DeLucia, E.H. Biotic Stress Globally Downregulates Photosynthesis Genes. Plant, Cell Environ. 2010, 33, 1597–1613. [Google Scholar] [CrossRef]
- Savage, Z.; Duggan, C.; Toufexi, A.; Pandey, P.; Liang, Y.; Segretin, M.E.; Yuen, L.H.; Gaboriau, D.C.A.; Leary, A.Y.; Tumtas, Y.; et al. Chloroplasts Alter Their Morphology and Accumulate at the Pathogen Interface During Infection by Phytophthora infestans. Plant J. 2021, 107, 1771–1787. [Google Scholar] [CrossRef]
- Swarbrick, P.J.; Schulze-Lefert, P.; Scholes, J.D. Metabolic Consequences of Susceptibility and Resistance (Race-Specific and Broad-Spectrum) in Barley Leaves Challenged with Powdery Mildew. Plant Cell Environ. 2006, 29, 1061–1076. [Google Scholar] [CrossRef]
- Kopczewski, T.; Kuźniak, E.; Kornaś, A.; Rut, G.; Nosek, M.; Ciereszko, I.; Szczepaniak, L. Local and Systemic Changes in Photosynthetic Parameters and Antioxidant Activity in Cucumber Challenged with Pseudomonas syringae Pv. Lachrymans. Int. J. Mol. Sci. 2020, 21, 6378. [Google Scholar] [CrossRef]
- Kanwar, P.; Jha, G. Alterations in Plant Sugar Metabolism: Signatory of Pathogen Attack. Planta 2019, 249, 305–318. [Google Scholar] [CrossRef]
- Kangasjärvi, S.; Neukermans, J.; Li, S.; Aro, E.M.; Noctor, G. Photosynthesis, Photorespiration, and Light Signalling in Defence Responses. J. Exp. Bot. 2012, 63, 1619–1636. [Google Scholar] [CrossRef]
- Foissner, I.; Wendehenne, D.; Langebartels, C.; Durner, J. In Vivo Imaging of Elicitor-Induced Nitric Oxide Burst in Tobacco. Plant J. 2000, 23, 817–824. [Google Scholar] [CrossRef]
- Giulietti, S.; Bigini, V.; Savatin, D.V. ROS and RNS Production, Subcellular Localization, and Signaling Triggered by Immunogenic Danger Signals. J. Exp. Bot. 2024, 75, 4512–4534. [Google Scholar] [CrossRef]
- Liu, J.; Gong, P.; Lu, R.; Lozano-Durán, R.; Zhou, X.; Li, F. Chloroplast Immunity: A Cornerstone of Plant Defense. Mol. Plant 2024, 17, 686–688. [Google Scholar] [CrossRef] [PubMed]
- Misra, A.N.; Vladkova, R.; Singh, R.; Misra, M.; Dobrikova, A.G.; Apostolova, E.L. Action and Target Sites of Nitric Oxide in Chloroplasts. Nitric Oxide-Biol. Chem. 2014, 39, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; El-Shetehy, M.; Shine, M.B.; Yu, K.; Navarre, D.; Wendehenne, D.; Kachroo, A.; Kachroo, P. Free Radicals Mediate Systemic Acquired Resistance. Cell Rep. 2014, 7, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Tanou, G.; Job, C.; Belghazi, M.; Molassiotis, A.; Diamantidis, G.; Job, D. Proteomic Signatures Uncover Hydrogen Peroxide and Nitric Oxide Cross-Talk Signaling Network in Citrus Plants. J. Proteome Res. 2010, 9, 5994–6006. [Google Scholar] [CrossRef]
- Hu, J.; Huang, X.; Chen, L.; Sun, X.; Lu, C.; Zhang, L.; Wang, Y.; Zuo, J. Site-Specific Nitrosoproteomic Identification of Endogenously S-Nitrosylated Proteins in Arabidopsis. Plant Physiol. 2015, 167, 1731–1746. [Google Scholar] [CrossRef]
- Karpiński, S.; Szechyńska-Hebda, M.; Wituszyńska, W.; Burdiak, P. Light Acclimation, Retrograde Signalling, Cell Death and Immune Defences in Plants. Plant Cell Environ. 2013, 36, 736–744. [Google Scholar] [CrossRef]
- Ballaré, C.L. Light Regulation of Plant Defense. Annu. Rev. Plant Biol. 2014, 65, 335–363. [Google Scholar] [CrossRef]
- Kamran, M.; Burdiak, P.; Karpiński, S. Crosstalk Between Abiotic and Biotic Stresses Responses and the Role of Chloroplast Retrograde Signaling in the Cross-Tolerance Phenomena in Plants. Cells 2025, 14, 176. [Google Scholar] [CrossRef]
- Lee, M.S.; Methela, N.J.; Lee, G.H.; Mun, B.G. Nitric Oxide and Melatonin Cross Talk on Photosynthetic Machinery. Molecules 2025, 30, 2148. [Google Scholar] [CrossRef] [PubMed]
- Nosek, M.; Kornaś, A.; Kuźniak, E.; Miszalski, Z. Plastoquinone Redox State Modifies Plant Response to Pathogen. Plant Physiol. Biochem. 2015, 96, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Ageeva-Kieferle, A.; Georgii, E.; Winkler, B.; Ghirardo, A.; Albert, A.; Hüther, P.; Mengel, A.; Becker, C.; Schnitzler, J.P.; Durner, J.; et al. Nitric Oxide Coordinates Growth, Development, and Stress Response via Histone Modification and Gene Expression. Plant Physiol. 2021, 187, 336–360. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Oliveira, P.J.; Oliveira, H.C.; Kolbert, Z.; Freschi, L. The Light and Dark Sides of Nitric Oxide: Multifaceted Roles of Nitric Oxide in Plant Responses to Light. J. Exp. Bot. 2021, 72, 885–903. [Google Scholar] [CrossRef]
- Vladkova, R.; Dobrikova, A.G.; Singh, R.; Misra, A.N.; Apostolova, E.L. Photoelectron Transport Ability of Chloroplast Thylakoid Membranes Treated with NO Donor SNP: Changes in Flash Oxygen Evolution and Chlorophyll Fluorescence. Nitric Oxide 2011, 24, 84–90. [Google Scholar] [CrossRef]
- Takahashi, S.; Yamasaki, H. Reversible Inhibition of Photophosphorylation in Chloroplasts by Nitric Oxide. FEBS Lett. 2002, 512, 145–148. [Google Scholar] [CrossRef]
- Wodala, B.; Deák, Z.; Vass, I.; Erdei, L.; Altorjay, I.; Horváth, F. In Vivo Target Sites of Nitric Oxide in Photosynthetic Electron Transport as Studied by Chlorophyll Fluorescence in Pea Leaves. Plant Physiol. 2008, 146, 1920–1927. [Google Scholar] [CrossRef]
- Saini, D.; Bapatla, R.B.; Pandey, J.; Aswani, V.; Sunil, B.; Gahir, S.; Bharath, P.; Subramanyam, R.; Raghavendra, A.S. Modulation by S-Nitrosoglutathione (a Natural Nitric Oxide Donor) of Photosystem in Pisum Sativum Leaves, as Revealed by Chlorophyll Fluorescence: Light-Dependent Aggravation of Nitric Oxide Effects. Plant Sci. Today 2023, 10, 393–398. [Google Scholar] [CrossRef]
- Sunil, B.; Rajsheel, P.; Aswani, V.; Bapatla, R.B.; Talla, S.K.; Raghavendra, A.S. Photosynthesis Is Sensitive to Nitric Oxide and Respiration Sensitive to Hydrogen Peroxide: Studies with Pea Mesophyll Protoplasts. J. Plant Physiol. 2020, 246–247, 153133. [Google Scholar] [CrossRef] [PubMed]
- Rashkov, G.D.; Stefanov, M.A.; Yotsova, E.K.; Borisova, P.B.; Dobrikova, A.G.; Apostolova, E.L. Impact of Sodium Nitroprusside on the Photosynthetic Performance of Maize and Sorghum. Plants 2024, 13, 118. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Oku, H.; Nahar, K.; Bhuyan, M.H.M.B.; Mahmud, J.A.; Baluska, F.; Fujita, M. Nitric Oxide-Induced Salt Stress Tolerance in Plants: ROS Metabolism, Signaling, and Molecular Interactions. Plant Biotechnol. Rep. 2018, 12, 77–92. [Google Scholar] [CrossRef]
- Hu, L.; Gao, X.; Li, Y.; Lyu, J.; Xiao, X.; Zhang, G.; Yu, J. Nitric Oxide Induced by Ammonium/Nitrate Ratio Ameliorates Low-Light Stress in Brassica Pekinesis: Regulation of Photosynthesis and Root Architecture. Int. J. Mol. Sci. 2023, 24, 7271. [Google Scholar] [CrossRef]
- Fatma, M.; Masood, A.; Per, T.S.; Khan, N.A. Nitric Oxide Alleviates Salt Stress Inhibited Photosynthetic Performance by Interacting with Sulfur Assimilation in Mustard. Front. Plant Sci. 2016, 7, 521. [Google Scholar] [CrossRef]
- Rashkov, G.D.; Stefanov, M.A.; Yotsova, E.K.; Borisova, P.B.; Dobrikova, A.G.; Apostolova, E.L. Exploring Nitric Oxide as a Regulator in Salt Tolerance: Insights into Photosynthetic Efficiency in Maize. Plants 2024, 13, 1312. [Google Scholar] [CrossRef]
- Afzal, M.K.; Habib, N.; Ashraf, M.A.; Ali, Q.; Ali, S. Nitric Oxide and Hydrogen Peroxide Coordinate to Improve Photosynthesis, Oxidative Defense, Osmoregulation, and Ions Homeostasis in Pea (Pisum sativum L.) Under Drought. J. Soil Sci. Plant Nutr. 2025, 25, 2534–2558. [Google Scholar] [CrossRef]
- Fejes, G.; Bodor, T.; Szőllősi, R.; Kondak, S.; Kutasi, K.; Fotopoulos, V.; Kolbert, Z. Nitric Oxide as an Integral Element in Priming-Induced Tolerance and Plant Stress Memory. J. Exp. Bot. 2025, eraf033. [Google Scholar] [CrossRef]
- Martínez-Medina, A.; Pescador, L.; Terrón-Camero, L.C.; Pozo, M.J.; Romero-Puertas, M.C. Nitric Oxide in Plant-Fungal Interactions. J. Exp. Bot. 2019, 70, 4489–4503. [Google Scholar] [CrossRef]
- Lum, H.K.; Lee, C.H.; Butt, Y.K.C.; Lo, S.C.L. Sodium Nitroprusside Affects the Level of Photosynthetic Enzymes and Glucose Metabolism in Phaseolus aureus (Mung bean). Nitric Oxide-Biol. Chem. 2005, 12, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Abat, J.K.; Mattoo, A.K.; Deswal, R. S-Nitrosylated Proteins of a Medicinal CAM Plant Kalanchoe pinnata—Ribulose-1,5-Bisphosphate Carboxylase/Oxygenase Activity Targeted for Inhibition. FEBS J. 2008, 275, 2862–2872. [Google Scholar] [CrossRef] [PubMed]
- Michelet, L.; Zaffagnini, M.; Morisse, S.; Sparla, F.; Pérez-Pérez, M.E.; Francia, F.; Danon, A.; Marchand, C.H.; Fermani, S.; Trost, P.; et al. Redox Regulation of the Calvin-Benson Cycle: Something Old, Something New. Front. Plant Sci. 2013, 4, 470. [Google Scholar] [CrossRef] [PubMed]
- Lindermayr, C.; Saalbach, G.; Durner, J. Proteomic Identification of S-Nitrosylated Proteins in Arabidopsis. Plant Physiol. 2005, 137, 921–930. [Google Scholar] [CrossRef]
- Mattioli, E.J.; Rossi, J.; Meloni, M.; De Mia, M.; Marchand, C.H.; Tagliani, A.; Fanti, S.; Falini, G.; Trost, P.; Lemaire, S.D.; et al. Structural Snapshots of Nitrosoglutathione Binding and Reactivity Underlying S-Nitrosylation of Photosynthetic GAPDH. Redox Biol. 2022, 54, 102387. [Google Scholar] [CrossRef]
- Jedelská, T.; Sedlářová, M.; Lochman, J.; Činčalová, L.; Luhová, L.; Petřivalský, M. Protein S-Nitrosation Differentially Modulates Tomato Responses to Infection by Hemi-Biotrophic Oomycetes of Phytophthora spp. Hortic. Res. 2021, 8, 34. [Google Scholar] [CrossRef]
- Biemelt, S.; Sonnewald, U. Plant-Microbe Interactions to Probe Regulation of Plant Carbon Metabolism. J. Plant Physiol. 2006, 163, 307–318. [Google Scholar] [CrossRef]
- Lemoine, R.; La Camera, S.; Atanassova, R.; Dédaldéchamp, F.; Allario, T.; Pourtau, N.; Bonnemain, J.L.; Laloi, M.; Coutos-Thévenot, P.; Maurousset, L.; et al. Source-to-Sink Transport of Sugar and Regulation by Environmental Factors. Front. Plant Sci. 2013, 4, 272. [Google Scholar] [CrossRef]
- Lapin, D.; Van den Ackerveken, G. Susceptibility to Plant Disease: More than a Failure of Host Immunity. Trends Plant Sci. 2013, 18, 546–554. [Google Scholar] [CrossRef]
- Tauzin, A.S.; Giardina, T. Sucrose and Invertases, a Part of the Plant Defense Response to the Biotic Stresses. Front. Plant Sci. 2014, 5, 293. [Google Scholar] [CrossRef]
- Kopczewski, T.; Kuźniak, E.; Ciereszko, I.; Kornaś, A. Alterations in Primary Carbon Metabolism in Cucumber Infected with Pseudomonas syringae Pv. Lachrymans: Local and Systemic Responses. Int. J. Mol. Sci. 2022, 23, 12418. [Google Scholar] [CrossRef]
- Tanotra, S.; Zhawar, V.K.; Sharma, S. Regulation of Antioxidant Enzymes and Invertases by Hydrogen Peroxide and Nitric Oxide Under ABA and Water-Deficit Stress in Wheat. Agric. Res. 2019, 8, 441–451. [Google Scholar] [CrossRef]
- Ganjewala, D.; Nagaraja, C.; Nayak, M.R.; Asha Devi, S. Effects of Sodium Nitroprusside on Activity of Acid and Alkaline Invertases and Alkaline Phosphatase in Lemongrass (Cymbopogon flexuosus steud) Wats. Int. J. Plant Biol. 2010, 1, e2. [Google Scholar] [CrossRef][Green Version]
- Wu, P.; Xiao, C.; Cui, J.; Hao, B.; Zhang, W.; Yang, Z.; Ahammed, G.J.; Liu, H.; Cui, H. Nitric Oxide and Its Interaction with Hydrogen Peroxide Enhance Plant Tolerance to Low Temperatures by Improving the Efficiency of the Calvin Cycle and the Ascorbate–Glutathione Cycle in Cucumber Seedlings. J. Plant Growth Regul. 2021, 40, 2390–2408. [Google Scholar] [CrossRef]
- Zhang, Z.W.; Luo, S.; Zhang, G.C.; Feng, L.Y.; Zheng, C.; Zhou, Y.H.; Du, J.B.; Yuan, M.; Chen, Y.E.; Wang, C.Q.; et al. Nitric Oxide Induces Monosaccharide Accumulation Through Enzyme S-Nitrosylation. Plant Cell Environ. 2017, 40, 1834–1848. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Z.; Wu, X.; Fang, H.; Huang, D.; Pan, X.; Liao, W. Role of Protein S-Nitrosylation in Plant Growth and Development. Plant Cell Rep. 2024, 43, 204. [Google Scholar] [CrossRef]
- Jahnová, J.; Luhová, L.; Petřivalský, M. S-Nitrosoglutathione Reductase-the Master Regulator of Protein S-Nitrosation in Plant NO Signaling. Plants 2019, 8, 48. [Google Scholar] [CrossRef]
- Jiménez, A.; Martí, M.C.; Sevilla, F. Oxidative Post-Translational Modifications of Plant Antioxidant Systems Under Environmental Stress. Physiol. Plant. 2025, 177, e70118. [Google Scholar] [CrossRef]
- Pande, A.; Mun, B.G.; Rahim, W.; Khan, M.; Lee, D.S.; Lee, G.M.; Al Azzawi, T.N.I.; Hussain, A.; Kim, C.K.; Yun, B.W. Phytohormonal Regulation Through Protein S-Nitrosylation Under Stress. Front. Plant Sci. 2022, 13, 865542. [Google Scholar] [CrossRef]
- Machchhu, F.; Wany, A. Protein S-Nitrosylation in Plants Under Biotic Stress. Theor. Exp. Plant Physiol. 2023, 35, 331–339. [Google Scholar] [CrossRef]
- Khator, K.; Parihar, S.; Jasik, J.; Shekhawat, G.S. Nitric Oxide in Plants: An Insight on Redox Activity and Responses Toward Abiotic Stress Signaling. Plant Signal. Behav. 2024, 19, 2298053. [Google Scholar] [CrossRef]
- Romero-Puertas, M.C.; Campostrini, N.; Mattè, A.; Righetti, P.G.; Perazzolli, M.; Zolla, L.; Roepstorff, P.; Delledonne, M. Proteomic Analysis of S-Nitrosylated Proteins in Arabidopsis thaliana Undergoing Hypersensitive Response. Proteomics 2008, 8, 1459–1469. [Google Scholar] [CrossRef]
- Hurley, B.; Subramaniam, R.; Guttman, D.S.; Desveaux, D. Proteomics of Effector-Triggered Immunity (ETI) in Plants. Virulence 2014, 5, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Ling, T.; Bellin, D.; Vandelle, E.; Imanifard, Z.; Delledonne, M. Host-Mediated S-Nitrosylation Disarms the Bacterial Effector HopAI1 to Reestablish Immunity. Plant Cell 2017, 29, 2871–2881. [Google Scholar] [CrossRef] [PubMed]
- Withers, J.; Dong, X. Posttranslational Modifications of NPR1: A Single Protein Playing Multiple Roles in Plant Immunity and Physiology. PLoS Pathog. 2016, 12, e1005707. [Google Scholar] [CrossRef] [PubMed]
- Roychowdhury, R.; Mishra, S.; Anand, G.; Dalal, D.; Gupta, R.; Kumar, A.; Gupta, R. Decoding the Molecular Mechanism Underlying Salicylic Acid (SA)-Mediated Plant Immunity: An Integrated Overview from Its Biosynthesis to the Mode of Action. Physiol. Plant. 2024, 176, e14399. [Google Scholar] [CrossRef]
- Tada, Y.; Spoel, S.H.; Pajerowska-Mukhtar, K.; Mou, Z.; Song, J.; Wang, C.; Zuo, J.; Dong, X. Plant Immunity Requires Conformational Charges of NPR1 via S-Nitrosylation and Thioredoxins. Science 2008, 321, 952–956. [Google Scholar] [CrossRef]
- Kohli, S.K.; Khanna, K.; Bhardwaj, R.; Corpas, F.J.; Ahmad, P. Nitric Oxide, Salicylic Acid and Oxidative Stress: Is It a Perfect Equilateral Triangle? Plant Physiol. Biochem. 2022, 184, 56–64. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Feechan, A.; Yun, B.W.; Shafiei, R.; Hofmann, A.; Taylor, P.; Xue, P.; Yang, F.Q.; Xie, Z.S.; Pallas, J.A.; et al. S-Nitrosylation of AtSABP3 Antagonizes the Expression of Plant Immunity. J. Biol. Chem. 2009, 284, 2131–2137. [Google Scholar] [CrossRef]
- Pande, A.; Mun, B.G.; Khan, M.; Rahim, W.; Lee, D.S.; Lee, G.M.; Al Azawi, T.N.I.; Hussain, A.; Yun, B.W. Nitric Oxide Signaling and Its Association with Ubiquitin-Mediated Proteasomal Degradation in Plants. Int. J. Mol. Sci. 2022, 23, 1657. [Google Scholar] [CrossRef]
- De Marchi, R.; Sorel, M.; Mooney, B.; Fudal, I.; Goslin, K.; Kwaśniewska, K.; Ryan, P.T.; Pfalz, M.; Kroymann, J.; Pollmann, S.; et al. The N-End Rule Pathway Regulates Pathogen Responses in Plants. Sci. Rep. 2016, 6, 26020. [Google Scholar] [CrossRef]
- Skelly, M.J.; Malik, S.I.; Le Bihan, T.; Bo, Y.; Jiang, J.; Spoel, S.H.; Loake, G.J. A Role for S-Nitrosylation of the SUMO-Conjugating Enzyme SCE1 in Plant Immunity. Proc. Natl. Acad. Sci. USA 2019, 116, 17090–17095. [Google Scholar] [CrossRef]
- Cui, B.; Pan, Q.; Clarke, D.; Villarreal, M.O.; Umbreen, S.; Yuan, B.; Shan, W.; Jiang, J.; Loake, G.J. S-Nitrosylation of the Zinc Finger Protein SRG1 Regulates Plant Immunity. Nat. Commun. 2018, 9, 4226. [Google Scholar] [CrossRef]
- Rosnoblet, C.; Bègue, H.; Blanchard, C.; Pichereaux, C.; Besson-Bard, A.; Aimé, S.; Wendehenne, D. Functional Characterization of the Chaperon-like Protein Cdc48 in Cryptogein-Induced Immune Response in Tobacco. Plant Cell Environ. 2017, 40, 491–508. [Google Scholar] [CrossRef]
- Shan, Q.; Zhao, D.; Cao, B.; Zhu, X.; Wang, C.; Deng, L.; Li, C.; Zhang, Y.; Shi, Q.; Gong, B. Jasmonic Acid and Nitric Oxide Orchestrate a Hierarchical Melatonin Cascade for Botrytis cinerea Resistance in Tomato. Plant Physiol. 2025, 197, kiaf078. [Google Scholar] [CrossRef]
- Palmieri, C.M.; Lindermayr, C.; Bauwe, H.; Steinhauser, C.; Durner, J. Regulation of Plant Glycine Decarboxylase by S-Nitrosylation and Glutathionylation. Plant Physiol. 2010, 152, 1514–1528. [Google Scholar] [CrossRef]
- Maldonado-Alconada, A.M.; Echevarría-Zomeño, S.; Lindermayr, C.; Redondo-López, I.; Durner, J.; Jorrín-Novo, J.V. Proteomic Analysis of Arabidopsis Protein S-Nitrosylation in Response to Inoculation with Pseudomonas syringae. Acta Physiol. Plant. 2011, 33, 1493–1514. [Google Scholar] [CrossRef]
- Romero-Puertas, M.C.; Laxa, M.; Mattè, A.; Zaninotto, F.; Finkemeier, I.; Jones, A.M.E.; Perazzolli, M.; Vandelle, E.; Dietz, K.J.; Delledonne, M. S-Nitrosylation of Peroxiredoxin II E Promotes Peroxynitrite-Mediated Tyrosine Nitration. Plant Cell 2007, 19, 4120–4130. [Google Scholar] [CrossRef] [PubMed]
- Serrato, A.J.; Romero-Puertas, M.C.; Lázaro-Payo, A.; Sahrawy, M. Regulation by S-Nitrosylation of the Calvin-Benson Cycle Fructose-1,6-Bisphosphatase in Pisum sativum. Redox Biol. 2018, 14, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Holzmeister, C.; Fröhlich, A.; Sarioglu, H.; Bauer, N.; Durner, J.; Lindermayr, C. Proteomic Analysis of Defense Response of Wildtype Arabidopsis thaliana and Plants with Impaired NO- Homeostasis. Proteomics 2011, 11, 1664–1683. [Google Scholar] [CrossRef]
- Rui, L.; Kang, P.; Shao, J.; Lu, M.; Cui, B.; Zhao, Y.; Wang, W.; Cai, H.; Tang, D.; Loake, G.J.; et al. The Chloroplast-localized Casein Kinase II α Subunit, CPCK2, Negatively Regulates Plant Innate Immunity Through Promoting S-nitrosylation of SABP3. Plant J. 2024, 120, 552–568. [Google Scholar] [CrossRef]
- Wang, D.; Guo, H.; Gong, X.; Chen, L.; Lin, H.; Wang, S.; Feng, T.; Yi, Y.; Wang, W.; Yang, S.; et al. Nitric Oxide Controls Stomatal Development and Stress Responses by Inhibiting MPK6 Phosphorylation via S-Nitrosylation in Arabidopsis. Dev. Cell 2025, 60, 1–14. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuźniak, E.; Ciereszko, I. Nitric Oxide and Photosynthesis Interplay in Plant Interactions with Pathogens. Int. J. Mol. Sci. 2025, 26, 6964. https://doi.org/10.3390/ijms26146964
Kuźniak E, Ciereszko I. Nitric Oxide and Photosynthesis Interplay in Plant Interactions with Pathogens. International Journal of Molecular Sciences. 2025; 26(14):6964. https://doi.org/10.3390/ijms26146964
Chicago/Turabian StyleKuźniak, Elżbieta, and Iwona Ciereszko. 2025. "Nitric Oxide and Photosynthesis Interplay in Plant Interactions with Pathogens" International Journal of Molecular Sciences 26, no. 14: 6964. https://doi.org/10.3390/ijms26146964
APA StyleKuźniak, E., & Ciereszko, I. (2025). Nitric Oxide and Photosynthesis Interplay in Plant Interactions with Pathogens. International Journal of Molecular Sciences, 26(14), 6964. https://doi.org/10.3390/ijms26146964






