Rethinking Osteoporosis Drugs: Can We Simultaneously Address Sarcopenia?
Abstract
1. Introduction
2. The Bone–Muscle System
3. Preventive Measures and Non-Pharmacological Approaches for Osteosarcopenia
4. Therapeutic Treatment Approaches
4.1. Denosumab
| Cohort | Study | Regime | Effects on Bone | Effects on Muscle | Ref. |
|---|---|---|---|---|---|
| 135 elderly osteoporotic patients without fractures | Longitudinal, multicenter, controlled, prospective study | 60 mg every 6 months for 5 years (+ Ca and vitamin D) | Increase in BMD (spine and hip) and decreased fraction risk | Improved grip strength (+4.3 kg), TUG test (1.5 s) and gait speed (0.1 m/s), which significantly worsened after discontinuation of Denosumab treatment | [88] |
| 60 postmenopausal osteoporotic Korean women | Prospective multicenter cohort study | 60 mg every 6 months for 3 years (+ Ca and vitamin D) | Increase in BMD in lumbar spine (9.7%) and hip (5.1%) | Significant increase in fat-free mass (3.6%) | [89] |
| 18 postmenopausal osteoporotic women (mean age 65.0 ± 1.5 years) | Retrospective—GERICO | 60 mg every 6 months for an average of 3 years | Increase in lumbar spine BMD (0.12 ± 0.29 g/cm2) | Significant increase in ALM (0.66 ± 2.2 kg) and in handgrip strength (3.22 ± 10.0 kg) | [83] |
| 60 osteoporotic or osteopenic patients | Retrospective, propensity-score-matched cohort study | 60 mg every 6 months for an average of 1.5 years (+ vitamin D) | Annual increase in femoral (+1.83%) and spinal BMD (3.30%) | Significant annual increase in grip strength (+5.14%) and in chair-rising test force (+8.20%); no change in chair-rising test time | [85] |
| 51 community-dwelling elderly patients (≥65 yo) with history or risk of falls and/or fractures | Longitudinal, prospective | 60 mg, follow up after 6 months (+ vitamin D) | / | Improved gait speed (0.06 m/s), TUG (1.7 s) and FSST (1.7 s); slight improvement in SPPB score (1.1 points) | [84] |
| 78 men and 123 women with osteoporosis aged ≥ 65 years | Two-year, double-blind, placebo-controlled, randomized trial—PROUD trial | 60 mg every 6 months for 2 years (+ Ca and vitamin D) | / | No statistically significant differences between the Denosumab and placebo groups in appendicular lean mass, chair stand performance, SPPB scores and gait speed | [86] |
4.2. Bisphosphonates
| Drug | Cohort | Study | Regime | Effects on Bone | Effects on Muscle | Ref. |
|---|---|---|---|---|---|---|
| Alendronate | 58 community-dwelling osteoporotic women ≥ 65 yo | Randomized, open-labelled, active-comparator | 35 mg/week, for 24 weeks | Increased lumbar BMD (3.9%) and femoral BMD (1.9%); decreased bone turnover markers (compared to baseline) | Increased dynamic balance, increased knee extension force (19%) and power (15%) and increased gait speed (2.6%); no effect on TUG, grip strength or appendicular muscle mass index (compared to baseline) | [102] |
| 199 osteoporotic patients (233 control patients) | Retrospective, case-controlled | 35 mg or 5 mg/week for 1 year | Retained bone mineral content (significantly decreased in control patients) | Increased skeletal muscle mass index (2.5-fold), appendicular skeletal muscle mass (2.5-fold), lower limb muscle mass (4.4-fold) and total fat mass | [104] | |
| 17 osteoporotic postmenopausal women ≥ 63 yo | Open-label, randomized, controlled | 35 mg/week for 6 months | / | No differences compared to baseline in alendronate-alone group in grip strength, back extensor strength, Iliopsoas muscle strength, static or dynamic postural balance or TUG test | [73] | |
| 38 osteoporotic postmenopausal women | Double-blind, placebo-controlled, randomized | 5 mg/day for 1 year | Increase in BMD in lumbar spine (3.5%) and femoral neck (1.3%) compared to placebo; no effect on radial bone mineral content | No effect on physical performance parameters, leg extensor power, dynamic balance and cardiorespiratory fitness (VO2max) | [111] | |
| 62 community-dwelling osteoporotic patients ≥ 80 yo (61 control patients) | Randomized, controlled, non-blind | 70 mg/week (+ Ca and alfacalcidol) for 18 months | Increased BMD in lumbar spine and femoral neck compared to baseline | No increase in muscle strength; decrease in TUG and gait speed | [112] | |
| 36 postmenopausal women with osteosarcopenia | Longitudinal study | 5 mg/day (+ calcitrol) for 6 months | Increased lumbar BMD (2.62%); no change in femur BMD | Improved handgrip strength (33.5%) | [103] | |
| 136 older patients | Longitudinal, multicenter, controlled, prospective study | 70 mg ALD/week for 5 years (+ Ca and vitamin D) | Improved spine and hip BMD; no significant change in falls risk | Improved TUG (0.8 s), 4 m walk test and gait speed (0.07 m/s), which persisted for up to 1 year after treatment discontinuation | [88] | |
| Risedronate | 91 osteopenic postmenopausal women (93 control) | Randomized, controlled | 150 mg/every 4 weeks (+ Ca and vitamin D) for 1 year | Increase in spine (1.9%), hip (0.9%) and femoral neck (0.09%) BMD compared to baseline | Increased body fat; small increase in total LBM (control patients lost total LBM) | [113,114] |
| Zoledronic acid | 62 older osteoporotic women ≥ 70 yo in long-term care communities | Double-blind, randomized, placebo-controlled | One 5 mg i.v. (+ Ca and vitamin D) | Increased spine (6%) and total hip (2.8%) BMD compared to baseline | No change in appendicular lean mass compared to control; slight decrease compared to baseline (−0.75%) | [115] |
| 136 older patients | Longitudinal, multicenter, controlled, prospective study | 5 mg/year for 3 years (+ Ca and vitamin D) | Improved spine and hip BMD; no significant change in falls risk | Improved TUG (0.7 s), 4 m walk test and gait speed (0.07 m/s), which persisted for up to 1 year after treatment discontinuation | [88] | |
| 1000 ambulant osteoporotic postmenopausal women > 65 yo | Double-blind, placebo-controlled | 4 5 mg i.v. in 18-month intervals for 6 years | Reduced risk of fractures | Reduced weight loss, no change in fat mass and higher loss of LBM compared to placebo | [116,117] | |
| 113 treated and 118 controls (both with osteoporosis) | Case–control retrospective cohort study | 5 mg/year for 3 years | Significantly improved BMD | Significantly improved appendicular skeletal muscle mass and appendicular skeletal muscle index | [101] | |
| 28 community-dwelling elderly patients (≥65 yo) with history or risk of falls and/or fractures | Longitudinal, prospective | 5 mg i.v., follow up after 6 months (+ vitamin D) | / | Improved gait speed (0.1 m/s) and TUG (1.6 s) | [84] | |
| Ibandronate | Children and adolescents (7–16 yo) with osteogenesis imperfecta | Longitudinal | 3 mg/kg body weight i.v. every 4 months for 2–4 years | Increase in lumbar spine BMD and vertebral area and decreased fracture rate | Increased grip force, median mobility score and self-care score | [118,119,120] |
4.3. Steroid Hormones and Hormone Replacement Therapies
4.3.1. Testosterone
4.3.2. Estrogens
4.3.3. Selective Estrogen Receptor Modulators
4.4. Teriparatide and Abaloparatide
4.5. Romosozumab
5. Myostatin Inhibitors
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 25(OH)D | 25-Hydroxy-vitamin D (calciferol) |
| ABL | Abaloparatide |
| BMD | Bone mineral density |
| BMI | Body mass index |
| BZD | Bazedoxifene (SERM drug) |
| DHEA | Dehydroepiandrosterone |
| EMA | European Medicines Agency |
| ERα/β | Estrogen receptor-alpha/beta |
| FDA | Food and Drug Administration |
| FGF | Basic fibroblast growth factor |
| FSST | Four-Square Step Test |
| HRT | Hormone replacement therapy |
| LBM | Lean body mass |
| IL-6 | Interleukin 6 |
| IGF-1 | Insulin-like growth factor-1 |
| MSCs | Mesenchymal stem cells |
| NF-κB | Nuclear factor-kappa B transcription factor |
| OPG | Osteoprotegerin |
| PTH | Teriparatide |
| RANKL | Nuclear factor-kappa B (NF-κB) receptor activator ligand |
| SERMs | Selective estrogen receptor modulators |
| SMA | Spinal muscle atrophy |
| SOST | Sclerostin |
| SPPB | Short physical performance battery |
| TUG | Timed Up and Go muscle performance test |
References
- Binkley, N.; Buehring, B. Beyond FRAX®: It’s Time to Consider “Sarco-Osteopenia”. J. Clin. Densitom. 2009, 12, 413–416. [Google Scholar] [CrossRef]
- Kirk, B.; Zanker, J.; Duque, G. Osteosarcopenia: Epidemiology, diagnosis, and treatment—Facts and numbers. J. Cachexia Sarcopenia Muscle 2020, 11, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Li, C.; Chen, F.; Xie, D.; Yang, C.; Chen, Y.; Wang, J.; Li, J.; Zheng, F. Prevalence and risk factors of osteosarcopenia: A systematic review and meta-analysis. BMC Geriatr. 2023, 23, 369. [Google Scholar] [CrossRef] [PubMed]
- Hernlund, E.; Svedbom, A.; Ivergård, M.; Compston, J.; Cooper, C.; Stenmark, J.; McCloskey, E.V.; Jönsson, B.; Kanis, J.A. Osteoporosis in the European Union: Medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch. Osteoporos. 2013, 8, 136. [Google Scholar] [CrossRef]
- Inoue, T.; Maeda, K.; Nagano, A.; Shimizu, A.; Ueshima, J.; Murotani, K.; Sato, K.; Hotta, K.; Morishita, S.; Tsubaki, A. Related Factors and Clinical Outcomes of Osteosarcopenia: A Narrative Review. Nutrients 2021, 13, 291. [Google Scholar] [CrossRef]
- Greco, E.A.; Pietschmann, P.; Migliaccio, S. Osteoporosis and Sarcopenia Increase Frailty Syndrome in the Elderly. Front. Endocrinol. 2019, 10, 255. [Google Scholar] [CrossRef]
- Zanker, J.; Duque, G. Osteoporosis in Older Persons: Old and New Players. J. Am. Geriatr. Soc. 2019, 67, 831–840. [Google Scholar] [CrossRef]
- Anker, S.D.; Morley, J.E.; von Haehling, S. Welcome to the ICD-10 code for sarcopenia. J. Cachexia Sarcopenia Muscle 2016, 7, 512–514. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Buckinx, F.; Landi, F.; Cesari, M.; Fielding, R.A.; Visser, M.; Engelke, K.; Maggi, S.; Dennison, E.; Al-Daghri, N.M.; Allepaerts, S.; et al. Pitfalls in the measurement of muscle mass: A need for a reference standard. J. Cachexia Sarcopenia Muscle 2018, 9, 269–278. [Google Scholar] [CrossRef]
- McGregor, R.A.; Cameron-Smith, D.; Poppitt, S.D. It is not just muscle mass: A review of muscle quality, composition and metabolism during ageing as determinants of muscle function and mobility in later life. Longev. Heal. 2014, 3, 9. [Google Scholar] [CrossRef]
- Calvani, R.; Marini, F.; Cesari, M.; Tosato, M.; Picca, A.; Anker, S.D.; von Haehling, S.; Miller, R.R.; Bernabei, R.; Landi, F.; et al. Biomarkers for physical frailty and sarcopenia. Aging Clin. Exp. Res. 2017, 29, 29–34. [Google Scholar] [CrossRef]
- Kaji, H. Interaction between Muscle and Bone. J. Bone Metab. 2014, 21, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Khrystoforova, I.; Liu, C.-T.; Karasik, D. Genetics of osteosarcopenia. In Osteosarcopenia; Duque, G., Troen, B.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; Chapter 13; pp. 217–238. ISBN 978-0-12-820088-9. [Google Scholar]
- Kirk, B.; Feehan, J.; Lombardi, G.; Duque, G. Muscle, Bone, and Fat Crosstalk: The Biological Role of Myokines, Osteokines, and Adipokines. Curr. Osteoporos. Rep. 2020, 18, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Dankbar, B.; Fennen, M.; Brunert, D.; Hayer, S.; Frank, S.; Wehmeyer, C.; Beckmann, D.; Paruzel, P.; Bertrand, J.; Redlich, K.; et al. Myostatin is a direct regulator of osteoclast differentiation and its inhibition reduces inflammatory joint destruction in mice. Nat. Med. 2015, 21, 1085–1090. [Google Scholar] [CrossRef]
- Qin, Y.; Peng, Y.; Zhao, W.; Pan, J.; Ksiezak-Reding, H.; Cardozo, C.; Wu, Y.; Divieti Pajevic, P.; Bonewald, L.F.; Bauman, W.A.; et al. Myostatin inhibits osteoblastic differentiation by suppressing osteocyte-derived exosomal microRNA-218: A novel mechanism in muscle-bone communication. J. Biol. Chem. 2017, 292, 11021–11033. [Google Scholar] [CrossRef]
- Crane, J.L.; Zhao, L.; Frye, J.S.; Xian, L.; Qiu, T.; Cao, X. IGF-1 Signaling is Essential for Differentiation of Mesenchymal Stem Cells for Peak Bone Mass. Bone Res. 2013, 1, 186–194. [Google Scholar] [CrossRef]
- Charoenlarp, P.; Rajendran, A.K.; Iseki, S. Role of fibroblast growth factors in bone regeneration. Inflamm. Regen. 2017, 37, 10. [Google Scholar] [CrossRef]
- Liu, L.; Guo, J.; Chen, X.; Tong, X.; Xu, J.; Zou, J. The Role of Irisin in Exercise-Mediated Bone Health. Front. Cell Dev. Biol. 2021, 9, 668759. [Google Scholar] [CrossRef]
- Lu, W.; Xiao, W.; Xie, W.; Fu, X.; Pan, L.; Jin, H.; Yu, Y.; Zhang, Y.; Li, Y. The Role of Osteokines in Sarcopenia: Therapeutic Directions and Application Prospects. Front. Cell Dev. Biol. 2021, 9, 735374. [Google Scholar] [CrossRef]
- Verma, S.; Rajaratnam, J.H.; Denton, J.; Hoyland, J.A.; Byers, R.J. Adipocytic proportion of bone marrow is inversely related to bone formation in osteoporosis. J. Clin. Pathol. 2002, 55, 693–698. [Google Scholar] [CrossRef]
- Patsch, J.M.; Li, X.; Baum, T.; Yap, S.P.; Karampinos, D.C.; Schwartz, A.V.; Link, T.M. Bone marrow fat composition as a novel imaging biomarker in postmenopausal women with prevalent fragility fractures. J. Bone Miner. Res. 2013, 28, 1721–1728. [Google Scholar] [CrossRef]
- Hamrick, M.W.; McGee-Lawrence, M.E.; Frechette, D.M. Fatty Infiltration of Skeletal Muscle: Mechanisms and Comparisons with Bone Marrow Adiposity. Front. Endocrinol. 2016, 7, 69. [Google Scholar] [CrossRef]
- Rivas, D.A.; McDonald, D.J.; Rice, N.P.; Haran, P.H.; Dolnikowski, G.G.; Fielding, R.A. Diminished anabolic signaling response to insulin induced by intramuscular lipid accumulation is associated with inflammation in aging but not obesity. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2016, 310, R561–R569. [Google Scholar] [CrossRef]
- Nuttall, M.E.; Gimble, J.M. Is there a therapeutic opportunity to either prevent or treat osteopenic disorders by inhibiting marrow adipogenesis? Bone 2000, 27, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Al Saedi, A.; Bermeo, S.; Plotkin, L.; Myers, D.E.; Duque, G. Mechanisms of palmitate-induced lipotoxicity in osteocytes. Bone 2019, 127, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Ormsbee, M.J.; Prado, C.M.; Ilich, J.Z.; Purcell, S.; Siervo, M.; Folsom, A.; Panton, L. Osteosarcopenic obesity: The role of bone, muscle, and fat on health. J. Cachexia Sarcopenia Muscle 2014, 5, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Ren, R.; Ocampo, A.; Liu, G.-H.; Izpisua Belmonte, J.C. Regulation of Stem Cell Aging by Metabolism and Epigenetics. Cell Metab. 2017, 26, 460–474. [Google Scholar] [CrossRef]
- Teixeira, L.A.C.; Soares, L.A.; Parentoni, A.N.; Nobre, J.N.P.; Figueiredo, P.H.S.; Leopoldino, A.A.O.; Avelar, N.C.P.; Mendonça, V.A.; Lacerda, A.C.R. Inflammatory biomarkers of osteosarcopenia in community-dwelling older woman. Clin. Nutr. Open Sci. 2024, 55, 173–182. [Google Scholar] [CrossRef]
- Zha, L.; He, L.; Liang, Y.; Qin, H.; Yu, B.; Chang, L.; Xue, L. TNF-α contributes to postmenopausal osteoporosis by synergistically promoting RANKL-induced osteoclast formation. Biomed. Pharmacother. 2018, 102, 369–374. [Google Scholar] [CrossRef]
- Cai, L.; Lv, Y.; Yan, Q.; Guo, W. Cytokines: The links between bone and the immune system. Injury 2024, 55, 111203. [Google Scholar] [CrossRef]
- Englund, D.A.; Zhang, X.; Aversa, Z.; LeBrasseur, N.K. Skeletal Muscle Aging, Cellular Senescence, and Senotherapeutics: Current Knowledge and Future Directions. Mech. Ageing Dev. 2021, 200, 111595. [Google Scholar] [CrossRef]
- Eastell, R.; Rosen, C.J.; Black, D.M.; Cheung, A.M.; Murad, M.H.; Shoback, D. Pharmacological Management of Osteoporosis in Postmenopausal Women: An Endocrine Society. Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2019, 104, 1595–1622. [Google Scholar] [CrossRef]
- Dent, E.; Morley, J.E.; Cruz-Jentoft, A.J.; Arai, H.; Kritchevsky, S.B.; Guralnik, J.; Bauer, J.M.; Pahor, M.; Clark, B.C.; Cesari, M.; et al. International Clinical Practice Guidelines for Sarcopenia (ICFSR): Screening, Diagnosis and Management. J. Nutr. Health Aging 2018, 22, 1148–1161. [Google Scholar] [CrossRef] [PubMed]
- Cosman, F.; de Beur, S.J.; LeBoff, M.S.; Lewiecki, E.M.; Tanner, B.; Randall, S.; Lindsay, R. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporos. Int. 2014, 25, 2359–2381. [Google Scholar] [CrossRef] [PubMed]
- Qaseem, A.; Hicks, L.A.; Etxeandia-Ikobaltzeta, I.; Shamliyan, T.; Cooney, T.G. Clinical Guidelines Committee of the American College of Physicians Pharmacologic Treatment of Primary Osteoporosis or Low Bone Mass to Prevent Fractures in Adults: A Living Clinical Guideline from the American College of Physicians. Ann. Intern. Med. 2023, 176, 224–238. [Google Scholar] [CrossRef] [PubMed]
- Nowlan, N.C.; Bourdon, C.; Dumas, G.; Tajbakhsh, S.; Prendergast, P.J.; Murphy, P. Developing bones are differentially affected by compromised skeletal muscle formation. Bone 2010, 46, 1275–1285. [Google Scholar] [CrossRef]
- Wang, L.; You, X.; Zhang, L.; Zhang, C.; Zou, W. Mechanical regulation of bone remodeling. Bone Res. 2022, 10, 16. [Google Scholar] [CrossRef]
- Attwaters, M.; Hughes, S.M. Cellular and molecular pathways controlling muscle size in response to exercise. FEBS J. 2022, 289, 1428–1456. [Google Scholar] [CrossRef]
- Hirschfeld, H.P.; Kinsella, R.; Duque, G. Osteosarcopenia: Where bone, muscle, and fat collide. Osteoporos. Int. 2017, 28, 2781–2790. [Google Scholar] [CrossRef]
- Erlich, A.T.; Tryon, L.D.; Crilly, M.J.; Memme, J.M.; Moosavi, Z.S.M.; Oliveira, A.N.; Beyfuss, K.; Hood, D.A. Function of specialized regulatory proteins and signaling pathways in exercise-induced muscle mitochondrial biogenesis. Integr. Med. Res. 2016, 5, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Lippi, L.; de Sire, A.; Mezian, K.; Curci, C.; Perrero, L.; Turco, A.; Andaloro, S.; Ammendolia, A.; Fusco, N.; Invernizzi, M. Impact of exercise training on muscle mitochondria modifications in older adults: A systematic review of randomized controlled trials. Aging Clin. Exp. Res. 2022, 34, 1495–1510. [Google Scholar] [CrossRef] [PubMed]
- Konopka, A.R.; Douglass, M.D.; Kaminsky, L.A.; Jemiolo, B.; Trappe, T.A.; Trappe, S.; Harber, M.P. Molecular Adaptations to Aerobic Exercise Training in Skeletal Muscle of Older Women. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65A, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Harber, M.P.; Konopka, A.R.; Undem, M.K.; Hinkley, J.M.; Minchev, K.; Kaminsky, L.A.; Trappe, T.A.; Trappe, S. Aerobic exercise training induces skeletal muscle hypertrophy and age-dependent adaptations in myofiber function in young and older men. J. Appl. Physiol. 2012, 113, 1495–1504. [Google Scholar] [CrossRef]
- Lopez, P.; Pinto, R.S.; Radaelli, R.; Rech, A.; Grazioli, R.; Izquierdo, M.; Cadore, E.L. Benefits of resistance training in physically frail elderly: A systematic review. Aging Clin. Exp. Res. 2018, 30, 889–899. [Google Scholar] [CrossRef]
- Rodrigues, F.; Domingos, C.; Monteiro, D.; Morouço, P. A Review on Aging, Sarcopenia, Falls, and Resistance Training in Community-Dwelling Older Adults. Int. J. Environ. Res. Public Health 2022, 19, 874. [Google Scholar] [CrossRef]
- Talar, K.; Hernández-Belmonte, A.; Vetrovsky, T.; Steffl, M.; Kałamacka, E.; Courel-Ibáñez, J. Benefits of Resistance Training in Early and Late Stages of Frailty and Sarcopenia: A Systematic Review and Meta-Analysis of Randomized Controlled Studies. J. Clin. Med. 2021, 10, 1630. [Google Scholar] [CrossRef]
- Michaëlsson, K.; Olofsson, H.; Jensevik, K.; Larsson, S.; Mallmin, H.; Berglund, L.; Vessby, B.; Melhus, H. Leisure Physical Activity and the Risk of Fracture in Men. PLoS Med. 2007, 4, e199. [Google Scholar] [CrossRef]
- LaMonte, M.J.; Wactawski-Wende, J.; Larson, J.C.; Mai, X.; Robbins, J.A.; LeBoff, M.S.; Chen, Z.; Jackson, R.D.; LaCroix, A.Z.; Ockene, J.K.; et al. Association of Physical Activity and Fracture Risk Among Postmenopausal Women. JAMA Netw. Open 2019, 2, e1914084. [Google Scholar] [CrossRef]
- Mathis, S.L.; Caputo, J.L. Resistance Training Is Associated with Higher Lumbar Spine and Hip Bone Mineral Density in Competitive Male Cyclists. J. Strength Cond. Res. 2018, 32, 274–279. [Google Scholar] [CrossRef]
- Nichols, J.F.; Rauh, M.J. Longitudinal changes in bone mineral density in male master cyclists and nonathletes. J. Strength Cond. Res. 2011, 25, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Wysocki, A.; Butler, M.; Shamliyan, T.; Kane, R.L. Whole-body vibration therapy for osteoporosis: State of the science. Ann. Intern. Med. 2011, 155, 680–686, W206–W213. [Google Scholar] [CrossRef] [PubMed]
- Kannus, P.; Haapasalo, H.; Sankelo, M.; Sievänen, H.; Pasanen, M.; Heinonen, A.; Oja, P.; Vuori, I. Effect of starting age of physical activity on bone mass in the dominant arm of tennis and squash players. Ann. Intern. Med. 1995, 123, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Kontulainen, S.; Sievänen, H.; Kannus, P.; Pasanen, M.; Vuori, I. Effect of long-term impact-loading on mass, size, and estimated strength of humerus and radius of female racquet-sports players: A peripheral quantitative computed tomography study between young and old starters and controls. J. Bone Miner. Res. 2003, 18, 352–359. [Google Scholar] [CrossRef]
- Tański, W.; Kosiorowska, J.; Szymańska-Chabowska, A. Osteoporosis-risk factors, pharmaceutical and non-pharmaceutical treatment. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 3557–3566. [Google Scholar] [CrossRef]
- Zhao, R.; Xu, Z.; Zhao, M. Antiresorptive agents increase the effects of exercise on preventing postmenopausal bone loss in women: A meta-analysis. PLoS ONE 2015, 10, e0116729. [Google Scholar] [CrossRef]
- Siddique, N.; O’Donoghue, M.; Casey, M.C.; Walsh, J.B. Malnutrition in the elderly and its effects on bone health–A review. Clin. Nutr. ESPEN 2017, 21, 31–39. [Google Scholar] [CrossRef]
- Verlaan, S.; Maier, A.B.; Bauer, J.M.; Bautmans, I.; Brandt, K.; Donini, L.M.; Maggio, M.; McMurdo, M.E.T.; Mets, T.; Seal, C.; et al. Sufficient levels of 25-hydroxyvitamin D and protein intake required to increase muscle mass in sarcopenic older adults–The PROVIDE study. Clin. Nutr. 2018, 37, 551–557. [Google Scholar] [CrossRef]
- Ganapathy, A.; Nieves, J.W. Nutrition and Sarcopenia—What Do We Know? Nutrients 2020, 12, 1755. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Dietary reference values for vitamin D. EFSA J. 2016, 14, e04547. [Google Scholar] [CrossRef]
- Cashman, K.D. Vitamin D: Dietary requirements and food fortification as a means of helping achieve adequate vitamin D status. J. Steroid Biochem. Mol. Biol. 2015, 148, 19–26. [Google Scholar] [CrossRef]
- Tang, B.M.P.; Eslick, G.D.; Nowson, C.; Smith, C.; Bensoussan, A. Use of calcium or calcium in combination with vitamin D supplementation to prevent fractures and bone loss in people aged 50 years and older: A meta-analysis. Lancet 2007, 370, 657–666. [Google Scholar] [CrossRef]
- van Dronkelaar, C.; van Velzen, A.; Abdelrazek, M.; van der Steen, A.; Weijs, P.J.M.; Tieland, M. Minerals and Sarcopenia; The Role of Calcium, Iron, Magnesium, Phosphorus, Potassium, Selenium, Sodium, and Zinc on Muscle Mass, Muscle Strength, and Physical Performance in Older Adults: A Systematic Review. J. Am. Med. Dir. Assoc. 2018, 19, 6–11.e3. [Google Scholar] [CrossRef]
- Groenendijk, I.; den Boeft, L.; van Loon, L.J.C.; de Groot, L.C.P.G.M. High Versus low Dietary Protein Intake and Bone Health in Older Adults: A Systematic Review and Meta-Analysis. Comput. Struct. Biotechnol. J. 2019, 17, 1101–1112. [Google Scholar] [CrossRef]
- Shams-White, M.M.; Chung, M.; Du, M.; Fu, Z.; Insogna, K.L.; Karlsen, M.C.; LeBoff, M.S.; Shapses, S.A.; Sackey, J.; Wallace, T.C.; et al. Dietary protein and bone health: A systematic review and meta-analysis from the National Osteoporosis Foundation. Am. J. Clin. Nutr. 2017, 105, 1528–1543. [Google Scholar] [CrossRef] [PubMed]
- Darling, A.L.; Millward, D.J.; Lanham-New, S.A. Dietary protein and bone health: Towards a synthesised view. Proc. Nutr. Soc. 2021, 80, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-Jentoft, A.J.; Morley, J.E.; Phillips, S.; Sieber, C.; Stehle, P.; Teta, D.; et al. Evidence-based recommendations for optimal dietary protein intake in older people: A position paper from the PROT-AGE Study Group. J. Am. Med. Dir. Assoc. 2013, 14, 542–559. [Google Scholar] [CrossRef]
- Deutz, N.E.P.; Bauer, J.M.; Barazzoni, R.; Biolo, G.; Boirie, Y.; Bosy-Westphal, A.; Cederholm, T.; Cruz-Jentoft, A.; Krznariç, Z.; Nair, K.S.; et al. Protein intake and exercise for optimal muscle function with aging: Recommendations from the ESPEN Expert Group. Clin. Nutr. Edinb. Scotl. 2014, 33, 929–936. [Google Scholar] [CrossRef]
- Wu, P.-Y.; Huang, K.-S.; Chen, K.-M.; Chou, C.-P.; Tu, Y.-K. Exercise, Nutrition, and Combined Exercise and Nutrition in Older Adults with Sarcopenia: A Systematic Review and Network Meta-analysis. Maturitas 2021, 145, 38–48. [Google Scholar] [CrossRef]
- Iwamoto, J.; Sato, Y. Eldecalcitol improves chair-rising time in postmenopausal osteoporotic women treated with bisphosphonates. Ther. Clin. Risk Manag. 2014, 10, 51–59. [Google Scholar] [CrossRef][Green Version]
- Ringe, J.D.; Farahmand, P.; Schacht, E.; Rozehnal, A. Superiority of a combined treatment of Alendronate and Alfacalcidol compared to the combination of Alendronate and plain vitamin D or Alfacalcidol alone in established postmenopausal or male osteoporosis (AAC-Trial). Rheumatol. Int. 2007, 27, 425–434. [Google Scholar] [CrossRef]
- Saito, K.; Miyakoshi, N.; Matsunaga, T.; Hongo, M.; Kasukawa, Y.; Shimada, Y. Eldecalcitol improves muscle strength and dynamic balance in postmenopausal women with osteoporosis: An open-label randomized controlled study. J. Bone Miner. Metab. 2016, 34, 547–554. [Google Scholar] [CrossRef]
- Kanis, J.A.; Cooper, C.; Rizzoli, R.; Reginster, J.-Y. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos. Int. 2019, 30, 3–44. [Google Scholar] [CrossRef] [PubMed]
- Dickens, L.T.; Jain, R.K. An Update on the Fracture Risk Assessment Tool: What Have We Learned over 15+ years? Endocrinol. Metab. Clin. N. Am. 2024, 53, 531–545. [Google Scholar] [CrossRef] [PubMed]
- Lewiecki, E.M.; Miller, P.D.; McClung, M.R.; Cohen, S.B.; Bolognese, M.A.; Liu, Y.; Wang, A.; Siddhanti, S.; Fitzpatrick, L.A. AMG 162 Bone Loss Study Group Two-year treatment with denosumab (AMG 162) in a randomized phase 2 study of postmenopausal women with low BMD. J. Bone Miner. Res. 2007, 22, 1832–1841. [Google Scholar] [CrossRef] [PubMed]
- Bekker, P.J.; Holloway, D.L.; Rasmussen, A.S.; Murphy, R.; Martin, S.W.; Leese, P.T.; Holmes, G.B.; Dunstan, C.R.; DePaoli, A.M. A single-dose placebo-controlled study of AMG 162, a fully human monoclonal antibody to RANKL, in postmenopausal women. J. Bone Miner. Res. 2004, 19, 1059–1066. [Google Scholar] [CrossRef]
- Cummings, S.R.; Martin, J.S.; McClung, M.R.; Siris, E.S.; Eastell, R.; Reid, I.R.; Delmas, P.; Zoog, H.B.; Austin, M.; Wang, A.; et al. Denosumab for Prevention of Fractures in Postmenopausal Women with Osteoporosis. N. Engl. J. Med. 2009, 361, 756–765. [Google Scholar] [CrossRef]
- Eghbali-Fatourechi, G.; Khosla, S.; Sanyal, A.; Boyle, W.J.; Lacey, D.L.; Riggs, B.L. Role of RANK ligand in mediating increased bone resorption in early postmenopausal women. J. Clin. Investig. 2003, 111, 1221–1230. [Google Scholar] [CrossRef]
- Cummings, S.R.; McClung, M.; Christiansen, C. A phase III study of the effects of denosumab on vertebral, nonvertebral, and hip fracture in women with osteoporosis: Results from the FREEDOM trial. J. Bone Miner. Res. 2008, 23, S80. [Google Scholar]
- Chen, Y.; Zhu, J.; Zhou, Y.; Peng, J.; Wang, B. Efficacy and Safety of Denosumab in Osteoporosis or Low Bone Mineral Density Postmenopausal Women. Front. Pharmacol. 2021, 12, 588095. [Google Scholar] [CrossRef]
- Bone, H.G.; Wagman, R.B.; Brandi, M.L.; Brown, J.P.; Chapurlat, R.; Cummings, S.R.; Czerwiński, E.; Fahrleitner-Pammer, A.; Kendler, D.L.; Lippuner, K.; et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: Results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017, 5, 513–523. [Google Scholar] [CrossRef]
- Bonnet, N.; Bourgoin, L.; Biver, E.; Douni, E.; Ferrari, S. RANKL inhibition improves muscle strength and insulin sensitivity and restores bone mass. J. Clin. Investig. 2019, 129, 3214–3223. [Google Scholar] [CrossRef]
- Phu, S.; Bani Hassan, E.; Vogrin, S.; Kirk, B.; Duque, G. Effect of Denosumab on Falls, Muscle Strength, and Function in Community-Dwelling Older Adults. J. Am. Geriatr. Soc. 2019, 67, 2660–2661. [Google Scholar] [CrossRef]
- Rupp, T.; von Vopelius, E.; Strahl, A.; Oheim, R.; Barvencik, F.; Amling, M.; Rolvien, T. Beneficial effects of denosumab on muscle performance in patients with low BMD: A retrospective, propensity score-matched study. Osteoporos. Int. 2022, 33, 2177–2184. [Google Scholar] [CrossRef]
- Haeri, N.S.; Perera, S.; Greenspan, S.L. Impact of denosumab on muscle health in older adults in long-term care. Bone 2025, 198, 117552. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.D. Denosumab: Anti-RANKL antibody. Curr. Osteoporos. Rep. 2009, 7, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Miedany, Y.E.; Gaafary, M.E.; Toth, M.; Hegazi, M.O.; Aroussy, N.E.; Hassan, W.; Almedany, S.; Nasr, A.; Bahlas, S.; Galal, S.; et al. Is there a potential dual effect of denosumab for treatment of osteoporosis and sarcopenia? Clin. Rheumatol. 2021, 40, 4225–4232. [Google Scholar] [CrossRef]
- Ha, J.; Kim, J.; Jeong, C.; Lee, J.; Lim, Y.; Baek, K.-H. Catholic Medical Center Bone Research Group Effects of denosumab and zoledronic acid on postmenopausal osteoporosis, bone density, and fat-free mass. Arch. Osteoporos. 2025, 20, 17. [Google Scholar] [CrossRef]
- Cremers, S.; Drake, M.T.; Ebetino, F.H.; Bilezikian, J.P.; Russell, R.G.G. Pharmacology of bisphosphonates. Br. J. Clin. Pharmacol. 2019, 85, 1052–1062. [Google Scholar] [CrossRef]
- Liberman, U.A.; Weiss, S.R.; Bröll, J.; Minne, H.W.; Quan, H.; Bell, N.H.; Rodriguez-Portales, J.; Downs, R.W.; Dequeker, J.; Favus, M.; et al. Effect of Oral Alendronate on Bone Mineral Density and the Incidence of Fractures in Postmenopausal Osteoporosis. N. Engl. J. Med. 1995, 333, 1437–1444. [Google Scholar] [CrossRef]
- Wang, C. Efficacy and Safety of Zoledronic Acid for Treatment of Postmenopausal Osteoporosis: A Meta-Analysis of Randomized Controlled Trials. Am. J. Ther. 2017, 24, e544–e552. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.A.; Hsieh, S.-C.; Zheng, C.; Peterson, J.; Tugwell, P.; Liu, W. Risedronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women. Cochrane Database Syst. Rev. 2022, 5, CD004523. [Google Scholar] [CrossRef] [PubMed]
- Mendes, D.; Penedones, A.; Alves, C.; Oliveira, T.; Donato, A.; Batel-Marques, F. Ibandronate in the Prevention of Vertebral and Nonvertebral Osteoporotic Fractures: A Systematic Review of Experimental and Observational Studies. JCR J. Clin. Rheumatol. 2023, 29, 78–83. [Google Scholar] [CrossRef]
- Chiu, H.; Chiu, C.; Yang, R.; Chan, D.; Liu, S.; Chiang, C. Preventing muscle wasting by osteoporosis drug alendronate in vitro and in myopathy models via sirtuin-3 down-regulation. J. Cachexia Sarcopenia Muscle 2018, 9, 585–602. [Google Scholar] [CrossRef]
- Watanabe, R.; Fujita, N.; Takeda, S.; Sato, Y.; Kobayashi, T.; Morita, M.; Oike, T.; Miyamoto, K.; Matsumoto, Y.; Matsumoto, M.; et al. Ibandronate concomitantly blocks immobilization-induced bone and muscle atrophy. Biochem. Biophys. Res. Commun. 2016, 480, 662–668. [Google Scholar] [CrossRef]
- Trivedi, T.; Guise, T.A. Systemic effects of abnormal bone resorption on muscle, metabolism, and cognition. Bone 2022, 154, 116245. [Google Scholar] [CrossRef]
- Hain, B.A.; Jude, B.; Xu, H.; Smuin, D.M.; Fox, E.J.; Elfar, J.C.; Waning, D.L. Zoledronic Acid Improves Muscle Function in Healthy Mice Treated with Chemotherapy. J. Bone Miner. Res. 2020, 35, 368–381. [Google Scholar] [CrossRef]
- Urano, T.; Shiraki, M.; Kuroda, T.; Tanaka, S.; Urano, F.; Uenishi, K.; Inoue, S. Bisphosphonates prevent age-related weight loss in Japanese postmenopausal women. J. Bone Miner. Metab. 2018, 36, 734–740. [Google Scholar] [CrossRef]
- Pin, F.; Bonetto, A.; Bonewald, L.F.; Klein, G.L. Molecular Mechanisms Responsible for the Rescue Effects of Pamidronate on Muscle Atrophy in Pediatric Burn Patients. Front. Endocrinol. 2019, 10, 543. [Google Scholar] [CrossRef]
- Huang, C.-F.; Shiao, M.-S.; Mao, T.-Y. Retrospective Study of the Effects of Zoledronic Acid on Muscle Mass in Osteoporosis Patients. Drug Des. Dev. Ther. 2021, 15, 3711–3715. [Google Scholar] [CrossRef]
- Suzuki, T.; Harada, A.; Shimada, H.; Hosoi, T.; Kawata, Y.; Inoue, T.; Saito, H. Assessment of eldecalcitol and alendronate effect on postural balance control in aged women with osteoporosis. J. Bone Miner. Metab. 2020, 38, 859–867. [Google Scholar] [CrossRef]
- Park, J.H.; Park, K.H.; Cho, S.; Choi, Y.S.; Seo, S.K.; Lee, B.S.; Park, H.S. Concomitant increase in muscle strength and bone mineral density with decreasing IL-6 levels after combination therapy with alendronate and calcitriol in postmenopausal women. Menopause 2013, 20, 747–753. [Google Scholar] [CrossRef]
- Harada, A.; Ito, S.; Matsui, Y.; Sakai, Y.; Takemura, M.; Tokuda, H.; Hida, T.; Shimokata, H. Effect of alendronate on muscle mass: Investigation in patients with osteoporosis. Osteoporos. Sarcopenia 2015, 1, 53–58. [Google Scholar] [CrossRef]
- Uchiyama, S.; Ikegami, S.; Kamimura, M.; Mukaiyama, K.; Nakamura, Y.; Nonaka, K.; Kato, H. The skeletal muscle cross sectional area in long-term bisphosphonate users is smaller than that of bone mineral density-matched controls with increased serum pentosidine concentrations. Bone 2015, 75, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Goodbrand, J.A.; Hughes, L.D.; Cochrane, L.; Donnan, P.T.; McGilchrist, M.; Frost, H.; McMurdo, M.E.T.; Witham, M.D. Association between bisphosphonate therapy and outcomes from rehabilitation in older people. Arch. Gerontol. Geriatr. 2017, 70, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Maraka, S.; Kennel, K.A. Bisphosphonates for the prevention and treatment of osteoporosis. BMJ 2015, 351, h3783. [Google Scholar] [CrossRef]
- The U.S. Food and Drug Administration (FDA). FDA Drug Safety Communication: Safety Update for Osteoporosis Drugs, Bisphosphonates, and Atypical Fractures; The U.S. Food and Drug Administration (FDA): Silver Spring, MD, USA, 2019. [Google Scholar]
- Kawada, S.; Harada, A.; Hashimoto, N. Impairment of cold injury-induced muscle regeneration in mice receiving a combination of bone fracture and alendronate treatment. PLoS ONE 2017, 12, e0181457. [Google Scholar] [CrossRef]
- Shiomi, K.; Nagata, Y.; Kiyono, T.; Harada, A.; Hashimoto, N. Differential impact of the bisphosphonate alendronate on undifferentiated and terminally differentiated human myogenic cells. J. Pharm. Pharmacol. 2014, 66, 418–427. [Google Scholar] [CrossRef]
- Uusi-Rasi, K.; Kannus, P.; Cheng, S.; Sievänen, H.; Pasanen, M.; Heinonen, A.; Nenonen, A.; Halleen, J.; Fuerst, T.; Genant, H.; et al. Effect of alendronate and exercise on bone and physical performance of postmenopausal women: A randomized controlled trial. Bone 2003, 33, 132–143. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, B.; Qin, M.-Z.; Liu, J.-P. Fall Prevention and Anti-Osteoporosis in Osteopenia Patients of 80 Years of Age and Older: A Randomized Controlled Study. Orthop. Surg. 2020, 12, 890–899. [Google Scholar] [CrossRef]
- Flores, L.E.; Kupzyk, K.; Waltman, N.; Beavers, K.M.; Bilek, L. Risedronate or exercise for lean mass preservation during menopause: Secondary analysis of a randomized controlled trial. JCSM Rapid Commun. 2022, 5, 154–161. [Google Scholar] [CrossRef]
- Waltman, N.; Kupzyk, K.A.; Flores, L.E.; Mack, L.R.; Lappe, J.M.; Bilek, L.D. Bone-loading exercises versus risedronate for the prevention of osteoporosis in postmenopausal women with low bone mass: A randomized controlled trial. Osteoporos. Int. 2022, 33, 475–486. [Google Scholar] [CrossRef]
- Haeri, N.S.; Perera, S.; Greenspan, S.L. Does Zoledronic Acid Improve Appendicular Lean Mass in Older Women with Osteoporosis? A Sub-Analysis of a Randomized Clinical Trial. J. Frailty Aging 2022, 11, 420–425. [Google Scholar] [CrossRef]
- Reid, I.R.; Horne, A.M.; Mihov, B.; Stewart, A.; Garratt, E.; Wong, S.; Wiessing, K.R.; Bolland, M.J.; Bastin, S.; Gamble, G.D. Fracture Prevention with Zoledronate in Older Women with Osteopenia. N. Engl. J. Med. 2018, 379, 2407–2416. [Google Scholar] [CrossRef] [PubMed]
- Reid, I.R.; Horne, A.M.; Mihov, B.; Stewart, A.; Bastin, S.; Gamble, G.D. Zoledronate Slows Weight Loss and Maintains Fat Mass in Osteopenic Older Women: Secondary Analysis of a Randomized Controlled Trial. Calcif. Tissue Int. 2020, 106, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Glorieux, F.H.; Bishop, N.J.; Plotkin, H.; Chabot, G.; Lanoue, G.; Travers, R. Cyclic administration of pamidronate in children with severe osteogenesis imperfecta. N. Engl. J. Med. 1998, 339, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Land, C.; Rauch, F.; Montpetit, K.; Ruck-Gibis, J.; Glorieux, F.H. Effect of intravenous pamidronate therapy on functional abilities and level of ambulation in children with osteogenesis imperfecta. J. Pediatr. 2006, 148, 456–460. [Google Scholar] [CrossRef]
- Land, C.; Rauch, F.; Travers, R.; Glorieux, F.H. Osteogenesis imperfecta type VI in childhood and adolescence: Effects of cyclical intravenous pamidronate treatment. Bone 2007, 40, 638–644. [Google Scholar] [CrossRef]
- Fink, H.A.; Ewing, S.K.; Ensrud, K.E.; Barrett-Connor, E.; Taylor, B.C.; Cauley, J.A.; Orwoll, E.S. Association of testosterone and estradiol deficiency with osteoporosis and rapid bone loss in older men. J. Clin. Endocrinol. Metab. 2006, 91, 3908–3915. [Google Scholar] [CrossRef]
- Feldman, H.A.; Longcope, C.; Derby, C.A.; Johannes, C.B.; Araujo, A.B.; Coviello, A.D.; Bremner, W.J.; McKinlay, J.B. Age trends in the level of serum testosterone and other hormones in middle-aged men: Longitudinal results from the Massachusetts male aging study. J. Clin. Endocrinol. Metab. 2002, 87, 589–598. [Google Scholar] [CrossRef]
- Cauley, J.A.; Robbins, J.; Chen, Z.; Cummings, S.R.; Jackson, R.D.; LaCroix, A.Z.; LeBoff, M.; Lewis, C.E.; McGowan, J.; Neuner, J.; et al. Effects of estrogen plus progestin on risk of fracture and bone mineral density: The Women’s Health Initiative randomized trial. JAMA 2003, 290, 1729–1738. [Google Scholar] [CrossRef]
- de Villiers, T.J.; Pines, A.; Panay, N.; Gambacciani, M.; Archer, D.F.; Baber, R.J.; Davis, S.R.; Gompel, A.A.; Henderson, V.W.; Langer, R.; et al. Updated 2013 International Menopause Society recommendations on menopausal hormone therapy and preventive strategies for midlife health. Climacteric J. Int. Menopause Soc. 2013, 16, 316–337. [Google Scholar] [CrossRef] [PubMed]
- Rossouw, J.E.; Anderson, G.L.; Prentice, R.L.; LaCroix, A.Z.; Kooperberg, C.; Stefanick, M.L.; Jackson, R.D.; Beresford, S.A.A.; Howard, B.V.; Johnson, K.C.; et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results From the Women’s Health Initiative randomized controlled trial. JAMA 2002, 288, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.L.; Auchus, R.J. The Molecular Biology, Biochemistry, and Physiology of Human Steroidogenesis and Its Disorders. Endocr. Rev. 2011, 32, 81–151. [Google Scholar] [CrossRef]
- Falahati-Nini, A.; Riggs, B.L.; Atkinson, E.J.; O’Fallon, W.M.; Eastell, R.; Khosla, S. Relative contributions of testosterone and estrogen in regulating bone resorption and formation in normal elderly men. J. Clin. Investig. 2000, 106, 1553–1560. [Google Scholar] [CrossRef]
- Raisz, L.G.; Wiita, B.; Artis, A.; Bowen, A.; Schwartz, S.; Trahiotis, M.; Shoukri, K.; Smith, J. Comparison of the effects of estrogen alone and estrogen plus androgen on biochemical markers of bone formation and resorption in postmenopausal women. J. Clin. Endocrinol. Metab. 1996, 81, 37–43. [Google Scholar] [CrossRef]
- Carani, C.; Qin, K.; Simoni, M.; Faustini-Fustini, M.; Serpente, S.; Boyd, J.; Korach, K.S.; Simpson, E.R. Effect of testosterone and estradiol in a man with aromatase deficiency. N. Engl. J. Med. 1997, 337, 91–95. [Google Scholar] [CrossRef]
- Rochira, V.; Zirilli, L.; Madeo, B.; Aranda, C.; Caffagni, G.; Fabre, B.; Montangero, V.E.; Roldan, E.J.A.; Maffei, L.; Carani, C. Skeletal effects of long-term estrogen and testosterone replacement treatment in a man with congenital aromatase deficiency: Evidences of a priming effect of estrogen for sex steroids action on bone. Bone 2007, 40, 1662–1668. [Google Scholar] [CrossRef]
- Bilezikian, J.P.; Morishima, A.; Bell, J.; Grumbach, M.M. Increased bone mass as a result of estrogen therapy in a man with aromatase deficiency. N. Engl. J. Med. 1998, 339, 599–603. [Google Scholar] [CrossRef]
- Hofbauer, L.C.; Khosla, S. Androgen effects on bone metabolism: Recent progress and controversies. Eur. J. Endocrinol. 1999, 140, 271–286. [Google Scholar] [CrossRef]
- Davis, S.R.; Baber, R.; Panay, N.; Bitzer, J.; Perez, S.C.; Islam, R.M.; Kaunitz, A.M.; Kingsberg, S.A.; Lambrinoudaki, I.; Liu, J.; et al. Global Consensus Position Statement on the use of Testosterone Therapy for Women. Maturitas 2019, 128, 89–93. [Google Scholar] [CrossRef]
- Bhasin, S.; Brito, J.P.; Cunningham, G.R.; Hayes, F.J.; Hodis, H.N.; Matsumoto, A.M.; Snyder, P.J.; Swerdloff, R.S.; Wu, F.C.; Yialamas, M.A. Testosterone Therapy in Men with Hypogonadism: An Endocrine Society* Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2018, 103, 1715–1744. [Google Scholar] [CrossRef]
- Zhang, Z.; Kang, D.; Li, H. The effects of testosterone on bone health in males with testosterone deficiency: A systematic review and meta-analysis. BMC Endocr. Disord. 2020, 20, 33. [Google Scholar] [CrossRef]
- Corona, G.; Vena, W.; Pizzocaro, A.; Giagulli, V.A.; Francomano, D.; Rastrelli, G.; Mazziotti, G.; Aversa, A.; Isidori, A.M.; Pivonello, R.; et al. Testosterone supplementation and bone parameters: A systematic review and meta-analysis study. J. Endocrinol. Investig. 2022, 45, 911–926. [Google Scholar] [CrossRef] [PubMed]
- Junjie, W.; Dongsheng, H.; Lei, S.; Hongzhuo, L.; Changying, S. Testosterone Replacement Therapy Has Limited Effect on Increasing Bone Mass Density in Older Men: A Meta-analysis. Curr. Pharm. Des. 2019, 25, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Buratto, J.; Kirk, B.; Phu, S.; Vogrin, S.; Duque, G. Safety and Efficacy of Testosterone Therapy on Musculoskeletal Health and Clinical Outcomes in Men: A Systematic Review and Meta-Analysis of Randomized Placebo-Controlled Trials. Endocr. Pract. 2023, 29, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Snyder, P.J.; Peachey, H.; Hannoush, P.; Berlin, J.A.; Loh, L.; Holmes, J.H.; Dlewati, A.; Staley, J.; Santanna, J.; Kapoor, S.C.; et al. Effect of Testosterone Treatment on Bone Mineral Density in Men Over 65 Years of Age1. J. Clin. Endocrinol. Metab. 1999, 84, 1966–1972. [Google Scholar] [CrossRef]
- Amory, J.K.; Watts, N.B.; Easley, K.A.; Sutton, P.R.; Anawalt, B.D.; Matsumoto, A.M.; Bremner, W.J.; Tenover, J.L. Exogenous testosterone or testosterone with finasteride increases bone mineral density in older men with low serum testosterone. J. Clin. Endocrinol. Metab. 2004, 89, 503–510. [Google Scholar] [CrossRef]
- Bouloux, P.M.G.; Legros, J.-J.; Elbers, J.M.H.; Geurts, T.B.P.; Kaspers, M.J.G.H.; Meehan, A.G.; Meuleman, E.J.H.; Study 43203 Investigators. Effects of oral testosterone undecanoate therapy on bone mineral density and body composition in 322 aging men with symptomatic testosterone deficiency: A 1-year, randomized, placebo-controlled, dose-ranging study. Aging Male 2013, 16, 38–47. [Google Scholar] [CrossRef]
- Deepika, F.; Ballato, E.; Colleluori, G.; Aguirre, L.; Chen, R.; Qualls, C.; Villareal, D.T.; Armamento-Villareal, R. Baseline Testosterone Predicts Body Composition and Metabolic Response to Testosterone Therapy. Front. Endocrinol. 2022, 13, 915309. [Google Scholar] [CrossRef]
- Basurto, L.; Zarate, A.; Gomez, R.; Vargas, C.; Saucedo, R.; Galván, R. Effect of testosterone therapy on lumbar spine and hip mineral density in elderly men. Aging Male 2008, 11, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Wiren, K.M.; Zhang, X.-W.; Toombs, A.R.; Kasparcova, V.; Gentile, M.A.; Harada, S.; Jepsen, K.J. Targeted overexpression of androgen receptor in osteoblasts: Unexpected complex bone phenotype in growing animals. Endocrinology 2004, 145, 3507–3522. [Google Scholar] [CrossRef] [PubMed]
- Wiren, K.M.; Semirale, A.A.; Zhang, X.-W.; Woo, A.; Tommasini, S.M.; Price, C.; Schaffler, M.B.; Jepsen, K.J. Targeting of androgen receptor in bone reveals a lack of androgen anabolic action and inhibition of osteogenesis A model for compartment-specific androgen action in the skeleton. Bone 2008, 43, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Burnett-Bowie, S.-A.M.; McKay, E.A.; Lee, H.; Leder, B.Z. Effects of Aromatase Inhibition on Bone Mineral Density and Bone Turnover in Older Men with Low Testosterone Levels. J. Clin. Endocrinol. Metab. 2009, 94, 4785–4792. [Google Scholar] [CrossRef]
- Sjögren, K.; Lagerquist, M.; Moverare-Skrtic, S.; Andersson, N.; Windahl, S.H.; Swanson, C.; Mohan, S.; Poutanen, M.; Ohlsson, C. Elevated aromatase expression in osteoblasts leads to increased bone mass without systemic adverse effects. J. Bone Miner. Res. 2009, 24, 1263–1270. [Google Scholar] [CrossRef]
- Dubois, V.; Laurent, M.; Boonen, S.; Vanderschueren, D.; Claessens, F. Androgens and skeletal muscle: Cellular and molecular action mechanisms underlying the anabolic actions. Cell. Mol. Life Sci. 2012, 69, 1651–1667. [Google Scholar] [CrossRef]
- Parahiba, S.M.; Ribeiro, É.C.T.; Corrêa, C.; Bieger, P.; Perry, I.S.; Souza, G.C. Effect of testosterone supplementation on sarcopenic components in middle-aged and elderly men: A systematic review and meta-analysis. Exp. Gerontol. 2020, 142, 111106. [Google Scholar] [CrossRef]
- Kenny, A.M.; Kleppinger, A.; Annis, K.; Rathier, M.; Browner, B.; Judge, J.O.; McGee, D. Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels, low bone mass, and physical frailty. J. Am. Geriatr. Soc. 2010, 58, 1134–1143. [Google Scholar] [CrossRef]
- Neto, W.K.; Gama, E.F.; Rocha, L.Y.; Ramos, C.C.; Taets, W.; Scapini, K.B.; Ferreira, J.B.; Rodrigues, B.; Caperuto, É. Effects of testosterone on lean mass gain in elderly men: Systematic review with meta-analysis of controlled and randomized studies. Age Dordr. Neth. 2015, 37, 9742. [Google Scholar] [CrossRef]
- Skinner, J.W.; Otzel, D.M.; Bowser, A.; Nargi, D.; Agarwal, S.; Peterson, M.D.; Zou, B.; Borst, S.E.; Yarrow, J.F. Muscular responses to testosterone replacement vary by administration route: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2018, 9, 465–481. [Google Scholar] [CrossRef]
- Lee, T.-W.; Kao, P.-Y.; Chen, Y.-C.; Wang, S.-T. Effects of Testosterone Replacement Therapy on Muscle Strength in Older Men with Low to Low-Normal Testosterone Levels: A Systematic Review and Meta-Analysis. Gerontology 2023, 69, 1157–1166. [Google Scholar] [CrossRef]
- Page, S.T.; Amory, J.K.; Bowman, F.D.; Anawalt, B.D.; Matsumoto, A.M.; Bremner, W.J.; Tenover, J.L. Exogenous testosterone (T) alone or with finasteride increases physical performance, grip strength, and lean body mass in older men with low serum T. J. Clin. Endocrinol. Metab. 2005, 90, 1502–1510. [Google Scholar] [CrossRef] [PubMed]
- Correa, C.; Bieger, P.; Perry, I.S.; Souza, G.C. Testosterone Supplementation on Sarcopenia Components in Chronic Patients: A Systematic Review and Meta-Analysis. Curr. Pharm. Des. 2022, 28, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Storer, T.W.; Woodhouse, L.; Magliano, L.; Singh, A.B.; Dzekov, C.; Dzekov, J.; Bhasin, S. Changes in Muscle Mass, Muscle Strength and Power, but not Physical Function are Related to Testosterone Dose in Healthy Older Men. J. Am. Geriatr. Soc. 2008, 56, 1991–1999. [Google Scholar] [CrossRef] [PubMed]
- Falqueto, H.; Júnior, J.L.R.; Silvério, M.N.O.; Farias, J.C.H.; Schoenfeld, B.J.; Manfredi, L.H. Can conditions of skeletal muscle loss be improved by combining exercise with anabolic-androgenic steroids? A systematic review and meta-analysis of testosterone-based interventions. Rev. Endocr. Metab. Disord. 2021, 22, 161–178. [Google Scholar] [CrossRef]
- Bhasin, S.; Ellenberg, S.S.; Storer, T.W.; Basaria, S.; Pahor, M.; Cauley, J.A.; Ensrud, K.E.; Farrar, J.T.; Cella, D.; Matsumoto, A.M.; et al. The Effects of Testosterone Replacement on Self-reported and Performance-based Measures of Mobility in Older Men with Mobility Limitation and Low Testosterone Levels: A Placebo-Controlled Trial. Lancet Diabetes Endocrinol. 2018, 6, 879–890. [Google Scholar] [CrossRef]
- Katznelson, L.; Robinson, M.W.; Coyle, C.L.; Lee, H.; Farrell, C.E. Effects of modest testosterone supplementation and exercise for 12 weeks on body composition and quality of life in elderly men. Eur. J. Endocrinol. 2006, 155, 867–875. [Google Scholar] [CrossRef]
- Basaria, S.; Coviello, A.D.; Travison, T.G.; Storer, T.W.; Farwell, W.R.; Jette, A.M.; Eder, R.; Tennstedt, S.; Ulloor, J.; Zhang, A.; et al. Adverse events associated with testosterone administration. N. Engl. J. Med. 2010, 363, 109–122. [Google Scholar] [CrossRef]
- Watts, N.B.; Adler, R.A.; Bilezikian, J.P.; Drake, M.T.; Eastell, R.; Orwoll, E.S.; Finkelstein, J.S. Endocrine Society Osteoporosis in men: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2012, 97, 1802–1822. [Google Scholar] [CrossRef]
- Faubion, S.S.; Crandall, C.J.; Davis, L.; El Khoudary, S.R.; Hodis, H.N.; Lobo, R.A.; Maki, P.M.; Manson, J.E.; Pinkerton, J.V.; Santoro, N.F.; et al. The 2022 Hormone Therapy Position Statement of The North American Menopause Society. Menopause 2022, 29, 767–794. [Google Scholar] [CrossRef]
- Abdi, F.; Mobedi, H.; Bayat, F.; Mosaffa, N.; Dolatian, M.; Ramezani Tehrani, F. The Effects of Transdermal Estrogen Delivery on Bone Mineral Density in Postmenopausal Women: A Meta-analysis. Iran. J. Pharm. Res. 2017, 16, 380–389. [Google Scholar]
- Wells, G.; Tugwell, P.; Shea, B.; Guyatt, G.; Peterson, J.; Zytaruk, N.; Robinson, V.; Henry, D.; O’Connell, D.; Cranney, A.; et al. Meta-analyses of therapies for postmenopausal osteoporosis. V. Meta-analysis of the efficacy of hormone replacement therapy in treating and preventing osteoporosis in postmenopausal women. Endocr. Rev. 2002, 23, 529–539. [Google Scholar] [CrossRef]
- Zhu, L.; Jiang, X.; Sun, Y.; Shu, W. Effect of hormone therapy on the risk of bone fractures: A systematic review and meta-analysis of randomized controlled trials. Menopause 2016, 23, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Wiik, A.; Ekman, M.; Johansson, O.; Jansson, E.; Esbjörnsson, M. Expression of both oestrogen receptor alpha and beta in human skeletal muscle tissue. Histochem. Cell Biol. 2009, 131, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Kyle, U.G.; Genton, L.; Hans, D.; Karsegard, L.; Slosman, D.O.; Pichard, C. Age-related differences in fat-free mass, skeletal muscle, body cell mass and fat mass between 18 and 94 years. Eur. J. Clin. Nutr. 2001, 55, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Sipilä, S.; Törmäkangas, T.; Sillanpää, E.; Aukee, P.; Kujala, U.M.; Kovanen, V.; Laakkonen, E.K. Muscle and bone mass in middle-aged women: Role of menopausal status and physical activity. J. Cachexia Sarcopenia Muscle 2020, 11, 698–709. [Google Scholar] [CrossRef]
- Smith, G.I.; Reeds, D.N.; Okunade, A.L.; Patterson, B.W.; Mittendorfer, B. Systemic delivery of estradiol, but not testosterone or progesterone, alters very low density lipoprotein-triglyceride kinetics in postmenopausal women. J. Clin. Endocrinol. Metab. 2014, 99, E1306–E1310. [Google Scholar] [CrossRef]
- Raue, U.; Slivka, D.; Jemiolo, B.; Hollon, C.; Trappe, S. Proteolytic Gene Expression Differs at Rest and After Resistance Exercise Between Young and Old Women. J. Gerontol. Ser. A 2007, 62, 1407–1412. [Google Scholar] [CrossRef]
- Smith, G.I.; Villareal, D.T.; Sinacore, D.R.; Shah, K.; Mittendorfer, B. Muscle protein synthesis response to exercise training in obese, older men and women. Med. Sci. Sports Exerc. 2012, 44, 1259–1266. [Google Scholar] [CrossRef]
- Greising, S.M.; Baltgalvis, K.A.; Lowe, D.A.; Warren, G.L. Hormone therapy and skeletal muscle strength: A meta-analysis. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64, 1071–1081. [Google Scholar] [CrossRef]
- Javed, A.A.; Mayhew, A.J.; Shea, A.K.; Raina, P. Association Between Hormone Therapy and Muscle Mass in Postmenopausal Women: A Systematic Review and Meta-analysis. JAMA Netw. Open 2019, 2, e1910154. [Google Scholar] [CrossRef]
- Bea, J.W.; Zhao, Q.; Cauley, J.A.; LaCroix, A.Z.; Bassford, T.; Lewis, C.E.; Jackson, R.D.; Tylavsky, F.A.; Chen, Z. Effect of hormone therapy on lean body mass, falls, and fractures: Six-year results from the Women’s Health Initiative Hormone Trials. Menopause 2011, 18, 44–52. [Google Scholar] [CrossRef]
- Chen, Z.; Bassford, T.; Green, S.B.; Cauley, J.A.; Jackson, R.D.; LaCroix, A.Z.; Leboff, M.; Stefanick, M.L.; Margolis, K.L. Postmenopausal hormone therapy and body composition—A substudy of the estrogen plus progestin trial of the Women’s Health Initiative. Am. J. Clin. Nutr. 2005, 82, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Taaffe, D.R.; Sipilä, S.; Cheng, S.; Puolakka, J.; Toivanen, J.; Suominen, H. The effect of hormone replacement therapy and/or exercise on skeletal muscle attenuation in postmenopausal women: A yearlong intervention. Clin. Physiol. Funct. Imaging 2005, 25, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Ronkainen, P.H.A.; Kovanen, V.; Alén, M.; Pöllänen, E.; Palonen, E.-M.; Ankarberg-Lindgren, C.; Hämäläinen, E.; Turpeinen, U.; Kujala, U.M.; Puolakka, J.; et al. Postmenopausal hormone replacement therapy modifies skeletal muscle composition and function: A study with monozygotic twin pairs. J. Appl. Physiol. 2009, 107, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Taaffe, D.R.; Newman, A.B.; Haggerty, C.L.; Colbert, L.H.; de Rekeneire, N.; Visser, M.; Goodpaster, B.H.; Nevitt, M.C.; Tylavsky, F.A.; Harris, T.B. Estrogen replacement, muscle composition, and physical function: The Health ABC Study. Med. Sci. Sports Exerc. 2005, 37, 1741–1747. [Google Scholar] [CrossRef]
- Sipilä, S.; Taaffe, D.R.; Cheng, S.; Puolakka, J.; Toivanen, J.; Suominen, H. Effects of hormone replacement therapy and high-impact physical exercise on skeletal muscle in post-menopausal women: A randomized placebo-controlled study. Clin. Sci. 1979 2001, 101, 147–157. [Google Scholar] [CrossRef]
- Kenny, A.M.; Dawson, L.; Kleppinger, A.; Iannuzzi-Sucich, M.; Judge, J.O. Prevalence of sarcopenia and predictors of skeletal muscle mass in nonobese women who are long-term users of estrogen-replacement therapy. J. Gerontol. A Biol. Sci. Med. Sci. 2003, 58, M436–M440. [Google Scholar] [CrossRef]
- Chlebowski, R.T.; Hendrix, S.L.; Langer, R.D.; Stefanick, M.L.; Gass, M.; Lane, D.; Rodabough, R.J.; Gilligan, M.A.; Cyr, M.G.; Thomson, C.A.; et al. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: The Women’s Health Initiative Randomized Trial. JAMA 2003, 289, 3243–3253. [Google Scholar] [CrossRef]
- Wassertheil-Smoller, S.; Hendrix, S.L.; Limacher, M.; Heiss, G.; Kooperberg, C.; Baird, A.; Kotchen, T.; Curb, J.D.; Black, H.; Rossouw, J.E.; et al. Effect of estrogen plus progestin on stroke in postmenopausal women: The Women’s Health Initiative: A randomized trial. JAMA 2003, 289, 2673–2684. [Google Scholar] [CrossRef]
- Gurney, E.P.; Nachtigall, M.J.; Nachtigall, L.E.; Naftolin, F. The Women’s Health Initiative trial and related studies: 10 years later: A clinician’s view. J. Steroid Biochem. Mol. Biol. 2014, 142, 4–11. [Google Scholar] [CrossRef]
- Wasnich, R.D.; Bagger, Y.Z.; Hosking, D.J.; McClung, M.R.; Wu, M.; Mantz, A.M.; Yates, J.J.; Ross, P.D.; Alexandersen, P.; Ravn, P.; et al. Changes in bone density and turnover after alendronate or estrogen withdrawal. Menopause 2004, 11, 622–630. [Google Scholar] [CrossRef]
- Barrett-Connor, E.; Wehren, L.E.; Siris, E.S.; Miller, P.; Chen, Y.-T.; Abbott, T.A.; Berger, M.L.; Santora, A.C.; Sherwood, L.M. Recency and duration of postmenopausal hormone therapy: Effects on bone mineral density and fracture risk in the National Osteoporosis Risk Assessment (NORA) study. Menopause 2003, 10, 412–419. [Google Scholar] [CrossRef]
- Maximov, P.Y.; Lee, T.M.; Jordan, V.C. The Discovery and Development of Selective Estrogen Receptor Modulators (SERMs) for Clinical Practice. Curr. Clin. Pharmacol. 2013, 8, 135–155. [Google Scholar] [CrossRef]
- Grey, A.B.; Stapleton, J.P.; Evans, M.C.; Tatnell, M.A.; Ames, R.W.; Reid, I.R. The effect of the antiestrogen tamoxifen on bone mineral density in normal late postmenopausal women. Am. J. Med. 1995, 99, 636–641. [Google Scholar] [CrossRef]
- Ryu, K.-J.; Kim, M.S.; Lee, J.Y.; Nam, S.; Jeong, H.G.; Kim, T.; Park, H. Risk of Endometrial Polyps, Hyperplasia, Carcinoma, and Uterine Cancer After Tamoxifen Treatment in Premenopausal Women with Breast Cancer. JAMA Netw. Open 2022, 5, e2243951. [Google Scholar] [CrossRef] [PubMed]
- Peterson, G.M.; Naunton, M.; Tichelaar, L.K.; Gennari, L. Lasofoxifene: Selective estrogen receptor modulator for the prevention and treatment of postmenopausal osteoporosis. Ann. Pharmacother. 2011, 45, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Cummings, S.R.; Ensrud, K.; Delmas, P.D.; LaCroix, A.Z.; Vukicevic, S.; Reid, D.M.; Goldstein, S.; Sriram, U.; Lee, A.; Thompson, J.; et al. Lasofoxifene in postmenopausal women with osteoporosis. N. Engl. J. Med. 2010, 362, 686–696. [Google Scholar] [CrossRef] [PubMed]
- de Villiers, T.J. The quest for new drugs to prevent osteoporosis-related fractures. Climacteric J. Int. Menopause Soc. 2017, 20, 103–106. [Google Scholar] [CrossRef]
- Ettinger, B.; Black, D.M.; Mitlak, B.H.; Knickerbocker, R.K.; Nickelsen, T.; Genant, H.K.; Christiansen, C.; Delmas, P.D.; Zanchetta, J.R.; Stakkestad, J.; et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: Results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA 1999, 282, 637–645. [Google Scholar] [CrossRef]
- Kanis, J.A.; Johnell, O.; Black, D.M.; Downs, R.W.; Sarkar, S.; Fuerst, T.; Secrest, R.J.; Pavo, I. Effect of raloxifene on the risk of new vertebral fracture in postmenopausal women with osteopenia or osteoporosis: A reanalysis of the Multiple Outcomes of Raloxifene Evaluation trial. Bone 2003, 33, 293–300. [Google Scholar] [CrossRef]
- Delmas, P.D.; Ensrud, K.E.; Adachi, J.D.; Harper, K.D.; Sarkar, S.; Gennari, C.; Reginster, J.-Y.; Pols, H.A.P.; Recker, R.R.; Harris, S.T.; et al. Efficacy of raloxifene on vertebral fracture risk reduction in postmenopausal women with osteoporosis: Four-year results from a randomized clinical trial. J. Clin. Endocrinol. Metab. 2002, 87, 3609–3617. [Google Scholar] [CrossRef]
- Qu, Y.; Wong, M.; Thiebaud, D.; Stock, J.L. The effect of raloxifene therapy on the risk of new clinical vertebral fractures at three and six months: A secondary analysis of the MORE trial. Curr. Med. Res. Opin. 2005, 21, 1955–1959. [Google Scholar] [CrossRef]
- Siris, E.S.; Harris, S.T.; Eastell, R.; Zanchetta, J.R.; Goemaere, S.; Diez-Perez, A.; Stock, J.L.; Song, J.; Qu, Y.; Kulkarni, P.M.; et al. Skeletal effects of raloxifene after 8 years: Results from the continuing outcomes relevant to Evista (CORE) study. J. Bone Miner. Res. 2005, 20, 1514–1524. [Google Scholar] [CrossRef]
- Dane, C.; Dane, B.; Cetin, A.; Erginbas, M. Comparison of the effects of raloxifene and low-dose hormone replacement therapy on bone mineral density and bone turnover in the treatment of postmenopausal osteoporosis. Gynecol. Endocrinol. 2007, 23, 398–403. [Google Scholar] [CrossRef]
- Reid, I.R.; Eastell, R.; Fogelman, I.; Adachi, J.D.; Rosen, A.; Netelenbos, C.; Watts, N.B.; Seeman, E.; Ciaccia, A.V.; Draper, M.W. A comparison of the effects of raloxifene and conjugated equine estrogen on bone and lipids in healthy postmenopausal women. Arch. Intern. Med. 2004, 164, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Neele, S.J.M.; Evertz, R.; De Valk-De Roo, G.; Roos, J.C.; Netelenbos, J.C. Effect of 1 year of discontinuation of raloxifene or estrogen therapy on bone mineral density after 5 years of treatment in healthy postmenopausal women. Bone 2002, 30, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Naylor, K.E.; Clowes, J.A.; Finigan, J.; Paggiosi, M.A.; Peel, N.F.A.; Eastell, R. The effect of cessation of raloxifene treatment on bone turnover in postmenopausal women. Bone 2010, 46, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.D.; Chines, A.A.; Christiansen, C.; Hoeck, H.C.; Kendler, D.L.; Lewiecki, E.M.; Woodson, G.; Levine, A.B.; Constantine, G.; Delmas, P.D. Effects of bazedoxifene on BMD and bone turnover in postmenopausal women: 2-yr results of a randomized, double-blind, placebo-, and active-controlled study. J. Bone Miner. Res. 2008, 23, 525–535. [Google Scholar] [CrossRef]
- Palacios, S.; Silverman, S.L.; de Villiers, T.J.; Levine, A.B.; Goemaere, S.; Brown, J.P.; De Cicco Nardone, F.; Williams, R.; Hines, T.L.; Mirkin, S.; et al. A 7-year randomized, placebo-controlled trial assessing the long-term efficacy and safety of bazedoxifene in postmenopausal women with osteoporosis: Effects on bone density and fracture. Menopause 2015, 22, 806. [Google Scholar] [CrossRef]
- Silverman, S.L.; Christiansen, C.; Genant, H.K.; Vukicevic, S.; Zanchetta, J.R.; de Villiers, T.J.; Constantine, G.D.; Chines, A.A. Efficacy of bazedoxifene in reducing new vertebral fracture risk in postmenopausal women with osteoporosis: Results from a 3-year, randomized, placebo-, and active-controlled clinical trial. J. Bone Miner. Res. 2008, 23, 1923–1934. [Google Scholar] [CrossRef]
- Silverman, S.L.; Chines, A.A.; Kendler, D.L.; Kung, A.W.C.; Teglbjærg, C.S.; Felsenberg, D.; Mairon, N.; Constantine, G.D.; Adachi, J.D. Bazedoxifene Study Group Sustained efficacy and safety of bazedoxifene in preventing fractures in postmenopausal women with osteoporosis: Results of a 5-year, randomized, placebo-controlled study. Osteoporos. Int. 2012, 23, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Itabashi, A.; Yoh, K.; Chines, A.A.; Miki, T.; Takada, M.; Sato, H.; Gorai, I.; Sugimoto, T.; Mizunuma, H.; Ochi, H.; et al. Effects of bazedoxifene on bone mineral density, bone turnover, and safety in postmenopausal japanese women with osteoporosis. J. Bone Miner. Res. 2011, 26, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; Johansson, H.; Oden, A.; McCloskey, E.V. Bazedoxifene reduces vertebral and clinical fractures in postmenopausal women at high risk assessed with FRAX. Bone 2009, 44, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- Yavropoulou, M.P.; Makras, P.; Anastasilakis, A.D. Bazedoxifene for the treatment of osteoporosis. Expert Opin. Pharmacother. 2019, 20, 1201–1210. [Google Scholar] [CrossRef]
- Lindsay, R.; Gallagher, J.C.; Kagan, R.; Pickar, J.H.; Constantine, G. Efficacy of tissue-selective estrogen complex of bazedoxifene/conjugated estrogens for osteoporosis prevention in at-risk postmenopausal women. Fertil. Steril. 2009, 92, 1045–1052. [Google Scholar] [CrossRef]
- Pinkerton, J.V.; Harvey, J.A.; Lindsay, R.; Pan, K.; Chines, A.A.; Mirkin, S.; Archer, D.F. SMART-5 Investigators Effects of bazedoxifene/conjugated estrogens on the endometrium and bone: A randomized trial. J. Clin. Endocrinol. Metab. 2014, 99, E189–E198. [Google Scholar] [CrossRef]
- Gallagher, J.C.; Palacios, S.; Ryan, K.A.; Yu, C.-R.; Pan, K.; Kendler, D.L.; Mirkin, S.; Komm, B.S. Effect of conjugated estrogens/bazedoxifene on postmenopausal bone loss: Pooled analysis of two randomized trials. Menopause 2016, 23, 1083–1091. [Google Scholar] [CrossRef]
- Mirkin, S.; Komm, B.S.; Pan, K.; Chines, A.A. Effects of bazedoxifene/conjugated estrogens on endometrial safety and bone in postmenopausal women. Climacteric J. Int. Menopause Soc. 2013, 16, 338–346. [Google Scholar] [CrossRef]
- Goldberg, T.; Fidler, B. Conjugated Estrogens/Bazedoxifene (Duavee): A Novel Agent for the Treatment of Moderate-to-Severe Vasomotor Symptoms Associated with Menopause and the Prevention of Postmenopausal Osteoporosis. P T Peer-Rev. J. Formul. Manag. 2015, 40, 178–182. [Google Scholar]
- Anastasilakis, A.D.; Polyzos, S.A.; Makras, P. THERAPY OF ENDOCRINE DISEASE: Denosumab vs bisphosphonates for the treatment of postmenopausal osteoporosis. Eur. J. Endocrinol. 2018, 179, R31–R45. [Google Scholar] [CrossRef]
- Jacobsen, D.E.; Samson, M.M.; Emmelot-Vonk, M.H.; Verhaar, H.J.J. Raloxifene and body composition and muscle strength in postmenopausal women: A randomized, double-blind, placebo-controlled trial. Eur. J. Endocrinol. 2010, 162, 371–376. [Google Scholar] [CrossRef]
- Jacobsen, D.E.; Melis, R.J.F.; Verhaar, H.J.J.; Olde Rikkert, M.G.M. Raloxifene and tibolone in elderly women: A randomized, double-blind, double-dummy, placebo-controlled trial. J. Am. Med. Dir. Assoc. 2012, 13, 189.e1–189.e7. [Google Scholar] [CrossRef] [PubMed]
- Tommaselli, G.A.; Di Carlo, C.; Di Spiezio Sardo, A.; Bifulco, G.; Cirillo, D.; Guida, M.; Capasso, R.; Nappi, C. Serum leptin levels and body composition in postmenopausal women treated with tibolone and raloxifene. Menopause 2006, 13, 660–668. [Google Scholar] [CrossRef] [PubMed]
- Dalton, J.T. The long and winding road for selective androgen receptor modulators. Br. J. Clin. Pharmacol. 2017, 83, 2131–2133. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Tian, Y.; Zhang, Y.; Zhang, Z.; Chen, R.; Li, M.; Tang, J.; Bian, J.; Li, Z.; Xu, X. Overview of the development of selective androgen receptor modulators (SARMs) as pharmacological treatment for osteoporosis (1998–2021). Eur. J. Med. Chem. 2022, 230, 114119. [Google Scholar] [CrossRef]
- Akel, M.; Parmar, M. Abaloparatide. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Vall, H.; Parmar, M. Teriparatide. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Hong, P.; Liu, R.; Rai, S.; Liu, J.; Zhou, Y.; Zheng, Y.; Li, J. Is abaloparatide more efficacious on increasing bone mineral density than teriparatide for women with postmenopausal osteoporosis? An updated meta-analysis. J. Orthop. Surg. 2023, 18, 116. [Google Scholar] [CrossRef]
- Neer, R.M.; Arnaud, C.D.; Zanchetta, J.R.; Prince, R.; Gaich, G.A.; Reginster, J.Y.; Hodsman, A.B.; Eriksen, E.F.; Ish-Shalom, S.; Genant, H.K.; et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N. Engl. J. Med. 2001, 344, 1434–1441. [Google Scholar] [CrossRef]
- Orwoll, E.S.; Scheele, W.H.; Paul, S.; Adami, S.; Syversen, U.; Diez-Perez, A.; Kaufman, J.M.; Clancy, A.D.; Gaich, G.A. The effect of teriparatide [human parathyroid hormone (1-34)] therapy on bone density in men with osteoporosis. J. Bone Miner. Res. 2003, 18, 9–17. [Google Scholar] [CrossRef]
- Black, D.M.; Greenspan, S.L.; Ensrud, K.E.; Palermo, L.; McGowan, J.A.; Lang, T.F.; Garnero, P.; Bouxsein, M.L.; Bilezikian, J.P.; Rosen, C.J. The Effects of Parathyroid Hormone and Alendronate Alone or in Combination in Postmenopausal Osteoporosis. N. Engl. J. Med. 2003, 349, 1207–1215. [Google Scholar] [CrossRef]
- Kendler, D.L.; Marin, F.; Zerbini, C.A.F.; Russo, L.A.; Greenspan, S.L.; Zikan, V.; Bagur, A.; Malouf-Sierra, J.; Lakatos, P.; Fahrleitner-Pammer, A.; et al. Effects of teriparatide and risedronate on new fractures in post-menopausal women with severe osteoporosis (VERO): A multicentre, double-blind, double-dummy, randomised controlled trial. Lancet 2018, 391, 230–240. [Google Scholar] [CrossRef]
- Fan, G.; Zhao, Q.; Lu, P.; Chen, H.; Tan, W.; Guo, W.; Liu, C.; Liu, J. Comparison between teriparatide and bisphosphonates for improving bone mineral density in postmenopausal osteoporosis patients. Medicine 2020, 99, e18964. [Google Scholar] [CrossRef]
- Black, D.M.; Bilezikian, J.P.; Ensrud, K.E.; Greenspan, S.L.; Palermo, L.; Hue, T.; Lang, T.F.; McGowan, J.A.; Rosen, C.J. PaTH Study Investigators One year of alendronate after one year of parathyroid hormone (1-84) for osteoporosis. N. Engl. J. Med. 2005, 353, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Kontogeorgos, G.; Krantz, E.; Trimpou, P.; Laine, C.M.; Landin-Wilhelmsen, K. Teriparatide treatment in severe osteoporosis-a controlled 10-year follow-up study. BMC Musculoskelet. Disord. 2022, 23, 1011. [Google Scholar] [CrossRef]
- Baczynski, R.; Massry, S.G.; Magott, M.; El-Belbessi, S.; Kohan, R.; Brautbar, N. Effect of parathyroid hormone on energy metabolism ofskeletal muscle. Kidney Int. 1985, 28, 722–727. [Google Scholar] [CrossRef]
- Garber, A.J. Effects of Parathyroid Hormone on Skeletal Muscle Protein and Amino Acid Metabolism in the Rat. J. Clin. Investig. 1983, 71, 1806–1821. [Google Scholar] [CrossRef]
- Aspenberg, P.; Malouf, J.; Tarantino, U.; García-Hernández, P.A.; Corradini, C.; Overgaard, S.; Stepan, J.J.; Borris, L.; Lespessailles, E.; Frihagen, F.; et al. Effects of Teriparatide Compared with Risedronate on Recovery After Pertrochanteric Hip Fracture. J. Bone Joint Surg. Am. 2016, 98, 1868–1878. [Google Scholar] [CrossRef] [PubMed]
- Malouf-Sierra, J.; Tarantino, U.; García-Hernández, P.A.; Corradini, C.; Overgaard, S.; Stepan, J.J.; Borris, L.; Lespessailles, E.; Frihagen, F.; Papavasiliou, K.; et al. Effect of Teriparatide or Risedronate in Elderly Patients with a Recent Pertrochanteric Hip Fracture: Final Results of a 78-Week Randomized Clinical Trial. J. Bone Miner. Res. 2017, 32, 1040–1051. [Google Scholar] [CrossRef] [PubMed]
- Peichl, P.; Holzer, L.A.; Maier, R.; Holzer, G. Parathyroid hormone 1-84 accelerates fracture-healing in pubic bones of elderly osteoporotic women. J. Bone Joint Surg. Am. 2011, 93, 1583–1587. [Google Scholar] [CrossRef]
- Bhandari, M.; Jin, L.; See, K.; Burge, R.; Gilchrist, N.; Witvrouw, R.; Krohn, K.D.; Warner, M.R.; Ahmad, Q.I.; Mitlak, B. Does Teriparatide Improve Femoral Neck Fracture Healing: Results from a Randomized Placebo-controlled Trial. Clin. Orthop. 2016, 474, 1234–1244. [Google Scholar] [CrossRef]
- Jepsen, D.B.; Masud, T.; Holsgaard-Larsen, A.; Hansen, S.; Jørgensen, N.R.; Ryg, J. The combined effect of parathyroid hormone (1–34) and whole-body vibration exercise on physical performance in OSteoporotic women (PaVOS study): A secondary analysis from a randomised controlled trial. BMC Sports Sci. Med. Rehabil. 2020, 12, 54. [Google Scholar] [CrossRef] [PubMed]
- Aspenberg, P.; Genant, H.K.; Johansson, T.; Nino, A.J.; See, K.; Krohn, K.; García-Hernández, P.A.; Recknor, C.P.; Einhorn, T.A.; Dalsky, G.P.; et al. Teriparatide for acceleration of fracture repair in humans: A prospective, randomized, double-blind study of 102 postmenopausal women with distal radial fractures. J. Bone Miner. Res. 2010, 25, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, D.B.; Ryg, J.; Hansen, S.; Jørgensen, N.R.; Gram, J.; Masud, T. The combined effect of Parathyroid hormone (1-34) and whole-body Vibration exercise in the treatment of postmenopausal OSteoporosis (PaVOS study): A randomized controlled trial. Osteoporos. Int. 2019, 30, 1827–1836. [Google Scholar] [CrossRef] [PubMed]
- Tu, X.; Delgado-Calle, J.; Condon, K.W.; Maycas, M.; Zhang, H.; Carlesso, N.; Taketo, M.M.; Burr, D.B.; Plotkin, L.I.; Bellido, T. Osteocytes mediate the anabolic actions of canonical Wnt/β-catenin signaling in bone. Proc. Natl. Acad. Sci. USA 2015, 112, E478–E486. [Google Scholar] [CrossRef]
- Shoback, D.; Rosen, C.J.; Black, D.M.; Cheung, A.M.; Murad, M.H.; Eastell, R. Pharmacological Management of Osteoporosis in Postmenopausal Women: An Endocrine Society Guideline Update. J. Clin. Endocrinol. Metab. 2020, 105, dgaa048. [Google Scholar] [CrossRef]
- Camacho, P.M.; Petak, S.M.; Binkley, N.; Diab, D.L.; Eldeiry, L.S.; Farooki, A.; Harris, S.T.; Hurley, D.L.; Kelly, J.; Lewiecki, E.M.; et al. American Association of Clinical Endocrinologists/American College of Endocrinology Clinical Practice Guidelines for the Diagnosis and Treatment of Postmenopausal Osteoporosis—2020 Update. Endocr. Pract. 2020, 26, 1–46. [Google Scholar] [CrossRef]
- Ishibashi, H.; Crittenden, D.B.; Miyauchi, A.; Libanati, C.; Maddox, J.; Fan, M.; Chen, L.; Grauer, A. Romosozumab increases bone mineral density in postmenopausal Japanese women with osteoporosis: A phase 2 study. Bone 2017, 103, 209–215. [Google Scholar] [CrossRef]
- Graeff, C.; Campbell, G.M.; Peña, J.; Borggrefe, J.; Padhi, D.; Kaufman, A.; Chang, S.; Libanati, C.; Glüer, C.-C. Administration of romosozumab improves vertebral trabecular and cortical bone as assessed with quantitative computed tomography and finite element analysis. Bone 2015, 81, 364–369. [Google Scholar] [CrossRef]
- Cosman, F.; Crittenden, D.B.; Adachi, J.D.; Binkley, N.; Czerwinski, E.; Ferrari, S.; Hofbauer, L.C.; Lau, E.; Lewiecki, E.M.; Miyauchi, A.; et al. Romosozumab Treatment in Postmenopausal Women with Osteoporosis. N. Engl. J. Med. 2016, 375, 1532–1543. [Google Scholar] [CrossRef]
- Genant, H.K.; Engelke, K.; Bolognese, M.A.; Mautalen, C.; Brown, J.P.; Recknor, C.; Goemaere, S.; Fuerst, T.; Yang, Y.-C.; Grauer, A.; et al. Effects of Romosozumab Compared with Teriparatide on Bone Density and Mass at the Spine and Hip in Postmenopausal Women with Low Bone Mass. J. Bone Miner. Res. 2017, 32, 181–187. [Google Scholar] [CrossRef]
- McClung, M.R.; Grauer, A.; Boonen, S.; Bolognese, M.A.; Brown, J.P.; Diez-Perez, A.; Langdahl, B.L.; Reginster, J.-Y.; Zanchetta, J.R.; Wasserman, S.M.; et al. Romosozumab in postmenopausal women with low bone mineral density. N. Engl. J. Med. 2014, 370, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Saag, K.G.; Petersen, J.; Brandi, M.L.; Karaplis, A.C.; Lorentzon, M.; Thomas, T.; Maddox, J.; Fan, M.; Meisner, P.D.; Grauer, A. Romosozumab or Alendronate for Fracture Prevention in Women with Osteoporosis. N. Engl. J. Med. 2017, 377, 1417–1427. [Google Scholar] [CrossRef] [PubMed]
- Magarò, M.S.; Bertacchini, J.; Florio, F.; Zavatti, M.; Potì, F.; Cavani, F.; Amore, E.; De Santis, I.; Bevilacqua, A.; Reggiani Bonetti, L.; et al. Identification of Sclerostin as a Putative New Myokine Involved in the Muscle-to-Bone Crosstalk. Biomedicines 2021, 9, 71. [Google Scholar] [CrossRef] [PubMed]
- Girardi, F.; Le Grand, F. Chapter Five—Wnt Signaling in Skeletal Muscle Development and Regeneration. In Progress in Molecular Biology and Translational Science; Larraín, J., Olivares, G., Eds.; WNT Signaling in Health and Disease; Academic Press: Cambridge, MA, USA, 2018; Volume 153, pp. 157–179. [Google Scholar]
- Fairfield, H.; Rosen, C.J.; Reagan, M.R. Connecting Bone and Fat: The Potential Role for Sclerostin. Curr. Mol. Biol. Rep. 2017, 3, 114–121. [Google Scholar] [CrossRef]
- Ahn, S.H.; Jung, H.-W.; Lee, E.; Baek, J.Y.; Jang, I.-Y.; Park, S.J.; Lee, J.Y.; Choi, E.; Lee, Y.S.; Hong, S.; et al. Decreased Serum Level of Sclerostin in Older Adults with Sarcopenia. Endocrinol. Metab. 2022, 37, 487–496. [Google Scholar] [CrossRef]
- Kim, J.A.; Roh, E.; Hong, S.-H.; Lee, Y.-B.; Kim, N.H.; Yoo, H.J.; Seo, J.A.; Kim, N.H.; Kim, S.G.; Baik, S.H.; et al. Association of serum sclerostin levels with low skeletal muscle mass: The Korean Sarcopenic Obesity Study (KSOS). Bone 2019, 128, 115053. [Google Scholar] [CrossRef]
- Medeiros, M.C.; Rocha, N.; Bandeira, E.; Dantas, I.; Chaves, C.; Oliveira, M.; Bandeira, F. Serum Sclerostin, Body Composition, and Sarcopenia in Hemodialysis Patients with Diabetes. Int. J. Nephrol. 2020, 2020, 4596920. [Google Scholar] [CrossRef]
- Möckel, L.; Bartneck, M.; Möckel, C. Risk of falls in postmenopausal women treated with romosozumab: Preliminary indices from a meta-analysis of randomized, controlled trials. Osteoporos. Sarcopenia 2020, 6, 20–26. [Google Scholar] [CrossRef]
- Schemitsch, E.H.; Miclau, T.; Karachalios, T.; Nowak, L.L.; Sancheti, P.; Poolman, R.W.; Caminis, J.; Daizadeh, N.; Dent-Acosta, R.E.; Egbuna, O.; et al. A Randomized, Placebo-Controlled Study of Romosozumab for the Treatment of Hip Fractures. J. Bone Joint Surg. Am. 2020, 102, 693–702. [Google Scholar] [CrossRef]
- Lair, L.; Qureshi, I.; Bechtold, C.; Heller, L.; Durham, S.; Campbell, D.; Marin, J.; Chen, K.; Coric, V. Taldefgrobep Alfa: Preclinical and Clinical Data Supporting the Phase 3 RESILIENT Study in Spinal Muscular Atrophy. Neuromuscul. Disord. 2023, 33 (Suppl. S1), S163. [Google Scholar] [CrossRef]
- Biohaven Ltd. Biohaven’s Taldefgrobep Alfa Receives FDA Fast Track Designation for Spinal Muscular Atrophy; Biohaven Ltd.: New Haven, CT, USA, 2023. [Google Scholar]
- Lee, S.-J. Myostatin: A Skeletal Muscle Chalone. Annu. Rev. Physiol. 2023, 85, 269–291. [Google Scholar] [CrossRef]
- Schuelke, M.; Wagner, K.R.; Stolz, L.E.; Hübner, C.; Riebel, T.; Kömen, W.; Braun, T.; Tobin, J.F.; Lee, S.-J. Myostatin Mutation Associated with Gross Muscle Hypertrophy in a Child. N. Engl. J. Med. 2004, 350, 2682–2688. [Google Scholar] [CrossRef]
- Camporez, J.-P.G.; Petersen, M.C.; Abudukadier, A.; Moreira, G.V.; Jurczak, M.J.; Friedman, G.; Haqq, C.M.; Petersen, K.F.; Shulman, G.I. Anti-myostatin antibody increases muscle mass and strength and improves insulin sensitivity in old mice. Proc. Natl. Acad. Sci. USA 2016, 113, 2212–2217. [Google Scholar] [CrossRef]
- Lee, S.-J.; Bhasin, S.; Klickstein, L.; Krishnan, V.; Rooks, D. Challenges and Future Prospects of Targeting Myostatin/Activin A Signaling to Treat Diseases of Muscle Loss and Metabolic Dysfunction. J. Gerontol. Ser. A 2023, 78, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, L.; Bechtold, C.; Tirucherai, G.; Ahlijanian, M.; Luo, F. BMS-986089: A novel adnectin protein that dose dependently lowers free myostatin and increases muscle volume and lean body mass. Neuromuscul. Disord. 2016, 26, S95. [Google Scholar] [CrossRef]
- Wagner, K.R.; Wong, B.L.; Byrne, B.J.; Tian, C.; Jacobsen, L.K.; Tirucherai, G.S.; Rabbia, M.; Kletzl, H.; Dukart, J.; Ong, R.; et al. A Phase 1b/2 Study of the Anti-Myostatin Adnectin RG6206 (BMS-986089) in Ambulatory Boys with Duchenne Muscular Dystrophy: A 72-Week Treatment Update (P1.6-062). Neurology 2019, 92. [Google Scholar] [CrossRef]
- Rooks, D.; Praestgaard, J.; Hariry, S.; Laurent, D.; Petricoul, O.; Perry, R.G.; Lach-Trifilieff, E.; Roubenoff, R. Treatment of Sarcopenia with Bimagrumab: Results from a Phase II, Randomized, Controlled, Proof-of-Concept Study. J. Am. Geriatr. Soc. 2017, 65, 1988–1995. [Google Scholar] [CrossRef] [PubMed]
- Rooks, D.; Swan, T.; Goswami, B.; Filosa, L.A.; Bunte, O.; Panchaud, N.; Coleman, L.A.; Miller, R.R.; Garcia Garayoa, E.; Praestgaard, J.; et al. Bimagrumab vs Optimized Standard of Care for Treatment of Sarcopenia in Community-Dwelling Older Adults. JAMA Netw. Open 2020, 3, e2020836. [Google Scholar] [CrossRef]
- Hofbauer, L.C.; Witvrouw, R.; Varga, Z.; Shiota, N.; Cremer, M.; Tanko, L.B.; Rooks, D.; Auberson, L.Z.; Arkuszewski, M.; Fretault, N.; et al. Bimagrumab to improve recovery after hip fracture in older adults: A multicentre, double-blind, randomised, parallel-group, placebo-controlled, phase 2a/b trial. Lancet Healthy Longev. 2021, 2, e263–e274. [Google Scholar] [CrossRef]
- Rooks, D.; Petricoul, O.; Praestgaard, J.; Bartlett, M.; Laurent, D.; Roubenoff, R. Safety and pharmacokinetics of bimagrumab in healthy older and obese adults with body composition changes in the older cohort. J. Cachexia Sarcopenia Muscle 2020, 11, 1525–1534. [Google Scholar] [CrossRef]
- Woodhouse, L.; Gandhi, R.; Warden, S.J.; Poiraudeau, S.; Myers, S.L.; Benson, C.T.; Hu, L.; Ahmad, Q.I.; Linnemeier, P.; Gomez, E.V.; et al. A Phase 2 Randomized Study Investigating the Efficacy and Safety of Myostatin Antibody LY2495655 versus Placebo in Patients Undergoing Elective Total Hip Arthroplasty. J. Frailty Aging 2016, 5, 62–70. [Google Scholar] [CrossRef]
- Becker, C.; Lord, S.R.; Studenski, S.A.; Warden, S.J.; Fielding, R.A.; Recknor, C.P.; Hochberg, M.C.; Ferrari, S.L.; Blain, H.; Binder, E.F.; et al. Myostatin antibody (LY2495655) in older weak fallers: A proof-of-concept, randomised, phase 2 trial. Lancet Diabetes Endocrinol. 2015, 3, 948–957. [Google Scholar] [CrossRef]
- Wagner, K.R.; Abdel-Hamid, H.Z.; Mah, J.K.; Campbell, C.; Guglieri, M.; Muntoni, F.; Takeshima, Y.; McDonald, C.M.; Kostera-Pruszczyk, A.; Karachunski, P.; et al. Randomized phase 2 trial and open-label extension of domagrozumab in Duchenne muscular dystrophy. Neuromuscul. Disord. 2020, 30, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Glasser, C.E.; Gartner, M.R.; Wilson, D.; Miller, B.; Sherman, M.L.; Attie, K.M. Locally acting ACE-083 increases muscle volume in healthy volunteers. Muscle Nerve 2018, 57, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Statland, J.M.; Campbell, C.; Desai, U.; Karam, C.; Díaz-Manera, J.; Guptill, J.T.; Korngut, L.; Genge, A.; Tawil, R.N.; Elman, L.; et al. Randomized phase 2 study of ACE-083, a muscle-promoting agent, in facioscapulohumeral muscular dystrophy. Muscle Nerve 2022, 66, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Elkasrawy, M.N.; Hamrick, M.W. Myostatin (GDF-8) as a key factor linking muscle mass and bone structure. J. Musculoskelet. Neuronal Interact. 2010, 10, 56–63. [Google Scholar]
- Cui, Y.; Yi, Q.; Sun, W.; Huang, D.; Zhang, H.; Duan, L.; Shang, H.; Wang, D.; Xiong, J. Molecular basis and therapeutic potential of myostatin on bone formation and metabolism in orthopedic disease. BioFactors 2023, 49, 21–31. [Google Scholar] [CrossRef]
- Morissette, M.R.; Stricker, J.C.; Rosenberg, M.A.; Buranasombati, C.; Levitan, E.B.; Mittleman, M.A.; Rosenzweig, A. Effects of Myostatin Deletion in Aging Mice. Aging Cell 2009, 8, 573–583. [Google Scholar] [CrossRef]
- Goh, B.C.; Singhal, V.; Herrera, A.J.; Tomlinson, R.E.; Kim, S.; Faugere, M.-C.; Germain-Lee, E.L.; Clemens, T.L.; Lee, S.-J.; DiGirolamo, D.J. Activin receptor type 2A (ACVR2A) functions directly in osteoblasts as a negative regulator of bone mass. J. Biol. Chem. 2017, 292, 13809–13822. [Google Scholar] [CrossRef]
- DiGirolamo, D.J.; Singhal, V.; Chang, X.; Lee, S.-J.; Germain-Lee, E.L. Administration of soluble activin receptor 2B increases bone and muscle mass in a mouse model of osteogenesis imperfecta. Bone Res. 2015, 3, 14042. [Google Scholar] [CrossRef]
- Pearsall, R.S.; Canalis, E.; Cornwall-Brady, M.; Underwood, K.W.; Haigis, B.; Ucran, J.; Kumar, R.; Pobre, E.; Grinberg, A.; Werner, E.D.; et al. A soluble activin type IIA receptor induces bone formation and improves skeletal integrity. Proc. Natl. Acad. Sci. USA 2008, 105, 7082–7087. [Google Scholar] [CrossRef]
- Bialek, P.; Parkington, J.; Li, X.; Gavin, D.; Wallace, C.; Zhang, J.; Root, A.; Yan, G.; Warner, L.; Seeherman, H.J.; et al. A myostatin and activin decoy receptor enhances bone formation in mice. Bone 2014, 60, 162–171. [Google Scholar] [CrossRef]
- Ruckle, J.; Jacobs, M.; Kramer, W.; Pearsall, A.E.; Kumar, R.; Underwood, K.W.; Seehra, J.; Yang, Y.; Condon, C.H.; Sherman, M.L. Single-dose, randomized, double-blind, placebo-controlled study of ACE-011 (ActRIIA-IgG1) in postmenopausal women. J. Bone Miner. Res. 2009, 24, 744–752. [Google Scholar] [CrossRef]
- Campbell, C.; McMillan, H.J.; Mah, J.K.; Tarnopolsky, M.; Selby, K.; McClure, T.; Wilson, D.M.; Sherman, M.L.; Escolar, D.; Attie, K.M. Myostatin inhibitor ACE-031 treatment of ambulatory boys with Duchenne muscular dystrophy: Results of a randomized, placebo-controlled clinical trial. Muscle Nerve 2017, 55, 458–464. [Google Scholar] [CrossRef]
- Suh, J.; Kim, N.-K.; Lee, S.-H.; Eom, J.-H.; Lee, Y.; Park, J.-C.; Woo, K.M.; Baek, J.-H.; Kim, J.-E.; Ryoo, H.-M.; et al. GDF11 promotes osteogenesis as opposed to MSTN, and follistatin, a MSTN/GDF11 inhibitor, increases muscle mass but weakens bone. Proc. Natl. Acad. Sci. USA 2020, 117, 4910–4920. [Google Scholar] [CrossRef]
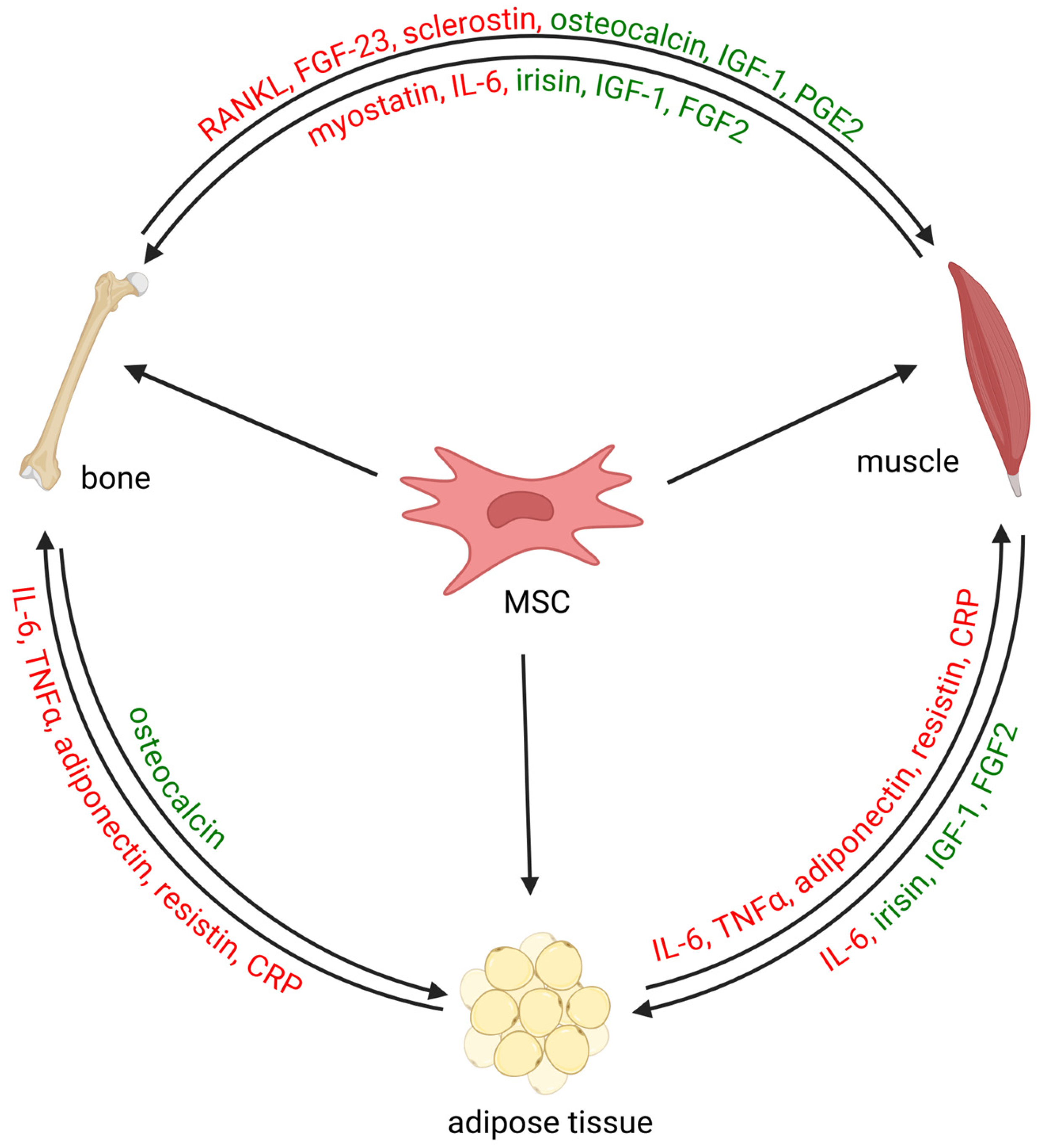
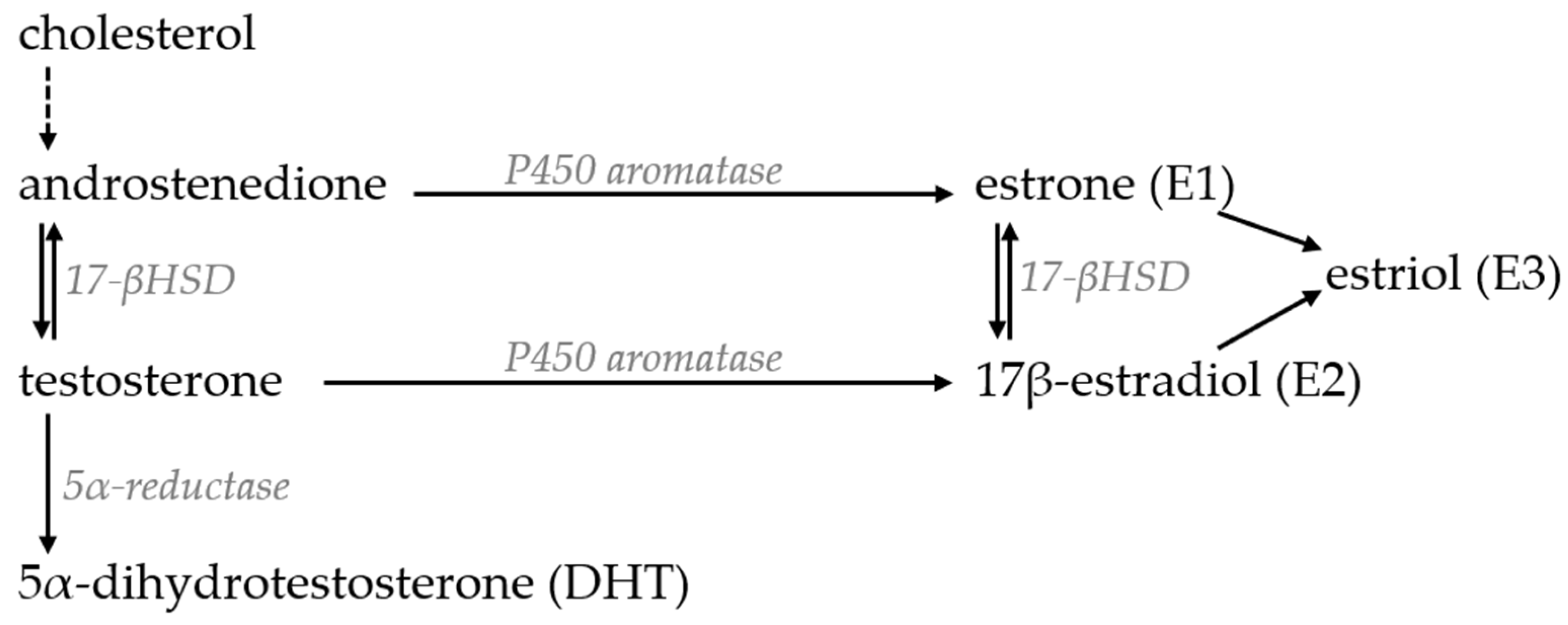
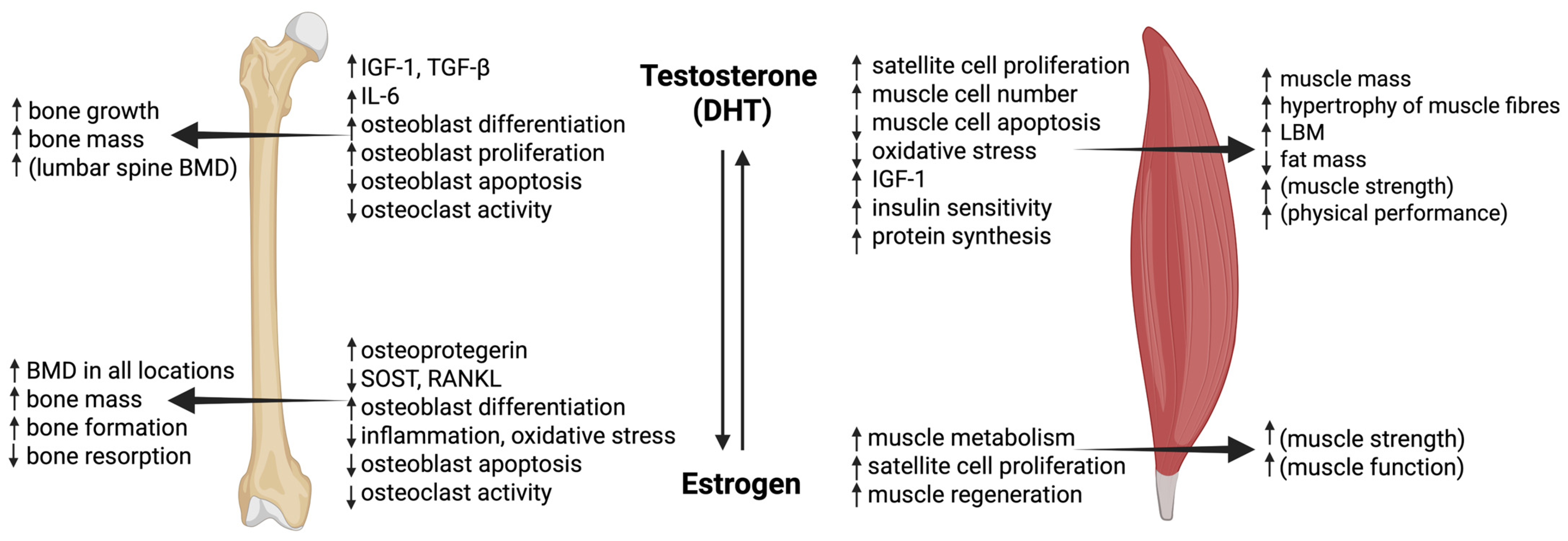
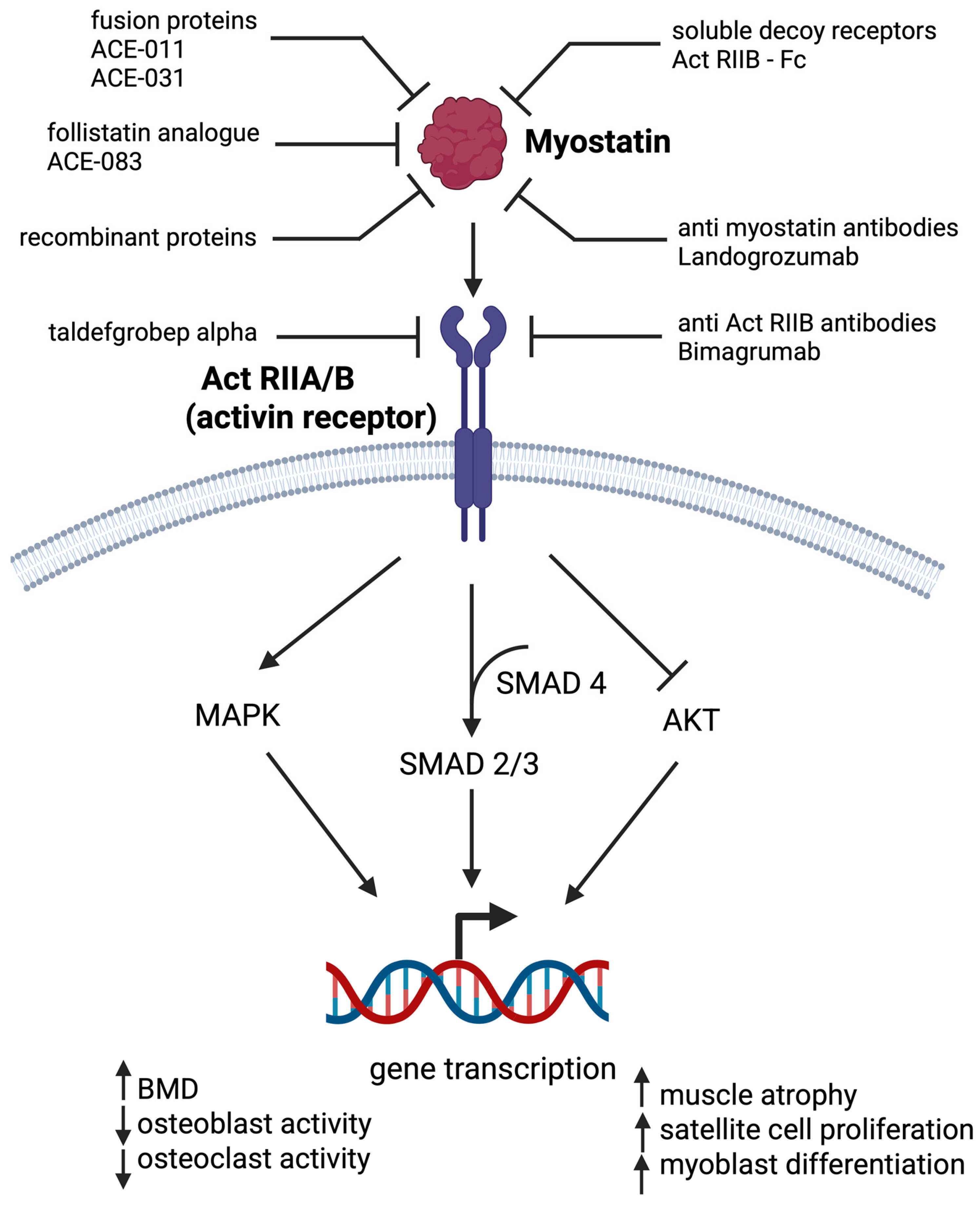
| Cohort | Study | Regime | Effects on Bone | Effects on Muscle | Ref. |
|---|---|---|---|---|---|
| 198 women aged 70–80 years | Randomized, double-blind, placebo-controlled | 60 mg/day for 12 months | / | Increase in fat-free mass and total body water; no differences in BMI and fat mass. No significant differences in muscle strength or muscle power. | [214] |
| 198 community-dwelling women aged > 70 | Randomized, double-blind, placebo-controlled | 60 mg/day for 24 months | Increased hip (0.011 g/cm3) and lumbar spine (0.02 g/cm3) BMD compared to placebo | No effect on handgrip muscle strength, muscle power, mobility measures and body composition compared to placebo. | [215] |
| 45 postmenopausal women | Prospective, randomized, controlled | 60 mg/day for 12 months | / | No changes in LBM; retained baseline fat mass (increased in control group). | [216] |
| Cohort | Study | Regime | Effects on Bone | Effects on Muscle | Ref. |
|---|---|---|---|---|---|
| 86 osteoporotic patients with hip fracture aged ≥ 50 years (+85 patients on risendronate) | Phase IV, randomized, multicenter, active-controlled trial | 20 μg/day subcutaneous (+ calcium and vitamin D) for 6 months | Similar hip fracture healing rate compared to risendronate group (radiographic evidence) | Shorter time to complete TUG test and reduced hip pain compared to risendronate group | [231] |
| 389 osteoporotic patients with pertrochanteric fracture (+85 patients on risendronate) | Multinational, multicenter, prospective, randomized, active-controlled | 20 μg/day subcutaneous (+ calcium and vitamin D) for 1.5 years | Increased lumbar BMD compared to risendronate group (mean difference, 0.040 g/cm2), increased femoral neck BMD compared to baseline and no change in total hip BMD from baseline or risendronate group | Shorter time to complete TUG test and reported pain with VAS score at early time points; no difference compared to risendronate group when the fracture healed | [232] |
| 21 patients with pelvic fracture (44 controls) | Prospective, randomized, controlled | 100 μg of PTH 1–84/day (+ calcium and vitamin D) | Faster fracture healing compared to control (radiographic evidence) | Shorter time to complete TUG test and improved VAS score for pain compared to control group | [233] |
| 78 patients with femoral neck fracture aged ≥ 50 years (81 control group) | Prospective, randomized, double-blind, placebo-controlled phase III | 20 μg/day subcutaneous (+ calcium and vitamin D) for 6 months | No differences in radiographic healing between the teriparatide and placebo groups at 10 weeks, 6 months or 12 months | No statistical difference in pain scores, gait speed time or recovery to pre-fracture ambulatory status but with a trend of better recovery in teriparatide group | [234] |
| 35 osteoporotic postmenopausal women aged ≥ 50 years with history of fracture | Randomized, controlled trial | 20 μg/day subcutaneous alone or in combination with WBV for 1 year | Significant increase in lumbar spine BMD in teriparatide group (6.65% ± 5.51) and teriparatide + WBV (8.90% ± 5.47), increase in bone turnover markers in both groups and no change bone microarchitecture parameters | Improved SPPB, 5TSTS, leg extension power and time to walk three meters in teriparatide + WBV compared to baseline; teriparatide-only group showed an increase in leg extension power compared to baseline; no significant change in handgrip strength, TUG or total or appendicular LBM | [235,237] |
| 102 postmenopausal women with distal radius fracture | Multinational, multicenter, prospective, randomized, controlled, double-blind study | 20 or 40 µg or placebo/day for 8 weeks (+ calcium and vitamin D) | Radiographic evidence of healing detected at 9.1, 7.4 and 8.8 weeks in the placebo, teriparatide 20 µg and teriparatide 40 µg groups, respectively (not statistically different); no differences in radiologic and anatomic deformities | Significant improvement in pain scores, grip strength and functional test (patient-rated wrist evaluation score) in both treatment groups but no significant differences compared to placebo | [236] |
| Drug Type | Effects on Bone | Effects on Muscle |
|---|---|---|
| Denosumab | Rapid increase in femoral neck, lumbar spine, hip, trochanter and total body BMD through inhibition of osteoclast activity and reduction in vertebral and non-vertebral fractures | Indications of increased muscle mass, strength (improved handgrip strength) and function (gait speed, TUG, FSST) |
| Bisphosphonates | Prevention of further bone loss and potential gradual increase in BMD through suppression of osteoclast activity | Conflicting evidence |
| Testosterone | Suggested lumbar spine BMD improvement in men with low serum testosterone concentrations | Increase in LBM and decrease in fat mass; indicated increase in strength and physical performance if combined with physical exercise |
| Estrogen | Increase in hip, lumbar spine and total body BMD and reduced risk of fractures | Indicated preservation of or increase in LBM and muscle performance in younger women if treated immediately upon menopause onset |
| SERMs | Prevention of or increase in BMD loss in spine and femoral neck and reduction in vertebral fractures | Maintenance of or reduction in fat mass; no indicated effect on LBM, muscle strength or performance |
| Teriparatide, Abaloparatide | Increase in cancellous bone, reduced risk of vertebral and non-vertebral fractures and improved fracture healing | Better early functional outcomes following fracture healing |
| Romosozumab | SOST inhibition leading to effective increase in bone formation and decrease in bone resorption | No evidence |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gavrilov, Z.; Lojk, J. Rethinking Osteoporosis Drugs: Can We Simultaneously Address Sarcopenia? Int. J. Mol. Sci. 2025, 26, 6924. https://doi.org/10.3390/ijms26146924
Gavrilov Z, Lojk J. Rethinking Osteoporosis Drugs: Can We Simultaneously Address Sarcopenia? International Journal of Molecular Sciences. 2025; 26(14):6924. https://doi.org/10.3390/ijms26146924
Chicago/Turabian StyleGavrilov, Zoran, and Jasna Lojk. 2025. "Rethinking Osteoporosis Drugs: Can We Simultaneously Address Sarcopenia?" International Journal of Molecular Sciences 26, no. 14: 6924. https://doi.org/10.3390/ijms26146924
APA StyleGavrilov, Z., & Lojk, J. (2025). Rethinking Osteoporosis Drugs: Can We Simultaneously Address Sarcopenia? International Journal of Molecular Sciences, 26(14), 6924. https://doi.org/10.3390/ijms26146924




