Csn5 Depletion Reverses Mitochondrial Defects in GCN5-Null Saccharomyces cerevisiae
Abstract
1. Introduction
2. Results
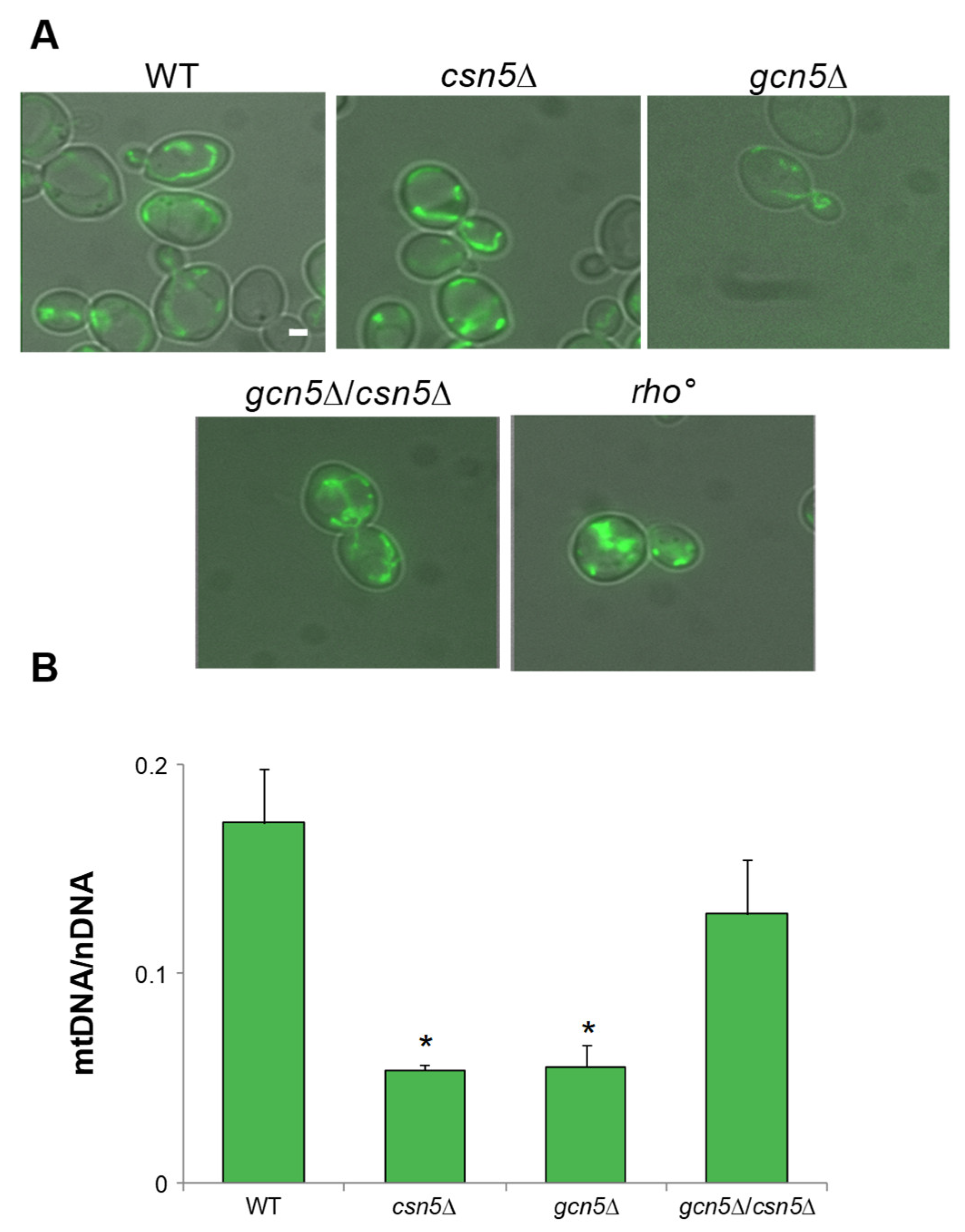
2.1. GCN5 Gene Deletion Is Associated with mtDNA Instability
2.1.1. GCN5 Deletion Impairs mtDNA Migration into the Bud During Cell Division
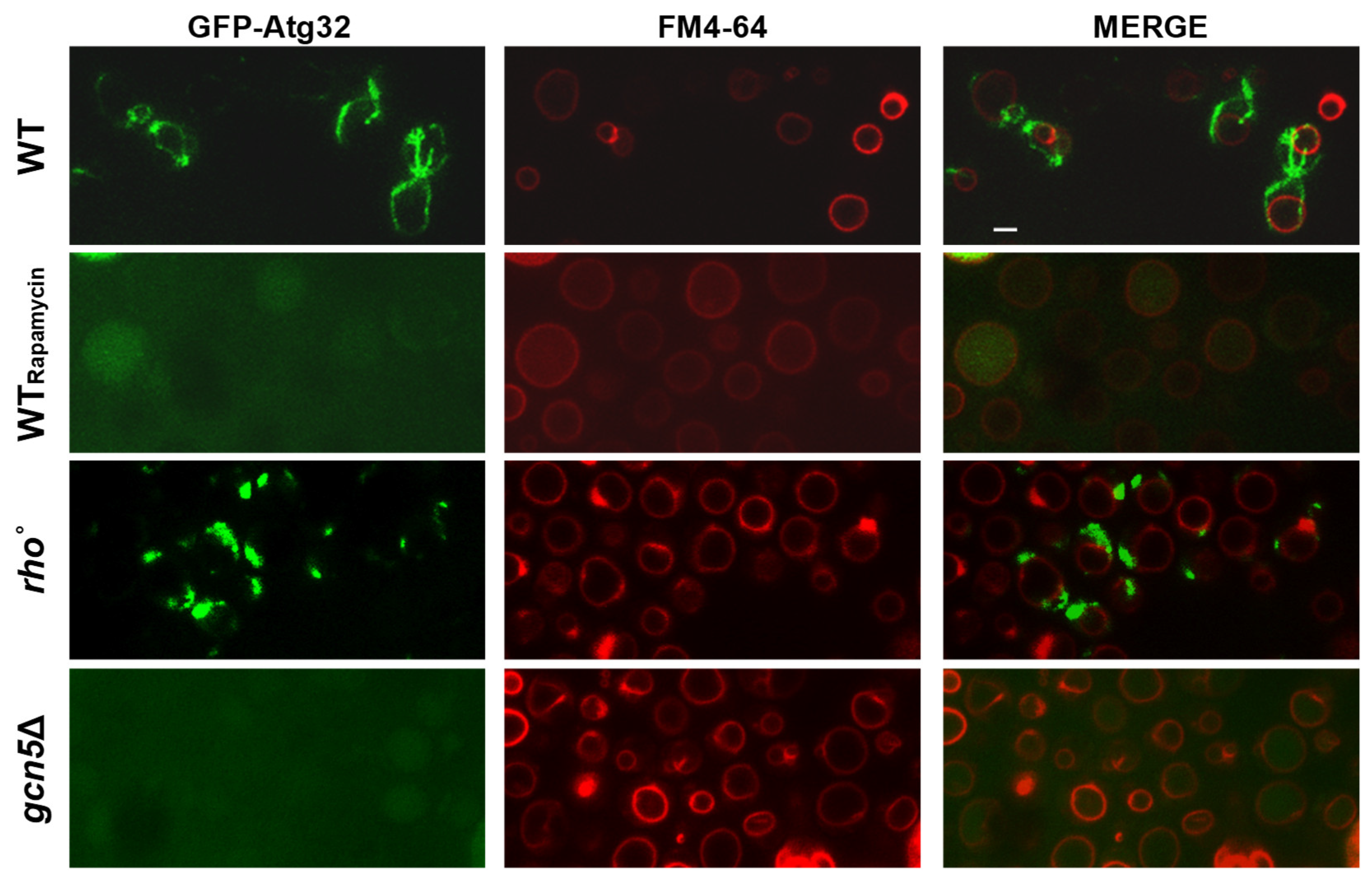
2.1.2. The gcn5Δ Strain Undergoes the Mitophagy Process
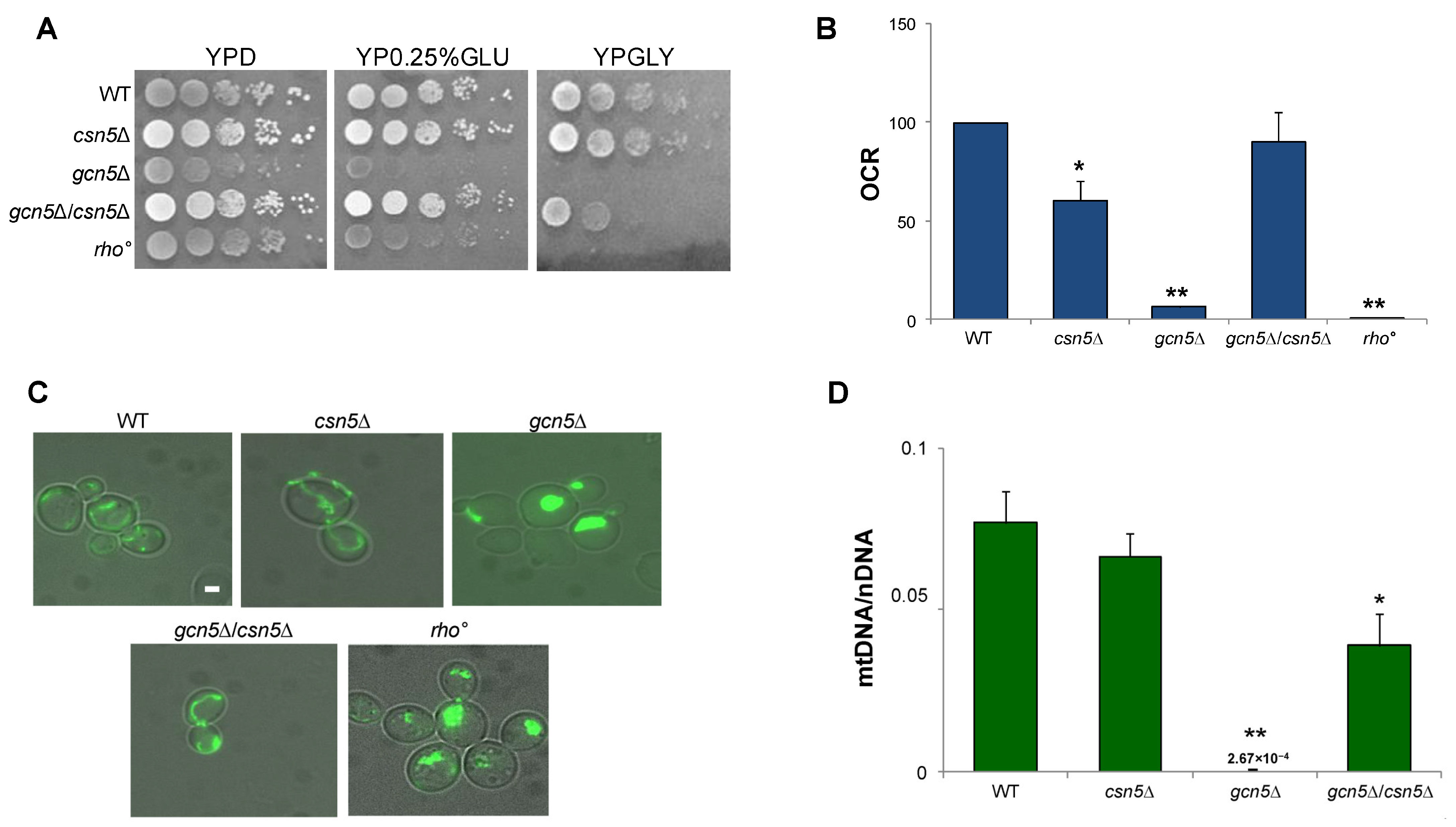
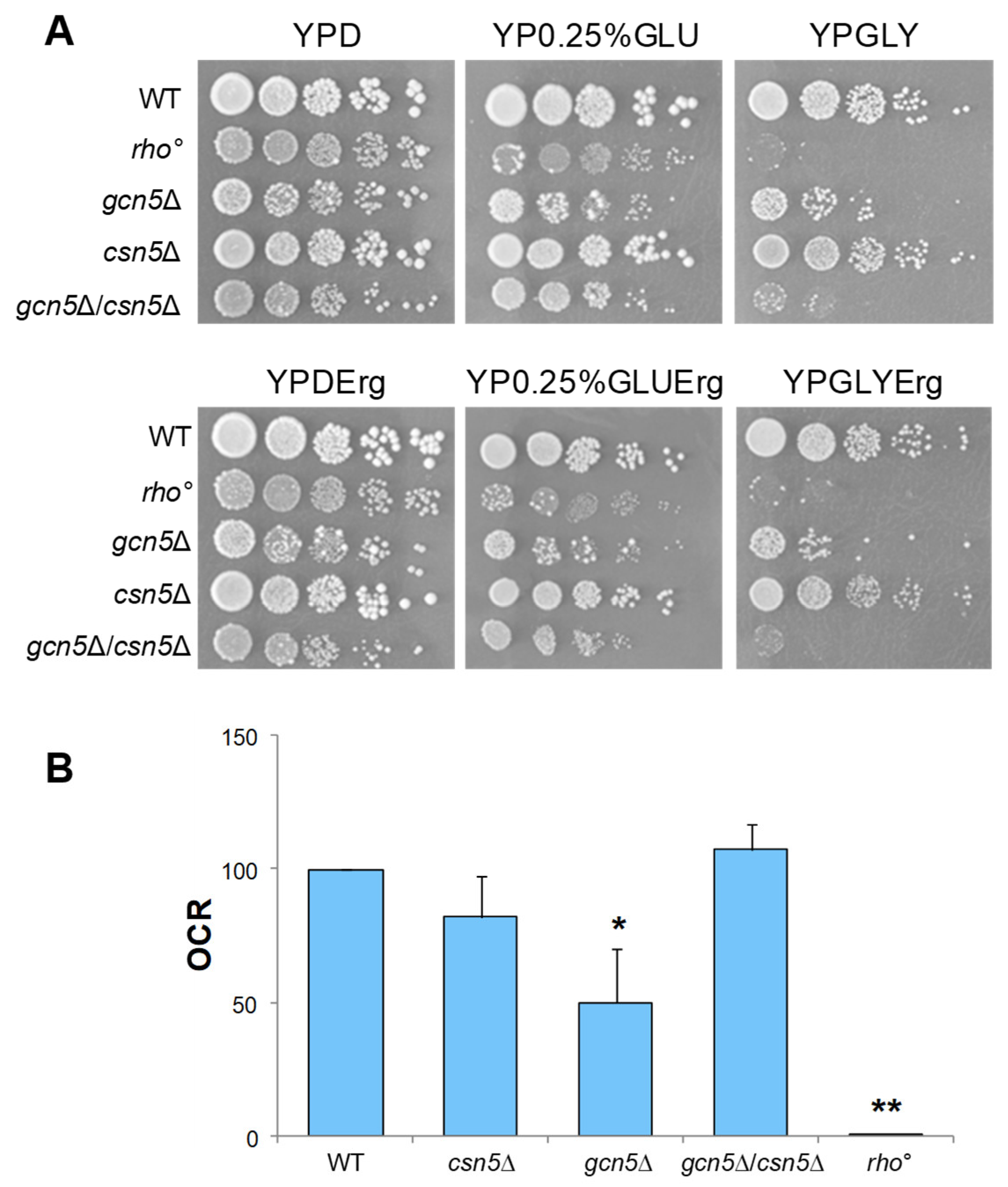
2.2. CSN5 Deletion Rescues the Mitochondrial Defects of gcn5Δ Strain
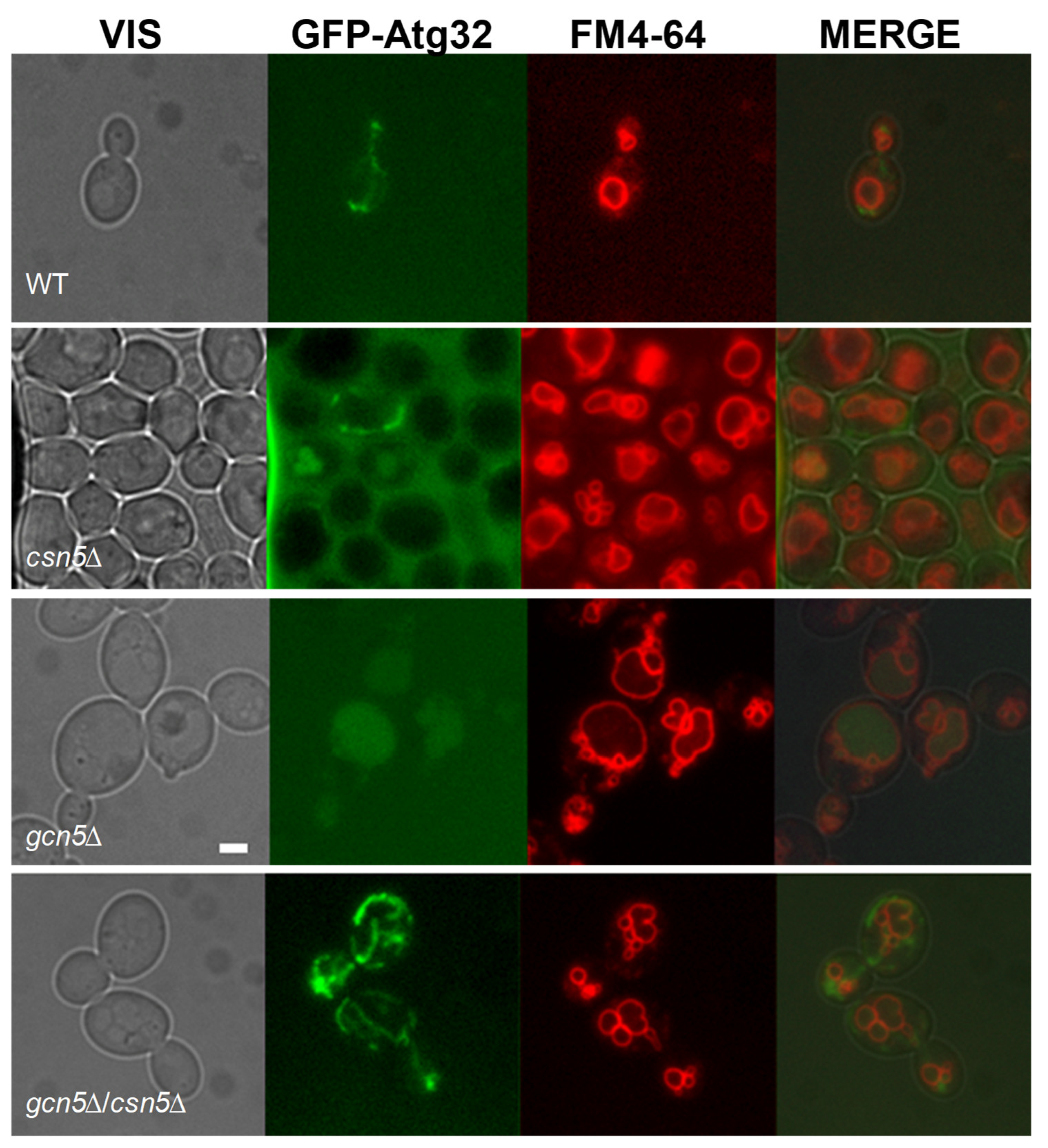
2.2.1. Mitochondrial Phenotype of csn5Δ/gcn5Δ Double Mutant
2.2.2. CSN5 Deletion Enhances Mitochondrial Stability and Suppresses Mitophagy in the gcn5Δ Background
2.3. Ergosterol Supplementation Restores Mitochondrial Functionality
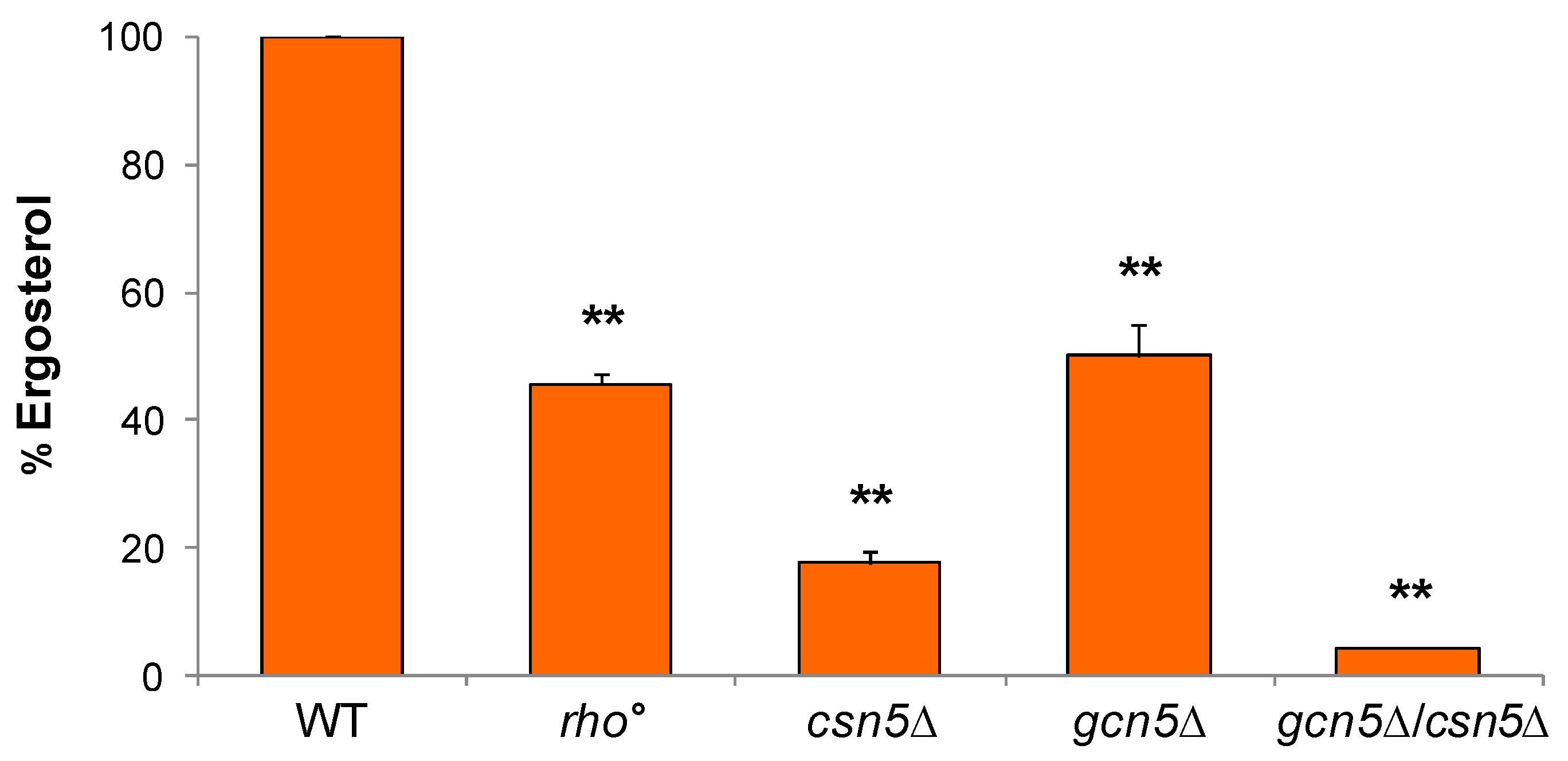
2.3.1. Ergosterol Content Is Decreased in Mutant Cells
2.3.2. Ergosterol Rescues Mitochondrial Defects of gcn5Δ Mutant and Acts Additively with CSN5 Deletion
3. Discussion
4. Materials and Methods
4.1. Yeast Strains and Growth Conditions
4.2. Oxygen Consumption Measurement
4.3. Plasmids and Transformation Experiments
4.4. Quantification of mtDNA
4.5. Actin Detection
4.6. Mitophagy Detection
4.7. Ergosterol Extraction
4.8. High-Performance Liquid Chromatography (HPLC) Analysis
4.9. Fluorescence Imaging
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| SAGA | Spt-Ada-Gcn5 Acetyltransferase |
| KAT | Lysine-Acetyltransferase |
| HAT | Histone Acetyltransferase |
| DUB | Deubiquitinase |
| SLIK | SAGA-like |
| OMM | Outer Mitochondrial Membrane |
| IMM | Inner Mitochondrial Membrane |
| mtDNA | Mitochondrial DNA |
| nDNA | Nuclear DNA |
| CSN | COP9 Signalosome |
| CRLs | Cullin RING E3 Ligases |
| UFA | Unsaturated Fatty Acids |
| ER | Endoplasmic Reticulum |
| YPD | Yeast Extract + Peptone + Glucose |
| mtGFP | Mitochondrial Green Fluorescent Protein |
| qRT-PCR | Quantitative Real Time Polymerase Chain Reaction |
| DAPI | 4′,6-diamidino-2-phenylindole |
| FM4-64 | N-(3-Triethylammoniumpropyl)-4-(6-(4-(Diethylamino) Phenyl) Hexatrienyl) Pyridinium Dibromide |
| OD600 | Optical Density at 600 nm |
| HPLC | High Performance Liquid Chromatography |
| DAD | Diode Array Detector |
| BHT | Butylated Hydroxytoluene |
| SE | Standard Error |
| OCR | Oxygen Consumption Rate |
References
- Soffers, J.H.M.; Workman, J.L. The SAGA Chromatin-Modifying Complex: The Sum of Its Parts Is Greater than the Whole. Genes Dev. 2020, 34, 1287–1303. [Google Scholar] [CrossRef] [PubMed]
- Chou, K.Y.; Lee, J.-Y.; Kim, K.-B.; Kim, E.; Lee, H.-S.; Ryu, H.-Y. Histone Modification in Saccharomyces cerevisiae: A Review of the Current Status. Comput. Struct. Biotechnol. J. 2023, 21, 1843–1850. [Google Scholar] [CrossRef]
- Patel, J.H.; Du, Y.; Ard, P.G.; Phillips, C.; Carella, B.; Chen, C.-J.; Rakowski, C.; Chatterjee, C.; Lieberman, P.M.; Lane, W.S.; et al. The C-MYC Oncoprotein Is a Substrate of the Acetyltransferases hGCN5/PCAF and TIP60. Mol. Cell. Biol. 2004, 24, 10826–10834. [Google Scholar] [CrossRef] [PubMed]
- Canzonetta, C.; Leo, M.; Guarino, S.R.; Montanari, A.; Francisci, S.; Filetici, P. SAGA Complex and Gcn5 Are Necessary for Respiration in Budding Yeast. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2016, 1863, 3160–3168. [Google Scholar] [CrossRef] [PubMed]
- Montanari, A.; Leo, M.; De Luca, V.; Filetici, P.; Francisci, S. Gcn5 Histone Acetyltransferase Is Present in the Mitoplasts. Biol. Open 2019, 8, bio041244. [Google Scholar] [CrossRef] [PubMed]
- De Luca, V.; Leo, M.; Cretella, E.; Montanari, A.; Saliola, M.; Ciaffi, G.; Vecchione, A.; Stoppacciaro, A.; Filetici, P. Role of yUbp8 in Mitochondria and Hypoxia Entangles the Finding of Human Ortholog Usp22 in the Glioblastoma Pseudo-Palisade Microlayer. Cells 2022, 11, 1682. [Google Scholar] [CrossRef] [PubMed]
- Leo, M.; Fanelli, G.; Di Vito, S.; Traversetti, B.; La Greca, M.; Palladino, R.A.; Montanari, A.; Francisci, S.; Filetici, P. Ubiquitin Protease Ubp8 Is Necessary for S. Cerevisiae Respiration. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2018, 1865, 1491–1500. [Google Scholar] [CrossRef] [PubMed]
- Hoke, S.M.; Guzzo, J.; Andrews, B.; Brandl, C.J. Systematic Genetic Array Analysis Links the Saccharomyces cerevisiae SAGA/SLIK and NuA4 Component Tra1 to Multiple Cellular Processes. BMC Genet. 2008, 9, 46. [Google Scholar] [CrossRef] [PubMed]
- Bari, K.A.; Berg, M.D.; Genereaux, J.; Brandl, C.J.; Lajoie, P. Tra1 Controls the Transcriptional Landscape of the Aging Cell. G3 2023, 13, jkac287. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Dai, W.; Xiao, D.; Xiong, Q.; Liu, C.; Hu, J.; Ge, F.; Yu, X.; Li, S. Acetylation-Dependent SAGA Complex Dimerization Promotes Nucleosome Acetylation and Gene Transcription. Nat. Struct. Mol. Biol. 2022, 29, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Cirigliano, A.; Macone, A.; Bianchi, M.M.; Oliaro-Bosso, S.; Balliano, G.; Negri, R.; Rinaldi, T. Ergosterol Reduction Impairs Mitochondrial DNA Maintenance in S. Cerevisiae. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2019, 1864, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Cirigliano, A.; Amelina, A.; Biferali, B.; Macone, A.; Mozzetta, C.; Bianchi, M.M.; Mori, M.; Botta, B.; Pick, E.; Negri, R.; et al. Statins Interfere with the Attachment of S. Cerevisiae mtDNA to the Inner Mitochondrial Membrane. J. Enzym. Inhib. Med. Chem. 2020, 35, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Boldogh, I.R.; Pon, L.A. Mitochondria on the Move. Trends Cell Biol. 2007, 17, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, L.; Rust, M.B.; Culmsee, C. Actin(g) on Mitochondria—A Role for Cofilin1 in Neuronal Cell Death Pathways. Biol. Chem. 2019, 400, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, T.; Pick, E.; Gambadoro, A.; Zilli, S.; Maytal-Kivity, V.; Frontali, L.; Glickman, M.H. Participation of the Proteasomal Lid Subunit Rpn11 in Mitochondrial Morphology and Function Is Mapped to a Distinct C-Terminal Domain. Biochem. J. 2004, 381, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Livnat-Levanon, N.; Glickman, M.H. Ubiquitin–Proteasome System and Mitochondria—Reciprocity. Biochim. Biophys. Acta (BBA)—Gene Regul. Mech. 2011, 1809, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Bramasole, L.; Sinha, A.; Gurevich, S.; Radzinski, M.; Klein, Y.; Panat, N.; Gefen, E.; Rinaldi, T.; Jimenez-Morales, D.; Johnson, J.; et al. Proteasome Lid Bridges Mitochondrial Stress with Cdc53/Cullin1 NEDDylation Status. Redox Biol. 2019, 20, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Barth, E.; Hübler, R.; Baniahmad, A.; Marz, M. The Evolution of COP9 Signalosome in Unicellular and Multicellular Organisms. Genome Biol. Evol. 2016, 8, 1279–1289. [Google Scholar] [CrossRef] [PubMed]
- Wee, S.; Hetfeld, B.; Dubiel, W.; Wolf, D.A. Conservation of the COP9/signalosome in budding yeast. BMC Genet. 2002, 3, 15. [Google Scholar] [CrossRef]
- Maytal-Kivity, V.; Pick, E.; Piran, R.; Hofmann, K.; Glickman, M.H. The COP9 Signalosome-like Complex in S. Cerevisiae and Links to Other PCI Complexes. Int. J. Biochem. Cell Biol. 2003, 35, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Licursi, V.; Salvi, C.; De Cesare, V.; Rinaldi, T.; Mattei, B.; Fabbri, C.; Serino, G.; Bramasole, L.; Zimbler, J.Z.; Pick, E.; et al. The COP9 Signalosome Is Involved in the Regulation of Lipid Metabolism and of Transition Metals Uptake in Saccharomyces cerevisiae. FEBS J. 2014, 281, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.; Israeli, R.; Cirigliano, A.; Gihaz, S.; Trabelcy, B.; Braus, G.H.; Gerchman, Y.; Fishman, A.; Negri, R.; Rinaldi, T.; et al. The COP9 Signalosome Mediates the Spt23 Regulated Fatty Acid Desaturation and Ergosterol Biosynthesis. FASEB J. 2020, 34, 4870–4889. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhang, Y.; Wang, W.; Cao, X.; Xu, H.; Liu, H.; Qi, J.; Jiang, C.; Wang, C. FgCsn12 Is Involved in the Regulation of Ascosporogenesis in the Wheat Scab Fungus Fusarium Graminearum. Int. J. Mol. Sci. 2022, 23, 10445. [Google Scholar] [CrossRef]
- Chen, A.; Ren, Y.; Han, X.; Liu, C.; Zhou, Y.; Xu, C.; Qi, H.; Ma, Z.; Chen, Y. The COP9 Signalosome Complex Regulates Fungal Development and Virulence in the Wheat Scab Fungus Fusarium Graminearum. Front. Microbiol. 2023, 14, 1179676. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Dai, H.; Bashir, A.; Wang, Z.; An, Y.; Yu, X.; Xu, L.; Li, L. Nematicidal Activity and Action Mode of a Methyl-Accepting Chemotaxis Protein from Pseudomonas Syringae against Caenorhabditis Elegans. Heliyon 2024, 10, e30366. [Google Scholar] [CrossRef] [PubMed]
- Franciosini, A.; Lombardi, B.; Iafrate, S.; Pecce, V.; Mele, G.; Lupacchini, L.; Rinaldi, G.; Kondou, Y.; Gusmaroli, G.; Aki, S.; et al. The Arabidopsis COP9 SIGNALOSOME INTERACTING F-BOX KELCH 1 Protein Forms an SCF Ubiquitin Ligase and Regulates Hypocotyl Elongation. Mol. Plant 2013, 6, 1616–1629. [Google Scholar] [CrossRef] [PubMed]
- Franciosini, A.; Moubayidin, L.; Du, K.; Matari, N.H.; Boccaccini, A.; Butera, S.; Vittorioso, P.; Sabatini, S.; Jenik, P.D.; Costantino, P.; et al. The COP9 SIGNALOSOME Is Required for Postembryonic Meristem Maintenance in Arabidopsis Thaliana. Mol. Plant 2015, 8, 1623–1634. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, Y.; Xu, Y.; Li, R.; Huang, S.; Wu, Q.; Yan, J.; Jiang, Z.; Wu, X.; Li, F.; Wang, Y.; et al. Transcriptomic and Metabolomic Analysis Reveals Multifaceted Impact of Gcn5 Knockdown in Drosophila Development. Metabolites 2024, 14, 680. [Google Scholar] [CrossRef] [PubMed]
- Koutelou, E.; Farria, A.T.; Dent, S.Y.R. Complex Functions of Gcn5 and Pcaf in Development and Disease. Biochim. Biophys. Acta (BBA)—Gene Regul. Mech. 2021, 1864, 194609. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Y.; Wang, B.; Ma, Y.; Chen, P. CSN5/Jab1 Facilitates Non-Small Cell Lung Cancer Cell Growth through Stabilizing Survivin. Biochem. Biophys. Res. Commun. 2018, 500, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Wang, Y.; Zhao, Y.; Shu, Y.; Liu, Z.; Zhou, H.; Wang, H.; Zhang, W. The Pivotal Oncogenic Role of Jab1/CSN5 and Its Therapeutic Implications in Human Cancer. Gene 2019, 687, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.-T.; Jin, J.; Zheng, Z.-G. Emerging Role of GCN5 in Human Diseases and Its Therapeutic Potential. Biomed. Pharmacother. 2023, 165, 114835. [Google Scholar] [CrossRef] [PubMed]
- Dewson, G.; Eichhorn, P.J.A.; Komander, D. Deubiquitinases in Cancer. Nat. Rev. Cancer 2023, 23, 842–862. [Google Scholar] [CrossRef] [PubMed]
- Bhatia-Kissova, I.; Camougrand, N. Mitophagy in Yeast: Decades of Research. Cells 2021, 10, 3541. [Google Scholar] [CrossRef] [PubMed]
- Miyakawa, I. Organization and Dynamics of Yeast Mitochondrial Nucleoids. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2017, 93, 339–359. [Google Scholar] [CrossRef] [PubMed]
- Vernarecci, S.; Ornaghi, P.; Bâgu, A.; Cundari, E.; Ballario, P.; Filetici, P. Gcn5p Plays an Important Role in Centromere Kinetochore Function in Budding Yeast. Mol. Cell. Biol. 2008, 28, 988–996. [Google Scholar] [CrossRef] [PubMed]
- Innokentev, A.; Kanki, T. Mitophagy in Yeast: Molecular Mechanism and Regulation. Cells 2021, 10, 3569. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, T.; Ricci, C.; Porro, D.; Bolotin-Fukuhara, M.; Frontali, L. A Mutation in a Novel Yeast Proteasomal Gene, RPN11/MPR1, Produces a Cell Cycle Arrest, Overreplication of Nuclear and Mitochondrial DNA, and an Altered Mitochondrial Morphology. MBoC 1998, 9, 2917–2931. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Kleifeld, O.; Lande-Atir, A.; Bsoul, M.; Kleiman, M.; Krutauz, D.; Book, A.; Vierstra, R.D.; Hofmann, K.; Reis, N.; et al. Dual Function of Rpn5 in Two PCI Complexes, the 26S Proteasome and COP9 Signalosome. MBoC 2011, 22, 911–920. [Google Scholar] [CrossRef]
- Pray-Grant, M.G.; Schieltz, D.; McMahon, S.J.; Wood, J.M.; Kennedy, E.L.; Cook, R.G.; Workman, J.L.; Yates, J.R.; Grant, P.A. The Novel SLIK Histone Acetyltransferase Complex Functions in the Yeast Retrograde Response Pathway. Mol. Cell. Biol. 2002, 22, 8774–8786. [Google Scholar] [CrossRef] [PubMed]
- Stoppacciaro, A.; Di Vito, S.; Filetici, P. Epigenetic Factors and Mitochondrial Biology in Yeast: A New Paradigm for the Study of Cancer Metabolism? Front. Pharmacol. 2018, 9, 1349. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, Q.; Yang, X.; Tang, Q.; Han, Y.; Meng, J.; Zhang, J.; Lu, X.; Wang, D.; Liu, J.; et al. Mitochondrial GCN5L1 Coordinates with YME1L and MICOS to Remodel Mitochondrial Cristae in White Adipocytes and Modulate Obesity. Cell Rep. 2025, 44, 115682. [Google Scholar] [CrossRef] [PubMed]
- Zinser, E.; Sperka-Gottlieb, C.D.; Fasch, E.V.; Kohlwein, S.D.; Paltauf, F.; Daum, G. Phospholipid Synthesis and Lipid Composition of Subcellular Membranes in the Unicellular Eukaryote Saccharomyces cerevisiae. J. Bacteriol. 1991, 173, 2026–2034. [Google Scholar] [CrossRef] [PubMed]
- Mollinedo, F. Lipid Raft Involvement in Yeast Cell Growth and Death. Front. Oncol. 2012, 2. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, T.; Büttner, S. Lipids and Cell Death in Yeast. FEMS Yeast Res. 2014, 14, 179–197. [Google Scholar] [CrossRef] [PubMed]
- Jordá, T.; Puig, S. Regulation of Ergosterol Biosynthesis in Saccharomyces cerevisiae. Genes 2020, 11, 795. [Google Scholar] [CrossRef] [PubMed]
- Dewhurst-Maridor, G.; Abegg, D.; David, F.P.A.; Rougemont, J.; Scott, C.C.; Adibekian, A.; Riezman, H. The SAGA Complex, Together with Transcription Factors and the Endocytic Protein Rvs167p, Coordinates the Reprofiling of Gene Expression in Response to Changes in Sterol Composition in Saccharomyces cerevisiae. MBoC 2017, 28, 2637–2649. [Google Scholar] [CrossRef]
- Hu, Z.; He, B.; Ma, L.; Sun, Y.; Niu, Y.; Zeng, B. Recent Advances in Ergosterol Biosynthesis and Regulation Mechanisms in Saccharomyces cerevisiae. Indian J. Microbiol. 2017, 57, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Albaugh, B.N.; Denu, J.M. Catalysis by Protein Acetyltransferase Gcn5. Biochim. Biophys. Acta (BBA)—Gene Regul. Mech. 2021, 1864, 194627. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Graf, L.G.; Striska, K.; Janetzky, M.; Geist, N.; Specht, R.; Schulze, S.; Palm, G.J.; Girbardt, B.; Dörre, B.; et al. Acetyl-CoA Synthetase Activity Is Enzymatically Regulated by Lysine Acetylation Using Acetyl-CoA or Acetyl-Phosphate as Donor Molecule. Nat. Commun. 2024, 15, 6002. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.-S.; Shemorry, A.; Varshavsky, A. N-Terminal Acetylation of Cellular Proteins Creates Specific Degradation Signals. Science 2010, 327, 973–977. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, J.; Dujon, B.; Netter, P.; Petrochilo, E.; Slonimski, P.P.; Bolotin-Fukuhara, M.; Coen, D. MITOCHONDRIAL GENETICS. VI THE PETITE MUTATION IN Saccharomyces cerevisiae: INTERRELATIONS BETWEEN THE LOSS OF THE Ρ+ FACTOR AND THE LOSS OF THE DRUG RESISTANCE MITOCHONDRIAL GENETIC MARKERS. Genetics 1974, 76, 195–219. [Google Scholar] [CrossRef] [PubMed]
- Thomas, B.J.; Rothstein, R. Elevated Recombination Rates in Transcriptionally Active DNA. Cell 1989, 56, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Ornaghi, P.; Rotili, D.; Sbardella, G.; Mai, A.; Filetici, P. A Novel Gcn5p Inhibitor Represses Cell Growth, Gene Transcription and Histone Acetylation in Budding Yeast. Biochem. Pharmacol. 2005, 70, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Agrimi, G.; Brambilla, L.; Frascotti, G.; Pisano, I.; Porro, D.; Vai, M.; Palmieri, L. Deletion or Overexpression of Mitochondrial NAD+ Carriers in Saccharomyces cerevisiae Alters Cellular NAD and ATP Contents and Affects Mitochondrial Metabolism and the Rate of Glycolysis. Appl. Environ. Microbiol. 2011, 77, 2239–2246. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T.; Russell, D.W.; Green, M.R. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1989; ISBN 978-0-87969-309-1. [Google Scholar]
- Westermann, B.; Neupert, W. Mitochondria-Targeted Green Fluorescent Proteins: Convenient Tools for the Study of Organelle Biogenesis in Saccharomyces cerevisiae. Yeast 2000, 16, 1421–1427. [Google Scholar] [CrossRef] [PubMed]
- Kanki, T.; Wang, K.; Cao, Y.; Baba, M.; Klionsky, D.J. Atg32 Is a Mitochondrial Protein That Confers Selectivity during Mitophagy. Dev. Cell 2009, 17, 98–109. [Google Scholar] [CrossRef]
- Meng, S.; Chao, S.; Xiong, M.; Cheng, L.; Sun, Y.; Wang, L.; Chen, Y.; Jane, S.J.; Luo, C.; Chen, J. CaSun1, a SUN Family Protein, Governs the Pathogenicity of Colletotrichum camelliae by Recruiting CaAtg8 to Promote Mitophagy. Hortic. Res. 2025, 12, uhaf121. [Google Scholar] [CrossRef] [PubMed]
- Breivik, O.N.; Owades, J.L. Yeast Analysis, Spectrophotometric Semimicrodetermination of Ergosterol in Yeast. J. Agric. Food Chem. 1957, 5, 360–363. [Google Scholar] [CrossRef]
- Patnana, D.P.; Biswal, R.P.; Dandamudi, R.B.; S, C.; Pandey, M. Simple HPLC-DAD-Based Method for Determination of Ergosterol Content in Lichens and Mushrooms. J. Liq. Chromatogr. Relat. Technol. 2021, 44, 229–234. [Google Scholar] [CrossRef]

| Strain | Genotype | Reference |
|---|---|---|
| W303-1A rho+ | MATa ade2-1 trp1-1 leu2-3,112 his3-11,15 ura3-1 can1-100 ssd1 rho+ | [53] |
| W303-1A rho° | MATa ade2-1 trp1-1 leu2-3,112 his3-11,15 ura3-1 can1-100 ssd1 rho° | [5] |
| gcn5Δ rho+ | MATa ade2-1 trp1-1 leu2-3,112 his3-11,15 ura3-1 can1-100 ssd1 gcn5::KanMX4 rho+ | [54] |
| csn5Δ rho+ | MATa ade2-1 trp1-1 leu2-3,112 his3-11,15 ura3-1 can1-100 ssd1 csn5::KanMX4 rho+ | [21] |
| csn5Δ rho+ | MATα ade2-1 trp1-1 leu2-3,112 his3-11,15 ura3-1 can1-100 ssd1 csn5::KanMX4 rho+ | This work |
| csn5Δ/gcn5Δ rho+ | ade2-1 trp1-1 leu2-3,112 his3-11,15 ura3-1 can1-100 ssd1 csn5Δ/gcn5Δ rho+ | This work |
| Oligonucleotides | Sequences |
|---|---|
| OXI1 For | GTACCAACACCTTATGCAT |
| OXI1 Rev | CATTCAAGATACTAAACCTAA |
| ACT1 For | ACGTTCCAGCCTTCTACGTTTCCA |
| ACT1 Rev | AGTCAGTCAAATCTCTACCGGCCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cirigliano, A.; Schifano, E.; Ricelli, A.; Bianchi, M.M.; Pick, E.; Rinaldi, T.; Montanari, A. Csn5 Depletion Reverses Mitochondrial Defects in GCN5-Null Saccharomyces cerevisiae. Int. J. Mol. Sci. 2025, 26, 6916. https://doi.org/10.3390/ijms26146916
Cirigliano A, Schifano E, Ricelli A, Bianchi MM, Pick E, Rinaldi T, Montanari A. Csn5 Depletion Reverses Mitochondrial Defects in GCN5-Null Saccharomyces cerevisiae. International Journal of Molecular Sciences. 2025; 26(14):6916. https://doi.org/10.3390/ijms26146916
Chicago/Turabian StyleCirigliano, Angela, Emily Schifano, Alessandra Ricelli, Michele M. Bianchi, Elah Pick, Teresa Rinaldi, and Arianna Montanari. 2025. "Csn5 Depletion Reverses Mitochondrial Defects in GCN5-Null Saccharomyces cerevisiae" International Journal of Molecular Sciences 26, no. 14: 6916. https://doi.org/10.3390/ijms26146916
APA StyleCirigliano, A., Schifano, E., Ricelli, A., Bianchi, M. M., Pick, E., Rinaldi, T., & Montanari, A. (2025). Csn5 Depletion Reverses Mitochondrial Defects in GCN5-Null Saccharomyces cerevisiae. International Journal of Molecular Sciences, 26(14), 6916. https://doi.org/10.3390/ijms26146916






