Abstract
In this study, we investigated the mitochondrial defects resulting from the deletion of GCN5, a lysine-acetyltransferase, in the yeast Saccharomyces cerevisiae. Gcn5 serves as the catalytic subunit of the SAGA acetylation complex and functions as an epigenetic regulator, primarily acetylating N-terminal lysine residues on histones H2B and H3 to modulate gene expression. The loss of GCN5 leads to mitochondrial abnormalities, including defects in mitochondrial morphology, a reduced mitochondrial DNA copy number, and defective mitochondrial inheritance due to the depolarization of actin filaments. These defects collectively trigger the activation of the mitophagy pathway. Interestingly, deleting CSN5, which encodes to Csn5/Rri1 (Csn5), the catalytic subunit of the COP9 signalosome complex, rescues the mitochondrial phenotypes observed in the gcn5Δ strain. Furthermore, these defects are suppressed by exogenous ergosterol supplementation, suggesting a link between the rescue effect mediated by CSN5 deletion and the regulatory role of Csn5 in the ergosterol biosynthetic pathway.
1. Introduction
The lysine-acetyltransferase (KAT) Gcn5 of Saccharomyces cerevisiae is a key component of the Spt-Ada-Gcn5 acetyltransferase (SAGA) complex, which is a multi-subunit chromatin-modifying enzymatic complex comprising distinct functional and structural modules. Among these, the histone acetyltransferase (HAT) and deubiquitinase (DUB) modules play a central role in regulating chromatin dynamics. The activities of the HAT and DUB modules are coordinated through interactions among subunits, notably between Gcn5 and Ubp8. These two proteins associate with additional proteins to assemble the HAT module [1]. Gcn5 primarily acetylates the lysine residues present on histone H3 with established target sites including K9, K14, K18, K23, K27, and K36 [2]. In addition to histone H3, Gcn5 also modifies histones H2B and H4, as well as several non-histone substrates, such as the oncoprotein c-Myc and the metabolic coactivator PGC1α [3].
In addition to its well-established role as a nuclear epigenetic factor regulator mediating the lysine acetylation of histones H3 and H4, we have previously demonstrated that Gcn5 also contributes to mitochondrial function and is essential for efficient respiration in S. cerevisiae [4,5,6]. Gcn5 has been localized to the mitoplasm, the mitochondrial compartment deprived of the outer mitochondrial membrane (OMM), and is associated with the inner mitochondrial membrane (IMM) [5]. Under respiratory growth conditions, GCN5 expression is upregulated, and accordingly, the Gcn5 protein level is elevated compared to the fermentative growth [4]. The gcn5Δ mutant strain exhibits compromised mitochondrial function, including abnormal mitochondrial membrane morphology, instability of mitochondrial DNA (mtDNA), and loss of mtDNA-specific regions [5,6]. It has been shown that other SAGA subunits play a role in mitochondrial function. Ubp8, a ubiquitin-specific protease belonging to the DUB module, is essential for respiration, and its depletion has been associated with mitochondrial metabolic defects [7]. Tra1 belongs to the phosphoinositide-3-kinase (PIKK) family and is an essential component of the SAGA/SLIK and NuA4 histone acetyltransferase complexes. Its genetic association with mitochondria has been demonstrated [8]. Furthermore, a TRA1 mutant was unable to grow on a glycerol-containing medium, indicating its involvement in mitochondrial function [9].
The role of Gcn5 in epigenetic regulation is highly conserved across eukaryotic evolution [10]. Therefore, using simple model organisms such as S. cerevisiae offers a valuable approach for uncovering the fundamental mechanisms by which Gcn5 contributes to mitochondrial function. This could provide insight into analogous processes in higher eukaryotic organisms.
To further investigate the mitochondrial role of Gcn5, we examined the mitochondrial stability of the gcn5Δ mutant by monitoring the mtDNA distribution during the budding process. In yeast, mtDNA is organized into protein-associated structures known as nucleoids, which are tethered to the inner mitochondrial membrane [11,12]. During cell division, mitochondria and their associated nucleoids are actively transported from the mother cell to the bud (anterograde movement), a process that is mediated by interactions between the outer mitochondrial membrane and actin filaments [13,14].
Given that the SAGA complex is functionally connected to the ubiquitin–proteasome system, primarily through its Ubp8-containing DUB module, we explored potential genetic interactions between GCN5 and components of the protein degradation machinery. Indeed, Ubp8 has previously been implicated in mitochondrial regulation [7], suggesting that other SAGA complex components, such as Gcn5, may similarly influence mitochondrial function through interactions with the ubiquitin–proteasome system. Notably, various proteasome subunits have been found to be involved in mitochondrial function and dynamics [11,15,16,17]. A subset of E3 ubiquitin ligases, the Cullin RING E3 ligases (CRLs), is regulated by the COP9 signalosome (CSN), a multi-subunit complex highly conserved from yeast to mammals. The CSN modulates CRL activity through deneddylation, whereby the ubiquitin-like modifier Nedd8 is removed from the Cullin subunits. This influences the degradation of numerous substrates [18]. We focused on the CSN5 gene, which encodes the catalytic subunit of the CSN complex, for several reasons. Firstly, in contrast to higher eukaryotes, deletion of CSN subunits in S. cerevisiae is not lethal, which enables genetic interaction studies [19]. Secondly, it has been shown to respond to the cellular redox status during mitochondrial respiration [17]. Thirdly, it participates in a variety of cellular pathways, including the response to mating pheromones and the uptake of metal ions [20,21], as well as the regulation of genes that control amino acids and lipid metabolism [20]. Most notably, CSN5 is involved in the ergosterol biosynthetic pathway, which is essential for the integrity and function of the mitochondrial membranes [21]. Disruption of Csn5 activity has been associated with reduced levels of ergosterol and unsaturated fatty acids (UFA), vacuolar defects, and the accumulation of endoplasmic reticulum (ER) stress [22]. Recently, Csn5 has been proposed as a potential target for fungicidal and nematocidal strategies [23,24,25].
The eukaryotic CSN and SAGA complexes play a role in development and differentiation. The Arabidopsis thaliana COP9 signalosome is involved in various developmental processes [26,27]. In Drosophila melanogaster, the loss of GCN5 is lethal, as it prevents the progression of metamorphosis [28], whereas in mice it interferes with normal development [29]. Furthermore, both complexes are implicated in human diseases. CSN5/JAB1 is overexpressed in various cancers [30,31], while GCN5/KAT2 plays a role in the development of cancer, diabetes, and osteoporosis [32]. Additionally, deubiquitinase USP22, the human homologue of yeast UBP8, and CSN5/JAB1 have been associated with tumour immune evasion due to their direct involvement in regulating cell surface immune receptors [33].
Here, we demonstrated that the deletion of CSN5 suppressed the mitochondrial defects of the gcn5Δ mutant. Given the involvement of the Csn5 in the ergosterol biosynthesis and its relation to alterations in organelle membrane structure, the addition of ergosterol suppresses the mitochondrial phenotypes of the gcn5Δ mutant.
2. Results
2.1. GCN5 Gene Deletion Is Associated with mtDNA Instability
2.1.1. GCN5 Deletion Impairs mtDNA Migration into the Bud During Cell Division
Deletion of the GCN5 gene has previously been linked to mtDNA instability in Saccharomyces cerevisiae [5]. In budding yeast, mitochondrial inheritance during cell division depends on the active transport of mitochondria along actin filaments [11,12]. To assess whether GCN5 deletion affects mitochondrial and mtDNA migration, we used fluorescence microscopy to simultaneously visualize mitochondria, actin cytoskeleton, and mtDNA. Mitochondria were labelled with mtGFP, actin filaments were stained with Rhodamine-Phalloidin, and the DNA was visualized with DAPI (Figure 1). Wild-type, rho°, and gcn5Δ strains were transformed with the mtGFP plasmid and stained as described. During the exponential growth phase, we monitored the intracellular distribution of mitochondria and mtDNA from mother to daughter cells. In wild-type cells, actin filaments (red) displayed a polarized organization toward the budding daughter cell, with associated mitochondrial structures (green) and mtDNA nucleoids (blue) localized along this axis. In contrast, the gcn5Δ mutant exhibited disrupted actin polarization with a consequent collapse of the mitochondrial network, resulting in defective mitochondrial distribution and mtDNA inheritance. Notably, rho° cells (lacking mtDNA) showed normal actin polarization and successful mitochondria migration into the bud, despite the absence of DNA within the organelle.
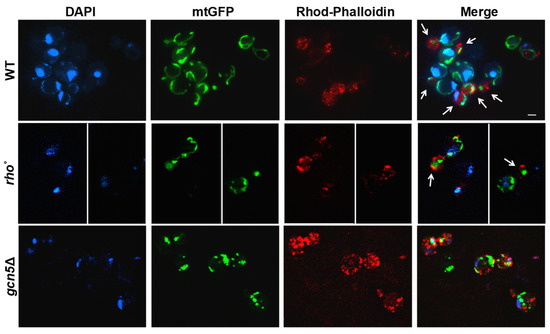
Figure 1.
Mitochondrial morphology and movement are altered in gcn5Δ cells. Fluorescence microscopy of wild-type (WT), gcn5Δ, and rho° cells transformed with mtGFP (green), stained with DAPI (blue), and Rhodamine-Phalloidin (red). Fluorescent signals show mitochondria (green), DNA (blue), and the actin cytoskeleton (red). The actin polarization into the buds is indicated by arrows. In gcn5Δ the mitochondrial migration into the bud is reduced. Scale bars: 2 μm.
2.1.2. The gcn5Δ Strain Undergoes the Mitophagy Process
In several yeast mutants, mitochondrial dysfunction and morphological abnormalities can trigger the selective degradation of mitochondria via mitophagy. To investigate whether the gcn5Δ strain activates this pathway, we assessed mitophagy in the gcn5Δ under exponential growth conditions. As a positive control, wild-type cells were treated with Rapamycin, which is a known inducer of the mitophagy pathway in the exponential phase even in the presence of a fermentable carbon source [34].
Cells were transformed with a plasmid encoding GFP-Atg32, in which the mitophagy marker ATG32 is fused to GFP. Upon mitophagy induction, Atg32 localized to the mitochondrial outer membrane, and the subsequent degradation of mitochondria results in the accumulation of free GFP within the vacuole. Vacuoles were labelled with the lipophilic dye FM4-64 (red), which allowed visualization of the GFP signal (green) co-localizing with vacuolar structures.
As shown in Figure 2, the gcn5Δ mutant cells displayed clear co-localization of the green and red signals, indicating active mitophagy in the cells. This pattern closely resembled that of the Rapamycin-treated wild-type cells control. In contrast, the rho° strain, which lacks mtDNA, showed no detectable accumulation of GFP in the vacuole, suggesting an absence of mitophagic activity under the same conditions.
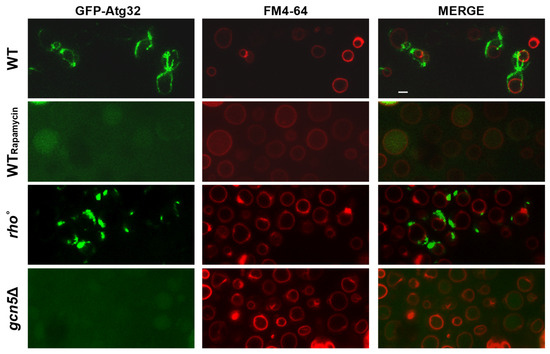
Figure 2.
In gcn5Δ, the mitochondrial defects induce the mitophagy detected by fluorescence microscopy. Wild-type (WT), Rapamycin-treated WT, and gcn5Δ and rho° strains have been transformed with GFP-Atg32, which highlights mitochondrial membranes (green), and stained with FM4-64, which stains vacuolar membranes (red). Mitophagy is visible where the green GFP signal is localized within the vacuoles. Scale bars: 2 μm.
These results suggest that the mitochondrial defects in the gcn5Δ strain are sufficient to activate the mitophagy pathway, highlighting a role for Gcn5 in maintaining mitochondrial integrity and preventing organelle turnover.
2.2. CSN5 Deletion Rescues the Mitochondrial Defects of gcn5Δ Strain
2.2.1. Mitochondrial Phenotype of csn5Δ/gcn5Δ Double Mutant
To test the hypothesis that the CSN complex plays a role in suppressing the mitochondrial defects observed in the gcn5Δ strain, we constructed a double mutant by crossing isogenic strains deleted for GCN5 and CSN5 (see Section 4). As reported in Figure 3A, growth of the gcn5Δ mutant on a glycerol-containing medium, a non-fermentable carbon source requiring mitochondrial respiration, was partially rescued by the deletion of CSN5. Furthermore, growth on low glucose (0.25%), which favours respiratory metabolism by alleviating glucose repression, was fully restored in the double mutant, indicating a suppression of the respiratory growth defect. Oxygen consumption measurements (Figure 3B and Figure S1) revealed that the respiratory activity of the csn5Δ/gcn5Δ double mutant was comparable to that of the wild-type strain, supporting this observation. These findings demonstrate that the deletion of CSN5 restores mitochondrial respiratory function in the gcn5Δ background. Mitochondrial morphology was also examined by fluorescence microscopy (Figure 3C). While the gcn5Δ mutant displayed a collapsed and fragmented mitochondrial network, reminiscent of the morphology observed in rho° cells, the csn5Δ/gcn5Δ double mutant exhibited a restored, wild-type-like mitochondrial structure.
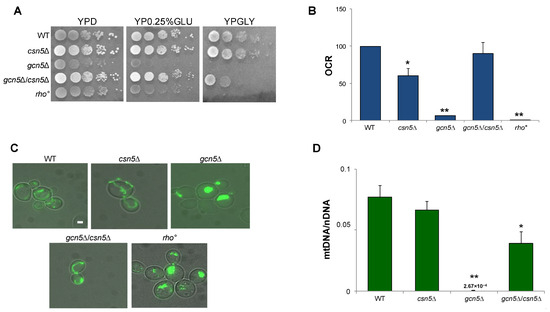
Figure 3.
The deletion of CSN5 gene suppresses the mitochondrial phenotypes of gcn5Δ. (A) Growth of serial dilutions of wild-type (WT) and csn5Δ, gcn5Δ, gcn5Δ/csn5Δ, and rho° strains in solid medium containing 2% glucose (fermentation), 0.25% glucose (intermediate), or 3% glycerol (respiration) as a carbon source. Images were taken after three days of growth at 28 °C. (B) Oxygen consumption rate (OCR) of strains grown overnight in 2% glucose-containing medium. (C) Mitochondrial network of strains transformed with mtGFP was observed by fluorescence microscopy (green). Scale bars: 1 μm. (D) qRT-PCR analysis of mtDNA level of the WT and derivative deleted mutants grown in 2% glucose-containing medium. The ratio between nuclear DNA (nDNA) mean value and mtDNA mean value (OXI1/ACT1) was used to overcome the variability among samples caused by total DNA quality. Data derive from at least three independent experiments and statistical significance by Student’s t-test is indicated. ** p < 0.01; * p < 0.05 for mutants versus WT strain.
Finally, qRT-PCR analysis of the mtDNA copy number (Figure 3D) showed a significant increase in the csn5Δ/gcn5Δ double mutant compared to the gcn5Δ mutant alone. This indicates partial recovery of mitochondrial genome stability.
Taken together, these results demonstrate that the deletion of CSN5 compensates for multiple mitochondrial defects associated with the loss of the GCN5 gene, including impaired respiration, defective morphology, and mtDNA instability.
2.2.2. CSN5 Deletion Enhances Mitochondrial Stability and Suppresses Mitophagy in the gcn5Δ Background
As shown in Figure 2, the severe mitochondrial dysfunction in the gcn5Δ strain activates the mitophagy pathway. To determine whether deletion of CSN5 can suppress this response, we analyzed mitophagy in the csn5Δ/gcn5Δ double mutant using the GFP-Atg32 reporter system. As shown in Figure 4, the double mutant exhibited no evidence of mitophagy activation under the conditions tested, in contrast to the pronounced mitophagy observed in gcn5Δ cells. The absence of the GFP signal co-localization with the vacuolar marker FM4-64 indicates that mitochondrial turnover is no longer triggered in the double mutant. These findings demonstrate that loss of the CSN5 function, which affects the ubiquitin–proteasome system, is sufficient to suppress the morphological and functional mitochondrial defects, as well as the downstream activation of mitophagy in the gcn5Δ mutant. This suggests that CSN5 plays a regulatory role in mitochondrial quality control and stability, particularly under conditions of Gcn5 deficiency.
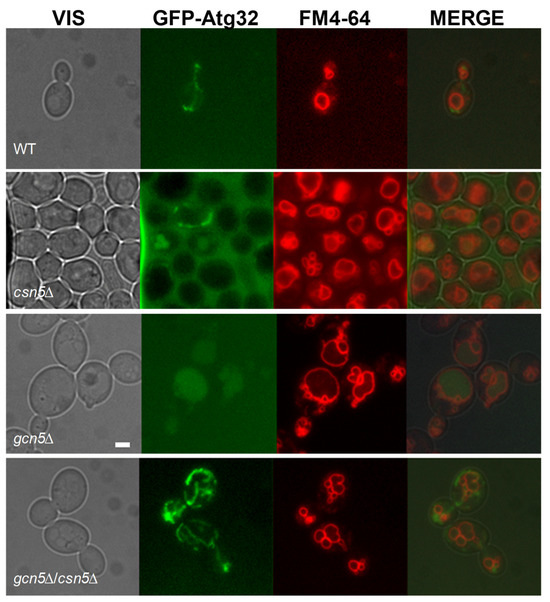
Figure 4.
The deletion of CSN5 suppresses the mitophagy in gcn5Δ. Fluorescence microscopy of wild-type (WT) and csn5Δ, gcn5Δ, and gcn5Δ/csn5Δ strains transformed with GFP-Atg32, which highlights mitochondrial membranes (green), and stained with FM4-64 (vacuolar membranes in red). Scale bars: 2 μm.
2.3. Ergosterol Supplementation Restores Mitochondrial Functionality
2.3.1. Ergosterol Content Is Decreased in Mutant Cells
Previous studies have demonstrated that ergosterol levels are significantly reduced in csn5Δ compared with the wild-type strain, and that ergosterol is essential for anchoring mtDNA in the lipid rafts microdomains within the inner mitochondrial membrane [22]. To evaluate whether alterations in ergosterol biosynthesis contribute to the mitochondrial phenotypes observed in our mutants, we quantified the ergosterol content of wild-type and mutant strains using High-Performance Liquid Chromatography (HPLC). As shown in Figure 5, all mutant strains displayed a significant reduction in ergosterol content relative to the wild-type. Consistent with prior findings [22], ergosterol levels were reduced to approximately 20% in csn5Δ cells and to around 50% in rho° cells. Notably, the gcn5Δ mutant also exhibited a ~50% reduction in ergosterol levels. Interestingly, the csn5Δ/gcn5Δ mutant showed an approximately 95% decrease in ergosterol content compared to the wild-type, indicating a possible additive or synergistic effect of the combined gene deletions on ergosterol biosynthesis.
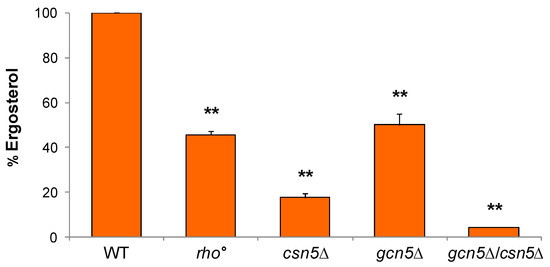
Figure 5.
Ergosterol content in mutant strains. The esterified ergosterol was measured by Hig- Performance Liquid Chromatography (HPLC) analysis in wild-type (WT) and rho°, gcn5Δ, csn5Δ, and gcn5Δ/csn5Δ cells. Values were reported as ergosterol percentage in the mutants compared to that of wild-type cells. Data derive from at least three independent experiments and statistical significance by Student’s t-test is indicated. ** p < 0.01; for mutants versus WT strain.
2.3.2. Ergosterol Rescues Mitochondrial Defects of gcn5Δ Mutant and Acts Additively with CSN5 Deletion
The markedly reduced ergosterol content observed in the gcn5Δ/csn5Δ double mutant prompted us to further explore the contribution of ergosterol to mitochondrial function in these strains. To this end, we assessed the respiratory phenotypes of the mutant strains following ergosterol supplementation. Cells were grown in a glucose-containing liquid medium supplemented with 0.02 mg/mL ergosterol, as described in the Section 4 [11]. Figure 6A (upper panels) shows the growth of the mutants in a solid medium under fermentative, respiratory, or intermediate condition. In the bottom panels, the same cells were grown on the same solid medium as in the upper panels, but with additional ergosterol supplementation. In both cases, we observed that the ergosterol treatment increased the growth capability of the gcn5Δ mutant in both low glucose and glycerol respiratory media. Growth recovery was evident when compared with the growth of the untreated gcn5Δ cells (Figure 2), indicating a positive effect of ergosterol on respiratory growth. Ergosterol supplementation also improved the respiration capability of all deleted mutants (Figure 6B and Figure S2A).
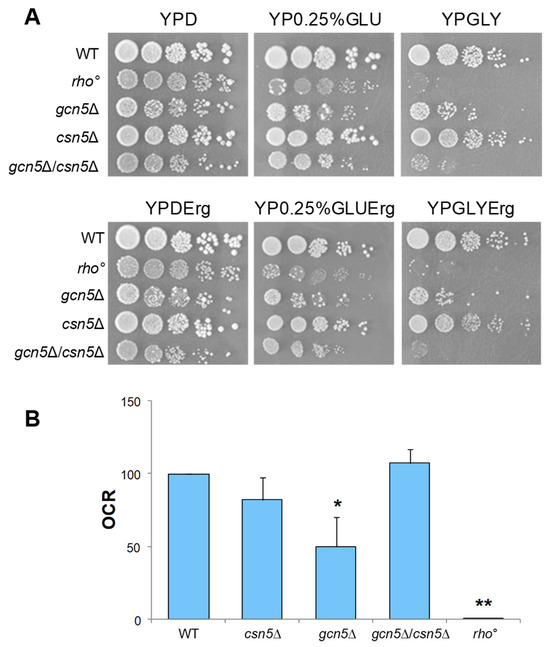
Figure 6.
Respiration phenotypes of the mutant strains treated with ergosterol. (A) Growth of wild-type (WT), rho°, gcn5Δ, csn5Δ, and gcn5Δ/csn5Δ mutants in a solid medium (serial dilutions) containing 2% glucose, 0.25% glucose, or 3% glycerol as a carbon source (upper panels) and on the same media supplemented with 0.02 mg/mL ergosterol. All strains were grown overnight in 2% glucose-liquid-containing medium with the addition of 0.02 mg/mL ergosterol before being plated on solid media. Images were taken after three days of growth at 28 °C. (B) Oxygen consumption rate (OCR) of indicated strains grown in 2% glucose-containing medium with the addition of 0.02 mg/mL ergosterol. Data derive from at least three independent experiments and statistical significance by Student’s t-test is indicated. ** p < 0.01; * p < 0.05 for mutants versus WT strain.
Ergosterol also improved the mitochondrial morphology of the gcn5Δ strain, resulting in a tubular mitochondrial network similar to that of the wild-type strain (Figure 7A). Additionally, stabilization of the mtDNA content was also observed in the mutants after treatment (Figure 7B and Figure S2B).
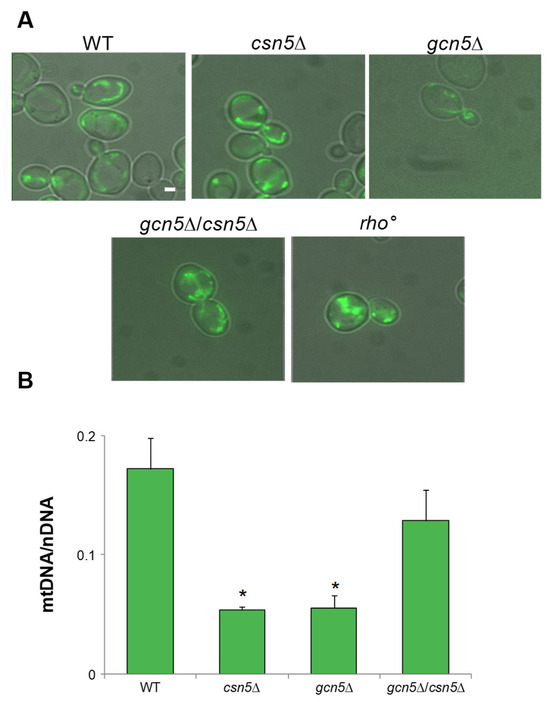
Figure 7.
Ergosterol supplementation suppresses the mitochondrial morphology defects and the mtDNA instability. (A) Fluorescence microscopy of wild-type (WT) and rho°, gcn5Δ, csn5Δ, and gcn5Δ/csn5Δ mutants grown in 2% glucose medium with the addition of 0.02 mg/mL ergosterol. The strains were transformed with mtGFP to visualize the mitochondrial network (green). Scale bars: 1 μm. (B) qRT-PCR analysis of mtDNA level of the wild-type and the mutants grown as in (A). The ratio between nuclear DNA (nDNA) mean value and mtDNA mean value (OXI1/ACT1) was used to overcome the variability among samples caused by total DNA quality. Data derive from at least three independent experiments and statistical significance by Student’s t-test is indicated. * p < 0.05 for deleted versus WT strain.
These results demonstrate that ergosterol exerts a suppressive effect on gcn5Δ mitochondrial defects that is similar to that of CSN5 gene deletion. Furthermore, the improvement in mitochondrial function observed in all mutants following ergosterol treatment further supports the hypothesis that ergosterol may have an additive effect with the CSN5 deletion.
3. Discussion
In this study we initially characterized the mitochondrial phenotypes associated with the deletion of the GCN5 gene in the yeast Saccharomyces cerevisiae, with the aim of identifying the underlying mechanism of mtDNA instability. We observed significant defects in mitochondrial inheritance due to the depolarization of the actin filaments in the gcn5Δ mutant. This prevented the proper migration of mitochondria, and consequently of the mtDNA, from the mother cell to the daughter cell during mitosis. Actin filament polarization is crucial for mitochondrial inheritance in yeast [35]; notably, rho° cells, which lack mtDNA, exhibit normal actin polarization and mitochondrial distribution, emphasizing that actin depolarization is specifically linked to GCN5 deletion [36]. Furthermore, gcn5Δ cells exhibit disrupted mitochondrial morphology and progressive mtDNA loss, which correlates with increasingly severe respiratory dysfunction over successive cell divisions [5,6]. These overall mitochondrial defects collectively trigger the activation of the mitophagy pathway, which involves the selective removal of impaired mitochondria via vacuolar degradation in yeast (lysosomal in mammals) [37]. Thus, our findings underscore the critical role of the GCN5 gene in maintaining mitochondrial integrity and function in yeast.
In order to identify pathways that can suppress mitochondrial defects in the gcn5Δ strain, we examined the genetic interactions between GCN5 and the components of the ubiquitin–proteasome pathway, with a particular focus on the COP9 signalosome (CSN) complex. The ubiquitin–proteasome system is intricately connected with epigenetic regulation, protein stability, and the modulation of cellular responses. However, dissecting its specific mitochondrial roles remains challenging due to pathway complexity [2]. We targeted CSN5, encoding a non-essential subunit of the CSN complex which does not exhibit mitochondrial dysfunction upon deletion, unlike many other proteasomal mutants [15,38]. Earlier studies have suggested possible genetic and functional interactions between the SAGA complex and the CSN, particularly involving GCN5. One proposed link arose from observations involving Csn12, a protein originally annotated as a putative CSN subunit in yeast [20]. However, more recent biochemical analyses have demonstrated that Csn12 does not stably associate with the canonical CSN complex [39]. Instead, Csn12 has been implicated in mRNA splicing and RNA processing, which may explain its genetic interactions with transcription-related factors such as Gcn5. These findings highlight the complexity of regulatory networks governing gene expression and the importance of revisiting earlier subunit assignments based on updated functional and structural data.
Overall, the results demonstrated that the csn5Δ/gcn5Δ double mutant no longer exhibited the mitochondrial defects observed in the gcn5Δ single mutant. Therefore, the GCN5 and CSN5 genes are both involved in the regulation of mitochondrial functionality, albeit in different ways. GCN5, coding for an acetyltransferase, can influence the expression of genes involved in mitochondrial processes through chromatin modification, favouring the activation of genes necessary for mitochondrial physiology. Gcn5 is required to activate nuclear genes that compensate for impaired mitochondrial function. This process, known as retrograde regulation, is critical for communication between mitochondria and the nucleus in order to regulate gene expression in the case of mitochondrial dysfunctions. Indeed, Gcn5 is also a part of the SAGA/SLIK transcriptional activator complex, which includes the retrograde factor Rtg2. This factor is involved in regulating the expression of genes that code for mitochondrial, cytoplasmic, and peroxisomal proteins, as well as cellular responses to mitochondrial damage [40]. In human cells, the post-translational modifications of mitochondrial proteins due to epigenetic factors of the SAGA complex, represent an interesting regulatory mechanism that links mitochondria and re-programmed metabolism in cancer cells [41]. Furthermore, the mitochondrial homologue of the nuclear GCN5 (GCN5L1) has been demonstrated to regulate mitochondrial proteins through acetylation. It is also involved in mitochondrial metabolism, biogenesis, and mitophagy [42].
The role of ergosterol in mitochondrial function was also investigated in this work. Previous studies have demonstrated the involvement of Csn5 in the ergosterol biosynthetic pathway [21], as well as the key role of the ergosterol content in the cells in mtDNA stability and maintenance [11]. There is an interplay between mitochondrial function and the ergosterol biosynthetic pathway. In S. cerevisiae, ergosterol is an essential component of all cell membranes, including mitochondrial membranes, and it is found at its highest concentration in the inner mitochondrial membrane [43]. The adhesion of mtDNA, inside the nucleoids, to this membrane is related to lipid raft-like mitochondrial microdomains, that are enriched in ergosterol, sphingolipids, and proteins, which are constantly exchanged via the endoplasmic reticulum–mitochondria complex [44,45]. This ensures the correct mitochondrial morphology and the maintenance of mtDNA [11]. It is therefore possible that the low ergosterol content found in the gcn5Δ mutant contributes to the instability of its mtDNA. The SAGA complex has also been shown to play a role in ergosterol biosynthesis [46]. Indeed, it has been reported that the SAGA complex acts as a coactivator of ergosterol transcription, inducing specific changes to the transcriptional profile, in response to variations in cellular sterol composition. This could account for the low ergosterol levels observed in the gcn5Δ mutant [47]. The addition of ergosterol to the growth medium improved the mitochondrial functionality of the gcn5Δ strain. The improvement in respiratory growth and oxygen consumption rate were associated with greater mtDNA stability and the restoration of a normal mitochondrial network. These data are consistent with a strong link existing between ergosterol and acetyl-CoA and, thus, with Gcn5 activity. Indeed, the first step of ergosterol biosynthesis involves the formation of mevalonate from acetyl-CoA itself [48]. Furthermore, the cellular acetyl-CoA availability efficiently regulates the Gcn5 lysine-acetyltransferase activity [49,50]. In the csn5Δ/gcn5Δ double mutant, the ergosterol content was significantly lower than in the wild-type strain. We hypothesize that this low amount may be due to the combined effect of deleting the CSN5 and GCN5 genes and the synergistic involvement of these two genes in regulating ergosterol biosynthesis. The CSN5 deletion rescued the defective phenotype caused by the GCN5 deletion, improving the mitochondrial defects despite the low level of ergosterol in the double mutant. Adding ergosterol then worked in synergy with the double deletion, improving the mitochondrial phenotypes analyzed here.
In conclusion, our results demonstrated a genetic interaction between GCN5 and CSN5 in the yeast S. cerevisiae. This interaction links the mechanisms of epigenetic regulation and protein stability, which are mediated by the SAGA and COP9 signalosome complexes, respectively, in order to ensure mitochondrial integrity. Recovery of mitochondrial function in the absence of the two genes is also favoured by ergosterol supplementation, suggesting that Csn5-mediated restoration is associated with its regulatory function in the ergosterol biosynthetic pathway. Further studies will be needed to elucidate the roles and the mechanisms of the interaction between GCN5 and CSN5, as well as the potential involvement of additional factors within their respective complexes. Future research will also focus on studying the link between protein acetylation and degradation. It has been reported that proteins with free α-amino groups can undergo ATP-dependent ubiquitin degradation, a process that can be prevented by acetylating the N-terminal α-amino group [51]. It would be interesting to determine whether non-acetylated proteins could undergo ubiquitylation for degradation in the absence of Gcn5. Furthermore, it should be clarified whether the absence of Csn5 in the double mutant could prevent the activation of E3 ligases, resulting in the accumulation of undegraded proteins inside the cell. Therefore, further research will be needed to establish whether Gcn5 and Csn5-regulated E3 ligases act on the same substrates.
4. Materials and Methods
4.1. Yeast Strains and Growth Conditions
Saccharomyces cerevisiae strains used in this work are derived from a W303-1A nuclear background. The complete list is reported in Table 1. The rho° strain (completely lacking mtDNA) was obtained from isogenic rho+ strain (having a wild-type mtDNA) by Ethidium Bromide treatment [52].

Table 1.
Saccharomyces cerevisiae strains used in this work.
The csn5Δ/gcn5Δ mutant was generated by mating, sporulation, and tetrad dissection. The single csn5Δ and gcn5Δ strains were crossed and, after sporulation of the diploid strain, tetrad dissection was performed. The double deleted mutant was obtained by selection on the G148 plate of the tetrad in which two spores were kanamycin sensitive and two spores showed a kanamycin-resistant recombinant phenotype.
Yeast cells were grown at 28 °C in YP medium (1% yeast extract, 2% bactopeptone) containing 2% glucose (YPD) or 0.25% glucose, either with or without 0.02 mg/mL ergosterol supplementation (Sigma-Aldrich, St. Louis, MO, USA), or 3% glycerol. For the selection of the double mutant, a YPD medium was supplemented with 0.1 mg/mL G418 (Sigma-Aldrich, St. Louis, MO, USA). Agarose was added in the solid medium at 2% concentration.
Comparison of growth capability was investigated by serial dilutions of concentrated suspensions (5–9 × 106 cell/mL), prepared from fresh single colonies after overnight incubation at 28 °C and spotted onto a plate. Pictures were acquired after 3 days of growth at 28 °C.
4.2. Oxygen Consumption Measurement
Respiration studies were performed using Clark Oxygen Electrode (Hansatech Instruments, King’s Lynn, UK) [55]. A total of 0.03 g of yeast cells (wet weight) were collected after growth in the YPD medium (with or without 0.02 mg/mL ergosterol), washed with 1 mL sterile water, and resuspended in 1 mL of 10 mM Na-phosphate buffer pH 7.4 containing 20 mM glucose. Samples were loaded in the Reaction Vessel of a previously calibrated Oxygen Electrode Chamber.
4.3. Plasmids and Transformation Experiments
DNA manipulation, restriction enzyme digestion, plasmid preparations, as well as Escherichia coli and yeast transformations, were performed as described in [56].
Plasmids used to transform the yeast strains were: (i) mtGFP, the episomal plasmid pVT100U in which the gene coding for the Green Fluorescent Protein (fused at the 3′end with the mitochondrial targeting sequence of subunit 9 of the Neurospora crassa ATP-ase) is cloned [57]; (ii) GFP-Atg32, the centromeric plasmid pRS416 in which the gene ATG32 is fused at 5′end with the GFP gene [58].
4.4. Quantification of mtDNA
Quantitative Real Time-PCR (qRT-PCR) was performed as previously described to quantify the mtDNA copy number [5]. For DNA extraction yeast cells were collected after overnight growth in the YPD medium. The same samples were also grown in the YPD medium with 0.02 mg/mL ergosterol. The sequences of the oligonucleotides OXI1 For and OXI1 Rev for mitochondrial gene amplification or ACT1 For and ACT1 Rev for nuclear gene amplification are reported in Table 2.

Table 2.
Oligonucleotides used in this work.
4.5. Actin Detection
To visualize the cellular actin filaments into the cells, 100 μL of transformed cells with the mtGFP plasmid were collected at the exponential phase after overnight growth in the YPD medium. The cells were fixed for 45 min at room temperature by adding two volumes of 1% formaldehyde. The samples were centrifuged for 5 min at 3500 rpm and the cells were resuspended in 50 μL of Triton X-100 (0.1%). After 10 min at room temperature, 6.6 μM Rhodamine-Phalloidin dye (Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA) was added. After 45 min of incubation at room temperature, the cells were washed with sterile water and resuspended in 5 μL of DAPI staining (1 μg/mL, Sigma-Aldrich, St. Louis, MO, USA). The observation was carried out by fluorescence microscope.
4.6. Mitophagy Detection
To selectively detect the mitophagy, 1 mL of transformed cells with the GFP-Atg32 plasmid were collected at the exponential phase after overnight growth in the YPD liquid medium, and the FM4-64 vacuolar lipophilic dye (Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA) was added to 16 μM final concentration. After 1 h of incubation at 28 °C, the cells were washed with 1 mL YPD medium twice. The cells were resuspended in 2 mL YPD and incubated for 2 h at 28 °C before observation by fluorescence microscope [59]. To obtain a positive control, a sample of wild-type cells was treated with Rapamycin, an inducer of mitophagy: the cells are resuspended in 2 mL YPD + 30 μL of Rapamycin (3 μg/mL, Sigma-Aldrich, St. Louis, MO, USA) before incubation for 2 h at 28 °C.
4.7. Ergosterol Extraction
Evaluation of the ergosterol amount in yeast cells was performed as previously described with minor changes [60]. For each yeast strain, 20 OD600 of an overnight-grown culture in a glucose-containing medium was harvested. The cell suspension was then collected into a 2 mL screw cap tube and washed once with sterile distilled water. Finally, the cell pellet was suspended in 600 μL of an ethanol/KOH solution. The hydroxide solution was prepared by mixing 2.5 g KOH with 3.6 mL DW and adding ethanol up to 10 mL. An amount of 50 mL of a solution of ethanolic butylated hydroxytoluene (BHT) (10 mg BHT in 1 mL ethanol) was added to prevent ergosterol oxidation. The suspension was incubated at 80 °C in a heat block under constant shaking for 1 h. Next, 600 μL of n-heptane and 55 μL of distilled water were added serially to the mix, followed by vigorous vortexing for 3 min. The samples were left for 5 min at room temperature for phase separation; then the upper phase was transferred to a new Eppendorf and dried under a nitrogen flow; and the samples were resuspended in methanol (MeOH) for analysis in HPLC.
4.8. High-Performance Liquid Chromatography (HPLC) Analysis
The quantification of ergosterol extracted from yeast cells as described in the previous paragraph was performed by a HPLC-UV/diode array detector (DAD). The analyses were performed following the method described by [61] with minor modifications. The liquid chromatography (Agilent 1260 Infinity, Agilent Technologies, Santa Clara, CA, USA) was equipped with a quaternary gradient pump, an autosampler injection system, a column oven set at 25 °C, a DAD, and a chromatography data system (ChemStation version C.01.07). A Poroshell-120 C18 column (4.6 × 50 mm, 2.7 μm particle sizes, Agilent Technologies, Santa Clara, CA, USA) was used. The mobile phase was an isocratic mixture of MeOH and acetonitrile (CH3CN) (20:80 v/v) eluting at a flow rate of 1.0 mL/min for 10 min. Ergosterol was identified in cell extracts by comparing the retention time and the UV spectra of the peak areas recorded in the chromatogram with those of the authentic standard. UV spectra were recorded in the range of 200 to 450 nm. Quantification of ergosterol was performed according to the external standard method, integrating peak areas acquired at the wavelength corresponding to its maximum absorbance (280 nm), at the retention time of the corresponding ergosterol standard. Stock solution of ergosterol was prepared in amber glass at a concentration of 50 ng/mL, and work solutions were prepared by appropriately diluting the stock solution with MeOH. After use, the solutions were stored at −25 °C.
4.9. Fluorescence Imaging
Cells were visualized by fluorescence microscopy on Zeiss Axio Imager Z1 Fluorescence Microscope (Carl Zeiss, Oberkochen, Germany). Images were acquired using Axio-Vision 4.8 Digital Image Processing System (Carl Zeiss, Oberkochen, Germany) and objective lens 63× oil.
4.10. Statistical Analysis
Experiments were performed at least in triplicate. Data are presented as mean ± SE and Student’s t test were used to determine the statistical significance between experimental groups. Statistical significance was defined as * p < 0.05, ** p < 0.01.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms26146916/s1.
Author Contributions
Conceptualization, A.M., T.R., M.M.B. and E.P.; methodology, A.C., E.S., A.R. and A.M.; validation, A.M., A.C., A.R. and E.S.; formal analysis, M.M.B. and E.P.; investigation, A.M. and T.R.; data curation, A.M., T.R., M.M.B. and E.P.; writing—original draft preparation, A.M. and T.R.; writing—review and editing A.C., E.S., A.R., M.M.B., E.P., T.R. and A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article and Supplementary Materials. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| SAGA | Spt-Ada-Gcn5 Acetyltransferase |
| KAT | Lysine-Acetyltransferase |
| HAT | Histone Acetyltransferase |
| DUB | Deubiquitinase |
| SLIK | SAGA-like |
| OMM | Outer Mitochondrial Membrane |
| IMM | Inner Mitochondrial Membrane |
| mtDNA | Mitochondrial DNA |
| nDNA | Nuclear DNA |
| CSN | COP9 Signalosome |
| CRLs | Cullin RING E3 Ligases |
| UFA | Unsaturated Fatty Acids |
| ER | Endoplasmic Reticulum |
| YPD | Yeast Extract + Peptone + Glucose |
| mtGFP | Mitochondrial Green Fluorescent Protein |
| qRT-PCR | Quantitative Real Time Polymerase Chain Reaction |
| DAPI | 4′,6-diamidino-2-phenylindole |
| FM4-64 | N-(3-Triethylammoniumpropyl)-4-(6-(4-(Diethylamino) Phenyl) Hexatrienyl) Pyridinium Dibromide |
| OD600 | Optical Density at 600 nm |
| HPLC | High Performance Liquid Chromatography |
| DAD | Diode Array Detector |
| BHT | Butylated Hydroxytoluene |
| SE | Standard Error |
| OCR | Oxygen Consumption Rate |
References
- Soffers, J.H.M.; Workman, J.L. The SAGA Chromatin-Modifying Complex: The Sum of Its Parts Is Greater than the Whole. Genes Dev. 2020, 34, 1287–1303. [Google Scholar] [CrossRef] [PubMed]
- Chou, K.Y.; Lee, J.-Y.; Kim, K.-B.; Kim, E.; Lee, H.-S.; Ryu, H.-Y. Histone Modification in Saccharomyces cerevisiae: A Review of the Current Status. Comput. Struct. Biotechnol. J. 2023, 21, 1843–1850. [Google Scholar] [CrossRef]
- Patel, J.H.; Du, Y.; Ard, P.G.; Phillips, C.; Carella, B.; Chen, C.-J.; Rakowski, C.; Chatterjee, C.; Lieberman, P.M.; Lane, W.S.; et al. The C-MYC Oncoprotein Is a Substrate of the Acetyltransferases hGCN5/PCAF and TIP60. Mol. Cell. Biol. 2004, 24, 10826–10834. [Google Scholar] [CrossRef] [PubMed]
- Canzonetta, C.; Leo, M.; Guarino, S.R.; Montanari, A.; Francisci, S.; Filetici, P. SAGA Complex and Gcn5 Are Necessary for Respiration in Budding Yeast. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2016, 1863, 3160–3168. [Google Scholar] [CrossRef] [PubMed]
- Montanari, A.; Leo, M.; De Luca, V.; Filetici, P.; Francisci, S. Gcn5 Histone Acetyltransferase Is Present in the Mitoplasts. Biol. Open 2019, 8, bio041244. [Google Scholar] [CrossRef] [PubMed]
- De Luca, V.; Leo, M.; Cretella, E.; Montanari, A.; Saliola, M.; Ciaffi, G.; Vecchione, A.; Stoppacciaro, A.; Filetici, P. Role of yUbp8 in Mitochondria and Hypoxia Entangles the Finding of Human Ortholog Usp22 in the Glioblastoma Pseudo-Palisade Microlayer. Cells 2022, 11, 1682. [Google Scholar] [CrossRef] [PubMed]
- Leo, M.; Fanelli, G.; Di Vito, S.; Traversetti, B.; La Greca, M.; Palladino, R.A.; Montanari, A.; Francisci, S.; Filetici, P. Ubiquitin Protease Ubp8 Is Necessary for S. Cerevisiae Respiration. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2018, 1865, 1491–1500. [Google Scholar] [CrossRef] [PubMed]
- Hoke, S.M.; Guzzo, J.; Andrews, B.; Brandl, C.J. Systematic Genetic Array Analysis Links the Saccharomyces cerevisiae SAGA/SLIK and NuA4 Component Tra1 to Multiple Cellular Processes. BMC Genet. 2008, 9, 46. [Google Scholar] [CrossRef] [PubMed]
- Bari, K.A.; Berg, M.D.; Genereaux, J.; Brandl, C.J.; Lajoie, P. Tra1 Controls the Transcriptional Landscape of the Aging Cell. G3 2023, 13, jkac287. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Dai, W.; Xiao, D.; Xiong, Q.; Liu, C.; Hu, J.; Ge, F.; Yu, X.; Li, S. Acetylation-Dependent SAGA Complex Dimerization Promotes Nucleosome Acetylation and Gene Transcription. Nat. Struct. Mol. Biol. 2022, 29, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Cirigliano, A.; Macone, A.; Bianchi, M.M.; Oliaro-Bosso, S.; Balliano, G.; Negri, R.; Rinaldi, T. Ergosterol Reduction Impairs Mitochondrial DNA Maintenance in S. Cerevisiae. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2019, 1864, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Cirigliano, A.; Amelina, A.; Biferali, B.; Macone, A.; Mozzetta, C.; Bianchi, M.M.; Mori, M.; Botta, B.; Pick, E.; Negri, R.; et al. Statins Interfere with the Attachment of S. Cerevisiae mtDNA to the Inner Mitochondrial Membrane. J. Enzym. Inhib. Med. Chem. 2020, 35, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Boldogh, I.R.; Pon, L.A. Mitochondria on the Move. Trends Cell Biol. 2007, 17, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, L.; Rust, M.B.; Culmsee, C. Actin(g) on Mitochondria—A Role for Cofilin1 in Neuronal Cell Death Pathways. Biol. Chem. 2019, 400, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, T.; Pick, E.; Gambadoro, A.; Zilli, S.; Maytal-Kivity, V.; Frontali, L.; Glickman, M.H. Participation of the Proteasomal Lid Subunit Rpn11 in Mitochondrial Morphology and Function Is Mapped to a Distinct C-Terminal Domain. Biochem. J. 2004, 381, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Livnat-Levanon, N.; Glickman, M.H. Ubiquitin–Proteasome System and Mitochondria—Reciprocity. Biochim. Biophys. Acta (BBA)—Gene Regul. Mech. 2011, 1809, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Bramasole, L.; Sinha, A.; Gurevich, S.; Radzinski, M.; Klein, Y.; Panat, N.; Gefen, E.; Rinaldi, T.; Jimenez-Morales, D.; Johnson, J.; et al. Proteasome Lid Bridges Mitochondrial Stress with Cdc53/Cullin1 NEDDylation Status. Redox Biol. 2019, 20, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Barth, E.; Hübler, R.; Baniahmad, A.; Marz, M. The Evolution of COP9 Signalosome in Unicellular and Multicellular Organisms. Genome Biol. Evol. 2016, 8, 1279–1289. [Google Scholar] [CrossRef] [PubMed]
- Wee, S.; Hetfeld, B.; Dubiel, W.; Wolf, D.A. Conservation of the COP9/signalosome in budding yeast. BMC Genet. 2002, 3, 15. [Google Scholar] [CrossRef]
- Maytal-Kivity, V.; Pick, E.; Piran, R.; Hofmann, K.; Glickman, M.H. The COP9 Signalosome-like Complex in S. Cerevisiae and Links to Other PCI Complexes. Int. J. Biochem. Cell Biol. 2003, 35, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Licursi, V.; Salvi, C.; De Cesare, V.; Rinaldi, T.; Mattei, B.; Fabbri, C.; Serino, G.; Bramasole, L.; Zimbler, J.Z.; Pick, E.; et al. The COP9 Signalosome Is Involved in the Regulation of Lipid Metabolism and of Transition Metals Uptake in Saccharomyces cerevisiae. FEBS J. 2014, 281, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.; Israeli, R.; Cirigliano, A.; Gihaz, S.; Trabelcy, B.; Braus, G.H.; Gerchman, Y.; Fishman, A.; Negri, R.; Rinaldi, T.; et al. The COP9 Signalosome Mediates the Spt23 Regulated Fatty Acid Desaturation and Ergosterol Biosynthesis. FASEB J. 2020, 34, 4870–4889. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhang, Y.; Wang, W.; Cao, X.; Xu, H.; Liu, H.; Qi, J.; Jiang, C.; Wang, C. FgCsn12 Is Involved in the Regulation of Ascosporogenesis in the Wheat Scab Fungus Fusarium Graminearum. Int. J. Mol. Sci. 2022, 23, 10445. [Google Scholar] [CrossRef]
- Chen, A.; Ren, Y.; Han, X.; Liu, C.; Zhou, Y.; Xu, C.; Qi, H.; Ma, Z.; Chen, Y. The COP9 Signalosome Complex Regulates Fungal Development and Virulence in the Wheat Scab Fungus Fusarium Graminearum. Front. Microbiol. 2023, 14, 1179676. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Dai, H.; Bashir, A.; Wang, Z.; An, Y.; Yu, X.; Xu, L.; Li, L. Nematicidal Activity and Action Mode of a Methyl-Accepting Chemotaxis Protein from Pseudomonas Syringae against Caenorhabditis Elegans. Heliyon 2024, 10, e30366. [Google Scholar] [CrossRef] [PubMed]
- Franciosini, A.; Lombardi, B.; Iafrate, S.; Pecce, V.; Mele, G.; Lupacchini, L.; Rinaldi, G.; Kondou, Y.; Gusmaroli, G.; Aki, S.; et al. The Arabidopsis COP9 SIGNALOSOME INTERACTING F-BOX KELCH 1 Protein Forms an SCF Ubiquitin Ligase and Regulates Hypocotyl Elongation. Mol. Plant 2013, 6, 1616–1629. [Google Scholar] [CrossRef] [PubMed]
- Franciosini, A.; Moubayidin, L.; Du, K.; Matari, N.H.; Boccaccini, A.; Butera, S.; Vittorioso, P.; Sabatini, S.; Jenik, P.D.; Costantino, P.; et al. The COP9 SIGNALOSOME Is Required for Postembryonic Meristem Maintenance in Arabidopsis Thaliana. Mol. Plant 2015, 8, 1623–1634. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, Y.; Xu, Y.; Li, R.; Huang, S.; Wu, Q.; Yan, J.; Jiang, Z.; Wu, X.; Li, F.; Wang, Y.; et al. Transcriptomic and Metabolomic Analysis Reveals Multifaceted Impact of Gcn5 Knockdown in Drosophila Development. Metabolites 2024, 14, 680. [Google Scholar] [CrossRef] [PubMed]
- Koutelou, E.; Farria, A.T.; Dent, S.Y.R. Complex Functions of Gcn5 and Pcaf in Development and Disease. Biochim. Biophys. Acta (BBA)—Gene Regul. Mech. 2021, 1864, 194609. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Y.; Wang, B.; Ma, Y.; Chen, P. CSN5/Jab1 Facilitates Non-Small Cell Lung Cancer Cell Growth through Stabilizing Survivin. Biochem. Biophys. Res. Commun. 2018, 500, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Wang, Y.; Zhao, Y.; Shu, Y.; Liu, Z.; Zhou, H.; Wang, H.; Zhang, W. The Pivotal Oncogenic Role of Jab1/CSN5 and Its Therapeutic Implications in Human Cancer. Gene 2019, 687, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.-T.; Jin, J.; Zheng, Z.-G. Emerging Role of GCN5 in Human Diseases and Its Therapeutic Potential. Biomed. Pharmacother. 2023, 165, 114835. [Google Scholar] [CrossRef] [PubMed]
- Dewson, G.; Eichhorn, P.J.A.; Komander, D. Deubiquitinases in Cancer. Nat. Rev. Cancer 2023, 23, 842–862. [Google Scholar] [CrossRef] [PubMed]
- Bhatia-Kissova, I.; Camougrand, N. Mitophagy in Yeast: Decades of Research. Cells 2021, 10, 3541. [Google Scholar] [CrossRef] [PubMed]
- Miyakawa, I. Organization and Dynamics of Yeast Mitochondrial Nucleoids. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2017, 93, 339–359. [Google Scholar] [CrossRef] [PubMed]
- Vernarecci, S.; Ornaghi, P.; Bâgu, A.; Cundari, E.; Ballario, P.; Filetici, P. Gcn5p Plays an Important Role in Centromere Kinetochore Function in Budding Yeast. Mol. Cell. Biol. 2008, 28, 988–996. [Google Scholar] [CrossRef] [PubMed]
- Innokentev, A.; Kanki, T. Mitophagy in Yeast: Molecular Mechanism and Regulation. Cells 2021, 10, 3569. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, T.; Ricci, C.; Porro, D.; Bolotin-Fukuhara, M.; Frontali, L. A Mutation in a Novel Yeast Proteasomal Gene, RPN11/MPR1, Produces a Cell Cycle Arrest, Overreplication of Nuclear and Mitochondrial DNA, and an Altered Mitochondrial Morphology. MBoC 1998, 9, 2917–2931. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Kleifeld, O.; Lande-Atir, A.; Bsoul, M.; Kleiman, M.; Krutauz, D.; Book, A.; Vierstra, R.D.; Hofmann, K.; Reis, N.; et al. Dual Function of Rpn5 in Two PCI Complexes, the 26S Proteasome and COP9 Signalosome. MBoC 2011, 22, 911–920. [Google Scholar] [CrossRef]
- Pray-Grant, M.G.; Schieltz, D.; McMahon, S.J.; Wood, J.M.; Kennedy, E.L.; Cook, R.G.; Workman, J.L.; Yates, J.R.; Grant, P.A. The Novel SLIK Histone Acetyltransferase Complex Functions in the Yeast Retrograde Response Pathway. Mol. Cell. Biol. 2002, 22, 8774–8786. [Google Scholar] [CrossRef] [PubMed]
- Stoppacciaro, A.; Di Vito, S.; Filetici, P. Epigenetic Factors and Mitochondrial Biology in Yeast: A New Paradigm for the Study of Cancer Metabolism? Front. Pharmacol. 2018, 9, 1349. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, Q.; Yang, X.; Tang, Q.; Han, Y.; Meng, J.; Zhang, J.; Lu, X.; Wang, D.; Liu, J.; et al. Mitochondrial GCN5L1 Coordinates with YME1L and MICOS to Remodel Mitochondrial Cristae in White Adipocytes and Modulate Obesity. Cell Rep. 2025, 44, 115682. [Google Scholar] [CrossRef] [PubMed]
- Zinser, E.; Sperka-Gottlieb, C.D.; Fasch, E.V.; Kohlwein, S.D.; Paltauf, F.; Daum, G. Phospholipid Synthesis and Lipid Composition of Subcellular Membranes in the Unicellular Eukaryote Saccharomyces cerevisiae. J. Bacteriol. 1991, 173, 2026–2034. [Google Scholar] [CrossRef] [PubMed]
- Mollinedo, F. Lipid Raft Involvement in Yeast Cell Growth and Death. Front. Oncol. 2012, 2. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, T.; Büttner, S. Lipids and Cell Death in Yeast. FEMS Yeast Res. 2014, 14, 179–197. [Google Scholar] [CrossRef] [PubMed]
- Jordá, T.; Puig, S. Regulation of Ergosterol Biosynthesis in Saccharomyces cerevisiae. Genes 2020, 11, 795. [Google Scholar] [CrossRef] [PubMed]
- Dewhurst-Maridor, G.; Abegg, D.; David, F.P.A.; Rougemont, J.; Scott, C.C.; Adibekian, A.; Riezman, H. The SAGA Complex, Together with Transcription Factors and the Endocytic Protein Rvs167p, Coordinates the Reprofiling of Gene Expression in Response to Changes in Sterol Composition in Saccharomyces cerevisiae. MBoC 2017, 28, 2637–2649. [Google Scholar] [CrossRef]
- Hu, Z.; He, B.; Ma, L.; Sun, Y.; Niu, Y.; Zeng, B. Recent Advances in Ergosterol Biosynthesis and Regulation Mechanisms in Saccharomyces cerevisiae. Indian J. Microbiol. 2017, 57, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Albaugh, B.N.; Denu, J.M. Catalysis by Protein Acetyltransferase Gcn5. Biochim. Biophys. Acta (BBA)—Gene Regul. Mech. 2021, 1864, 194627. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Graf, L.G.; Striska, K.; Janetzky, M.; Geist, N.; Specht, R.; Schulze, S.; Palm, G.J.; Girbardt, B.; Dörre, B.; et al. Acetyl-CoA Synthetase Activity Is Enzymatically Regulated by Lysine Acetylation Using Acetyl-CoA or Acetyl-Phosphate as Donor Molecule. Nat. Commun. 2024, 15, 6002. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.-S.; Shemorry, A.; Varshavsky, A. N-Terminal Acetylation of Cellular Proteins Creates Specific Degradation Signals. Science 2010, 327, 973–977. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, J.; Dujon, B.; Netter, P.; Petrochilo, E.; Slonimski, P.P.; Bolotin-Fukuhara, M.; Coen, D. MITOCHONDRIAL GENETICS. VI THE PETITE MUTATION IN Saccharomyces cerevisiae: INTERRELATIONS BETWEEN THE LOSS OF THE Ρ+ FACTOR AND THE LOSS OF THE DRUG RESISTANCE MITOCHONDRIAL GENETIC MARKERS. Genetics 1974, 76, 195–219. [Google Scholar] [CrossRef] [PubMed]
- Thomas, B.J.; Rothstein, R. Elevated Recombination Rates in Transcriptionally Active DNA. Cell 1989, 56, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Ornaghi, P.; Rotili, D.; Sbardella, G.; Mai, A.; Filetici, P. A Novel Gcn5p Inhibitor Represses Cell Growth, Gene Transcription and Histone Acetylation in Budding Yeast. Biochem. Pharmacol. 2005, 70, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Agrimi, G.; Brambilla, L.; Frascotti, G.; Pisano, I.; Porro, D.; Vai, M.; Palmieri, L. Deletion or Overexpression of Mitochondrial NAD+ Carriers in Saccharomyces cerevisiae Alters Cellular NAD and ATP Contents and Affects Mitochondrial Metabolism and the Rate of Glycolysis. Appl. Environ. Microbiol. 2011, 77, 2239–2246. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T.; Russell, D.W.; Green, M.R. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1989; ISBN 978-0-87969-309-1. [Google Scholar]
- Westermann, B.; Neupert, W. Mitochondria-Targeted Green Fluorescent Proteins: Convenient Tools for the Study of Organelle Biogenesis in Saccharomyces cerevisiae. Yeast 2000, 16, 1421–1427. [Google Scholar] [CrossRef] [PubMed]
- Kanki, T.; Wang, K.; Cao, Y.; Baba, M.; Klionsky, D.J. Atg32 Is a Mitochondrial Protein That Confers Selectivity during Mitophagy. Dev. Cell 2009, 17, 98–109. [Google Scholar] [CrossRef]
- Meng, S.; Chao, S.; Xiong, M.; Cheng, L.; Sun, Y.; Wang, L.; Chen, Y.; Jane, S.J.; Luo, C.; Chen, J. CaSun1, a SUN Family Protein, Governs the Pathogenicity of Colletotrichum camelliae by Recruiting CaAtg8 to Promote Mitophagy. Hortic. Res. 2025, 12, uhaf121. [Google Scholar] [CrossRef] [PubMed]
- Breivik, O.N.; Owades, J.L. Yeast Analysis, Spectrophotometric Semimicrodetermination of Ergosterol in Yeast. J. Agric. Food Chem. 1957, 5, 360–363. [Google Scholar] [CrossRef]
- Patnana, D.P.; Biswal, R.P.; Dandamudi, R.B.; S, C.; Pandey, M. Simple HPLC-DAD-Based Method for Determination of Ergosterol Content in Lichens and Mushrooms. J. Liq. Chromatogr. Relat. Technol. 2021, 44, 229–234. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).