Formation of an Amyloid-like Structure During In Vitro Interaction of Titin and Myosin-Binding Protein C
Abstract
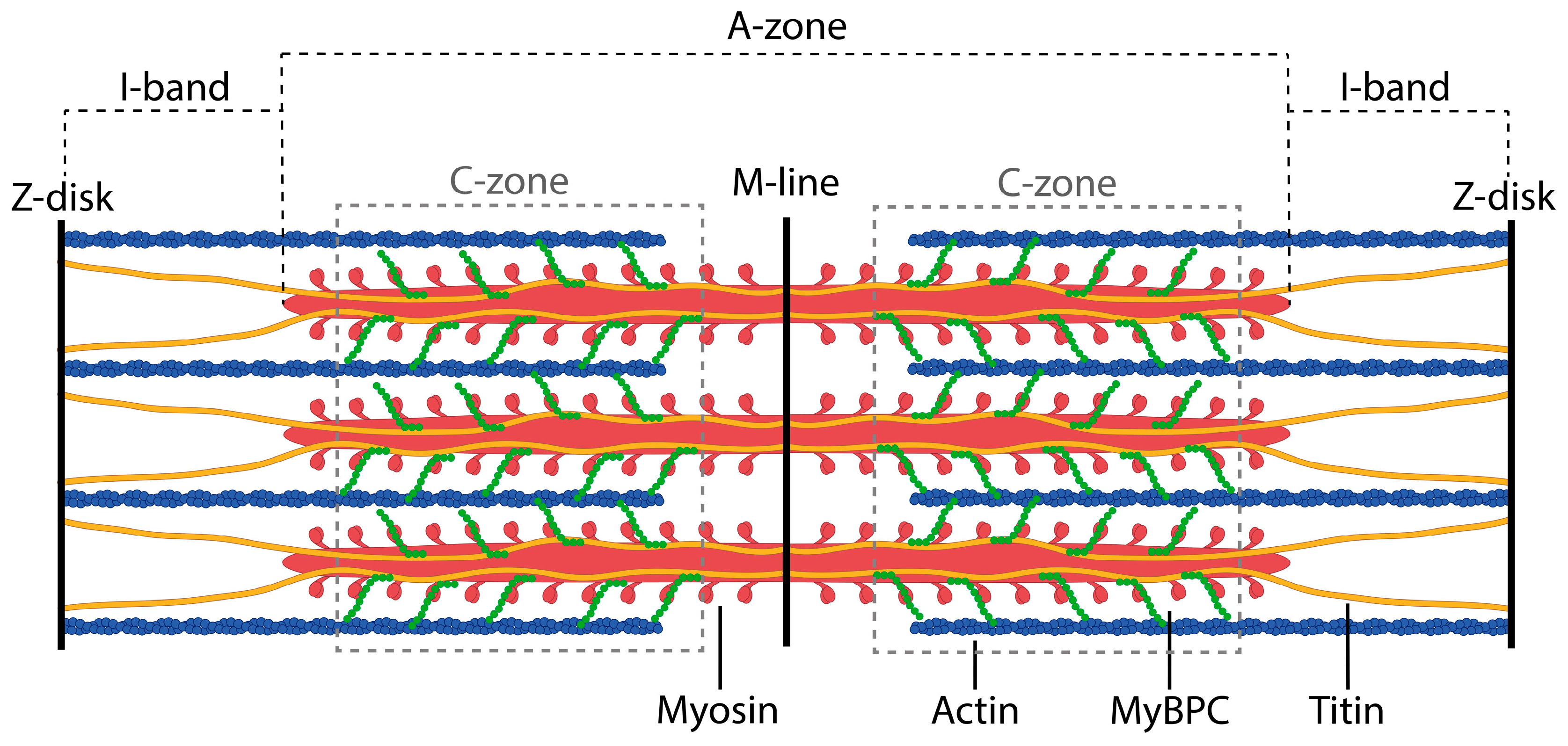
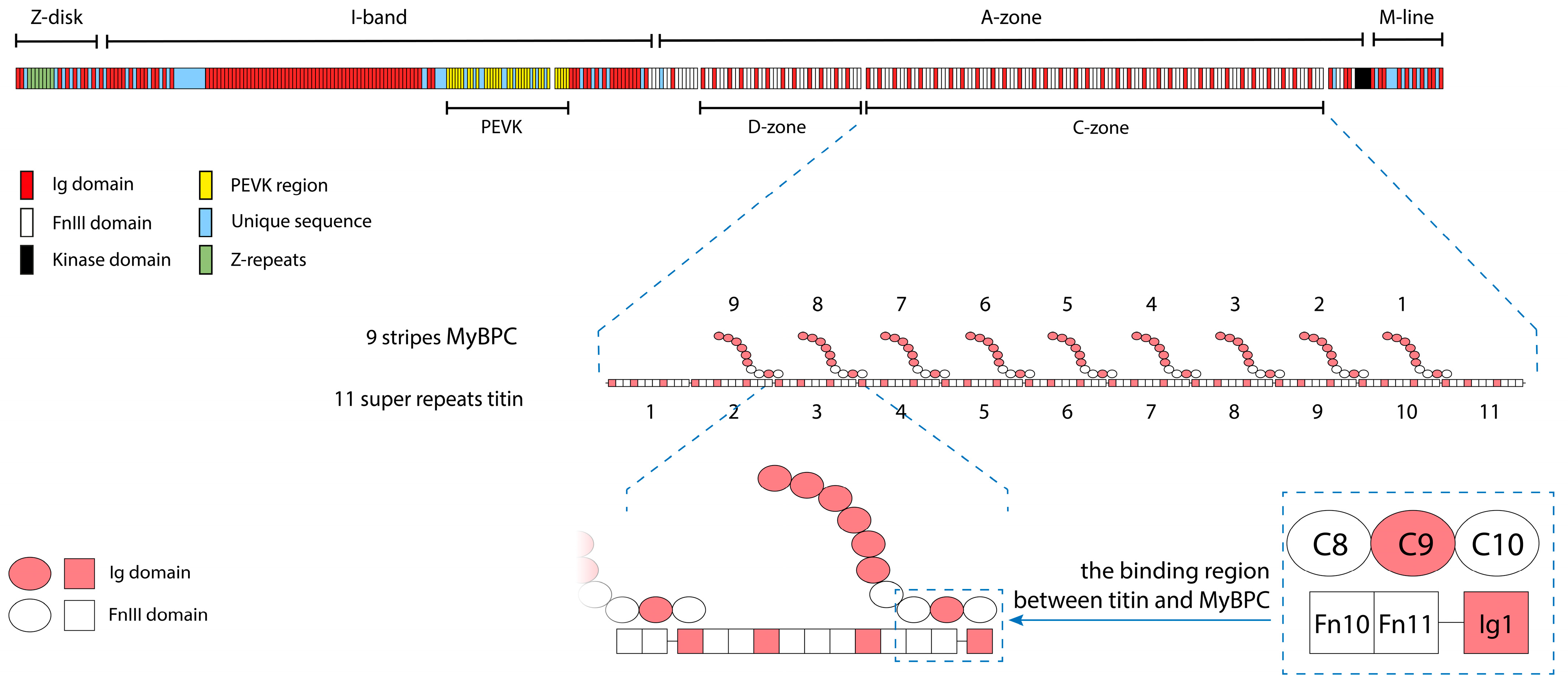
1. Introduction
2. Results
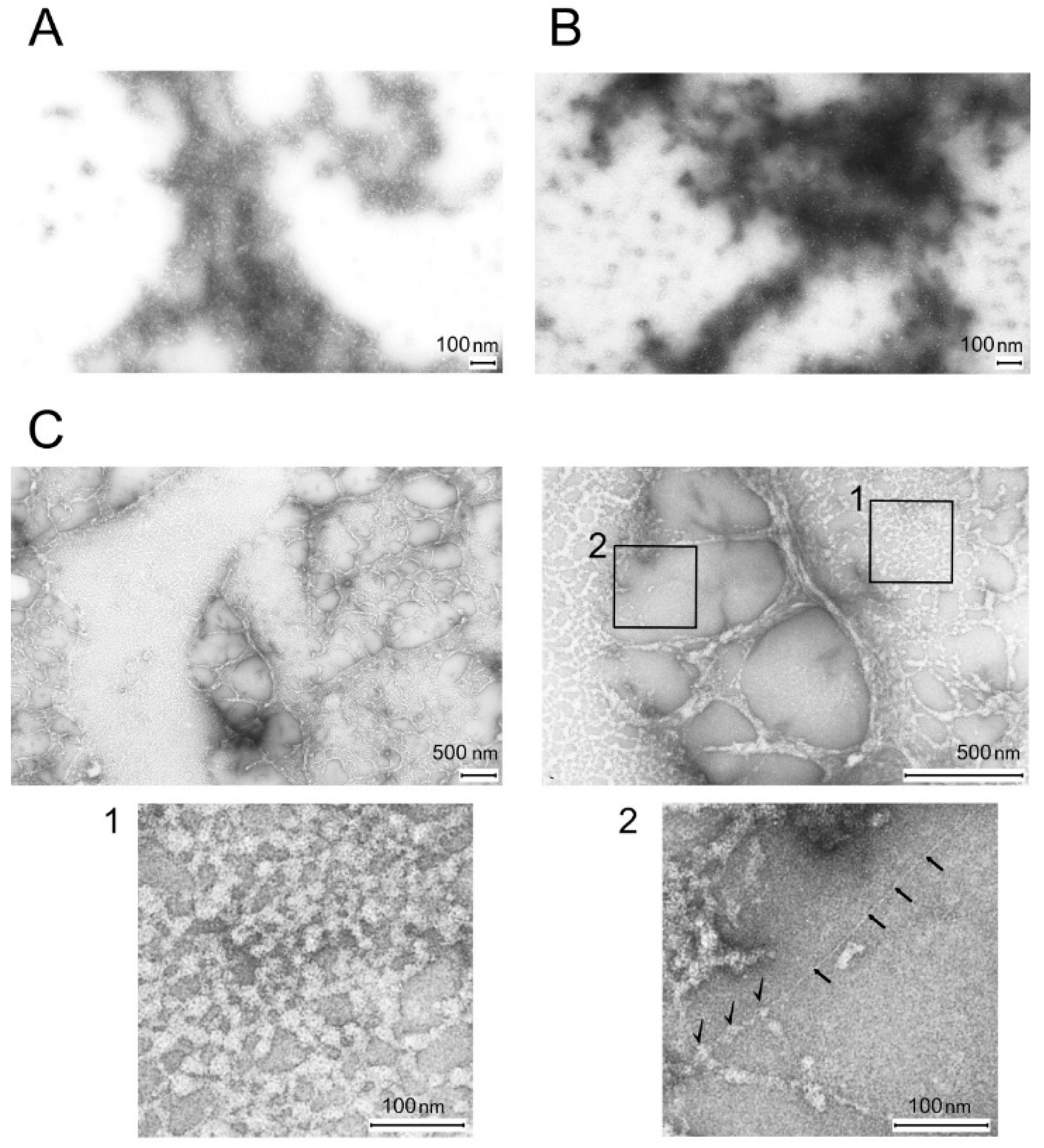
2.1. Electron Microscopy of Titin, MyBP-C, and Their Complexes
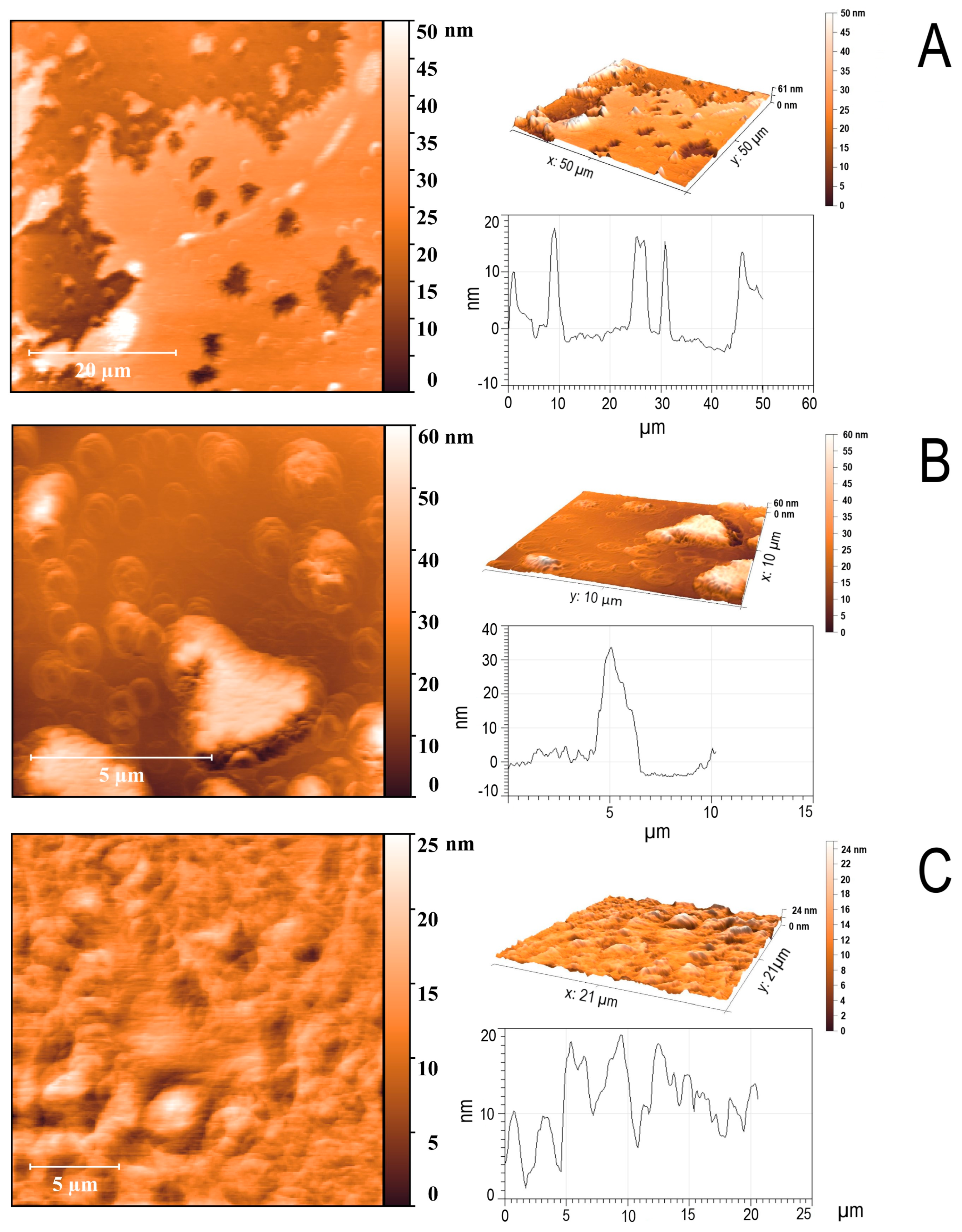
2.2. Atomic Force Microscopy
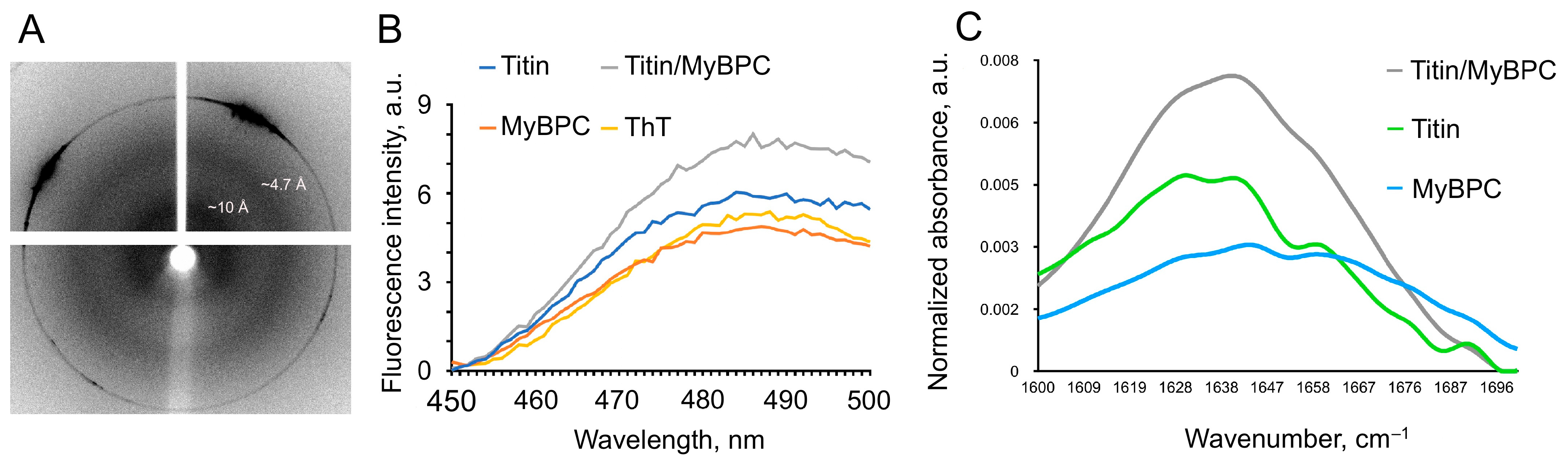
2.3. X-Ray Diffraction Study of the Amyloid Properties of Titin/MyBP-C Complexes
2.4. Binding of Titin/MyBP-C Complexes to Thioflavin T
2.5. Secondary Structure of the Complexes Obtained by Fourier Transform Infrared Spectroscopy
2.6. Prediction of Amyloidogenic Regions in Titin and MyBP-C
3. Discussion
4. Materials and Methods
4.1. Isolation of Titin and MyBP-C from Rabbit Skeletal Muscle
4.2. Electron Microscopy
4.3. High-Performance Liquid Chromatography–Mass Spectrometry
4.4. Lyophilization of Proteins
4.5. Conditions for the Formation of Titin and MyBP-C Complexes
4.6. Atomic Force Microscopy
4.7. X-Ray Diffraction
4.8. Thioflavin T Fluorimetric Assay
4.9. Fourier Transform Infrared Spectroscopy
4.10. Calculation of Amyloidogenic Regions
4.11. Amino Acid Sequence Identity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Ig | Titin immunoglobulin-like domain |
| FnIII | Titin fibronectin-like type III domains |
| FTIR | Fourier transform infrared spectroscopy |
| DTT | Dithiothreitol |
| SDS–PAGE | Sodium dodecyl sulfate polyacrylamide gel electrophoresis |
| ThT | Thioflavin T |
| TEM | Transmission electron microscopy |
| AFM | Atomic force microscopy |
References
- Labbadia, J.; Morimoto, R.I. The biology of proteostasis in aging and disease. Annu. Rev. Biochem. 2015, 84, 435–464. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, G.; Haran, G.; Zhou, H.X. Fundamental aspects of protein-protein association kinetics. Chem. Rev. 2009, 109, 839–860. [Google Scholar] [CrossRef] [PubMed]
- Thabault, L.; Liberelle, M.; Frédérick, R. Targeting protein self-association in drug design. Drug Discov. Today 2021, 26, 1148–1163. [Google Scholar] [CrossRef] [PubMed]
- Bennett, P.; Rees, M.; Gautel, M. The axial alignment of titin on the muscle thick filament supports its role as a molecular ruler. J. Mol. Biol. 2020, 432, 4815–4829. [Google Scholar] [CrossRef] [PubMed]
- Improta, S.; Krueger, J.K.; Gautel, M.; Atkinson, R.A.; Lefèvre, J.F.; Moulton, S.; Trewhella, J.; Pastore, A. The assembly of immunoglobulin-like modules in titin: Implications for muscle elasticity. J. Mol. Biol. 1998, 284, 761–777. [Google Scholar] [CrossRef] [PubMed]
- Goll, C.M.; Pastore, A.; Nilges, M. The three-dimensional structure of a type I module from titin: A prototype of intracellular fibronectin type III domains. Structure 1998, 6, 1291–1302. [Google Scholar] [CrossRef] [PubMed]
- Tonino, P.; Kiss, B.; Gohlke, J.; Smith, J.E.; Granzier, H. Fine mapping of titin–MyBP-C interaction sites using co-crystallization and cross-linking. Structure 2017, 25, 857–870.e4. [Google Scholar] [CrossRef]
- Schafer, S.; de Marvao, A.; Adami, E.; Fiedler, L.R.; Ng, B.; Khin, E.; Rackham, O.J.; van Heesch, S.; Pua, C.J.; Kui, M.; et al. Titin-truncating variants affect heart function in disease cohorts and the general population. Nat. Genet. 2017, 49, 46–53. [Google Scholar] [CrossRef]
- Haggerty, C.M.; Damrauer, S.M.; Levin, M.G.; Birtwell, D.; Carey, D.J.; Golden, A.M.; Hartzel, D.N.; Hu, Y.; Judy, R.; Kelly, M.A.; et al. Genomics-first evaluation of heart disease associated with titin-truncating variants. Circulation 2019, 140, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Hackman, P.; Vihola, A.; Haravuori, H.; Marchand, S.; Sarparanta, J.; De Seze, J.; Labeit, S.; Witt, C.; Peltonen, L.; Richard, I.; et al. Tibial muscular dystrophy is a titinopathy caused by mutations in TTN, the gene encoding the giant skeletal-muscle protein titin. Am. J. Hum. Genet. 2002, 71, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Richard, P.; Fressart, V.; Charron, P.; Hainque, B. Génétique des cardiomyopathies héréditaires. Pathol. Biol. 2010, 58, 343–352. [Google Scholar] [CrossRef]
- Harris, S.P.; Lyons, R.G.; Bezold, K.L. In the thick of it: HCM-causing mutations in myosin binding proteins of the thick filament. Circ. Res. 2011, 108, 751–764. [Google Scholar] [CrossRef] [PubMed]
- Desai, D.A.; Rao, V.J.; Jegga, A.G.; Dhandapany, P.S.; Sadayappan, S. Heterogeneous Distribution of Genetic Mutations in Myosin Binding Protein-C Paralogs. Front. Genet. 2022, 13, 896117. [Google Scholar] [CrossRef] [PubMed]
- Sliwa, K.; Hilfiker-Kleiner, D.; Petrie, M.C.; Mebazaa, A.; Pieske, B.; Buchmann, E.; Regitz-Zagrosek, V.; Schaufelberger, M.; Tavazzi, L.; van Veldhuisen, D.J.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of peripartum cardiomyopathy: A position statement from the Heart Failure Association of the European Society of Cardiology Working Group on peripartum cardiomyopathy. Eur. J. Heart Fail. 2010, 12, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Herman, D.S.; Lam, L.; Taylor, M.R.G.; Wang, L.; Teekakirikul, P.; Christodoulou, D.; Conner, L.; DePalma, S.R.; McDonough, B.; Sparks, E.; et al. Truncations of titin causing dilated cardiomyopathy. N. Engl. J. Med. 2012, 366, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Begay, R.L.; Graw, S.; Sinagra, G.; Asimaki, A.; Rowland, T.J.; Slavov, D.B.; Goolsby, H.A.; L’Italien, G.; McNally, E.M. Role of Titin Missense Variants in Dilated Cardiomyopathy. J. Am. Heart Assoc. 2015, 4, e002645. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.M.; Ware, J.S.; Herman, D.S.; Schafer, S.; Baksi, J.; Bick, A.G.; Buchan, R.J.; Walsh, R.; John, S.; Wilkinson, S.; et al. Integrated allelic, transcriptional, and phenomic dissection of the cardiac effects of titin truncations in health and disease. Sci. Transl. Med. 2015, 7, 270ra6. [Google Scholar] [CrossRef] [PubMed]
- Bobylev, A.G.; Galzitskaya, O.V.; Fadeev, R.S.; Bobyleva, L.G.; Yurshenas, D.A.; Molochkov, N.V.; Dovidchenko, N.V.; Selivanova, O.M.; Penkov, N.V.; Podlubnaya, Z.A.; et al. Smooth muscle titin forms in vitro amyloid aggregates. Biosci. Rep. 2016, 36, e00334. [Google Scholar] [CrossRef] [PubMed]
- Yakupova, E.I.; Vikhlyantsev, I.M.; Bobyleva, L.G.; Penkov, N.V.; Timchenko, A.A.; Timchenko, M.A.; Enin, G.A.; Khutzian, S.S.; Selivanova, O.M.; Bobylev, A.G. Different amyloid aggregation of smooth muscle titin in vitro. J. Biomol. Struct. Dyn. 2018, 36, 2237–2248. [Google Scholar] [CrossRef] [PubMed]
- Bobyleva, L.G.; Shumeyko, S.A.; Yakupova, E.I.; Galkin, A.V.; Stepanova, A.V.; Vikhlyantsev, I.M.; Gusev, N.B. Myosin Binding Protein-C Forms Amyloid-Like Aggregates In Vitro. Int. J. Mol. Sci. 2021, 22, 731. [Google Scholar] [CrossRef] [PubMed]
- Bobylev, A.G.; Yakupova, E.I.; Bobyleva, L.G.; Molochkov, N.V.; Timchenko, A.A.; Timchenko, M.A.; Kihara, H.; Nikulin, A.D.; Gabdulkhakov, A.G.; Melnik, T.N.; et al. Nonspecific Amyloid Aggregation of Chicken Smooth-Muscle Titin: In Vitro Investigations. Int. J. Mol. Sci. 2023, 24, 1056. [Google Scholar] [CrossRef] [PubMed]
- Bobyleva, L.G.; Uryupina, T.A.; Penkov, N.V.; Timchenko, M.A.; Ulanova, A.D.; Gabdulkhakov, A.G.; Vikhlyantsev, I.M.; Bobylev, A.G. The Structural Features of Skeletal Muscle Titin Aggregates. Mol. Biol. 2024, 58, 319–332. [Google Scholar] [CrossRef]
- Makin, O.S.; Serpell, L.C. Structural characterization of islet amyloid polypeptide fibrils. J. Mol. Biol. 2005, 356, 891–903. [Google Scholar] [CrossRef]
- Jahn, T.R.; Makin, O.S.; Morris, K.L.; Marshall, K.E.; Tian, P.; Sikorski, P.; Serpell, L.C. The common architecture of cross-β amyloid. J. Mol. Biol. 2010, 395, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Morris, K.L.; Serpell, L.C. X-ray fibre diffraction studies of amyloid fibrils. Methods Mol. Biol. 2012, 849, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Fowler, D.M.; Koulov, A.V.; Balch, W.E.; Kelly, J.W. Functional amyloid formation within mammalian tissue. PLoS Biol. 2006, 4, e6. [Google Scholar] [CrossRef] [PubMed]
- Barth, A. Infrared spectroscopy of proteins. Biochim. Biophys. Acta 2007, 1767, 1073–1101. [Google Scholar] [CrossRef] [PubMed]
- Sarroukh, R.; Goormaghtigh, E.; Ruysschaert, J.M.; Raussens, V. ATR-FTIR: A “rejuvenated” tool to investigate amyloid proteins. Biochim. Biophys. Acta 2013, 1828, 2328–2338. [Google Scholar] [CrossRef] [PubMed]
- Garbuzynskiy, S.O.; Lobanov, M.Y.; Galzitskaya, O.V. FoldAmyloid: A method of prediction of amyloidogenic regions from protein sequence. Bioinformatics 2010, 26, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Conchillo-Solé, O.; de Groot, N.S.; Avilés, F.X.; Vendrell, J.; Daura, X.; Ventura, S. AGGRESCAN: A server for the prediction and evaluation of “hot spots” of aggregation in polypeptides. BMC Bioinform. 2007, 8, 65. [Google Scholar] [CrossRef] [PubMed]
- Rivas-Pardo, J.A.; Eckels, E.C.; Popa, I.; Kosuri, P.; Linke, W.A.; Fernández, J.M. Work Done by Titin Protein Folding Assists Muscle Contraction. Cell Rep. 2016, 14, 1339–1347. [Google Scholar] [CrossRef] [PubMed]
- Eckels, E.C.; Haldar, S.; Tapia-Rojo, R.; Rivas-Pardo, J.A.; Fernández, J.M. The mechanical power of titin folding. Cell Rep. 2019, 27, 1836–1847.e4. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.F.; Teichmann, S.A.; Clarke, J.; Dobson, C.M. The importance of sequence diversity in the aggregation and evolution of proteins. Nature 2005, 438, 878–881. [Google Scholar] [CrossRef] [PubMed]
- Knowles, T.P.; Fitzpatrick, A.W.P.; Meehan, S.; Mott, H.R.; Vendruscolo, M.; Dobson, C.M.; Welland, M.E. Role of intermolecular forces in defining material properties of protein nanofibrils. Science 2007, 318, 1900–1903. [Google Scholar] [CrossRef] [PubMed]
- Keten, S.; Buehler, M.J. Geometric confinement governs the rupture strength of H-bond assemblies at a critical length scale. Nano Lett. 2008, 8, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, F.S.; Adamcik, J.; Jeong, J.S.; Lashuel, H.A.; Mezzenga, R.; Dietler, G. Influence of the β-sheet content on the mechanical properties of aggregates during amyloid fibrillization. Angew. Chem. Int. Ed. Engl. 2015, 54, 2462–2466. [Google Scholar] [CrossRef] [PubMed]
- Pedebos, C.; Smith, I.P.S.; Boags, A.; Khalid, S. The hitchhiker’s guide to the periplasm: Unexpected molecular interactions of polymyxin B1 in E. coli. Structure 2021, 29, 444–456.e2. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.S.; Srinivas, K.; Sujini, G.N.; Kumar, G.N. Protein–protein interaction detection: Methods and analysis. Int. J. Proteom. 2014, 2014, 147648. [Google Scholar] [CrossRef] [PubMed]
- Fraterman, S.; Zeiger, U.; Khurana, T.S.; Wilm, M.; Rubinstein, N.A. Quantitative proteomics profiling of sarcomere-associated proteins in limb and extraocular muscle allotypes. Mol. Cell. Proteom. 2007, 6, 728–737. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Flashman, E.; Redwood, C.; Moolman-Smook, J.; Watkins, H. Cardiac myosin binding protein C: Its role in physiology and disease. Circ. Res. 2004, 94, 1279–1289. [Google Scholar] [CrossRef] [PubMed]
- Fomin, A.; Gärtner, A.; Cyganek, L.; Tiburcy, M.; Tuleta, I.; Wellers, L.; Folsche, L.; Hobbach, A.J.; von Frieling-Salewsky, M.; Unger, A.; et al. Truncated titin proteins and titin haploinsufficiency are targets for functional recovery in human cardiomyopathy due to TTN mutations. Sci. Transl. Med. 2021, 13, eabd3079. [Google Scholar] [CrossRef] [PubMed]
- Kellermayer, D.; Tordai, H.; Kiss, B.; Török, G.; Péter, D.M.; Sayour, A.A.; Pólos, M.; Hartyánszky, I.; Szilveszter, B.; Labeit, S.; et al. Truncated titin is structurally integrated into the human dilated cardiomyopathic sarcomere. J. Clin. Investig. 2024, 134, e169753. [Google Scholar] [CrossRef] [PubMed]
- Mearini, G.; Schlossarek, S.; Willis, M.S.; Carrier, L. The ubiquitin–proteasome system in cardiac dysfunction. Biochim. Biophys. Acta 2008, 1782, 749–763. [Google Scholar] [CrossRef]
- Sarikas, A.; Carrier, L.; Schenke, C.; Doll, D.; Flavigny, J.; Lindenberg, K.S.; Eschenhagen, T.; Zolk, O. Impairment of the ubiquitin–proteasome system by truncated cardiac myosin binding protein C mutants. Cardiovasc. Res. 2005, 66, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Zolk, O.; Caroni, P.; Böhm, M. Decreased expression of the cardiac LIM domain protein MLP in chronic human heart failure. Circulation 2000, 101, 2674–2677. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.; Nguyen, V.; Campbell, K.S.; Padrón, R.; Craig, R. Cryo-EM structure of the human cardiac myosin filament. Nature 2023, 623, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Kötter, S.; Krüger, M. Protein quality control at the sarcomere: Titin protection and turnover and implications for disease development. Front. Physiol. 2022, 13, 914296. [Google Scholar] [CrossRef] [PubMed]
- Fernando, R.; Drescher, C.; Nowotny, K.; Grune, T.; Castro, J.P. Impaired proteostasis during skeletal muscle aging. Free Radic. Biol. Med. 2019, 132, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Ayyadevara, S.; Balasubramaniam, M.; Suri, P.; Mackintosh, S.G.; Tackett, A.J.; Sullivan, D.H.; Reis, R.J.S.; Dennis, R.A. Proteins that accumulate with age in human skeletal-muscle aggregates contribute to declines in muscle mass and function in Caenorhabditis elegans. Aging 2016, 8, 3486–3497. [Google Scholar] [CrossRef] [PubMed]
- Soteriou, A.; Clark, J.F.; Trinick, J. Titin folding energy and elasticity. Proc. R. Soc. Lond. B 1993, 254, 83–86. [Google Scholar] [CrossRef]
- Trinick, J.; Knight, P.; Whiting, A. Purification and properties of native titin. J. Mol. Biol. 1984, 180, 331–356. [Google Scholar] [CrossRef] [PubMed]
- Offer, G.; Moos, C.; Starr, R. A new protein of the thick filaments of vertebrate skeletal myofibrils. J. Mol. Biol. 1973, 74, 653–676. [Google Scholar] [CrossRef] [PubMed]
- Starr, R.; Offer, G. Preparation of C-protein, H-protein, X-protein, and phosphofructokinase. Methods Enzymol. 1982, 85 Pt B, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Fritz, J.D.; Swartz, D.R.; Greaser, M.L. Factors affecting polyacrylamide gel electrophoresis and electroblotting of high-molecular-weight myofibrillar proteins. Anal. Biochem. 1989, 180, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Venyaminov, S.; Prendergast, F.G. Water (H2O and D2O) molar absorptivity in the 1000–4000 cm−1 range and quantitative infrared spectroscopy of aqueous solutions. Anal. Biochem. 1997, 248, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yang, S.; Kong, J.; Dong, A.; Yu, S. Obtaining information about protein secondary structures in aqueous solution using Fourier transform IR spectroscopy. Nat. Protoc. 2015, 10, 382–396. [Google Scholar] [CrossRef] [PubMed]
- Borgia, A.; Kemplen, K.R.; Borgia, M.B.; Soranno, A.; Shammas, S.; Wunderlich, B.; Nettels, D.; Best, R.B.; Clarke, J.; Schuler, B. Transient misfolding dominates multidomain protein folding. Nat. Commun. 2015, 6, 8861. [Google Scholar] [CrossRef] [PubMed]
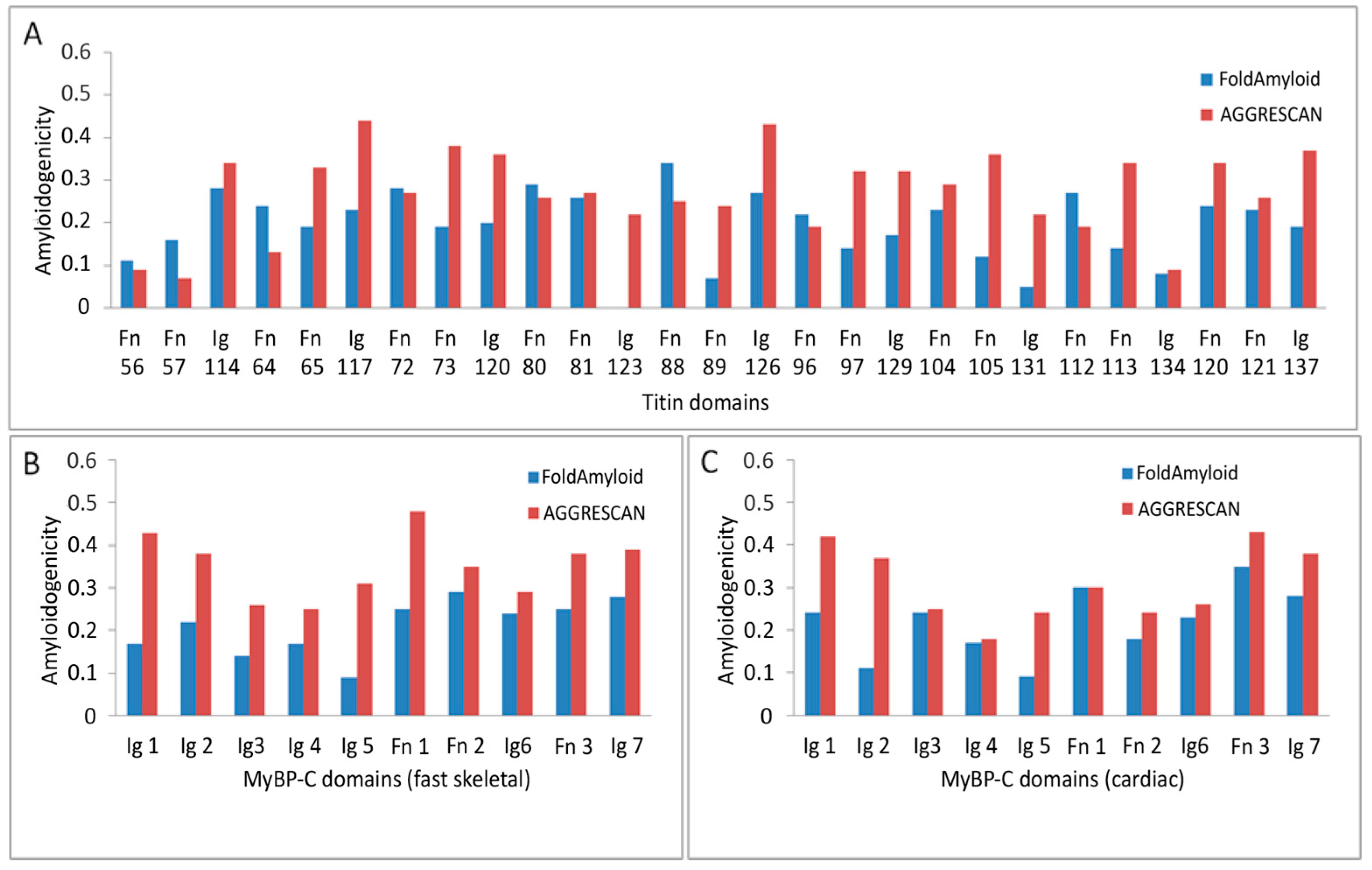
| Titin | ||
| Program | FoldAmyloid | AGGRESCAN * |
| Whole protein | 0.16 | 0.25 |
| Domain average | 0.19 | 0.27 |
| Ig domain average | 0.16 | 0.31 |
| FnIII domain average | 0.21 | 0.25 |
| MyBP-C (fast skeletal) | ||
| Program | FoldAmyloid | AGGRESCAN |
| Whole protein | 0.18 | 0.29 |
| Domain average | 0.21 | 0.35 |
| Ig domain average | 0.19 | 0.33 |
| FnIII domain average | 0.26 | 0.40 |
| MyBP-C (cardiac) | ||
| Program | FoldAmyloid | AGGRESCAN |
| Whole protein | 0.18 | 0.24 |
| Domain average | 0.21 | 0.31 |
| Ig domain average | 0.19 | 0.30 |
| FnIII domain average | 0.28 | 0.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uryupina, T.A.; Bobyleva, L.G.; Penkov, N.V.; Timchenko, M.A.; Gabdulkhakov, A.G.; Glyakina, A.V.; Rogachevsky, V.V.; Surin, A.K.; Galzitskaya, O.V.; Vikhlyantsev, I.M.; et al. Formation of an Amyloid-like Structure During In Vitro Interaction of Titin and Myosin-Binding Protein C. Int. J. Mol. Sci. 2025, 26, 6910. https://doi.org/10.3390/ijms26146910
Uryupina TA, Bobyleva LG, Penkov NV, Timchenko MA, Gabdulkhakov AG, Glyakina AV, Rogachevsky VV, Surin AK, Galzitskaya OV, Vikhlyantsev IM, et al. Formation of an Amyloid-like Structure During In Vitro Interaction of Titin and Myosin-Binding Protein C. International Journal of Molecular Sciences. 2025; 26(14):6910. https://doi.org/10.3390/ijms26146910
Chicago/Turabian StyleUryupina, Tatiana A., Liya G. Bobyleva, Nikita V. Penkov, Maria A. Timchenko, Azat G. Gabdulkhakov, Anna V. Glyakina, Vadim V. Rogachevsky, Alexey K. Surin, Oxana V. Galzitskaya, Ivan M. Vikhlyantsev, and et al. 2025. "Formation of an Amyloid-like Structure During In Vitro Interaction of Titin and Myosin-Binding Protein C" International Journal of Molecular Sciences 26, no. 14: 6910. https://doi.org/10.3390/ijms26146910
APA StyleUryupina, T. A., Bobyleva, L. G., Penkov, N. V., Timchenko, M. A., Gabdulkhakov, A. G., Glyakina, A. V., Rogachevsky, V. V., Surin, A. K., Galzitskaya, O. V., Vikhlyantsev, I. M., & Bobylev, A. G. (2025). Formation of an Amyloid-like Structure During In Vitro Interaction of Titin and Myosin-Binding Protein C. International Journal of Molecular Sciences, 26(14), 6910. https://doi.org/10.3390/ijms26146910










