Neuromuscular Activity Determines, at Least in Part, the Motoneuron, Nerve and Muscle Properties Under Normal Conditions and After Nerve Injury
Abstract
1. Introduction
2. Pattern of Neuromuscular Activity
2.1. Muscle Cross-Reinnervation
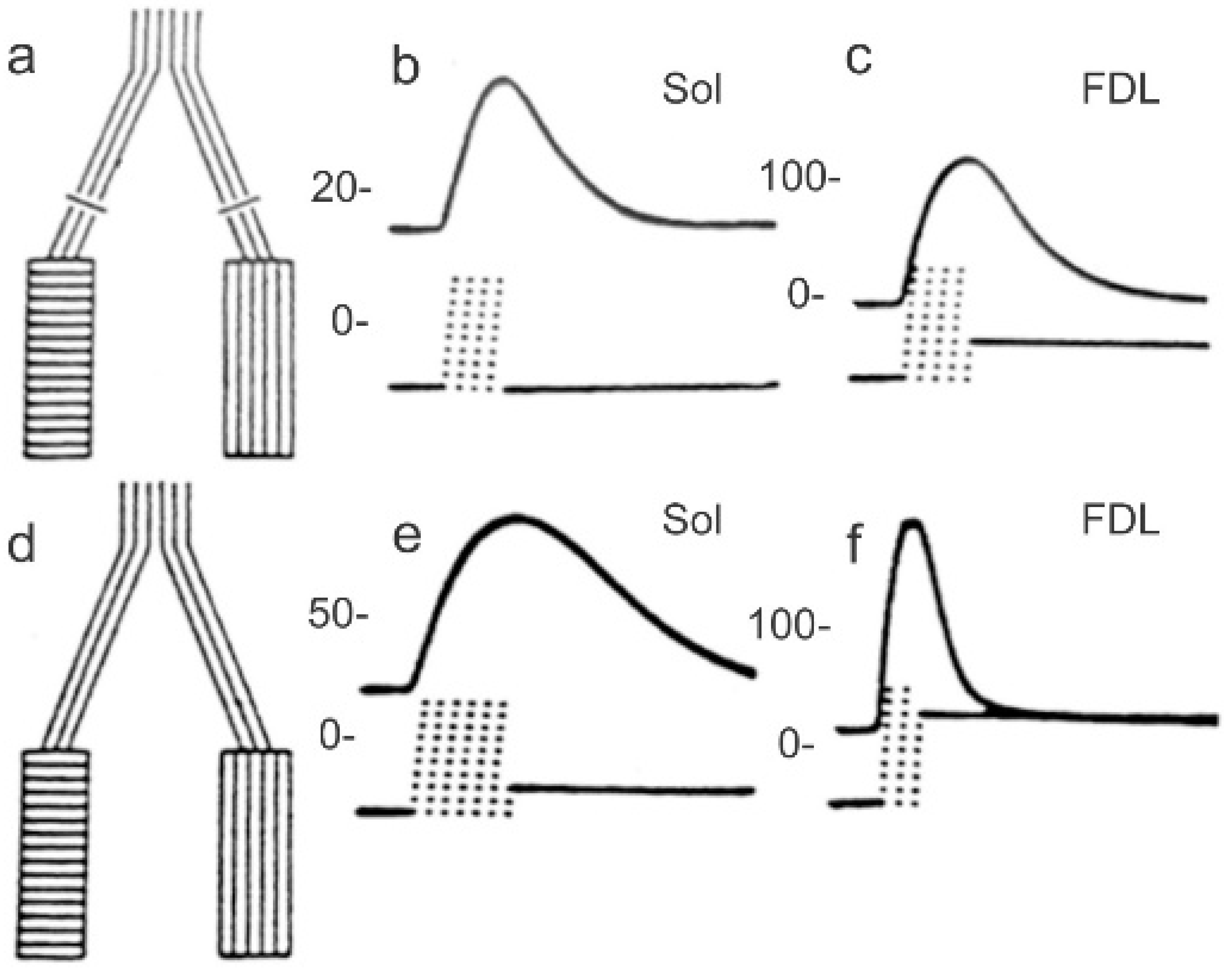
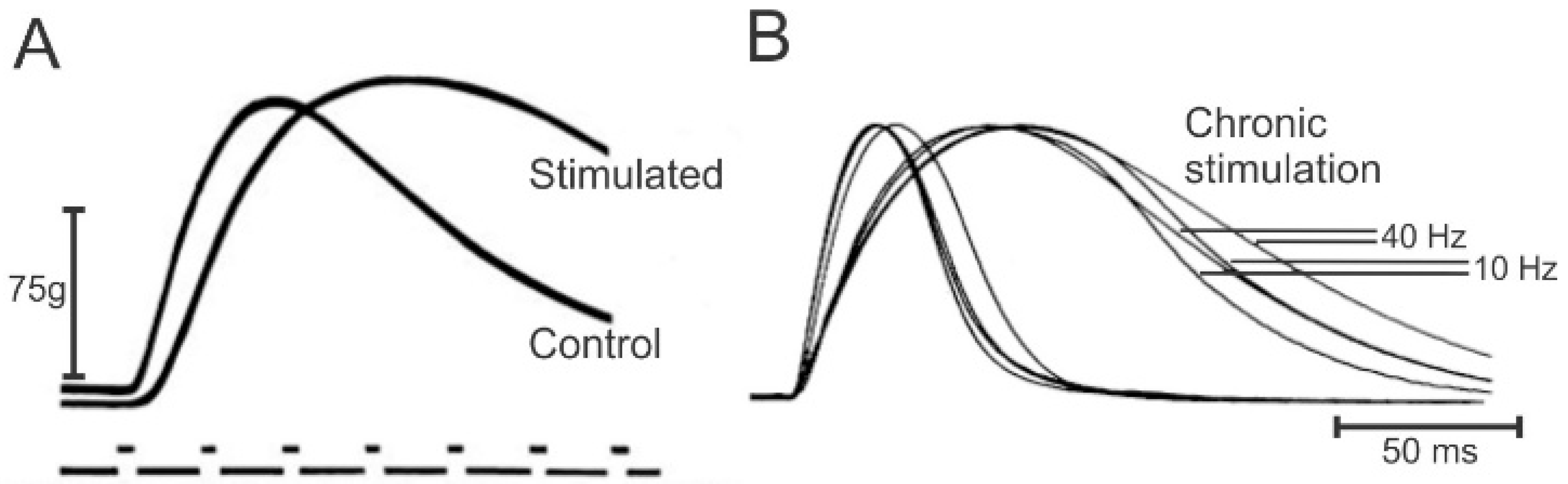
2.2. Electrical Stimulation
3. Amount of Neuromuscular Activity
3.1. Kernell’s Experiments
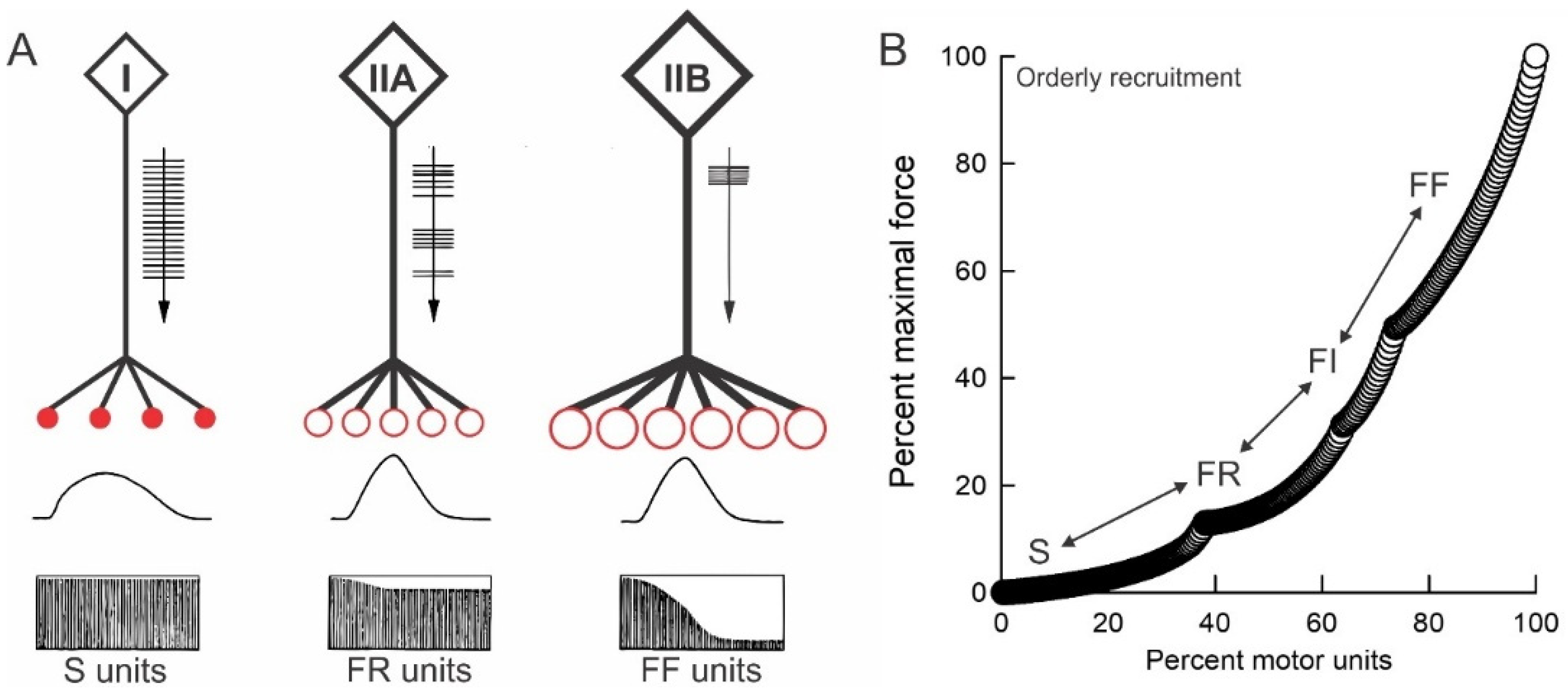
3.2. The Size Principle
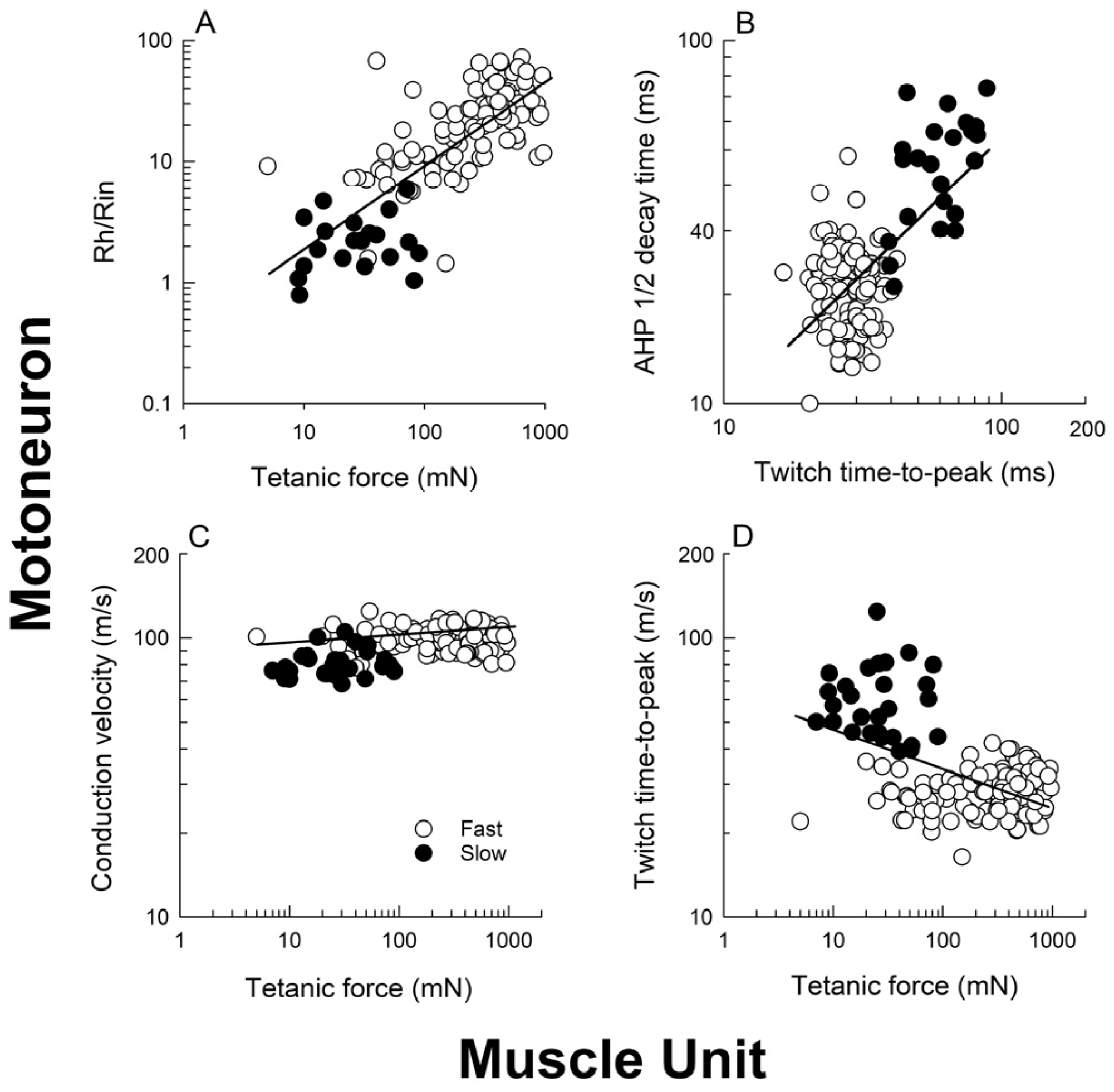
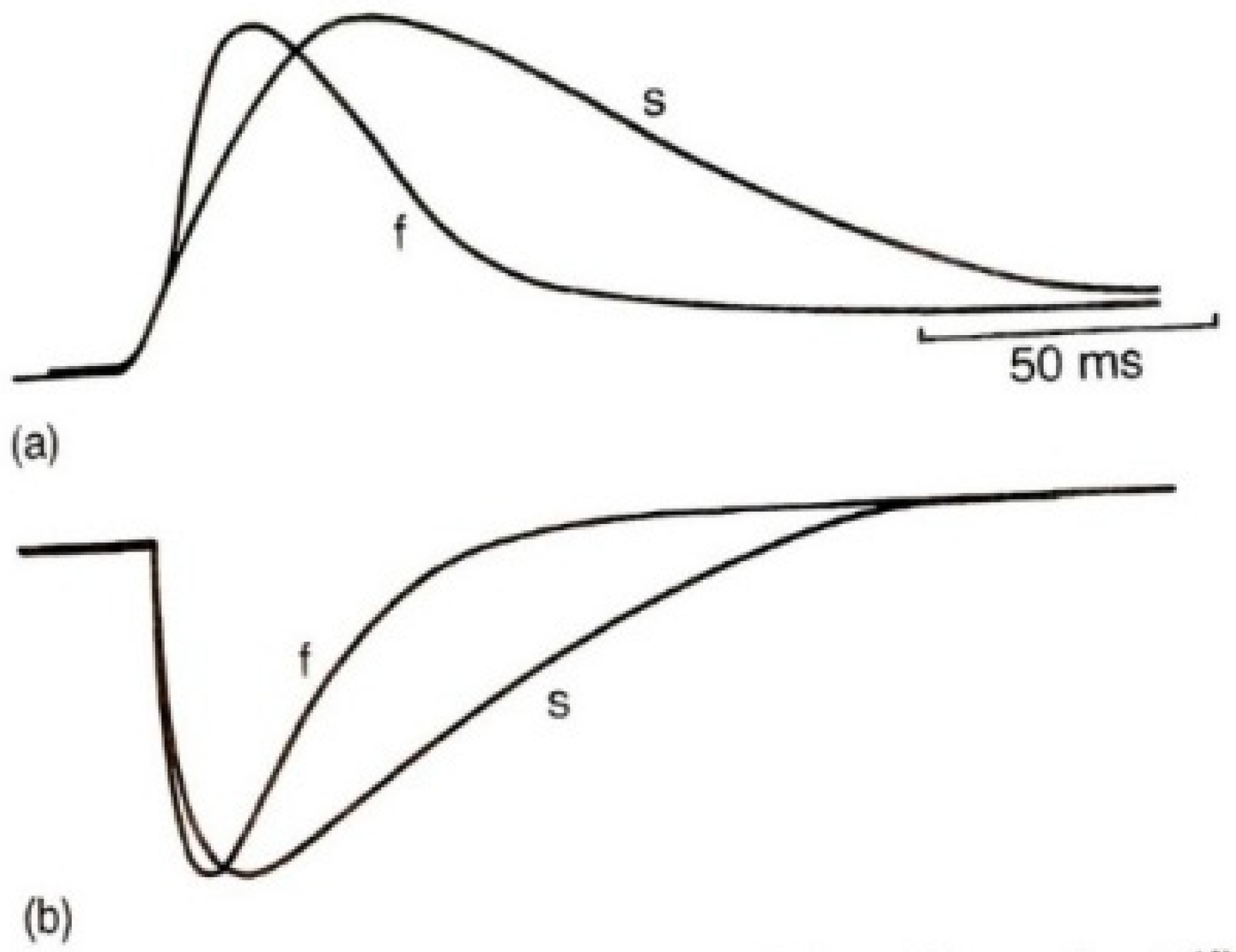
3.3. The “Speed” Match of Motoneurons and Their Muscles
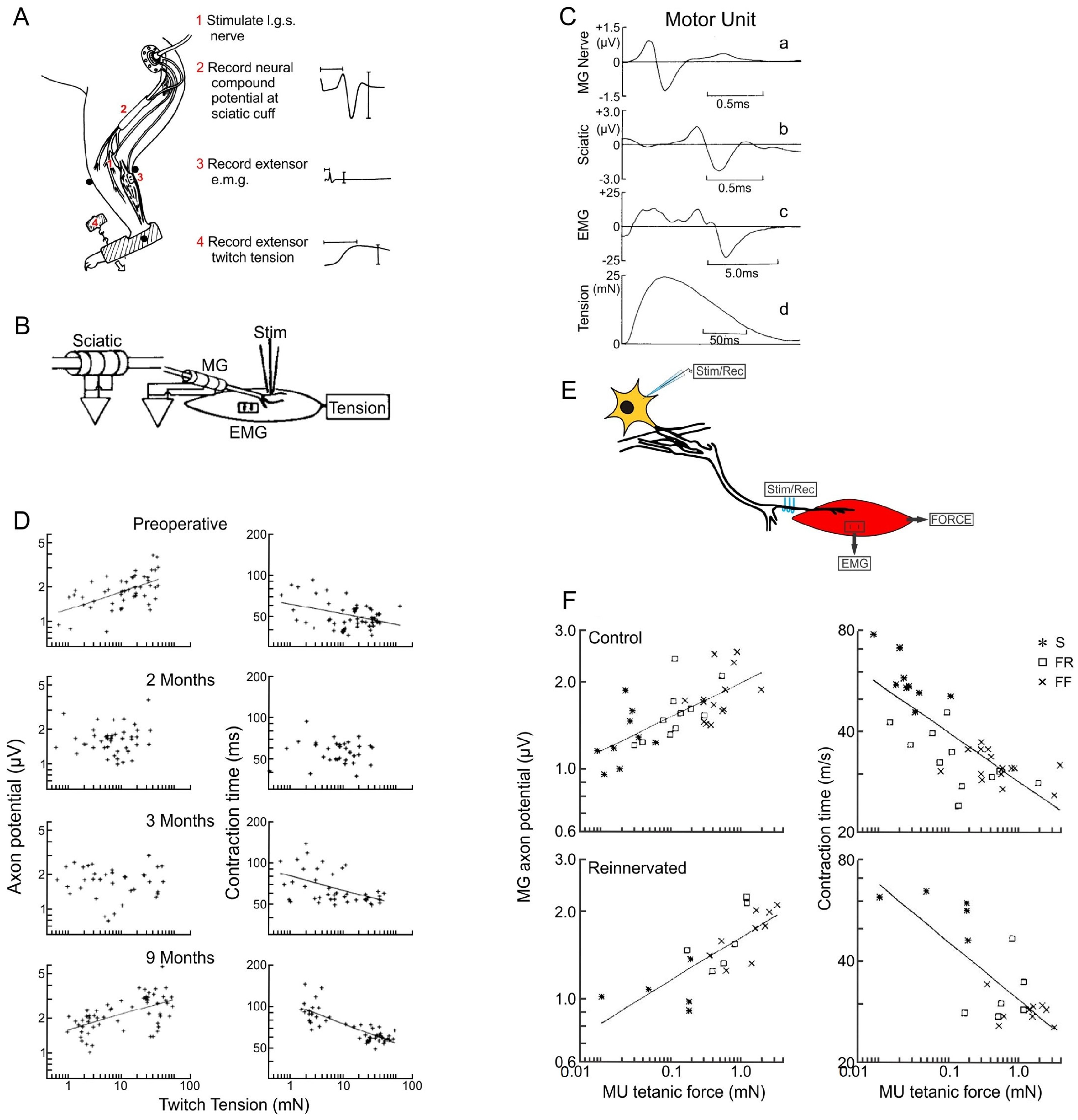
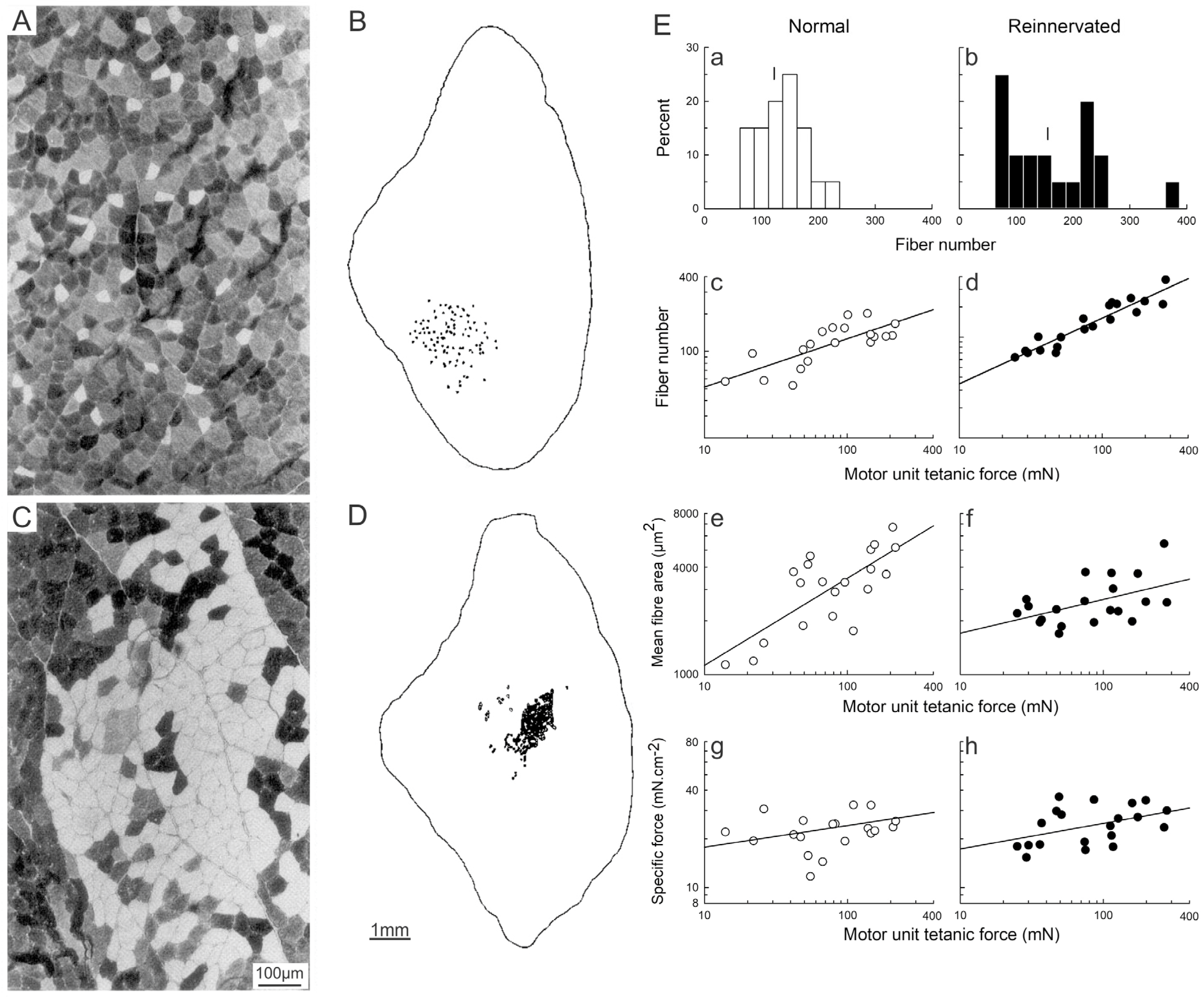
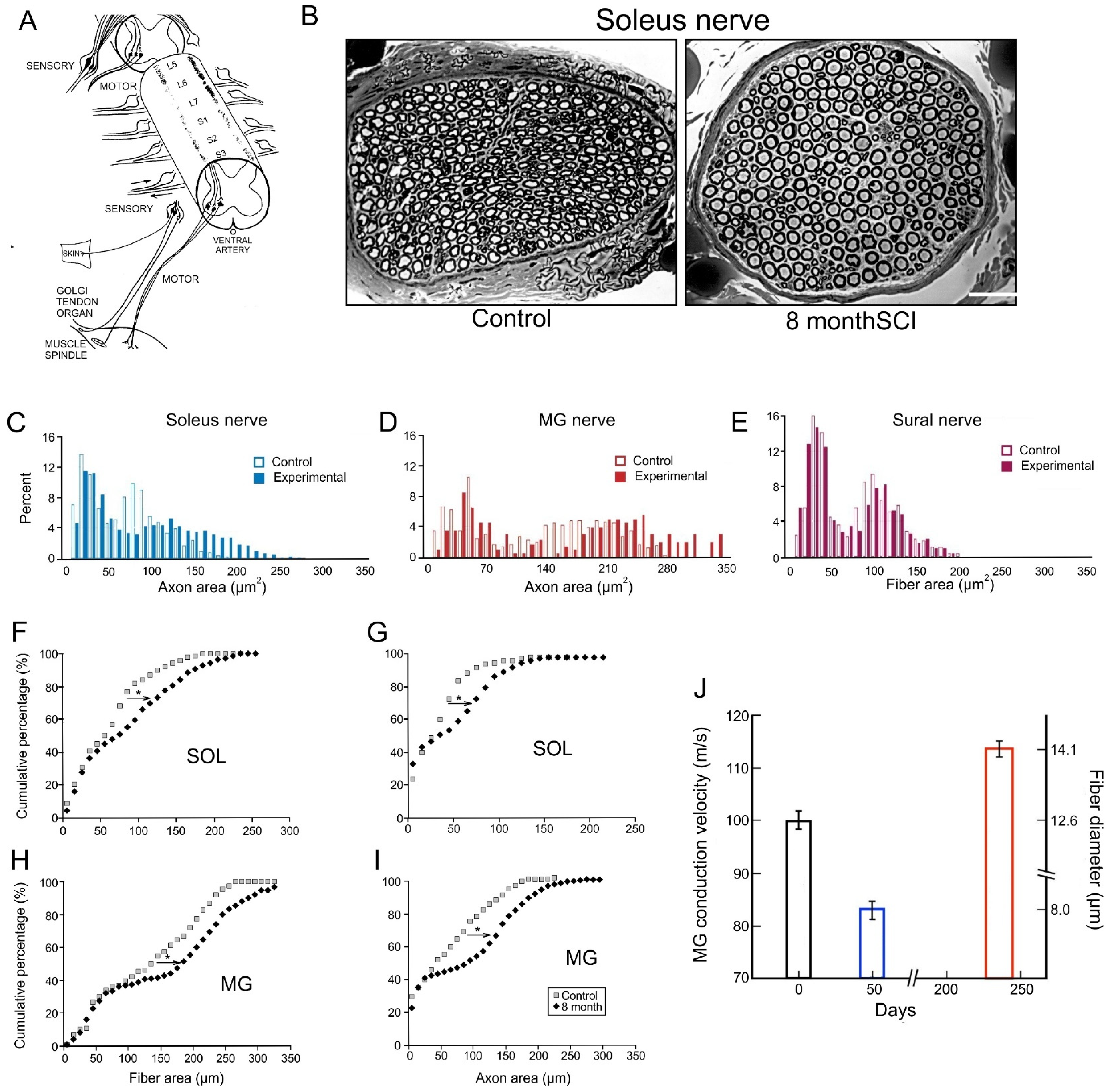
3.4. The Size Principle After Nerve Injury
3.5. Activity-Controlled Muscle and Motor Unit Properties
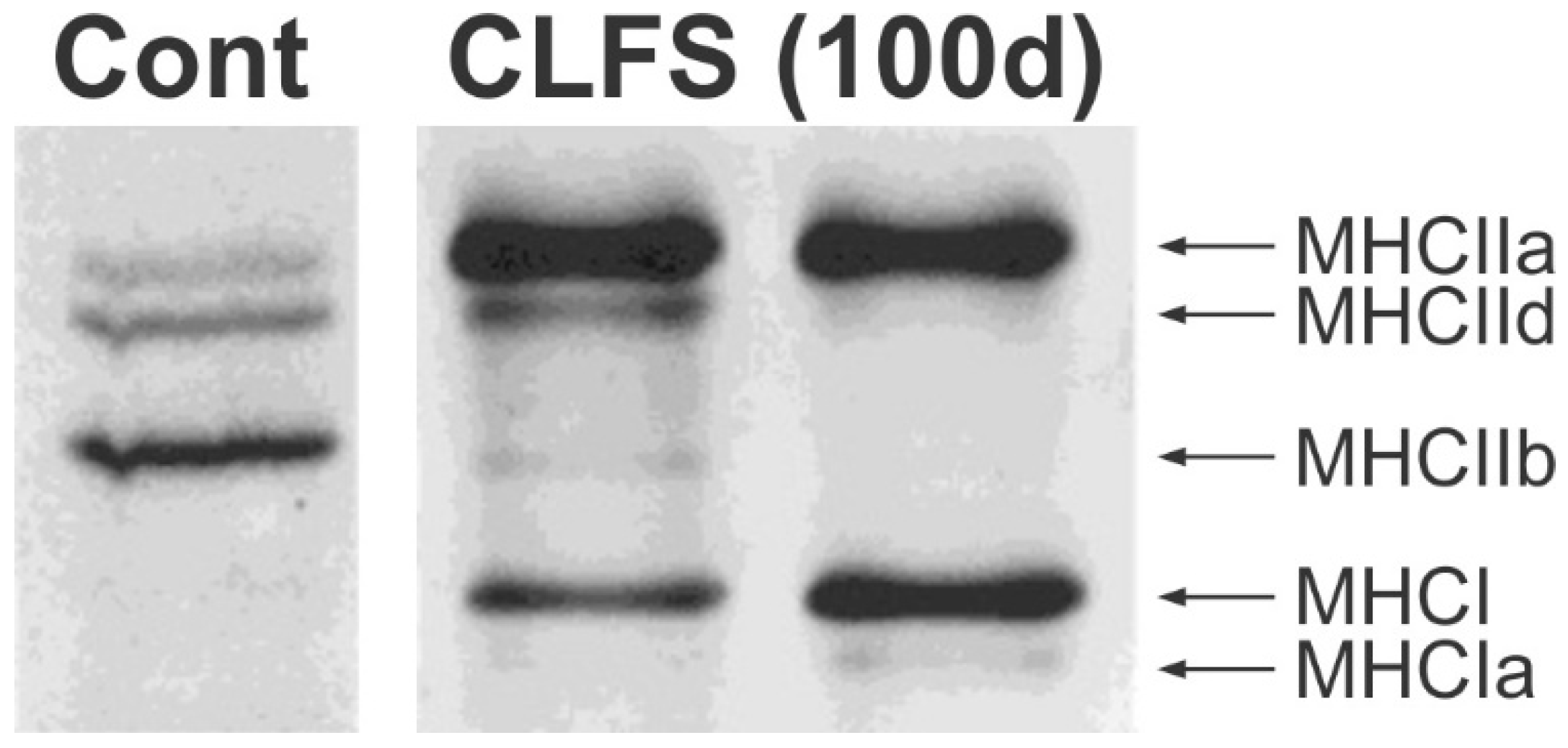
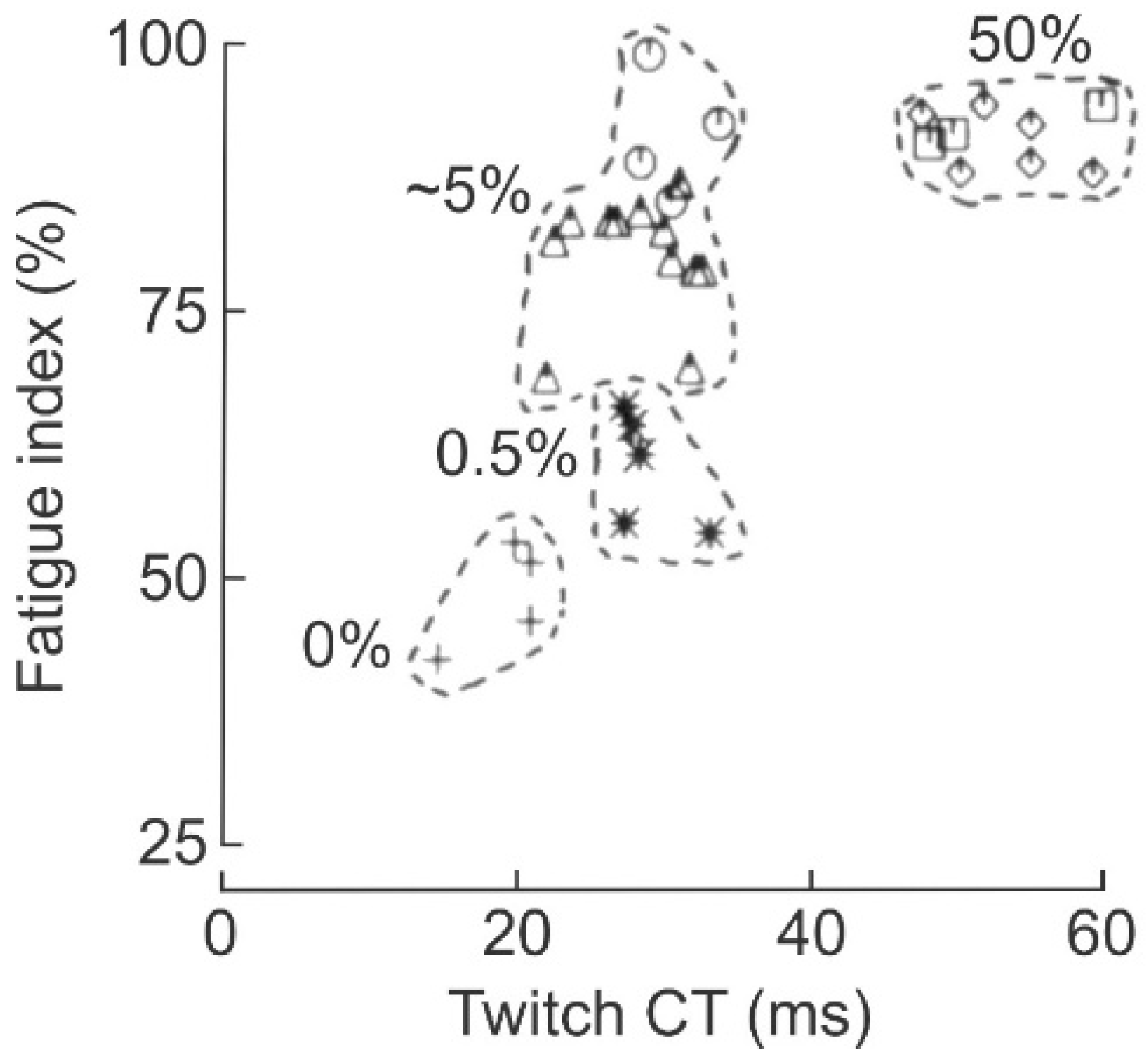
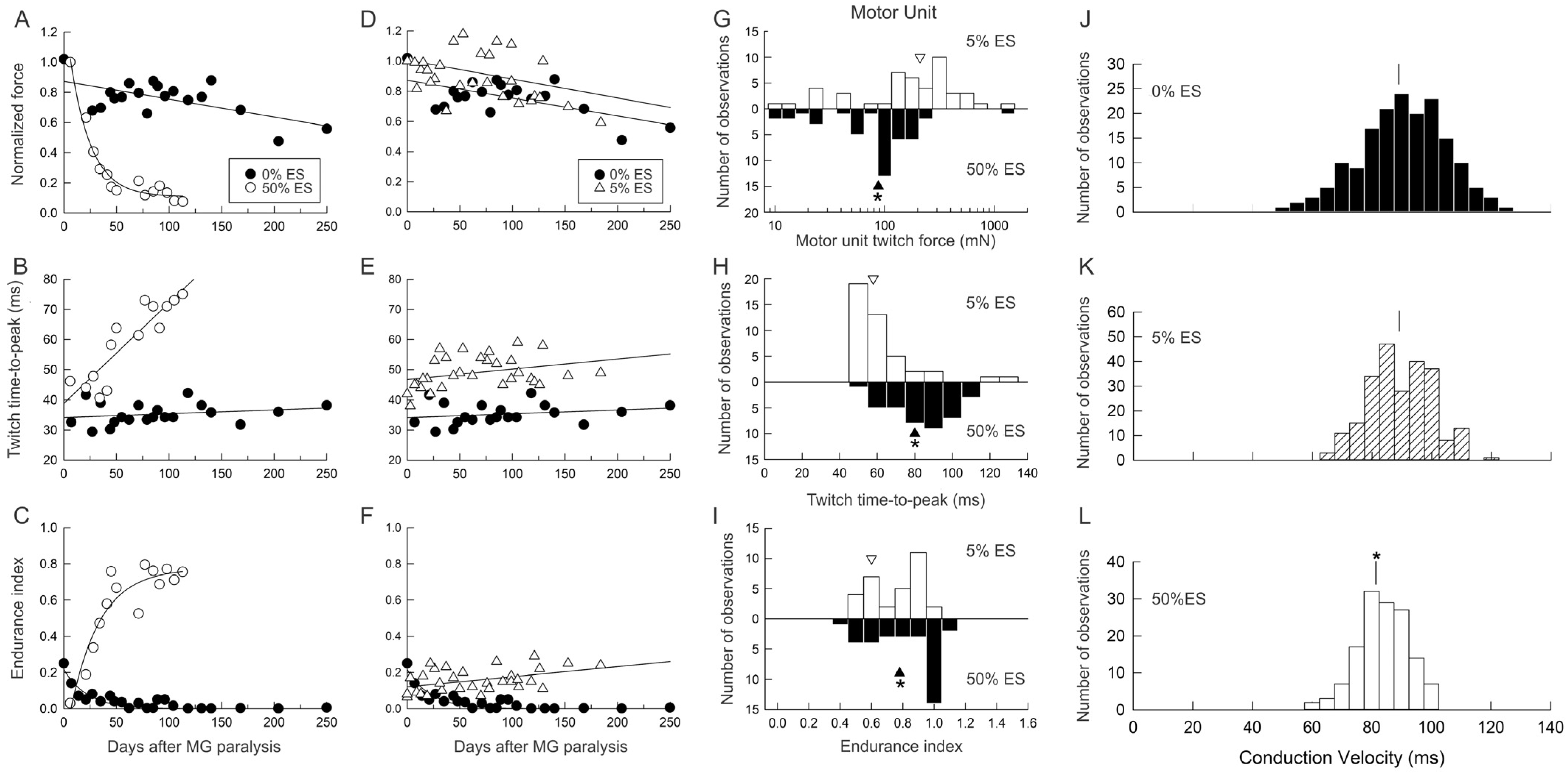
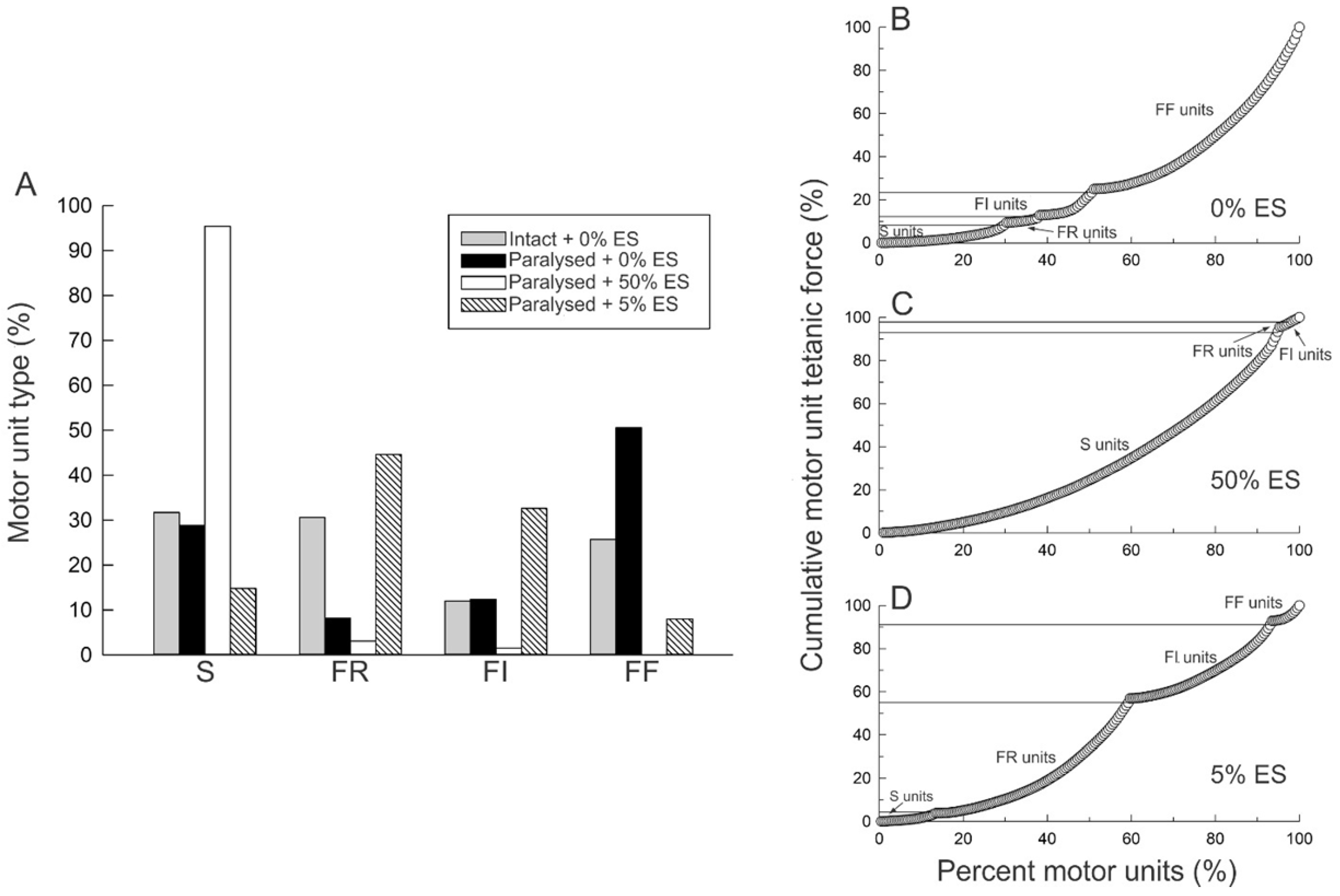
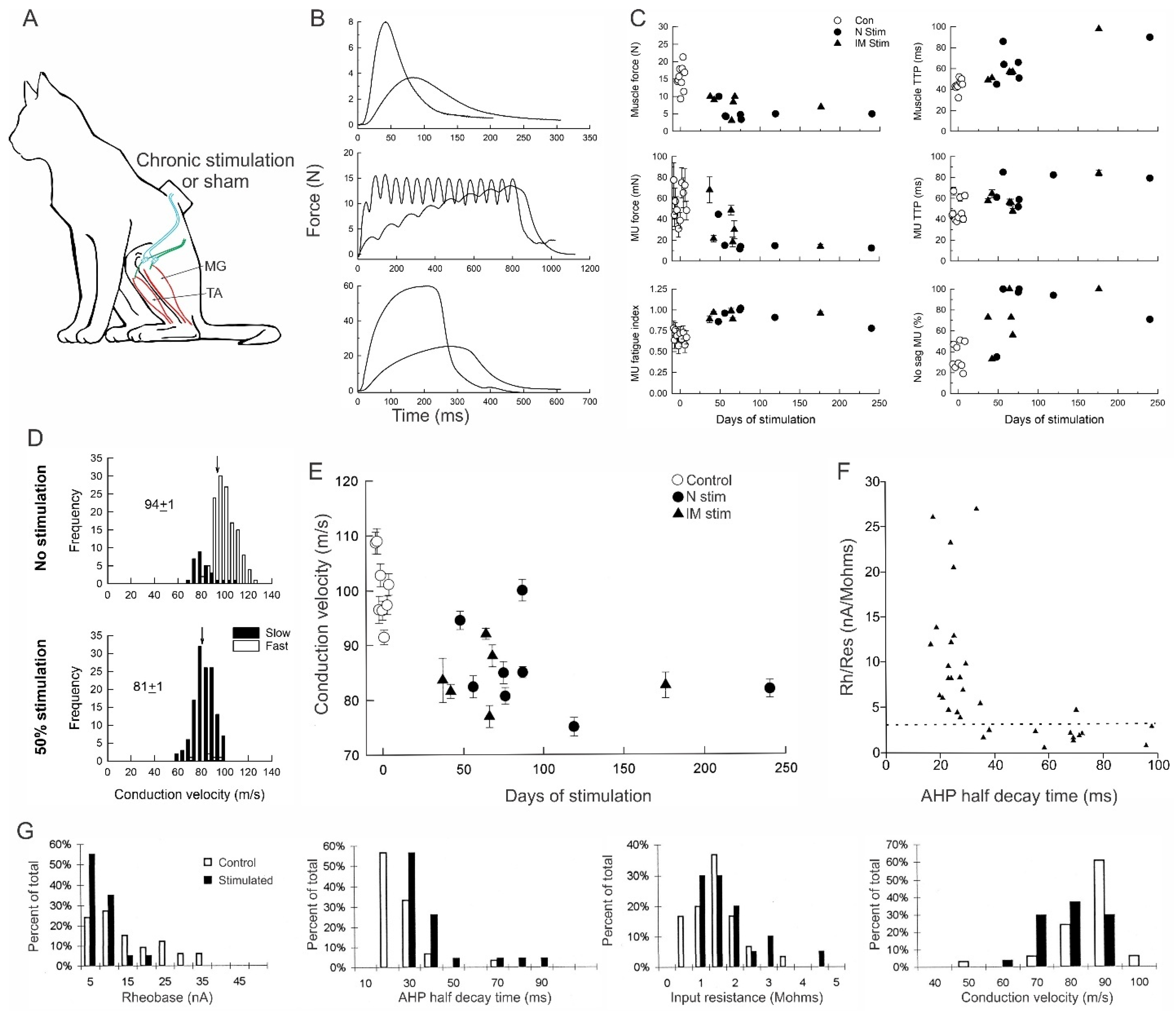
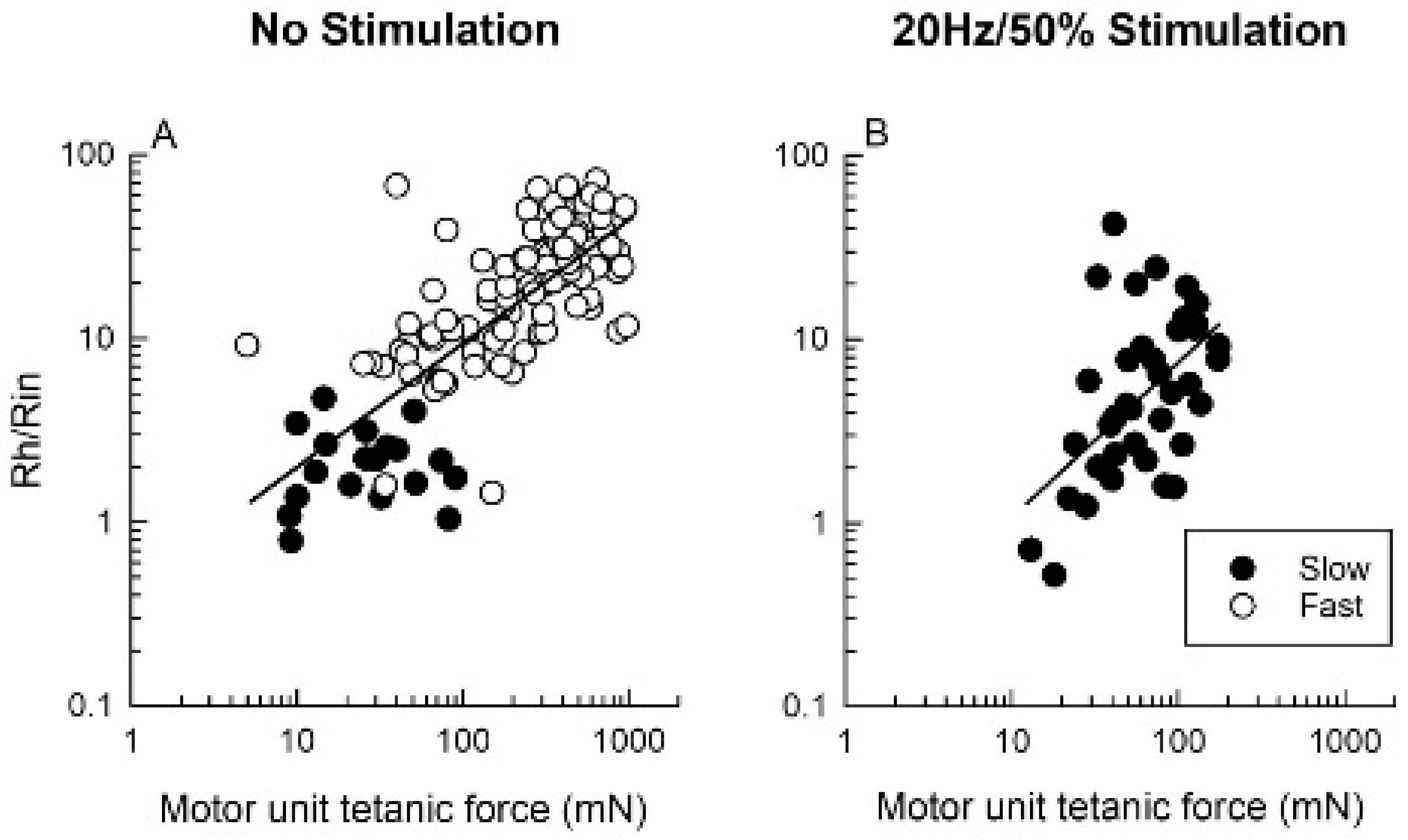
3.5.1. Reduction in Daily Neuromuscular Activity to 0%
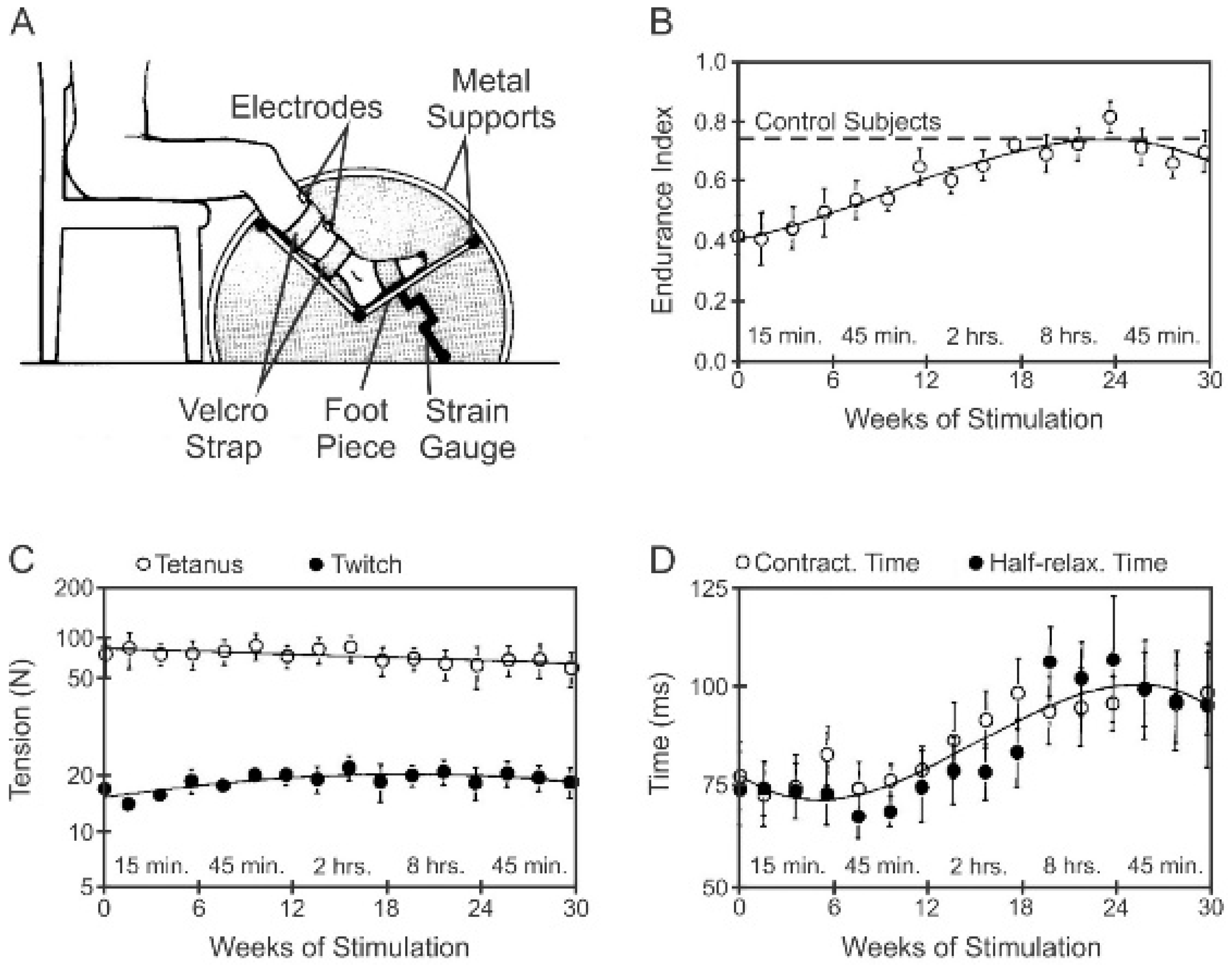
3.5.2. Low Daily Amounts of Neuromuscular Activity in Cat and Human Muscles
3.5.3. High Daily Amounts of Neuromuscular Activity
3.5.4. A Range of Properties Within MU Types
3.5.5. Plasticity of Human Muscle Fibers
4. Conclusions and Significance
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| MU | motor unit |
| S | slow |
| FR | fast-fatigue-resistant |
| FF | fast-fatigable |
| F | fast |
| SO | slow oxidative |
| FOG | fast-oxidative-glycolytic |
| FG | fast-glycolytic |
| MusU | muscle unit |
| FDL | flexor digitorum longus |
| SOL | soleus muscle |
| MG | medial gastrocnemius muscle |
| FDL | flexor digitorum longus muscle |
| TA | tibialis anterior muscle |
| ES | electrical stimulation |
| s | second |
| CP | common peroneal nerve |
| PerL | peroneus longus muscle |
| CT | twitch contraction time |
| FI | fatigue index |
| CV | nerve conduction velocity |
| TTP | time-to-peak force |
| Rh | Rheobase current |
| Rin | input resistance |
| AHP | after-hyperpolarization |
| EMG | electromyographic signal |
| T-f | relationship between T muscle tension and f the frequency of stimulation frequency |
| SCI | spinal cord isolation for 8 months |
| min | minutes |
| SE | standard error |
References
- Ranvier, L. De quelques faits relatifs a l’histologie et à la physiologie des muscles striès. Arch. Physiol. Norm. Pathol. 1874, 6, 1–15. [Google Scholar]
- Grűtzner, P. Zűr anatomie und physioslogie der querquestreiften muskeln. Rec. Zool. Suisse 1884, 1, 665–684. [Google Scholar]
- Kuhne, W. Under die Endignung der Nerven u deb Nyscjekb. Vischows Arch. 1863, 27, 1628–1632. [Google Scholar]
- Denny-Brown, D. On the nature of postural reflexes. Proc. R. Soc. (Biol.) 1929, 104, 252–301. [Google Scholar]
- Hilton, S.M.; Jeffries, M.G.; Vrbová, G. Functional specializations of the vascular bed of soleus. J. Physiol. 1970, 206, 543–562. [Google Scholar] [CrossRef] [PubMed]
- Hudlická, O.; Hoppeler, H.; Uhlmann, E. Relationship between the size of the capillary bed and oxidative capacity in various cat skeletal muscles. Pflug. Arch. 1987, 410, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Ariano, M.A.; Armstrong, R.B.; Edgerton, V.R. Hindlimb muscle fiber populations of five mammals. J. Histochem. Cytochem. 1973, 21, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R.B. Properties and distributions of the fibre types in the locomotory muscles of mammals. In Comparative Physiology: Primitive Mammals; Schmidt-Nielsen, K., Bolis, L., Taylor, C.R., Eds.; Cambridge University Press: Cambridge, UK, 1980; pp. 243–254. [Google Scholar]
- Armstrong, R.B.; Phelps, R.O. Muscle fiber type composition of the rat hindlimb. Am. J. Anat. 1984, 171, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T.; Pattullo, M. Plasticity of muscle fiber and motor unit types. Exerc. Sport Sci. Rev. 1993, 21, 331–362. [Google Scholar] [CrossRef] [PubMed]
- Kernell, D. Muscle regionalization. Can. J. Appl. Physiol. 1998, 23, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.C.; Kernell, D. Quantification of fibre type regionalisation: An analysis of lower hindlimb muscles in the rat. J. Anat. 2001, 198, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Sherrington, C. The correlation of reflexes and the principle of the common final path. Br. Assoc. 1939, 74, 728–741. [Google Scholar]
- Burke, R.E. Motor unit types of cat triceps surae. J. Physiol. 1967, 193, 141–160. [Google Scholar] [CrossRef] [PubMed]
- Burke, R.E.; Tsairis, P. The correlation of physiological properties with histochemical characteristics of single muscle units. Ann. N. Y. Acad. Sci. 1974, 228, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Burke, R.E.; Levine, D.N.; Tsairis, P.; Zajac, F.E. Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J. Physiol. 1973, 234, 723–748. [Google Scholar] [CrossRef] [PubMed]
- Edstrőm, L.; Kugelberg, E. Histochemical composition, distribution of fibres and fatigability of single motor units. J. Neurol. Neurosurg. Psychiatry 1968, 31, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Burke, R.E. Motor units: Anatomy, physiology, and functional organization. In Handbook of Physiology. Sect. I. Vol. II. The Nervous System: Motor Control. Part I; Brooks, V.B., Ed.; American Physiological Society: Rockville, MD, USA, 1981; pp. 345–422. [Google Scholar]
- Pette, D.; Staron, R.S. Myosin isoforms, muscle fiber types, and transitions. Microsc. Res. Tech. 2006, 50, 500–509. [Google Scholar] [CrossRef]
- Gauthier, G.F.; Burke, R.E.; Lowey, S.; Hobbs, A.W. Myosin isozymes in normal and reinnervated cat skeletal muscle fibers. J. Cell Biol. 1983, 97, 756–771. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, P.; Pette, D.; Vrbová, G. Comparison of enzyme activities among single muscle fibres within defined motor units. J. Physiol. 1981, 311, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Vrbová, G.; Jones, R.; Gordon, T. Nerve-Muscle Interaction, 2nd ed.; Chapman and Hall, John Wiley & Sons: New York, NY, USA, 1995. [Google Scholar]
- Pette, D.; Vrbová, G. Neural control of phenotypic expression in mammalian muscle fibers. Muscle Nerve 1985, 8, 676–689. [Google Scholar] [CrossRef] [PubMed]
- Pette, D.; Vrbová, G. Adaptations of muscle fibers to chronic electrical stimulation. Rev. Physiol. Biochem. Pharmacol. 1992, 120, 115–202. [Google Scholar] [PubMed]
- Pette, D.; Vrbová, G. The contribution of neuromuscular stimulation in elucidating muscle plasticity revisited. Eur. J. Transl. Myol. 2017, 27, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Eerbeek, O.; Kernell, D.; Verhey, B.A. Effects of fast and slow patterns of tonic long-term stimulation on contractile properties of fast muscle in the cat. J. Physiol. 1984, 352, 73–90. [Google Scholar] [CrossRef] [PubMed]
- Donselaar, Y.; Eerbeek, O.; Kernell, D.; Verhey, B.A. Fibre sizes and histochemical staining characteristics in normal and chronically stimulated fast muscle of the cat. J. Physiol. 1987, 382, 237–254. [Google Scholar] [CrossRef] [PubMed]
- Kernell, D.; Eerbeek, O.; Verhey, B.A.; Donselaar, Y. Effects of physiological amounts of high- and low-rate chronic stimulation on fast-twitch muscle of the cat hindlimb. I. Speed and force-related properties. J. Neurophysiol. 1987, 58, 598–613. [Google Scholar] [CrossRef] [PubMed]
- Kernell, D.; Donselaar, Y.; Eerbeek, O. Effects of physiological amounts of high- and low-rate chronic stimulation on fast-twitch muscle of the cat hindlimb. II. Fatigability-related properties. J. Neurophysiol. 1987, 58, 614–627. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T.; Mao, J. Muscle atrophy and procedures for training after spinal cord injury. Phys. Ther. 1994, 74, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T.; Tyreman, N.; Rafuse, V.F.; Munson, J.B. Fast-to-slow conversion following chronic low-frequency activation of medial gastrocnemius muscle in cats. I. Muscle and motor unit properties. J. Neurophysiol. 1997, 77, 2585–2604. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Munson, J.B.; Foehring, R.C.; Mendell, L.M.; Gordon, T. Fast-to-slow conversion following chronic low-frequency activation of medial gastrocnemius muscle in cats. II. Motoneuron properties. J. Neurophysiol. 1997, 77, 2005–2615. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T.; Pattullo, M.; Rafuse, V.F. Medial gastrocnemius muscles fatigue but do not atrophy in the paralyzed cat hindlimb after long-term hemisection and deafferentation. Exp. Neurol. 2020, 327, 113201. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T.; Eldridge, L.; Aurora, S. Enlargement of the nerve fibres of silenced lumbosacral motoneurons in cats. Biomedicines 2022, 10, 2022. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T.; Tyreman, N. Electrical stimulation to promote muscle and motor unit force and endurance after spinal cord injury. J. Physiol. 2023, 601, 1449–1466. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.E.; Cosmos, E.; Brierly, J. Differentiation of muscle fibre types in aneurogenic branchial muscles of the chick embryo. J. Exp. Zool. 1982, 224, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Condon, K.L.; Silberstein, H.M.; Blau, H.M.; Thompson, W.J. Differentiation of fiber types in aneural musculature of the prenatal rat hindlimb. Dev. Biol. 1990, 198, 275–295. [Google Scholar] [CrossRef] [PubMed]
- Fredette, B.J.; Landmesser, L.T. Relationship of primary and secondary myogenesis in fiber type development in embryonic chick muscle. Dev. Biol. 1990, 143, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.B.; Stockdale, F.E. Developmental regulation of the multiple myogenic cell lineages of the avian embryo. J. Cell Biol. 1986, 103, 2197–2208. [Google Scholar] [CrossRef] [PubMed]
- Buller, A.J.; Eccles, J.C.; Eccles, R.M. Interactions between motoneurons and muscles in respect of the characteristic speeds of their responses. J. Physiol. 1960, 150, 417–439. [Google Scholar] [CrossRef] [PubMed]
- Kernell, D. The repetitive discharge of motoneurons. In Muscular Afferents and Motor Control Nobel Symposium I; Granit, R., Ed.; Almqvist and Wilseli: Stockholm, Sweden, 1966; pp. 351–362. [Google Scholar]
- Granit, R. Mechanisms regulating the discharge of motoneurones. In The Sherrington Lectures, XI; Liverpool University Press: Liverpool, UK, 1970. [Google Scholar]
- Granit, R. The Basis of Motor Control; Academic Press: London, UK; New York, NY, USA, 1970. [Google Scholar]
- Kernell, D. Recruitment, rate modulation and the tonic stretch reflex. Prog. Brain Res. 1976, 44, 257–265. [Google Scholar] [PubMed]
- Kernell, D. Rhythmic properties of motoneurones innervating muscle fibres of different speed in m. gastrocnemius medialis of the cat. Brain Res. 1979, 160, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Vrbová, G. The effect of motoneurone activity on the speed of contraction of striated muscle. J. Physiol. 1963, 169, 513–526. [Google Scholar] [CrossRef] [PubMed]
- Salmons, S.; Vrbová, G. The influence of activity on some contractile characteristics of mammalian fast and slow muscles. J. Physiol. 1969, 201, 535–549. [Google Scholar] [CrossRef] [PubMed]
- Pette, D.; Smith, M.E.; Staudte, H.W.; Vrbová, G. Effects of long-term stimulation on some contractile and metabolic characteristics of fast rabbit muscles. Pflug. Arch. Eur. J. Physiol. 1973, 338, 257–272. [Google Scholar] [CrossRef] [PubMed]
- Pette, D.; Muller, W.; Leisner, E.; Vrbová, G. Time dependent effect on contractile properties, fibre population, myosin light chains and enzymes of energy metabolism in intermittently and continuously stimulated fast twitch muscles of the rabbit. Pflug. Arch. 1976, 364, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Nuhr, M.; Crevenna, R.; Gohlsch, H.; Bittner, C.; Pleiner, J.; Wiesinger, G.; Fialka-Moser, V.; Quittan, M.; Pette, D. Functional and biochemical properties of chronically stimulated human skeletal muscle. Eur. J. Appl. Physiol. 2003, 89, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Hendriksson, J.; Chi, M.M.-Y.; Hintz, C.S.; Young, D.A.; Kaiser, K.K.; Salmons, S.; Lowry, O.H. Chronic stimulation of mammalian muscle: Changes in enzymes of six metabolic pathways. Am. J. Physiol. 1986, 251, C614–C632. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.S.; Carein-Moll, M.; Mellor, J.; Salmons, S.; Harlan, W. Adaptation of skeletal muscle to increased contractile activity. J. Biol. Chem. 1987, 262, 2764–2767. [Google Scholar] [CrossRef] [PubMed]
- Hood, D.A.; Zak, R.; Pette, D. Chronic stimulation of rat skeletal muscle induces coordinate increases in mitochondrial and nuclear mRNAs of cytochrome-c-oxidase subunits. Eur. J. Biochem. 1989, 179, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Gorza, L.; Gundersen, K.; Lomo, S.; Schiaffino, S.; Westgaard, R.H. Slow-to-fast transformation of denervated soleus muscles by chronic high-frequency stimulation in the rat. J. Physiol. 1988, 402, 627–769. [Google Scholar] [CrossRef] [PubMed]
- Lomo, T.; Westgaard, R.H.; Dahl, H.A. Contractile properties of muscle: Control by pattern of muscle activity in the rat. Proc. R. Soc. (Lond.) Ser. B Biol. Sci. 1974, 187, 99–103. [Google Scholar]
- Hennig, R.; Lomo, T. Effects of chronic stimulation on the size and speed of long-term denervated and innervated rat fast and slow skeletal muscles. Acta Physiol. Scand. 1987, 130, 115–131. [Google Scholar] [CrossRef] [PubMed]
- Hennig, R.; Lomo, T. Firing patterns of motor units in normal rats. Nature 1985, 314, 164–166. [Google Scholar] [CrossRef] [PubMed]
- Fischbach, G.; Robbins, N. Changes in contractile properties of disused soleus muscles. J. Physiol. 1989, 201, 305–320. [Google Scholar] [CrossRef] [PubMed]
- Mayer, R.F.; Burke, R.E.; Toop, J.; Hodgson, A.; Kanda, K.; Walmsley, B. The effect of long-term immobilization on the motor unit population of the cat medial gastrocnemius muscle. Neuroscience 1981, 6, 725–739. [Google Scholar] [CrossRef] [PubMed]
- Mayer, R.F.; Burke, R.E.; Toop, J.; Walmsley, B.; Hodgson, J.A. The effect of spinal cord transection on motor units in cat medial gastrocnemius muscles. Muscle Nerve 1984, 7, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Guth, L.; Wells, J.B. Physiological and histochemical properties of the soleus muscle after denervation of its antagonists. Exp. Neurol. 1972, 36, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Hennig, R. Section of fibular nerve affects activity pattern and contractile properties of soleus motor units in adult rats. Acta Physiol. Scand. 1987, 130, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Henneman, E.; Olson, C.B. Relations between structure and function in the design of skeletal muscles. J. Neurophysiol. 1965, 28, 581–598. [Google Scholar] [CrossRef] [PubMed]
- Henneman, E.; Somjen, G.; Carpenter, D.O. Functional significance of cell size in spinal motoneurons. J. Neurophysiol. 1965, 28, 560–580. [Google Scholar] [CrossRef] [PubMed]
- McPhedran, A.M.; Wuerker, R.B.; Henneman, E. Properties of motor units in a homogeneous red muscle (soleus) of the cat. J. Neurophysiol. 1965, 28, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Wuerker, R.B.; McPhedran, A.M.; Henneman, E. Properties of motor units in a heterogeneous pale muscle (m. gastrocnemius) of the cat. J. Neurophysiol. 1965, 28, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Henneman, E.; Mendell, L.M. Functional organization of the motoneurone pool and its inputs. In Handbook of Physiology. Sect. I. Vol. II. The Nervous System: Motor Control Part I; Brooks, V.B., Ed.; American Physiology Society: Washington, DC, USA, 1981. [Google Scholar]
- Bawa, P.; Binder, M.D.; Ruenzel, P.; Henneman, E. Recruitment order of motoneurons in stretch reflexes is highly correlated with their axonal conduction velocity. J. Neurophysiol. 1984, 52, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Henneman, E.; Clamann, H.P.; Gillies, J.D.; Skinner, R.D. Rank order of motoneurons within a pool: Law of combination. J. Neurophysiol. 1974, 37, 1338–1349. [Google Scholar] [CrossRef] [PubMed]
- Milner-Brown, H.S.; Stein, R.B.; Yemm, R. The orderly recruitment of human motor units during linearly changing during voluntary isometric contractions. J. Physiol. 1993, 230, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Dengler, R.; Stein, R.B.; Thomas, C.K. Axonal conduction velocity and force of single human motor units. Muscle Nerve 1988, 11, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T.; Tyreman, N.; Rafuse, V.F.; Munson, J.B. Limited plasticity of adult motor units conserves recruitment order and rate coding. Prog. Brain Res. 1999, 123, 191–202. [Google Scholar] [PubMed]
- Jami, L.; Petit, J. Correlation between axonal conduction velocity and tetanic tension of motor units in four muscles of the cat hind limb. Brain Res. 1975, 96, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T.; Stein, R.B. Time course and extent of recovery in reinnervated motor units of cat triceps surae muscles. J. Physiol. 1982, 323, 307–323. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T.; Stein, R.B. Reorganization of motor-unit properties in reinnervated muscles of the cat. J. Neurophysiol. 1982, 48, 1175–1190. [Google Scholar] [CrossRef] [PubMed]
- Zengel, J.E.; Reid, S.R.; Sypert, G.W.; Munson, J.B. Membrane electrical properties and prediction of motor-unit type of medial gastrocnemius motoneurons in the cat. J. Neurophysiol. 1985, 53, 1323–1344. [Google Scholar] [CrossRef] [PubMed]
- Fleshman, J.W.; Munson, J.B.; Sypert, G.W.; Friedman, W.A. Rheobase, input resistance, and motor unit type in medial gastrocnemius motoneurons in the cat. J. Neurophysiol. 1981, 46, 1326–1338. [Google Scholar] [CrossRef] [PubMed]
- Tőtősy de Zepetnek, J.F.; Zung, H.V.; Erdebil, S.; Gordon, T. Innervation ratio is an important determinant of force in normal and reinnervated rat tibialis anterior muscles. J. Neurophysiol. 1992, 67, 1385–1403. [Google Scholar] [CrossRef] [PubMed]
- Cooper, E.; Eccles, J.C. The isometric responses of mammalian muscles. J. Physiol. 1930, 69, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Kernell, D.; Eerbeek, O.; Verhey, B.A. Relation between isometric force and stimulus rate in cat’s hindlimb motor units of different twitch contraction time. Exp. Brain Res. 1983, 50, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Kernell, D. The limits of firing frequency in cat lumbosacral motoneurones possessing different time course of afterhyperpolarization. Acta Physiol. Scand. 1965, 65, 87–100. [Google Scholar] [CrossRef]
- Eccles, J.C.; Eccles, R.M.; Lundberg, A. The action potentials of the alpha motoneurones supplying fast and slow muscles. J. Physiol. 1958, 142, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, P.F.; Kernell, D. The ‘fastness’ of rat motoneurones: Time course of afterhyperpolarization in relation to axonal conduction velocity and muscle contractile speed. Pflug. Arch. 1990, 415, 762–766. [Google Scholar] [CrossRef] [PubMed]
- Hammarberg, C.; Kellerth, J.-O. Studies of some twitch and fatigue properties of different motor unit types in the ankle muscles of the adult cat. Acta Physiol. Scand. 1975, 95, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Bakels, R.; Kernell, D. Matching between motoneurone and muscle unit properties in rat medial gastrocnemius. J. Physiol. 1993, 463, 307–324. [Google Scholar] [CrossRef] [PubMed]
- Bakels, R.; Kernell, D. Average but not continuous speed match between motoneurons and muscle units of rat tibialis anterior. J. Neurophysiol. 1993, 70, 1300–1306. [Google Scholar] [CrossRef] [PubMed]
- Bellemare, F.; Woods, J.J.; Johansson, R.; Bigland-Ritchie, B. Motor-unit discharge rates in maximal voluntary contractions of three human muscles. J. Neurophysiol. 1983, 50, 1380–1392. [Google Scholar] [CrossRef] [PubMed]
- Dalton, B.H.; Harwood, B.; Davidson, A.W.; Rice, C.L. Triceps surae contractile properties and firing rates in the soleus of young and old men. J. Appl. Physiol. 2009, 107, 1781–1788. [Google Scholar] [CrossRef] [PubMed]
- Graham, M.T.; Rice, C.L.; Dalton, B.H. MU firing rates of the gastrocnemii during maximal brief steady-state contractions in humans. J. Electromyogr. Kinesiol. 2016, 26, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Kirk, E.A.; Copithorne, D.B.; Dalton, B.H.; Rice, C.L. MU firing rates of the gastrocnemii during maximal and sub-maximal isometric contractions in young and old men. Neuroscience 2016, 330, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Bigland-Richie, B.; Johansson, R.; Lippold, O.C.J.; Woods, J.J. Contraction speed and EMG changes during fatigue of sustained maximal voluntary contractions. J. Neurophysiol. 1983, 50, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Hali, K.; Kirk, E.A.; Rice, C.L. Effect of knee joint position on triceps surae motor unit recruitment and firing rates. Exp. Brain Res. 2019, 237, 2345–2352. [Google Scholar] [CrossRef] [PubMed]
- Hali, K.; Dalton, B.H.; Harwood, B.; Fessler, A.F.; Power, G.A.; Rice, C.L. Differential modulation of motor unit properties from the separate components of the triceps surae in humans. Neuroscience 2020, 428, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Kernell, D. Principles of force gradation in skeletal muscles. Neural Plast. 2003, 10, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Enoka, R.M.; Duchateau, J. Rate coding and the control of muscle force. Cold Spring Harb. Perspect. Med. 2017, 7, a029702. [Google Scholar] [CrossRef] [PubMed]
- Kernell, D.; Sjoholm, H. Recruitment and firing rate modulation of motor unit tension in a small muscle of the cat’s foot. Brain Res. 1975, 98, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Granit, R. Neuromuscular interaction in postural tone of the cat’s isometric soleus muscle. J. Physiol. 1958, 143, 387–402. [Google Scholar] [CrossRef] [PubMed]
- Grillner, S.; Udo, M. Recruitment in the tonic stretch reflex. Acta Physiol. Scand. 1971, 81, 571–573. [Google Scholar] [CrossRef] [PubMed]
- Heroux, M.E.; Dakin, C.J.; Luu, B.L.; Iglis, J.T.; Blouin, J.S. Absence of lateral gastrocnemius activity and differential MU behavior in soleus and medial gastrocnemius during standing balance. J. Appl. Physiol. 2014, 116, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Oya, T.; Riek, S.; Cresswell, A.G. Recruitment and rate coding organisation for soleus MUs across entire range of voluntary isometric plantar flexions. J. Physiol. 2009, 587, 4737–4748. [Google Scholar] [CrossRef] [PubMed]
- Rafuse, V.F.; Gordon, T.; Orozco, R. Proportional enlargement of motor units after partial denervation of cat triceps surae muscles. J. Neurophysiol. 1992, 68, 1261–1276. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, M.J.; Gordon, T.; Murphy, P.R. Reinnervation of the lateral gastrocnemius and soleus muscles in the rat by their common nerve. J. Physiol. 1986, 372, 485–500. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T.; Stein, R.B.; Thomas, C.K. Organization of motor units following cross-reinnervation of antagonistic muscles in the cat hindlimb. J. Physiol. 1986, 374, 442–456. [Google Scholar] [CrossRef] [PubMed]
- Rafuse, V.F.; Gordon, T. Incomplete rematching of nerve and muscle properties in motor units after extensive nerve injuries in cat hindlimb muscle. J. Physiol. 1998, 509, 909–926. [Google Scholar] [CrossRef] [PubMed]
- Milner-Brown, H.S.; Stein, R.B.; Lee, R.G. Pattern of recruiting human motor units in neuropathies and motorneuron disease. J. Neurol. Neurosurg. Psychiatry 1974, 37, 665–669. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thomas, C.K.; Stein, R.B.; Gordon, T.; Lee, R.G.; Elleker, M.G. Patterns of reinnervation and motor unit recruitment in human hand muscles after complete ulnar and median nerve section and resuture. J. Neurol. Neurosurg. Psychiatry 1987, 54, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T.; Stein, R.B.; Thomas, C.K. Innervation and function of hind-limb muscles in the cat after cross-union of the tibial and peroneal nerves. J. Physiol. 1986, 374, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Tam, S.L.; Gordon, T. Mechanisms controlling axonal sprouting at the neuromuscular junction. J. Neurocytol. 2003, 32, 961–974. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T.; Hegedus, J.; Tam, S.L. Adaptive and maladaptive motor axonal sprouting in aging and motoneuron disease. Invited review. Neurol. Res. 2004, 26, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Tam, S.L.; Gordon, T. Axonal sprouting in health and disease. In Encyclopedia of Neuroscience; Springer: Berlin/Heidelberg, Germany, 2011; Part 1; pp. 322–328. [Google Scholar]
- Kugelberg, E.; Edström, L.; Abbruzzese, M. Mapping of motor units in experimentally reinnervated rat muscle. Interpretation of histochemical and atrophic fibre patterns in neurogenic lesions. J. Neurol. Neurosurg. Psychiatry 1970, 33, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Karpati, G.; Engel, W.K. “Type-grouping” in skeletal muscles after experimental reinnervation. Neurology 1988, 18, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Grimby, G.; Broberg, C.; Krotkiewska, I.; Krotkiewski, M. Muscle fiber composition in patients with traumatic cord lesion. Scand. J. Rehabil. Med. 1976, 8, 37–42. [Google Scholar] [PubMed]
- Rafuse, V.F.; Gordon, T. Self-reinnervated cat medial gastrocnemius muscles. II. analysis of the mechanisms and significance of fiber type grouping in reinnervated muscles. J. Neurophysiol. 1996, 75, 282–297. [Google Scholar] [CrossRef] [PubMed]
- Stein, R.B.; Gordon, T.; Jefferson, J.; Sharfenberger, A.; Yang, J.; Tőtősy de Zepetnek, J.; Belanger, M. Optimal stimulation of paralysed muscle in spinal cord patients. J. Appl. Physiol. 1992, 72, 1393–1400. [Google Scholar] [CrossRef] [PubMed]
- Eldridge, L. Lumbosacral spinal isolation in cat: Surgical preparation and health maintenance. Exp. Neurol. 1984, 83, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Shields, R.K. Fatigability, relaxation properties, and electromyographic responses of the human paralyzed soleus muscle. J. Neurophysiol. 1995, 173, 2195–2206. [Google Scholar] [CrossRef] [PubMed]
- Shields, R.K.; Chang, Y.J.; Ross, M. Neuromuscular propagation after fatiguing contractions of the paralyzed soleus muscle in humans. Muscle Nerve 1998, 21, 776–787. [Google Scholar] [CrossRef]
- Kralj, A.R.; Bajd, T. Functional Electrical Stimulation: Standing and Walking; CRC Press: Boca Raton, FL, USA, 1989. [Google Scholar]
- Gerrits, H.L.; De Haan, A.; Hopman, M.T.; van Der Woude, L.J.; Jones, D.A.; Sargeant, A.J. Contractile properties of the quadriceps in individuals with spinal cord injury. Muscle Nerve 1999, 22, 1249–1256. [Google Scholar] [CrossRef]
- Alaimo, M.A.; Smith, J.L.; Roy, R.R.; Edgerton, V.R. EMG activity of slow and fast ankle extensors following spinal cord transection. J. Appl. Physiol. 1984, 56, 1608–1613. [Google Scholar] [CrossRef] [PubMed]
- Burnham, R.; Martin, T.; Stein, R.; Bell, G.; MacLean, I.; Steadward, R. Skeletal muscle fibre type transformation following spinal cord injury. Spinal Cord 1997, 35, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Round, J.M.; Barr, F.M.; Moffat, B.; Jones, D.A. Fibre areas and histochemical fibre types in the quadriceps muscle of paraplegic subjects. J. Neurol. Sci. 1993, 116, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Talmadge, R.J. Myosin heavy chain isoform expression following reduced neuromuscular activity: Potential regulatory mechanisms. Muscle Nerve 2000, 23, 661–679. [Google Scholar] [CrossRef]
- Castro, M.J.; Apple, D.F., Jr.; Staron, R.S.; Gerson, E.R.; Campos, G.E.; Dudley, G.A. Influence of complete spinal cord injury on skeletal muscle within 6 mo of injury. J. Appl. Physiol. 1999, 86, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Scelsi, R.; Marchetti, C.; Poggi, P.; Lotta, S.; Lommi, G. Muscle fiber type morphology and distribution in paraplegic patient with traumatic cord lesion. Acta Neuropathol. 1982, 57, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.J.; Apple, D.F., Jr.; Hillegass, E.A.; Dudley, G.A. Influence of complete spinal cord injury on skeletal muscle cross-sectional area within the first 6 months of injury. Eur. J. Appl. Physiol. 1999, 80, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.K.; Zaidner, E.Y.; Calancie, B.; Broton, J.G.; Bigland-Richie, B.R. Muscle weakness, paralysis, and atrophy after human cervical spinal cord injury. Exp. Neurol. 1997, 148, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Burke, R.E.; Rymer, W.Z. Relative strength of synaptic input from short-latency pathways to motor units of defined type in cat medial gastrocnemius. J. Neurophysiol. 1976, 39, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Sciaffiino, S.; Reggiani, C. Fiber types in mammalian skeletal muscles. Physiol. Rev. 2011, 9, 1447–1531. [Google Scholar] [CrossRef] [PubMed]
- Esmarck, B.; Andersen, J.L.; Olsen, S.; Richter, E.A.; Mizuno, M.; Kjaer, M. Timing of postexercise protein intake is important for muscle hypertrophy with resistance training in elderly humans. J. Physiol. 2001, 535, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Flewwelling, L.D.; Hannaian, S.J.; Cao, V.; Chaillou, T.; Churchward-Venne, T.A.; Cheng, A.J. What are the potential mechanisms of fatigue-induced skeletal muscle hypertrophy with low-load resistance exercise training? Am. J. Physiol. Cell Physiol. 2025, 328, C1001–C1014. [Google Scholar] [CrossRef] [PubMed]
- Quisar, R.; Bhaskaran, S.; Van Remmen, H. Muscle fiber type diversification during exercise and regeneration. Free Radic. Biol. Med. 2016, 98, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Rampinini, E.; Coutts, A.J.; Castagna, C.; Sassi, R.; Impellizzeri, F.M. Variation in top level soccer match performance. Int. J. Sports Med. 2007, 28, 1018–1024. [Google Scholar] [CrossRef] [PubMed]
- Metaxas, T.; Mandroukas, A.; Michailidis, Y.; Koutlianos, N.; Christoulas, K.; Ekblom, B. Correlation of fiber-type composition and sprint performance in youth soccer players. J. Strength Cond. Res. 2019, 33, 2629–2634. [Google Scholar] [CrossRef] [PubMed]
- Hopwood, H.J.; Bellinger, P.M.; Compton, H.R.; Bourne, M.N.; Minahan, C. The Relevance of Muscle Fiber Type to Physical Characteristics and Performance in Team-Sport Athletes. Int. J. Sports Physiol. Perform. 2023, 18, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Y.; Okutsu, M.; Vitor, Y.N.; Lira, V.A. Regulation of exercise-induced fiber type transformation, mitochondrial biogenesis, and angiogenesis in skeletal muscle. J. Appl. Physiol. 1985, 110, 264–274. [Google Scholar]
- Hood, D.A. Mechanisms of exercise-induced mitochondrial biogenesis in skeletal muscle. Appl. Physiol. Nutr. Metab. 2009, 34, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Ljubicic, V.; Joseph, A.-M.; Saleem, A.; Uguccioni, G.; Collu-Marchese, M.; Lai, R.Y.J.; Nguyen, L.M.-D.; Hood, D.A. Transcriptional and post-transcriptional regulation of mitochondrial biogenesis in skeletal muscle: Effects of exercise and aging. Biochem. Biophys. Acta 2010, 1800, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, N. Effect of endurance training on muscle fiber type composition and capillary supply in rat diaphragm. Eur. J. Appl. Physiol. Occup. Physiol. 1987, 56, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.I.; Fournier, H.; Wang, H.; Storor, R.; Casaburi, R.; Kopple, J.D. Effect of endurance and/or strength training on muscle fiber size, oxidative capacity, and capillarity in hemodialysis patients. J. Appl. Physiol. 2015, 119, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Hikida, R.S.; Staron, R.S.; Hagerman, F.C.; Walsh, S.; Kaiser, E.; Shell, S.; Hervey, S. Effects of high-intensity resistance training on untrained older men. II. Muscle fiber characteristics and nucleo-cytoplasmic relationships. J. Gerontol. 2000, 55, B347–B354. [Google Scholar]
- Revero, J.L.; Talmadge, R.J.; Edgerton, V.R. Fibre size and metabolic properties of myosin heavy chain-based fibre types in rat skeletal muscle. J. Muscle Res. Cell Motil. 1998, 19, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Medler, S. Comparative trends in shortening velocity and force production in skeletal muscles. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 283, R368–R378. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.C.; Rosenthal, N. Different modes of hypertrophy in skeletal muscle fibers. J. Cell Biol. 2002, 156, 751–760. [Google Scholar] [CrossRef] [PubMed]
- West, D.W.; Burd, N.A.; Staples, A.W.; Phillips, S.M. Human exercise-mediated skeletal muscle hypertrophy is an intrinsic process. Int. J. Biochem. Cell Biol. 2010, 42, 1371–1375. [Google Scholar] [CrossRef] [PubMed]
- Fank, F.; Perieira, F.d.S.; Santos, L.D.; Tŭlio de Mello, M.; Mazo, G.Z. Effects of Exercise on Sleep in Older Adults: An Overview of Systematic Reviews and Meta-Analyses. J. Aging Phys. Act. 2022, 30, 1101–1117. [Google Scholar] [CrossRef] [PubMed]
- Lieber, R.L. Skeletal Muscle Structure, Function, and Plasticity: The Physiological Basis of Rehabilitation; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2009. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gordon, T. Neuromuscular Activity Determines, at Least in Part, the Motoneuron, Nerve and Muscle Properties Under Normal Conditions and After Nerve Injury. Int. J. Mol. Sci. 2025, 26, 6891. https://doi.org/10.3390/ijms26146891
Gordon T. Neuromuscular Activity Determines, at Least in Part, the Motoneuron, Nerve and Muscle Properties Under Normal Conditions and After Nerve Injury. International Journal of Molecular Sciences. 2025; 26(14):6891. https://doi.org/10.3390/ijms26146891
Chicago/Turabian StyleGordon, Tessa. 2025. "Neuromuscular Activity Determines, at Least in Part, the Motoneuron, Nerve and Muscle Properties Under Normal Conditions and After Nerve Injury" International Journal of Molecular Sciences 26, no. 14: 6891. https://doi.org/10.3390/ijms26146891
APA StyleGordon, T. (2025). Neuromuscular Activity Determines, at Least in Part, the Motoneuron, Nerve and Muscle Properties Under Normal Conditions and After Nerve Injury. International Journal of Molecular Sciences, 26(14), 6891. https://doi.org/10.3390/ijms26146891




