L-Lysine from Bacillus subtilis M320 Induces Salicylic-Acid–Dependent Systemic Resistance and Controls Cucumber Powdery Mildew
Abstract
1. Introduction
2. Results
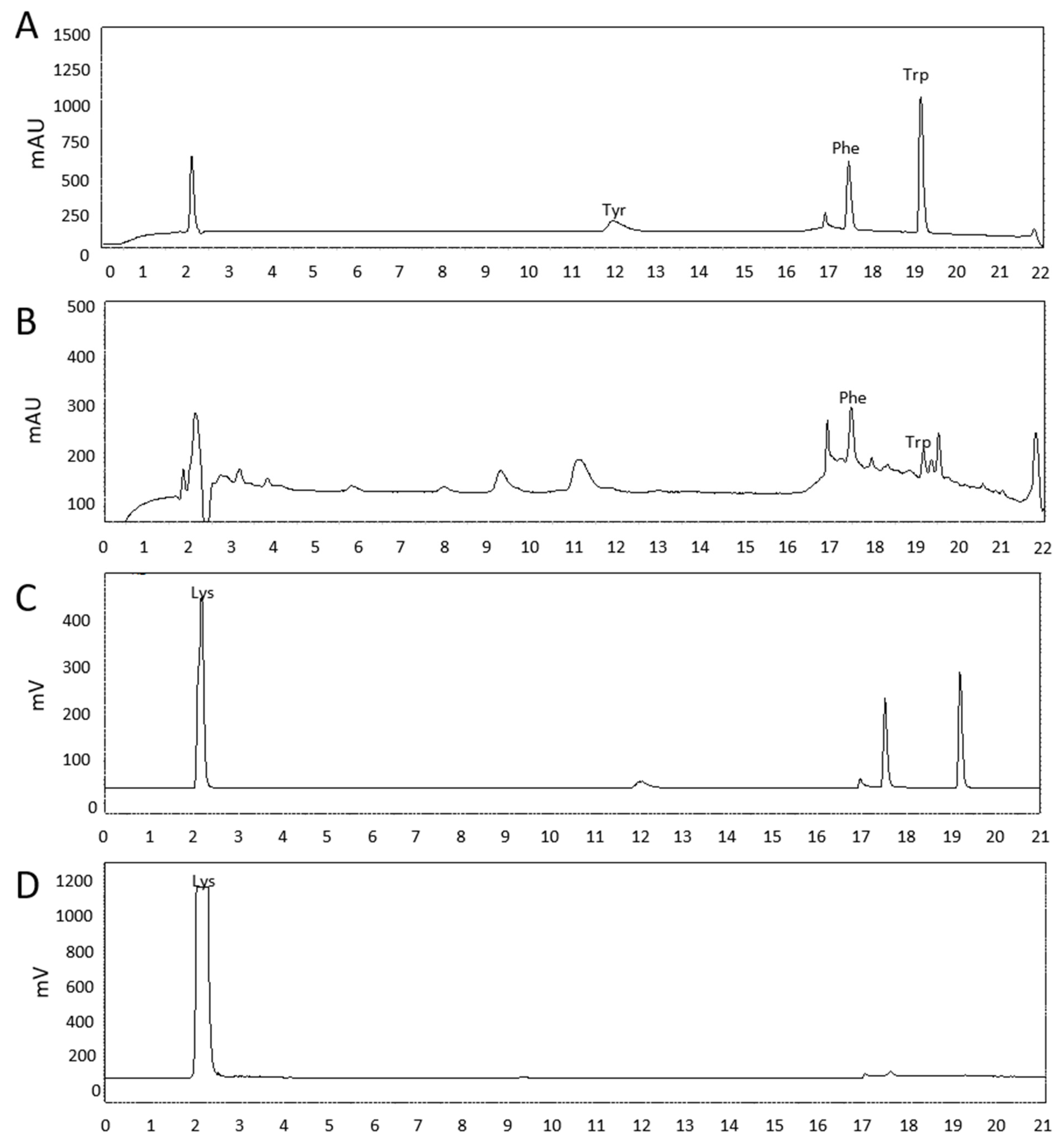
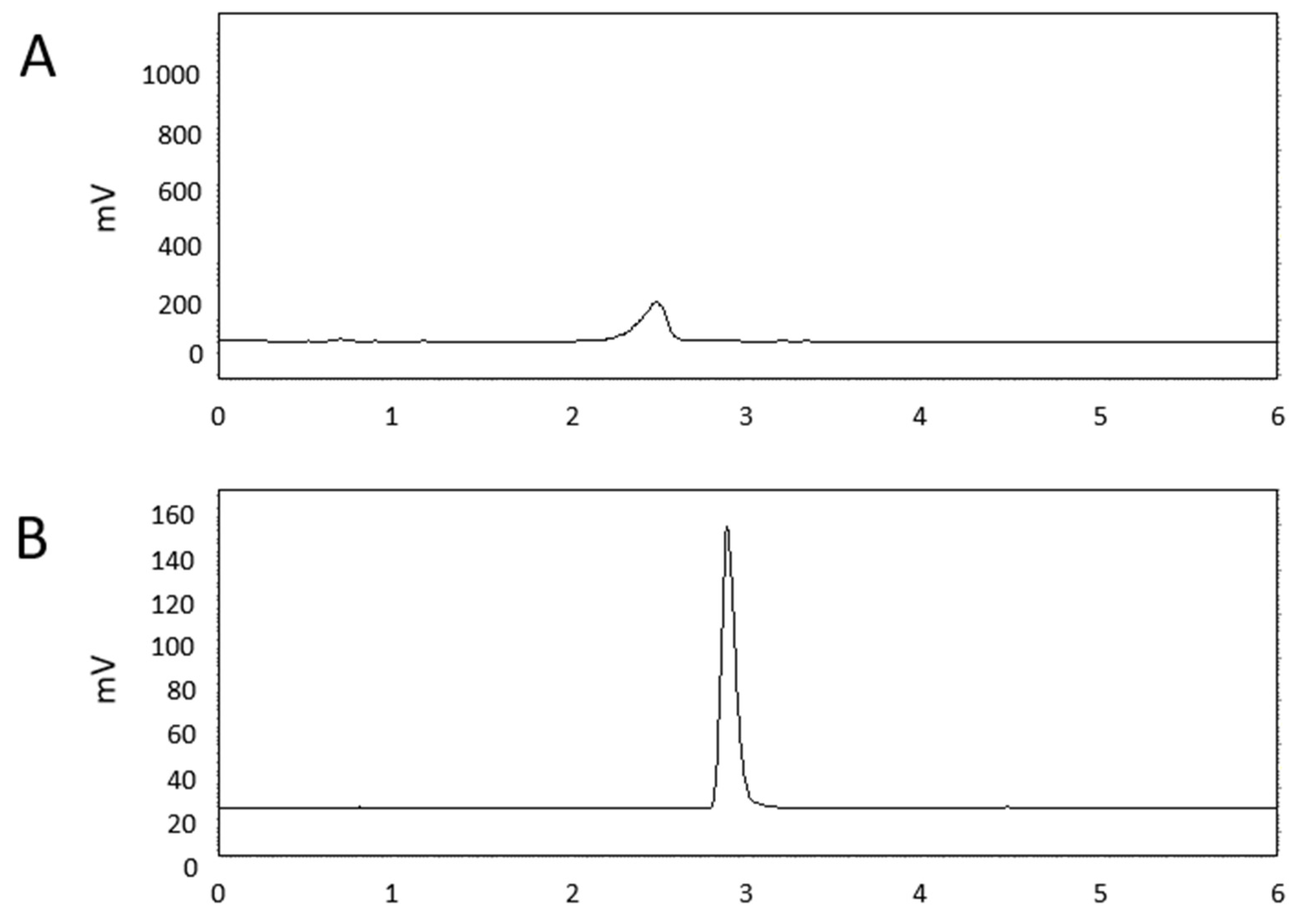
2.1. Identification of Antifungal Compounds from Culture Filtrate of BSM320
2.2. Effect of Lysine Against Cucumber Powdery Mildew
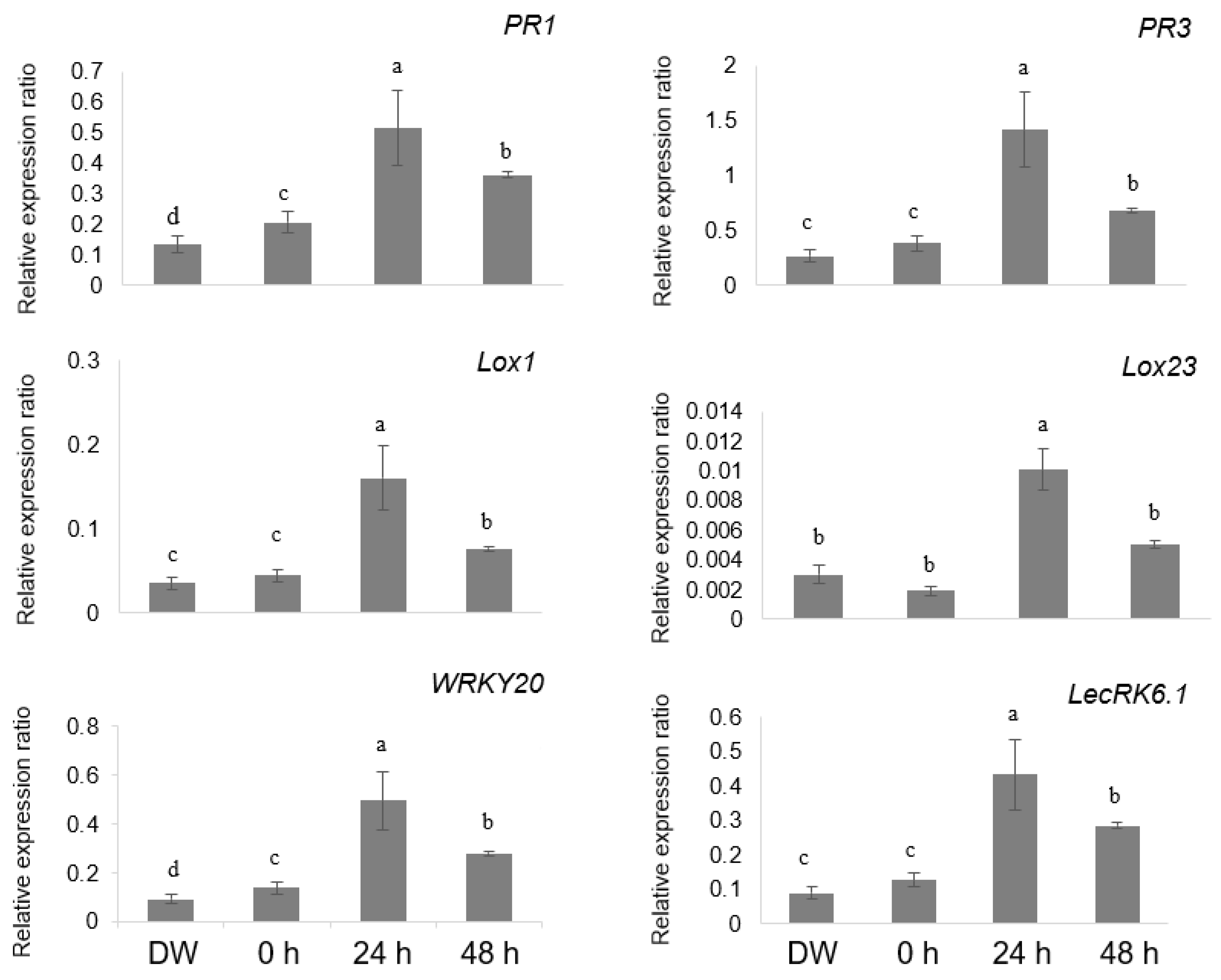
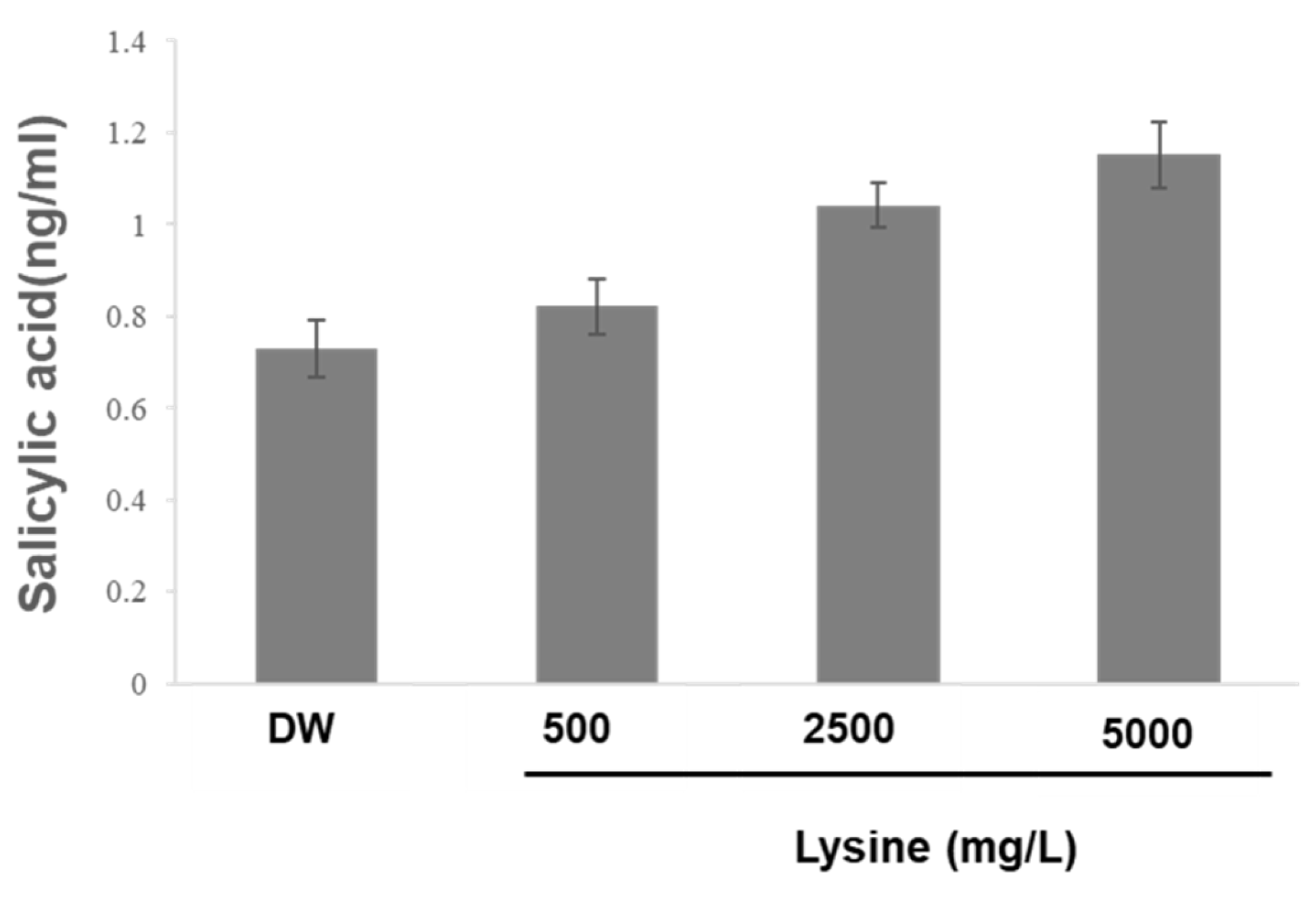
2.3. Lysine-Induced Activation of Plant Defense Responses
3. Discussion
4. Materials and Methods
4.1. Purification and Identification of Antimicrobial Compounds from BSM320 Culture Filtrate
4.2. Plant Materials and Powdery Mildew Inoculation
4.3. Spore Germination Rate of Cucumber Powdery Mildew Fungus
4.4. Quantitative Reverse Transcription PCR (qRT-PCR)
4.5. Extraction and Measurement of SA from Cucumber Leaves
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| BSM320 | Bacillus subtilis mutant 320 |
| C/N | Carbon/nitrogen |
| DW | Distilled water |
| EI–MS | Electrospray ionization mass spectrometry |
| HPLC | High-performance liquid chromatography |
| ISR | Induced systemic resistance |
| LB | Luria–Bertani |
| LC–MS | Liquid chromatography–mass spectrometry |
| LecRK6.1 | L-type lectin receptor kinase 6.1 |
| LOX1 | Lipoxygenase 1 |
| LOX23 | Lipoxygenase 23 |
| NMR | Nuclear magnetic resonance |
| PR1 | Pathogenesis-related protein 1 |
| PR3 | Pathogenesis-related protein 3 |
| qRT-PCR | Quantitative reverse transcription PCR |
| SA | Salicylic acid |
| SAR | Systemic acquired resistance |
| SD | Standard deviation |
| WRKY20 | WRKY transcription factor 20 |
References
- Xu, X.; Du, Y.; Li, S.; Tan, M.; Sohail, H.; Liu, X.; Qi, X.; Yang, X.; Chen, X. A genome-wide association study reveals molecular mechanism underlying powdery mildew resistance in cucumber. Genome Biol. 2024, 25, 252. [Google Scholar] [CrossRef] [PubMed]
- Lebeda, A.; Mieslerová, B. Taxonomy, distribution and biology of lettuce powdery mildew (Golovinomyces cichoracearum sensu stricto). Plant Pathol. 2011, 60, 400–415. [Google Scholar] [CrossRef]
- Keinath, A.P.; DuBose, V.B. Controlling powdery mildew on cucurbit rootstock seedlings in the greenhouse with fungicides and biofungicides. Crop Prot. 2012, 42, 338–344. [Google Scholar] [CrossRef]
- Hafez, Y.M.; Attia, K.A.; Kamel, S.; Alamery, S.F.; El-Gendy, S.; Al-Doss, A.A.; Mehiar, F.; Ghazy, A.I.; Ibrahim, E.I.; Abdelaal, K.A. Bacillus subtilis as a bio-agent combined with nano molecules can control powdery mildew disease through histochemical and physiobiochemical changes in cucumber plants. Physiol. Mol. Plant Pathol. 2020, 111, 101489. [Google Scholar] [CrossRef]
- Hashem, A.; Tabassum, B.; Abd_Allah, E.F. Bacillus subtilis: A plant-growth promoting rhizobacterium that also impacts biotic stress. Saudi J. Biol. Sci. 2019, 26, 1291–1297. [Google Scholar] [CrossRef]
- García-Gutiérrez, L.; Romero, D.; Zeriouh, H.; Cazorla, F.M.; Torés, J.A.; de Vicente, A.; Pérez-García, A. Isolation and selection of plant growth-promoting rhizobacteria as inducers of systemic resistance in melon. Plant Soil 2012, 358, 201–212. [Google Scholar] [CrossRef]
- Samaras, A.; Roumeliotis, E.; Ntasiou, P.; Karaoglanidis, G. Bacillus subtilis MBI600 promotes growth of tomato plants and induces systemic resistance contributing to the control of soilborne pathogens. Plants 2021, 10, 1113. [Google Scholar] [CrossRef]
- Amir, R.; Galili, G.; Cohen, H. The metabolic roles of free amino acids during seed development. Plant Sci. 2018, 275, 11–18. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Bi, Y.; Jiang, Q.; Mao, R.; Liu, Z.; Huang, Y.; Zhang, M.; Prusky, D.B. Induction of defense response against Alternaria rot in Zaosu pear fruit by exogenous L-lysine through regulating ROS metabolism and activating defense-related proteins. Postharvest Biol. Technol. 2021, 179, 111567. [Google Scholar] [CrossRef]
- Yang, Q.; Zhao, D.; Liu, Q. Connections between amino acid metabolisms in plants: Lysine as an example. Front. Plant Sci. 2020, 11, 928. [Google Scholar] [CrossRef]
- Návarová, H.; Bernsdorff, F.; Döring, A.-C.; Zeier, J. Pipecolic acid, an endogenous mediator of defense amplification and priming, is a critical regulator of inducible plant immunity. Plant Cell 2012, 24, 5123–5141. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.A.; Abo-Elyousr, K.A. Activation of tomato plant defence responses against bacterial wilt caused by Ralstonia solanacearum using DL-3-aminobutyric acid (BABA). Eur. J. Plant Pathol. 2013, 136, 145–157. [Google Scholar] [CrossRef]
- Wang, D.; Weaver, N.D.; Kesarwani, M.; Dong, X. Induction of protein secretory pathway is required for systemic acquired resistance. Science 2005, 308, 1036–1040. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.Q.; Dong, X. Systemic acquired resistance: Turning local infection into global defense. Annu. Rev. Plant Biol. 2013, 64, 839–863. [Google Scholar] [CrossRef]
- Pangesti, N.; Reichelt, M.; van de Mortel, J.E.; Kapsomenou, E.; Gershenzon, J.; van Loon, J.J.; Dicke, M.; Pineda, A. Jasmonic acid and ethylene signaling pathways regulate glucosinolate levels in plants during rhizobacteria-induced systemic resistance against a leaf-chewing herbivore. J. Chem. Ecol. 2016, 42, 1212–1225. [Google Scholar] [CrossRef]
- Yu, Y.; Gui, Y.; Li, Z.; Jiang, C.; Guo, J.; Niu, D. Induced systemic resistance for improving plant immunity by beneficial microbes. Plants 2022, 11, 386. [Google Scholar] [CrossRef]
- Manners, J.M.; Penninckx, I.A.; Vermaere, K.; Kazan, K.; Brown, R.L.; Morgan, A.; Maclean, D.J.; Curtis, M.D.; Cammue, B.P.; Broekaert, W.F. The promoter of the plant defensin gene PDF1.2 from Arabidopsis is systemically activated by fungal pathogens and responds to methyl jasmonate but not to salicylic acid. Plant Mol. Biol. 1998, 38, 1071–1080. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Kang, H.-W. β-Aminobutyric acid and powdery mildew infection enhanced the activation of defense-related genes and salicylic acid in cucumber (Cucumis sativus L.). Genes 2023, 14, 2087. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Park, S.-H.; Park, S.-J.; Bo, J.-H.H.; Kang, H.-W. High density culture of Bacillus subtilis BSM320 in aqueous extract of composted spent mushroom substrate of Lentinula edodes and biological control of green mold disease. J. Mushroom 2023, 21, 140–144. [Google Scholar]
- Galili, G.; Tang, G.; Zhu, X.; Gakiere, B. Lysine catabolism: A stress and development super-regulated metabolic pathway. Curr. Opin. Plant Biol. 2001, 4, 261–266. [Google Scholar] [CrossRef]
- Eskandari, S.; Khoshgoftarmanesh, A.; Sharifnabi, B. The effect of foliar-applied manganese in mineral and complex forms with amino acids on certain defense mechanisms of cucumber (Cucumis sativus L.) against powdery mildew. J. Plant Growth Regul. 2018, 37, 481–490. [Google Scholar] [CrossRef]
- Hashemi, L.; Golparvar, A.R.; Nasr-Esfahani, M.; Golabadi, M. Expression analysis of defense-related genes in cucumber (Cucumis sativus L.) against Phytophthora melonis. Mol. Biol. Rep. 2020, 47, 4933–4944. [Google Scholar] [CrossRef] [PubMed]
- Pu, X.; Xie, B.; Li, P.; Mao, Z.; Ling, J.; Shen, H.; Zhang, J.; Huang, N.; Lin, B. Analysis of the defence-related mechanism in cucumber seedlings in relation to root colonization by nonpathogenic Fusarium oxysporum CS-20. FEMS Microbiol. Lett. 2014, 355, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Van Andel, O. Amino acids and plant diseases. Annu. Rev. Phytopathol. 1966, 4, 349–368. [Google Scholar] [CrossRef]
- Posas, M.B.; Toyota, K. Mechanism of tomato bacterial wilt suppression in soil amended with lysine. Microbes Environ. 2010, 25, 83–94. [Google Scholar] [CrossRef]
- Yariyama, S.; Ando, S.; Seo, S.; Nakaho, K.; Miyashita, S.; Kanayama, Y.; Takahashi, H. Exogenous application of l-histidine suppresses bacterial diseases and enhances ethylene production in rice seedlings. Plant Pathol. 2019, 68, 1072–1078. [Google Scholar] [CrossRef]
- Kadotani, N.; Akagi, A.; Takatsuji, H.; Miwa, T.; Igarashi, D. Exogenous proteinogenic amino acids induce systemic resistance in rice. BMC Plant Biol. 2016, 16, 1–10. [Google Scholar] [CrossRef]
- Roblin, G.; Octave, S.; Faucher, M.; Fleurat-Lessard, P.; Berjeaud, J.-M. Cysteine: A multifaceted amino acid involved in signaling, plant resistance and antifungal development. Plant Physiol. Biochem. 2018, 129, 77–89. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Holmes, E.C.; Rajniak, J.; Kim, J.-G.; Tang, S.; Fischer, C.R.; Mudgett, M.B.; Sattely, E.S. N-hydroxy-pipecolic acid is a mobile metabolite that induces systemic disease resistance in Arabidopsis. Proc. Natl. Acad. Sci. USA 2018, 115, E4920–E4929. [Google Scholar] [CrossRef]
- Okrent, R.A.; Brooks, M.D.; Wildermuth, M.C. Arabidopsis GH3.12 (PBS3) conjugates amino acids to 4-substituted benzoates and is inhibited by salicylate. J. Biol. Chem. 2009, 284, 9742–9754. [Google Scholar] [CrossRef]
- Kishor, P.K.; Suravajhala, R.; Rajasheker, G.; Marka, N.; Shridhar, K.K.; Dhulala, D.; Scinthia, K.P.; Divya, K.; Doma, M.; Edupuganti, S. Lysine, lysine-rich, serine, and serine-rich proteins: Link between metabolism, development, and abiotic stress tolerance and the role of ncRNAs in their regulation. Front. Plant Sci. 2020, 11, 546213. [Google Scholar] [CrossRef]
- Boubakri, H.; Wahab, M.A.; Chong, J.; Gertz, C.; Gandoura, S.; Mliki, A.; Bertsch, C.; Soustre-Gacougnolle, I. Methionine elicits H2O2 generation and defense gene expression in grapevine and reduces Plasmopara viticola infection. J. Plant Physiol. 2013, 170, 1561–1568. [Google Scholar] [CrossRef]
- Holmes, E.C.; Chen, Y.-C.; Sattely, E.S.; Mudgett, M.B. An engineered pathway for N-hydroxy-pipecolic acid synthesis enhances systemic acquired resistance in tomato. Sci. Signal. 2019, 12, eaay3066. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-Y.; Choi, D.-C.; Yun, B.-S.; Kang, H.-W. L-Lysine from Bacillus subtilis M320 Induces Salicylic-Acid–Dependent Systemic Resistance and Controls Cucumber Powdery Mildew. Int. J. Mol. Sci. 2025, 26, 6882. https://doi.org/10.3390/ijms26146882
Kim J-Y, Choi D-C, Yun B-S, Kang H-W. L-Lysine from Bacillus subtilis M320 Induces Salicylic-Acid–Dependent Systemic Resistance and Controls Cucumber Powdery Mildew. International Journal of Molecular Sciences. 2025; 26(14):6882. https://doi.org/10.3390/ijms26146882
Chicago/Turabian StyleKim, Ja-Yoon, Dae-Cheol Choi, Bong-Sik Yun, and Hee-Wan Kang. 2025. "L-Lysine from Bacillus subtilis M320 Induces Salicylic-Acid–Dependent Systemic Resistance and Controls Cucumber Powdery Mildew" International Journal of Molecular Sciences 26, no. 14: 6882. https://doi.org/10.3390/ijms26146882
APA StyleKim, J.-Y., Choi, D.-C., Yun, B.-S., & Kang, H.-W. (2025). L-Lysine from Bacillus subtilis M320 Induces Salicylic-Acid–Dependent Systemic Resistance and Controls Cucumber Powdery Mildew. International Journal of Molecular Sciences, 26(14), 6882. https://doi.org/10.3390/ijms26146882





