Abstract
Brachial plexus root avulsion [BPRA] and concomitant spinal cord injury [SCI] represent devastating injuries that come with limited hope for recovery owing to the adult spinal cord’s loss of intrinsic ability to spontaneously regenerate. BPRA/SCI is an enormous public health issue the world over, and its catastrophic impact goes beyond the patient, the family, businesses, and national health budgets, draining billions of dollars annually. The rising population and economic growth have seen the incidence of SCI surging. Genomic, transcriptomic, and proteomic studies have yielded loads of information on the various molecular events that precede, regulate, and support both regenerative and degenerative pathways post-SCI. Metabolomics, on the other hand, comes in as the search for a cure and the objective monitoring of SCI severity and prognosis remains on the horizon. Despite the large number of review articles on metabolomics and its application fields such as in cancer and diabetes research, there is no comprehensive review on metabolite profiling to study disease mechanisms, biomarkers, or neuroprotection in SCI. First, we present a short review on BPRA/SCI. Second, we discuss potential benefits of metabolomics as applied in BPRA/SCI cases. Next, a look at the analytical techniques that are used in metabolomics. Next, we present an overview of the studies that have used metabolomics to reveal SCI metabolic fingerprints and point out areas of further investigation. Finally, we discuss future research directions.
1. Introduction
1.1. What Is Brachial Plexus Root Avulsion and Its Link with Spinal Cord Injury?
Brachial plexus root avulsion (BPRA) is a serious nerve injury accompanied by a concomitant reactive injury in the central nervous system, especially in the motor and sensory neurons of the spinal cord [1]. Avulsion of the brachial plexus roots leads to their detachment from the spinal cord, initiating a cascade of central pathological responses characterized by astrocytic and microglial activation, along with the degeneration and death of motor neurons whose axons have been severed [2,3]. It leads to the loss of sensation and motor function in the ipsilateral upper limb and often leaves the patients with chronic pain [4]. Numerous therapeutic strategies have been designed to address brachial plexus avulsion and concomitant spinal cord injuries in both humans and experimental animals [5]. These include surgery [6,7,8], cell transplantation [9], neurotrophin delivery [10], the removal of growth inhibition [11], immune modulation, and even the use of scaffolds for axonal guidance [12,13]. Despite all these attempts, there is still no cure or clinically effective therapy currently available for BPRA or spinal cord injuries [14,15]. There is now a consensus that, due to the complexity of the injured tissue environment, a multi-faceted treatment regime is necessary [16], and that the integration of multiple technologies is likely key to the effective treatment of spinal cord injuries. Accordingly, further extensive preclinical studies are indicated. Among the technologies poised to transform our understanding of the complex pathological environment of BPRA and SCI is the integration of metabolomics into both preclinical and clinical studies. However, there is a dearth of studies on the application of metabolomics in BPRA [17,18].
1.2. Enter Metabolomics and Metabonomics
Metabolomics is an emerging field, similar to proteomics, transcriptomics, and genomics, that is specifically meant for high-throughput global profiling of the collection of all low-molecular-weight (<1 kDa) metabolites in a biological system (i.e., the metabolome) [19,20]. These include amino acids, fatty acids, sugars, lipids, and steroids [21,22]. It provides a snapshot of the metabolic dynamics that reflect the response of living systems to both pathophysiological stimuli and/or genetic modification [23,24]. On the other hand, metabonomics, a subcategory of metabolomics, focuses on the temporal interactions between metabolites over time [24]. Each type of cell and tissue has a characteristic metabolic composition that is uniquely altered in response to physiological and pathophysiological stimuli, making knowledge of such dynamic compositions of great clinical and scientific interest [20]. These characteristic metabolic compositions are a result of the combined influence of epigenetic factors, heterogeneous distributions of molecules, and differential reaction rates [24]. Metabolomics, therefore, involves the examination of biofluids, including blood plasma/serum, urine, feces, blister fluid, saliva, and semen, as well as tissue extracts and intact tissue biopsies to study many diseases [25,26,27,28]. For example, metabolomics has seen many applications in diseases such as cancer [29,30], diabetes [31,32], tuberculosis [33], degenerative central nervous system disease [34], and even drug discovery [35]. Metabolomics is also currently being used to discover new biomarkers of various diseases and to identify biochemical pathways involved in their pathogenesis [36].
1.3. What Will Metabolomics Do to BPRA/SCI?
A range of metabolic derangements has been observed following brachial plexus root avulsion (BPRA) and spinal cord injury (SCI), including oxidative and nitrosative stress, disrupted glycolysis, and altered amino acid and lipid metabolism. Notably, even when axonal injury is peripheral, as in BPRA, significant metabolic responses occur both locally and within the spinal cord, underscoring a shared pathophysiological continuum between BPRA and SCI [17,37]. The primary injury phase in BPRA involves axonal severance, neuronal death, and vascular disruption, while secondary processes in the spinal cord include edema, ion imbalance, and apoptotic and necrotic cell death [14,38]. These changes result in the accumulation of reactive oxygen species, neurotransmitters, and cytotoxic debris, which further fuel inflammation and neuronal loss [3]. Despite this degenerative cascade, limited spontaneous improvement sometimes occurs, driven by endogenous repair mechanisms such as spinal tissue remodeling [39].
Understanding these metabolic disturbances is critical for uncovering injury mechanisms, monitoring progression, and guiding treatment strategies. While transcriptomic and proteomic studies have revealed the involvement of molecules like c-Jun and nNOS in spinal motor neurons following BPRA [40,41], metabolomics remains underexplored in this context [18]. This gap is significant, as both BPRA and SCI induce metabolic signatures with strong implications for neuroprotection and regeneration. Given the limitations of current clinical scales such as the ASIA Impairment Scale, which can be imprecise and often infeasible to use in acute trauma settings, there is an urgent need for objective biomarkers to stratify injury severity and predict outcomes [15,42,43,44].
Metabolomics offers a promising solution. By capturing a real-time chemical fingerprint of the body’s physiological and pathological states, it provides higher diagnostic sensitivity than protein-based analyses [45]. It enables the identification of disease-specific metabolic signatures essential for precision medicine [46], helping to elucidate injury mechanisms, discover neuroprotective biomarkers, and uncover therapeutic targets. Metabolite profiles reflect genome–environment interactions [47], making metabolomics especially powerful in the study of complex injuries like SCI. As such, metabolomics stands to redefine how we classify, monitor, and treat BPRA and SCI, marking a critical shift toward systems-level, personalized approaches in neurotrauma research.
2. The Literature Search Strategy
A comprehensive search of the literature was conducted to identify relevant peer-reviewed studies focusing on the application of metabolomics in spinal cord injury (SCI) and brachial plexus root avulsion (BPRA). The electronic databases PubMed, Scopus, and Google scholar, and specific journal publishers such as Wiley, MDPI, and Web of Science were searched for articles published between January 2017 and March 2025. The search strategy combined keywords and MeSH terms including: “metabolomics,” “metabonomics,” “spinal cord injury,” “brachial plexus injury or roots avulsion,” “neuroprotection,” “metabolic profiles,” and “biomarker discovery.” Boolean operators [“AND,” “OR”] and filters, e.g., animal studies, human clinical trials, were applied to refine results.
2.1. Inclusion and Exclusion Criteria
Studies were included if they:
Utilized metabolomic techniques (e.g., LC-MS, GC-MS, and NMR) to analyze tissue, blood, urine, feces or cerebrospinal fluid (CSF) samples.
Investigated either animal models or human subjects with SCI or BPRA.
Reported outcomes related to metabolic changes, biomarker identification, or therapeutic targets.
2.2. Exclusion Criteria Were
Non-metabolomics-based studies.
Conference abstracts, editorials, and commentaries without primary data.
Non-English publications.
2.3. Study Selection Process
Two independent reviewers screened the titles and abstracts of the retrieved articles for relevance. The full texts of potentially eligible studies were then assessed for inclusion. Disagreements were resolved through discussion or third-party adjudication. Figure 1 shows the PRISMA study flow diagram [48].
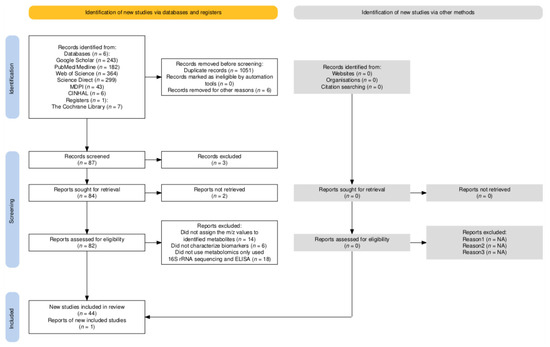
Figure 1.
PRISMA study flow diagram. NA—not applicable.
2.4. Data Extraction and Synthesis
Data were systematically extracted using a pre-defined template, including the following:
Author(s), year, and country of study; animal or human model used; tissue or biofluid analyzed; analytical technique employed (e.g., LC-MS, NMR); key identified metabolites; and clinical or experimental outcome relevance (e.g., injury severity markers, neuroprotection indicators). Quantitative synthesis was limited due to methodological heterogeneity; therefore, results were summarized descriptively. Table 1 and Table 2 were used to organize studies by species, sample source, technique, and biomarker application.

Table 1.
Overview of recent metabolomics studies in animal SCI models. The table summarizes key metabolites identified, associated biofluids/tissues, metabolomic techniques, and insights into biomarker potential related to spinal cord injury outcomes in rats and mice.

Table 2.
Overview of recent metabolomics studies in human SCI models. The table summarizes key metabolites identified, associated biofluids/tissues, metabolomic techniques, and insights into biomarker potential related to spinal cord injury outcomes.
3. Analytical Techniques Used in Metabolomics
Metabolites comprise a broad array of low-molecular-weight structures, such as lipids, amino acids, and carbohydrates, making their comprehensive analysis challenging. With the advancement in analytical technologies like Gas Chromatography, Ultra Performance Liquid Chromatography (UPLC), and Nuclear Magnetic Resonance (NMR), it is now feasible to identify, quantify, and study these metabolites and their metabolic pathways. These metabolomics technologies have been described in detail recently [90,91]. However, due to the vast diversity and complexity of the metabolome, no single technology can detect all metabolites in a given biological sample. This section delves into the latest methods used in metabolomics for metabolic profiling.
The most common analytical platforms used in metabolomics involve nuclear magnetic resonance (NMR) spectroscopy, mostly 1H-NMR, and mass spectrometry [MS]. Both permit the analysis of a multitude of small molecules coexisting in a sample including metabolite identification and quantitation, but each of them has its own strengths and limitations. Huang et al. [92] recently provided a succinct review of NMR-based metabolomics practices in human disease research. Briefly, NMR spectroscopy offers benefits such as minimal sample preparation and the ability to analyze without separating analytes. Its non-destructive approach ensures that specimens remain intact for other analyses, making it ideal for studying intact cells or tissue samples such as nervous tissue. Furthermore, NMR signal intensity is less impacted by sample matrix components compared to the MS technique, making it well-suited for complex biological samples like cerebrospinal fluid. However, NMR’s primary drawback is its low sensitivity. Numerous factors such as diet, stress, and age, can influence metabolic composition, posing challenges in biomarker discovery [45]. These elements are easier to control in lab animals compared to humans. For uniform outcomes and replicability, it is vital to optimize every experimental step, from gathering samples to analyzing data, in accordance with local standard guidelines [93].
Application of mass spectrometry in metabolomic investigation usually requires the separation of metabolites from analyzed biofluids. Thus, mass spectrometry is frequently coupled with high-resolution techniques, such as liquid chromatography [LC], gas chromatography (GC), or capillary electrophoresis (CE) [94]. Chromatography–mass spectrometry platforms have the advantage of higher sensitivity, hence their use over NMR. Although mass spectrometry is a destructive technique, it has the advantage of only requiring minute amounts (μL) of the sample for analysis. In GC-MS, substances are heated until they become gaseous, so non-volatile materials cannot be directly studied and need derivatization. With the right choice of mobile phases and column, LC-MS allows for the examination of an extensive array of metabolites, from hydrophilic to hydrophobic [45,95]. Upgrading of chromatography has seen the introduction of ultra-high-performance liquid chromatography (UHPLC). The UHPLC systems use columns with smaller particle sizes, which result in increased peak capacity, higher sensitivity, and the reduced time of analysis in comparison to HPLC [96]. The separation mechanism in capillary electrophoresis is different from that used in liquid and gas chromatography and enables analysis of polar and ionic molecules. Low repeatability is one of the major limitations of CE, whereas excellent separation capacity is regarded as the main advantage of this analytical technique [97].
4. Attempts to Get Down to Metabolomics in SCI
In recent years, the exploration of metabolomics has emerged as a promising frontier in understanding the intricate biochemical landscapes of spinal cord injuries. The scientific development of novel therapies for SCI treatment depends upon an understanding of the complex pathophysiologic mechanisms that are triggered after acute SCI. These underlying processes have been studied extensively at the level of proteomics, transcriptomics, and genomics (-omics). However, the contributions of metabolomics have not been on par in spite of the work in progress as outlined below [98].
4.1. SCI Animal Studies Involving Metabolomics
A growing body of metabolomics research in preclinical spinal cord injury animal models has elucidated a complex but increasingly coherent portrait of injury-induced biochemical disturbances. As summarized in Table 1, these studies employ a range of high-resolution analytical techniques—including untargeted and targeted liquid chromatography–mass spectrometry (LC-MS/MS), gas chromatography–mass spectrometry (GC-MS), and mass spectrometry imaging (MSI)—to interrogate the metabolic landscape of SCI across rat and mouse animal models, injury mechanisms, and tissue types.
The metabolomic analyses in Table 1 were typically conducted on n = 3 to n = 8 animals per group, across tissues like spinal cord, plasma, serum, CSF, urine, feces, skeletal muscle, lung, and exosomes, with time points ranging from 30 min to 28 days post-injury, reflecting a broad representation of SCI pathophysiology in preclinical animal models. A central and recurring theme is the dysregulation of lipid metabolism following SCI. Studies utilizing both targeted and untargeted LC-MS/MS approaches have consistently identified arachidonic acid derivatives (such as PGE2 and LTB4 [49,67]), lysophospholipids [76], and a spectrum of fatty acids and their derivatives [50,55,66,68,76] as robust biomarkers of acute neuroinflammation, membrane integrity, mitochondrial dysfunction, and secondary injury progression. For example, a study by Yang et al. [61] demonstrated that uric acid, phosphorylcholine, pyridoxine, and guanidoacetic acid, differentially altered across spinal cord, CSF, and plasma, serve as candidate biomarkers for spinal cord injury severity, reflecting disruptions in glyoxylate, dicarboxylate, glycine, serine, and threonine metabolism linked to neuroinflammation, oxidative stress, and membrane repair.
In addition, Liang et al. [55] further demonstrated that glycerophospholipid metabolites such as phosphatidylserines, phosphatidylethanolamines, and phosphatidylcholines were identified as biomarkers of neuropathic pain in rats, reflecting disrupted membrane homeostasis and neuroinflammation mediated via microglial P2 × 4/IRF8/P38 MAPK signaling and oxidative stress pathways. The prominence of these lipid biomarkers likely reflects the universal activation of phospholipases and lipid peroxidation signaling pathways in response to membrane disruption and oxidative stress [55]. These processes are rapidly engaged regardless of the specific injury mechanism and persist despite distinct metabolic profiles. These findings collectively underscore that lipidomic dysregulation post-SCI centers around phospholipid degradation, fatty acid oxidation, and inflammation. Lipid species such as lysophospholipids, oxylipins, and carnitines may serve as robust biomarkers or therapeutic targets in SCI pathology and recovery monitoring.
In parallel, multiple studies in Table 1 report significant disturbances in amino acid metabolism following SCI, with key neurotransmitter precursors and derivatives, including dopamine, tyrosine [51,68], tryptophan [56,60], glutamate [62,64,65,70], alanine [62,80], and branched-chain amino acids [73], emerging as functional biomarkers of neuropathic pain, neuroinflammation, and metabolic dysfunction [59,73]. Although the specific metabolite profiles vary between studies, dopamine, tyrosine, tryptophan, and their downstream derivatives have been consistently reported as altered following SCI, particularly in experimental models of neuropathic pain and opioid responsiveness [51,56,68]. Additionally, Liu et al. [59] demonstrated that metabolite alterations in the peripheral detrusor muscle, particularly in acetylcholine, histamine, and kynurenine pathways, reflect neuromodulatory and neuroimmune disruption beyond spinal cord or blood biomarkers, supporting their potential role in identifying neurogenic bladder dysfunction following SCI. These metabolites are not only implicated in pain modulation and central sensitization but are also linked to gut–brain axis signaling, with Kang et al., 2023, and Chen et al., 2021, independently demonstrating that alterations in amino acid metabolism are influenced by changes in the gut microbiota [68,73]. Disruption of the tyrosine metabolism is a shared feature in Rodgers et al. [51] and Yuan et al. [56], though the specific metabolites differ. Rodgers and colleagues linked altered dopamine-related metabolites to morphine resistance, identifying markers of opioid responsiveness. Yuan et al. found that ligustrazine and sinomenine modulated tyrosine pathway balance, highlighting different tyrosine-derived metabolites as therapeutic indicators [56]. Despite variation, both studies support tyrosine metabolism as a core disrupted pathway and a promising source of biomarkers for pain severity, neuroinflammation, and therapeutic efficacy.
Energy metabolism is another axis of convergence. SCI-induced energy metabolism disruption is consistently demonstrated across multiple tissues, with Liu et al. [59] identifying phosphocreatine, α-ketoglutaric acid, and fructose 1,6-bisphosphate in bladder muscle [54]; Zeng et al. [58] reported purine cycle markers [AMP, IMP, GMP, and guanidoacetic acid] in the spinal cord; Zhang et al. [54] detected pyruvate, lactate, and isocitrate in the serum; Shi et al. [72] confirmed lactate and glycolytic intermediates [glucose-6-phosphate, fructose-6-phosphate, and AMP] in the spinal tissue; and Graham et al. [72,79] and Potter et al. [78] revealed glucose, lactate, and pyruvate dysregulation in the skeletal muscle, all supporting impaired ATP production as a hallmark of SCI pathology and recovery potential. Despite this convergence in key metabolic pathways, the specific lists of altered metabolites often vary across studies due to differences in animal species [rats vs. mice], injury models [contusion, compression, or avulsion], sampling time points, and analytical conditions. These metabolites serve as sensitive markers of ATP depletion, mitochondrial dysfunction, and oxidative stress, and their detection is facilitated by the high sensitivity of both LC-MS/MS and GC-MS platforms. These metabolites not only reflect underlying mitochondrial and glycolytic dysfunction but also serve as candidate biomarkers for early diagnosis, severity stratification, and the evaluation of metabolic-targeted therapies in spinal cord injury, regardless of the model or tissue examined.
Despite these overarching similarities, important differences emerge that are closely tied to the mechanism of injury, the tissue or biofluid analyzed, and the analytical platform employed. Contusion models simulating blunt trauma emphasize acute inflammatory lipids and energy metabolites, such as prostaglandin E2 and leukotriene B4 [49] and purines and glycolytic intermediates like AMP and lactate [54,72]. In contrast, compression and CCI models highlight chronic osmotic and membrane stress, with elevated osmolytes like betaine and creatine [52] and metabolites such as taurine, ascorbic acid, and dopamine [58,68]. Transection and excitotoxic models reveal ionic imbalance and edema, marked by small osmolytes like carnosine and inosine [56,66]. By revealing how injury-type-specific metabolic profiles shape biomarker expression, this comparison supports a future stratified approach to biomarker discovery in SCI, ensuring relevance to both acute and chronic clinical scenarios.
The spatial resolution afforded by mass spectrometry imaging and region-specific sampling strategies has revealed that certain metabolites are localized to distinct pathological zones following SCI. For example, phosphatidylserine (PS) and related lipids were identified in inflamed spinal cord regions using AFADESI-MSI, marking sites of oxidative stress and neural damage [66], while nicotinamide was enriched in fibrotic scar regions and proposed as a marker of scar modulation and therapeutic efficacy [74]. Similarly, Pukale et al. [52] used MSI to detect osmolyte gradients, such as betaine and creatine, along the rostrocaudal axis of syrinx expansion. These findings underscore the importance of accounting for both anatomical location and injury phase when interpreting metabolomic profiles and selecting spatially relevant biomarkers.
The clinical and translational implications of these findings are substantial. Many of the identified metabolites are not only diagnostic or prognostic indicators, reflecting injury severity, progression, or comorbid risks such as neuropathic pain and depression, but they also point toward therapeutic potential. For instance, docosahexaenoic acid (DHA) was proposed by Jiang et al. [57] and Zhang et al. [60] as a neuroprotective and anti-inflammatory biomarker linked to repair and resolution signaling across species and platforms. Similarly, different types of short-chain fatty acids (SCFAs), including butyrate and propionate, were associated with the gut–brain axis recovery and anti-inflammatory activity [63,68,71,75]. Moreover, exosomal fatty acids linked to depression-like behavior post-SCI [63] and nicotinamide (NAM), implicated in fibrotic scar suppression [74], are highlighted as mechanistic biomarkers with therapeutic relevance. Integrating metabolomics with transcriptomics, proteomics, and spatial mapping strategies is likely to refine these biomarker panels, improving their specificity, contextual relevance, and utility in both experimental and clinical settings.
In summary, rat and mouse SCI models contribute distinct but complementary insights into biomarker discovery. Rat studies (e.g., [54,58]) primarily highlight acute-phase markers of inflammation, energy failure, and osmotic imbalance. In contrast, mouse studies (e.g., [57,72,73]) emphasize chronic metabolic remodeling, mitochondrial dysfunction, and gut–brain axis disruption. Together, these models support time-specific and system-wide biomarker identification, enhancing translational relevance in SCI. In conclusion, the collective evidence from Table 1 demonstrates that while certain metabolic disturbances, particularly in lipid, energy, and amino acid metabolism, are conserved across SCI models, the precise biomarker signatures are shaped by the mechanism of injury, tissue context, and analytical technique. Future studies should prioritize cross-model integration, longitudinal sampling, and validation in human cohorts to establish robust, phase-specific biomarker panels for diagnosis, prognosis, and therapeutic monitoring.
4.2. Human Studies Involving Metabolomics
The human SCI metabolomics studies listed in Table 2 consistently used advanced MS or NMR platforms on urine, serum, or plasma, and identify amino acids, energy metabolites, lipids, and gut-derived molecules as robust, non-invasive biomarkers. These metabolites track with injury severity, recovery, and intervention response, supporting their clinical utility for diagnosis, prognosis, and therapy monitoring in SCI.
The studies summarized in Table 2 collectively highlight the rapid progress and diversity of metabolomics approaches applied to biomarker discovery in human spinal cord injury patients. Across these investigations, a range of analytical techniques, including 1H NMR spectroscopy, LC-MS/MS, UHPLC-QTOF/MS, and GC-MS, were employed to interrogate metabolic changes in accessible biofluids such as urine, serum, plasma, and, in some cases, feces. The choice of technique and sample type not only influenced the breadth and specificity of metabolites detected but also shaped the clinical relevance and translational potential of the identified biomarkers. However, despite these advances, the routine clinical translation of these metabolomic biomarkers remains limited due to challenges in validation, standardization, and integration into existing diagnostic frameworks.
A consistent finding across clinical metabolomics studies is the disruption of amino acid metabolism following spinal cord injury, with several studies identifying amino acid-related metabolites as sensitive indicators of injury status and recovery. In serum samples, Singh et al. [82] reported significant alterations in glycine, valine, phenylalanine, alanine, tyrosine, isoleucine, and others, with glycine and lactate inversely correlating with neurological recovery scores. In a related longitudinal urine-based study, Singh et al. [84] again observed the significant regulation of alanine and phenylalanine, particularly in patients undergoing stem cell therapy, supporting their role as non-invasive biomarkers for monitoring functional improvement, as they were correlated with ASIA Impairment Scale scores. These consistent findings across time points and sample types highlight the translational potential of amino acid metabolites as accessible, reproducible indicators of neurological status, offering a path toward personalized monitoring tools in the clinical management of SCI patients. However, broader validation and standardization are still needed to establish their routine utility in clinical settings. Bykowski et al. [85] further identified L-valine in urine as a robust prognostic biomarker, highlighting its recurrence across studies; although not identical in context, its repeated detection underscores the relevance of branched-chain amino acids as a metabolite class in SCI biomarker discovery. Meanwhile, Li et al. [86] reported elevated phenylacetylglutamine and L-5-oxoproline in serum samples collected from chronic SCI patients, associating these metabolites with metabolic and cardiovascular risk, while Zhou et al. [77] detected the amino acid derivative S-allyl-L-cysteine in plasma following a rehabilitation program, suggesting links to neuroprotective processes. The authors of both studies integrated KEGG pathway analysis to interpret the biological relevance of their metabolomics findings. In Li et al. [86], phenylacetylglutamine and L-5-oxoproline, identified as elevated in SCI patients with prediabetes/type 2 diabetes, were linked to microbial metabolism and glutathione metabolism, respectively. These metabolites are implicated in cardiovascular and metabolic risk, reflecting host–microbe co-metabolism and oxidative stress responses. In contrast, Zhou et al. [77] reported S-allyl-L-cysteine following rehabilitation, mapping it to cysteine and methionine metabolism. This pathway supports neuroprotective mechanisms, including antioxidant defense and cellular repair. Despite variability in exact metabolite identities, the KEGG-mapped pathways converge on conserved mechanisms like oxidative balance, amino acid metabolism, and gut microbial contributions, reinforcing their pathophysiological relevance in SCI. These findings underscore the potential of targeting conserved metabolic pathways in biomarker discovery and highlight the need for longitudinal studies to validate their diagnostic and prognostic value in clinical settings.
The observed variations in metabolite profiles can be partly attributed to differences in biofluid type (serum vs. urine vs. plasma), time post-injury (acute vs. chronic), and therapeutic intervention status (e.g., stem cell therapy, exercise rehabilitation). Urinary and serum studies also differ in sensitivity and metabolite composition due to renal filtration and reabsorption dynamics. Additional clinical factors such as injury severity (e.g., AIS grade), presence of infections, particularly urinary tract infections common in SCI patients, medication use, comorbidities like diabetes or pressure ulcers, and nutritional status further complicate metabolic readouts, underscoring the need for careful interpretation and standardization in human SCI metabolomics research. Notably, L-valine and phenylalanine are the only amino acids consistently reported across both serum and urine studies [82,84,85], reinforcing their translational value as stable, non-invasive biomarkers for SCI monitoring.
Disruption of energy metabolism is a recurring and mechanistically relevant finding across multiple clinical SCI metabolomics studies. Serum-based analyses by Singh et al. [82] identified significant alterations in lactate, acetate, succinate, and glucose, all indicative of acute glycolytic flux and mitochondrial stress. These changes correlated with neurological recovery, especially as lactate and glycine levels decreased over time. In urine, Singh et al. [84] reported changes in acetate and β-hydroxybutyrate, a ketone body, alongside creatine and creatine phosphate, reflecting systemic metabolic adaptation during subacute recovery. Bykowski et al. [87] confirmed the serum elevation of lactate, acetic acid, and succinic acid up to six months post-injury, linking them to functional recovery (SCIM score). Further, Shi et al. [71] measured elevated lactate, glucose-6-phosphate, and fructose-6-phosphate in spinal cord tissue, emphasizing local neuronal energy failure and impaired axonal regeneration. Although not focused on traditional energy intermediates, Li et al. [86] also identified glutamine and L-5-oxoproline, linked to energy and redox balance, in chronic SCI serum samples, particularly in patients with metabolic comorbidities (e.g., prediabetes).
Lactate and acetate emerged as the most consistently altered energy metabolites across studies and sample types (e.g., [84,85,88]), suggesting their robustness as systemic biomarkers. However, differences in reported metabolites likely reflect variations in sample type (serum vs. urine vs. tissue), analytical platform (NMR vs. MS), injury chronicity, and cohort characteristics. For example, serum and tissue samples captured acute mitochondrial and glycolytic disturbances, while urine-based studies (e.g., Singh et al. [84]) may emphasize renal clearance-filtered metabolic byproducts. Chronic studies, e.g., Li et al. [86] further showed energy metabolites associated with oxidative stress and cardiometabolic risk, reflecting longer-term adaptation or dysfunction. Collectively, these findings highlight a core panel of energy-related metabolites, particularly lactate, acetate, succinate, and β-hydroxybutyrate, as promising biomarkers for metabolic dysfunction, therapeutic response, and longitudinal recovery monitoring in SCI.
Four studies in Table 2 highlighted lipid metabolism as a promising domain for biomarker discovery in SCI, particularly in relation to neuroinflammation, immune modulation, and rehabilitation outcomes. Using untargeted LC-MS/MS, Zhou et al. [77] identified elevated lysoglycerophospholipids (e.g., LPE[P-17:0]) and free fatty acids in plasma following a 4-week exercise program, with several correlating with immunoglobulin heavy-chain expression and functional recovery. Similarly, Kong et al. [88] reported increased levels of phosphatidylcholine PC [18:2/0:0] in serum from chronic SCI patients, suggesting persistent lipid remodeling linked to gut dysbiosis and systemic metabolic stress. Zhang et al. [89] extended these findings by detecting elevated fecal fatty acids, such as linoleic and butyric acid, implicating gut-derived lipid alterations in inflammation and recovery. Adding to this, Yarar-Fisher et al. [99] used untargeted metabolomics to show that a ketogenic diet increased serum levels of the anti-inflammatory lysophospholipid lysoPC [16:0] while decreasing pro-inflammatory fibrinogen peptides in acute SCI patients—changes that were associated with improved motor recovery. While these studies vary in biofluid type, patient chronicity, and intervention (e.g., diet vs. exercise), they converge on the dysregulation of lipid signaling, particularly phospholipids and fatty acids, as a core metabolic feature of SCI. Together, these findings support the translational potential of lipid-derived metabolites as mechanistic markers of neuroimmune regulation, metabolic recovery, and therapeutic response in spinal cord injury.
While Zhou et al. [77] focused on lipid changes associated with rehabilitation-induced neuroplasticity, Kong et al. [88] profiled lipid signatures at a later post-injury stage [~23 months], likely capturing chronic-phase metabolic adaptations. This difference in time post-injury, alongside distinct sample matrices (plasma vs. serum), may explain the variability in specific lipid species reported. Notably, both studies converge on the importance of glycerophospholipid metabolites, reinforcing their potential as common lipid biomarkers in SCI. Together, these studies support lipidomics, particularly the profiling of lysophospholipids and phosphatidylcholines, as a valuable approach for identifying biomarkers of chronic inflammation, repair, and rehabilitation response in human SCI.
Microbiota-derived metabolites and their interplay with systemic metabolism represent a rapidly emerging area, as exemplified by Li et al. [86], Kong et al. [88], and Jing et al. [81]. These studies combined metabolomics with microbiome profiling [using both MS and GC-MS for SCFAs] to show that metabolites such as indoxyl sulfate, phenylacetylglutamine, and short-chain fatty acids [butyric and valeric acid] are significantly altered in SCI patients, particularly those with metabolic comorbidities. The authors propose these metabolites as biomarkers for metabolic and cardiovascular risk as well as indicators of gut–brain axis disruption [71,81,86,89]. Notably, the reduction in beneficial SCFAs and the increase in gut-derived uremic toxins were linked to both injury severity and neurological outcome, highlighting the translational potential of these markers for risk stratification and therapeutic targeting [71,75,89].
Despite methodological differences, a consistent theme is the clinical utility of these biomarkers. Most studies emphasize the feasibility of using urine and serum for repeated, non-invasive sampling, thereby facilitating longitudinal monitoring in both acute and chronic SCI settings. The integration of metabolomics data with clinical outcome measures and, in some cases, machine learning approaches, has further refined the prognostic value of these markers. For instance, the combination of metabolite panels with SCIM scores [85,86] or with immunoregulatory protein levels [87] has yielded biomarker signatures with strong predictive power for neurological recovery and rehabilitation response.
While the human SCI metabolomics studies in Table 2 provide valuable insights, they are constrained by several methodological limitations that must be addressed to improve clinical utility and reproducibility. A primary concern is small sample sizes; for example, Bykowski et al. [85] included only six male patients, and their follow-up study [88] involved just seven male participants. Similarly, Singh et al. [82] and Singh et al. [100] enrolled approximately 20 SCI patients, limiting statistical power and increasing the risk of false-positive or false-negative findings. Zhou et al. [77] acknowledged that their small cohort reduced their ability to detect subtle multi-omic effects. Moreover, Bykowski et al. [87] highlighted substantial heterogeneity in injury level and severity within their cohort, reinforcing the need for larger, multi-center studies to enable subgroup analysis and validation.
Participant selection and control group design also varied significantly. Both Bykowski studies [85,87] enrolled only male participants, excluding females and potentially overlooking sex-specific metabolic signatures, an issue also noted by Zhou et al. [77] in relation to sex mismatches between human and animal subjects. Additionally, Singh et al. [82] focused on acute, complete injuries, whereas Kong et al. [88] included patients with a broad range of severities (AIS A–D) and chronicity (~23 months post-injury), introducing biological variability. Control group inclusion was also inconsistent: Kong et al. [88] and Jing et al. [81] included well-matched healthy controls, while Bykowski et al. [85,87] relied solely on within-subject longitudinal comparisons and lacked healthy or trauma-exposed controls, limiting their ability to distinguish SCI-specific effects from general post-trauma alterations.
Analytical platform limitations also influenced the findings. Several studies [82,84,85,87] used 1H-NMR, which provides high reproducibility but limited sensitivity, possibly missing low-abundance yet biologically important metabolites. In contrast, Kong et al. [88] applied high-resolution untargeted mass spectrometry (UHPLC-QTOF/MS), detecting over 5000 features but identifying only 41 named metabolites—highlighting the common challenge of poor annotation in untargeted MS studies. Future work could combine NMR and MS approaches to maximize coverage and follow up untargeted results with targeted validation using authentic standards.
Finally, study design and sample collection timing were suboptimal. Many studies used only two time points (e.g., baseline and 6 months in Singh et al. [84] and Bykowski et al. [85,87], missing intermediate dynamics between the acute, subacute, and chronic phases. Others, like Kong et al. [88] and Jing et al. [81], employed cross-sectional designs, limiting causal inference. Dietary intake, medications, and rehabilitation status were rarely controlled or recorded, although Bykowski et al. [85] acknowledged that caffeine levels were likely influenced by uncontrolled dietary factors. Moving forward, studies should adopt longitudinal sampling frameworks with more frequent time points, standardize collection conditions (e.g., fasting, morning sampling), and record potential confounders like diet, medication, and BMI.
4.3. Conserved Metabolic Pathways Across Animal and Human Studies
While animal studies offer mechanistic insights through controlled injury models and diverse tissue sampling, human SCI metabolomics reflects a broader spectrum of clinical variability, comorbidities, and interventions. Despite methodological differences, both domains converge on key pathways, energy metabolism, oxidative stress, amino acid dysregulation, and microbial metabolites, highlighting conserved biological responses to SCI and informing biomarker discovery across species.
Energy metabolism was the most consistently disrupted pathway. Shi et al. [71] showed that spinal cord injury-induced hypoxia suppresses the MCT1-mediated lactate transport, impairing neuronal energy supply and axonal regeneration in mice. Restoration of this shuttle improved recovery, highlighting lactate as a mechanistic and therapeutic target. In rats, studies by Ni et al. [53], Zhang et al. [54], and Lyu et al. [66] also highlighted elevated lactic acid, pyruvate, and inosine in the spinal cord and serum, indicating mitochondrial dysfunction and oxidative stress in acute and chronic phases. In human studies, Bykowski et al. [87] reported that lactate, acetic acid, and succinate levels in serum correlated with SCIM scores, while Singh et al. [84] found that urinary acetate and β-hydroxybutyrate tracked with functional recovery, demonstrating translational alignment in energy biomarkers across species.
Amino acid metabolism, especially branched-chain amino acids (BCAAs), also recurred as a conserved signature. In rats and mice, Kang et al. [73] and Zhang et al. [74] observed significant perturbations in valine, leucine, and isoleucine, correlating with neuroinflammation and gut–brain axis disruption. Human studies mirrored these findings: Singh et al. [82] reported that valine, isoleucine, and glycine levels were linked to neurological recovery, and Bykowski et al. [85] validated L-valine as a non-invasive urinary biomarker. These data suggest that BCAA dysregulation reflects metabolic stress as well as repair capacity post-injury and is conserved across species.
Purine metabolism emerged in both datasets. In animals, Zeng et al. [58] and Yang et al. [61] identified purine derivatives like AMP, IMP, and uric acid as indicators of energy disruption and oxidative stress. In humans, Bykowski et al. [87] detected 1,3,7-trimethyluric acid and hypoxanthine, linking purine breakdown with poor recovery trajectories. These metabolites may serve as markers of injury burden and mitochondrial dysfunction.
Gut-derived short-chain fatty acid (SCFA) metabolism was another conserved axis. Jing et al. [81] and Kong et al. [88] showed that SCI patients had reduced butyric, acetic, and propionic acids, accompanied by gut dysbiosis. This mirrored findings in animal models (e.g., Wang et al., [63]), where SCFA depletion was associated with neuroinflammation and functional decline. Notably, SCFA supplementation restored motor function in rodents, emphasizing their therapeutic relevance [63].
Lastly, lipid metabolism, including changes in phosphatidylcholines, lysoglycerophospholipids, and arachidonic acid derivatives, was frequently altered across species. Kong et al. [88] in humans and Liang et al. [55] in rats identified these lipids as markers of systemic inflammation and neuroimmune signaling. Zhou et al. [77] further linked exercise-induced lipid shifts to functional recovery, while Lyu et al. [66] mapped lipid species like phosphatidylserine [PS] to lesion regions using mass spectrometry imaging. Collectively, SCI produces a metabolic fingerprint marked by energetic disruption, amino acid and purine dysregulation, gut–brain axis breakdown, and lipid-mediated inflammation. These conserved pathways not only validate cross-species relevance but also represent modifiable targets for biomarker development and therapeutic intervention.
5. Potential and Future of Metabolomics in SCI
5.1. Potential of Metabolomics in SCI
5.1.1. Injury Profiling and Prognosis
In other clinical fields, metabolomics has already produced validated biomarkers that inform diagnosis, prognosis, and therapeutic decision-making. Maple syrup urine disease, for instance, is detected in newborns by measuring elevated branched-chain amino acids (BCAAs) in dried blood using tandem mass spectrometry [99]. Urinary creatinine–albumin ratios, quantified by mass spectrometry (MS), guide the staging of chronic kidney disease, while serum lactate, also routinely measured by MS, serves as a prognostic marker in sepsis and traumatic shock, reflecting hypoxia and mitochondrial dysfunction [101]. In addition, oncometabolites such as 2-HG are now in clinical use for diagnosing gliomas and assessing mutation status [100].
Building on these precedents, SCI metabolomics studies have begun to reveal injury- and outcome-specific signatures with a similar translational promise. Metabolites such as lactate, acetate, succinate, and β-hydroxybutyrate have been consistently identified in the serum and urine of SCI patients and have been shown to correlate with functional recovery scores (e.g., Spinal Cord Independence Measure) [84,87]. These potential biomarkers reflect core disruptions in mitochondrial function and systemic energy metabolism, common features of acute and subacute SCI.
In animal models, Shi et al. [71] mechanistically linked elevated lactate and glycolytic intermediates to suppressed MCT1-mediated neuronal transport, demonstrating both prognostic and therapeutic relevance. Amino acids such as L-valine and phenylalanine also show prognostic value in both humans and animals [60,73,82,85], echoing their future clinical use in metabolic disorders. Therefore, SCI metabolomics holds the potential to deliver clinically actionable biomarkers that inform early diagnosis, injury stratification, and personalized rehabilitation strategies, provided they undergo rigorous validation, standardization, and integration into clinical practice.
5.1.2. Tailored Therapies
Personalized treatments can be guided by each patient’s metabolic profile, enabling clinicians to target the most disrupted pathways with precision [102]. For example, if metabolomics shows increased reliance on glycolysis or oxidative stress, one strategy is metabolic reprogramming: ketogenic diets have been shown to improve motor recovery and reduce inflammation in SCI patients [83]. Conversely, amino acid or antioxidant supplementation can be adjusted to counteract deficits in individuals. Microbiome–metabolome analysis in BPRA also suggests targeted interventions: Zhang et al. [18] found that avulsion-induced neuropathic pain altered gut Lactobacillus and the bile acid–neurotransmitter–amino acid metabolism, contributing to anxiety-like behavior. Supplementing with a Lactobacillus reuteri, the dominated probiotic blend reversed these metabolomic changes and relieved anxiety in mice. In human SCI, personalized regimens are under study; for instance, a single-masked, longitudinal, randomized, parallel-controlled study introduced a ketogenic diet during acute SCI care expecting the detection of serum biomarkers linked to improvements in neurological recovery and functional independence [103]. By matching interventions to each patient’s metabolic abnormalities, metabolomics enables precision neurorehabilitation in SCI.
5.1.3. Understanding Secondary Complications
Metabolomics can trace the biochemical roots of post-injury inflammation, oxidative stress, and related complications. After SCI, for example, targeted lipidomics revealed dramatic upregulation of pro-inflammatory arachidonic acid metabolites, notably 5-lipoxygenase and COX-2 products, consistent with intense secondary inflammation [49]. Detection of elevated lactate and glycerol in CSF [104] likewise signals tissue hypoxia and membrane breakdown, linking metabolism to damage. Importantly, multi-omic studies have identified purine and amino acid pathways driving oxidative stress. Integrated transcriptome–metabolome analysis in rats showed that acute SCI caused the profound dysregulation of purine metabolism (e.g., xanthine, inosine), which severely compromised energy balance and increased oxidative stress in the injury microenvironment [58]. Similarly, a mouse SCI model found the accumulation of amino acids [leucine, methionine, phenylalanine, and valine] that correlated with gut dysbiosis and promoted reactive oxygen species generation and neuroinflammation [81]. Metabolomics has illuminated gut–brain axis disruption in SCI, with Jing et al. [81] reporting a 30–40% reduction in key short-chain fatty acids, acetate, propionate, and butyrate due to microbiome shifts in patients; in the same study, SCFA supplementation in mice enhanced neuronal survival, reduced astrogliosis, and improved locomotion, likely by dampening systemic inflammation. Thus, metabolite profiling links immune and metabolic changes after SCI, revealing how molecular byproducts (e.g., oxidized lipids, excitotoxins, and microbial metabolites) underlie secondary injury.
5.1.4. Drug Development and Mechanism-Based Therapeutics
By pinpointing disrupted metabolic pathways, metabolomics can inform the discovery of targeted drugs. Cutting-edge approaches such as dose–response metabolomics and stable isotope-resolved metabolomics are uncovering dysregulated metabolic pathways in diseases, directly informing target identification and validation [105,106]. For example, recent studies have used metabolomics to reveal that restoring phenylalanine levels and modulating glutathione metabolism can identify drug targets such as AKT2 and PDK2 in diabetes and cancer models, thereby guiding precision drug design and repurposing [101,106]. Moreover, artificial intelligence (AI)-driven models, including graph neural networks, are now being applied to predict pharmacokinetic and toxicity profiles with high accuracy, accelerating candidate optimization and reducing late-stage attrition [107]. Additionally, metabolomics is advancing personalized medicine by correlating metabolic signatures (for example, branched-chain amino acids, phosphatidylcholines, and long-chain acylcarnitines in metformin-treated cancers) with treatment efficacy, ensuring therapies align with individual metabolic profiles [108]. Collectively, these innovations are breaking traditional research and development bottlenecks and offering a pathway to overcome Eroom’s Law, delivering targeted, biomarker-driven therapies [101]. In summary, each aberrant metabolic signature uncovered in SCI offers a rational target for drug development, enabling therapies that address the injury’s biochemical mechanisms.
5.2. Future Perspectives in SCI Metabolomics
Metabolomics in SCI is at an early stage, facing challenges due to the complexity of metabolic networks and the need for study standardization. Collaborative research integrating various “omics” technologies and expertise from different fields is crucial for understanding SCI and translating findings into clinical practice.
5.2.1. Integrated Systems Biology Approach
A key future direction is to integrate metabolomics with other “omics” (genomics, transcriptomics, and proteomics) to build a holistic view of spinal cord injury pathology and recovery [109]. Single-omics studies capture only one layer of biology, whereas combined multi-omics approaches can reveal complex interactions and compensatory mechanisms that a single modality might miss [58]. Indeed, researchers have begun shifting from isolated analyses toward comprehensive multi-omics strategies to overcome the insufficiencies of any single dataset [110]. Recent SCI studies exemplify this trend: for instance, one clinical trial coupled plasma proteomics with immune cell transcriptomics and then added metabolomic and transcriptomic analysis in an animal model to map the molecular effects of exercise [77]. Such integrative analyses identified key pathways and molecules (e.g., immune and metabolic regulators) that underlie recovery benefits. Similarly, metabolomics combined with transcriptomics can elucidate mechanistic links—as shown by Shi et al. [71], who used metabolite profiling and a lactate sensor to discover a lactate shuttle between endothelial cells and neurons, then re-analyzed single-cell RNA sequencing data to find the corresponding transporter (MCT1) was downregulated after injury [71]. By converging insights from metabolites, gene expression, and proteins, future SCI research can pinpoint biomarker panels and therapeutic targets with far greater precision than metabolomics alone, ultimately providing a more complete “systems biology” understanding of injury and repair.
5.2.2. Technological Advancements
Continued advances in analytical technology are expected to dramatically improve the sensitivity, accuracy, and throughput of SCI metabolomics. Modern mass spectrometry [MS] offers detection limits in the picogram range, orders of magnitude more sensitive than NMR, enabling identification of many more low-abundance metabolites than was previously possible [111]. Emerging MS platforms also provide new capabilities such as spatially resolved metabolite mapping: for example, mass spectrometry imaging allows sensitive label-free detection, quantification, and characterization of metabolites in tissue, adding a spatial context to biochemical changes that conventional MS lacks [112]. In situ mass spectrometry imaging was shown to provide valuable information on the metabolite profiles across various anatomical regions of the spinal cord following SCI, which may support the development of precise treatment strategies for patients [66]. Furthermore, matrix-assisted laser desorption/ionization mass spectrometry imaging (MALDI-MSI), one of the most widely used and recently refined spatial metabolomics techniques, allows for the near single-cell resolution mapping of metabolite distributions across tissue sections, offering powerful insights into localized biochemical processes [113].
On the NMR front, hardware innovations are boosting sensitivity and throughput despite NMR’s lower intrinsic sensitivity. Ultra-high-field magnets (high-frequency spectrometers) and improved cryogenic probes now reveal peaks that were formerly undetectable, and hyperpolarization techniques can amplify NMR signals by >10,000-fold [114], greatly extending the range of observable metabolites. These improvements, combined with better automation and sample processing, are increasing metabolomic workflow throughput without sacrificing data quality. Notably, recent protocols have addressed prior challenges like ion suppression, reproducibility, and spectral complexity in high-throughput MS assays [115]. Both 1H-NMR and MS are already proven tools in SCI biomarker research; for instance, NMR spectroscopy has successfully discovered metabolite biomarkers correlating with recovery in patients [87], and ongoing enhancements in resolution and sensitivity will make future metabolomics studies in SCI even more precise and routinely used in SCI patient care. The net result will be metabolomic analyses that are faster, cover a broader metabolite range, and yield more reliable quantitative data, facilitating their translation into clinical and research settings.
5.2.3. Real-Time Monitoring
Another promising future avenue is the development of real-time metabolomic monitoring for SCI, enabling the dynamic tracking of biochemical changes to inform quick clinical decision-making. Currently, metabolomic analyses are typically performed on discrete samples [e.g., serum, CSF] in a lab setting, but real-time monitoring of endogenous metabolites in vivo could capture the rapid metabolic fluctuations during acute injury or rehabilitation interventions [116]. Such a capability would allow clinicians to detect secondary injury processes or responses to therapy in near real-time and adjust treatments accordingly. Achieving this is challenging due to the issues of sensitivity, speed, and invasiveness [116], but recent technological breakthroughs are starting to make it feasible. For example, new microfluidic and sensor systems are being designed to continuously sample and analyze metabolites from biological fluids, addressing the need for automated, continuous separation and detection of metabolites at the bedside [64,117]. Innovative ambient MS techniques like probe electrospray ionization coupled with tandem MS have already been used in vivo to instantly ionize and identify metabolites from living brain tissue [116], demonstrating the potential for real-time metabolomic readouts in the nervous system. In the context of SCI, researchers have shown that biosensors can directly monitor key injury-related metabolites: Shi et al. used an intracellular lactate sensor to demonstrate lactate shuttling in the cultured cortical cells, providing immediate insights into metabolic support for neurons [69]. In the future, similar biosensors or rapid MS platforms could be deployed to monitor biomarkers (e.g., lactate, glucose, and inflammatory mediators) in SCI patients in intensive care units or during surgery, enabling timely interventions. The ability to obtain metabolic feedback in real time, essentially “metabolic vital signs”, would represent a paradigm shift in SCI management, allowing preemptive adjustments to therapy and more personalized, responsive care [118]. As these technologies mature, real-time metabolomics holds promise for improving outcomes by catching pathological changes early and guiding interventions with unprecedented speed and precision.
5.2.4. Enhancing Rehabilitation (Personalized Nutrition and Exercise)
Metabolomic profiling also holds promise for optimizing rehabilitation interventions, such as tailoring dietary and exercise strategies to individual metabolic needs. Recent studies show that SCI disrupts systemic metabolism and the gut microbiome, leading to deficiencies in beneficial metabolites such as short-chain fatty acids (SCFAs), which play critical roles in neuroinflammation and repair [81,88]. Supplementation with SCFAs or the restoration of SCFA-producing gut bacteria significantly improved motor recovery and reduced inflammation in SCI mouse models [81]. On the exercise front, Zhou et al. [77] found that resistance training induced specific metabolic and immune signatures associated with improved locomotor function in SCI patients. Strikingly, plasma from exercised animals, when transferred to sedentary SCI mice, replicated some of these neuroprotective effects, demonstrating that exercise-induced metabolites mediate functional recovery. Future rehabilitation programs could incorporate metabolomic biomarkers to personalize nutritional support and tailor exercise protocols, thereby enhancing neuroplasticity and repair. This aligns with the growing movement toward precision rehabilitation, where interventions are matched to the individual’s molecular and metabolic state for optimal outcomes.
6. Conclusions
Looking ahead, the future of SCI metabolomics lies in scaling up current findings through multi-center, longitudinal studies that validate candidate metabolite panels and correlate them robustly with neurological outcomes. This step is essential for translating pilot data into clinically deployable diagnostic tools. Equally transformative is the integration of machine learning and artificial intelligence, which can manage the high-dimensional complexity of metabolomic datasets to uncover the predictive biosignatures of injury severity and recovery potential. These computational approaches already demonstrate high prognostic accuracy and hold promise for individualized treatment planning, such as patient stratification for clinical trials or tailoring rehabilitation intensity based on metabolic recovery trajectories. Together, these innovations signal a future where AI-enhanced metabolomics drives precision medicine in spinal cord injury.
Author Contributions
P.L.M.Z. contributed to the conceptualization, writing of the original draft, supervision, project administration, and final review and editing of the manuscript. A.B.G. was responsible for data collection, drafting the manuscript, visualization, and critical review. T.C. conducted the literature search, data curation, writing for review and editing, and formatting. L.Z. provided validation, contributed to the methodology, and performed the final review and critical editing of the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Ajman University Internal Research Grant, grant number 2022-IRG-DEN-12. The APC was funded by Ajman University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Rhee, P.C.; Pirola, E.; Hébert-Blouin, M.-N.; Kircher, M.F.; Spinner, R.J.; Bishop, A.T.; Shin, A.Y. Concomitant Traumatic Spinal Cord and Brachial Plexus Injuries in Adult Patients. J. Bone Jt. Surg. 2011, 93, 2271–2277. [Google Scholar] [CrossRef]
- Zhou, L.-H.; Han, S.; Xie, Y.-Y.; Wang, L.-L.; Yao, Z.-B. Differences in c-jun and nNOS expression levels in motoneurons following different kinds of axonal injury in adult rats. Brain Cell Biol. 2008, 36, 213–227. [Google Scholar] [CrossRef]
- Zilundu, P.L.M.; Xu, X.; Liaquat, Z.; Wang, Y.; Zhong, K.; Fu, R.; Zhou, L. Long-Term Suppression of c-Jun and nNOS Preserves Ultrastructural Features of Lower Motor Neurons and Forelimb Function after Brachial Plexus Roots Avulsion. Cells 2021, 10, 1614. [Google Scholar] [CrossRef]
- Eggers, R.; Tannemaat, M.R.; De Winter, F.; Malessy, M.J.A.; Verhaagen, J.; Kirik, D. Clinical and neurobiological advances in promoting regeneration of the ventral root avulsion lesion. Eur. J. Neurosci. 2016, 43, 318–335. [Google Scholar] [CrossRef]
- Wu, K.Y.; Spinner, R.J.; Shin, A.Y. Traumatic brachial plexus injury: Diagnosis and treatment. Curr. Opin. Neurol. 2022, 35, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Khani, M.; Mansoori, N.; Midha, R. Adult brachial plexus injuries: Surgical strategies and approaches. Neurol. India 2016, 64, 289. [Google Scholar] [CrossRef] [PubMed]
- Zelenski, N.A.; Oishi, T.; Shin, A.Y. Indications and Technique for the Use of Intraoperative Neuromonitoring in Brachial Plexus Surgery. J. Hand Surg. 2023, 48, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-W.; Tu, Y.-K.; Hsueh, Y.-H. Prespinal Versus Conventional Hemicontralateral C7 Nerve Transfer in the Treatment of Total Brachial Plexus Roots Avulsion Injuries: A Retrospective Study With a Minimum Follow-Up Period of 4 Years. J. Hand Surg. 2023, 48, 1175.e1–1175.e10. [Google Scholar] [CrossRef]
- Muheremu, A.; Peng, J.; Ao, Q. Stem cell based therapies for spinal cord injury. Tissue Cell 2016, 48, 328–333. [Google Scholar] [CrossRef]
- Zhou, L.-H.; Wu, W. Survival of Injured Spinal Motoneurons in Adult Rat upon Treatment with Glial Cell Line–Derived Neurotrophic Factor at 2 Weeks but Not at 4 Weeks after Root Avulsion. J. Neurotrauma 2006, 23, 920–927. [Google Scholar] [CrossRef]
- Lang, B.T.; Cregg, J.M.; DePaul, M.A.; Tran, A.P.; Xu, K.; Dyck, S.M.; Madalena, K.M.; Brown, B.P.; Weng, Y.-L.; Li, S.; et al. Modulation of the proteoglycan receptor PTPσ promotes recovery after spinal cord injury. Nature 2015, 518, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Niknami, N.; Moghaddam, A.; Mahand, S.N.; Moghaddam, A.S.; Arjmand, M.; Alt, F.; Kruppke, B.; Khonakdar, H.A. Application of stem cells, growth factors, small molecules, and biological macromolecules on nerve regeneration: A review and future direction. Int. J. Polym. Mater. Polym. Biomater. 2023, 73, 817–849. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, Q.; Li, W.; Su, H.; Li, S.; Zhu, Y.; Zhou, J.; Feng, Z.; Liu, Z.; Mao, S.; et al. Spinal cord conduits for spinal cord injury regeneration. Eng. Regen. 2023, 4, 68–80. [Google Scholar] [CrossRef]
- Alizadeh, A.; Dyck, S.M.; Karimi-Abdolrezaee, S. Traumatic Spinal Cord Injury: An Overview of Pathophysiology, Models and Acute Injury Mechanisms. Front. Neurol. 2019, 10, 282. [Google Scholar] [CrossRef]
- Failli, V.; Kleitman, N.; Lammertse, D.P.; Hsieh, J.T.; Steeves, J.D.; Fawcett, J.W.; Tuszynski, M.H.; Curt, A.; Fehlings, M.M.G.; Guest, J.D.; et al. Experimental Treatments for Spinal Cord Injury: What you Should Know. Top. Spinal Cord Inj. Rehabil. 2021, 27, 50–74. [Google Scholar] [CrossRef]
- Griffin, J.M.; Bradke, F. Therapeutic repair for spinal cord injury: Combinatory approaches to address a multifaceted problem. EMBO Mol. Med. 2020, 12, e11505. [Google Scholar] [CrossRef]
- Kachramanoglou, C.; De Vita, E.; Thomas, D.L.; Wheeler-Kingshott, C.A.M.; Balteau, E.; Carlstedt, T.; Choi, D.; Thompson, A.J.; Ciccarelli, O. Metabolic changes in the spinal cord after brachial plexus root re-implantation. Neurorehabilit. Neural Repair 2013, 27, 118–124. [Google Scholar] [CrossRef]
- Zhang, J.-L.; Xian, H.; Zhao, R.; Luo, C.; Xie, R.-G.; Tian, T.; Cong, R. Brachial plexus avulsion induced changes in gut microbiota promotes pain related anxiety-like behavior in mice. Front. Neurol. 2023, 14, 1084494. [Google Scholar] [CrossRef]
- Holmes, E.; Loo, R.L.; Stamler, J.; Bictash, M.; Yap, I.K.S.; Chan, Q.; Ebbels, T.; De Iorio, M.; Brown, I.J.; Veselkov, K.A.; et al. Human metabolic phenotype diversity and its association with diet and blood pressure. Nature 2008, 453, 396–400. [Google Scholar] [CrossRef]
- Wishart, D.S. Metabolomics for Investigating Physiological and Pathophysiological Processes. Physiol. Rev. 2019, 99, 1819–1875. [Google Scholar] [CrossRef]
- Turi, K.N.; Romick-Rosendale, L.; Ryckman, K.K.; Hartert, T.V. A review of metabolomics approaches and their application in identifying causal pathways of childhood asthma. J. Allergy Clin. Immunol. 2018, 141, 1191–1201. [Google Scholar] [CrossRef]
- Züllig, T.; Köfeler, H.C. High Resolution Mass Spectrometry in Lipidomics. Mass Spectrom. Rev. 2021, 40, 162–176. [Google Scholar] [CrossRef]
- Fujieda, Y.; Ueno, S.; Ogino, R.; Kuroda, M.; Jönsson, T.J.; Guo, L.; Bamba, T.; Fukusaki, E.; Di Giovanni, S. Metabolite Profiles Correlate Closely with Neurobehavioral Function in Experimental Spinal Cord Injury in Rats. PLoS ONE 2012, 7, e43152. [Google Scholar] [CrossRef]
- Kosmides, A.K.; Kamisoglu, K.; Calvano, S.E.; Corbett, S.A.; Androulakis, I.P. Metabolomic Fingerprinting: Challenges and Opportunities. Crit. Rev. Biomed. Eng. 2013, 41, 205–221. [Google Scholar] [CrossRef]
- Blaurock, J.; Baumann, S.; Grunewald, S.; Schiller, J.; Engel, K.M. Metabolomics of Human Semen: A Review of Different Analytical Methods to Unravel Biomarkers for Male Fertility Disorders. Int. J. Mol. Sci. 2022, 23, 9031. [Google Scholar] [CrossRef]
- Hyvärinen, E.; Savolainen, M.; Mikkonen, J.J.W.; Kullaa, A.M. Salivary Metabolomics for Diagnosis and Monitoring Diseases: Challenges and Possibilities. Metabolites 2021, 11, 587. [Google Scholar] [CrossRef] [PubMed]
- Kiseleva, O.; Kurbatov, I.; Ilgisonis, E.; Poverennaya, E. Defining Blood Plasma and Serum Metabolome by GC-MS. Metabolites 2021, 12, 15. [Google Scholar] [CrossRef] [PubMed]
- Saoi, M.; Britz-McKibbin, P. New Advances in Tissue Metabolomics: A Review. Metabolites 2021, 11, 672. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, D.R.; Patel, R.; Kirsch, D.G.; Lewis, C.A.; Vander Heiden, M.G.; Locasale, J.W. Metabolomics in cancer research and emerging applications in clinical oncology. CA Cancer J. Clin. 2021, 71, 333–358. [Google Scholar] [CrossRef]
- Suri, G.S.; Kaur, G.; Carbone, G.M.; Shinde, D. Metabolomics in oncology. Cancer Rep. 2023, 6, e1795. [Google Scholar] [CrossRef]
- Li, J.; Hu, J.; Liang, L.; Guasch, M.; Merino, J.; Ruiz-Canela, M.; Luo, K.; Rebholz, C.; Porneala, B.; Dupuis, J.; et al. 188-OR: Circulating metabolites and type 2 diabetes [T2D] risk—An integrated metabolomics and genetics study of ~23,000 adults. Diabetes 2023, 72 (Suppl. S1), 188-OR. [Google Scholar] [CrossRef]
- Shahisavandi, M.; Wang, K.; Ghanbari, M.; Ahmadizar, F. Exploring Metabolomic Patterns in Type 2 Diabetes Mellitus and Response to Glucose-Lowering Medications—Review. Genes 2023, 14, 1464. [Google Scholar] [CrossRef]
- Chaiyachat, P.; Kaewseekhao, B.; Chaiprasert, A.; Kamolwat, P.; Nonghanphithak, D.; Phetcharaburanin, J.; Sirichoat, A.; Ong, R.T.-H.; Faksri, K. Metabolomic analysis of Mycobacterium tuberculosis reveals metabolic profiles for identification of drug-resistant tuberculosis. Sci. Rep. 2023, 13, 8655. [Google Scholar] [CrossRef]
- Escobar, M.Q.; Pontes, J.G.d.M.; Tasic, L. Metabolomics in degenerative brain diseases. Brain Res. 2021, 1773, 147704. [Google Scholar] [CrossRef]
- Pang, H.; Hu, Z. Metabolomics in drug research and development: The recent advances in technologies and applications. Acta Pharm. Sin. B 2023, 13, 3238–3251. [Google Scholar] [CrossRef]
- Qiu, S.; Cai, Y.; Yao, H.; Lin, C.; Xie, Y.; Tang, S.; Zhang, A. Small molecule metabolites: Discovery of biomarkers and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 132. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, X.; Huang, Q.; Dou, Y.; Qu, C.; Xu, Q.; Yuan, Q.; Xian, Y.-F.; Lin, Z.-X. Quercetin enhances survival and axonal regeneration of motoneurons after spinal root avulsion and reimplantation: Experiments in a rat model of brachial plexus avulsion. Inflamm. Regen. 2022, 42, 56. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Y.; Al Mamun, A.; Yuan, Y.; Lu, Q.; Xiong, J.; Yang, S.; Wu, C.; Wu, Y.; Wang, J. Acute spinal cord injury: Pathophysiology and pharmacological intervention. Mol. Med. Rep. 2021, 23, 417. [Google Scholar] [CrossRef] [PubMed]
- Yip, P.K.; Malaspina, A. Spinal cord trauma and the molecular point of no return. Mol. Neurodegener. 2012, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Yuan, M.-Z.; Sun, T.-Y.; Xie, Y.-Y.; Liu, L.-L.; Tang, Y.; Ling, Z.-M.; Li, Y.-Q.; Yu, G.-Y.; Zhou, L.H. Identification of the Avulsion-Injured Spinal Motoneurons. J. Mol. Neurosci. 2015, 57, 142–151. [Google Scholar] [CrossRef][Green Version]
- Wang, L.-L.; Zhao, X.-C.; Yan, L.-F.; Wang, Y.-Q.; Cheng, X.; Fu, R.; Zhou, L.-H. C-jun phosphorylation contributes to down regulation of neuronal nitric oxide synthase protein and motoneurons death in injured spinal cords following root-avulsion of the brachial plexus. Neuroscience 2011, 189, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Heneghan, C.; Goldacre, B.; Mahtani, K.R. Why clinical trial outcomes fail to translate into benefits for patients. Trials 2017, 18, 122. [Google Scholar] [CrossRef] [PubMed]
- Guest, J.; Datta, N.; Jimsheleishvili, G.; Gater, D.R. Pathophysiology, Classification and Comorbidities after Traumatic Spinal Cord Injury. J. Pers. Med. 2022, 12, 1126. [Google Scholar] [CrossRef] [PubMed]
- Dietz, V.A.; Roberts, N.; Knox, K.; Moore, S.; Pitonak, M.; Barr, C.; Centeno, J.; Leininger, S.; New, K.C.; Nowell, P.; et al. Fighting for recovery on multiple fronts: The past, present, and future of clinical trials for spinal cord injury. Front. Cell. Neurosci. 2022, 16, 977679. [Google Scholar] [CrossRef]
- Emwas, A.-H. Theory and Applications of NMR-Based Metabolomics in Human Disease Diagnosis; Bentham Science Publishers: Sharjah, United Arab Emirates, 2015; pp. 93–130. [Google Scholar]
- Gonzalez-Covarrubias, V.; Martínez-Martínez, E.; del Bosque-Plata, L. The Potential of Metabolomics in Biomedical Applications. Metabolites 2022, 12, 194. [Google Scholar] [CrossRef]
- Cho, K.; Mahieu, N.G.; Johnson, S.L.; Patti, G.J. After the Feature Presentation: Technologies Bridging Untargeted Metabolomics and Biology. Curr. Opin. Biotechnol. 2014, 28, 143–148. [Google Scholar] [CrossRef]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and Open Synthesis. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef]
- Pang, Y.; Liu, X.; Zhao, C.; Shi, X.; Zhang, J.; Zhou, T.; Xiong, H.; Gao, X.; Zhao, X.; Yang, X.; et al. LC–MS/MS-based arachidonic acid metabolomics in acute spinal cord in’jury reveals the upregulation of 5-LOX and COX-2 products. Free Radic. Biol. Med. 2022, 193, 363–372. [Google Scholar] [CrossRef]
- Domenichiello, A.F.; Sapio, M.R.; Loydpierson, A.J.; Maric, D.; Goto, T.; Horowitz, M.S.; Keyes, G.S.; Yuan, Z.-X.; Majchrzak-Hong, S.F.; Mannes, A.J.; et al. Molecular Pathways Linking Oxylipins to Nociception in Rats. J. Pain 2021, 22, 275–299. [Google Scholar] [CrossRef]
- Rodgers, H.M.; Patton, R.; Yow, J.; Zeczycki, T.N.; Kew, K.; Clemens, S.; Brewer, K.L. Morphine resistance in spinal cord injury-related neuropathic pain in rats is associated with alterations in dopamine and dopamine-related metabolomics. J. Pain 2022, 23, 772–783. [Google Scholar] [CrossRef]
- Pukale, D.D.; Adkins-Travis, K.; Aryal, S.R.; Shriver, L.P.; Patti, G.J.; Leipzig, N.D. Investigating post-traumatic syringomyelia and local fluid osmoregulation via a rat model. Fluids Barriers CNS 2024, 21, 19. [Google Scholar] [CrossRef] [PubMed]
- Ni, S.; Yang, B.; Xia, L.; Zhang, H.; Xia, Y. EZH2 Mediates miR-146a-5p/HIF-1α to Alleviate Inflammation and Glycolysis after Acute Spinal Cord Injury. Mediat. Inflamm. 2021, 2021, 5591582. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, J.; Ying, X.; Hu, R.; Huang, Y.; Wang, R.; Wu, L.; Han, D.; Ma, R.; He, K. Metagenomic and metabolomic analysis of gut microbiome’s role in spinal cord injury recovery in rats. Biomol. Biomed. 2025; Online ahead of print. [Google Scholar] [CrossRef]
- Liang, W.; Zhang, T.; Zhang, M.; Gao, J.; Huang, R.; Huang, X.; Chen, J.; Cheng, L.; Zhang, L.; Huang, Z.; et al. Daphnetin Ameliorates Neuropathic Pain via Regulation of Microglial Responses and Glycerophospholipid Metabolism in the Spinal Cord. Pharmaceuticals 2024, 17, 789. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Zhao, X.; Zhang, Y.; Jiao, Y.; Liu, Y.; Gao, C.; Zhang, J.; Ma, Y.; Wang, Z.; Li, T. Using Integrated Network Pharmacology and Metabolomics to Reveal the Mechanisms of the Combined Intervention of Ligustrazine and Sinomenine in CCI-Induced Neuropathic Pain Rats. Int. J. Mol. Sci. 2025, 26, 2604. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Zeng, Z.; He, H.; Li, M.; Lan, Y.; Hui, J.; Bie, P.; Chen, Y.; Liu, H.; Fan, H.; et al. Lycium Barbarum Glycopeptide alleviates neuroinflammation in spinal cord injury via modulating docosahexaenoic acid to inhibiting MAPKs/NF-kB and pyroptosis pathways. J. Transl. Med. 2023, 21, 770. [Google Scholar] [CrossRef]
- Zeng, Z.; Li, M.; Jiang, Z.; Lan, Y.; Chen, L.; Chen, Y.; Li, H.; Hui, J.; Zhang, L.; Hu, X.; et al. Integrated Transcriptomic and Metabolomic Profiling Reveals Dysregulation of Purine Metabolism During the Acute Phase of Spinal Cord Injury in Rats. Front. Neurosci. 2022, 16, 1066528. [Google Scholar] [CrossRef]
- Liu, Y.-H.; Gong, Q.; Wang, Y.-K.; Shuang, W.-B. Time-Dependent Changes in the Bladder Muscle Metabolome After Traumatic Spinal Cord Injury in Rats Using Metabolomics. Int. Neurourol. J. 2023, 27, 88–98. [Google Scholar] [CrossRef]
- Zhang, B.; Yan, R.; Lu, J.; Ma, Z.; Zhang, X.; Li, L.; Gao, J.; Xia, L.; Zhang, K.; Zhang, F.; et al. Metabolic Changes in Serum in the Rat Model of Cauda Equina Injury. World Neurosurg. 2019, 130, e1051–e1060. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, P.; Xie, M.; Luo, J.; Zhang, J.; Zhang, G.; Wang, Y.; Lin, H.; Ji, Z. Parallel Metabolomic Profiling of Cerebrospinal Fluid, Plasma, and Spinal Cord to Identify Biomarkers for Spinal Cord Injury. J. Mol. Neurosci. 2022, 72, 126–135. [Google Scholar] [CrossRef]
- Huffman, E.E.; Dong, B.E.; Clarke, H.A.; Young, L.E.; Gentry, M.S.; Allison, D.B.; Sun, R.C.; Waters, C.M.; Alilain, W.J. Cervical spinal cord injury leads to injury and altered metabolism in the lungs. Brain Commun. 2023, 5, fcad091. [Google Scholar] [CrossRef]
- Wang, Z.; Xie, Z.; Zhang, Z.; Zhou, W.; Guo, B.; Li, M. Multi-platform omics sequencing dissects the atlas of plasma-derived exosomes in rats with or without depression-like behavior after traumatic spinal cord injury. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2024, 132, 110987. [Google Scholar] [CrossRef]
- Wang, C.; Wu, L.; Zhou, R.; Song, C.; Chen, P.; Huang, S.; Ali Khan, A.; Lu, D.; Hu, Y.; Chen, L. Integration of microbiota and metabolomics reveals the analgesic mechanisms of emodin against neuropathic pain. Int. Immunopharmacol. 2023, 125 Pt A, 111170. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Niu, Z.; Hu, X.; Liu, H.; Li, S.; Chen, L.; Zheng, D.; Liu, Z.; Liu, T.; Xu, F.; et al. Regional cerebral metabolic levels and turnover in awake rats after acute or chronic spinal cord injury. FASEB J. 2020, 34, 10547–10559. [Google Scholar] [CrossRef] [PubMed]
- Lyu, C.; Tang, Y.; Shang, F.-F. In situ mass spectrometry imaging reveals metabolite alterations in gray and white matter after spinal cord injury. Exp. Ther. Med. 2025, 29, 117. [Google Scholar] [CrossRef] [PubMed]
- Francos-Quijorna, I.; Santos-Nogueira, E.; Gronert, K.; Sullivan, A.B.; Kopp, M.A.; Brommer, B.; David, S.; Schwab, J.M.; López-Vales, R. Maresin 1 Promotes Inflammatory Resolution, Neuroprotection, and Functional Neurological Recovery After Spinal Cord Injury. J. Neurosci. 2017, 37, 11731–11743. [Google Scholar] [CrossRef]
- Chen, P.; Wang, C.; Ren, Y.-N.; Ye, Z.-J.; Jiang, C.; Wu, Z.-B. Alterations in the gut microbiota and metabolite profiles in the context of neuropathic pain. Mol. Brain 2021, 14, 50. [Google Scholar] [CrossRef]
- Shi, Y.; Zheng, M.; Luo, Y.; Li, J.; Ouyang, F.; Zhao, Y.; Wang, J.; Ma, Z.; Meng, C.; Bi, Y.; et al. Targeting transcription factor pu.1 for improving neurologic outcomes after spinal cord injury. Front. Neurosci. 2024, 18, 1418615. [Google Scholar] [CrossRef]
- Ohnishi, Y.; Yamamoto, M.; Sugiura, Y.; Setoyama, D.; Kishima, H. Rostro-caudal different energy metabolism leading to differences in degeneration in spinal cord injury. Brain Commun. 2021, 3, fcab058. [Google Scholar] [CrossRef]
- Shi, C.; Xu, J.; Ding, Y.; Chen, X.; Yuan, F.; Zhu, F.; Duan, C.; Hu, J.; Lu, H.; Wu, T.; et al. MCT1-mediated endothelial cell lactate shuttle as a target for promoting axon regeneration after spinal cord injury. Theranostics 2024, 14, 5662–5681. [Google Scholar] [CrossRef]
- Graham, Z.A.; Siedlik, J.A.; Harlow, L.; Sahbani, K.; Bauman, W.A.; Tawfeek, H.A.; Cardozo, C.P. Key Glycolytic Metabolites in Paralyzed Skeletal Muscle Are Altered Seven Days after Spinal Cord Injury in Mice. J. Neurotrauma 2019, 36, 2722–2731. [Google Scholar] [CrossRef]
- Kang, J.-N.; Sun, Z.-F.; Li, X.-Y.; Zhang, X.-D.; Jin, Z.-X.; Zhang, C.; Zhang, Y.; Wang, H.-Y.; Huang, N.-N.; Jiang, J.-H.; et al. Alterations in gut microbiota are related to metabolite profiles in spinal cord injury. Neural Regen. Res. 2023, 18, 1076–1083. [Google Scholar] [CrossRef]
- Zhang, C.; Shao, Q.; Zhang, Y.; Liu, W.; Kang, J.; Jin, Z.; Huang, N.; Ning, B. Therapeutic application of nicotinamide: As a potential target for inhibiting fibrotic scar formation following spinal cord injury. CNS Neurosci. Ther. 2024, 30, e14826. [Google Scholar] [CrossRef]
- Rong, Z.-J.; Cai, H.-H.; Wang, H.; Liu, G.-H.; Zhang, Z.-W.; Chen, M.; Huang, Y.-L. Ursolic Acid Ameliorates Spinal Cord Injury in Mice by Regulating Gut Microbiota and Metabolic Changes. Front. Cell. Neurosci. 2022, 16, 872935. [Google Scholar] [CrossRef] [PubMed]
- Scholpa, N.E.; Simmons, E.C.; Snider, J.M.; Barrett, K.; Buss, L.G.; Schnellmann, R.G. Evolution of Lipid Metabolism in the Injured Mouse Spinal Cord. J. Neurotrauma 2025, 42, 182–196. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Chen, J.; Tang, Y.; Wei, C.; Yu, P.; Ding, X.; Zhu, L.; Yao, J.; Ouyang, Z.; Qiao, J.; et al. Multi-omics uncovers immune-modulatory molecules in plasma contributing to resistance exercise-ameliorated locomotor disability after incomplete spinal cord injury. Genome Med. 2025, 17, 10. [Google Scholar] [CrossRef]
- Potter, L.A.; Toro, C.A.; Harlow, L.; Lavin, K.M.; Cardozo, C.P.; Wende, A.R.; Graham, Z.A. Assessing the impact of boldine on the gastrocnemius using multiomics profiling at 7 and 28 days post-complete spinal cord injury in young male mice. Physiol. Genom. 2023, 55, 297–313. [Google Scholar] [CrossRef] [PubMed]
- Graham, Z.A.; DeBerry, J.J.; Cardozo, C.P.; Bamman, M.M. SS-31 does not prevent or reduce muscle atrophy 7 days after a 65 kdyne contusion spinal cord injury in young male mice. Physiol. Rep. 2022, 10, e15266. [Google Scholar] [CrossRef]
- Zhao, Y.-Y.; Wu, Z.-J.; Hao, S.-J.; Dong, B.-B.; Zheng, Y.-X.; Liu, B.; Li, J. Common alterations in parallel metabolomic profiling of serum and spinal cord and mechanistic studies on neuropathic pain following PPARα administration. Neuropharmacology 2024, 254, 109988. [Google Scholar] [CrossRef]
- Jing, Y.; Yang, D.; Bai, F.; Wang, Q.; Zhang, C.; Yan, Y.; Li, Z.; Li, Y.; Chen, Z.; Li, J.; et al. Spinal cord injury-induced gut dysbiosis influences neurological recovery partly through short-chain fatty acids. NPJ Biofilms Microbio. 2023, 9, 99. [Google Scholar] [CrossRef]
- Singh, A.; Srivastava, R.N.; Agrahari, A.; Singh, S.; Raj, S.; Chatterji, T.; Mahdi, A.A.; Garg, R.K.; Roy, R. Proton NMR based serum metabolic profile correlates with the neurological recovery in treated acute spinal cord injury [ASCI] subjects: A pilot study. Clin. Chim. Acta 2018, 480, 150–160. [Google Scholar] [CrossRef]
- Yarar-Fisher, C.; Kulkarni, A.; Li, J.; Farley, P.; Renfro, C.; Aslam, H.; Bosarge, P.; Wilson, L.; Barnes, S. Evaluation of a ketogenic diet for improvement of neurological recovery in individuals with acute spinal cord injury: A pilot, randomized safety and feasibility trial. Spinal Cord Ser. Cases 2018, 4, 88. [Google Scholar] [CrossRef]
- Singh, A.; Srivastava, R.N.; Chatterji, T.; Singh, S.; Raj, L.; Mahdi, A.A.; Garg, R.K.; Roy, R. 1H NMR urine metabolomics is an effective prognostic indicator in acute spinal cord injury [ASCI]: A prospective case-control study. J. Metabolom. Syst. Biol. 2020, 4, 1–21. [Google Scholar]
- Bykowski, E.A.; Petersson, J.N.; Dukelow, S.; Ho, C.; Debert, C.T.; Montina, T.; Metz, G.A. Urinary biomarkers indicative of recovery from spinal cord injury: A pilot study. IBRO Neurosci. Rep. 2021, 10, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Morrow, C.; Barnes, S.; Wilson, L.; Womack, E.D.; McLain, A.; Yarar-Fisher, C. Gut Microbiome Composition and Serum Metabolome Profile Among Individuals With Spinal Cord Injury and Normal Glucose Tolerance or Prediabetes/Type 2 Diabetes. Arch. Phys. Med. Rehabil. 2022, 103, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Bykowski, E.A.; Petersson, J.N.; Dukelow, S.; Ho, C.; Debert, C.T.; Montina, T.; Metz, G.A.S. Identification of Serum Metabolites as Prognostic Biomarkers Following Spinal Cord Injury: A Pilot Study. Metabolites 2023, 13, 605. [Google Scholar] [CrossRef]
- Kong, G.; Zhang, W.; Zhang, S.; Chen, J.; He, K.; Zhang, C.; Yuan, X.; Xie, B. The gut microbiota and metabolite profiles are altered in patients with spinal cord injury. Mol. Brain 2023, 16, 26. [Google Scholar] [CrossRef]
- Zhang, D.; Peng, R.; Yang, D. A preliminary study on the changes of fecal short chain fatty acids in patients with traumatic spinal cord injury in the chronic phase. Spinal Cord Ser. Cases 2025, 11, 3. [Google Scholar] [CrossRef]
- David, A.; Rostkowski, P. Chapter 2—Analytical techniques in metabolomics. In Environmental Metabolomics; Álvarez-Muñoz, D., Farré, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 35–64. [Google Scholar] [CrossRef]
- Segers, K.; Declerck, S.; Mangelings, D.; Heyden, Y.V.; Eeckhaut, A.V. Analytical techniques for metabolomic studies: A review. Bioanalysis 2019, 11, 2297–2318. [Google Scholar] [CrossRef]
- Huang, K.; Thomas, N.; Gooley, P.R.; Armstrong, C.W. Systematic Review of NMR-Based Metabolomics Practices in Human Disease Research. Metabolites 2022, 12, 963. [Google Scholar] [CrossRef]
- Ivanisevic, J.; Want, E.J. From Samples to Insights into Metabolism: Uncovering Biologically Relevant Information in LC-HRMS Metabolomics Data. Metabolites 2019, 9, 308. [Google Scholar] [CrossRef]
- Danzi, F.; Pacchiana, R.; Mafficini, A.; Scupoli, M.T.; Scarpa, A.; Donadelli, M.; Fiore, A. To metabolomics and beyond: A technological portfolio to investigate cancer metabolism. Signal Transduct. Target. Ther. 2023, 8, 137. [Google Scholar] [CrossRef]
- Khalikova, M.; Jireš, J.; Horáček, O.; Douša, M.; Kučera, R.; Nováková, L. What is the role of current mass spectrometry in pharmaceutical analysis? Mass Spectrom. Rev. 2024, 43, 560–609. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.; Eticha, T.; Hymete, A.; Ashenef, A. Principles and Applications of Ultra-High-Performance Liquid Chromatography. IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Gao, Z.; Zhong, W. Recent [2018–2020] development in capillary electrophoresis. Anal. Bioanal. Chem. 2022, 414, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Doerr, A. Global metabolomics. Nat. Methods 2017, 14, 32. [Google Scholar] [CrossRef]
- Blackburn, P.R.; Gass, J.M.; e Vairo, F.P.; Farnham, K.M.; Atwal, H.K.; Macklin, S.; Klee, E.W.; Atwal, P.S. Maple syrup urine disease: Mechanisms and management. Appl. Clin. Genet. 2017, 10, 57–66. [Google Scholar] [CrossRef]
- Chou, F.-J.; Liu, Y.; Lang, F.; Yang, C. D-2-Hydroxyglutarate in glioma biology. Cells 2021, 10, 2345. [Google Scholar] [CrossRef]
- Wishart, D. Emerging applications of metabolomics in drug discovery and precision medicine. Nat. Rev. Drug Discov. 2016, 15, 473–484. [Google Scholar] [CrossRef]
- Jacob, M.; Lopata, A.L.; Dasouki, M.; Rahman, A.M.A. Metabolomics toward personalized medicine. Mass Spectrom. Rev. 2019, 38, 221–238. [Google Scholar] [CrossRef]
- Demirel, A.; Li, J.; Morrow, C.; Barnes, S.; Jansen, J.; Gower, B.; Kirksey, K.; Redden, D.; Yarar-Fisher, C. Evaluation of a ketogenic diet for improvement of neurological recovery in individuals with acute spinal cord injury: Study protocol for a randomized controlled trial. Trials 2020, 21, 372. [Google Scholar] [CrossRef]
- Wu, Y.; Streijger, F.; Wang, Y.; Lin, G.; Christie, S.; Mac-Thiong, J.-M.; Parent, S.; Bailey, C.S.; Paquette, S.; Boyd, M.C.; et al. Parallel Metabolomic Profiling of Cerebrospinal Fluid and Serum for Identifying Biomarkers of Injury Severity after Acute Human Spinal Cord Injury. Sci. Rep. 2016, 6, 38718. [Google Scholar] [CrossRef]
- Astarita, G.; Kelly, R.S.; Lasky-Su, J. Metabolomics and lipidomics strategies in modern drug discovery and development. Drug Discov. Today 2023, 28, 103751. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pan, S.; Yin, L.; Liu, J.; Tong, J.; Wang, Z.; Zhao, J.; Liu, X.; Chen, Y.; Miao, J.; Zhou, Y.; et al. Metabolomics-driven approaches for identifying therapeutic targets in drug discovery. Medcomm 2024, 5, e792. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhang, T.; Liu, Q.; Sutcharitchan, C.; Zhou, Z.; Zhang, D.; Li, S. Elucidating the role of artificial intelligence in drug development from the perspective of drug-target interactions. J. Pharm. Anal. 2025, 15, 101144. [Google Scholar] [CrossRef]
- Bellerba, F.; Chatziioannou, A.C.; Jasbi, P.; Robinot, N.; Keski-Rahkonen, P.; Trolat, A.; Vozar, B.; Hartman, S.J.; Scalbert, A.; Bonanni, B.; et al. Metabolomic profiles of metformin in breast cancer survivors: A pooled analysis of plasmas from two randomized placebo-controlled trials. J. Transl. Med. 2022, 20, 629. [Google Scholar] [CrossRef] [PubMed]
- Pinu, F.R.; Beale, D.J.; Paten, A.M.; Kouremenos, K.; Swarup, S.; Schirra, H.J.; Wishart, D. Systems Biology and Multi-Omics Integration: Viewpoints from the Metabolomics Research Community. Metabolites 2019, 9, 76. [Google Scholar] [CrossRef]
- Olivier, M.; Asmis, R.; Hawkins, G.A.; Howard, T.D.; Cox, L.A. The need for multi-omics biomarker signatures in precision medicine. Int. J. Mol. Sci. 2019, 20, 4781. [Google Scholar] [CrossRef]
- Gowda, G.A.N.; Raftery, D. NMR Metabolomics Methods for Investigating Disease. Anal. Chem. 2023, 95, 83–99. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hsieh, W.-C.; Budiarto, B.R.; Wang, Y.-F.; Lin, C.-Y.; Gwo, M.-C.; So, D.K.; Tzeng, Y.-S.; Chen, S.-Y. Spatial multi-omics analyses of the tumor immune microenvironment. J. Biomed. Sci. 2022, 29, 96. [Google Scholar] [CrossRef]
- Peng, R.; Zhang, L.; Xie, Y.; Guo, S.; Cao, X.; Yang, M. Spatial multi-omics analysis of the microenvironment in traumatic spinal cord injury: A narrative review. Front. Immunol. 2024, 15, 1432841. [Google Scholar] [CrossRef]
- Wishart, D.S.; Cheng, L.L.; Copié, V.; Edison, A.S.; Eghbalnia, H.R.; Hoch, J.C.; Gouveia, G.J.; Pathmasiri, W.; Powers, R.; Schock, T.B.; et al. NMR and Metabolomics-A Roadmap for the Future. Metabolites 2022, 12, 678. [Google Scholar] [CrossRef]
- Plekhova, V.; De Windt, K.; De Spiegeleer, M.; De Graeve, M.; Vanhaecke, L. Recent advances in high-throughput biofluid metabotyping by direct infusion and ambient ionization mass spectrometry. TrAC Trends Anal. Chem. 2023, 168, 117163. [Google Scholar] [CrossRef]
- Zaitsu, K.; Hayashi, Y.; Murata, T.; Yokota, K.; Ohara, T.; Kusano, M.; Tsuchihashi, H.; Ishikawa, T.; Ishii, A.; Ogata, K.; et al. In Vivo Real-Time Monitoring System Using Probe Electrospray Ionization/Tandem Mass Spectrometry for Metabolites in Mouse Brain. Anal. Chem. 2018, 90, 4695–4701. [Google Scholar] [CrossRef]
- Lombard-Banek, C.; Li, J.; Portero, E.P.; Onjiko, R.M.; Singer, C.D.; Plotnick, D.O.; Al Shabeeb, R.Q.; Nemes, P. In Vivo Subcellular Mass Spectrometry Enables Proteo-Metabolomic Single-Cell Systems Biology in a Chordate Embryo Developing to a Normally Behaving Tadpole [X. laevis]*. Angew. Chem. Int. Ed. Engl. 2021, 60, 12852–12858. [Google Scholar] [CrossRef]
- Richter, J.K.; Vallesi, V.; Zölch, N.; Chan, K.L.; Hunkeler, N.; Abramovic, M.; Hashagen, C.; Christiaanse, E.; Shetty, G.; Verma, R.K.; et al. Metabolic profile of complete spinal cord injury in pons and cerebellum: A 3T 1H MRS study. Sci. Rep. 2023, 13, 7245. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).