Longitudinal Dysregulation of Adiponectin and Leptin Following Blast-Induced Polytrauma in a Rat Model
Abstract
1. Introduction
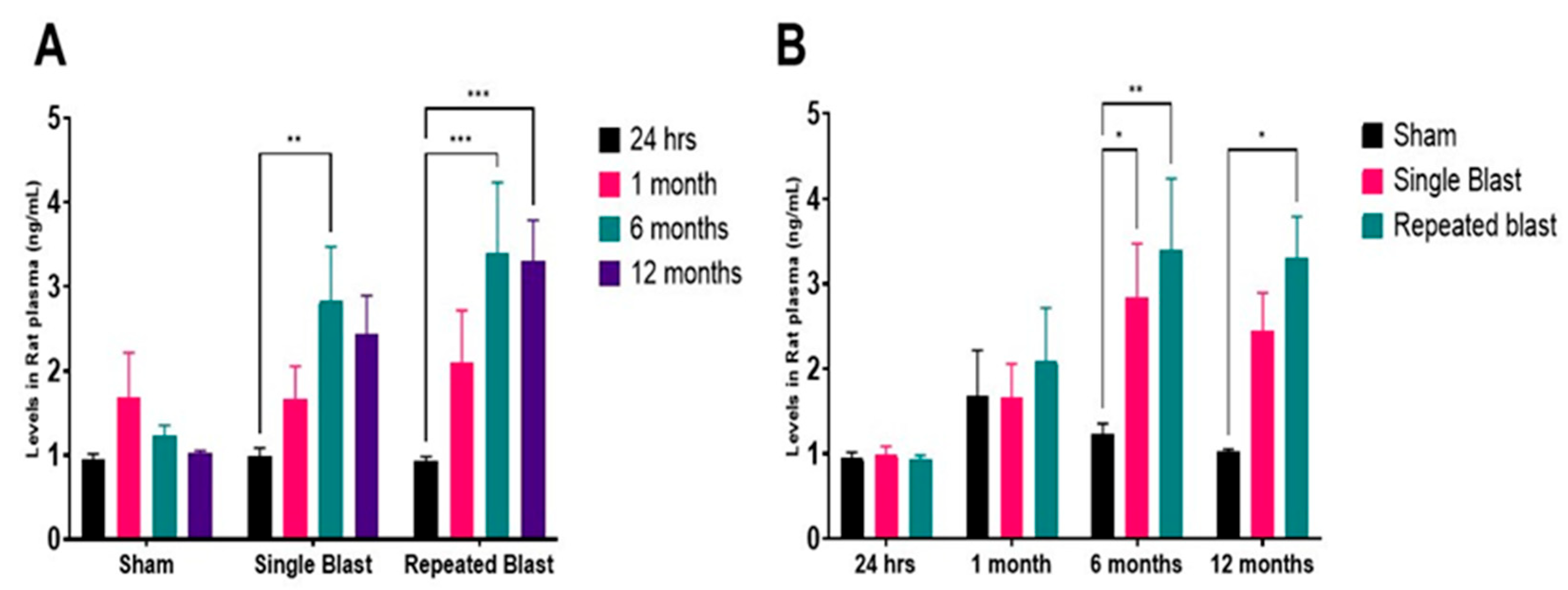
2. Results
3. Discussion
4. Conclusions
5. Methods
5.1. Animal Use and Ethical Approval
5.2. Animal Cohorts, Housing, and Diet Regulation
5.3. Experimental Grouping and Randomization
5.4. Blast Exposure
5.5. Blood Collection and Plasma Preparation
5.6. ELISA for Adipokine Quantification
5.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimer
References
- Sachdeva, T.; Ganpule, S.G. Twenty Years of Blast-Induced Neurotrauma: Current State of Knowledge. Neurotrauma Rep. 2024, 5, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wu, Y. Stress Disorder After Blast Injury. In Explosive Blast Injuries: Principles and Practices; Wang, Z., Jiang, J., Eds.; Springer Nature: Singapore, 2023; pp. 281–292. ISBN 978-981-19285-6-7. [Google Scholar]
- Chan, A.; Ouyang, J.; Nguyen, K.; Jones, A.; Basso, S.; Karasik, R. Traumatic Brain Injuries: A Neuropsychological Review. Front. Behav. Neurosci. 2024, 18, 1326115. [Google Scholar] [CrossRef] [PubMed]
- Kursancew, A.C.S.; Faller, C.J.; Piva-Uchida, E.M.; Benedet, I.B.; Maciel, P.M.; de Figueredo, S.M.; Petronilho, F.; Ceretta, L.B.; Streck, E.; Generoso, J.S. Metabolic Disorders after Traumatic Brain Injury: A Narrative Review of Systemic Consequences. Metab. Brain Dis. 2025, 40, 93. [Google Scholar] [CrossRef] [PubMed]
- Boleti, A.P.d.A.; Cardoso, P.H.d.O.; Frihling, B.E.F.; Silva, P.S.E.; de Moraes, L.F.R.N.; Migliolo, L. Adipose Tissue, Systematic Inflammation, and Neurodegenerative Diseases. Neural Regen. Res. 2023, 18, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Huber, K.; Szerenos, E.; Lewandowski, D.; Toczylowski, K.; Sulik, A. The Role of Adipokines in the Pathologies of the Central Nervous System. Int. J. Mol. Sci. 2023, 24, 14684. [Google Scholar] [CrossRef] [PubMed]
- Casado, M.E.; Collado-Pérez, R.; Frago, L.M.; Barrios, V. Recent Advances in the Knowledge of the Mechanisms of Leptin Physiology and Actions in Neurological and Metabolic Pathologies. Int. J. Mol. Sci. 2023, 24, 1422. [Google Scholar] [CrossRef] [PubMed]
- Baldelli, S.; Aiello, G.; Mansilla Di Martino, E.; Campaci, D.; Muthanna, F.M.S.; Lombardo, M. The Role of Adipose Tissue and Nutrition in the Regulation of Adiponectin. Nutrients 2024, 16, 2436. [Google Scholar] [CrossRef] [PubMed]
- Arora, P.; Sharma, A.; Trivedi, R.; Sharma, P.; Padhy, S.; Shah, S.; Dutta, S.K.; Manda, K.; Rana, P. Lipidomic Analysis Reveals Systemic Alterations in Servicemen Exposed to Repeated Occupational Low-Level Blast Waves. Mil. Med. 2025, 190, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Knoff, A.A.; Nowak, M.K.; Van Etten, E.J.; Andreu-Arasa, V.C.; Esterman, M.; Leritz, E.C.; Fortenbaugh, F.C.; Milberg, W.P.; Fortier, C.B.; Salat, D.H. Metabolic Syndrome Is Associated with Reduced Default Mode Network Functional Connectivity in Young Post-9/11 Veterans. Brain Imaging Behav. 2024, 18, 1499–1508. [Google Scholar] [CrossRef] [PubMed]
- Wrba, L.; Halbgebauer, R.; Roos, J.; Huber-Lang, M.; Fischer-Posovszky, P. Adipose Tissue: A Neglected Organ in the Response to Severe Trauma? Cell. Mol. Life Sci. 2022, 79, 207. [Google Scholar] [CrossRef] [PubMed]
- Gilani, A.; Stoll, L.; Homan, E.A.; Lo, J.C. Adipose Signals Regulating Distal Organ Health and Disease. Diabetes 2024, 73, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Khodashahi, R.; Tavakkoli, M.; Ferns, G.A.; Feyzmohammadi, L.; Mirzaei, A.H.; Aliakbarian, M.; Arjmand, M.-H. Adipose Tissue Dysfunction Following Trauma and Hypoxia Increases the Risk of Post-Surgical Adhesion: Potential for Therapeutic Interventions. Curr. Mol. Pharmacol. 2024, 17, e18761429308567. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Pinilla, F.; Myers, S.K. Traumatic Brain Injury from a Peripheral Axis Perspective: Uncovering the Roles of Liver and Adipose Tissue in Temperature Regulation. Prog. Neurobiol. 2025, 247, 102733. [Google Scholar] [CrossRef] [PubMed]
- Cree, M.G.; Wolfe, R.R. Postburn Trauma Insulin Resistance and Fat Metabolism. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E1–E9. [Google Scholar] [CrossRef] [PubMed]
- Lendvai, D.; Whittemore, R.; Womack, J.A.; Fortier, C.B.; Milberg, W.P.; Fonda, J.R. The Impact of Blast Exposure—With or without Traumatic Brain Injury—On Metabolic Abnormalities in Post-9/11 Veterans. J. Head Trauma Rehabil. 2023, 38, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Rosenberger, P.H.; Ning, Y.; Brandt, C.; Allore, H.; Haskell, S. BMI Trajectory Groups in Veterans of the Iraq and Afghanistan Wars. Prev. Med. 2011, 53, 149–154. [Google Scholar] [CrossRef] [PubMed]
- VandeVord, P.J.; Sajja, V.S.S.S.; Ereifej, E.; Hermundstad, A.; Mao, S.; Hadden, T.J. Chronic Hormonal Imbalance and Adipose Redistribution Is Associated with Hypothalamic Neuropathology Following Blast Exposure. J. Neurotrauma 2016, 33, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, L.E.; Fisher, A.M.; Tagge, C.A.; Zhang, X.-L.; Velisek, L.; Sullivan, J.A.; Upreti, C.; Kracht, J.M.; Ericsson, M.; Wojnarowicz, M.W.; et al. Chronic Traumatic Encephalopathy in Blast-Exposed Military Veterans and a Blast Neurotrauma Mouse Model. Sci. Transl. Med. 2012, 4, 134ra60. [Google Scholar] [CrossRef] [PubMed]
- Arun, P.; Wilder, D.M.; Eken, O.; Urioste, R.; Batuure, A.; Sajja, S.; Van Albert, S.; Wang, Y.; Gist, I.D.; Long, J.B. Long-Term Effects of Blast Exposure: A Functional Study in Rats Using an Advanced Blast Simulator. J. Neurotrauma 2020, 37, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Miura, J.; Lu, L.X.; Bernier, M.; DeCabo, R.; Lane, M.A.; Roth, G.S.; Ingram, D.K. Circulating Adiponectin Levels Increase in Rats on Caloric Restriction: The Potential for Insulin Sensitization. Exp. Gerontol. 2004, 39, 1049–1059. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, S.; Wada, K.; Nawashiro, H.; Uozumi, Y.; Otani, N.; Nagatani, K.; Kobayashi, H.; Shima, K. Decrease in Plasma Adiponectin Level and Increase in Adiponectin Immunoreactivity in Cortex and Hippocampus after Traumatic Brain Injury in Rats. Turk. Neurosurg. 2013, 23, 349–354. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yatomi, K.; Miyamoto, N.; Komine-Kobayashi, M.; Liu, M.; Oishi, H.; Arai, H.; Hattori, N.; Urabe, T. Pathophysiological Dual Action of Adiponectin after Transient Focal Ischemia in Mouse Brain. Brain Res. 2009, 1297, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Ilhan, N.; Susam, S.; Canpolat, O.; Belhan, O. The Emerging Role of Leptin, Adiponectin and Visfatin in Ischemic/Hemorrhagic Stroke. Br. J. Neurosurg. 2019, 33, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Ciancarelli, I.; Morone, G.; Iosa, M.; Paolucci, S.; Pignolo, L.; Tonin, P.; Cerasa, A.; Ciancarelli, M.G.T. Adipokines as Potential Biomarkers in the Neurorehabilitation of Obese Stroke Patients. Curr. Neurovasc. Res. 2020, 17, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Mulder, G.J.; Brouwer, S.; Weitering, J.G.; Scholtens, E.; Pang, K.S. Glucuronidation and Sulfation in the Rat in Vivo. The Role of the Liver and the Intestine in the in Vivo Clearance of 4-Methylumbelliferone. Biochem. Pharmacol. 1985, 34, 1325–1329. [Google Scholar] [CrossRef] [PubMed]
- Carbone, G.; Bencivenga, L.; Santoro, M.A.; De Lucia, N.; Palaia, M.E.; Ercolano, E.; Scognamiglio, F.; Edison, P.; Ferrara, N.; Vitale, D.F.; et al. Impact of Serum Leptin and Adiponectin Levels on Brain Infarcts in Patients with Mild Cognitive Impairment and Alzheimer’s Disease: A Longitudinal Analysis. Front. Endocrinol. 2024, 15, 1389014. [Google Scholar] [CrossRef] [PubMed]
- Khoramipour, K.; Chamari, K.; Hekmatikar, A.A.; Ziyaiyan, A.; Taherkhani, S.; Elguindy, N.M.; Bragazzi, N.L. Adiponectin: Structure, Physiological Functions, Role in Diseases, and Effects of Nutrition. Nutrients 2021, 13, 1180. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.-F.; Chen, Z.-H.; Huang, Q.; Dai, W.-M.; Jie, Y.-Q.; Yu, G.-F.; Wu, A.; Yan, X.-J.; Li, Y.-P. Leptin as a Marker for Severity and Prognosis of Aneurysmal Subarachnoid Hemorrhage. Peptides 2013, 48, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Huang, S.-J.; Wang, N.; Shen, Z.-P. Relationship between Plasma Leptin Levels and Clinical Outcomes of Pediatric Traumatic Brain Injury. Peptides 2012, 35, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Boeve, B.F.; Wijdicks, E.F.; Benarroch, E.E.; Schmidt, K.D. Paroxysmal Sympathetic Storms (“diencephalic Seizures”) after Severe Diffuse Axonal Head Injury. Mayo Clin. Proc. 1998, 73, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Mantzoros, C.S.; Frederich, R.C.; Flier, J.S.; Qu, D.; Susulic, V.S.; Lowell, B.B.; Maratos-Flier, E. Activation of Β3 Adrenergic Receptors Suppresses Leptin Expression and Mediates a Leptin-Independent Inhibition of Food Intake in Mice. Diabetes 1996, 45, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Jeevanandam, M.; Begay, C.K.; Petersen, S.R. Plasma Leptin Levels in Trauma Patients: Effect of Adjuvant Recombinant Human Growth Hormone in Intravenously Fed Multiple Trauma Patients. JPEN J. Parenter. Enter. Nutr. 1998, 22, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Keren, O.; Yupatov, S.; Radai, M.M.; Elad-Yarum, R.; Faraggi, D.; Abboud, S.; Ring, H.; Groswasser, Z. Heart Rate Variability (HRV) of Patients with Traumatic Brain Injury (TBI) during the Post-Insult Sub-Acute Period. Brain Inj. 2005, 19, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Trentz, O.A.; Handschin, A.E.; Bestmann, L.; Hoerstrup, S.P.; Trentz, O.L.; Platz, A. Influence of Brain Injury on Early Posttraumatic Bone Metabolism. Crit. Care Med. 2005, 33, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Toklu, H.Z.; Yang, Z.; Oktay, S.; Sakarya, Y.; Kirichenko, N.; Matheny, M.K.; Muller-Delp, J.; Strang, K.; Scarpace, P.J.; Wang, K.K.W.; et al. Overpressure Blast Injury-Induced Oxidative Stress and Neuroinflammation Response in Rat Frontal Cortex and Cerebellum. Behav. Brain Res. 2018, 340, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yuan, J.; Zhang, H.; Ding, H.; Tang, X.; Wei, Y. Effect of Leptin on Bone Metabolism in Rat Model of Traumatic Brain Injury and Femoral Fracture. Chin. J. Traumatol. (Engl. Ed.) 2011, 14, 7–13. [Google Scholar] [CrossRef]
- Liu, J.; Guo, M.; Zhang, D.; Cheng, S.-Y.; Liu, M.; Ding, J.; Scherer, P.E.; Liu, F.; Lu, X.-Y. Adiponectin Is Critical in Determining Susceptibility to Depressive Behaviors and Has Antidepressant-like Activity. Proc. Natl. Acad. Sci. USA 2012, 109, 12248–12253. [Google Scholar] [CrossRef] [PubMed]
- Elder, G.A.; Dorr, N.P.; Gasperi, R.D.; Sosa, M.A.G.; Shaughness, M.C.; Maudlin-Jeronimo, E.; Hall, A.A.; McCarron, R.M.; Ahlers, S.T. Blast Exposure Induces Post-Traumatic Stress Disorder-Related Traits in a Rat Model of Mild Traumatic Brain Injury. J. Neurotrauma 2012, 29, 2564. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, N.; Tseng, C.-E.J.; Maffei, C.; Tromly, S.L.; Deary, K.B.; McKinney, I.R.; Kelemen, J.N.; Healy, B.C.; Hu, C.G.; Ramos-Llordén, G.; et al. Impact of Repeated Blast Exposure on Active-Duty United States Special Operations Forces. Proc. Natl. Acad. Sci. USA 2024, 121, e2313568121. [Google Scholar] [CrossRef] [PubMed]
- Ravula, A.R.; Das, T.; Gosain, A.; Dolalas, T.; Padhi, S.; Chandra, N.; Pfister, B.J. An Update on Repeated Blast Traumatic Brain Injury. Curr. Opin. Biomed. Eng. 2022, 24, 100409. [Google Scholar] [CrossRef]
- Saeedi, M.; Rashidy-Pour, A. Association between Chronic Stress and Alzheimer’s Disease: Therapeutic Effects of Saffron. Biomed. Pharmacother. 2021, 133, 110995. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Oliveira, V.; Câmara, N.O.S.; Moraes-Vieira, P.M. Adipokines as Drug Targets in Diabetes and Underlying Disturbances. J. Diabetes Res. 2015, 2015, 681612. [Google Scholar] [CrossRef] [PubMed]
- Arjunan, A.; Song, J. Pharmacological and Physiological Roles of Adipokines and Myokines in Metabolic-Related Dementia. Biomed. Pharmacother. 2023, 163, 114847. [Google Scholar] [CrossRef] [PubMed]
- Zadgaonkar, U. The Interplay Between Adipokines and Body Composition in Obesity and Metabolic Diseases. Cureus 2025, 17, e78050. [Google Scholar] [CrossRef] [PubMed]
- National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DC, USA, 2011.

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thanapaul, R.J.R.S.; Govindarajulu, M.; Pundkar, C.; Phuyal, G.; Eken, O.; Long, J.B.; Arun, P. Longitudinal Dysregulation of Adiponectin and Leptin Following Blast-Induced Polytrauma in a Rat Model. Int. J. Mol. Sci. 2025, 26, 6860. https://doi.org/10.3390/ijms26146860
Thanapaul RJRS, Govindarajulu M, Pundkar C, Phuyal G, Eken O, Long JB, Arun P. Longitudinal Dysregulation of Adiponectin and Leptin Following Blast-Induced Polytrauma in a Rat Model. International Journal of Molecular Sciences. 2025; 26(14):6860. https://doi.org/10.3390/ijms26146860
Chicago/Turabian StyleThanapaul, Rex Jeya Rajkumar Samdavid, Manoj Govindarajulu, Chetan Pundkar, Gaurav Phuyal, Ondine Eken, Joseph B Long, and Peethambaran Arun. 2025. "Longitudinal Dysregulation of Adiponectin and Leptin Following Blast-Induced Polytrauma in a Rat Model" International Journal of Molecular Sciences 26, no. 14: 6860. https://doi.org/10.3390/ijms26146860
APA StyleThanapaul, R. J. R. S., Govindarajulu, M., Pundkar, C., Phuyal, G., Eken, O., Long, J. B., & Arun, P. (2025). Longitudinal Dysregulation of Adiponectin and Leptin Following Blast-Induced Polytrauma in a Rat Model. International Journal of Molecular Sciences, 26(14), 6860. https://doi.org/10.3390/ijms26146860





