Ceramide Synthase 2 Promotes Cardiac Very-Long-Chain Dihydroceramide Accumulation and Is Linked to Arrhythmias and Heart Failure in Humans
Abstract
1. Introduction
2. Results
2.1. Hypoxia Induces Marked Accumulation of VLC Dihydroceramides in HL-1 Cardiomyocytes
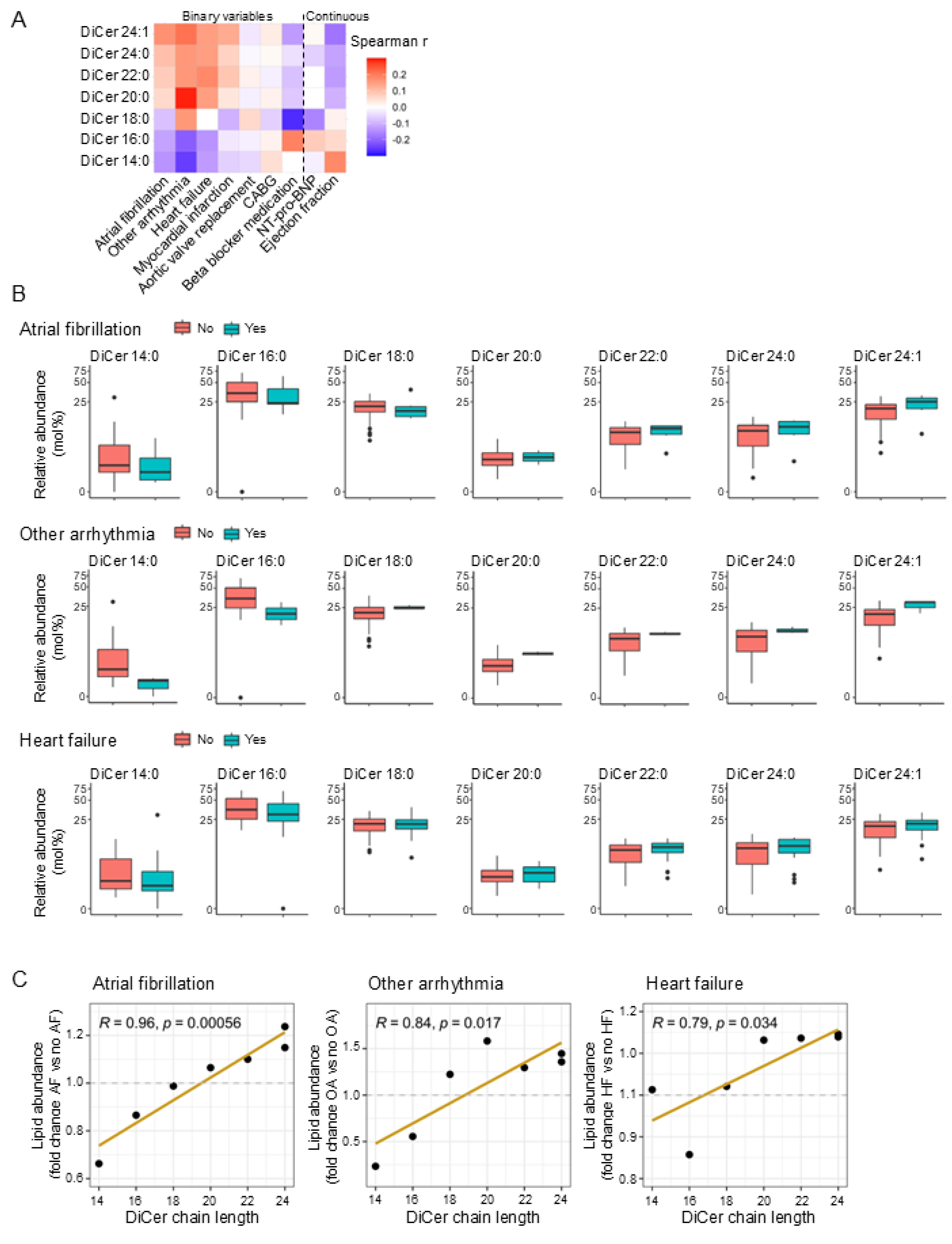
2.2. Dihydroceramide Chain Length Correlates with Cardiac Arrhythmias and Heart Failure in Humans
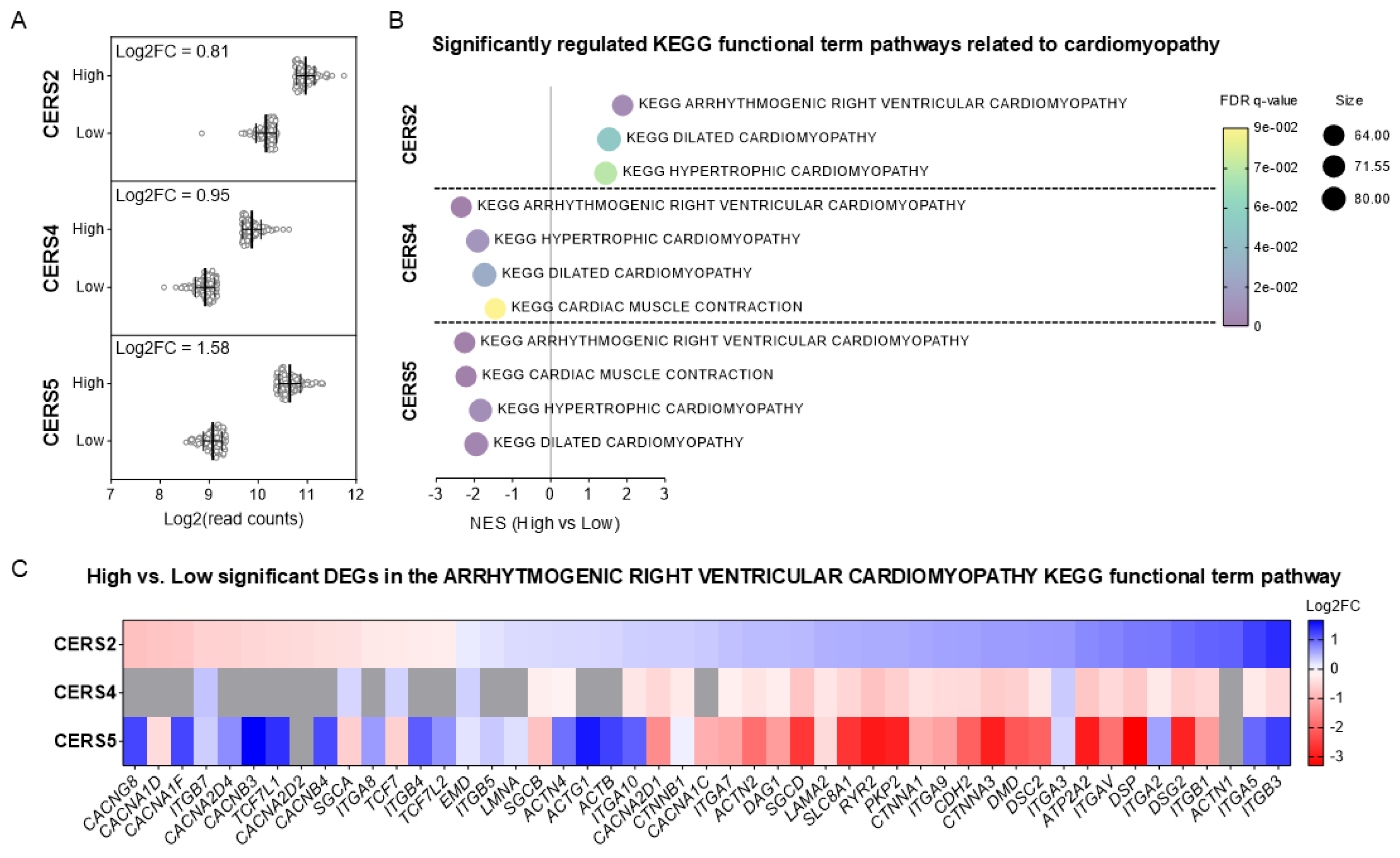
2.3. High CERS2 Expression Correlates with Functional Pathways for Arrhythmogenic Cardiomyopathy, Dilated Cardiomyopathy and Hypertrophic Cardiomyopathy in Humans
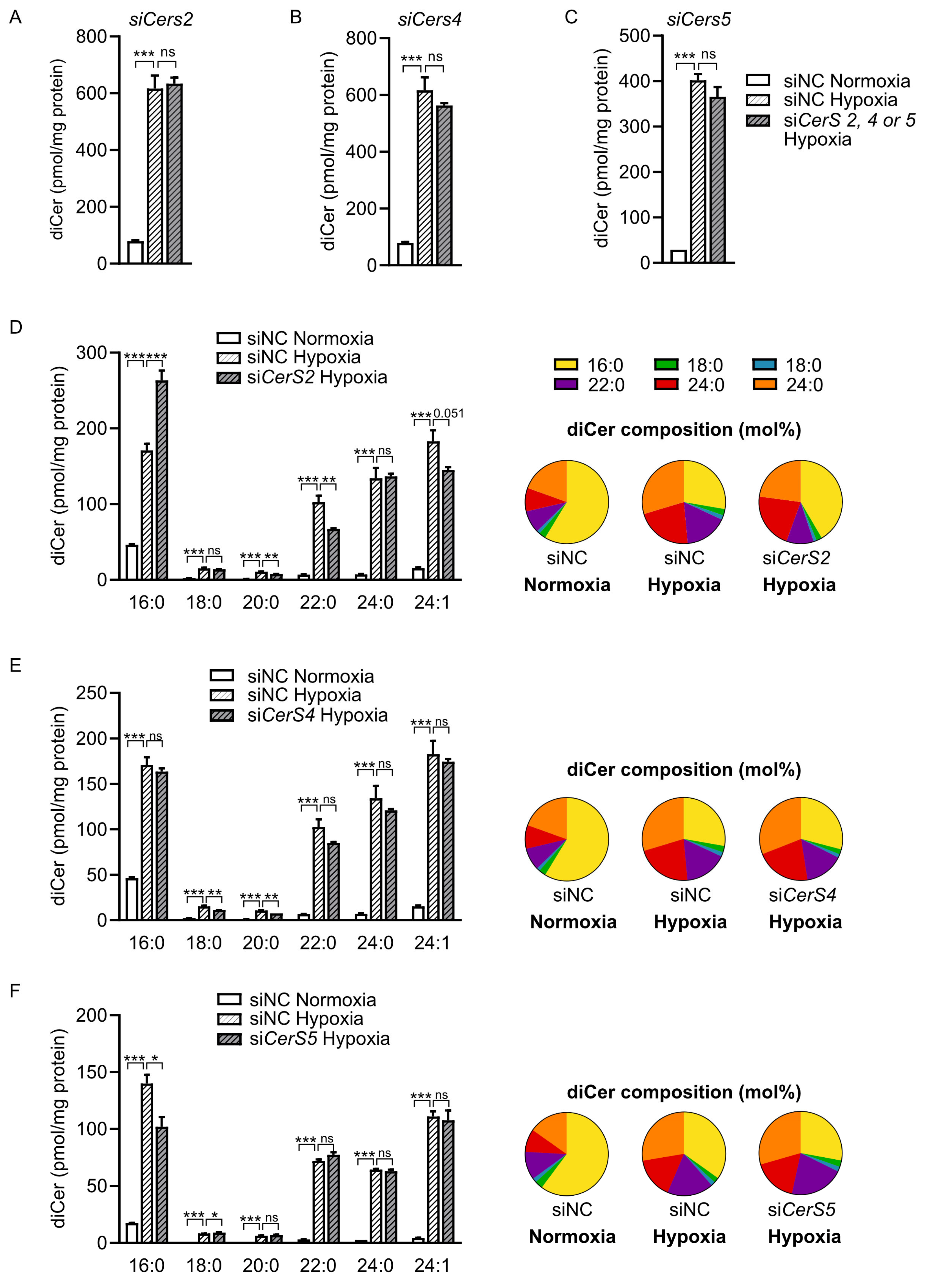
2.4. CerS2 Depletion Reduces Hypoxia-Induced Accumulation of VLC Dihydroceramides
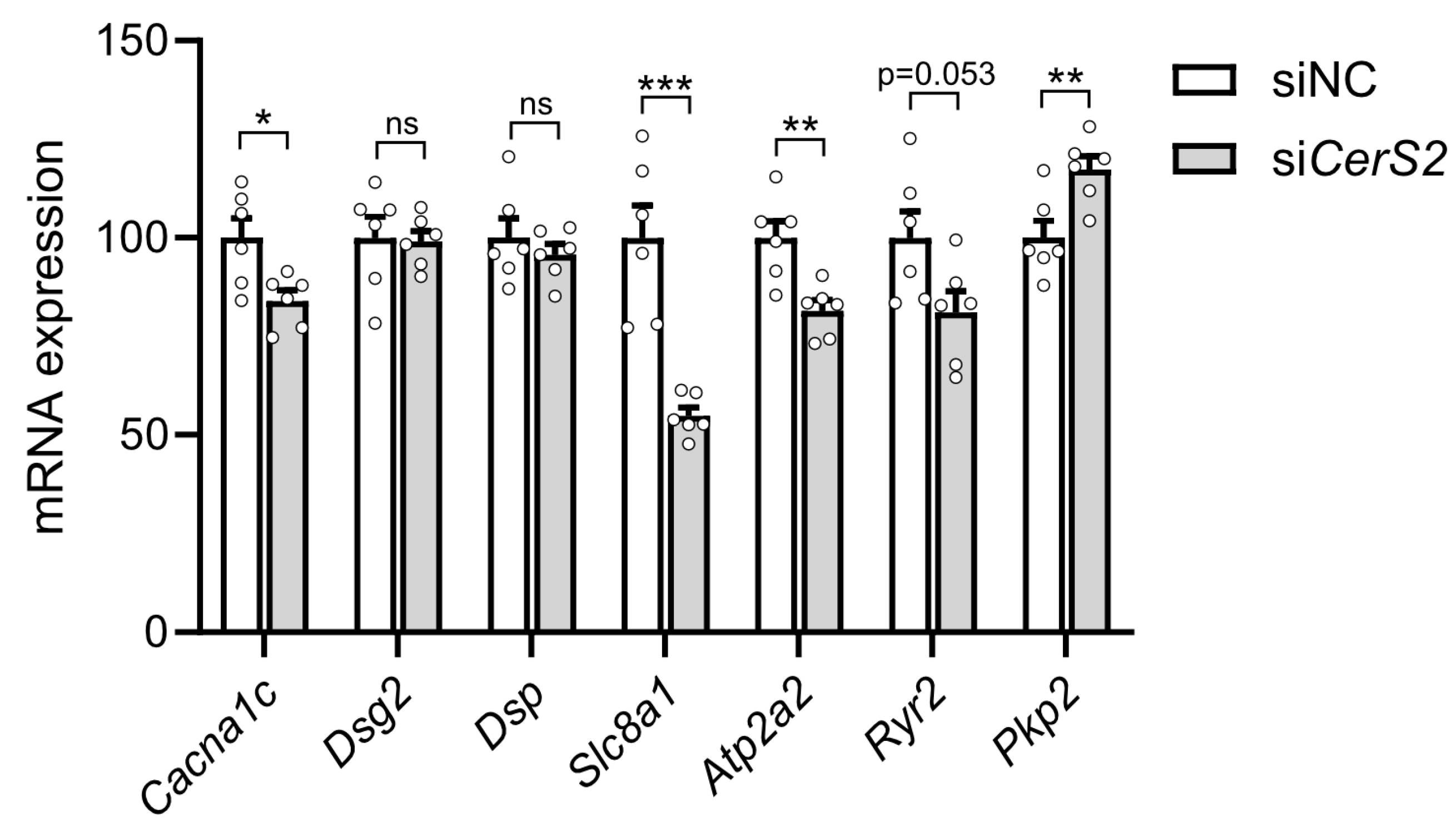
2.5. CerS2 Depletion Modulates Expression of Functional Markers in Cardiomyocytes
3. Discussion
4. Materials and Methods
4.1. Cell Culture Experiments
4.2. Human Myocardial Biopsies
4.3. Lipid Analysis
4.4. Analysis of Human RNA-Seq Data
4.5. Gene Expression Analysis
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| VLC | Very-long-chain |
| CerS | Ceramide synthase |
| DES | Dihydroceramide desaturase |
| LC | Long-chain |
| LVEF | Left ventricular ejection fraction |
| ARV | Aortic valve replacement |
| CABG | Coronary artery bypass graft |
| CPB | Cardiopulmonary bypass |
| AF | Atrial fibrillation |
| GSEA | Gene set enrichment analysis |
| FDR | False discovery rate |
| SEM | Standard error of the mean |
References
- Drevinge, C.; Karlsson, L.O.; Stahlman, M.; Larsson, T.; Perman Sundelin, J.; Grip, L.; Andersson, L.; Boren, J.; Levin, M.C. Cholesteryl esters accumulate in the heart in a porcine model of ischemia and reperfusion. PLoS ONE 2013, 8, e61942. [Google Scholar] [CrossRef] [PubMed]
- Perman, J.C.; Bostrom, P.; Lindbom, M.; Lidberg, U.; StAhlman, M.; Hagg, D.; Lindskog, H.; Scharin Tang, M.; Omerovic, E.; Mattsson Hulten, L.; et al. The VLDL receptor promotes lipotoxicity and increases mortality in mice following an acute myocardial infarction. J. Clin. Investig. 2011, 121, 2625–2640. [Google Scholar] [CrossRef] [PubMed]
- Klevstig, M.; Stahlman, M.; Lundqvist, A.; Scharin Tang, M.; Fogelstrand, P.; Adiels, M.; Andersson, L.; Kolesnick, R.; Jeppsson, A.; Boren, J.; et al. Targeting acid sphingomyelinase reduces cardiac ceramide accumulation in the post-ischemic heart. J. Mol. Cell. Cardiol. 2016, 93, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Bikman, B.T.; Summers, S.A. Ceramides as modulators of cellular and whole-body metabolism. J. Clin. Investig. 2011, 121, 4222–4230. [Google Scholar] [CrossRef] [PubMed]
- Summers, S.A.; Chaurasia, B.; Holland, W.L. Metabolic Messengers: Ceramides. Nat. Metab. 2019, 1, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Park, T.S.; Hu, Y.; Noh, H.L.; Drosatos, K.; Okajima, K.; Buchanan, J.; Tuinei, J.; Homma, S.; Jiang, X.C.; Abel, E.D.; et al. Ceramide is a cardiotoxin in lipotoxic cardiomyopathy. J. Lipid Res. 2008, 49, 2101–2112. [Google Scholar] [CrossRef] [PubMed]
- Russo, S.B.; Baicu, C.F.; Van Laer, A.; Geng, T.; Kasiganesan, H.; Zile, M.R.; Cowart, L.A. Ceramide synthase 5 mediates lipid-induced autophagy and hypertrophy in cardiomyocytes. J. Clin. Investig. 2012, 122, 3919–3930. [Google Scholar] [CrossRef] [PubMed]
- Nwabuo, C.C.; Duncan, M.; Xanthakis, V.; Peterson, L.R.; Mitchell, G.F.; McManus, D.; Cheng, S.; Vasan, R.S. Association of Circulating Ceramides With Cardiac Structure and Function in the Community: The Framingham Heart Study. J. Am. Heart Assoc. 2019, 8, e013050. [Google Scholar] [CrossRef] [PubMed]
- Peterson, L.R.; Xanthakis, V.; Duncan, M.S.; Gross, S.; Friedrich, N.; Volzke, H.; Felix, S.B.; Jiang, H.; Sidhu, R.; Nauck, M.; et al. Ceramide Remodeling and Risk of Cardiovascular Events and Mortality. J. Am. Heart Assoc. 2018, 7, e007931. [Google Scholar] [CrossRef] [PubMed]
- Breslow, D.K.; Weissman, J.S. Membranes in balance: Mechanisms of sphingolipid homeostasis. Mol. Cell 2010, 40, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Zelnik, I.D.; Rozman, B.; Rosenfeld-Gur, E.; Ben-Dor, S.; Futerman, A.H. A Stroll Down the CerS Lane. Adv. Exp. Med. Biol. 2019, 1159, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Geeraert, L.; Mannaerts, G.P.; van Veldhoven, P.P. Conversion of dihydroceramide into ceramide: Involvement of a desaturase. Biochem. J. 1997, 327 Pt 1, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Law, B.A.; Liao, X.; Moore, K.S.; Southard, A.; Roddy, P.; Ji, R.; Szulc, Z.; Bielawska, A.; Schulze, P.C.; Cowart, L.A. Lipotoxic very-long-chain ceramides cause mitochondrial dysfunction, oxidative stress, and cell death in cardiomyocytes. FASEB J. 2018, 32, 1403–1416. [Google Scholar] [CrossRef] [PubMed]
- Mullen, T.D.; Hannun, Y.A.; Obeid, L.M. Ceramide synthases at the centre of sphingolipid metabolism and biology. Biochem. J. 2012, 441, 789–802. [Google Scholar] [CrossRef] [PubMed]
- Pewzner-Jung, Y.; Ben-Dor, S.; Futerman, A.H. When do Lasses (longevity assurance genes) become CerS (ceramide synthases)?: Insights into the regulation of ceramide synthesis. J. Biol. Chem. 2006, 281, 25001–25005. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.; Futerman, A.H. Mammalian ceramide synthases. IUBMB Life 2010, 62, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Crowder, C.M. Cell biology. Ceramides--friend or foe in hypoxia? Science 2009, 324, 343–344. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lachkar, F.; Ferre, P.; Foufelle, F.; Papaioannou, A. Dihydroceramides: Their emerging physiological roles and functions in cancer and metabolic diseases. Am. J. Physiol. Endocrinol. Metab. 2021, 320, E122–E130. [Google Scholar] [CrossRef] [PubMed]
- Devlin, C.M.; Lahm, T.; Hubbard, W.C.; Van Demark, M.; Wang, K.C.; Wu, X.; Bielawska, A.; Obeid, L.M.; Ivan, M.; Petrache, I. Dihydroceramide-based response to hypoxia. J. Biol. Chem. 2011, 286, 38069–38078. [Google Scholar] [CrossRef] [PubMed]
- Plant, L.D.; Xiong, D.; Romero, J.; Dai, H.; Goldstein, S.A.N. Hypoxia Produces Pro-arrhythmic Late Sodium Current in Cardiac Myocytes by SUMOylation of Na(V)1.5 Channels. Cell Rep. 2020, 30, 2225–2236 e4. [Google Scholar] [CrossRef] [PubMed]
- Specterman, M.J.; Aziz, Q.; Li, Y.; Anderson, N.A.; Ojake, L.; Ng, K.E.; Thomas, A.M.; Finlay, M.C.; Schilling, R.J.; Lambiase, P.D.; et al. Hypoxia Promotes Atrial Tachyarrhythmias via Opening of ATP-Sensitive Potassium Channels. Circ. Arrhythm. Electrophysiol. 2023, 16, e011870. [Google Scholar] [CrossRef] [PubMed]
- Steels, E.L.; Learmonth, R.P.; Watson, K. Stress tolerance and membrane lipid unsaturation in Saccharomyces cerevisiae grown aerobically or anaerobically. Microbiology 1994, 140 Pt 3, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Jain, I.H.; Calvo, S.E.; Markhard, A.L.; Skinner, O.S.; To, T.L.; Ast, T.; Mootha, V.K. Genetic Screen for Cell Fitness in High or Low Oxygen Highlights Mitochondrial and Lipid Metabolism. Cell 2020, 181, 716–727 e11. [Google Scholar] [CrossRef] [PubMed]
- Menuz, V.; Howell, K.S.; Gentina, S.; Epstein, S.; Riezman, I.; Fornallaz-Mulhauser, M.; Hengartner, M.O.; Gomez, M.; Riezman, H.; Martinou, J.C. Protection of C. elegans from anoxia by HYL-2 ceramide synthase. Science 2009, 324, 381–384. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Rojo, N.; Sot, J.; Viguera, A.R.; Collado, M.I.; Torrecillas, A.; Gomez-Fernandez, J.C.; Goni, F.M.; Alonso, A. Membrane permeabilization induced by sphingosine: Effect of negatively charged lipids. Biophys. J. 2014, 106, 2577–2584. [Google Scholar] [CrossRef] [PubMed]
- Contreras, F.X.; Sot, J.; Alonso, A.; Goni, F.M. Sphingosine increases the permeability of model and cell membranes. Biophys. J. 2006, 90, 4085–4092. [Google Scholar] [CrossRef] [PubMed]
- Levitan, I.; Fang, Y.; Rosenhouse-Dantsker, A.; Romanenko, V. Cholesterol and ion channels. Subcell. Biochem. 2010, 51, 509–549. [Google Scholar] [CrossRef] [PubMed]
- Leifert, W.R.; Jahangiri, A.; McMurchie, E.J. Membrane fluidity changes are associated with the antiarrhythmic effects of docosahexaenoic acid in adult rat cardiomyocytes. J. Nutr. Biochem. 2000, 11, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Sassa, T.; Hirayama, T.; Kihara, A. Enzyme Activities of the Ceramide Synthases CERS2-6 Are Regulated by Phosphorylation in the C-terminal Region. J. Biol. Chem. 2016, 291, 7477–7487. [Google Scholar] [CrossRef] [PubMed]
- Laviad, E.L.; Kelly, S.; Merrill, A.H., Jr.; Futerman, A.H. Modulation of ceramide synthase activity via dimerization. J. Biol. Chem. 2012, 287, 21025–21033. [Google Scholar] [CrossRef] [PubMed]
- Ohno, Y.; Suto, S.; Yamanaka, M.; Mizutani, Y.; Mitsutake, S.; Igarashi, Y.; Sassa, T.; Kihara, A. ELOVL1 production of C24 acyl-CoAs is linked to C24 sphingolipid synthesis. Proc. Natl. Acad. Sci. USA 2010, 107, 18439–18444. [Google Scholar] [CrossRef] [PubMed]
- Kretzschmar, T.; Bekhite, M.M.; Wu, J.M.F.; Haase, D.; Forster, M.; Muller, T.; Nietzsche, S.; Westermann, M.; Franz, M.; Graler, M.H.; et al. Long-Chain and Very Long-Chain Ceramides Mediate Doxorubicin-Induced Toxicity and Fibrosis. Int. J. Mol. Sci. 2021, 22, 11852. [Google Scholar] [CrossRef] [PubMed]
- Bers, D.M.; Despa, S. Cardiac myocytes Ca2+ and Na+ regulation in normal and failing hearts. J. Pharmacol. Sci. 2006, 100, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, J.; Qin, Y.; Wang, J.; Zhou, L. Mutations in voltage-gated L-type calcium channel: Implications in cardiac arrhythmia. Channels 2018, 12, 201–218. [Google Scholar] [CrossRef] [PubMed]
- Kistamas, K.; Veress, R.; Horvath, B.; Banyasz, T.; Nanasi, P.P.; Eisner, D.A. Calcium Handling Defects and Cardiac Arrhythmia Syndromes. Front. Pharmacol. 2020, 11, 72. [Google Scholar] [CrossRef] [PubMed]
- Scranton, K.; John, S.; Angelini, M.; Steccanella, F.; Umar, S.; Zhang, R.; Goldhaber, J.I.; Olcese, R.; Ottolia, M. Cardiac function is regulated by the sodium-dependent inhibition of the sodium-calcium exchanger NCX1. Nat. Commun. 2024, 15, 3831. [Google Scholar] [CrossRef] [PubMed]
- Chua, C.J.; Morrissette-McAlmon, J.; Tung, L.; Boheler, K.R. Understanding Arrhythmogenic Cardiomyopathy: Advances through the Use of Human Pluripotent Stem Cell Models. Genes 2023, 14, 1864. [Google Scholar] [CrossRef] [PubMed]
- Claycomb, W.C.; Lanson, N.A., Jr.; Stallworth, B.S.; Egeland, D.B.; Delcarpio, J.B.; Bahinski, A.; Izzo, N.J., Jr. HL-1 cells: A cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc. Natl. Acad. Sci. USA 1998, 95, 2979–2984. [Google Scholar] [CrossRef] [PubMed]
- Lofgren, L.; Forsberg, G.B.; Stahlman, M. The BUME method: A new rapid and simple chloroform-free method for total lipid extraction of animal tissue. Sci. Rep. 2016, 6, 27688. [Google Scholar] [CrossRef] [PubMed]
- Amrutkar, M.; Cansby, E.; Nunez-Duran, E.; Pirazzi, C.; Stahlman, M.; Stenfeldt, E.; Smith, U.; Boren, J.; Mahlapuu, M. Protein kinase STK25 regulates hepatic lipid partitioning and progression of liver steatosis and NASH. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2015, 29, 1564–1576. [Google Scholar] [CrossRef] [PubMed]
- Stahlman, M.; Fagerberg, B.; Adiels, M.; Ekroos, K.; Chapman, J.M.; Kontush, A.; Boren, J. Dyslipidemia, but not hyperglycemia and insulin resistance, is associated with marked alterations in the HDL lipidome in type 2 diabetic subjects in the DIWA cohort: Impact on small HDL particles. Biochim. Et Biophys. Acta 2013, 1831, 1609–1617. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andersson, L.; Cinato, M.; Björnson, E.; Lundqvist, A.; Miljanovic, A.; Henricsson, M.; Bergh, P.-O.; Adiels, M.; Jeppsson, A.; Borén, J.; et al. Ceramide Synthase 2 Promotes Cardiac Very-Long-Chain Dihydroceramide Accumulation and Is Linked to Arrhythmias and Heart Failure in Humans. Int. J. Mol. Sci. 2025, 26, 6859. https://doi.org/10.3390/ijms26146859
Andersson L, Cinato M, Björnson E, Lundqvist A, Miljanovic A, Henricsson M, Bergh P-O, Adiels M, Jeppsson A, Borén J, et al. Ceramide Synthase 2 Promotes Cardiac Very-Long-Chain Dihydroceramide Accumulation and Is Linked to Arrhythmias and Heart Failure in Humans. International Journal of Molecular Sciences. 2025; 26(14):6859. https://doi.org/10.3390/ijms26146859
Chicago/Turabian StyleAndersson, Linda, Mathieu Cinato, Elias Björnson, Annika Lundqvist, Azra Miljanovic, Marcus Henricsson, Per-Olof Bergh, Martin Adiels, Anders Jeppsson, Jan Borén, and et al. 2025. "Ceramide Synthase 2 Promotes Cardiac Very-Long-Chain Dihydroceramide Accumulation and Is Linked to Arrhythmias and Heart Failure in Humans" International Journal of Molecular Sciences 26, no. 14: 6859. https://doi.org/10.3390/ijms26146859
APA StyleAndersson, L., Cinato, M., Björnson, E., Lundqvist, A., Miljanovic, A., Henricsson, M., Bergh, P.-O., Adiels, M., Jeppsson, A., Borén, J., & Levin, M. C. (2025). Ceramide Synthase 2 Promotes Cardiac Very-Long-Chain Dihydroceramide Accumulation and Is Linked to Arrhythmias and Heart Failure in Humans. International Journal of Molecular Sciences, 26(14), 6859. https://doi.org/10.3390/ijms26146859






