Abstract
Hypoxic pulmonary vasoconstriction (HPV) optimizes gas exchange but, when impaired, can result in life-threatening hypoxemia. Moreover, under conditions of generalized alveolar hypoxia, HPV can result in pulmonary hypertension. Voltage-gated K+ channels (Kv channels) are key to HPV: a change in the intracellular hydrogen peroxide (H2O2) levels during acute hypoxia is assumed to modulate these channels’ activity to trigger HPV. However, there are longstanding conflicting findings on whether H2O2 inhibits or activates Kv channels. Therefore, we hypothesized that H2O2 affects Kv channels depending on the experimental conditions, i.e., the H2O2 concentration, the channel’s subunit configuration or the experimental clamping potential in electrophysiological recordings. Therefore, cRNAs encoding the Kv1.5 channel and the auxiliary Kvβ subunits (Kvβ1.1, Kvβ1.4) were generated via in vitro transcription before being injected into Xenopus laevis oocytes for heterologous expression. The K+ currents of homomeric (Kv1.5) or heteromeric (Kv1.5/Kvβ1.1 or Kv1.5/Kvβ1.4) channels were assessed by two-electrode voltage clamp. The response of the Kv channels to H2O2 was markedly dependent on (a) the clamping potential, (b) the H2O2 concentration, and (c) the Kv channel’s subunit composition. In conclusion, our data highlight the importance of the choice of experimental conditions when assessing the H2O2 sensitivity of Kv channels in the context of HPV, thus providing an explanation for the long-lasting controversial findings reported in the literature.
1. Introduction
Hypoxic pulmonary vasoconstriction (HPV) is a physiological mechanism of the mammalian lung that optimizes gas exchange. By rapidly matching perfusion to ventilation, HPV ensures optimal oxygenation of the blood during local alveolar O2 shortage, i.e., hypoxia [1,2,3]. Impairment of this vital mechanism can result in insufficient blood oxygenation and thus O2 undersupply to the organs. Such fatal hypoxemia due to disturbed HPV can occur in various clinical scenarios. This includes patients afflicted with pneumonia, those with acute respiratory distress syndrome (ARDS), or those requiring mechanical ventilation, e.g., COVID-19 patients during the recent pandemic [4]. Additionally, systemic administration of pulmonary vasodilatory agents, such as prostacyclin, has been associated with similar adverse outcomes due to impaired HPV [2,5]. Moreover, imbalanced HPV is considered to provoke high-altitude pulmonary edema (HAPE), a life-threatening condition [5,6,7], and also acquires pathophysiological significance in situations with prolonged hypoxia, e.g., at high altitudes or in lung diseases such as sleep apnea or chronic obstructive pulmonary disease (COPD) [8,9,10]. In these conditions, generalized and consistent alveolar hypoxia can occur, leading to the constriction of the blood vessels in the entire lung. If hypoxia persists, the subsequent onset of vascular remodeling processes leads—in addition to vasoconstriction—to the manifestation of pulmonary hypertension, which can ultimately result in right heart failure.
Although HPV was already described in 1946 by von Euler and Liljestrand, the underlying molecular mechanisms are not yet fully known [3,11]. A mandatory step in HPV is the hypoxia-induced inhibition of O2-sensitive voltage-gated potassium (Kv) channels in pulmonary artery smooth muscle cells (PASMCs) [12,13,14,15,16]. Kv channels are composed of four α subunits forming a central pore in the membrane and four auxiliary β subunits to fine-tune the channel’s activity [13,17]. By mediating constant K+ leakage, Kv channels significantly contribute to maintaining the cellular membrane potential [16,18,19]. It is undisputed that hypoxia induces the closure of Kv channels, which in turn depolarizes the membrane potential of PASMCs, thereby increasing the Ca2+ influx via the activation of voltage-gated Ca2+ channels [2,20,21]. The resulting increase in intracellular Ca2+ induces the constriction of the individual cells and thus the pulmonary arterial vessels. Although much progress has been made in understanding the signaling pathways underlying O2 sensing in HPV, the molecular mechanisms by which hypoxia affects the Kv channel activity are still not fully resolved [13].
A proposed signaling mechanism linking hypoxia to the Kv channel function in PASMCs involves reactive oxygen species (ROS) and hydrogen peroxide (H2O2) in particular [3,13,22,23,24]. Although there is a consensus on the involvement of ROS in hypoxia-induced Kv channel inhibition, there is a long-lasting disagreement as to whether hypoxia induces an increase or decrease in ROS [2,13]. The original redox hypothesis of Archer, Weir, and colleagues proposes that under normoxic conditions, a certain level of intracellular ROS, i.e., H2O2, maintains the Kv channels in an oxidized open state. Conversely, acute hypoxia causes an increase in the NADH/NAD+ ratio and a concomitant decrease in the H2O2 level that shifts the cellular redox state to a more reduced state, resulting in Kv channel inhibition and subsequent vasoconstriction [12,17,25,26,27]. In contrast, Paul Schumacker’s lab presented an opposing concept, often referred to as the mitochondrial ROS theory, which describes a hypoxia-induced increase in the intracellular ROS levels being causative for the inhibition of Kv channels [13,28]. This theory was not only confirmed by previous studies by our research group, but we could also identify the signaling molecule responsible as H2O2 [3] and further decipher the role of mitochondria in this regard [3,22]. Although there is a large body of evidence for increased ROS levels during acute hypoxia underlying HPV, both theories emphasize that the Kv channels are inhibited by the indisputable hypoxia-induced change in the intracellular H2O2 levels [3,13].
Consequently, several studies investigated the influence of H2O2 on Kv channel activity. In line with the discrepancies between the two distinct theories, contrary results were also reported by distinct research groups in this context. While some studies showed an activation of the Kv channels in response to H2O2 [29,30,31], other groups observed an H2O2-induced inhibition of these channels [3,32,33,34]. Upon thorough examination of these reports, discrepancies in the experimental conditions became apparent, specifically the widely varying and occasionally nonphysiologically high concentrations of H2O2 utilized. Furthermore, the studies were conducted at distinct experimental clamping potentials and on very different cell types, including various primary cells as well as overexpression systems that may be featured by different subunit compositions of Kv channels. In particular, the auxiliary Kvβ subunits are differentially expressed in the pulmonary circulation [35]. The reported correlation between their expression level and the strength of HPV suggests a role for these subunits in O2 sensitivity [35]. Thus, the presence or absence of Kvβ subunits could be responsible for the different responses of Kv channels to H2O2, thereby delivering a potential explanation for the contrary results described in the literature.
Considering the controversy as to whether H2O2 inhibits or activates Kv channels, we hypothesized that H2O2 regulates Kv channel activity (a) in a concentration-dependent manner and/or (b) in a manner dependent on the molecular composition of the channel, i.e., the presence or absence of auxiliary Kvβ subunits.
2. Results
2.1. Homomeric Kvβ Subunits Do Not Form Functional Kv Channels
To address these questions, we selected the Kv1.5 channel as a suitable candidate. Kv1.5 has long been recognized as a channel involved in HPV [36,37]. In line with this, mice lacking Kv1.5 exhibited impaired HPV [38], which could be fully restored by its subsequent introduction via gene transfer in vivo [39]. To address the H2O2 sensitivity of Kv1.5, this subunit was heterologously expressed either as a homomeric or heteromeric channel, i.e., in combination with either Kvβ1.1 or Kvβ1.4 subunits in Xenopus laevis oocytes, which are supposed to not express endogenous Kv channels [40,41]. The potassium (K+) currents were assessed via the two-electrode voltage clamp technique (TEVC) by applying depolarizing 200 ms voltage pulses from a holding potential of −60 mV to +50 mV in 10 mV steps. Neither water-injected control oocytes (Figure 1a) nor oocytes injected with the auxiliary subunits Kvβ1.1 (Figure 1b) or Kvβ1.4 (Figure 1c) alone exhibited voltage-dependent currents when depolarized. In contrast, oocytes injected with cRNA-encoding Kv1.5 subunits showed typical rapid outward K+ currents upon depolarization to potentials more positive than −20 mV (Figure 1d). In agreement with previous studies, the homomeric Kv1.5 currents exhibited no detectable inactivation in response to such short pressure pulses [42]. Co-expression with Kvβ1.1 altered the Kv1.5 activity by inducing a rapid voltage-dependent inactivation (Figure 1e), while co-expression with Kvβ1.4 lowered the currents’ amplitude (Figure 1f). The final application of 4-aminopyridine (4-AP) largely inhibited the K+ currents (Figure 1d–f), which confirmed the successful expression of the three Kv channel combinations.
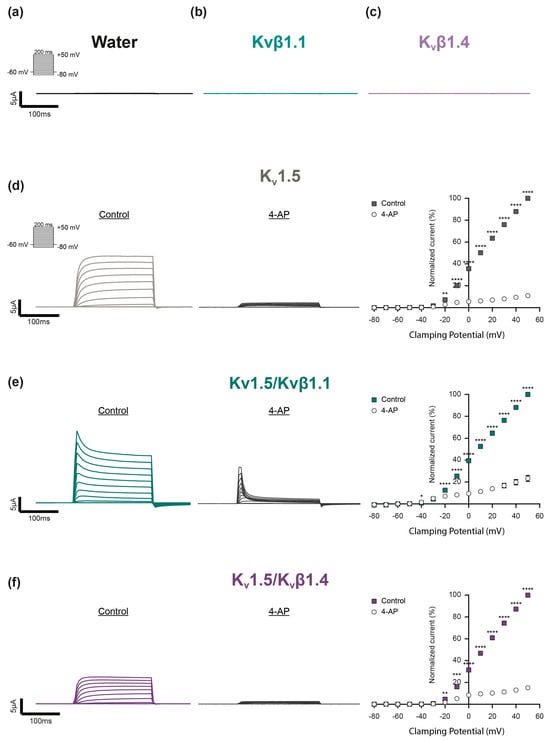
Figure 1.
Expression and electrophysiological characterization of distinct Kv channel combinations in Xenopus laevis oocytes. (a–c) Representative current traces of control oocytes exposed to depolarizing voltage steps. Neither (a) water (black) nor (b) homomeric Kvβ1.1 (light turquois) or (c) Kvβ1.4 subunits (light purple) built functional channels. (d–f) Electrophysiological characterization of (d) homomeric Kv1.5 channels (gray), (e) Kv1.5 co-expressed with the auxiliary subunits Kvβ1.1 (Kv1.5/Kvβ1.1, dark turquoise) or (f) together with Kvβ1.4 (Kv1.5/Kvβ1.4, dark purple). Current traces were elicited by depolarizing voltage pulses (200 ms) between −80 mV and +50 mV in 10 mV steps from a holding potential of −60 mV before (Control) and after application of the Kv channel inhibitor 4-aminopyridine (4-AP) to validate the successful Kv channel expression. The current–voltage relationship (I–V curve) was determined by plotting the averaged current amplitude (plateau) against the corresponding voltage step (Kv1.5: n = 13; Kv1.5/Kvβ1.1: n = 12; Kv1.5/Kvβ1.4: n = 13). The mean Kv current amplitudes were each normalized relatively to the current value at + 50 mV under control conditions (no 4-AP). Data were statistically analyzed by 2-way ANOVA and the uncorrected Fisher’s least significant difference test for multiple comparisons (*: p ≤ 0.05; **: p ≤ 0.01, ***: p ≤ 0.001; ****: p ≤ 0.0001).
2.2. Evaluation of H2O2 Decomposition in the Experimental Setup
Beside the temperature and the presence of decomposing impurities, the stability of H2O2 in aqueous solution is affected by the pH value [43]. For optimum stability, the pH of pure H2O2 is below pH 4.5, while increasing it to above pH 5 sharply increases the decomposition of H2O2 into water and oxygen. Since the pH value of the oocyte Ringer’s solution (ORi) used in our experiments was at a physiological value of pH 7.4, we first tested the decomposition of H2O2 in our experimental setup via an Amplex Red Hydrogen Peroxide/Peroxidase Assay. As schematically shown in Figure 2a, the ORi in the reservoir was analyzed directly after adding H2O2 to the perfusate (input). Afterwards, the perfusion was started to deliver the H2O2-containing ORi to the recording chamber. The second sample was then taken from the recording chamber 90 s post starting the perfusion—which corresponds to the time point at which the K+ currents were recorded in the electrophysiological experiments. We observed that at the time point of current recording, approximately 50% of the H2O2 of the distinct input concentrations was decomposed (Figure 2b).
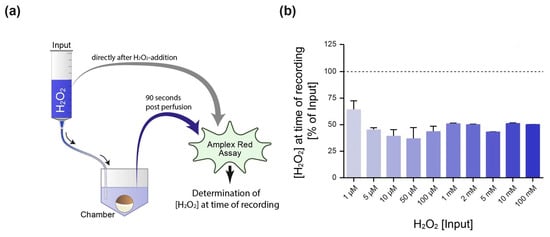
Figure 2.
Determination of the H2O2 concentration present at the time point of electrophysiological recording. (a) Schematical illustration of the experimental setup. Oocyte Ringer’s solution (ORi) was supplemented with H2O2 within the perfusion reservoir (input). One sample was taken directly after H2O2 addition (input), while the second sample was extracted from the recording chamber 90 s post starting the perfusion—which represents the time point at which the electrophysiological recording was performed. Both samples were subjected to an Amplex Red Hydrogen Peroxide/Peroxidase Assay to determine the H2O2 decomposition for distinct concentrations within the experimental setup, i.e., the H2O2 concentration at the time of recording (b). The dotted line represents the input concentration (100%).
2.3. Experimental Approach to Evaluate the Effect of H2O2 on Kv Channel Activity
For evaluation of the effect of H2O2 on Kv channel activity, Kv currents were elicited by depolarizing voltage pulses between −80 mV and +50 mV in 10 mV steps from a holding potential of −60 mV in the absence (Control, Figure 3a) and after the addition of H2O2 (Figure 3b). As shown in Figure 3c, H2O2 (10 mM) significantly activated K+ currents at all the clamping potentials of −30 mV and more positive—with a prominent effect at −20 mV, a value within the physiological range of the membrane potential of PASMCs under hypoxia [3,23]. Therefore, two distinct voltage steps were chosen for statistical analysis in all the following experiments: the one that lies within the physiological range of the membrane potential of PASMCs (−20 mV) and one at a positive membrane potential, which was characterized by the strongest H2O2-induced activation (+50 mV).
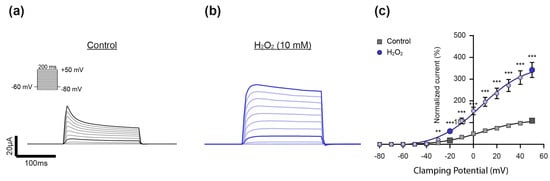
Figure 3.
Experimental approach to evaluate the effect of H2O2 on Kv channel activity. (a) Representative current trace of a Kv-channel-expressing oocyte that was exposed to depolarizing voltage steps in the (a) absence (Control, gray) and (b) presence of H2O2 (10 mM, blue), as well as (c) the corresponding statistical analysis. The current–voltage relationship (I–V curve) was determined by plotting the averaged current amplitude (plateau) against the corresponding voltage step (control: n = 12; H2O2: n = 11). The I–V curve was fitted using the Boltzmann equation. No differences in the V50 (Control: 7.1 ± 1.6 mV, H2O2; 6.1 ± 1.4 mV; p = 0.6523) and gating charge (q) (control: 1.4 ± 0.038 e0, H2O2: 1.4 ± 0.028 e0; p = 0.4252) were observed in response to H2O2. The mean Kv current amplitudes were each normalized relatively to the current amplitude in the control condition at +50 mV. H2O2 increased the channel’s activity at clamping potentials more positive than −30 mV, with a prominent effect at −20 mV, a value within the physiological range of the membrane potential of PASMCs. Therefore, clamping potentials of −20 mV and +50 mV (characterized by the strongest H2O2-induced activation) were chosen as the clamping potentials for analysis in all the following experiments (respective current traces and data points are highlighted in darker colors). Data were statistically analyzed by 2-way ANOVA and uncorrected Fisher’s least significant difference test for multiple comparisons (**: p ≤ 0.01, ***: p ≤ 0.001).
2.4. The Effect of H2O2 on Homomeric Kv1.5 Channels Is Voltage- and Concentration-Dependent
To assess the effect of distinct H2O2 concentrations on the activity of homomeric Kv1.5 channels, H2O2 was applied in concentrations between 0.01 µM and 100 mM (Figure 4a,b), using a new oocyte and freshly prepared H2O2 for each individual experiment. As previously described, the K+ current amplitudes were analyzed at step potentials of −20 mV (Figure 4a) and +50 mV (Figure 4b) to determine the potential voltage-dependency of the H2O2 effect. In our investigation, we observed dependencies on both voltage and concentration: while low H2O2 concentrations (ranging from 0.1 µM to 1 mM) did not affect the activity of Kv1.5 channels at a physiological step potential of −20 mV (Figure 4a), these same concentrations were found to inhibit the channels at a positive step potential (+50 mV, Figure 4b). Interestingly, higher H2O2 concentrations (10 mM and above) were observed to enhance the K+ currents at −20 mV (Figure 4a), yet no such effect was noted for the same concentrations at +50 mV (Figure 4b). These findings underscore a dual relationship involving both the voltage- and concentration-dependency of H2O2 modulation on Kv1.5 channel activity.
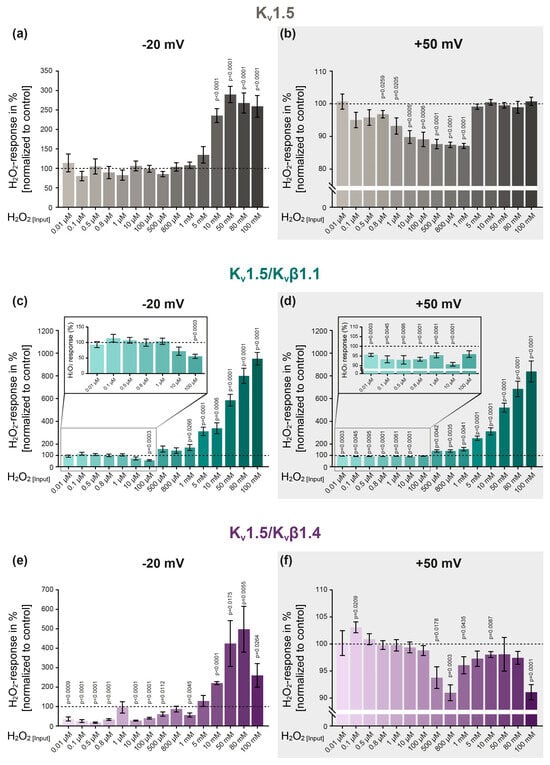
Figure 4.
The effect of H2O2 on Kv channels is dependent on the concentration, step potential and ion channel subunit composition. (a) The effect of increasing H2O2 concentrations on homomeric Kv1.5 channels at step potentials of −20 mV and (b) +50mV (light gray background). (c,d) Assessment of the H2O2 response of heteromeric Kv1.5/Kvβ1.1 as well as (e,f) Kv1.5/Kvβ1.4 channels. The H2O2 response was assessed by normalizing the current amplitude upon H2O2 application to the control conditions (represented by the dotted line). A new oocyte and freshly prepared H2O2 were used for each experiment. Number of experiments per H2O2 concentration: n = 8–13 (Kv1.5); n = 8–13 (Kv1.5/Kvβ1.1); n = 6–13 (Kv1.5/Kvβ1.4). For statistical analysis, one-sample t-tests were performed, with p ≤ 0.05 being considered indicative of significance. Group data are expressed as the mean ± SEM.
2.5. Co-Expression of Kv1.5 with Kvβ Subunits Modulates the Channel’s Response to H2O2
To determine whether co-expression with accessory β subunits alters the channel’s response to H2O2, Kv1.5 was co-expressed with the β-isoform Kvβ1.1 and the channels’ response to H2O2 was assessed by TEVC (Figure 4c,d). While low H2O2 concentrations (0.01 µM–10 µM) did not affect the activity of the Kv1.5/Kvβ1.1 channels at a step potential of −20 mV (Figure 4c), the same concentrations significantly inhibited the Kv1.5/Kvβ1.1 currents at +50 mV (see enlargement in Figure 4d). Importantly, this inhibition was induced even with the lowest H2O2 concentration, i.e., 0.01 µM. In contrast, application of higher H2O2 concentrations (1 mM to 100 mM) significantly and concentration-dependently increased the K+ currents at both step potentials, i.e., −20 mV and +50 mV (Figure 4c,d).
To assess whether distinct isoforms differently modulate the response of Kv1.5 to H2O2, an additional Kvβ isoform (Kvβ1.4) was co-expressed with Kv1.5 (Figure 4e,f). In contrast to the homomeric Kv1.5 and heteromeric Kv1.5/Kvβ1.1 channels, this channel combination was significantly inhibited by low H2O2 concentrations at −20 mV (Figure 4e). Importantly, this inhibition occurred under physiologically relevant conditions in terms of both the clamping potential and the H2O2 concentration. Notably, Kv1.5/Kvβ1.4 was the only channel combination that remained unaffected by low H2O2 concentrations at a depolarized clamping potential (+50 mV) while being inhibited when exposed to high concentrations of 500 µM and above (Figure 4f).
3. Discussion
It is well accepted that a shift in the intracellular ROS levels in response to acute hypoxia inhibits Kv channels in PASMCs, thereby triggering HPV. However, although the signaling molecule responsible could be identified as H2O2 [3], long-lasting divergences and contradictory reports exist in the literature on whether H2O2 inhibits or activates Kv channels. We hypothesized that these contrary observations depend on (a) the experimental clamping potential, (b) the H2O2 concentration utilized and/or (c) the Kv channel subunit composition, i.e., the presence of auxiliary Kvβ subunits. Therefore, the aim of the present study was to determine whether H2O2 modulates Kv channel activity in a concentration-dependent manner, and at the same time, to address the potential role of the accessory β subunits Kvβ1.1 and Kvβ1.4 as well as the experimental clamping potential in H2O2 sensitivity. For our studies, we heterologously expressed Kv1.5 as a homomeric channel and in combination with either Kvβ1.1 or Kvβ1.4, respectively, before exposing them to H2O2 at various concentrations between 0.01 µM and 100 mM. We then analyzed the Kv currents at two distinct clamping potentials, i.e., (a) at −20 mV, which falls within the physiological resting membrane potential of PASMCs and (b) at +50 mV, which was characterized by the highest voltage-induced current.
As an expression system, we used Xenopus laevis oocytes that are supposed to not express endogenous 4-AP-sensitive Kv channels [40,41]. This assumption was confirmed in our study as neither the water-injected control oocytes, nor the oocytes injected with Kvβ subunits alone exhibited voltage-induced currents upon depolarization, thereby confirming the choice of the expression system as ideally suited for investigating the effect of H2O2 on defined Kv channel combinations.
3.1. The Reaction of Kv Channels to H2O2 Depends on the Experimental Clamping Potential
Kv currents are typically measured at positive clamping potentials of up to +70 mV [29,34], which not only ensures channel activation but also maximizes the driving force for K+ efflux, thereby facilitating current detection and analysis. However, such positive clamping potentials do not reflect physiological conditions, as the membrane potentials in PASMCs typically range from approximately −50 mV to −10 mV [3,44]. As a result, the Kv channel behavior observed at such strongly positive (depolarized) voltages may not fully reflect their natural activation, inactivation, or response to pharmacological modulation under physiological conditions. In line with this, we observed a clear voltage-dependency in the response to H2O2 for all three channel combinations, i.e., Kv1.5, Kv1.5/Kvβ1.1 and Kv1.5/Kvβ1.4 (see Figure 4). For example, at a depolarizing voltage step of +50 mV, the homomeric Kv1.5 channels were inhibited by low concentrations of H2O2 (0.8 µM–1 mM), while higher H2O2 concentrations (5 mM–100 mM) had no effect on the channels’ activity. In contrast, at a physiological clamping potential of −20 mV, the channels exhibited a completely different response pattern, being unaffected by low H2O2 concentrations but strongly activated by H2O2 concentrations of 10 mM and above. Similar observations of voltage-dependent effects were also made for the heteromeric channels, i.e., Kv1.5/Kvβ1.1 and Kv1.5/Kvβ1.4. These data clearly indicate that the response of the Kv channels to H2O2 is markedly dependent on the experimental clamping potential.
However, it is important to consider that—in contrast to Xenopus laevis oocytes—in both primary cells and overexpression systems (particularly mammalian cell lines), endogenous ion channels might be active at physiological clamping potentials and contribute to the recorded currents, thus potentially confounding the detection and analysis of Kv-channel-specific responses. Furthermore, at physiological clamping potentials, the electrochemical driving force for K+ is diminished, resulting in reduced current amplitudes and less pronounced differences, thereby potentially masking subtle differences that are more apparent under depolarized conditions. Thus, the choice of clamping protocol constitutes a critical experimental parameter that can profoundly affect the biophysical properties and pharmacological response of Kv channels to H2O2.
3.2. The Effect of H2O2 on Kv Channel Activity Is Concentration-Dependent
The estimation of the intracellular concentration of H2O2 is based on the kinetics of production and elimination, while its determination is still technically difficult [45,46]. The physiological estimate of the cytosolic H2O2 concentration has been reported to be in the pico- to nanomolar range, e.g., 2.2 nM [47], 1–700 nM [48], 1–10 nM [46] or even in the picomolar range [47,49]. Consequently, H2O2 concentrations above the nanomolar range are regarded as pathological levels that are related to oxidative stress [46].
In previous studies investigating the effect of H2O2 on Kv channel activity, various concentrations of H2O2 were applied, resulting in contradictory outcomes. Higher concentrations, ranging from 0.1 to 10 mM, were reported to activate Kv channels [29,30,31]. In contrast, other studies employing lower concentrations, i.e., between 5 µM and 400 µM, observed an inhibitory effect on Kv channel activity [32,33,34]. These divergent observations prompted us to hypothesize that the concentration of H2O2 used under experimental conditions underlies the reported inconsistencies in H2O2-mediated Kv channel modulation, i.e., whether H2O2 inhibits or activates Kv channels. Based on this rationale, we applied a broad range of H2O2 concentrations, spanning from physiological to pathophysiological levels (10 nM to 100 mM).
At a physiological clamping potential of −20 mV, we observed the clear concentration-dependency of H2O2 on the homomeric Kv1.5 channel activity: whereas H2O2 did not affect the Kv1.5 activity at physiological concentrations, an activation of up to 200% was observed in response to pathophysiological concentrations of 10 mM and above. As discussed in the previous paragraph, a different response pattern was observed at a depolarized clamping potential (+50 mV), although there was still a clear difference between the physiological and pathophysiological H2O2 concentrations. Interestingly, the channel’s response to high doses of H2O2 appeared to be an “all or nothing” effect as no difference was observed between the concentrations applied. This indicates that oxidation by H2O2 above a certain level might induce a conformational change within the channel structure that increases the channel’s activity. However, although Kv1.5 has been implicated in HPV and is therefore presumed to be inhibited by hypoxia-induced H2O2 release, we did not observe an inhibition of homomeric Kv1.5 in response to H2O2 at physiological clamping potentials—regardless of the H2O2 concentration applied. Given that the accessory β subunits are reported to exert regulatory functions on Kv channels, including modulation of their redox sensitivity [50], we next investigated their influence on the H2O2 sensitivity of Kv1.5 channels.
3.3. Kvβ Subunits Modulate the Response of Kv1.5 to H2O2
Interestingly, the H2O2-induced activation in response to pathophysiological concentrations above 10 mM could be further potentiated by the co-expression of Kv1.5 with Kvβ1.1. In these heteromeric channels (Kv1.5/Kvβ1.1), H2O2 of 1 mM and above induced a channel activation of up to 900%, which in contrast to the homomeric Kv1.5 channels, was clearly concentration-dependent. This observation suggests that the accessory β subunits not only alter the channel’s activity per se, as described in several studies [51,52,53], but also equip them with the ability to fine-tune the kinetics of Kv1.5 channels in response to H2O2, most likely as a result of their oxidoreductase properties [50]. However, an H2O2-induced inhibition of the channel, as would be necessary for HPV initiation, was only observed in response to an H2O2 concentration of 100 µM—which, however, is not in the assumed physiological range of intracellular H2O2 concentrations [46].
The co-expression of Kv1.5 with the auxiliary isoform Kvβ1.4 rendered the channel sensitive to inhibition by physiological H2O2 concentrations—even as low as 10 nM. Most interestingly, this inhibition was observed at a physiological clamping potential—thereby closely reflecting the physiological conditions present in PASMCs under hypoxia. Given that H2O2 was decomposed by approximately 50% under our experimental conditions, this suggests that Kv1.5/Kvβ1.4 channels can be inhibited by H2O2 concentrations of less than 10 nM. Sommer et al. reported in a study from our laboratory that 124 nM H2O2 inhibits the Kv currents in primary mouse PASMCs [3], thereby contributing to the initiation of HPV. This finding is consistent with the present data, which demonstrate that even lower concentrations of H2O2 can significantly inhibit Kv currents, an effect that is—at least for Kv1.5—critically dependent on the clamping potential and the ion channel composition, particularly the presence of the auxiliary subunit Kvβ1.4.
It should be noted, however, that in addition to Kv1.5, a variety of other Kv channel subunits contribute to HPV initiation and further studies would be required to investigate the effect of H2O2 on these channels as well. However, our study clearly highlights the importance of carefully selecting experimental parameters—particularly the necessity of utilizing physiological conditions—when attempting to elucidate a physiological process.
3.4. Conclusions
In conclusion, our findings indicate that the response of Kv channels to H2O2 is markedly dependent on (a) the experimental clamping potential, (b) the H2O2 concentration and (c) the Kv channel subunit composition. Thus, our data clearly highlight the importance of the choice of experimental conditions when assessing the H2O2 sensitivity of Kv channels in the context of HPV, thus providing an explanation for the long-lasting controversial findings reported in the literature.
4. Materials and Methods
4.1. Generation of cRNA for Heterologous Expression of Kv Channels
Plasmids encoding murine Kv1.5 (NM_145983.2), Kvβ1.1 (NM_010597.4) or Kvβ1.4 (NM_001403872.1) in a pTNT expression vector (Promega, Madison, WI, USA) were purchased from the Eurofins gene synthesis service (Eurofins, Ebersberg, Germany) and transformed into competent JM109 E. coli cells (Promega, Madison, WI, USA) for amplification. Upon subsequent plasmid purification using the GenElute Plasmid Miniprep Kit (Sigma Aldrich, St Louis, MO, USA) according to the manufacturer’s instructions, the purified plasmids were used for cRNA synthesis via in vitro transcription using the mMESSAGE mMACHINE T7 kit (Invitrogen, Waltham, MA, USA). The resulting full-length capped cRNAs were then purified using the MEGAclear transcription cleaning kit (Invitrogen, Waltham, MA, USA). The cRNAs were stored at –80 °C and diluted in nuclease-free water immediately prior to injection.
4.2. Preparation, Storage and Injection of Xenopus Laevis Oocytes
Xenopus laevis oocytes at stages V–VI were purchased from Ecocyte Bioscience (Dr. Lohmann Diaclean GmbH, Dortmund, Germany) and plated individually in a 96 Conical Bottom MicroWell Plate (Fischer Scientific, Schwerte, Germany) filled with modified Barth’s solution (MBS, in mM: NaCl 88, KCl 1, CaCl2 0.4, Ca(NO3)2 0.33, MgSO4 0.8, TRIS-HCl 5, NaHCO3 2.4, pH 7.4, Dr. Lohmann Diaclean GmbH, Dortmund, Germany) that was supplemented with sodium pyruvate (2.5 mM, Sigma Aldrich, St Louis, MO, USA), penicillin (20 µg/mL, Sigma Aldrich, St Louis, MO, USA) and streptomycin (25 µg/mL, Sigma Aldrich, St Louis, MO, USA). The oocytes were stored in a digital mini-incubator (Gilson, Middleton, WI, USA) at 5–8 °C until use.
At 24–48 h prior to the electrophysiological recordings, the oocytes were injected with Kv1.5: 0.1 ng/oocyte; Kvβ1.1: 1.75 ng/oocyte; Kvβ1.4: 0.1 ng/oocyte in a total injection volume of 10 nL/oocyte (for Kv1.5 and Kv1.5/Kvβ1.4, resp.) and 17.5 nL/oocyte (for Kv1.5/Kvβ1.1) using the Roboinject fully automated injection system (Multichannel Systems, Reutlingen, Germany). Therefore, the injection needle was filled with mineral oil (Sigma Aldrich, St Louis, MO, USA) before being mounted onto the device. Afterwards, the cRNAs that were diluted in nuclease-free water were drawn into the injection needle and injected into the oocytes. The impalement and injection depth for the cRNAs were defined for all the samples as 550 µm and 450 µm, respectively. The injected oocytes were incubated for 24 h at 18 °C for heterologous expression.
4.3. Electrophysiological Recordings via Two-Electrode Voltage Clamp (TEVC)
The Kv currents of the cRNA injected oocytes were recorded via the two-electrode voltage clamp (TEVC) technique using an automated TEVC system (Roboocyte2, Multichannel Systems, Reutlingen, Germany). Therefore, the glass capillaries of the measure head were filled with 1 M KCl. Only electrodes with tip resistances between 300 and 800 kΩ were used for the experiments. All the electrophysiological recordings were carried out at room temperature (20–22 °C) under continuous perfusion with oocyte Ringer’s solution (ORi; Normal Frog Ringer, Ecocyte Biosciences, composition in mM: NaCl 64, KCl 2, CaCl2 2, MgCl2 1, NaHCO3 26). The ORi inside the perfusion reservoir was continuously gassed with 21% O2, 5.3% CO2, 73.7 N2 for pH stabilization at pH 7.4. Due to the rapid decomposition of H2O2 at physiological pH values, the H2O2 (Sigma Aldrich, St Louis, MO, USA) was freshly prepared directly before the start of each individual experiment. Being a strong oxidizing agent, H2O2 has the potential to corrode Ag/AgCl electrodes upon direct contact, which would manifest in a shift of the baseline current. Anticipating this potential issue, a rigorous maintenance protocol involving regular and frequent re-chlorination and replacement of the electrodes was implemented. Additionally, the baseline current was continuously monitored to ensure stability throughout the experiments.
For the electrophysiological recordings, the oocytes were voltage-clamped at a holding potential of −60 mV. The current amplitudes were elicited by depolarizing voltage pulses in 10 mV increment steps starting from −80 mV to +50 mV and 200 ms duration.
4.4. Experimental Design and Analysis
As a current rundown was already observed in the control recordings (two recordings in succession with an interval of 90 s between the two recordings), a different procedure was used. Two sets of experiments were performed for each experiment. As a control, two recordings were performed in the absence of H2O2 (control), separated by 90 s. In the second set of experiments, only one control recording was performed, before H2O2 was delivered to the recording chamber for 90 s. The recording taken after the 90 s (+H2O2) was then compared with the second current trace from the control measurements (−H2O2). The recordings +/−H2O2 were always performed on oocytes obtained from the same individual. The mean Kv current amplitudes were normalized relatively to the current at +50 mV in the control experiment. All the current traces were leak-subtracted prior to statistical evaluation. The current–voltage relationship (I–V curve) was obtained by plotting the averaged plateau current against the respective clamping potential. The specific blocker 4-aminopyrimidine (4-AP, 10 mM) was added at the end of experiment to validate the Kv channel expression.
4.5. Amplex Red Hydrogen Peroxide/Peroxidase Assay
To determine the decomposition of H2O2 inside the experimental system, H2O2 was added to the perfusate and delivered to the recording chamber via the perfusion system. At 90 s post starting the perfusion, a sample was taken from the recording chamber, representing the time point where the electrophysiological recording (+H2O2) was performed. The H2O2 concentration (input, i.e., [H2O2] present at time of recording) was determined by Amplex® Red Hydrogen Peroxide/Peroxidase Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA). The assay was performed according to the supplier’s instructions. The absorbance was measured at 560/590 nm using a microplate reader TECAN Spark 10M (Tecan Trading AG, Männedorf, Switzerland). The manuscript consistently reports the input H2O2 concentration. However, it should be considered that—due to the decomposition of H2O2—the effective concentration at the oocyte during the electrophysiological recordings is lower (see Figure 2).
4.6. Statistical Analysis
The statistical differences were assessed via two-way ANOVA and the uncorrected Fisher’s least significant difference test for multiple comparisons or one-sample t-tests. All the analyses were considered statistically significant at p ≤ 0.05. Data are expressed as the mean ± SEM. The statistical analysis was performed using Prism 10 (GraphPad Software Inc., San Diego, CA, USA).
Author Contributions
Conceptualization, F.K.; methodology, O.T.Y., A.B. and F.K.; software, O.T.Y., A.B. and F.K.; validation, O.T.Y., A.B., N.S., M.D., N.W. and F.K.; formal analysis, O.T.Y., A.B., N.S. and F.K.; investigation, O.T.Y., A.B. and F.K.; resources, F.K.; writing—original draft preparation, F.K.; writing—review and editing, O.T.Y., A.B., N.S., M.D. and N.W.; visualization, O.T.Y., A.B. and F.K.; supervision, N.W., M.D. and F.K.; project administration, F.K.; funding acquisition, F.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the German Research Foundation (DFG), project ID 452531259.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on reasonable request.
Acknowledgments
The authors thank Ingrid Breitenborn-Müller, Nils Schupp and Monika Brosien for their technical assistance, as well as Christine Veith and Simone Kraut for the careful proofreading of the article. Portions of the doctoral theses of Ornella Tchokondu Yamdjeu and Anouk Begerow are incorporated into this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ARDS | acute respiratory distress syndrome |
| cRNA | complementary ribonucleic acid |
| HPV | hypoxic pulmonary vasoconstriction |
| I–V curve | current–voltage relationship |
| Kv channel | voltage-gated potassium channel |
| nM | nanomolar |
| ORi | oocyte Ringer’s solution |
| PASMC | pulmonary arterial smooth muscle cell |
| ROS | reactive oxygen species |
| TEVC | two-electrode voltage clamp |
| 4-AP | 4-aminopyridine |
References
- Moreno-Domínguez, A.; Colinas, O.; Smani, T.; Ureña, J.; López-Barneo, J. Acute oxygen sensing by vascular smooth muscle cells. Front. Physiol. 2023, 14, 1142354. [Google Scholar] [CrossRef] [PubMed]
- Sylvester, J.T.; Shimoda, L.A.; Aaronson, P.I.; Ward, J.P. Hypoxic pulmonary vasoconstriction. Physiol. Rev. 2012, 92, 367–520. [Google Scholar] [CrossRef] [PubMed]
- Sommer, N.; Hüttemann, M.; Pak, O.; Scheibe, S.; Knoepp, F.; Sinkler, C.; Malczyk, M.; Gierhardt, M.; Esfandiary, A.; Kraut, S.; et al. Mitochondrial Complex IV Subunit 4 Isoform 2 Is Essential for Acute Pulmonary Oxygen Sensing. Circ. Res. 2017, 121, 424–438. [Google Scholar] [CrossRef] [PubMed]
- Lang, M.; Som, A.; Mendoza, D.P.; Flores, E.J.; Reid, N.; Carey, D.; Li, M.D.; Witkin, A.; Rodriguez-Lopez, J.M.; Shepard, J.-A.O.; et al. Hypoxaemia related to COVID-19: Vascular and perfusion abnormalities on dual-energy CT. Lancet Infect. Dis. 2020, 20, 1365–1366. [Google Scholar] [CrossRef] [PubMed]
- Dunham-Snary, K.J.; Wu, D.; Sykes, E.A.; Thakrar, A.; Parlow, L.R.G.; Mewburn, J.D.; Parlow, J.L.; Archer, S.L. Hypoxic Pulmonary Vasoconstriction: From Molecular Mechanisms to Medicine. Chest 2017, 151, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Hackett, P.H.; Creagh, C.E.; Grover, R.F.; Honigman, B.; Houston, C.S.; Reeves, J.T.; Sophocles, A.M.; Van Hardenbroek, M. High-altitude pulmonary edema in persons without the right pulmonary artery. N. Engl. J. Med. 1980, 302, 1070–1073. [Google Scholar] [CrossRef] [PubMed]
- Hultgren, H.N. High-altitude pulmonary edema: Current concepts. Annu. Rev. Med. 1996, 47, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Chaouat, A.; Naeije, R.; Weitzenblum, E. Pulmonary hypertension in COPD. Eur. Respir. J. 2008, 32, 1371–1385. [Google Scholar] [CrossRef] [PubMed]
- Blanco, I.; Tura-Ceide, O.; Peinado, V.I.; Barberà, J.A. Updated Perspectives on Pulmonary Hypertension in COPD. Int. J. Chron. Obstruct. Pulm. Dis. 2020, 15, 1315–1324. [Google Scholar] [CrossRef] [PubMed]
- Kholdani, C.; Fares, W.H.; Mohsenin, V. Pulmonary hypertension in obstructive sleep apnea: Is it clinically significant? A critical analysis of the association and pathophysiology. Pulm. Circ. 2015, 5, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Dunham-Snary, K.J.; Wu, D.; Potus, F.; Sykes, E.A.; Mewburn, J.D.; Charles, R.L.; Eaton, P.; Sultanian, R.A.; Archer, S.L. Ndufs2, a Core Subunit of Mitochondrial Complex I, Is Essential for Acute Oxygen-Sensing and Hypoxic Pulmonary Vasoconstriction. Cir. Res. 2019, 124, 1727–1746. [Google Scholar] [CrossRef] [PubMed]
- Weir, E.K.; Archer, S.L. The mechanism of acute hypoxic pulmonary vasoconstriction: The tale of two channels. FASEB J. 1995, 9, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Rocher, A.; Aaronson, P.I. The Thirty-Fifth Anniversary of K+ Channels in O2 Sensing: What We Know and What We Don’t Know. Oxygen 2024, 4, 53–89. [Google Scholar] [CrossRef]
- Archer, S.L.; Wu, X.C.; Thébaud, B.; Nsair, A.; Bonnet, S.; Tyrrell, B.; McMurtry, M.S.; Hashimoto, K.; Harry, G.; Michelakis, E.D. Preferential expression and function of voltage-gated, O2-sensitive K+ channels in resistance pulmonary arteries explains regional heterogeneity in hypoxic pulmonary vasoconstriction: Ionic diversity in smooth muscle cells. Circ. Res. 2004, 95, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Post, J.M.; Hume, J.R.; Archer, S.L.; Weir, E.K. Direct role for potassium channel inhibition in hypoxic pulmonary vasoconstriction. Am. J. Physiol. 1992, 262, C882–C890. [Google Scholar] [CrossRef] [PubMed]
- Redel-Traub, G.; Sampson, K.J.; Kass, R.S.; Bohnen, M.S. Potassium Channels as Therapeutic Targets in Pulmonary Arterial Hypertension. Biomolecules 2022, 12, 1341. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.J.; Honoré, E. Molecular physiology of oxygen-sensitive potassium channels. Eur. Respir. J. 2001, 18, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Boucherat, O.; Chabot, S.; Antigny, F.; Perros, F.; Provencher, S.; Bonnet, S. Potassium channels in pulmonary arterial hypertension. Eur. Respir. J. 2015, 46, 1167–1177. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.; Weir, E.K.; Nelson, D.P.; Olschewski, A. Subacute Hypoxia Decreases Voltage-Activated Potassium Channel Expression and Function in Pulmonary Artery Myocytes. Am. J. Respir. Cell Mol. Biol. 2004, 31, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Dipp, M.; Nye, P.C.G.; Evans, A.M. Hypoxic release of calcium from the sarcoplasmic reticulum of pulmonary artery smooth muscle. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001, 281, L318–L325. [Google Scholar] [CrossRef] [PubMed]
- Waypa, G.B.; Marks, J.D.; Mack, M.M.; Boriboun, C.; Mungai, P.T.; Schumacker, P.T. Mitochondrial reactive oxygen species trigger calcium increases during hypoxia in pulmonary arterial myocytes. Circ. Res. 2002, 91, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Sommer, N.; Alebrahimdehkordi, N.; Pak, O.; Knoepp, F.; Strielkov, I.; Scheibe, S.; Dufour, E.; Andjelković, A.; Sydykov, A.; Saraji, A.; et al. Bypassing mitochondrial complex III using alternative oxidase inhibits acute pulmonary oxygen sensing. Sci. Adv. 2020, 6, eaba0694. [Google Scholar] [CrossRef] [PubMed]
- Knoepp, F.; Wahl, J.; Andersson, A.; Kraut, S.; Sommer, N.; Weissmann, N.; Ramser, K. A Microfluidic System for Simultaneous Raman Spectroscopy, Patch-Clamp Electrophysiology, and Live-Cell Imaging to Study Key Cellular Events of Single Living Cells in Response to Acute Hypoxia. Small Methods 2021, 5, e2100470. [Google Scholar] [CrossRef] [PubMed]
- Sommer, N.; Strielkov, I.; Pak, O.; Weissmann, N. Oxygen sensing and signal transduction in hypoxic pulmonary vasoconstriction. Eur. Respir. J. 2016, 47, 288–303. [Google Scholar] [CrossRef] [PubMed]
- Michelakis, E.D.; Thébaud, B.; Weir, E.K.; Archer, S.L. Hypoxic pulmonary vasoconstriction: Redox regulation of O2-sensitive K+ channels by a mitochondrial O2-sensor in resistance artery smooth muscle cells. J. Mol. Cell Cardiol. 2004, 37, 1119–1136. [Google Scholar] [CrossRef] [PubMed]
- Archer, S.L.; Huang, J.; Henry, T.; Peterson, D.; Weir, E.K. A redox-based O2 sensor in rat pulmonary vasculature. Circ. Res. 1993, 73, 1100–1112. [Google Scholar] [CrossRef] [PubMed]
- Pak, O.; Nolte, A.; Knoepp, F.; Giordano, L.; Pecina, P.; Hüttemann, M.; Grossman, L.I.; Weissmann, N.; Sommer, N. Mitochondrial oxygen sensing of acute hypoxia in specialized cells—Is there a unifying mechanism? Biochim. Biophys. Acta Bioenerg. 2022, 1863, 148911. [Google Scholar] [CrossRef] [PubMed]
- Waypa, G.B.; Chandel, N.S.; Schumacker, P.T. Model for Hypoxic Pulmonary Vasoconstriction Involving Mitochondrial Oxygen Sensing. Circ. Res. 2001, 88, 1259–1266. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Youngson, C.; Wong, V.; Yeger, H.; Dinauer, M.C.; de Miera, E.V.-S.; Rudy, B.; Cutz, E. NADPH-oxidase and a hydrogen peroxide-sensitive K+ channel may function as an oxygen sensor complex in airway chemoreceptors and small cell lung carcinoma cell lines. Proc. Natl. Acad. Sci. USA 1996, 93, 13182–13187. [Google Scholar] [CrossRef] [PubMed]
- Rogers, P.A.; Chilian, W.M.; Bratz, I.N.; Bryan, R.M., Jr.; Dick, G.M. H2O2 activates redox- and 4-aminopyridine-sensitive Kv channels in coronary vascular smooth muscle. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H1404–H1411. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Noh, H.J.; Sung, D.J.; Kim, J.G.; Kim, J.M.; Ryu, S.-Y.; Kang, K.; Kim, B.; Bae, Y.M.; Cho, H. Hydrogen peroxide induces vasorelaxation by enhancing 4-aminopyridine-sensitive Kv currents through S-glutathionylation. Pflüg Arch. Eur. J. Physiol. 2015, 467, 285–297. [Google Scholar] [CrossRef] [PubMed]
- You, N.; Li, W.; Guo, J.; Yang, T.; Zhang, S. Hypoxia Inhibits Kv1.5 Currents Through Reactive Oxygen Species-Mediated Disulfide Bond Formation. Biophys. J. 2020, 118, 109a. [Google Scholar] [CrossRef]
- Cogolludo, A.; Frazziano, G.; Cobeño, L.; Moreno, L.; Lodi, F.; Villamor, E.; Tamargo, J.; Perez-Vizcaino, F. Role of Reactive Oxygen Species in Kv Channel Inhibition and Vasoconstriction Induced by TP Receptor Activation in Rat Pulmonary Arteries. Ann. N. Y. Acad. Sci. 2006, 1091, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Michelakis, E.D.; Rebeyka, I.; Wu, X.; Nsair, A.; Thébaud, B.; Hashimoto, K.; Dyck, J.R.B.; Haromy, A.; Harry, G.; Barr, A.; et al. O2 Sensing in the Human Ductus Arteriosus. Circ. Res. 2002, 91, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Coppock, E.A.; Tamkun, M.M. Differential expression of KV channel α- and β-subunits in the bovine pulmonary arterial circulation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001, 281, L1350–L1360. [Google Scholar] [CrossRef] [PubMed]
- Archer, S.L.; Souil, E.; Dinh-Xuan, A.T.; Schremmer, B.; Mercier, J.C.; El Yaagoubi, A.; Nguyen-Huu, L.; Reeve, H.L.; Hampl, V. Molecular identification of the role of voltage-gated K+ channels, Kv1.5 and Kv2.1, in hypoxic pulmonary vasoconstriction and control of resting membrane potential in rat pulmonary artery myocytes. J. Clin. Investig. 1998, 101, 2319–2330. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.X.-J. Oxygen-sensitive K+ channel(s): Where and what? Am. J. Physiol. Lung Cell. Mol. Physiol. 2001, 281, L1345–L1349. [Google Scholar] [CrossRef] [PubMed]
- Archer, S.L.; London, B.; Hampl, V.; Wu, X.; Nsair, A.; Puttagunta, L.; Hashimoto, K.; Waite, R.E.; Michelakis, E.D. Impairment of hypoxic pulmonary vasoconstriction in mice lacking the voltage-gated potassium channel Kv1.5. FASEB J. 2001, 15, 1801–1803. [Google Scholar] [CrossRef] [PubMed]
- Pozeg, Z.I.; Michelakis, E.D.; McMurtry, M.S.; Thébaud, B.; Wu, X.-C.; Dyck, J.R.B.; Hashimoto, K.; Wang, S.; Moudgil, R.; Harry, G.; et al. In Vivo Gene Transfer of the O2-Sensitive Potassium Channel Kv1.5 Reduces Pulmonary Hypertension and Restores Hypoxic Pulmonary Vasoconstriction in Chronically Hypoxic Rats. Circulation 2003, 107, 2037–2044. [Google Scholar] [CrossRef] [PubMed]
- Terhag, J.; Cavara, N.A.; Hollmann, M. Cave Canalem: How endogenous ion channels may interfere with heterologous expression in Xenopus oocytes. Methods 2010, 51, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Weber, W.-M. Ion currents of Xenopus laevis oocytes: State of the art. Biochim. Biophys. Acta Biomembr. 1999, 1421, 213–233. [Google Scholar] [CrossRef] [PubMed]
- Decher, N.; Kumar, P.; Gonzalez, T.; Renigunta, V.; Sanguinetti, M.C. Structural Basis for Competition between Drug Binding and Kvβ1.3 Accessory Subunit-Induced N-Type Inactivation of Kv1.5 Channels. Mol. Pharmacol. 2005, 68, 995–1005. [Google Scholar] [CrossRef] [PubMed]
- Yazıcı, E.; Deveci, H. Factors Affecting Decomposition of Hydrogen Peroxide. In Proceedings of the XIIth International Mineral Processing Symposium, Cappadocia-Nevsehir, Turkey, 6–8 October 2010. [Google Scholar] [CrossRef]
- Olschewski, A.; Hong, Z.; Linden, B.C.; Porter, V.A.; Weir, E.K.; Cornfield, D.N. Contribution of the KCa channel to membrane potential and O2 sensitivity is decreased in an ovine PPHN model. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002, 283, L1103–L1109. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Forman, H.J.; Bernardo, A.; Davies, K.J. What is the concentration of hydrogen peroxide in blood and plasma? Arch. Biochem. Biophys. 2016, 603, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Lyublinskaya, O.; Antunes, F. Measuring intracellular concentration of hydrogen peroxide with the use of genetically encoded H2O2 biosensor HyPer. Redox Biol. 2019, 24, 101200. [Google Scholar] [CrossRef] [PubMed]
- Stone, J.R.; Yang, S. Hydrogen Peroxide: A Signaling Messenger. Antioxid. Redox Signal. 2006, 8, 243–270. [Google Scholar] [CrossRef] [PubMed]
- Ezeriņa, D.; Morgan, B.; Dick, T.P. Imaging dynamic redox processes with genetically encoded probes. J. Mol. Cell. Cardiol. 2014, 73, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Pongs, O.; Leicher, T.; Berger, M.; Roeper, J.; Bähring, R.; Wray, D.; Giese, K.P.; Silva, A.J.; Storm, J.F. Functional and Molecular Aspects of Voltage-Gated K+ Channel β Subunits. Ann. N. Y. Acad. Sci. 1999, 868, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.M.; Sahoo, N.; Dennhardt, S.; Schönherr, R.; Heinemann, S.H. Ca2+/calmodulin regulates Kvβ1.1-mediated inactivation of voltage-gated K+ channels. Sci. Rep. 2015, 5, 15509. [Google Scholar] [CrossRef] [PubMed]
- Tipparaju, S.M.; Liu, S.-Q.; Barski, O.A.; Bhatnagar, A. NADPH binding to β-subunit regulates inactivation of voltage-gated K+ channels. Biochem. Biophys. Res. Commun. 2007, 359, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Raph, S.M.; Bhatnagar, A.; Nystoriak, M.A. Biochemical and physiological properties of K(+) channel-associated AKR6A (Kvβ) proteins. Chem. Biol. Interact. 2019, 305, 21–27. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).