Pectin and Its Beneficial Effect on Health: New Contributions in Research and the Need to Increase Fruits and Vegetables Consumption—A Review
Abstract
1. Introduction
2. Effect of Pectin on Starch Digestion
2.1. Starch
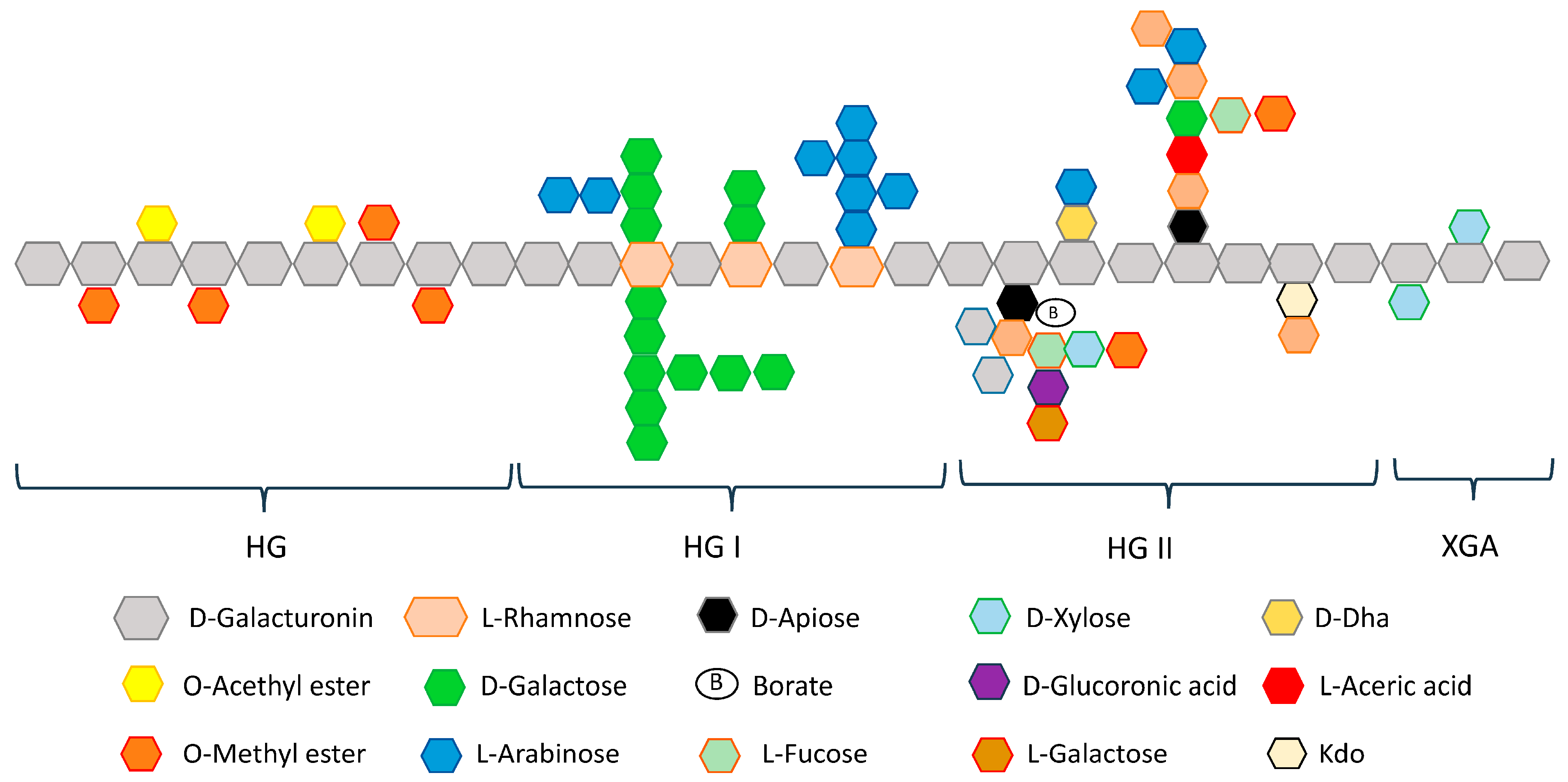
2.2. Pectin
2.3. Physicochemical Changes Caused by Pectin
2.4. Impact of Pectin on the Activity of Enzymes Amylases
3. Pectin, Microbiota, and Production of Short-Chain Fatty Acids
3.1. Impact of Dietary Fiber on Gut Microbiota
3.2. Production of SCFAs
3.3. SCFAs and Gut–Heart Axis
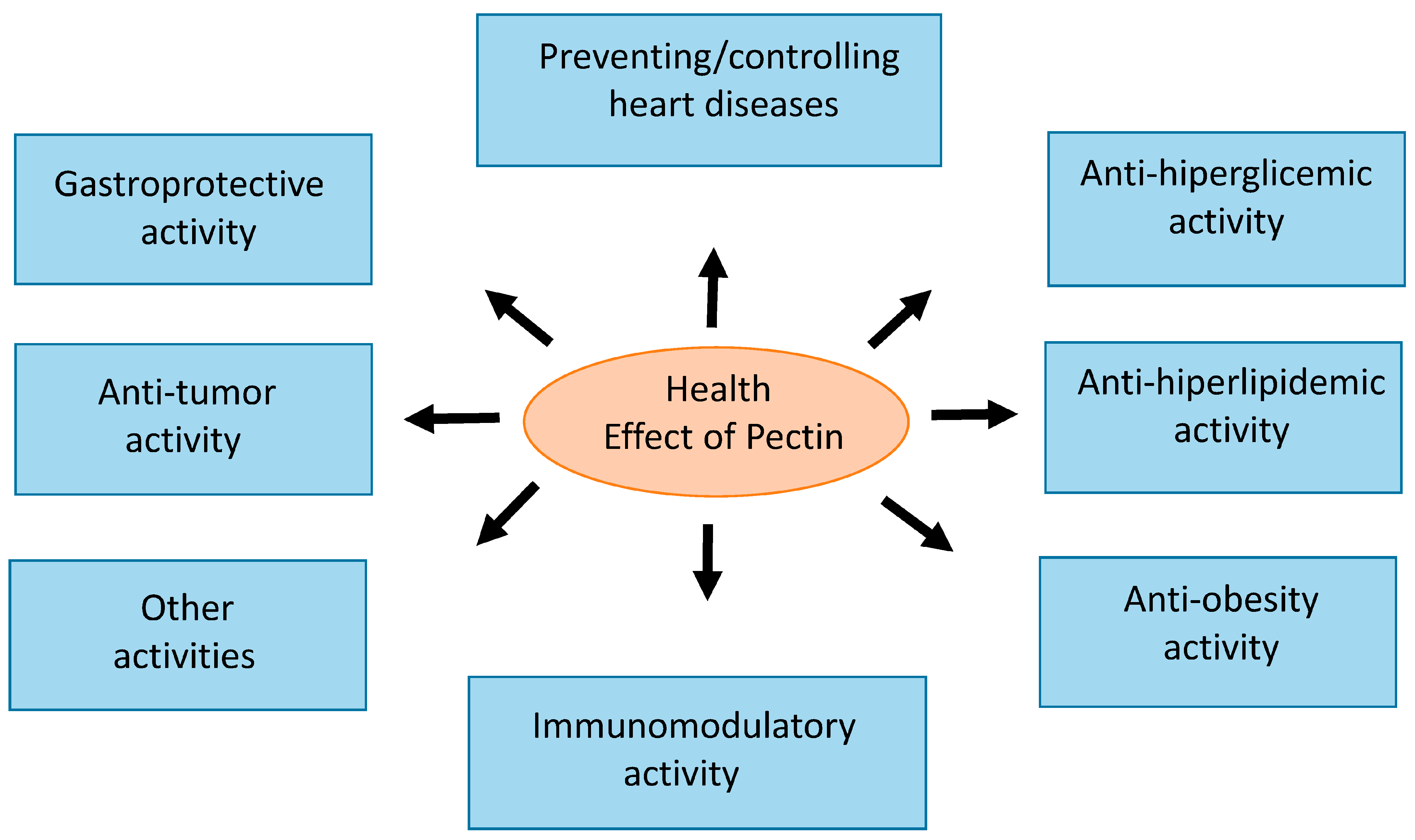
4. Pectin and Health Benefits
4.1. Anti-Hyperglycemic Activity
4.2. Anti-Hyperlipidemic Activity
4.3. Anti-Obesity Effect
4.4. Preventing/Controlling Heart Disease
4.5. Butirate in Regulating the Myocardial Ischemia–Reperfusion Injury (MIRI)
4.6. Anti-Tumor Activity
4.7. Gastroprotective Activity
4.8. Immunomodulatory Activity
5. Discussion
6. Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Willett, W.C. Vitamin A and lung cancer. Nutr. Rev. 1990, 48, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Negri, E.; La Vecchia, C.; Franceschi, S.; D’Avanzo, B.; Parazzini, F. Vegetable and fruit consumption and cancer risk. Int. J. Cancer 1991, 48, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, K.A.; Potter, J.D. Vegetables, fruit, and cancer: II. Mechanisms. Cancer Causes Control 1991, 2, 427–442. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, R.G. A review of epidemiologic evidence that carotenoids reduce the risk of cancer. J. Nutr. 1989, 119, 116–122. [Google Scholar] [CrossRef]
- Ziegler, R.G. Vegetables, fruits, and carotenoids and the risk of cancer. Am. J. Clin. Nutr. 1991, 53, 251S–259S. [Google Scholar] [CrossRef]
- Hertog, M.G.; Feskens, E.J.; Hollman, P.C.; Katan, M.B.; Kromhout, D. Dietary antioxidant flavonoids and risk of coronary heart disease: The Zutphen Elderly Study. Lancet 1993, 342, 1007–1011. [Google Scholar] [CrossRef]
- Gillman, M.W.; Cupples, L.A.; Gagnon, D.; Posner, B.M.; Ellison, R.C.; Castelli, W.P.; Wolf, P.A. Protective Effect of Fruits and Vegetables on Development of Stroke in Men. JAMA 1995, 273, 1113–1117. [Google Scholar] [CrossRef]
- NHANES2001–2002.2001. Available online: https://wwwn.cdc.gov/Nchs/Nhanes/Search/DataPage.aspx?Component=Questionnaire& (accessed on 28 January 2025).
- World Health Organization. Diet, Nutrition and the Prevention of Chronic Diseases: Report of a Joint WHO/FAO Expert Consultation; WHO Technical Report Series 916; World Health Organization: Geneva, Switzerland, 2002. [Google Scholar]
- Wang, D.D.; Li, Y.; Bhupathiraju, S.N.; Rosner, B.A.; Sun, Q.; Giovannucci, E.L.; Rimm, E.B.; Manson, J.E.; Willett, W.C.; Stampfer, M.J.; et al. Fruit and Vegetable Intake and Mortality: Results from 2 Prospective Cohort Studies of US Men and Women and a Meta-Analysis of 26 Cohort Studies. Circulation 2021, 143, 1642–1654. [Google Scholar] [CrossRef]
- Goff, H.D.; Repin, N.; Fabek, H.; El Khoury, D.; Gidley, M.J. Dietary fibre for glycaemia control: Towards a mechanistic understanding. Bioact. Carbohydr. Diet. Fibre 2018, 14, 39–53. [Google Scholar] [CrossRef]
- He, H.; Chi, C.; Xie, F.; Li, X.; Liang, Y.I.; Chen, L. Improving the in vitro digestibility of rice starch by thermomechanically assisted complexation with guar gum. Food Hydrocoll. 2020, 102, 105637. [Google Scholar] [CrossRef]
- Tappy, L. Metabolism of sugars: A window to the regulation of glucose and lipid homeostasis by splanchnic organs. Clin. Nutr. 2021, 40, 1691–1698. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Bai, Y.; Gilbert, R.G. Effects of the Molecular Structure of Starch in Foods on Human Health. Foods 2023, 12, 2263. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Wu, J.; Liu, X.; Shi, J.; Xu, J. Physiological effects of resistant starch and its applications in food: A review. Food Prod. Process. Nutr. 2023, 5, 48. [Google Scholar] [CrossRef]
- Kumar, A.A.; Satheesh, G.; Vijayakumar, G.; Chandran, M.; Prabhu, P.R.; Simon, L.; Kutty, V.R.; Kartha, C.C.; Jaleel, A. Postprandial Metabolism is Impaired in Overweight Normoglycemic Young Adults without Family History of Diabetes. Sci. Rep. 2020, 10, 353. [Google Scholar] [CrossRef]
- Kamruzzaman, M.; Horowitz, M.; Jones, K.L.; Marathe, C.S. Gut-Based Strategies to Reduce Postprandial Glycaemia in Type 2 Diabetes. Front. Endocrinol. 2021, 12, 661877. [Google Scholar] [CrossRef]
- Shibib, L.; Al-Qaisi, M.; Guess, N.; Miras, A.D.; Greenwald, S.; Pelling, M.; Ahmed, A. Manipulation of Post-Prandial Hyperglycaemia in Type 2 Diabetes: An Update for Practitioners. Diabetes Metab. Syndr. Obes. 2024, 17, 3111–3130. [Google Scholar] [CrossRef]
- Hershon, K.S.; Hirsch, B.R.; Odugbesan, O. Importance of Postprandial Glucose in Relation to A1C and Cardiovascular Disease. Clin. Diabetes 2019, 37, 250–259. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Y.; Wu, Y.; Ouyang, J. Factors influencing the starch digestibility of starchy foods: A review. Food Chem. 2023, 406, 135009. [Google Scholar] [CrossRef]
- Chi, C.; Li, X.; Huang, S.; Chen, L.; Zhang, Y.; Li, L.; Miao, S. Basic principles in starch multi-scale structuration to mitigate digestibility: A review. Trends Food Sci. Technol. 2021, 109, 154–168. [Google Scholar] [CrossRef]
- Dong, S.; Fang, G.; Luo, Z.; Gao, Q. Effect of granule size on the structure and digestibility of jackfruit seed starch. Food Hydrocoll. 2021, 120, 106964. [Google Scholar] [CrossRef]
- Gong, B.; Cheng, L.; Gilbert, R.G.; Li, C. Distribution of short to medium amylose chains are major controllers of in vitro digestion of retrograded rice starch. Food Hydrocoll. 2019, 96, 634–643. [Google Scholar] [CrossRef]
- Ouyang, Q.; Wang, X.; Xiao, Y.; Luo, F.; Lin, Q.; Ding, Y. Structural changes of A-, B- and C-type starches of corn, potato and pea as influenced by sonication temperature and their relationships with digestibility. Food Chem. 2021, 358, 129858. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yeats, T.H.; Uluisik, S.; Rose, J.K.C.; Seymour, G.B. Fruit Softening: Revisiting the Role of Pectin. Trends Plant Sci. 2018, 23, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Cheng, L.; Yang, Q.; Shi, K.; Han, F.; Luo, W.; Duan, S. Source, Extraction, Properties, and Multifunctional Applications of Pectin: A Short Review. Polymers 2024, 16, 2883. [Google Scholar] [CrossRef]
- Shahin, L.; Zhang, L.; Mohnen, D.; Urbanowicz, B.R. Insights into pectin O-acetylation in the plant cell wall: Structure, synthesis, and modification. Cell Surf. 2023, 9, 100099. [Google Scholar] [CrossRef]
- Gawkowska, D.; Cybulska, J.; Zdunek, A. Structure-Related Gelling of Pectins and Linking with Other Natural Compounds: A Review. Polymers 2018, 10, 762. [Google Scholar] [CrossRef]
- Bai, Y.; Gilbert, R.G. Mechanistic Understanding of the Effects of Pectin on In Vivo Starch Digestion: A Review. Nutrients 2022, 14, 5107. [Google Scholar] [CrossRef]
- Ioniță-Mîndrican, C.-B.; Ziani, K.; Mititelu, M.; Oprea, E.; Neacșu, S.M.; Moroșan, E.; Dumitrescu, D.-E.; Roșca, A.C.; Drăgănescu, D.; Negrei, C. Therapeutic Benefits and Dietary Restrictions of Fiber Intake: A State of the Art Review. Nutrients 2022, 14, 2641. [Google Scholar] [CrossRef]
- Ma, M.; Gu, Z.; Cheng, L.; Li, Z.; Li, C.; Hong, Y. Effect of hydrocolloids on starch digestion: A review. Food Chem. 2024, 444, 138636. [Google Scholar] [CrossRef]
- Yu, Y.; Lu, P.; Yang, Y.; Ji, H.; Zhou, H.; Chen, S.; Qiu, Y.; Chen, H. Differences in physicochemical properties of pectin extracted from pomelo peel with different extraction techniques. Sci. Rep. 2024, 14, 9182. [Google Scholar] [CrossRef]
- Iftikhar, S.Y.; Washington, N.; Wilson, C.G.; Macdonald, I.A.; Homer-Ward, M.D. The effect of pectin on the gastric emptying rates and blood glucose levels after a test meal. J. Pharm. Pharmacol. 1994, 46, 851–853. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.A.; Gassull, M.A.; Leeds, A.R.; Metz, G.; Dilawari, J.B.; Slavin, B.; Blendis, L.M. Effect of dietary fiber on complications of gastric surgery: Prevention of postprandial hypoglycemia by pectin. Gastroenterology 1977, 73, 215–217. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Sotome, I.; Okadome, H. In vitro starch digestibility and in vivo glucose response of gelatinized potato starch in the presence of non-starch polysaccharides. Starch-Starke 2015, 67, 415–423. [Google Scholar] [CrossRef]
- Flourie, B.; Vidon, N.; Florent, C.; Bernier, J.J. Effect of pectin on jejunal glucose absorption and unstirred layer thickness in normal man. Gut 1984, 25, 936–941. [Google Scholar] [CrossRef]
- Macagnan, F.T.; dos Santos, L.R.; Roberto, B.S.; de Moura, F.A.; Bizzani, M.; da Silva, L.P. Biological properties of apple pomace, orange bagasse and passion fruit peel as alternative sources of dietary fibre. Bioact. Carbohydr. Diet. Fibre 2015, 6, 1–6. [Google Scholar] [CrossRef]
- Lin, P.; Shih, B.; Hsu, J. Effects of different sources of dietary non-starch polysaccharides on the growth performance, development of digestive tract and activities of pancreatic enzymes in goslings. Br. Poult. Sci. 2010, 51, 270–277. [Google Scholar] [CrossRef]
- Ranganathan, S.; Champ, M.; Pechard, C.; Blanchard, P.; Nguyen, M.; Colonna, P.; Krempf, M. Comparative study of the acute effects of resistant starch and dietary fibers on metabolic indexes in men. Am. J. Clin. Nutr. 1994, 59, 879–883. [Google Scholar] [CrossRef]
- Forman, L.P.; Schneeman, B.O. Dietary pectin’s effect on starch utilization in rats. J. Nutr. 1982, 112, 528–533. [Google Scholar] [CrossRef]
- Date, K.; Satoh, A.; Iida, K.; Ogawa, H. Pancreatic α-Amylase Controls Glucose Assimilation by Duodenal Retrieval through N-Glycan-specific Binding, Endocytosis, and Degradation. J. Biol. Chem. 2015, 290, 17439–17450. [Google Scholar] [CrossRef]
- Baron, A.D. Postprandial hyperglycaemia and α-glucosidase inhibitors. Diabetes Res. Clin. Pract. 1998, 40, S51–S55. [Google Scholar] [CrossRef]
- Ogunyemi, O.M.; Gyebi, G.A.; Ibrahim, I.M.; Esan, A.M.; Olaiya, C.O.; Soliman, M.M.; Batiha, G.E.-S. Identification of promising multi-targeting inhibitors of obesity from Vernonia amygdalina through computational analysis. Mol. Divers. 2022, 27, 1–25. [Google Scholar] [CrossRef]
- Kalinovskii, A.P.; Sintsova, O.V.; Gladkikh, I.N.; Leychenko, E.V. Natural Inhibitors of Mammalian α-Amylases as Promising Drugs for the Treatment of Metabolic Diseases. Int. J. Mol. Sci. 2023, 24, 16514. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Atluri, S.; Zhang, Z.; Gidley, M.J.; Li, E.; Gilbert, R.G. Structural reasons for inhibitory effects of pectin on α-amylase enzyme activity and in-vitro digestibility of starch. Food Hydrocoll. 2021, 114, 106581. [Google Scholar] [CrossRef]
- Jiang, W.; Gao, J.; Ming, J.; Li, F. Influence of mulberry, pectin, rutin, and their combinations on α-amylase activity and glucose absorption during starch digestion. Food Chem. 2025, 465 Pt 2, 142136. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Guo, L.; Nsor-Atindana, J.; Douglas Goff, H.; Zhang, W.; Mao, J.; Zhong, F. The effect of viscous soluble dietary fiber on nutrient digestion and metabolic responses Ⅰ: In vitro digestion process. Food Hydrocoll. 2020, 107, 105971. [Google Scholar] [CrossRef]
- Schwartz, S.E.; Levine, R.A.; Singh, A.; Scheidecker, J.R.; Track, N.S. Sustained pectin ingestion delays gastric emptying. Gastroenterology 1982, 83, 812–817. [Google Scholar] [CrossRef]
- Maljaars, P.; Peters, H.; Mela, D.; Masclee, A. Ileal brake: A sensible food target for appetite control. A review. Physiol. Behav. 2008, 95, 271–281. [Google Scholar] [CrossRef]
- Layer, P.; Peschel, S.; Schlesinger, T.; Goebell, H. Human pancreatic secretion and intestinal motility: Effects of ileal nutrient perfusion. Am. J. Physiol. Liver Physiol. 1990, 258, G196–G201. [Google Scholar] [CrossRef]
- Wei, H.-Y.; Qi, J.-R.; Liao, J.-S.; Zhuo, T.; Xiao, R. Co-influence of the degree of esterification and degree of amidation on the properties of acid-sugar-calcium amidated pectin gels. Food Hydrocoll. 2025, 159, 110707. [Google Scholar] [CrossRef]
- Liu, Y.; Chao, C.; Yu, J.; Wang, S.; Wang, S.; Copeland, L. New insights into starch gelatinization by high pressure: Comparison with heat-gelatinization. Food Chem. 2020, 318, 126493. [Google Scholar] [CrossRef]
- Chen, R.; Williams, P.A.; Shu, J.; Luo, S.; Chen, J.; Liu, C. Pectin adsorption onto and penetration into starch granules and the effect on the gelatinization process and rheological properties. Food Hydrocoll. 2022, 129, 107618. [Google Scholar] [CrossRef]
- Zhuang, J.; Zhu, J.; Cheung, P.C.; Li, C. The physical and chemical interactions between starch and dietary fiber: Their impact on the physicochemical and nutritional properties of starch. Trends Food Sci. Technol. 2024, 149, 104566. [Google Scholar] [CrossRef]
- Ishwarya, S.P.; Nisha, P. Advances and prospects in the food applications of pectin hydrogels. Crit. Rev. Food Sci. Nutr. 2021, 62, 4393–4417. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, W.; Gong, S.; Gu, X.; Yu, Y.; Wu, J.; Wang, Z. Low and high methoxyl pectin lowers on structural change and digestibility of fried potato starch. LWT 2020, 132, 109853. [Google Scholar] [CrossRef]
- Yin, X.; Zheng, Y.; Kong, X.; Cao, S.; Chen, S.; Liu, D.; Ye, X.; Tian, J. RG-I pectin affects the physicochemical properties and digestibility of potato starch. Food Hydrocoll. 2021, 117, 106687. [Google Scholar] [CrossRef]
- Ye, S.; Shah, B.R.; Li, J.; Liang, H.; Zhan, F.; Geng, F.; Li, B. A critical review on interplay between dietary fibers and gut microbiota. Trends Food Sci. Technol. 2022, 124, 237–249. [Google Scholar] [CrossRef]
- Malard, F.; Dore, J.; Gaugler, B.; Mohty, M. Introduction to host microbiome symbiosis in health and disease. Mucosal Immunol. 2021, 14, 547–554. [Google Scholar] [CrossRef]
- Zhang, W.-Y.; Liao, J.-S.; Qi, J.-R. Modulation of gut microbiota by pectin: The critical role of degree of esterification and rhamnogalacturonan-I ratios. Food Biosci. 2025, 64, 105763. [Google Scholar] [CrossRef]
- Pascale, N.; Gu, F.; Larsen, N.; Jespersen, L.; Respondek, F. The Potential of Pectins to Modulate the Human Gut Microbiota Evaluated by In Vitro Fermentation: A Systematic Review. Nutrients 2022, 14, 3629. [Google Scholar] [CrossRef] [PubMed Central]
- Blanco-Pérez, F.H.; Steigerwald, S.; Schülke, S.; Vieths, M. The dietary fiber pectin: Health benefits and potential for the treatment of allergies by modulation of gut microbiota. Curr. Allergy Asthma Rep. 2021, 21, 43. [Google Scholar] [CrossRef]
- Tan, H.; Nie, S. Deciphering diet-gut microbiota-host interplay: Investigations of pectin. Trends Food Sci. Technol. 2020, 106, 171–181. [Google Scholar] [CrossRef]
- Meyers, G.R.; Samouda, H.; Bohn, T. Short Chain Fatty Acid Metabolism in Relation to Gut Microbiota and Genetic Variability. Nutrients 2022, 14, 5361. [Google Scholar] [CrossRef] [PubMed]
- Thriene, K.; Michels, K.B. Human Gut Microbiota Plasticity throughout the Life Course. Int. J. Environ. Res. Public Health 2023, 20, 1463. [Google Scholar] [CrossRef] [PubMed]
- Drula, E.; Garron, M.-L.; Dogan, S.; Lombard, V.; Henrissat, B.; Terrapon, N. The carbohydrate-active enzyme database: Functions and literature. Nucleic Acids Res. 2022, 50, D571–D577. [Google Scholar] [CrossRef] [PubMed]
- Tingirikari, J.M.R. Microbiota-accessible pectic poly- and oligosaccharides in gut health. Food Funct. 2018, 9, 5059–5073. [Google Scholar] [CrossRef]
- Palko-Łabuz, A.; Maksymowicz, J.; Sobieszczańska, B.; Wikiera, A.; Skonieczna, M.; Wesołowska, O.; Środa-Pomianek, K. Newly obtained apple pectin as an adjunct to irinotecan therapy of colorectal cancer reducing E. coli Adherence and β-glucuronidase activity. Cancers 2021, 13, 2952. [Google Scholar] [CrossRef]
- Wardman, J.F.; Bains, R.K.; Rahfeld, P.; Withers, S.G. Carbohydrate-active enzymes (CAZymes) in the gut microbiome. Nat. Rev. Microbiol. 2022, 20, 542–556. [Google Scholar] [CrossRef]
- Clarke, G.; Stilling, R.M.; Kennedy, P.J.; Stanton, C.; Cryan, J.F.; Dinan, T.G. Minireview: Gut Microbiota: The Neglected Endocrine Organ. Mol. Endocrinol. 2014, 28, 1221–1238. [Google Scholar] [CrossRef]
- Palm, C.L.; Nijholt, K.T.; Bakker, B.M.; Westenbrink, B.D. Short-chain fatty acids in the metabolism of heart failure—Rethinking the fat stigma. Front. Cardiovasc. Med. 2022, 9, 915102. [Google Scholar] [CrossRef]
- Fernandes, J.; Su, W.; Rahat-Rozenbloom, S.; Wolever, T.M.S.; Comelli, E.M. Adiposity, gut microbiota and faecal short chain fatty acids are linked in adult humans. Nutr. Diabetes 2014, 4, e121. [Google Scholar] [CrossRef]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; et al. Short-Chain Fatty-Acid-Producing Bacteria: Key Components of the Human Gut Microbiota. Nutrients 2023, 15, 2211. [Google Scholar] [CrossRef] [PubMed]
- Topping, D.L.; Clifton, P.M. Short-Chain Fatty Acids and Human Colonic Function: Roles of Resistant Starch and Nonstarch Polysaccharides. Physiol. Rev. 2001, 81, 1031–1064. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Scott, K.P.; Duncan, S.H.; Flint, H.J. Understanding the Effects of Diet on Bacterial Metabolism in the Large Intestine. J. Appl. Microbiol. 2007, 102, 1197–1208. [Google Scholar] [CrossRef] [PubMed]
- Fredstrom, S.B.; Lampe, J.W.; Jung, H.G.; Slavin, J.L. Apparent Fiber Digestibility and Fecal Short-Chain Fatty Acid Concentrations with Ingestion of Two Types of Dietary Fiber. J. Parenter. Enter. Nutr. 1994, 18, 14–19. [Google Scholar] [CrossRef]
- Zhang, D.; Jian, Y.-P.; Zhang, Y.-N.; Li, Y.; Gu, L.-T.; Sun, H.-H.; Liu, M.-D.; Zhou, H.-L.; Wang, Y.-S.; Xu, Z.-X. Short-chain fatty acids in diseases. Cell Commun. Signal. 2023, 21, 212. [Google Scholar] [CrossRef]
- Du, Y.; He, C.; An, Y.; Huang, Y.; Zhang, H.; Fu, W.; Wang, M.; Shan, Z.; Xie, J.; Yang, Y.; et al. The Role of Short Chain Fatty Acids in Inflammation and Body Health. Int. J. Mol. Sci. 2024, 25, 7379. [Google Scholar] [CrossRef]
- Hu, T.; Wu, Q.; Yao, Q.; Jiang, K.; Yu, J.; Tang, Q. Short-chain fatty acid metabolism and multiple effects on cardiovascular diseases. Ageing Res. Rev. 2022, 81, 101706. [Google Scholar] [CrossRef]
- Hanssen, N.M.J.; Kraakman, M.J.; Flynn, M.C.; Nagareddy, P.R.; Schalkwijk, C.G.; Murphy, A.J. Postprandial Glucose Spikes, an Important Contributor to Cardiovascular Disease in Diabetes? Front. Cardiovasc. Med. 2020, 7, 570553. [Google Scholar] [CrossRef]
- Alssema, M.; Ruijgrok, C.; Blaak, E.E.; Egli, L.; Dussort, P.; Vinoy, S.; Dekker, J.M.; Robertson, M.D. Effects of alpha-glucosidase-inhibiting drugs on acute postprandial glucose and insulin responses: A systematic review and meta-analysis. Nutr. Diabetes 2021, 11, 11. [Google Scholar] [CrossRef]
- Jones, M.; Gu, X.; Stebbins, N.; Crandall, P.; Ricke, S.; Lee, S. Effects of soybean pectin on blood glucose and insulin responses in healthy men. FASEB J. 2015, 29, 596.16. [Google Scholar] [CrossRef]
- Bianchi, F.; Larsen, N.; Tieghi, T.d.M.; Adorno, M.A.T.; Kot, W.; Saad, S.M.I.; Jespersen, L.; Sivieri, K. Modulation of gut microbiota from obese individuals by in vitro fermentation of citrus pectin in combination with Bifidobacterium longum BB-46. Appl. Microbiol. Biotechnol. 2018, 102, 8827–8840. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Martínez, E.; López-Ándres, N.; Jurado-López, R.; Rousseau, E.; Bartolomé, M.V.; Fernández-Celis, A.; Rossignol, P.; Islas, F.; Antequera, A.; Prieto, S.; et al. Galectin-3 participates in cardiovascular remodeling associated with obesity. Hypertension 2015, 66, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Seropian, I.M.; Cassaglia, P.; Miksztowicz, V.; González, G.E. Unraveling the role of galectin-3 in cardiac pathology and physiology. Front. Physiol. 2023, 14, 1304735. [Google Scholar] [CrossRef] [PubMed]
- Lou, J.; Zhang, B.; Zheng, Y.; Liu, M.; Qu, Y. Hawthorn pectin plays a protective role in myocardial ischaemia by regulating intestinal flora and short chain fatty acids. Curr. Res. Food Sci. 2024, 9, 100863. [Google Scholar] [CrossRef]
- Yellon, D.M.; Hausenloy, D.J. Myocardial reperfusion injury. N. Engl. J. Med. 2007, 357, 1121–1135. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, K.; Xu, C.; Chen, Z.; Jiang, H. Anti-inflammatory effect of sodium butyrate preconditioning during myocardial ischemia/reperfusion. Exp. Ther. Med. 2014, 8, 229–232. [Google Scholar] [CrossRef]
- Hu, X.; Fu, W.; Jiang, H. HMGB1: A potential therapeutic target for myocardial ischemia and reperfusion injury. Int. J. Cardiol. 2012, 155, 489. [Google Scholar] [CrossRef]
- Onyszkiewicz, M.; Gawrys-Kopczynska, M.; Konopelski, P.; Aleksandrowicz, M.; Sawicka, A.; Koźniewska, E.; Samborowska, E.; Ufnal, M. Butyric acid, a gut bacteria metabolite, lowers arterial blood pressure via colon-vagus nerve signaling and GPR41/43 receptors. Pflug. Arch. 2019, 471, 1441–1453. [Google Scholar] [CrossRef]
- Yu, C.D.; Xu, Q.J.; Chang, R.B. Vagal sensory neurons and gut-brain signaling. Curr. Opin. Neurobiol. 2020, 62, 133–140. [Google Scholar] [CrossRef]
- Yu, Z.; Han, J.; Chen, H.; Wang, Y.; Zhou, L.; Wang, M.; Zhang, R.; Jin, X.; Zhang, G.; Wang, C.; et al. Oral supplementation with butyrate improves myocardial ischemia/reperfusion injury via a gut-brain neural circuit. Front. Cardiovasc. Med. 2021, 8, 718674. [Google Scholar] [CrossRef]
- Xue, H.; Zhao, Z.; Lin, Z.; Geng, J.; Guan, Y.; Song, C.; Zhou, Y.; Tai, G. Selective effects of ginseng pectins on galectin-3-mediated T cell activation and apoptosis. Carbohydr. Polym. 2019, 219, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Emran, T.B.; Islam, F.; Mitra, S.; Paul, S.; Nath, N.; Khan, Z.; Das, R.; Chandran, D.; Sharma, R.; Lima, C.M.G.; et al. Pectin: A Bioactive Food Polysaccharide with Cancer Preventive Potential. Molecules 2022, 27, 7405. [Google Scholar] [CrossRef] [PubMed]
- Picot-Allain, M.C.N.; Neergheen, V.S. Pectin a multifaceted biopolymer in the management of cancer: A review. Heliyon 2023, 9, e22236. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.; Dou, Z.; Hou, K.; Wang, W.; Chen, X.; Chen, X.; Chen, H.; Fu, X. A critical review of RG-I pectin: Sources, extraction methods, structure, and applications. Crit. Rev. Food Sci. Nutr. 2024, 64, 8911–8931. [Google Scholar] [CrossRef]
- Jin, H.; Li, M.; Tian, F.; Yu, F.; Zhao, W. An Overview of Antitumour Activity of Polysaccharides. Molecules 2022, 27, 8083. [Google Scholar] [CrossRef]
- Parada Venegas, D.; De La Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]
- Vio, F.; Domper, A.; González, C.G.; Fonseca, L.; Barrios, L.; Zacarías, I. (Eds.) 20 Años Programa 5 al día en Chile. Promoviendo el Consumo de Frutas y Verduras; Mamarracho Comunicaciones; Universidad de Chile: Santiago, Chile, 2023; ISBN 978-956-416-182-2. [Google Scholar]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to pectins and reduction of post-prandial glycaemic responses (ID 786), maintenance of normal blood cholesterol concentrations (ID 818) and increase in satiety leading to a reduction in energy intake (ID 4692) pursuant to Article 13(1) of Regulation (EC) No1924/2006. Eur. Food Saf. Auth. 2010, 8, 1747. [Google Scholar]
- Bersamin, A.; Hernández-Garbanzo, Y.; Atoloye, A.T.; Gonzalez, J.U.; Ríos-Castillo, I.; Oo, T.T.M.; Banna, J.; El Shikieri, A.; Bonsi, E. Growing Our Commitment to Promoting Fruits and Vegetables: Looking Beyond the International Year of Fruits and Vegetables. J. Nutr. Educ. Behav. 2021, 53, 909–910. [Google Scholar] [CrossRef]
- Vio, F. Año internacional de frutas y verduras 2021 [International year of fruits and vegetables 2021]. Rev. Med. Chile 2022, 150, 131–132. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.; Mytton, O.; White, M.; Monsivais, P. Why are some population interventions for diet and obesity more equitable and effective than others? The role of individual agency. PLoS Med. 2016, 13, e1001990. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valladares, L.; Vio, F. Pectin and Its Beneficial Effect on Health: New Contributions in Research and the Need to Increase Fruits and Vegetables Consumption—A Review. Int. J. Mol. Sci. 2025, 26, 6852. https://doi.org/10.3390/ijms26146852
Valladares L, Vio F. Pectin and Its Beneficial Effect on Health: New Contributions in Research and the Need to Increase Fruits and Vegetables Consumption—A Review. International Journal of Molecular Sciences. 2025; 26(14):6852. https://doi.org/10.3390/ijms26146852
Chicago/Turabian StyleValladares, Luis, and Fernando Vio. 2025. "Pectin and Its Beneficial Effect on Health: New Contributions in Research and the Need to Increase Fruits and Vegetables Consumption—A Review" International Journal of Molecular Sciences 26, no. 14: 6852. https://doi.org/10.3390/ijms26146852
APA StyleValladares, L., & Vio, F. (2025). Pectin and Its Beneficial Effect on Health: New Contributions in Research and the Need to Increase Fruits and Vegetables Consumption—A Review. International Journal of Molecular Sciences, 26(14), 6852. https://doi.org/10.3390/ijms26146852





