Screening, Characterization and Comparison of Endoglucanases/Xylanases from Thermophilic Fungi: A Thielavia terrestris Xylanase with High Activity-Stability Properties
Abstract
1. Introduction
2. Results
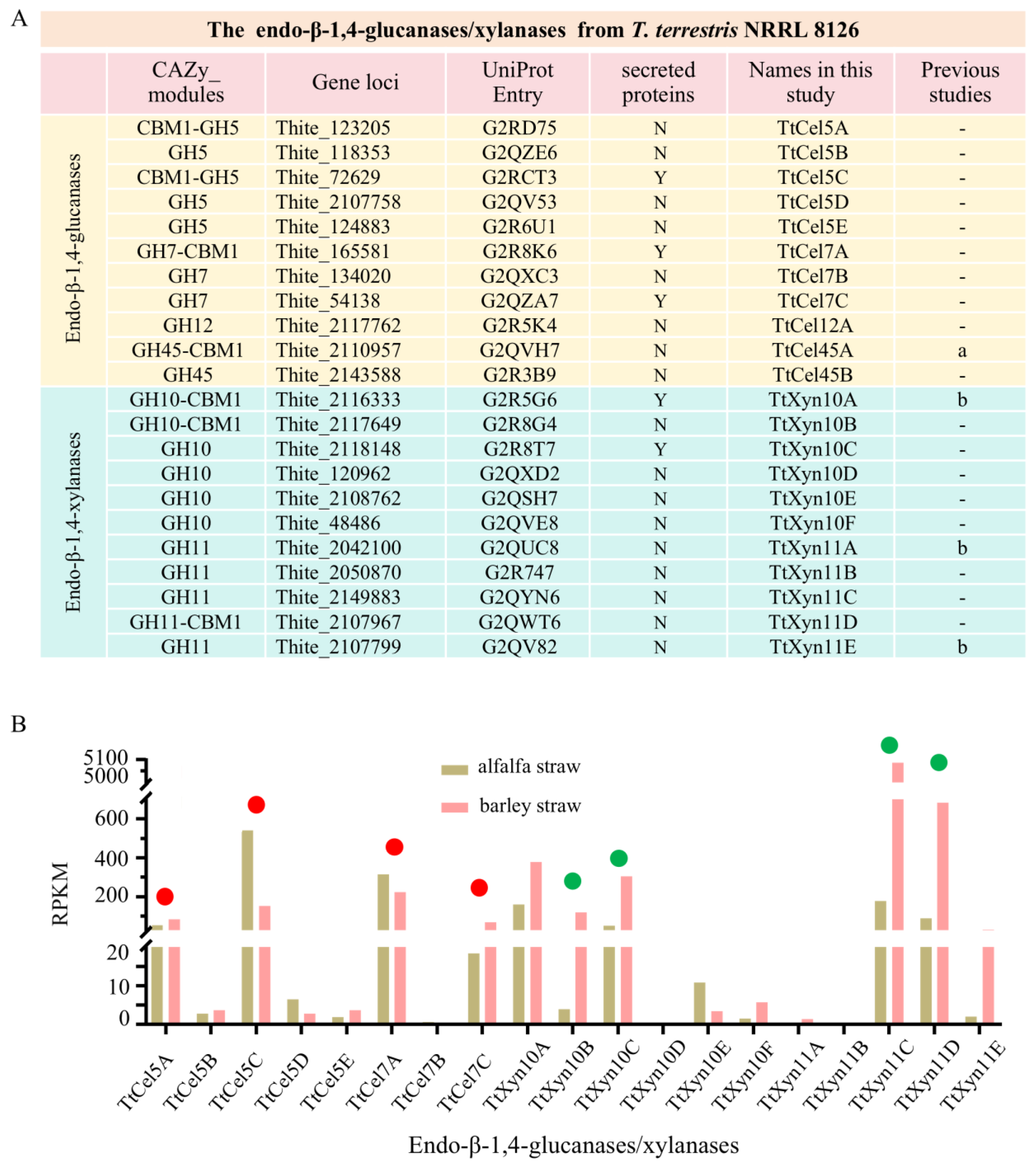
2.1. The Selection of Target Endo-β-1,4-Endoglucanases/Xylanases in T. terrestris
2.2. Recombinant TtCel5A and TtXyn10B/10C Exhibit High Optimum Temperatures
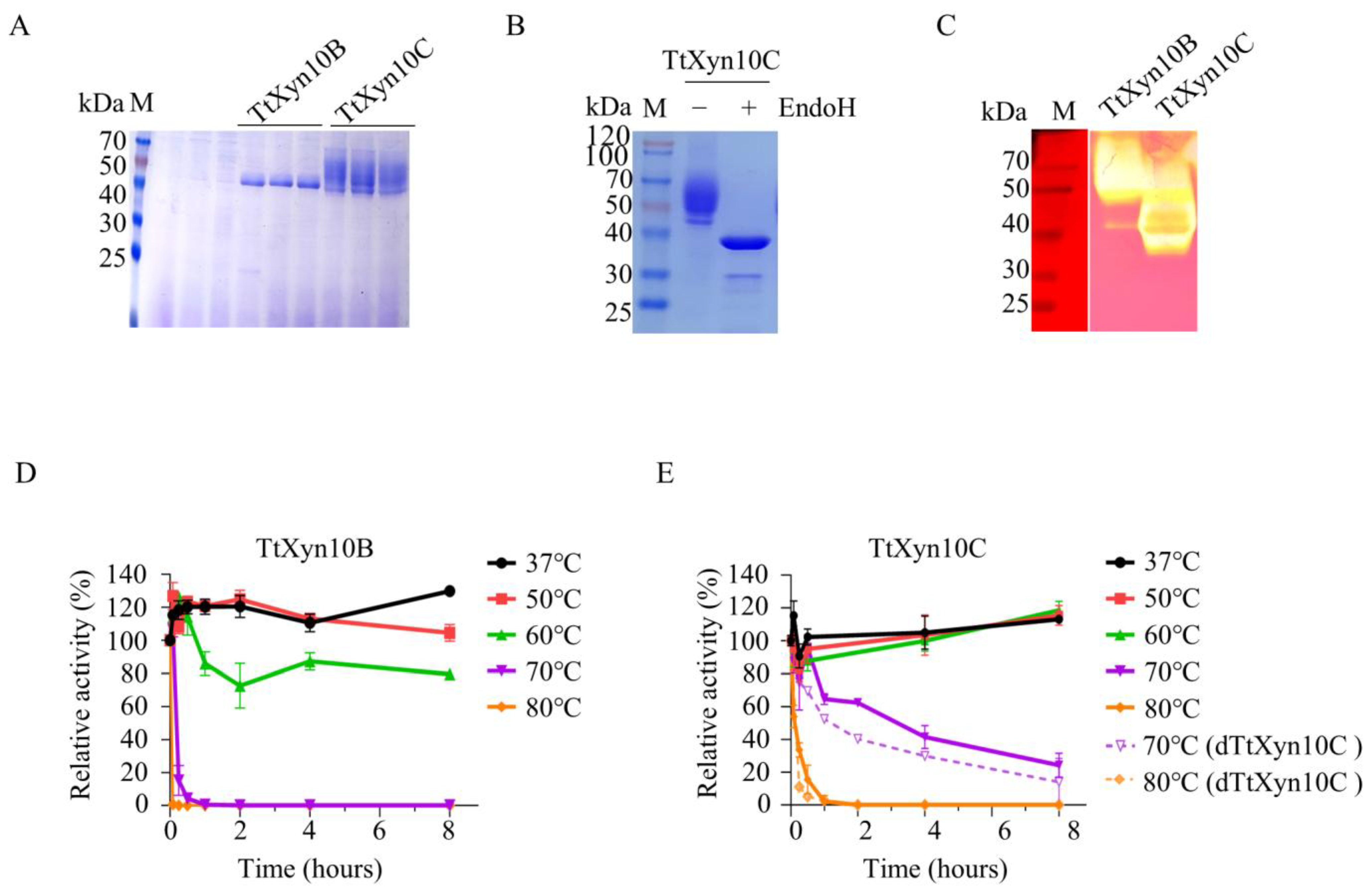
2.3. Recombinant TtXyn10C Exhibits Outstanding Thermostability Properties
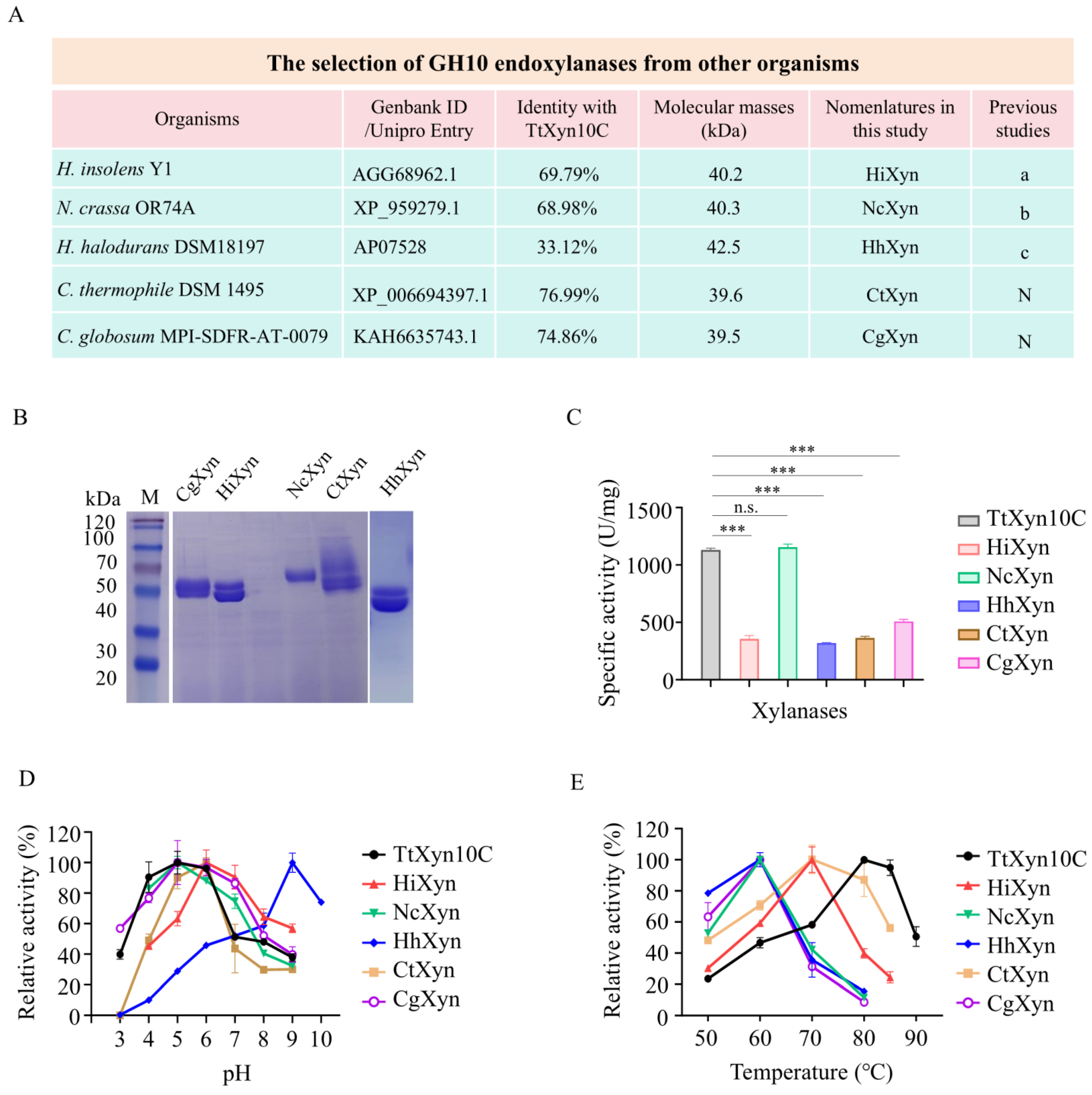
2.4. Recombinant TtXyn10C Shows High Enzymatic Activity
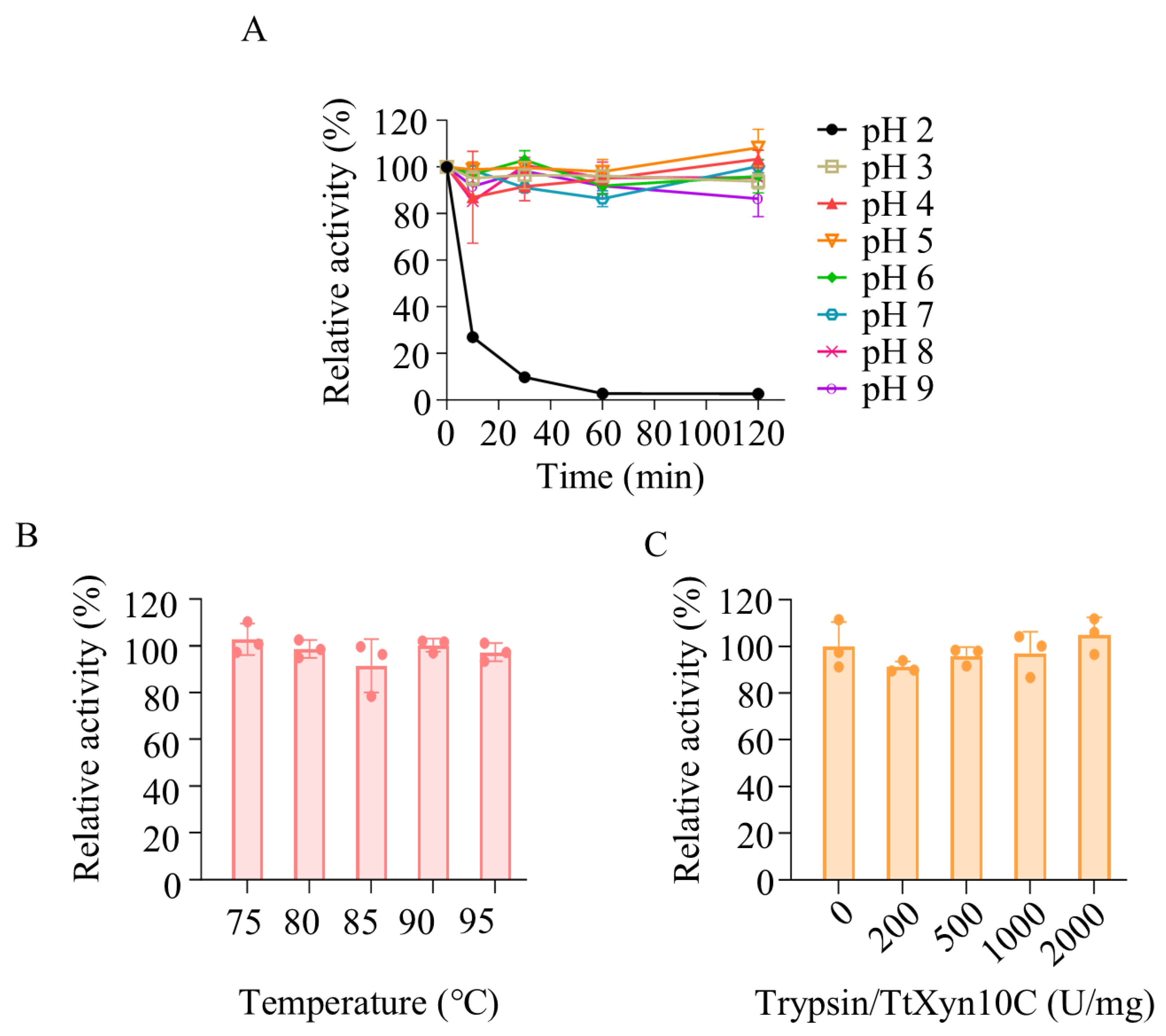
2.5. Recombinant TtXyn10C Exhibits Stability Across a Broad pH Range, Brief High-Temperature Exposure, and Trypsin
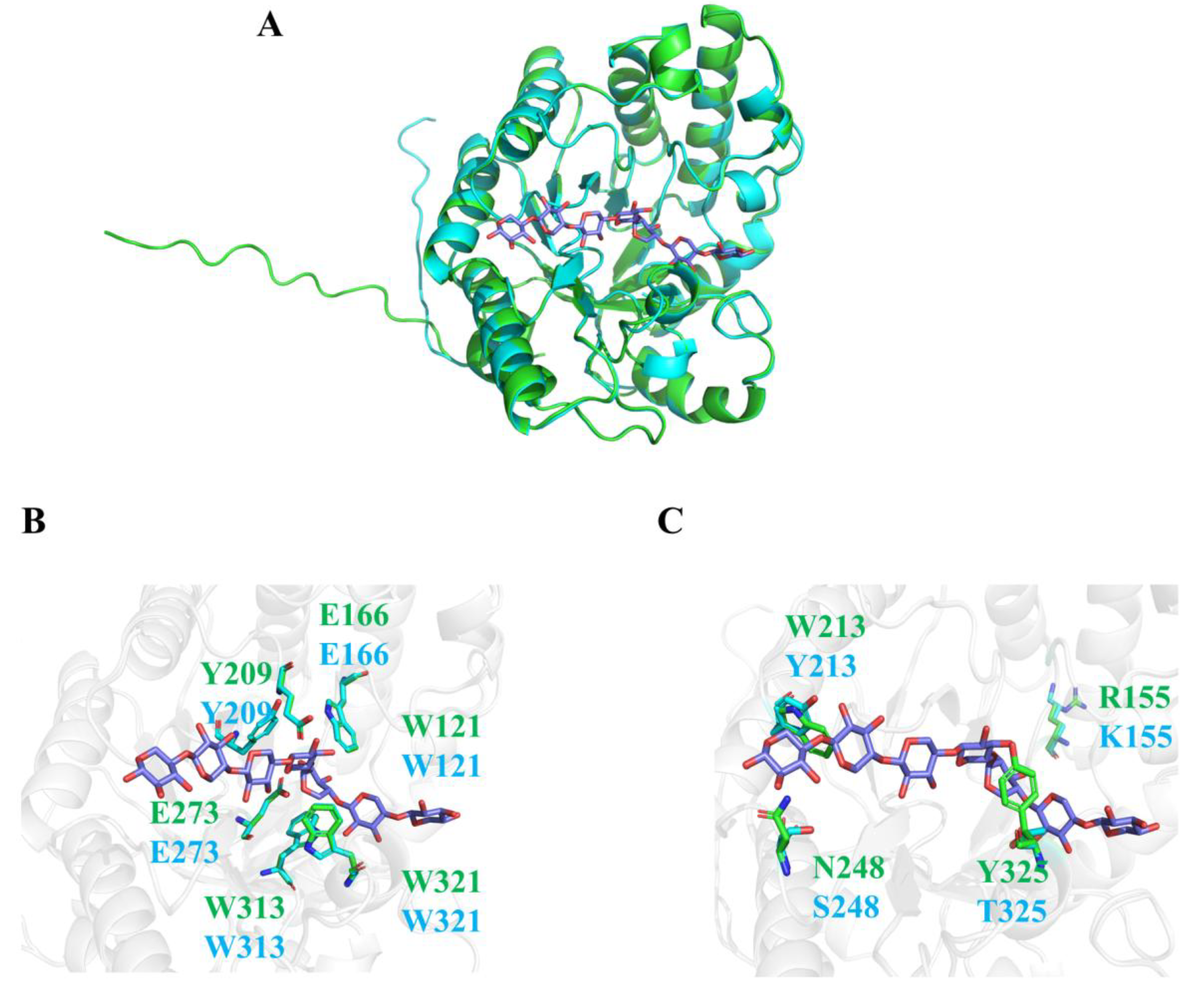
2.6. Structural Comparison of TtXyn10C and CtXyn Proteins
3. Discussion
4. Materials and Methods
4.1. Strains and Plasmids Construction
4.2. Protein Expression and Purification
4.3. In-Gel Xylanase Activity Staining
4.4. Activity Assay of Endoglucanases and Endoxylanases
4.5. pH Profiles of Endoglucanases and Endoxylanases
4.6. Temperature Profiles of Endoglucanases and Endoxylanases
4.7. Trypsin Resistance Assays
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lynd, L.R.; Weimer, P.J.; Van Zyl, W.H.; Pretorius, I.S. Microbial cellulose utilization: Fundamentals and biotechnology. Microbiol. Mol. Biol. Rev. 2002, 66, 506–577. [Google Scholar] [CrossRef]
- Qaseem, M.F.; Zhang, W.; Dupree, P.; Wu, A.M. Xylan structural diversity, biosynthesis, and functional regulation in plants. Int. J. Biol. Macromol. 2025, 291, 138866. [Google Scholar] [CrossRef]
- Li, X.; Dilokpimol, A.; Kabel, M.A.; De Vries, R.P. Fungal xylanolytic enzymes: Diversity and applications. Bioresour. Technol. 2022, 344, 126290. [Google Scholar] [CrossRef]
- Wilson, D.B. Cellulases and biofuels. Curr. Opin. Biotechnol. 2009, 20, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, U.; Sohail, M.; Ghanemi, A. Cellulases: From Bioactivity to a Variety of Industrial Applications. Biomimetics 2021, 6, 44. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Singh, S.; Singh, O.V. Bioconversion of lignocellulosic biomass: Biochemical and molecular perspectives. J. Ind. Microbiol. Biotechnol. 2008, 35, 377–391. [Google Scholar] [CrossRef] [PubMed]
- Billard, H.; Faraj, A.; Lopes Ferreira, N.; Menir, S.; Heiss-Blanquet, S. Optimization of a synthetic mixture composed of major Trichoderma reesei enzymes for the hydrolysis of steam-exploded wheat straw. Biotechnol. Biofuels. 2012, 5, 9. [Google Scholar] [CrossRef]
- Kaur, D.; Joshi, A.; Sharma, V.; Batra, N.; Sharma, A.K. An insight into microbial sources, classification, and industrial applications of xylanases: A rapid review. Biotechnol. Appl. Biochem. 2023, 70, 1489–1503. [Google Scholar] [CrossRef]
- Alnoch, R.C.; Alves, G.S.; Salgado, J.C.S.; de Andrades, D.; Freitas, E.N.; Nogueira, K.M.V.; Vici, A.C.; Oliveira, D.P.; Carvalho-Jr, V.P.; Silva, R.N.; et al. Immobilization and Application of the Recombinant Xylanase GH10 of Malbranchea pulchella in the Production of Xylooligosaccharides from Hydrothermal Liquor of the Eucalyptus (Eucalyptus grandis) Wood Chips. Int. J. Mol. Sci. 2022, 23, 13329. [Google Scholar] [CrossRef]
- Mendonca, M.; Barroca, M.; Collins, T. Endo-1,4-beta-xylanase-containing glycoside hydrolase families: Characteristics, singularities and similarities. Biotechnol. Adv. 2023, 65, 108148. [Google Scholar] [CrossRef]
- Lo Leggio, L.; Kalogiannis, S.; Eckert, K.; Teixeira, S.C.; Bhat, M.K.; Andrei, C.; Pickersgill, R.W.; Larsen, S. Substrate specificity and subsite mobility in T. aurantiacus xylanase 10A. FEBS Lett. 2001, 509, 303–308. [Google Scholar] [CrossRef]
- Zolotnitsky, G.; Cogan, U.; Adir, N.; Solomon, V.; Shoham, G.; Shoham, Y. Mapping glycoside hydrolase substrate subsites by isothermal titration calorimetry. Proc. Natl. Acad. Sci. USA 2004, 101, 11275–11280. [Google Scholar] [CrossRef]
- Vandermarliere, E.; Bourgois, T.M.; Rombouts, S.; van Campenhout, S.; Volckaert, G.; Strelkov, S.V.; Delcour, J.A.; Rabijns, A.; Courtin, C.M. Crystallographic analysis shows substrate binding at the −3 to +1 active-site subsites and at the surface of glycoside hydrolase family 11 endo-1,4-beta-xylanases. Biochem. J. 2008, 410, 71–79. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, X.; Li, C.; Kovalevsky, A.; Wan, Q. Studying the Role of a Single Mutation of a Family 11 Glycoside Hydrolase Using High-Resolution X-ray Crystallography. Protein J. 2020, 39, 671–680. [Google Scholar] [CrossRef]
- Paes, G.; Berrin, J.G.; Beaugrand, J. GH11 xylanases: Structure/function/properties relationships and applications. Biotechnol. Adv. 2012, 30, 564–592. [Google Scholar] [CrossRef] [PubMed]
- Berka, R.M.; Grigoriev, I.V.; Otillar, R.; Salamov, A.; Grimwood, J.; Reid, I.; Ishmael, N.; John, T.; Darmond, C.; Moisan, M.C.; et al. Comparative genomic analysis of the thermophilic biomass-degrading fungi Myceliophthora thermophila and Thielavia terrestris. Nat. Biotechnol. 2011, 29, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Ajeje, S.B.; Hu, Y.; Song, G.; Peter, S.B.; Afful, R.G.; Sun, F.; Asadollahi, M.A.; Amiri, H.; Abdulkhani, A.; Sun, H. Thermostable Cellulases/Xylanases From Thermophilic and Hyperthermophilic Microorganisms: Current Perspective. Front. Bioeng. Biotechnol. 2021, 9, 794304. [Google Scholar] [CrossRef]
- Xu, K.; Fu, H.; Chen, Q.; Sun, R.; Li, R.; Zhao, X.; Zhou, J.; Wang, X. Engineering thermostability of industrial enzymes for enhanced application performance. Int. J. Biol. Macromol. 2025, 291, 139067. [Google Scholar] [CrossRef]
- Karaoglu, H.; Ramadan, K.M.A.; Al Hashedi, S.A.; AlShoaibi, A.; Iqbal, Z.; Aydın, R.; Secgin, B.A.; Secgin, Z.; Bendary, E.S.A.; Mahmoud, M.A.A.; et al. Selection, heterologous production, and functional characterization of a thermostable xylanase from anoxybacillus for dough and bread quality enhancement. Int. J. Biol. Macromol. 2025, 312, 144000. [Google Scholar] [CrossRef]
- Patel, D.K.; Rawat, R.; Sharma, S.; Shah, K.; Borsadiya, N.; Dave, G. Linker-assisted engineering of chimeric xylanase-phytase for improved thermal tolerance of feed enzymes. J. Biomol. Struct. Dyn. 2024, 42, 8114–8124. [Google Scholar] [CrossRef]
- Singh, B. Myceliophthora thermophila syn. Sporotrichum thermophile: A thermophilic mould of biotechnological potential. Crit. Rev. Biotechnol. 2016, 36, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.R.M.; Mohamed, S.G.A.; Altyar, A.E.; Mohamed, G.A. Natural Products of the Fungal Genus Humicola: Diversity, Biological Activity, and Industrial Importance. Curr. Microbiol. 2021, 78, 2488–2509. [Google Scholar] [CrossRef] [PubMed]
- Amlacher, S.; Sarges, P.; Flemming, D.; Van Noort, V.; Kunze, R.; Devos, D.P.; Arumugam, M.; Bork, P.; Hurt, E. Insight into structure and assembly of the nuclear pore complex by utilizing the genome of a eukaryotic thermophile. Cell 2011, 146, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Ghaffar, A.; Khan, S.A.; Mukhtar, Z.; Rajoka, M.I.; Latif, F. Heterologous expression of a gene for thermostable xylanase from Chaetomium thermophilum in Pichia pastoris GS115. Mol. Biol. Rep. 2011, 38, 3227–3233. [Google Scholar] [CrossRef]
- Zhou, Q.; Ji, P.; Zhang, J.; Li, X.; Han, C. Characterization of a novel thermostable GH45 endoglucanase from Chaetomium thermophilum and its biodegradation of pectin. J. Biosci. Bioeng. 2017, 124, 271–276. [Google Scholar] [CrossRef]
- Hua, C.; Li, W.; Han, W.; Wang, Q.; Bi, P.; Han, C.; Zhu, L. Characterization of a novel thermostable GH7 endoglucanase from Chaetomium thermophilum capable of xylan hydrolysis. Int. J. Biol. Macromol. 2018, 117, 342–349. [Google Scholar] [CrossRef]
- Caputo, F.; Siaperas, R.; Dias, C.; Nikolaivits, E.; Olsson, L. Elucidating Thermothielavioides terrestris secretome changes for improved saccharification of mild steam-pretreated spruce. Biotechnol. Biofuels Bioprod. 2024, 17, 127. [Google Scholar] [CrossRef]
- Velasco, J.; Oliva, B.; Goncalves, A.L.; Lima, A.S.; Ferreira, G.; Franca, B.A.; Mulinari, E.J.; Goncalves, T.A.; Squina, F.M.; Kadowaki, M.A.S.; et al. Functional characterization of a novel thermophilic exo-arabinanase from Thermothielavioides terrestris. Appl. Microbiol. Biotechnol. 2020, 104, 8309–8326. [Google Scholar] [CrossRef]
- Meng, Z.; Yang, Q.Z.; Wang, J.Z.; Hou, Y.H. Cloning, Characterization, and Functional Expression of a Thermostable Type B Feruloyl Esterase from Thermophilic Thielavia Terrestris. Appl. Biochem. Biotechnol. 2019, 189, 1304–1317. [Google Scholar] [CrossRef]
- Gao, J.; Huang, J.W.; Li, Q.; Liu, W.; Ko, T.P.; Zheng, Y.; Xiao, X.; Kuo, C.J.; Chen, C.C.; Guo, R.T. Characterization and crystal structure of a thermostable glycoside hydrolase family 45 1,4-beta-endoglucanase from Thielavia terrestris. Enzyme Microb. Technol. 2017, 99, 32–37. [Google Scholar] [CrossRef]
- Du, Y.; Shi, P.; Huang, H.; Zhang, X.; Luo, H.; Wang, Y.; Yao, B. Characterization of three novel thermophilic xylanases from Humicola insolens Y1 with application potentials in the brewing industry. Bioresour. Technol. 2013, 130, 161–167. [Google Scholar] [CrossRef]
- Nakamura, S.; Nakai, R.; Wakabayashi, K.; Ishiguro, Y.; Aono, R.; Horikoshi, K. Thermophilic Alkaline Xylanase from Newly Isolated Alkaliphilic and Thermophilic Bacillus sp. Strain TAR-1. Biosci. Biotechnol. Biochem. 1994, 58, 78–81. [Google Scholar] [CrossRef]
- Wang, R.; Arioka, M. Functional analyses of xylanolytic enzymes involved in xylan degradation and utilization in Neurospora crassa. Int. J. Biol. Macromol. 2021, 169, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Takita, T.; Kuwata, K.; Nakatani, K.; Li, T.; Katano, Y.; Kojima, K.; Mizutani, K.; Mikami, B.; Yatsunami, R.; et al. Insight into the mechanism of thermostabilization of GH10 xylanase from Bacillus sp. strain TAR-1 by the mutation of S92 to E. Biosci. Biotechnol. Biochem. 2021, 85, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Future Market Insights. Xylanase Market Analysis—Size, Share & Forecast 2025 to 2035. 2023. Available online: https://www.futuremarketinsights.com/reports/xylanase-market (accessed on 11 June 2025).
- You, S.; Chen, C.C.; Tu, T.; Wang, X.; Ma, R.; Cai, H.Y.; Guo, R.T.; Luo, H.Y.; Yao, B. Insight into the functional roles of Glu175 in the hyperthermostable xylanase XYL10C-ΔN through structural analysis and site-saturation mutagenesis. Biotechnol. Biofuels 2018, 11, 159. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Huante, Y.; Cayetano-Cruz, M.; Santiago-Hernandez, A.; Cano-Ramirez, C.; Marsch-Moreno, R.; Campos, J.E.; Aguilar-Osorio, G.; Benitez-Cardoza, C.G.; Trejo-Estrada, S.; Hidalgo-Lara, M.E. The thermophilic biomass-degrading fungus Thielavia terrestris Co3Bag1 produces a hyperthermophilic and thermostable beta-1,4-xylanase with exo- and endo-activity. Extremophiles 2017, 21, 175–186. [Google Scholar] [CrossRef]
- Lopez-Lopez, A.; Santiago-Hernandez, A.; Cayetano-Cruz, M.; Garcia-Huante, Y.; Campos, J.E.; Bustos-Jaimes, I.; Marsch-Moreno, R.; Cano-Ramirez, C.; Benitez-Cardoza, C.G.; Hidalgo-Lara, M.E. TtCel7A: A Native Thermophilic Bifunctional Cellulose/Xylanase Exogluclanase from the Thermophilic Biomass-Degrading Fungus Thielavia terrestris Co3Bag1, and Its Application in Enzymatic Hydrolysis of Agroindustrial Derivatives. J. Fungi 2023, 9, 152. [Google Scholar] [CrossRef]
- Zerva, A.; Limnaios, A.; Kritikou, A.S.; Thomaidis, N.S.; Taoukis, P.; Topakas, E. A novel thermophile beta-galactosidase from Thermothielavioides terrestris producing galactooligosaccharides from acid whey. N. Biotechnol. 2021, 63, 45–53. [Google Scholar] [CrossRef]
- Long, L.; Sun, L.; Lin, Q.; Ding, S.; St John, F.J. Characterization and functional analysis of two novel thermotolerant alpha-L-arabinofuranosidases belonging to glycoside hydrolase family 51 from Thielavia terrestris and family 62 from Eupenicillium parvum. Appl. Microbiol. Biotechnol. 2020, 104, 8719–8733. [Google Scholar] [CrossRef]
- Woon, J.S.; Mackeen, M.M.; Mahadi, N.M.; Illias, R.M.; Abdul Murad, A.M.; Abu Bakar, F.D. Expression and characterization of a cellobiohydrolase (CBH7B) from the thermophilic fungus Thielavia terrestris in Pichia pastoris. Biotechnol. Appl. Biochem. 2016, 63, 690–698. [Google Scholar] [CrossRef]
- Rey, M.W.; Brown, K.M.; Golightly, E.J.; Fuglsang, C.C.; Nielsen, B.R.; Hendriksen, H.V.; Butterworth, A.; Xu, F. Cloning, heterologous expression, and characterization of Thielavia terrestris glucoamylase. Appl. Biochem. Biotechnol. 2003, 111, 153–166. [Google Scholar] [CrossRef]
- Xu, H.; Yan, Q.; Duan, X.; Yang, S.; Jiang, Z. Characterization of an acidic cold-adapted cutinase from Thielavia terrestris and its application in flavor ester synthesis. Food Chem. 2015, 188, 439–445. [Google Scholar] [CrossRef]
- Tõlgo, M.; Hegnar, O.A.; Østby, H.; Várnai, A.; Vilaplana, F.; Eijsink, V.G.H.; Olsson, L. Comparison of Six Lytic Polysaccharide Monooxygenases from Thermothielavioides terrestris Shows That Functional Variation Underlies the Multiplicity of LPMO Genes in Filamentous Fungi. Appl. Environ. Microbiol. 2022, 88, e0009622. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, M.; Lakizadeh, A.; Agha-Golzadeh, P.; Ebrahimie, E.; Ebrahimi, M. Prediction of thermostability from amino acid attributes by combination of clustering with attribute weighting: A new vista in engineering enzymes. PLoS ONE 2011, 6, e23146. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.T.; Duarte, M.E.; Holanda, D.M.; Kim, S.W. Friend or Foe? Impacts of Dietary Xylans, Xylooligosaccharides, and Xylanases on Intestinal Health and Growth Performance of Monogastric Animals. Animals 2021, 11, 609. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Wan, X. Engineering a Trypsin-Resistant Thermophilic alpha-Galactosidase to Enhance Pepsin Resistance, Acidic Tolerance, Catalytic Performance, and Potential in the Food and Feed Industry. J. Agric. Food Chem. 2020, 68, 10560–10573. [Google Scholar] [CrossRef]
- Dhaver, P.; Pletschke, B.; Sithole, B.; Govinden, R. Optimization of Xylooligosaccharides Production by Native and Recombinant Xylanase Hydrolysis of Chicken Feed Substrates. Int. J. Mol. Sci. 2023, 24, 17110. [Google Scholar] [CrossRef]
- Gonçalves, G.A.; Takasugi, Y.; Jia, L.; Mori, Y.; Noda, S.; Tanaka, T.; Ichinose, H.; Kamiya, N. Synergistic effect and application of xylanases as accessory enzymes to enhance the hydrolysis of pretreated bagasse. Enzym. Microb. Technol. 2015, 72, 16–24. [Google Scholar] [CrossRef]
- Zhang, J.; Tuomainen, P.; Siika-Aho, M.; Viikari, L. Comparison of the synergistic action of two thermostable xylanases from GH families 10 and 11 with thermostable cellulases in lignocellulose hydrolysis. Bioresour. Technol. 2011, 102, 9090–9095. [Google Scholar] [CrossRef]
- Wang, S.; Han, H.; Dong, J.; Li, M.; Wang, Q.; Wang, L.; Wu, X.; Cui, H.; Tian, Y.; Han, C. A novel GH11 β-1,4-xylanase from Fusarium verticillioides: Its eukaryotic expression, biochemical characterization and synergistic effect with cellulase on lignocellulosic biomass degradation. Int. J. Biol. Macromol. 2025, 305 Pt. 1, 141169. [Google Scholar] [CrossRef]
- Luo, H.; Li, J.; Yang, J.; Wang, H.; Yang, Y.; Huang, H.; Shi, P.; Yuan, T.; Fan, Y.; Yao, B. A thermophilic and acid stable family-10 xylanase from the acidophilic fungus Bispora sp. MEY-1. Extremophiles 2009, 13, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, K.S. Defying the activity-stability trade-off in enzymes: Taking advantage of entropy to enhance activity and thermostability. Crit. Rev. Biotechnol. 2017, 37, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Kurahashi, R.; Tanaka, S.I.; Takano, K. Activity-stability trade-off in random mutant proteins. J. Biosci. Bioeng. 2019, 128, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Enkhbaatar, B.; Lee, C.R.; Hong, Y.S.; Hong, S.K. Molecular Characterization of Xylobiose- and Xylopentaose-Producing beta-1,4-Endoxylanase SCO5931 from Streptomyces coelicolor A3(2). Appl. Biochem. Biotechnol. 2016, 180, 349–360. [Google Scholar] [CrossRef]
- Hou, Q.; Rooman, M.; Pucci, F. Enzyme Stability-Activity Trade-Off: New Insights from Protein Stability Weaknesses and Evolutionary Conservation. J. Chem. Theory Comput. 2023, 19, 3664–3671. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, T.; Zhai, Q.; Chen, Q.; Zhang, X.; Chen, Y.; He, W.; Li, J.; Fan, J.; Tao, J.; et al. Two-step computational redesign of Bacillus subtilis cellulase and beta-glucanase for enhanced thermostability and activity. Int. J. Biol. Macromol. 2025, 285, 138274. [Google Scholar] [CrossRef]
- Li, T.; Wang, R.; Hua, B.; Cao, L.; Zhang, Q.; Zhai, Y.; Ling, S.; Wang, M.; Li, E. Improving the Thermal Stability of GH11 Xylanase XynASP through Cord Region Engineering. J. Agric. Food Chem. 2025, 73, 1516–1528. [Google Scholar] [CrossRef]
- Li, Z.; Li, X.; Zhou, Y.; Lu, M.; Li, M.; Zhu, Y.; Wan, Q. Rational design of GH11 xylanase to balance the activity-stability trade-off. Int. J. Biol. Macromol. 2025, 311 Pt. 1, 143063. [Google Scholar] [CrossRef]
- Zheng, N.; Cai, Y.; Zhang, Z.; Zhou, H.; Deng, Y.; Du, S.; Tu, M.; Fang, W.; Xia, X. Tailoring industrial enzymes for thermostability and activity evolution by the machine learning-based iCASE strategy. Nat. Commun. 2025, 16, 604. [Google Scholar] [CrossRef]
- Vucinic, J.; Novikov, G.; Montanier, C.Y.; Dumon, C.; Schiex, T.; Barbe, S. A Comparative Study to Decipher the Structural and Dynamics Determinants Underlying the Activity and Thermal Stability of GH-11 Xylanases. Int. J. Mol. Sci. 2021, 22, 5961. [Google Scholar] [CrossRef]
- Li, Z.; Yao, G.; Wu, R.; Gao, L.; Kan, Q.; Liu, M.; Yang, P.; Liu, G.; Qin, Y.; Song, X.; et al. Synergistic and Dose-Controlled Regulation of Cellulase Gene Expression in Penicillium oxalicum. PLoS Genet. 2015, 11, e1005509. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, S.; Ma, K.; Chen, Z.; Zhao, J.; Song, X.; Qin, Y. Screening, Characterization and Comparison of Endoglucanases/Xylanases from Thermophilic Fungi: A Thielavia terrestris Xylanase with High Activity-Stability Properties. Int. J. Mol. Sci. 2025, 26, 6849. https://doi.org/10.3390/ijms26146849
Xu S, Ma K, Chen Z, Zhao J, Song X, Qin Y. Screening, Characterization and Comparison of Endoglucanases/Xylanases from Thermophilic Fungi: A Thielavia terrestris Xylanase with High Activity-Stability Properties. International Journal of Molecular Sciences. 2025; 26(14):6849. https://doi.org/10.3390/ijms26146849
Chicago/Turabian StyleXu, Shaohua, Kexuan Ma, Zixiang Chen, Jian Zhao, Xin Song, and Yuqi Qin. 2025. "Screening, Characterization and Comparison of Endoglucanases/Xylanases from Thermophilic Fungi: A Thielavia terrestris Xylanase with High Activity-Stability Properties" International Journal of Molecular Sciences 26, no. 14: 6849. https://doi.org/10.3390/ijms26146849
APA StyleXu, S., Ma, K., Chen, Z., Zhao, J., Song, X., & Qin, Y. (2025). Screening, Characterization and Comparison of Endoglucanases/Xylanases from Thermophilic Fungi: A Thielavia terrestris Xylanase with High Activity-Stability Properties. International Journal of Molecular Sciences, 26(14), 6849. https://doi.org/10.3390/ijms26146849






