Abstract
Oral squamous cell carcinoma (OSCC) remains a significant global health challenge, representing 90% of oral malignancies. Despite therapeutic advances, patient outcomes remain poor, highlighting the need for novel prognostic biomarkers and treatment targets. We investigated the expression patterns of NTRK genes and their corresponding proteins (TrkA, TrkB, and TrkC) in OSCC, analyzing their relationships with clinical outcomes and potential as therapeutic targets. We examined 93 OSCC tissue samples using immunohistochemistry and quantitative real-time PCR. Protein expression was quantified using the H-score method. We analyzed correlations between Trk expression, clinicopathological parameters, and 2-year survival rates using chi-square tests, Mann–Whitney U tests, and Kaplan–Meier survival analysis. TrkA showed near-universal expression (97.8%—91 patients) in OSCC samples, with high expression levels significantly correlating with lower tumor grade (p = 0.014) and improved 2-year survival (p = 0.011). While TrkB and TrkC were expressed in 65.5% and 84.9% of cases, respectively, neither showed significant associations with clinical parameters. NTRK2 and NTRK3 mRNA levels demonstrated a strong positive correlation (R = 0.64, p = 0.002), suggesting coordinated regulation. Our findings establish TrkA as a promising positive prognostic marker in OSCC, warranting investigation as a therapeutic target. The strong correlation between NTRK2 and NTRK3 expression suggests shared regulatory mechanisms in OSCC pathogenesis. Further studies with larger cohorts and longer follow-up periods are needed to validate these findings and explore their therapeutic implications.
1. Introduction
Oral squamous cell carcinoma (OSCC), encompassing malignancies of the lip, floor of the mouth, tongue, gingiva, and buccal mucosa, represents approximately 90% of oral malignant neoplasms [1]. While other malignant tumors, such as mucoepidermoid carcinoma, adenoid cystic carcinoma, and myoepithelial carcinoma, occur in this region, they are considerably less frequent [2,3,4].
As the 16th most common malignant tumor worldwide, OSCC poses a significant global health challenge. The 2020 GLOBOCAN report documented 476,125 new cases and 225,900 deaths globally, highlighting its substantial public health impact [5].
The pathogenesis of OSCC involves multiple environmental and genetic factors. Key risk factors include tobacco use, alcohol consumption, betel quid chewing, viral infections, notably human papilloma virus (HPV), and Ebstein–Barr virus (EBV), and genetic predispositions, such as Fanconi anemia, xeroderma pigmentosum, and dyskeratosis congenita [6,7]. Tobacco use is a particularly significant risk factor, increasing the likelihood of developing oral cancer by approximately 8.4-fold, while users of smokeless tobacco face a 5- to 7-fold elevated risk [7]. Feghali et al. [8] identified smoking as an independent predictor of poor survival, alongside advanced age and higher pathological risk scores. Alcohol consumption synergistically amplifies tobacco’s carcinogenic effects, potentially increasing oral cancer risk up to 35-fold. Viral infections, particularly HPV16 and HPV18, contribute to approximately 6% of OSCC cases globally, predominantly in oropharyngeal tumors [9]. EBV infection, detected in 22.67–52% of cases, shows a notable association with buccal mucosa lesions in smokers [10,11,12]. OSCC has also been rarely reported in proximity to dental implants. While no direct causal relationship has been established, lesions in these areas are occasionally misdiagnosed as peri-implantitis, leading to delays in cancer diagnosis [13,14]. Ramos et al. [13] noted that OSCC near implants was more frequently observed in women without traditional risk factors such as smoking or alcohol consumption but with a history of leukoplakia.
The molecular pathogenesis of OSCC involves complex interactions of genetic and epigenetic alterations. Key mechanisms include activation of oncogenic pathways (PI3K/AKT/mTOR, MAPK, and Wnt-signaling) [7,15,16], DNA methylation changes, histone modifications, non-coding RNA dysregulation, and alterations in the tumor microenvironment and oral microbiome [16].
Prognostic factors in OSCC encompass various clinical and pathological parameters. Poor prognostic indicators include low socioeconomic status, diagnosis before age 40, single-modality treatment, advanced disease stage, lymph node metastases, tongue base localization, HPV-negative status, and positive surgical margins [17,18,19].
Adverse histological prognostic factors in OSCC include invasion depth > 5 mm, high tumor budding (poorly differentiated clusters or single cancer cells at the invasive front), perineural invasion, lymphovascular invasion, bone invasion, a multifocal invasive pattern (pattern 5), and a stromal-to-tumor ratio > 50% in deeply invasive tumors [2].
Molecular markers, including COX2, EGFR, cyclin D1, and p21 overexpression, have emerged as significant prognostic indicators [17].
The prognostic significance of programmed death-ligand 1 (PD-L1) depends on tumor location and the cell type expressing this immune checkpoint protein—either tumor cells or tumor-infiltrating lymphocytes [20]. High PD-L1 expression on tumor-infiltrating lymphocytes was associated with the N0 status and improved patient survival, regardless of tumor location. Conversely, PD-L1 expression on tumor cells appears to have variable effects on survival depending on the tumor’s location [20]. However, a meta-analysis by Nocini et al. [21] did not confirm the prognostic value of PD-L1 in OSCC, emphasizing challenges in standardizing its assessment.
NTRK1, NTRK2, and NTRK3 (neurotrophic tropomyosin receptor kinase) refer to a group of genes that encode the TrkA, TrkB, and TrkC proteins, respectively, a family of receptor tyrosine kinases (RTKs). Trk receptors bind neurotrophins, regulating cellular processes such as proliferation, differentiation, metabolism, and apoptosis through phosphorylation of intracellular targets [22]. They play crucial roles in cellular regulation through neurotrophin binding and subsequent activation of MAPK, PI3K, and PKC pathways [23].
The most common oncogenic mechanism of Trk activation involves NTRK gene fusions, resulting from intra- or interchromosomal rearrangements. These fusions juxtapose the 3′ kinase domain-containing regions of NTRK1, NTRK2, or NTRK3 with the 5′ region of another gene, creating a chimeric oncoprotein. This fusion protein leads to constitutive Trk activation, independent of ligand binding, driving uncontrolled signaling pathway activation and promoting oncogenesis [23,24].
Other mechanisms of Trk activation in cancer include NTRK mutations, alternative splicing variants, and Trk overexpression [23]. These alterations further underscore the pivotal role of NTRK genes in tumorigenesis and their potential as therapeutic targets.
Given the incomplete understanding of the etiopathogenesis of OSCC and the unsatisfactory treatment outcomes, the identification of new biomarkers and potential therapeutic targets remains a priority.
In this study, we additionally aim to compare the immunohistochemically assessed protein expression levels of NTRK1, NTRK2, and NTRK3 genes in OSCC tissues and to analyze their associations with clinicopathological parameters, including age, sex, risk factors (alcohol consumption and tobacco use), tumor stage, treatment response, and two-year survival rates.
Furthermore, we intend to evaluate the correlation between the mRNA expression levels of the NTRK1, NTRK2, and NTRK3 genes and these clinicopathological features.
2. Results
2.1. Expression of Trk Proteins and Clinicopathological Correlations
Assessment of Trk protein expression was carried applying the H-score approach, which integrates both the intensity of staining (graded on a scale from 0 to 3) and the proportion of positively stained cells (ranging from 0% to 100%). TrkA protein expression was detected in 91 cases (97.8%), with a mean H-score of 187 (median 205, range 0–300, IQR 115–260). Analysis revealed a significant correlation between TrkA expression and histological grade (p = 0.014). However, when analyzed as a categorical variable (low/high expression), TrkA showed no significant associations with clinicopathological features apart from histological grade (p = 0.024) (Table 1 and Table 2).

Table 1.
TrkA, TrkB, and TrkC expression (low vs. high) in relation to clinicopathological features. p-values were calculated using the chi-square test.

Table 2.
Correlations between TrkA, TrkB and TrkC expression and clinicopathological variables, treating expression as a continuous variable (non-parametric test—Wilcoxon/Mann–Whitney U test).
TrkB protein was expressed in 61 cases (65.5%), with a mean H-score of 33.5 (median 5, range 0–300, IQR 0–25). No significant correlations were found between TrkB expression and clinicopathological variables, whether analyzed as continuous or categorical data (Table 2 and Table 3).

Table 3.
Analyzed clinical and pathological features.
TrkC protein expression was observed in 79 cases (84.9%), with a mean H-score of 0.833 (median 0, range 0–112.5, IQR 0). Analysis of TrkC expression as a categorical variable showed no significant associations with clinicopathological parameters, although a trend toward an association between TrkC expression and pN status was observed (p = 0.060) (Table 2).
2.2. Survival Analysis
High TrkA expression in primary oral cancer lesions correlated significantly with improved prognosis compared to cases with low expression (p = 0.011). Conversely, low expression of TrkB and TrkC showed a trend toward better prognosis, although these associations did not reach statistical significance (p = 0.150 and p = 0.300, respectively). Survival curves are presented in Figure 1.
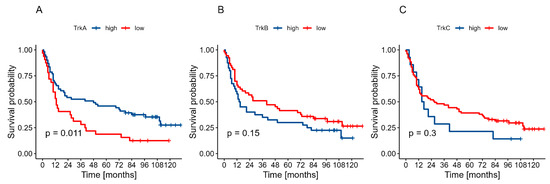
Figure 1.
Two-year OS in relation to expression levels: (A)—TrkA, (B)—TrkB, (C)—TrkC.
2.3. NTRK Gene Expression Correlations
Analysis of NTRK mRNA levels revealed distinct correlation patterns. NTRK1 mRNA level showed weak negative correlations with both NTRK2 mRNA level (R = −0.12, p = 0.570) and NTRK3 mRNA level (R = −0.06, p = 0.790), neither reaching statistical significance. In contrast, NTRK2 and NTRK3 demonstrated a strong positive correlation (R = 0.64, p = 0.002). Correlation plots with 95% confidence intervals are shown in Figure 2.
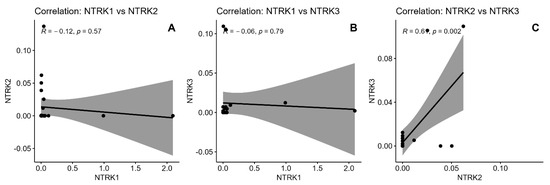
Figure 2.
Graphical correlation between mRNA levels of NTRK genes. (A)—Correlation: NTRK1 vs. NTRK2, (B)—Correlation: NTRK1 vs. NTRK3, (C)—Correlation: NTRK1 vs. NTRK2.
3. Discussion
The current literature provides limited data regarding the expression and clinical significance of NTRK1/2/3 genes in OSCC. Our immunohistochemical analysis revealed differential expression patterns of Trk proteins, with TrkA, TrkB, and TrkC detected in 97.8%, 65.5%, and 84.9% of cases, respectively. High TrkA expression demonstrated a significant correlation with lower tumor grade (p = 0.014), while TrkB and TrkC showed no significant associations with clinicopathological parameters. Notably, patients with high TrkA expression exhibited significantly improved OS rates compared to those lacking TrkA expression (p = 0.011). In contrast, TrkB and TrkC expression showed trends towards poorer survival outcomes, although these associations did not reach statistical significance (p = 0.15 and p = 0.300, respectively). One possible explanation for the limited prognostic value of TrkB and TrkC in our study is their complex and context-dependent biological role. The literature suggests that both TrkB and TrkC can exhibit dual functions depending on the tumor type, stage, or presence of specific ligands. For instance, TrkB has been linked to aggressive behavior and chemoresistance in several cancers due to its role in promoting epithelial-mesenchymal transition (EMT) [25]. As for TrkC, its role is even more ambiguous. While some studies have identified TrkC as a potential tumor suppressor in the absence of its ligand neurotrophin-3 (NT3) [26], other reports show it may support tumor growth under different conditions [27]. This dichotomy might explain the lack of association with clinical outcomes in our cohort, particularly in a heterogeneous tumor like OSCC, where co-factors such as neurotrophin availability, receptor isoforms, and tumor microenvironmental context play a critical role.
A comparative Mexican study using PanTrk immunostaining found positive expression in only 42% of cases (n = 95) [28]. Direct comparison with our findings is limited due to the non-specific nature of PanTrk antibodies, which cannot distinguish between individual Trk proteins. Their analysis revealed age-dependent associations between PanTrk expression and tumor location, with higher prevalence in gingival tissues among patients over 60 years and lateral tongue among those under 60 years (p = 0.017). In contrast, our study found no correlation between TrkA expression and tumor location, identifying a significant association only with tumor grade (p = 0.014). Additional insights come from Escoto-Vasquez et al. [29], who reported differential expression patterns with high NTRK2 mRNA levels in poorly differentiated OSCC and high NTRK3 mRNA in well-differentiated cases, suggesting distinct roles for TrkB and TrkC in OSCC pathogenesis. However, their small sample size (n = 24) necessitates cautious interpretation of these findings.
Collectively, our findings support the role of Trk proteins in OSCC pathogenesis and highlight their potential as both prognostic biomarkers and therapeutic targets. Molecular analysis revealed a strong positive correlation between NTRK2 and NTRK3 mRNA expression (R = 0.64, p = 0.002). The strength and exclusivity of this correlation suggest potential shared regulatory mechanisms governing these genes in OSCC tissue. TrkA, a high-affinity receptor for nerve growth factor (NGF), is known to activate several key signaling cascades—most notably the PI3K/AKT, RAS/MAPK, and PLCγ pathways—resulting in enhanced cell survival, proliferation, and differentiation [30,31]. These pathways are frequently implicated in head and neck SCCs and could plausibly mediate the observed association between high TrkA expression and improved differentiation and survival in our cohort. We now explicitly state that further functional studies, including in vitro and in vivo models, are needed to confirm these mechanistic links and to clarify whether TrkA exerts a causal role in tumor suppression or reflects a more differentiated tumor phenotype [23].
Further functional studies are needed to explore these regulatory pathways and their implications for tumor biology.
NTRK gene fusions are predominantly associated with rare, aggressive malignancies, including infantile fibrosarcoma, secretory breast carcinoma, and salivary gland secretory carcinoma [32,33]. These genetic alterations occur less frequently in common cancers such as colorectal adenocarcinoma, lung adenocarcinoma, non-small cell lung cancer, and papillary thyroid carcinoma. However, emerging evidence demonstrates the significance of NTRK gene expression and Trk protein function across various squamous cell carcinomas (SCCs), including those of the oropharynx, esophagus, uterine cervix, and lung.
In their study of oropharyngeal cancer, Cho et al. [34] demonstrated a significant association between high TrkA expression and improved OS and recurrence-free survival (RFS). However, they observed contrasting results in OSCC, where PanTrk expression correlated with shorter RFS. Their research also suggested a potential mechanistic link between HPV infection and TrkA expression, as demonstrated by increased TrkA expression in SCC cell lines following HPV 16 E6/E7 gene transfer. These findings underline the importance of context-specific factors, such as HPV status, in interpreting Trk protein roles across SCC subtypes.
Studies of cervical malignancies provide additional insights into Trk protein expression patterns. Cervical SCC showed a higher prevalence of TrkA overexpression compared to cervical adenocarcinomas [35]. This pattern differed from our OSCC findings, as cervical SCC showed no association between TrkA expression and tumor grade, contrasting with the significant grade correlation we observed in OSCC. In esophageal SCC, Yu et al. [36] identified a distinct pattern, where increased NTRK1 copy numbers correlated with poorer clinical outcomes, providing further evidence for the tissue-specific roles of NTRK alterations in different SCC types.
A comprehensive study by Terry et al. [37], analyzing TrkA and TrkB expression in 686 lung cancer cases, revealed distinctive patterns in SCC. They found TrkA or TrkB expression in over 90% of SCC cases, markedly higher than in other cancer types, where expression was sporadic. Furthermore, TrkB expression in lung SCC correlated with improved OS (p = 0.047) and disease-free survival (p < 0.001), paralleling our observations regarding the prognostic significance of Trk proteins in OSCC.
These findings have important clinical implications for OSCC management. The strong association between TrkA expression and improved survival suggests its potential use as a prognostic marker in clinical practice. Additionally, the tissue-specific expression patterns and functional roles of Trk proteins across different SCC types highlight the need for personalized therapeutic approaches. Overall, these findings emphasize the complexity and tissue-specific nature of NTRK gene alterations and Trk protein expression across SCCs. Further research is warranted to validate these results and explore the mechanisms underlying these associations, with the goal of advancing personalized therapeutic approaches in OSCC and related malignancies.
4. Materials and Methods
4.1. Study Population and Clinical Data
The approval of the independent bioethics committee was granted for conducting this study. The study cohort comprised 93 OSCC patients (31 women and 62 men) who underwent surgical treatment in the University Clinical Centre in Gdańsk, Poland. Patient survival data were obtained from clinical records and the Social Insurance Institution in Gdańsk database. The median follow-up period was 24 months, with a 2-year overall survival rate of 36.6%.
Comprehensive clinicopathological data were collected for each patient, including demographic factors, disease characteristics (tumor stage according to the 8th TNM classification, histological grade, tumor location), treatment modalities, and risk factors such as alcohol consumption and smoking status. Mortality data were also recorded throughout the follow-up period. All patient characteristics are summarized in Table 3.
4.2. Tissue Processing and Immunohistochemistry
Fresh tumor specimens were fixed in 10% formalin for 48 h, followed by dehydration and paraffin embedding. Hematoxylin and eosin-stained sections were reviewed to confirm diagnosis and disease staging. Representative tissue areas were selected based on typical tumor morphology, preserved architecture, and absence of necrosis, thermal damage, or significant inflammatory infiltrates.
Tissue microarrays (TMAs) were constructed using a Manual Tissue Arrayer I (Beecher Instruments, MTAI, K7 BioSystems) with 1.5 mm diameter cores. Each tumor was represented by at least two tissue cores, resulting in seven microarray blocks. Control tissues (palatine tonsils and stomach) were included in each block for orientation. Immunohistochemical analysis was performed using specific monoclonal antibodies (Cell Signaling, Danvers, MA, USA: TrkA 12G8, TrkB 80G2, TrkC C44H5) following the manufacturer’s protocols. Two pathologists (AC and RP) independently evaluated receptor expression using the H-score method, which combines staining intensity (0–3) and percentage of positive cells (0–100%), using an Olympus BX43 microscope (Tokyo, Japan) (Figure 3).
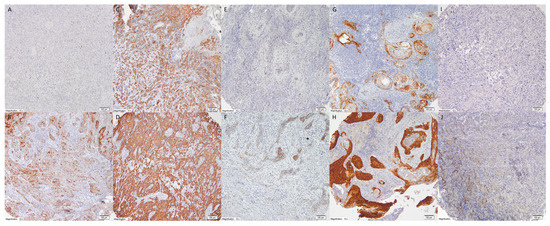
Figure 3.
Expression of TrkA in OSCC—membranous and cytoplasmic staining intensity according to H-score: (A). 0, (B). 1, (C). 2, (D). 3, expression of TrkB in OSCC—membranous and cytoplasmic staining intensity according to H-score: (E). 0, (F). 1, (G). 2, (H). 3, expression of TrkC in OSCC—membranous and cytoplasmic staining intensity according to H-score: (I). 0, (J). 1. (magnification: 10×).
4.3. Molecular Analysis
From paraffin blocks of 30 patients with oral cancer, 10 μm × 2 μm sections were cut, which were then subjected to deparaffinization and RNA extraction. Total RNA isolation from FFPE sections was performed using a commercial kit, the RNeasy FFPE Mini Kit (QIAGEN, Hilden, Germany), following the manufacturer’s instructions.
The concentration of the isolated RNA was determined using fluorometric methods with a Qubit fluorometer (Invitrogen, Waltham, MA, USA) and the commercial Qubit RNA HS assay kit, in accordance with the manufacturer’s protocols.
The mRNA levels of the NTRK1, NTRK2, and NTRK3 genes were determined relative to the transcript level of the reference gene beta-actin (ACTB) using real-time RT-qPCR with TaqMan probes. The RT-qPCR reactions were carried out using the Path-ID™ Multiplex One-Step RT-PCR Kit (Applied Biosystems, Foster City, CA, USA) and Universal ProbeLibrary Probes for Human (Roche) on the LightCycler 480 II system (Roche), with specific primers for both the target and reference transcripts. Each measurement was performed in triplicate, and the final result was reported as the mean of these replicates. The reproducibility of the obtained results was assessed based on the standard deviation and coefficient of variation for each sample. Each RT-PCR reaction included control samples: a negative control (no RNA template) and a positive control, to exclude contamination and confirm the efficiency of the reaction.
The relative expression level of each transcription factor gene was presented as a normalized ratio of the expression level of the target transcript to that of the reference gene transcript. Data were processed using LightCycler® 480 II Software, version 2.0 (Roche Diagnostics, Basel, Switzerland).
4.4. Statistical Analysis
Data analysis was performed using Statistica 13 (TIBCO Software Inc., Palo Alto, CA, USA) and the R programming environment, version 4.3.2. The chi-square test was used for categorical variables, with Fisher’s exact test applied to small sample sizes. The Mann–Whitney U test was employed for quantitative variables due to non-normal distribution. Survival analysis utilized the Kaplan–Meier method with the log-rank test for curve comparison. Statistical significance was set at p < 0.05.
5. Conclusions
Our comprehensive analysis of NTRK1, NTRK2, and NTRK3 expression in OSCC reveals distinct patterns with potential clinical implications. High TrkA protein expression emerged as a favorable prognostic indicator, significantly correlating with lower tumor grade and improved survival outcomes. While elevated TrkB and TrkC expression showed trends toward poorer outcomes, these associations require validation through larger studies.
The strong positive correlation between NTRK2 and NTRK3 mRNA levels suggests shared regulatory mechanisms in OSCC pathogenesis, opening new avenues for molecular research. These findings collectively support the role of Trk proteins in OSCC development and highlight their potential as both prognostic biomarkers and therapeutic targets, with TrkA particularly promising for clinical applications.
Limitations of This Study
Several important limitations should be considered when interpreting our findings. First, while our cohort of 93 patients provided sufficient statistical power for primary analyses, the sample size and single-center nature of this study may limit the generalizability of the results to broader OSCC populations. An additional limitation regarding the significance of Trk protein expression in our OSCC cohort is the lack of HPV and EBV status assessment in the cancer cells, as these factors can influence tumor biology and potentially affect Trk expression patterns.
Our analysis focused on Trk proteins and mRNA expression levels without exploring functional aspects of NTRK gene alterations, such as fusions, mutations, or splicing variants. This limitation prevents definitive conclusions about the mechanistic role of these genes in OSCC pathogenesis. Similarly, while we identified a significant correlation between NTRK2 and NTRK3 mRNA levels, the underlying regulatory mechanisms remain unexplored due to a lack of functional validation studies. Furthermore, this study did not include data on HPV and EBV status in cancer cells, which may have influenced survival outcomes and clinicopathological factors. Additionally, despite the use of standardized protocols, the inherent subjectivity of immunohistochemical H-score evaluation could compromise the reproducibility of the results.
Although we discussed comparisons with SCCs from other anatomical sites, the absence of direct comparative analysis between OSCC and other SCC subtypes limits our ability to establish tissue-specific patterns of NTRK expression.
Future research addressing these limitations through larger multicenter studies, extended follow-up periods, functional analyses, and direct comparative studies will enhance our understanding of NTRK genes’ role in OSCC and strengthen the clinical applicability of these findings.
Author Contributions
A.C.: conceptualization, methodology, investigation, writing—original draft; F.S., P.M., R.B. and B.J.-F.: writing—original draft, visualization; M.K.: statistical analysis, visualization; M.S.-B.: genetic testing; A.S. and R.P.: formal analysis, supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical approval for this study was obtained from the Independent Bioethics Committee for Scientific Research at the Medical University of Gdańsk, Poland (Approval No. NKBBN/240/2019, 15 May 2019–2022).
Informed Consent Statement
Patient consent was waived due to the retrospective nature of the study using anonymized archival tissue samples. Written informed consent for publication was not required as no patients can be identified.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Nijakowski, K.; Gruszczyński, D.; Kopała, D.; Surdacka, A. Salivary Metabolomics for Oral Squamous Cell Carcinoma Diagnosis: A Systematic Review. Metabolites 2022, 12, 294. [Google Scholar] [CrossRef] [PubMed]
- Muller, S.; Tilakaratne, W.M. Update from the 5th Edition of the World Health Organization Classification of Head and Neck Tumors: Tumours of the Oral Cavity and Mobile Tongue. Head Neck Pathol. 2022, 16, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Maymone, M.B.C.; Greer, R.O.; Kesecker, J.; Sahitya, P.C.; Burdine, L.K.; Cheng, A.-D.; Maymone, A.C.; Vashi, N.A. Premalignant and malignant oral mucosal lesions: Clinical and pathological findings. J. Am. Acad. Dermatol. 2019, 81, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Telugu, R.B.; Gaikwad, P.; Baitule, A.; Michael, R.C.; Mani, T.; Thomas, M. Myoepithelial Tumors of Salivary Gland: A Clinicopathologic and Immunohistochemical Study of 15 Patients with MIB-1 Correlation. Head Neck Pathol. 2021, 15, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Chamoli, A.; Gosavi, A.S.; Shirwadkar, U.P.; Wangdale, K.V.; Behera, S.K.; Kurrey, N.K.; Kalia, K.; Mandoli, A. Overview of oral cavity squamous cell carcinoma: Risk factors, mechanisms, and diagnostics. Oral Oncol. 2021, 121, 105451. [Google Scholar] [CrossRef] [PubMed]
- Al Feghali, K.A.; Ghanem, A.I.; Burmeister, C.; Chang, S.S.; Ghanem, T.; Keller, C.; Siddiqui, F. Impact of smoking on pathological features in oral cavity squamous cell carcinoma. J. Cancer Res. Ther. 2019, 15, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Katirachi, S.K.; Grønlund, M.P.; Jakobsen, K.K.; Grønhøj, C.; von Buchwald, C. The Prevalence of HPV in Oral Cavity Squamous Cell Carcinoma. Viruses 2023, 15, 451. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.W.; Baig, F.A.; Hadi, N.I. A novel comparison of Epstein-Barr virus with broad histological spectrum of oral squamous cell carcinoma. Pak. J. Med. Sci. 2019, 35, 1192. [Google Scholar] [CrossRef] [PubMed]
- Zebardast, A.; Yahyapour, Y.; Majidi, M.S.; Chehrazi, M.; Sadeghi, F. Detection of Epstein-Barr virus encoded small RNA genes in oral squamous cell carcinoma and non-cancerous oral cavity samples. BMC Oral Health 2021, 21, 502. [Google Scholar] [CrossRef] [PubMed]
- Khalesi, S.; Khalesi, S.; Ghapani, F. Evaluation of Epstein-Barr virus expression in oral squamous cell carcinomas. Dent. Res. J. 2022, 19, 58. [Google Scholar]
- Ramos, J.C.; Alves, F.A.; Kowalski, L.P.; dos Santos-Silva, A.R.; Vargas, P.A.; Lopes, M.A. Epidemiological profile and clinical implications of oral squamous cell carcinoma adjacent to dental implants. Oral Dis. 2021, 27, 1687–1698. [Google Scholar] [CrossRef] [PubMed]
- Afrashtehfar, K.I.; Almomani, M.M.; Momani, M. Lack of association between dental implants and oral squamous cell carcinoma. Evid.-Based Dent. 2022, 23, 40–42. [Google Scholar] [CrossRef] [PubMed]
- Zanoni, D.K.; Demétrio De Souza França, P.; Valero, C.; Peterson, G.; Ardigo, M.; Ghossein, R.; Dusza, S.W.; Matsuura, D.; Scholfield, D.W.; Adilbay, D.; et al. A Prospective Double-Blinded Comparison of Reflectance Confocal Microscopy with Conventional Histopathology for In Vivo Assessment in Oral Cancer. Clin. Cancer Res. 2024, 30, 2486–2496. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Wang, Z.; Xu, M.; Li, B.; Huang, Z.; Qin, S.; Nice, E.C.; Tang, J.; Huang, C. Oral squamous cell carcinomas: State of the field and emerging directions. Int. J. Oral Sci. 2023, 15, 14. [Google Scholar] [CrossRef] [PubMed]
- Massano, J.; Regateiro, F.S.; Januário, G.; Ferreira, A. Oral squamous cell carcinoma: Review of prognostic and predictive factors. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 102, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.K.; Carvalho, S.; Granville-Garcia, A.; Sarmento, D.; Agripino, G.; Abreu, M.; Melo, M.; Caldas, A.D., Jr.; Godoy, G. Survival and prognostic factors in patients with oral squamous cell carcinoma. Med. Oral Patol. Oral Cir. Bucal 2021, 26, e387. [Google Scholar] [CrossRef] [PubMed]
- Eljabo, N.; Nikolic, N.; Carkic, J.; Jelovac, D.; Lazarevic, M.; Tanic, N.; Milasin, J. Genetic and epigenetic alterations in the tumour, tumour margins, and normal buccal mucosa of patients with oral cancer. Int. J. Oral Maxillofac. Surg. 2018, 47, 976–982. [Google Scholar] [CrossRef] [PubMed]
- Adamski, Ł.J.; Starzyńska, A.; Adamska, P.; Kunc, M.; Sakowicz-Burkiewicz, M.; Marvaso, G.; Alterio, D.; Korwat, A.; Jereczek-Fossa, B.A.; Pęksa, R. High PD-L1 Expression on Tumor Cells Indicates Worse Overall Survival in Advanced Oral Squamous Cell Carcinomas of the Tongue and the Floor of the Mouth but Not in Other Oral Compartments. Biomedicines 2021, 9, 1132. [Google Scholar] [CrossRef] [PubMed]
- Nocini, R.; Vianini, M.; Girolami, I.; Calabrese, L.; Scarpa, A.; Martini, M.; Morbini, P.; Marletta, S.; Brunelli, M.; Molteni, G.; et al. PD-L1 in oral squamous cell carcinoma: A key biomarker from the laboratory to the bedside. Clin. Exp. Dent. Res. 2022, 8, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Wang, G.; Liu, Y.; Feng, L.; Wang, M.; Liu, J.; Chen, Y.; Ouyang, L. Development of small-molecule tropomyosin receptor kinase (TRK) inhibitors for NTRK fusion cancers. Acta Pharm. Sin. B 2021, 11, 355–372. [Google Scholar] [CrossRef] [PubMed]
- Cocco, E.; Scaltriti, M.; Drilon, A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat. Rev. Clin. Oncol. 2018, 15, 731–747. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.A.; Colebatch, A.J.; Van Beek, D.; Tierney, G.; Gupta, R.; Cooper, W.A. NTRK fusions in solid tumours: What every pathologist needs to know. Pathology 2023, 55, 596–609. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Zhang, Z.; Qin, W.; Sun, W. Neurotrophic receptor TrkB: Is it a predictor of poor prognosis for carcinoma patients? Med. Hypotheses 2007, 68, 407–409. [Google Scholar] [CrossRef] [PubMed]
- Segal, R.A. Selectivity in neurotrophin signaling: Theme and variations. Annu. Rev. Neurosci. 2003, 26, 299–330. [Google Scholar] [CrossRef] [PubMed]
- Jin, W. Roles of TrkC Signaling in the Regulation of Tumorigenicity and Metastasis of Cancer. Cancers 2020, 12, 147. [Google Scholar] [CrossRef] [PubMed]
- Sasahira, T.; Ueda, N.; Yamamoto, K.; Bhawal, U.K.; Kurihara, M.; Kirita, T.; Kuniyasu, H. Trks are novel oncogenes involved in the induction of neovascularization, tumor progression, and nodal metastasis in oral squamous cell carcinoma. Clin. Exp. Metastasis 2013, 30, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Escoto-Vasquez, L.S.; Portilla-Robertson, J.; Ramírez-Jarquín, J.O.; Jacinto-Alemán, L.F.; Alonso-Moctezuma, A.; Ramírez-Martínez, C.M.; Chanes-Cuevas, O.A.; Salgado-Chavarria, F. NTRK Gene Expression Analysis in Oral Squamous Cell Carcinoma Mexican Population. Dent. J. 2024, 12, 327. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.J.; Reichardt, L.F. Trk receptors: Roles in neuronal signal transduction. Annu. Rev. Biochem. 2003, 72, 609–642. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, D.R.; Miller, F.D. Neurotrophin signal transduction in the nervous system. Curr. Opin. Neurobiol. 2000, 10, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Conde, E.; Sanchez, E.; Regojo, R.M.; Camacho, C.; Alonso, M.; Martinez, R.; Lopez-Rios, F. Pan-TRK immunohistochemistry an example-based practical approach to efficiently identify patients with NTRK fusion cancer. Arch. Pathol. Lab. Med. 2021, 145, 1031–1040. [Google Scholar] [CrossRef] [PubMed]
- Durzyńska, M.; Michałek, I.M. Pan-TRK immunohistochemistry as a tool in the screening for NTRK gene fusions in cancer patients. Oncol. Clin. Pract. 2024, 20, 15–21. [Google Scholar] [CrossRef]
- Cho, Y.A.; Chung, J.M.; Ryu, H.; Kim, E.K.; Cho, B.C.; Yoon, S.O. Investigating Trk Protein Expression between Oropharyngeal and Non-oropharyngeal Squamous Cell Carcinoma: Clinical implications and possible roles of human papillomavirus infection. Cancer Res. Treat. 2019, 51, 1052–1063. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, S.; Griffin, N.; Rowe, C.W.; Jobling, P.; Lombard, J.M.; Oliveira, S.M.; Walker, M.M.; Hondermarck, H. Nerve growth factor and its receptor tyrosine kinase TrkA are overexpressed in cervical squamous cell carcinoma. FASEB BioAdv. 2020, 2, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Wang, H.; Song, Q.; Huang, J.; Xu, J.; Su, J.; Wang, H.; Tan, L.; Wang, X.; Jiang, Z.; et al. Prognostic value and characterization of NTRK1 variation by fluorescence in situ hybridization in esophageal squamous cell carcinoma. J. Cancer Res. Clin. Oncol. 2021, 147, 3113–3121. [Google Scholar] [CrossRef] [PubMed]
- Terry, J.; De Luca, A.; Leung, S.; Peacock, G.; Wang, Y.; Elliot, W.M.; Huntsman, D. Immunohistochemical expression of neurotrophic tyrosine kinase receptors 1 and 2 in lung carcinoma: Potential discriminators between squamous and nonsquamous subtypes. Arch. Pathol. Lab. Med. 2011, 135, 433–439. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).