The Effect of the Ethanolic Extracts from Syzygium aromaticum and Syzygium nervosum on Antiproliferative Activity and Apoptosis in HCT116 and HT-29 Cells
Abstract
1. Introduction
2. Results
2.1. Quality Control of the Raw Materials
2.2. Quality Control of the Extracts
2.2.1. Identification and Quantification of Major Components
2.2.2. Antioxidant Properties of SA and SN Extracts
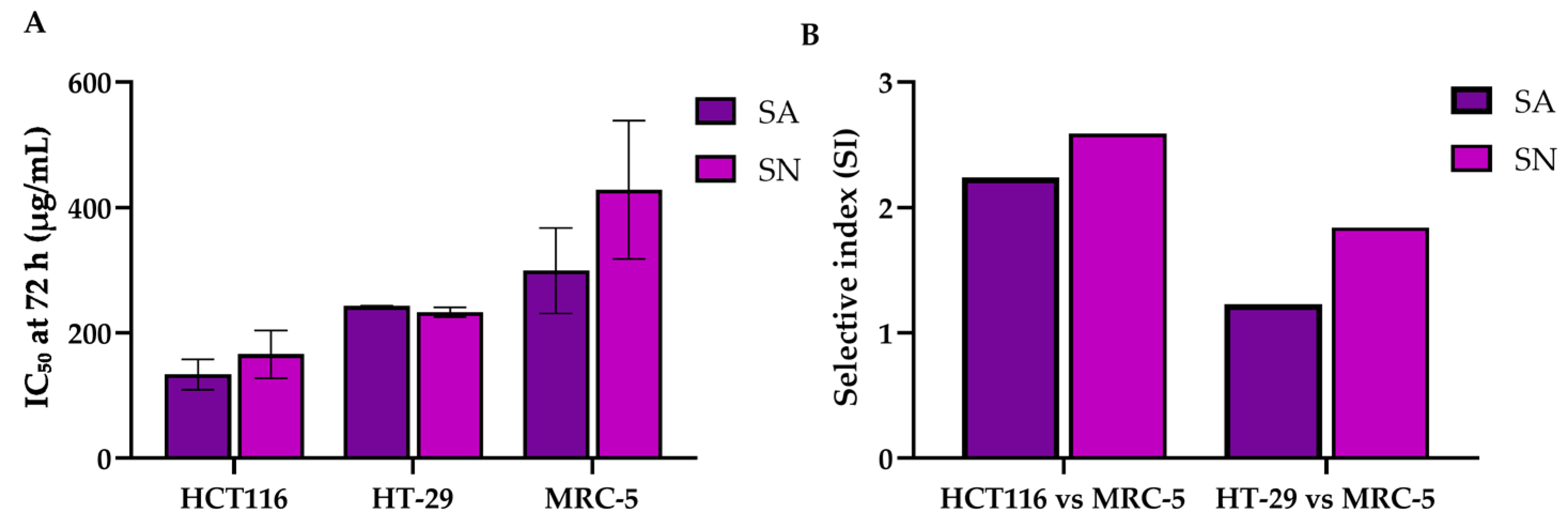
2.3. Cytotoxic Effects of SA and SN Extracts on Colorectal Cancer and Normal Cells
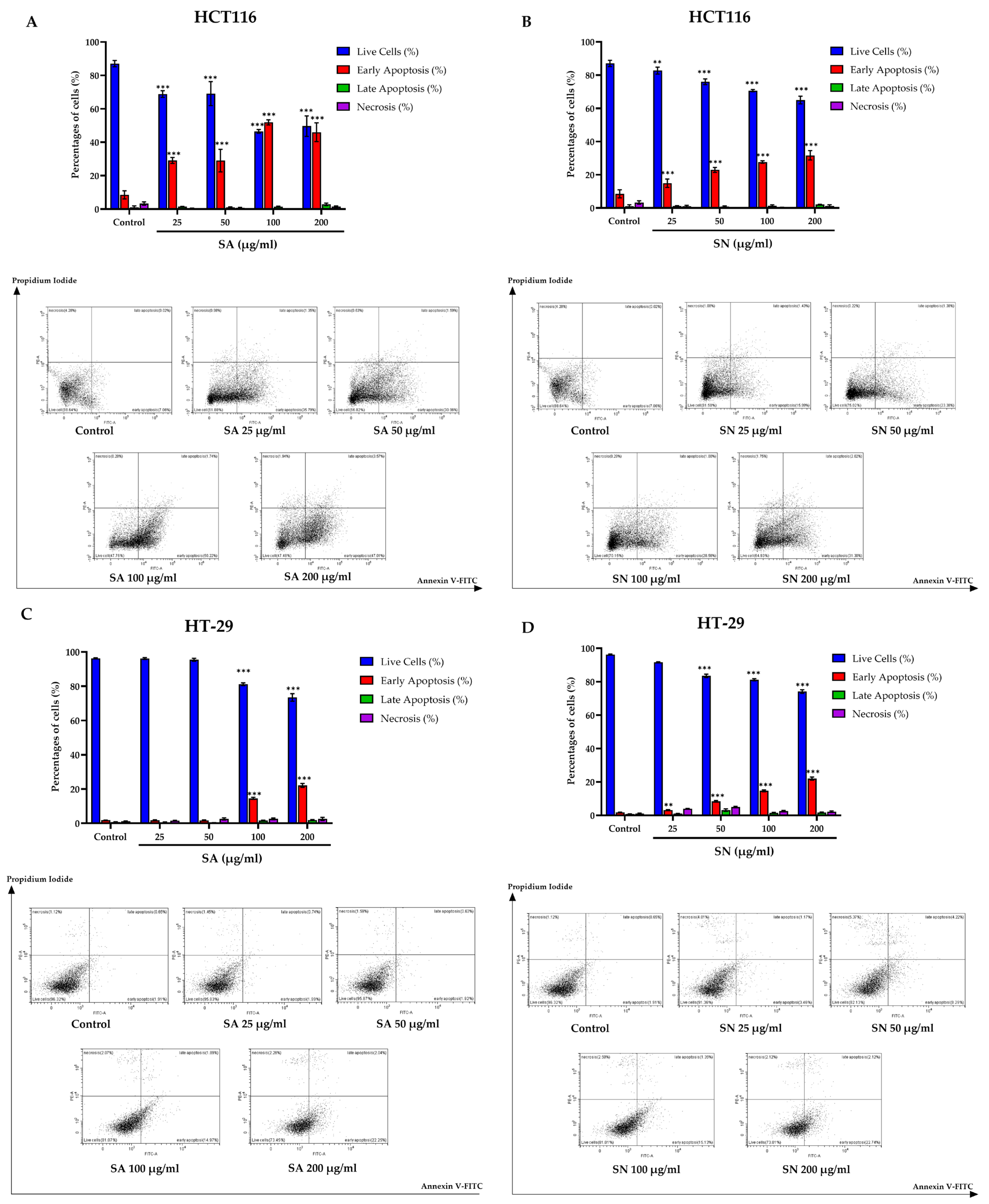
2.4. SA and SN Extracts Induce Apoptosis in Colorectal Cancer Cells
2.4.1. Flow Cytometer for Apoptosis Assay Annexin V-FITC/PI
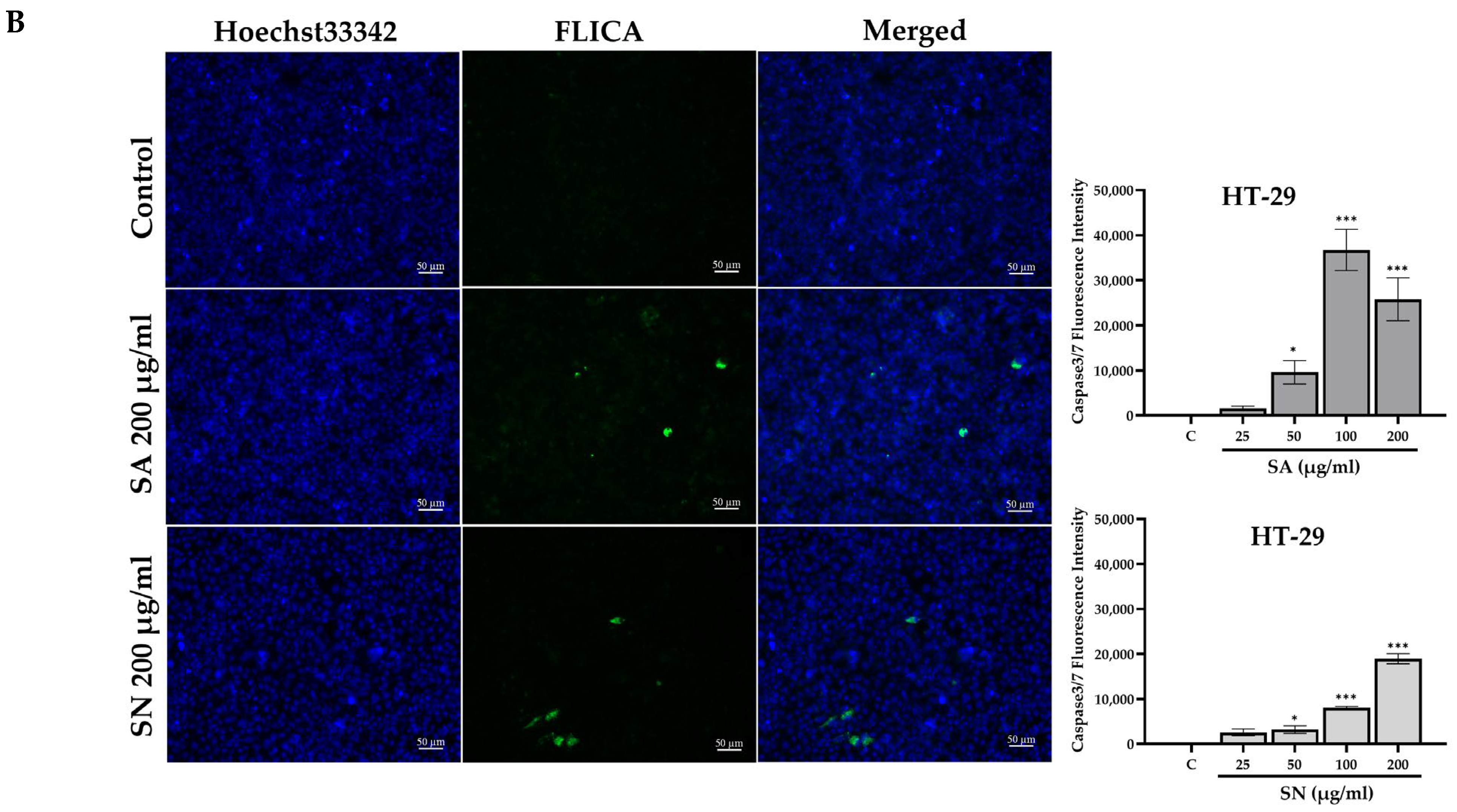
2.4.2. Caspase 3/7 In Situ Fluorescence Microscope
2.5. Inhibition of Cell Growth and Induction of Cell Cycle Arrest by SA and SN Extracts in Colorectal Cancer Cells
2.5.1. Colony Formation Assay
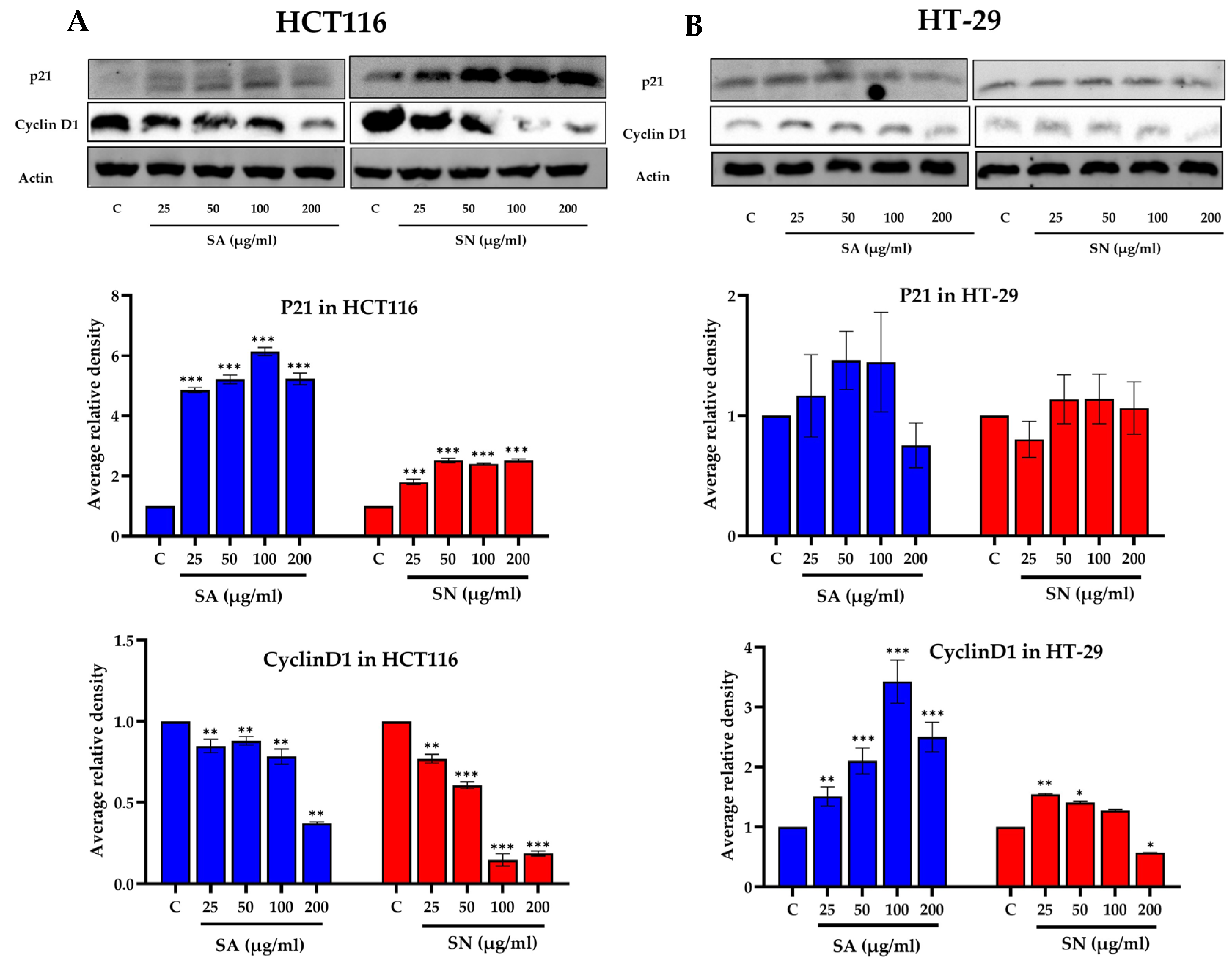
2.5.2. Cell Cycle Analysis by Flow Cytometry and Western Blotting
3. Discussion
4. Materials and Methods
4.1. Plant Sample Preparation and Raw Material Quality Control
4.1.1. Plant Identification
4.1.2. Plant Extraction
4.2. Quality Control of the Extracts
4.2.1. Identification and Quantification of Major Phytochemicals Using LC-DAD-Q-Orbitrap-MS/MS
4.2.2. Quantification of Total Phenolic Compounds Using the Folin–Ciocalteu Assay
4.2.3. Evaluating Potential of Plant Extracts Using Antioxidant Assays
4.3. In Vitro Assay
4.3.1. Cell Culture
4.3.2. Cell Viability Assay
4.3.3. Apoptosis Assay
4.3.4. Caspase 3/7 In Situ Fluorescence Microscope
4.3.5. Colony Formation Assay
4.3.6. Cell Cycle Assay
4.3.7. Western Blotting
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SA | Residue from clove (Syzygium aromaticum) hydrodistillation |
| SN | Seed extract from Syzygium nervosum |
| IC50 | Approximate concentration causing 50% cell death |
References
- Tiankanon, K.; Aniwan, S.; Rerknimitr, R. Current Status of Colorectal Cancer and Its Public Health Burden in Thailand. Clin. Endosc. 2021, 54, 499–504. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Biller, L.H.; Schrag, D. Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review. JAMA 2021, 325, 669–685. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, M.; Zou, Y.; Jin, L.; Zhao, Z.; Liu, Q.; Wang, S.; Li, J. Mechanisms of chemotherapeutic resistance and the application of targeted nanoparticles for enhanced chemotherapy in colorectal cancer. J. Nanobiotechnol. 2022, 20, 371. [Google Scholar] [CrossRef]
- Wang, Q.; Shen, X.; Chen, G.; Du, J. Drug Resistance in Colorectal Cancer: From Mechanism to Clinic. Cancers 2022, 14, 2928. [Google Scholar] [CrossRef]
- Hu, W.; Kavanagh, J.J. Anticancer therapy targeting the apoptotic pathway. Lancet Oncol. 2003, 4, 721–729. [Google Scholar] [CrossRef]
- Ghobrial, I.M.; Witzig, T.E.; Adjei, A.A. Targeting apoptosis pathways in cancer therapy. CA Cancer J. Clin. 2005, 55, 178–194. [Google Scholar] [CrossRef] [PubMed]
- Tolomeo, M.; Simoni, D. Drug resistance and apoptosis in cancer treatment: Development of new apoptosis-inducing agents active in drug resistant malignancies. Curr. Med. Chem. Anti Cancer Agents 2002, 2, 387–401. [Google Scholar] [CrossRef]
- Sanchez-Martinez, C.; Gelbert, L.M.; Lallena, M.J.; de Dios, A. Cyclin dependent kinase (CDK) inhibitors as anticancer drugs. Bioorganic Med. Chem. Lett. 2015, 25, 3420–3435. [Google Scholar] [CrossRef]
- Fantini, M.; Benvenuto, M.; Masuelli, L.; Frajese, G.V.; Tresoldi, I.; Modesti, A.; Bei, R. In vitro and in vivo antitumoral effects of combinations of polyphenols, or polyphenols and anticancer drugs: Perspectives on cancer treatment. Int. J. Mol. Sci. 2015, 16, 9236–9282. [Google Scholar] [CrossRef]
- Liu, H.; Schmitz, J.C.; Wei, J.; Cao, S.; Beumer, J.H.; Strychor, S.; Cheng, L.; Liu, M.; Wang, C.; Wu, N.; et al. Clove extract inhibits tumor growth and promotes cell cycle arrest and apoptosis. Oncol. Res. 2014, 21, 247–259. [Google Scholar] [CrossRef]
- Hadidi, M.; Linan-Atero, R.; Tarahi, M.; Christodoulou, M.C.; Aghababaei, F. The Potential Health Benefits of Gallic Acid: Therapeutic and Food Applications. Antioxidants 2024, 13, 1001. [Google Scholar] [CrossRef] [PubMed]
- Chailungka, A.; Junpirom, T.; Pompimon, W.; Nuntasaen, N.; Meepowpan, P. Two flavonoids first isolated from the seed of Syzygium nervosum and preliminary study of their anticancer and anti- HIV-1 reverse transcriptase activities. Maejo Int. J. Sci. Technol. 2017, 11, 58–67. [Google Scholar]
- Vachiraarunwong, A.; Tuntiwechapikul, W.; Wongnoppavich, A.; Meepowpan, P.; Wongpoomchai, R. 2,4′-dihydroxy-6′-methoxy-3′,5′-dimethylchalcone from Cleistocalyx nervosum var. paniala seeds attenuated the early stage of diethylnitrosamine and 1,2-dimethylhydrazine-induced colorectal carcinogenesis. Biomed. Pharmacother. 2023, 165, 115221. [Google Scholar] [CrossRef] [PubMed]
- Bureau of Drug and Narcotic, Department of Medical Sciences (D.o.M.S.), Ministry of Public Health. Thai Herbal Pharmacopoeia; Department of Medical Sciences: Nonthaburi, Thailand, 2021. [Google Scholar]
- Sinosaki, N.; Tonin, A.; Ribeiro, M.; Poliseli, C.; Roberto, S.; da Silveira, R.; Visentainer, J.; Santos, O.; Meurer, E. Structural Study of Phenolic Acids by Triple Quadrupole Mass Spectrometry with Electrospray Ionization in Negative Mode and H/D Isotopic Exchange. J. Braz. Chem. Soc. 2020, 31. [Google Scholar] [CrossRef]
- Cai, L.; Wu, C.D. Compounds from Syzygium aromaticum possessing growth inhibitory activity against oral pathogens. J. Nat. Prod. 1996, 59, 987–990. [Google Scholar] [CrossRef]
- Tanaka, T.; Orii, Y.; Nonaka, G.I.; Nishioka, I. Tannins and Related Compounds. CXXIII. Chromone, Acetophenone and Phenylpropanoid Glycosides and Their Galloyl and/or Hexahydroxydiphenoyl Esters from the Leaves of Syzygium aromaticum MERR. et PERRY. Chem. Pharm. Bull. 1993, 41, 1232–1237. [Google Scholar] [CrossRef][Green Version]
- Snarska, J.; Jakimiuk, K.; Strawa, J.W.; Tomczyk, T.M.; Tomczykowa, M.; Piwowarski, J.P.; Tomczyk, M. A Comprehensive Review of Pedunculagin: Sources, Chemistry, Biological and Pharmacological Insights. Int. J. Mol. Sci. 2024, 25, 11511. [Google Scholar] [CrossRef]
- Khamto, N.; Chaichuang, L.; Rithchumpon, P.; Phupong, W.; Bhoopong, P.; Tateing, S.; Pompimon, W.; Semakul, N.; Chomsri, N.O.; Meepowpan, P. Synthesis, cytotoxicity evaluation and molecular docking studies on 2′,4′-dihydroxy-6′-methoxy-3′,5′-dimethylchalcone derivatives. RSC Adv. 2021, 11, 31433–31447. [Google Scholar] [CrossRef]
- Choommongkol, V.; Punturee, K.; Klumphu, P.; Rattanaburi, P.; Meepowpan, P.; Suttiarporn, P. Microwave-Assisted Extraction of Anticancer Flavonoid, 2′,4′-Dihydroxy-6′-methoxy-3′,5′-dimethyl Chalcone (DMC), Rich Extract from Syzygium nervosum Fruits. Molecules 2022, 27, 1397. [Google Scholar] [CrossRef]
- Subramanian, A.P.; John, A.A.; Vellayappan, M.V.; Balaji, A.; Jaganathan, S.K.; Supriyanto, E.; Yusof, M. Gallic acid: Prospects and molecular mechanisms of its anticancer activity. RSC Adv. 2015, 5, 35608–35621. [Google Scholar] [CrossRef]
- Anitha, P.; Priyadarsini, R.V.; Kavitha, K.; Thiyagarajan, P.; Nagini, S. Ellagic acid coordinately attenuates Wnt/beta-catenin and NF-kappaB signaling pathways to induce intrinsic apoptosis in an animal model of oral oncogenesis. Eur. J. Nutr. 2013, 52, 75–84. [Google Scholar] [CrossRef]
- Lin, X.; Wang, G.; Liu, P.; Han, L.; Wang, T.; Chen, K.; Gao, Y. Gallic acid suppresses colon cancer proliferation by inhibiting SRC and EGFR phosphorylation. Exp. Ther. Med. 2021, 21, 638. [Google Scholar] [CrossRef]
- Ahmed, Z.S.O.; Khan, E.; Elias, N.; Elshebiny, A.; Dou, Q. Updated Review on Natural Polyphenols: Molecular Mechanisms, Biological Effects, and Clinical Applications for Cancer Management. Biomolecules 2025, 15, 629. [Google Scholar] [CrossRef]
- Zhao, J.; Li, G.; Wei, J.; Dang, S.; Yu, X.; Ding, L.; Shang, C.; Zhang, H.; Zhang, Z.; Chen, H.; et al. Ellagic acid induces cell cycle arrest and apoptosis via the TGF-beta1/Smad3 signaling pathway in human colon cancer HCT-116 cells. Oncol. Rep. 2020, 44, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, G.; Bo, W.; Zhou, Y.; Dang, S.; Wei, J.; Li, X.; Liu, M. Multiple effects of ellagic acid on human colorectal carcinoma cells identified by gene expression profile analysis. Int. J. Oncol. 2017, 50, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi Ekbatan, S.; Li, X.Q.; Ghorbani, M.; Azadi, B.; Kubow, S. Chlorogenic Acid and Its Microbial Metabolites Exert Anti-Proliferative Effects, S-Phase Cell-Cycle Arrest and Apoptosis in Human Colon Cancer Caco-2 Cells. Int. J. Mol. Sci. 2018, 19, 723. [Google Scholar] [CrossRef] [PubMed]
- Utama, K.; Khamto, N.; Meepowpan, P.; Aobchey, P.; Kantapan, J.; Sringarm, K.; Roytrakul, S.; Sangthong, P. Effects of 2′,4′-Dihydroxy-6′-methoxy-3′,5′-dimethylchalcone from Syzygium nervosum Seeds on Antiproliferative, DNA Damage, Cell Cycle Arrest, and Apoptosis in Human Cervical Cancer Cell Lines. Molecules 2022, 27, 1154. [Google Scholar] [CrossRef]
- Utama, K.; Khamto, N.; Meepowpan, P.; Aobchey, P.; Kantapan, J.; Meerak, J.; Roytrakul, S.; Sangthong, P. 2′,4′-Dihydroxy-6′-methoxy-3′,5′-dimethylchalcone and its amino acid-conjugated derivatives induce G0/G1 cell cycle arrest and apoptosis via BAX/BCL2 ratio upregulation and in silico insight in SiHa cell lines. Eur. J. Pharm. Sci. 2023, 184, 106390. [Google Scholar] [CrossRef]
- Taya, S.; Punvittayagul, C.; Meepowpan, P.; Wongpoomchai, R. Cancer Chemopreventive Effect of 2′,4′-Dihydroxy-6′-methoxy-3′,5′-dimethylchalcone on Diethylnitrosamine-Induced Early Stages of Hepatocarcinogenesis in Rats. Plants 2024, 13, 1975. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef] [PubMed]
- Zeb, A. Concept, mechanism, and applications of phenolic antioxidants in foods. J. Food Biochem. 2020, 44, e13394. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.; Dominguez-Lopez, I.; Lamuela-Raventos, R.M. The Chemistry Behind the Folin-Ciocalteu Method for the Estimation of (Poly)phenol Content in Food: Total Phenolic Intake in a Mediterranean Dietary Pattern. J. Agric. Food Chem. 2023, 71, 17543–17553. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Mittal, M.; Gupta, N.; Parashar, P.; Mehra, V.; Khatri, M. Phytochemical evaluation and pharmacological activity of Syzygium aromaticum: A comprehensive review. Int. J. Pharm. Pharm. Sci. 2014, 6, 67–72. [Google Scholar]
- Samavardhana, K.; Supawititpattana, P.; Jittrepotch, N.; Rojsuntornkitti, K.; Kongbangkerd, T. Effects of extracting conditions on phenolic compounds and antioxidant activity from different grape processing byproducts. Int. Food Res. J. 2015, 22, 1169–1179. [Google Scholar]
- Molole, G.J.; Gure, A.; Abdissa, N. Determination of total phenolic content and antioxidant activity of Commiphora mollis (Oliv.) Engl. resin. BMC Chem. 2022, 16, 48. [Google Scholar] [CrossRef]
- Gulcin, İ.; Alwasel, S.H. DPPH Radical Scavenging Assay. Processes 2023, 11, 2248. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Jumina, J.; Siswanta, D.; Zulkarnain, A.K.; Triono, S.; Priatmoko, P.; Yuanita, E.; Fatmasari, N.; Nursalim, I. Development of C-Arylcalix[4]resorcinarenes and C-Arylcalix[4]pyrogallolarenes as Antioxidant and UV-B Protector. Indones. J. Chem. 2019, 19, 273. [Google Scholar] [CrossRef]
- Minarti, M.; Ariani, N.; Megawati, M.; Hidayat, A.; Hendra, M.; Primahana, G.; Darmawan, A. Potential Antioxidant Activity Methods DPPH, ABTS, FRAP, Total Phenol and Total Flavonoid Levels of Macaranga hypoleuca (Reichb. f. & Zoll.) Leaves Extract and Fractions. E3S Web Conf. 2024, 503, 07005. [Google Scholar] [CrossRef]
- Al-Rimawi, F.; Rishmawi, S.; Ariqat, S.H.; Khalid, M.F.; Warad, I.; Salah, Z. Anticancer Activity, Antioxidant Activity, and Phenolic and Flavonoids Content of Wild Tragopogon porrifolius Plant Extracts. Evid.-Based Complement. Altern. Med. 2016, 2016, 9612490. [Google Scholar] [CrossRef]
- Flis, S.; Jastrzebski, Z.; Namiesnik, J.; Arancibia-Avila, P.; Toledo, F.; Leontowicz, H.; Leontowicz, M.; Suhaj, M.; Trakhtenberg, S.; Gorinstein, S. Evaluation of inhibition of cancer cell proliferation in vitro with different berries and correlation with their antioxidant levels by advanced analytical methods. J. Pharm. Biomed. Anal. 2012, 62, 68–78. [Google Scholar] [CrossRef]
- Okafor, J.N.C.; Rautenbauch, F.; Meyer, M.; Le Roes-Hill, M.; Harris, T.; Jideani, V.A. Phenolic content, antioxidant, cytotoxic and antiproliferative effects of fractions of Vigna subterraenea (L.) verdc from Mpumalanga, South Africa. Heliyon 2021, 7, e08397. [Google Scholar] [CrossRef] [PubMed]
- Radha Abbas Hasoon, M.; Jawad Kadhim, N. Improvement of the Selectivity Index (SI) and Cytotoxicity Activity of Doxorubicin Drug by Panax ginseng Plant Extract. Arch. Razi Inst. 2021, 76, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, M.; Seghatoleslam, A.; Namavari, M.; Muhammadi, A.; Fahmidehkar, M.A.; Ramezani, A.; Eftekhar, E.; Hosseini, A.; Erfani, N.; Fakher, S. Selective Cytotoxicity and Apoptosis-Induction of Cyrtopodion Scabrum Extract Against Digestive Cancer Cell Lines. Int. J. Cancer Manag. 2017, 10, e8633. [Google Scholar] [CrossRef]
- Munteanu, A.; Gogulescu, A.; Șoica, C.; Mioc, A.; Mioc, M.; Milan, A.; Lukinich-Gruia, A.T.; Pricop, M.-A.; Jianu, C.; Banciu, C.; et al. In Vitro and In Silico Evaluation of Syzygium aromaticum Essential Oil: Effects on Mitochondrial Function and Cytotoxic Potential Against Cancer Cells. Plants 2024, 13, 3443. [Google Scholar] [CrossRef]
- Abu El Maaty, M.A.; Strassburger, W.; Qaiser, T.; Dabiri, Y.; Wolfl, S. Differences in p53 status significantly influence the cellular response and cell survival to 1,25-dihydroxyvitamin D3-metformin cotreatment in colorectal cancer cells. Mol. Carcinog. 2017, 56, 2486–2498. [Google Scholar] [CrossRef]
- Lakshmanan, I.; Batra, S.K. Protocol for Apoptosis Assay by Flow Cytometry Using Annexin V Staining Method. Bio Protoc. 2013, 3, e374. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, G.; Zhang, D.; An, W.; Lai, H.; Li, X.; Cao, S.; Lin, X. Active fraction of clove induces apoptosis via PI3K/Akt/mTOR-mediated autophagy in human colorectal cancer HCT-116 cells. Int. J. Oncol. 2018, 53, 1363–1373. [Google Scholar] [CrossRef]
- Thermo Fisher Scientific Inc. No-wash imaging of apoptosis: CellEvent™ Caspase-3/7 Green Detection Reagent. BioProbes 2011, 64. [Google Scholar]
- Utsumi, F.; Kajiyama, H.; Nakamura, K.; Tanaka, H.; Mizuno, M.; Ishikawa, K.; Kondo, H.; Kano, H.; Hori, M.; Kikkawa, F. Effect of indirect nonequilibrium atmospheric pressure plasma on anti-proliferative activity against chronic chemo-resistant ovarian cancer cells in vitro and in vivo. PLoS ONE 2013, 8, e81576. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Zhang, D.; Yang, X.H.; Li, X.F.; Liu, M.H. Inhibition effect of active fraction from clove on PI3K/Akt/mTOR signaling pathway to induce apoptosis of human colon cancer HCT116 cells. Zhongguo Zhong Yao Za Zhi 2021, 46, 1197–1204. [Google Scholar] [CrossRef] [PubMed]
- Franken, N.A.; Rodermond, H.M.; Stap, J.; Haveman, J.; van Bree, C. Clonogenic assay of cells in vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xing, G.; Guo, X.; Chen, H.; Li, J.; Wang, J.; Li, Y.; Liang, G.; Liu, M. Inhibition of colorectal cancer cell growth by downregulation of M2-PK and reduction of aerobic glycolysis by clove active ingredients. Front. Pharmacol. 2025, 16, 1552486. [Google Scholar] [CrossRef]
- Shen, A.; Liu, L.; Chen, H.; Qi, F.; Huang, Y.; Lin, J.; Sferra, T.J.; Sankararaman, S.; Wei, L.; Chu, J.; et al. Cell division cycle associated 5 promotes colorectal cancer progression by activating the ERK signaling pathway. Oncogenesis 2019, 8, 19. [Google Scholar] [CrossRef]
- Chien, J.H.; Chang, K.F.; Lee, S.C.; Lee, C.J.; Chen, Y.T.; Lai, H.C.; Lu, Y.C.; Tsai, N.M. Cedrol restricts the growth of colorectal cancer in vitro and in vivo by inducing cell cycle arrest and caspase-dependent apoptotic cell death. Int. J. Med. Sci. 2022, 19, 1953–1964. [Google Scholar] [CrossRef]
- Abbas, T.; Dutta, A. p21 in cancer: Intricate networks and multiple activities. Nat. Rev. Cancer 2009, 9, 400–414. [Google Scholar] [CrossRef]
- Bunz, F.; Dutriaux, A.; Lengauer, C.; Waldman, T.; Zhou, S.; Brown, J.P.; Sedivy, J.M.; Kinzler, K.W.; Vogelstein, B. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science 1998, 282, 1497–1501. [Google Scholar] [CrossRef]
- Haddad, A.Q.; Venkateswaran, V.; Viswanathan, L.; Teahan, S.J.; Fleshner, N.E.; Klotz, L.H. Novel antiproliferative flavonoids induce cell cycle arrest in human prostate cancer cell lines. Prostate Cancer Prostatic Dis. 2006, 9, 68–76. [Google Scholar] [CrossRef]
- Hazafa, A.; Rehman, K.U.; Jahan, N.; Jabeen, Z. The Role of Polyphenol (Flavonoids) Compounds in the Treatment of Cancer Cells. Nutr. Cancer 2020, 72, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, L.R.; Mazza, G. Assessing antioxidant and prooxidant activities of phenolic compounds. J. Agric. Food Chem. 2000, 48, 3597–3604. [Google Scholar] [CrossRef]
- Vongsak, B.; Sithisarn, P.; Mangmool, S.; Thongpraditchote, S.; Wongkrajang, Y.; Gritsanapan, W. Maximizing total phenolics, total flavonoids contents and antioxidant activity of Moringa oleifera leaf extract by the appropriate extraction method. Ind. Crops Prod. 2013, 44, 566–571. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Acosta, M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 2001, 73, 239–244. [Google Scholar] [CrossRef]
- Chen, Y.-F.; Yang, J.-S.; Chang, W.-S.; Tsai, S.-C.; Peng, S.-F.; Zhou, Y.-R. Houttuynia cordata Thunb extract modulates G 0/G 1 arrest and Fas/CD95-mediated death receptor apoptotic cell death in human lung cancer A549 cells. J. Biomed. Sci. 2013, 20, 18. [Google Scholar] [CrossRef]
- Subhawa, S.; Chewonarin, T.; Banjerdpongchai, R. The Effects of Houttuynia cordata Thunb and Piper ribesioides Wall Extracts on Breast Carcinoma Cell Proliferation, Migration, Invasion and Apoptosis. Molecules 2020, 25, 1196. [Google Scholar] [CrossRef]
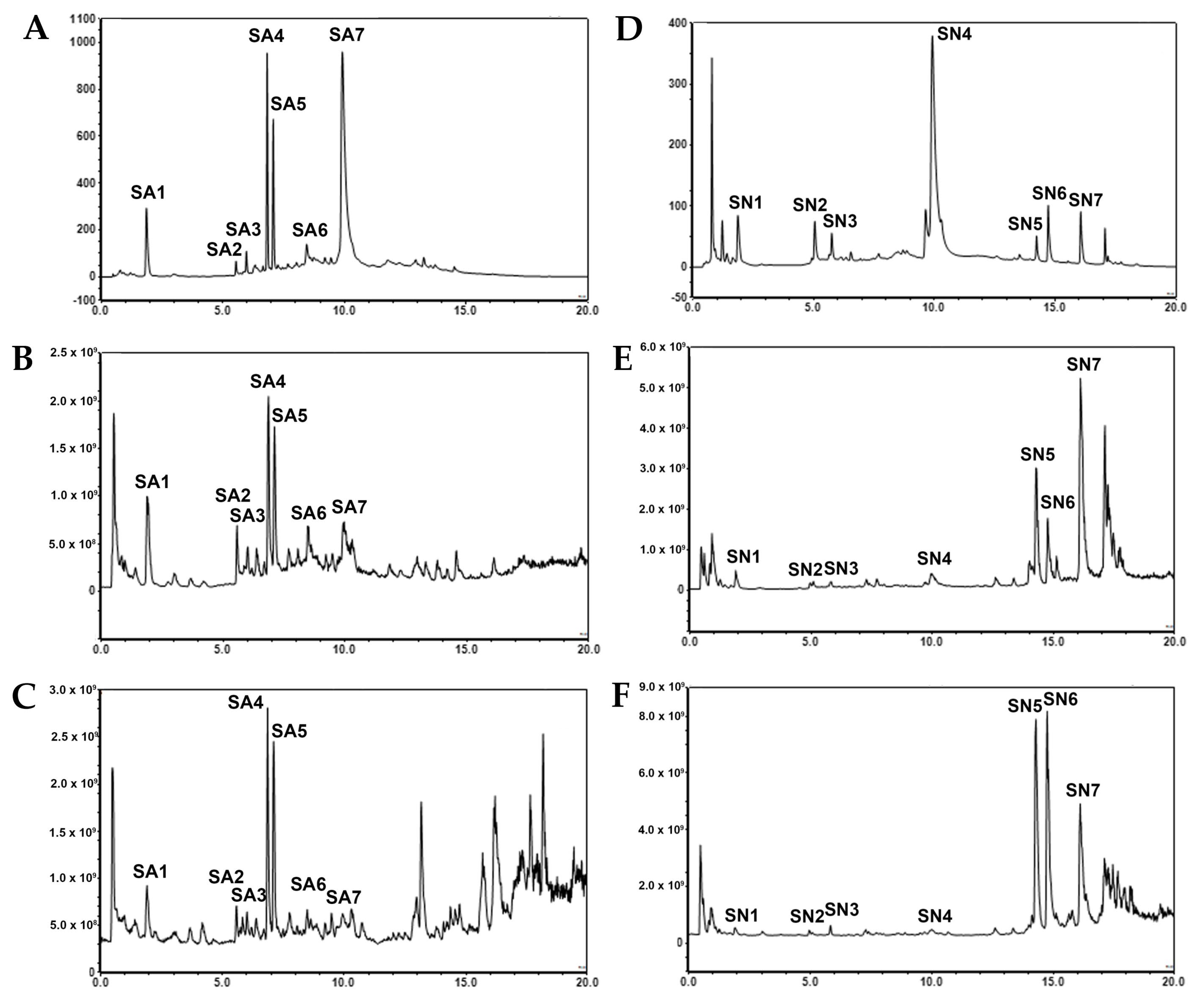






| Peak No. | Retention Time (min) | Compound | Mode | Parent Ion (MS1) | Fragment Ion (MS2) |
|---|---|---|---|---|---|
| SA1 | 1.91 | Gallic acid C7H6O5, MW = 170 | Negative | 169.0140 (Calc for [M−H]− = 169.01315) | 125.0245 |
| Positive | 171.0288 (Calc for [M+H]+ = 171.02880) | 153.0183, 127.0390, 125.0234, 109.0284, 107.0127, 81.0335 | |||
| SA2 | 5.58 | Unknown C20H20O14 MW = 484 | Negative | 483.0773 (Calc for [M−H]− = 483.07693) | 331.0677, 313.0566, 169.0142, 125.0245 |
| Positive | 485.0925 (Calc for [M+H]+ = 485.09258) | 202.0779, 153.0182 | |||
| 507.0744 (Calc for [M+Na]+ = 507.07453) | 337.0526, 202.0778, 153.0185 | ||||
| SA3 | 6.02 | Stictinin C27H22O18 MW = 634 | Negative | 633.0729 (Calc for [M−H]− = 633.07224) | 300.9988, 275.0199, 202.0787, 169.0141 |
| Positive | 657.0695 (Calc for [M+Na]+ = 657.06983) | N/A | |||
| SA4 | 6.87 | Biflorin or isobiflorin C16H18O9 MW = 354 | Negative | 353.0870 (Calc for [M−H]− = 353.08671) | 233.0456, 205.0506, 202.0789 |
| Positive | 355.1019 (Calc for [M+H]+ = 355.10236) | 337.0921, 319.0810, 301.0715, 289.0709, 273.0757, 259.0605, 245.0802, 235.0603, 205.0498, 202.0780 | |||
| SA5 | 7.11 | Biflorin or isobiflorin C16H18O9 MW = 354 | Negative | 353.0869 (Calc for [M−H]− = 353.08671) | 263.0563, 245.0458, 233.0455, 205.0506, 202.0790 |
| Positive | 355.1020 (Calc for [M+H]+ = 355.10236) | 337.0906, 319.0818, 301.0713, 283.0608, 271.0605, 259.0602, 245.0805, 235.0602, 231.0653, 205.0496, 202.0779 | |||
| SA6 | 8.47 | Unknown C13H8O7 MW = 276 | Negative | 247.0247 | 219.0298, 202.0789, 191.0350 |
| 275.0196 (Calc for [M−H]− = 275.01863) | 257.0090, 229.0142, 202.0788 | ||||
| Positive | 249.1119 | 207.0287, 202.0778 | |||
| 277.0342 (Calc for [M+H]+ = 277.03428) | 259.0236, 231.0287, 215.0338, 202.0778, 187.0390 | ||||
| SA7 | 9.97 | Ellagic acid C14H6O8 MW = 302 | Negative | 300.9986 (Calc for [M−H]− = 300.99789) | 283.9955, 257.0091, 245.0097, 229.0150, 202.0789, 185.0245 |
| Positive | 303.0134 (Calc for [M+H]+ = 303.01354) | 285.0031, 275.0186, 257.0082, 202.0777 | |||
| 324.9954 (Calc for [M+Na]+ = 344.99549) | 202.0777 |
| Peak No. | Retention Time (min) | Compound | Mode | Parent Ion (MS1) | Fragment Ion (MS2) |
|---|---|---|---|---|---|
| SN1 | 1.90 | Gallic acid C7H6O5 MW = 170 | Negative | 169.0141 (Calc for [M−H]− = 169.01315) | 125.0244 |
| Positive | 171.0282 (Calc for [M+H]+ = 171.02880) | 153.0177, 135.0073, 127.0385, 125.0229, 109.0280, 107.0123, 81.0332 | |||
| SN2 | 5.10 | Pedunculagin or its isomer C34H24O22 MW = 784 | Negative | 391.0303 (Calc for [M−2H]2− = 391.03068) | 300.9984, 275.0197, 202.0786 |
| 783.0677 (Calc for [M−H]− = 783.06755) | 300.9988, 275.0197 | ||||
| Positive | 785.0827 (Calc for [M+H]+ = 785.08320) | N/A | |||
| 799.0986 | 303.0134, 276.0264 | ||||
| 802.1094 (Calc for [M+NH4]+ = 802.10975) | 303.0133, 277.0341, 259.0237 | ||||
| SN3 | 5.84 | Pedunculagin or its isomer C34H24O22 MW = 784 | Negative | 391.0302 (Calc for [M−2H]2− = 391.03068) | 300.9985, 275.0196, 202.0788 |
| 783.0677 (Calc for [M−H]− = 783.06755) | 300.9989, 275.0197 | ||||
| Positive | 243.0473 | 202.0777, 203.0853 | |||
| 463.1057 | 243.0476, 202.0777, 203.0847 | ||||
| 802.1097 (Calc for [M+NH4]+ = 802.10975) | N/A | ||||
| SN4 | 9.96 | Ellagic acid C14H6O8 MW = 302 | Negative | 300.9987 (Calc for [M−H]− = 300.99789) | 283.9963, 202.0789 |
| Positive | 303.0134 (Calc for [M+H]+ = 303.01354) | 285.0031, 275.0188, 257.0082 | |||
| 324.9954 (Calc for [M+Na]+ = 344.99549) | 202.0777, 203.0843, 227.9203 | ||||
| SN5 | 14.28 | Unknown Dimer of 2′,4′-Dihydroxy-6′-methoxy-3′,5′-dimethylchalcone C36H36O8 MW = 596 | Negative | 297.1129 (Calc for [M−2H]2− = 297.11323) | 282.0896, 255.1027, 240.0792, 202.0788, 193.0507, 178.0272, 150.0322 |
| Positive | 299.1275 (Calc for [M+2H]2+ = 299.12779) | 284.1057, 202.0777, 195.0652 | |||
| 321.1093 | 306.0862, 202.0778, 203.0846 | ||||
| 619.2296 (Calc for [M+Na]+ = 619.23024) | 321.1097, 202.0780, 203.0837 | ||||
| SN6 | 14.76 | Unknown Dimer of 2′,4′-Dihydroxy-6′-methoxy-3′,5′-dimethylchalcone C36H36O8 MW = 596 | Negative | 297.1130 (Calc for [M−2H]2− = 297.11323) | 255.1028, 211.1129, 202.0789, 193.0507, 149.0609, 79.0190 |
| 617.2155 | 297.1131, 255.1028, 193.0506 | ||||
| Positive | 299.1275 (Calc for [M+2H]2+ = 299.12779) | 213.0758, 195.0652 | |||
| 321.1095 | 202.0776, 203.0847, 217.0467, 233.3068 | ||||
| 619.2299 (Calc for [M+Na]+ = 619.23024) | 321.1097, 202.0780, 203.0837 | ||||
| SN7 | 16.10 | 2′,4′-Dihydroxy-6′-methoxy-3′,5′-dimethylchalcone (DMC) C18H18O4 MW = 298 | Negative | 297.1127 (Calc for [M−H]− = 297.11323) | 253.1234, 202.0789, 193.0506 |
| Positive | 299.1275 (Calc for [M+H]+ = 299.12779) | 213.0757, 195.0652 | |||
| 321.1094 (Calc for [M+Na]+ = 321.10973) | 202.0778, 203.0840, 235.0583 |
| Compounds/Time | Cell Lines | ||
|---|---|---|---|
| HCT116 | HT-29 | MRC-5 | |
| Syzygium aromaticum (SA) | IC50 values (µg/mL) | ||
| 24 h | 352.85 ± 51.40 | N/A | N/A |
| 48 h | 140.11 ± 5.89 | 346.50 ± 14.19 | N/A |
| 72 h | 133.65 ± 24.01 | 242.94 ± 0.70 | 299.3 ± 68.2 |
| SI * | 2.24 | 1.23 | - |
| Syzygium nervosum (SN) | IC50 values (µg/mL) | ||
| 24 h | 386.99 ± 4.97 | N/A | N/A |
| 48 h | 265.01 ± 12.26 | 354.54 ± 17.50 | N/A |
| 72 h | 165.64 ± 37.79 | 232.92 ± 8.08 | 428.3 ± 110.6 |
| SI * | 2.59 | 1.84 | - |
| Extract Compounds | Colony-Forming Efficiency (%) | ||||
|---|---|---|---|---|---|
| Control | 25 µg/mL | 50 µg/mL | 100 µg/mL | 200 µg/mL | |
| HCT116 | |||||
| Syzygium aromaticum (SA) | 100.00 ± 0 | 77.67 ± 12.73 | 62.44 ± 5.98 ** | 52.61 ± 3.69 *** | 10.80 ± 5.84 *** |
| Syzygium nervosum (SN) | 100.00 ± 0 | 91.05 ± 12.66 | 82.28 ± 10.29 | 90.30 ± 13.72 | 72.58 ± 3.43 * |
| HT-29 | |||||
| Syzygium aromaticum (SA) | 100.00 ± 0 | 91.01 ± 5.53 | 83.21 ± 5.25 ** | 78.71 ± 4.43 *** | 60.05 ± 4.11 *** |
| Syzygium nervosum (SN) | 100.00 ± 0 | 102.30 ± 2.59 | 104.50 ± 0.49 | 110.00 ± 4.02 | 106.80 ± 3.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yimsoo, T.; Taychaworaditsakul, W.; Chansakaow, S.; Kongkiatpaiboon, S.; Tayana, N.; Chewonarin, T.; Khonsung, P.; Sireeratawong, S. The Effect of the Ethanolic Extracts from Syzygium aromaticum and Syzygium nervosum on Antiproliferative Activity and Apoptosis in HCT116 and HT-29 Cells. Int. J. Mol. Sci. 2025, 26, 6826. https://doi.org/10.3390/ijms26146826
Yimsoo T, Taychaworaditsakul W, Chansakaow S, Kongkiatpaiboon S, Tayana N, Chewonarin T, Khonsung P, Sireeratawong S. The Effect of the Ethanolic Extracts from Syzygium aromaticum and Syzygium nervosum on Antiproliferative Activity and Apoptosis in HCT116 and HT-29 Cells. International Journal of Molecular Sciences. 2025; 26(14):6826. https://doi.org/10.3390/ijms26146826
Chicago/Turabian StyleYimsoo, Thunyatorn, Weerakit Taychaworaditsakul, Sunee Chansakaow, Sumet Kongkiatpaiboon, Ngampuk Tayana, Teera Chewonarin, Parirat Khonsung, and Seewaboon Sireeratawong. 2025. "The Effect of the Ethanolic Extracts from Syzygium aromaticum and Syzygium nervosum on Antiproliferative Activity and Apoptosis in HCT116 and HT-29 Cells" International Journal of Molecular Sciences 26, no. 14: 6826. https://doi.org/10.3390/ijms26146826
APA StyleYimsoo, T., Taychaworaditsakul, W., Chansakaow, S., Kongkiatpaiboon, S., Tayana, N., Chewonarin, T., Khonsung, P., & Sireeratawong, S. (2025). The Effect of the Ethanolic Extracts from Syzygium aromaticum and Syzygium nervosum on Antiproliferative Activity and Apoptosis in HCT116 and HT-29 Cells. International Journal of Molecular Sciences, 26(14), 6826. https://doi.org/10.3390/ijms26146826





