Lactate in Heart Failure
Abstract
1. Introduction
2. Acute Heart Failure (AHF)
3. Chronic Heart Failure (CHF)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Braunwald, E. Heart failure. JACC Heart Fail. 2013, 1, 1–20. [Google Scholar] [CrossRef]
- Braunwald, E. The war against heart failure: The Lancet lecture. Lancet 2015, 385, 812–824. [Google Scholar] [CrossRef]
- La Franca, E.; Manno, G.; Ajello, L.; Di Gesaro, G.; Minà, C.; Visconti, C.; Bellavia, D.; Falletta, C.; Romano, G.; Dell’ Oglio, S.; et al. Physiopathology and Diagnosis of Congestive Heart Failure: Consolidated Certainties and New Perspectives. Curr. Probl. Cardiol. 2021, 46, 100691. [Google Scholar] [CrossRef]
- Njoroge, J.N.; Teerlink, J.R. Pathophysiology and Therapeutic Approaches to Acute Decompensated Heart Failure. Circ. Res. 2021, 128, 1468–1486. [Google Scholar] [CrossRef]
- Desai, A.S.; Stevenson, L.W. Rehospitalization for Heart Failure. Circulation 2012, 126, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.S.; Chen, J.; Lin, Z.; Bueno, H.; Curtis, J.P.; Keenan, P.S.; Normand, S.-L.T.; Schreiner, G.; Spertus, J.A.; Vidán, M.T.; et al. Recent National Trends in Readmission Rates After Heart Failure Hospitalization. Circ. Heart Fail. 2010, 3, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Chioncel, O.; Mebazaa, A.; Maggioni, A.P.; Harjola, V.; Rosano, G.; Laroche, C.; Piepoli, M.F.; Crespo-Leiro, M.G.; Lainscak, M.; Ponikowski, P.; et al. Acute heart failure congestion and perfusion status—Impact of the clinical classification on in-hospital and long-term outcomes; insights from the ESC-EORP-HFA Heart Failure Long-Term Registry. Eur. J. Heart Fail. 2019, 21, 1338–1352. [Google Scholar] [CrossRef]
- Zymliński, R.; Biegus, J.; Sokolski, M.; Siwołowski, P.; Nawrocka-Millward, S.; Todd, J.; Jankowska, E.A.; Banasiak, W.; Cotter, G.; Cleland, J.G.; et al. Increased blood lactate is prevalent and identifies poor prognosis in patients with acute heart failure without overt peripheral hypoperfusion. Eur. J. Heart Fail. 2018, 20, 1011–1018. [Google Scholar] [CrossRef]
- Frea, S.; Pidello, S.; Canavosio, F.G.; Bovolo, V.; Botta, M.; Bergerone, S.; Gaita, F. Clinical Assessment of Hypoperfusion in Acute Heart Failure. Circ. J. 2015, 79, 398–405. [Google Scholar] [CrossRef]
- Shirakabe, A.; Hata, N.; Kobayashi, N.; Shinada, T.; Tomita, K.; Tsurumi, M.; Matsushita, M.; Okazaki, H.; Yamamoto, Y.; Yokoyama, S.; et al. Clinical significance of acid–base balance in an emergency setting in patients with acute heart failure. J. Cardiol. 2012, 60, 288–294. [Google Scholar] [CrossRef]
- Mebazaa, A.; Tolppanen, H.; Mueller, C.; Lassus, J.; DiSomma, S.; Baksyte, G.; Cecconi, M.; Choi, D.J.; Cohen Solal, A.; Christ, M.; et al. Acute heart failure and cardiogenic shock: A multidisciplinary practical guidance. Intensive Care Med. 2016, 42, 147–163. [Google Scholar] [CrossRef] [PubMed]
- Bertaina, M.; Morici, N.; Frea, S.; Garatti, L.; Briani, M.; Sorini, C.; Villanova, L.; Corrada, E.; Sacco, A.; Moltrasio, M.; et al. Differences between cardiogenic shock related to acute decompensated heart failure and acute myocardial infarction. ESC Heart Fail. 2023, 10, 3472–3482. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, N.; van Diepen, S.; So, D.; Lawler, P.R.; Fordyce, C.B. Cardiogenic shock teams and centres: A contemporary review of multidisciplinary care for cardiogenic shock. ESC Heart Fail. 2021, 8, 988–998. [Google Scholar] [CrossRef] [PubMed]
- James, J.H.; Luchette, F.A.; McCarter, F.D.; Fischer, J.E. Lactate is an unreliable indicator of tissue hypoxia in injury or sepsis. Lancet 1999, 354, 505–508. [Google Scholar] [CrossRef]
- Garcia-Alvarez, M.; Marik, P.; Bellomo, R. Sepsis-associated hyperlactatemia. Crit. Care 2014, 18, 503. [Google Scholar] [CrossRef]
- Kraut, J.A.; Madias, N.E. Lactic Acidosis. N. Engl. J. Med. 2014, 371, 2309–2319. [Google Scholar] [CrossRef]
- Adamo, L.; Nassif, M.E.; Novak, E.; LaRue, S.J.; Mann, D.L. Prevalence of lactic acidaemia in patients with advanced heart failure and depressed cardiac output. Eur. J. Heart Fail. 2017, 19, 1027–1033. [Google Scholar] [CrossRef]
- Andersen, L.W.; Mackenhauer, J.; Roberts, J.C.; Berg, K.M.; Cocchi, M.N.; Donnino, M.W. Etiology and Therapeutic Approach to Elevated Lactate Levels. Mayo Clin. Proc. 2013, 88, 1127–1140. [Google Scholar] [CrossRef]
- Kawase, T.; Toyofuku, M.; Higashihara, T.; Okubo, Y.; Takahashi, L.; Kagawa, Y.; Yamane, K.; Mito, S.; Tamekiyo, H.; Otsuka, M.; et al. Validation of lactate level as a predictor of early mortality in acute decompensated heart failure patients who entered intensive care unit. J. Cardiol. 2015, 65, 164–170. [Google Scholar] [CrossRef]
- Garcia, C.K.; Goldstein, J.L.; Pathak, R.K.; Anderson, R.G.W.; Brown, M.S. Molecular characterization of a membrane transporter for lactate, pyruvate, and other monocarboxylates: Implications for the Cori cycle. Cell 1994, 76, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A. Role of the Heart in Lactate Shuttling. Front. Nutr. 2021, 8, 663560. [Google Scholar] [CrossRef] [PubMed]
- Rabinowitz, J.D.; Enerbäck, S. Lactate: The ugly duckling of energy metabolism. Nat. Metab. 2020, 2, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, Y.; Zhang, B.; Lin, X.; Fu, X.; An, Y.; Zou, Y.; Wang, J.; Wang, Z.; Yu, T. Lactate metabolism in human health and disease. Signal Transduct. Target. Ther. 2022, 7, 305. [Google Scholar] [CrossRef]
- Bar, O.; Aronson, D. Hyperlactataemia and acid–base disturbances in normotensive patients with acute heart failure. Eur. Heart J. Acute Cardiovasc. Care 2022, 11, 242–251. [Google Scholar] [CrossRef]
- Biegus, J.; Zymliński, R.; Sokolski, M.; Gajewski, P.; Banasiak, W.; Ponikowski, P. Clinical, respiratory, haemodynamic, and metabolic determinants of lactate in heart failure. Kardiol. Pol. 2019, 77, 47–52. [Google Scholar] [CrossRef]
- Uyar, H.; Yesil, E.; Karadeniz, M.; Orscelik, O.; Ozkan, B.; Ozcan, T.; Yilmaz, D.C.; Celik, A. The Effect of High Lactate Level on Mortality in Acute Heart Failure Patients with Reduced Ejection Fraction Without Cardiogenic Shock. Cardiovasc. Toxicol. 2020, 20, 361–369. [Google Scholar] [CrossRef]
- Josiassen, J.; Helgestad, O.K.L.; Møller, J.E.; Schmidt, H.; Jensen, L.O.; Holmvang, L.; Ravn, H.B.; Hassager, C. Cardiogenic shock due to predominantly right ventricular failure complicating acute myocardial infarction. Eur. Heart J. Acute Cardiovasc. Care 2021, 10, 33–39. [Google Scholar] [CrossRef]
- Ebner, M.; Sentler, C.; Harjola, V.P.; Bueno, H.; Lerchbaumer, M.H.; Hasenfuß, G.; Eckardt, K.-U.; Konstantinides, S.V.; Lankeit, M. Outcome of patients with different clinical presentations of high-risk pulmonary embolism. Eur. Heart J. Acute Cardiovasc. Care 2021, 10, 787–796. [Google Scholar] [CrossRef]
- Brooks, G.A. The Science and Translation of Lactate Shuttle Theory. Cell Metab. 2018, 27, 757–785. [Google Scholar] [CrossRef]
- Glancy, B.; Kane, D.A.; Kavazis, A.N.; Goodwin, M.L.; Willis, W.T.; Gladden, L.B. Mitochondrial lactate metabolism: History and implications for exercise and disease. J. Physiol. 2021, 599, 863–888. [Google Scholar] [CrossRef] [PubMed]
- Rossello, X.; Bueno, H.; Gil, V.; Jacob, J.; Martín-Sánchez, F.J.; Llorens, P.; Puente, P.H.; Alquézar-Arbé, A.; Espinosa, B.; Raposeiras-Roubín, S.; et al. Synergistic Impact of Systolic Blood Pressure and Perfusion Status on Mortality in Acute Heart Failure. Circ. Heart Fail. 2021, 14, e007347. [Google Scholar] [CrossRef] [PubMed]
- Schulze, P.C.; Biolo, A.; Gopal, D.; Shahzad, K.; Balog, J.; Fish, M.; Siwik, D.; Colucci, W.S. Dynamics in Insulin Resistance and Plasma Levels of Adipokines in Patients with Acute Decompensated and Chronic Stable Heart Failure. J. Card. Fail. 2011, 17, 1004–1011. [Google Scholar] [CrossRef]
- Levy, B. Bench-to-bedside review: Is there a place for epinephrine in septic shock? Crit. Care 2005, 9, 561. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gjesdal, G.; Braun, O.Ö.; Smith, J.G.; Scherstén, F.; Tydén, P. Blood lactate is a predictor of short-term mortality in patients with myocardial infarction complicated by heart failure but without cardiogenic shock. BMC Cardiovasc. Disord. 2018, 18, 8. [Google Scholar] [CrossRef]
- Biegus, J.; Zymliński, R.; Gajewski, P.; Sokolski, M.; Siwołowski, P.; Sokolska, J.; Swoboda, K.; Banasiak, M.; Banasiak, W.; Ponikowski, P. Persistent hyperlactataemia is related to high rates of in-hospital adverse events and poor outcome in acute heart failure. Kardiol. Pol. 2019, 77, 355–362. [Google Scholar] [CrossRef]
- Biegus, J.; Zymliński, R.; Sokolski, M.; Jankowska, E.A.; Banasiak, W.; Ponikowski, P. Elevated lactate in acute heart failure patients with intracellular iron deficiency as identifier of poor outcome. Kardiol. Pol. 2019, 77, 347–354. [Google Scholar] [CrossRef]
- Hu, W.; Yuan, L.; Wang, X.; Zang, B.; Zhang, Y.; Yan, X.; Zhao, W.; Chao, Y. Predictive Value of Arterial Blood Lactic Acid Concentration on the Risk of in-Hospital All-Cause Death in Patients with Acute Heart Failure. Int. J. Clin. Pract. 2022, 2022, 7644535. [Google Scholar] [CrossRef]
- Nalos, M.; Leverve, X.; Huang, S.; Weisbrodt, L.; Parkin, R.; Seppelt, I.; Ting, I.; Mclean, A. Half-molar sodium lactate infusion improves cardiac performance in acute heart failure: A pilot randomised controlled clinical trial. Crit. Care 2014, 18, R48. [Google Scholar] [CrossRef]
- Drake, A.J.; Haines, J.R.; Noble, M.I. Preferential uptake of lactate by the normal myocardium in dogs. Cardiovasc. Res. 1980, 14, 65–72. [Google Scholar] [CrossRef]
- Weber, K.T.; Janicki, J.S. Cardiopulmonary exercise testing for evaluation of chronic cardiac failure. Am. J. Cardiol. 1985, 55, 22A–31A. [Google Scholar] [CrossRef]
- Wensel, R.; Francis, D.P.; Georgiadou, P.; Scott, A.; Genth-Zotz, S.; Anker, S.D.; Coats, A.J.S.; Piepoli, M.F. Exercise hyperventilation in chronic heart failure is not caused by systemic lactic acidosis. Eur. J. Heart Fail. 2005, 7, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Larsen, A. Assessing the effect of exercise training in men with heart failure. Comparison of maximal, submaximal and endurance exercise protocols. Eur. Heart J. 2001, 22, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Tyni-Lenné, R.; Gordon, A.; Jansson, E.; Bermann, G.; Sylvén, C. Skeletal muscle endurance training improves peripheral oxidative capacity, exercise tolerance, and health-related quality of life in women with chronic congestive heart failure secondary to either ischemic cardiomyopathy or idiopathic dilated cardiomyopathy. Am. J. Cardiol. 1997, 80, 1025–1029. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.J.; Green, H.J.; Cobb, F.R. Skeletal muscle biochemistry and histology in ambulatory patients with long-term heart failure. Circulation 1990, 81, 518–527. [Google Scholar] [CrossRef]
- Sullivan, M.J.; Higginbotham, M.B.; Cobb, F.R. Exercise training in patients with severe left ventricular dysfunction. Hemodynamic and metabolic effects. Circulation 1988, 78, 506–515. [Google Scholar] [CrossRef]
- Belardinelli, R.; Georgiou, D.; Scocco, V.; Barstow, T.J.; Purcaro, A. Low intensity exercise training in patients with chronic heart failure. J. Am. Coll. Cardiol. 1995, 26, 975–982. [Google Scholar] [CrossRef]
- Metra, M.; Raddino, R.; Cas, L.D.; Visioli, O. Assessment of peak oxygen consumption, lactate and ventilatory thresholds and correlation with resting and exercise hemodynamic data in chronic congestive heart failure. Am. J. Cardiol. 1990, 65, 1127–1133. [Google Scholar] [CrossRef]
- Delagardelle, C.; Feiereisen, P.; Autier, P.; Shita, R.; Krecke, R.; Beissel, J. Strength/endurance training versus endurance training in congestive heart failure. Med. Sci. Sports Exerc. 2002, 34, 1868–1872. [Google Scholar] [CrossRef]
- Scrutton, M.C. Clinical and biochemical aspects of lactic acidosis. FEBS Lett. 1976, 70, 294–295. [Google Scholar] [CrossRef]
- Nan Tie, E.; Wolsk, E.; Nanayakkara, S.; Vizi, D.; Mariani, J.; Moller, J.E.; Hassager, C.; Gustafsson, F.; Kaye, D.M. Hyperlactataemia is a marker of reduced exercise capacity in heart failure with preserved ejection fraction. ESC Heart Fail. 2024, 11, 2557–2565. [Google Scholar] [CrossRef]
- Armillotta, M.; Angeli, F.; Paolisso, P.; Belmonte, M.; Raschi, E.; Di Dalmazi, G.; Amicone, S.; Canton, L.; Fedele, D.; Suma, N.; et al. Cardiovascular therapeutic targets of sodium-glucose co-transporter 2 (SGLT2) inhibitors beyond heart failure. Pharmacol. Ther. 2025, 270, 108861. [Google Scholar] [CrossRef] [PubMed]
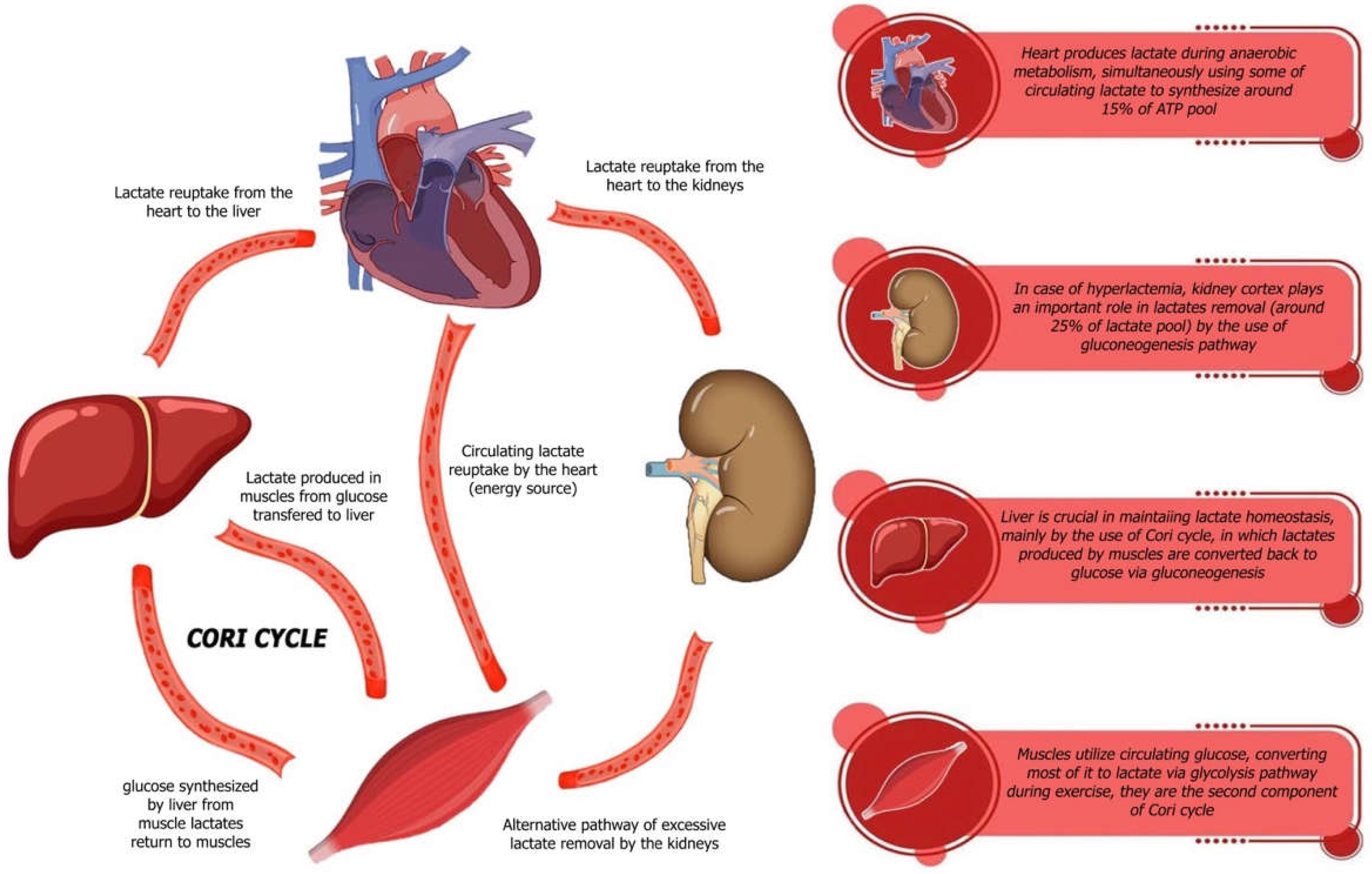
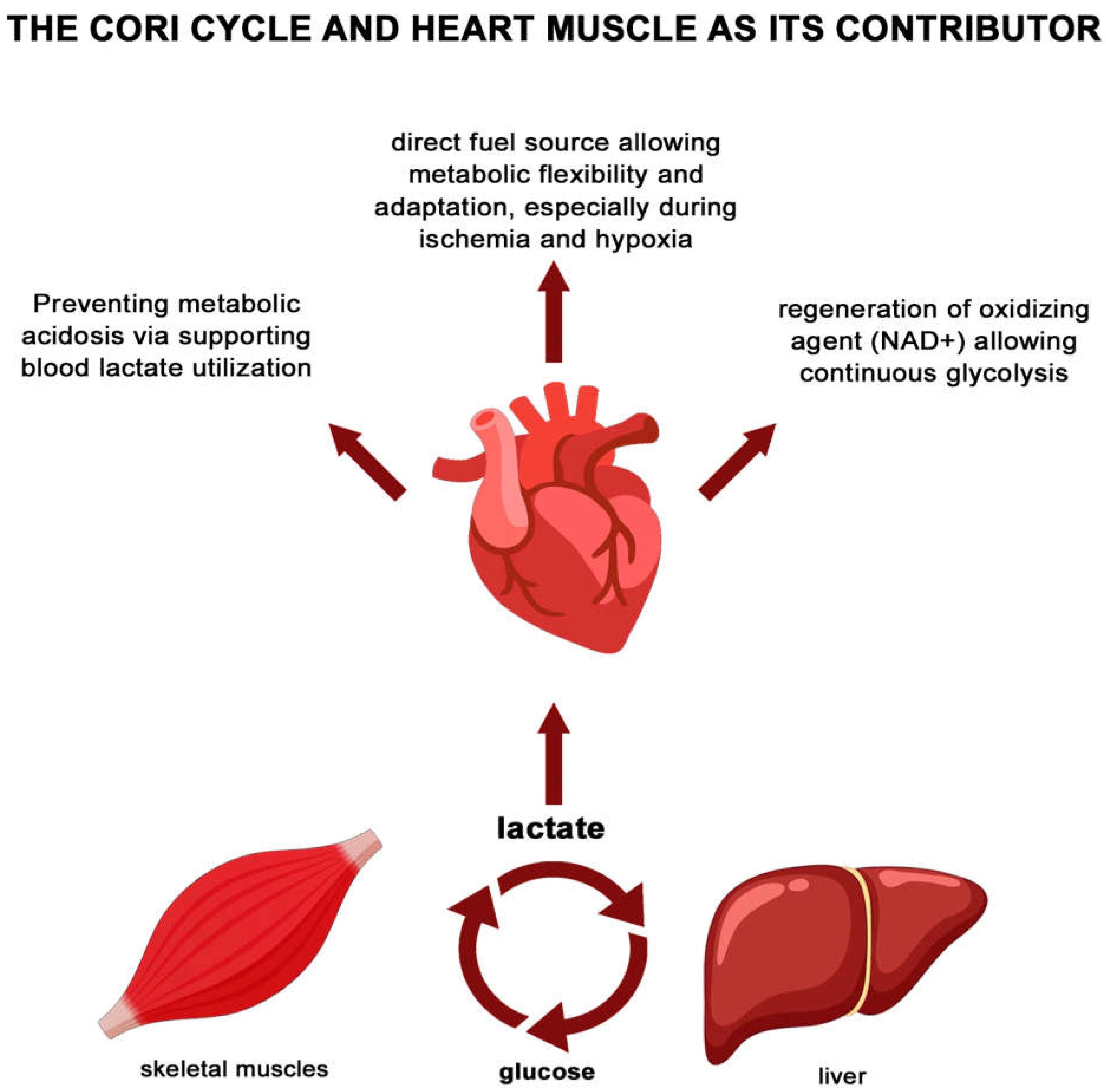
| Study (Author Year) | Hf Type | Sample Size/Population | Lactate Threshold | Key Clinical Outcomes/Findings |
|---|---|---|---|---|
| Weber et al., 1985 [41] | CHF | Patients with mild to severe CHF undergoing cardiopulmonary exercise testing; n = not stated | Anaerobic threshold at 60–70% of VO2 max | HF patients show earlier shift to anaerobic metabolism; lactate accumulates at lower workloads compared to healthy individuals |
| Nalos et al., 2014 [39] | AHF | Pilot RCT, n = 40 patients with acute heart failure receiving sodium lactate vs. control | Exogenous sodium lactate infusion | Sodium lactate infusion improved cardiac output and hemodynamics without negative effects on organ function |
| Kawase et al., 2015 [20] | AHF | ICU patients with acute decompensated HF; n = 113 | >3.2 mmol/L | High lactate at admission significantly predicted early mortality, independent of other clinical variables |
| Adamo et al., 2017 [18] | CHF (Advanced) | n = 89 patients with advanced HF and low cardiac output | ~25% had elevated lactate | Despite low cardiac output, only 25% had hyperlactatemia; suggests lactate elevation is a late metabolic event in CHF |
| Zymliński et al., 2018 [9] | AHF | n = 312 AHF patients, mostly normotensive, no overt shock | ≥2.0 mmol/L | Elevated lactate associated with higher 1-year mortality and evidence of cardiac/hepatic injury; useful for early risk stratification |
| Gjesdal et al., 2018 [35] | AHF | n = 188 patients with MI complicated by HF but without cardiogenic shock | >2.5 mmol/L | Increased lactate strongly predicted 30-day mortality; highlighted lactate’s role in post-MI prognosis |
| Biegus et al., 2019 [36] | AHF | n = 259 AHF patients admitted to ICU | Persistent elevation > 24 h | Patients with persistently elevated lactate had worse in-hospital and 1-year outcomes than those whose lactate normalized |
| Biegus et al., 2019 [37] | AHF | n = 405 AHF patients with assessment of iron status | Any elevation | Combined elevated lactate and intracellular iron deficiency dramatically worsened prognosis compared to patients without both risk factors |
| Hu et al., 2022 [38] | AHF | n = 1201 ICU patients with AHF | Continuous (range not specified) | Lactate was an independent predictor of in-hospital all-cause mortality; predictive value increased when combined with SAPS II and NT-proBNP |
| Nan Tie et al., 2024 [51] | HFpEF (CHF) | n = 36 HFpEF patients vs. 19 healthy controls in exercise testing | Elevated resting and exercise lactate | HFpEF patients showed early lactate rise during low workloads due to chronotropic incompetence and impaired oxygen extraction; reflects early anaerobic switch during daily activity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gajewski, P.; Wilk, M.M.; Aleksandrowicz, K.; Ponikowska, B.; Zymliński, R. Lactate in Heart Failure. Int. J. Mol. Sci. 2025, 26, 6810. https://doi.org/10.3390/ijms26146810
Gajewski P, Wilk MM, Aleksandrowicz K, Ponikowska B, Zymliński R. Lactate in Heart Failure. International Journal of Molecular Sciences. 2025; 26(14):6810. https://doi.org/10.3390/ijms26146810
Chicago/Turabian StyleGajewski, Piotr, Michał Maksymilian Wilk, Krzysztof Aleksandrowicz, Beata Ponikowska, and Robert Zymliński. 2025. "Lactate in Heart Failure" International Journal of Molecular Sciences 26, no. 14: 6810. https://doi.org/10.3390/ijms26146810
APA StyleGajewski, P., Wilk, M. M., Aleksandrowicz, K., Ponikowska, B., & Zymliński, R. (2025). Lactate in Heart Failure. International Journal of Molecular Sciences, 26(14), 6810. https://doi.org/10.3390/ijms26146810






