Leflunomide-Mediated Immunomodulation Inhibits Lesion Progression in a Vitiligo Mouse Model
Abstract
1. Introduction
2. Results
2.1. LEF Ameliorates Progressive Depigmentation in a Vitiligo Mouse Model
2.2. LEF Suppresses Cutaneous CD8+ T Lymphocyte Infiltration
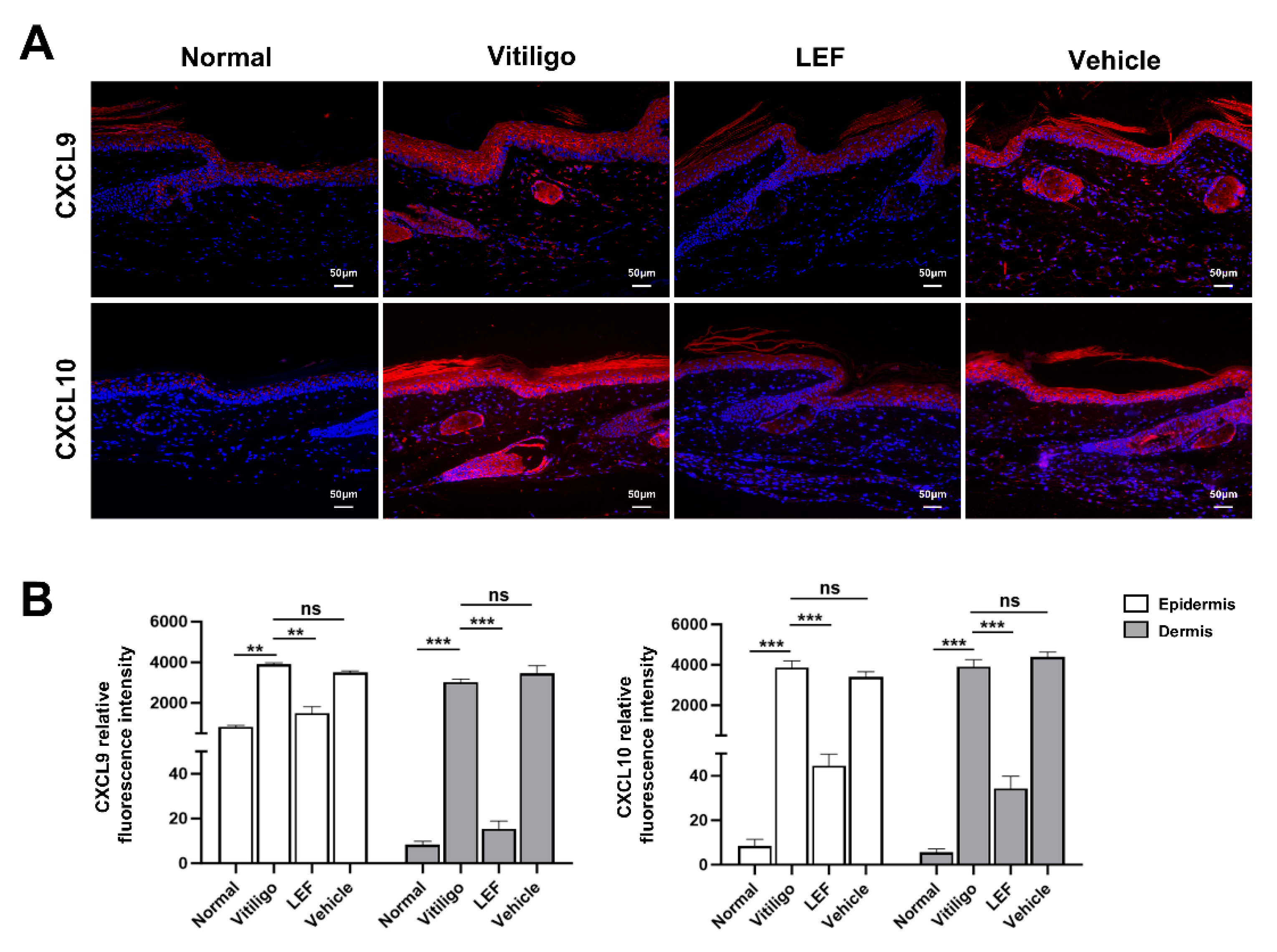
2.3. LEF Downregulates CXCL9 and 10 Chemokine Expression
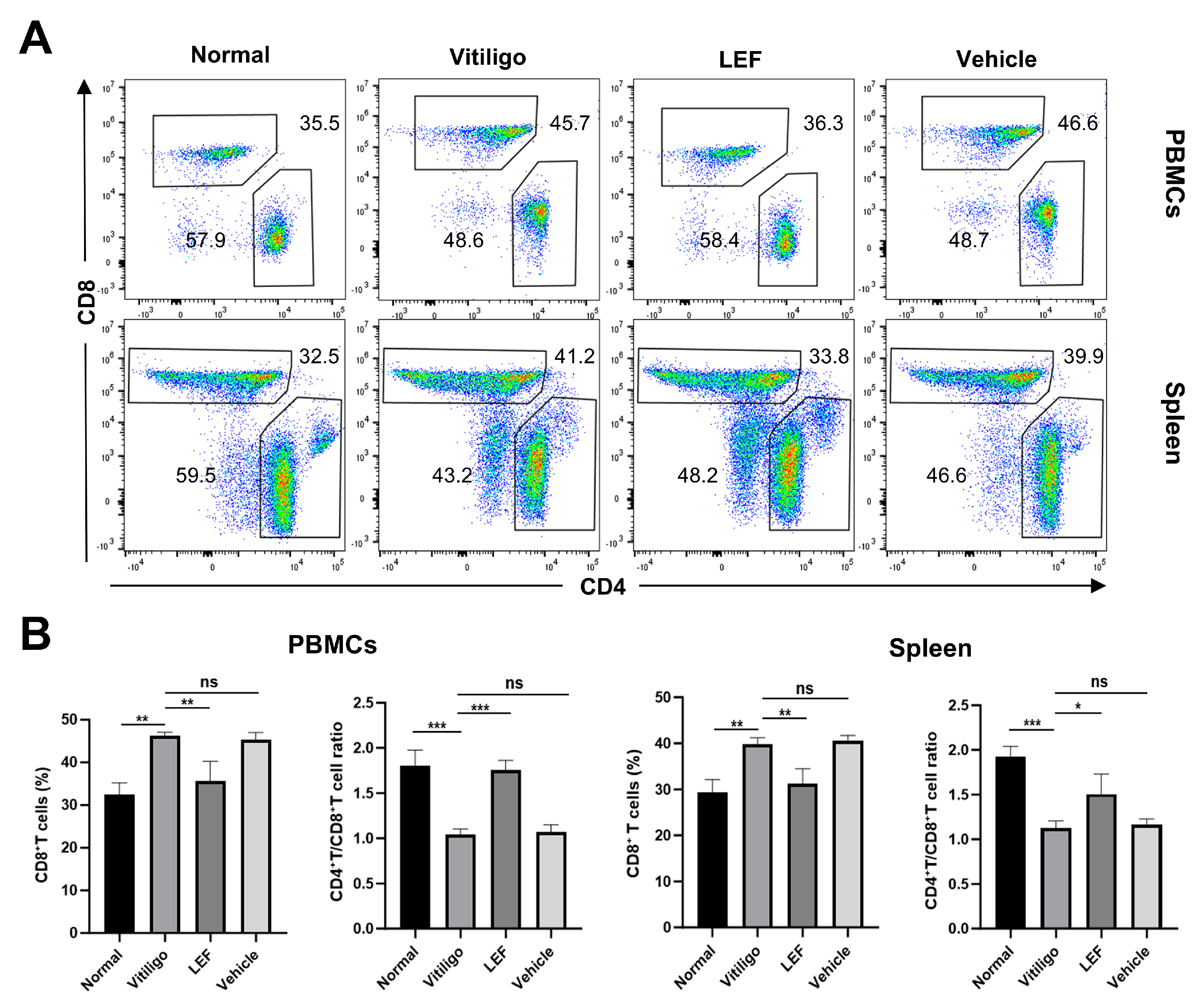
2.4. LEF Restores CD4+/CD8+ T Lymphocytes Homeostasis
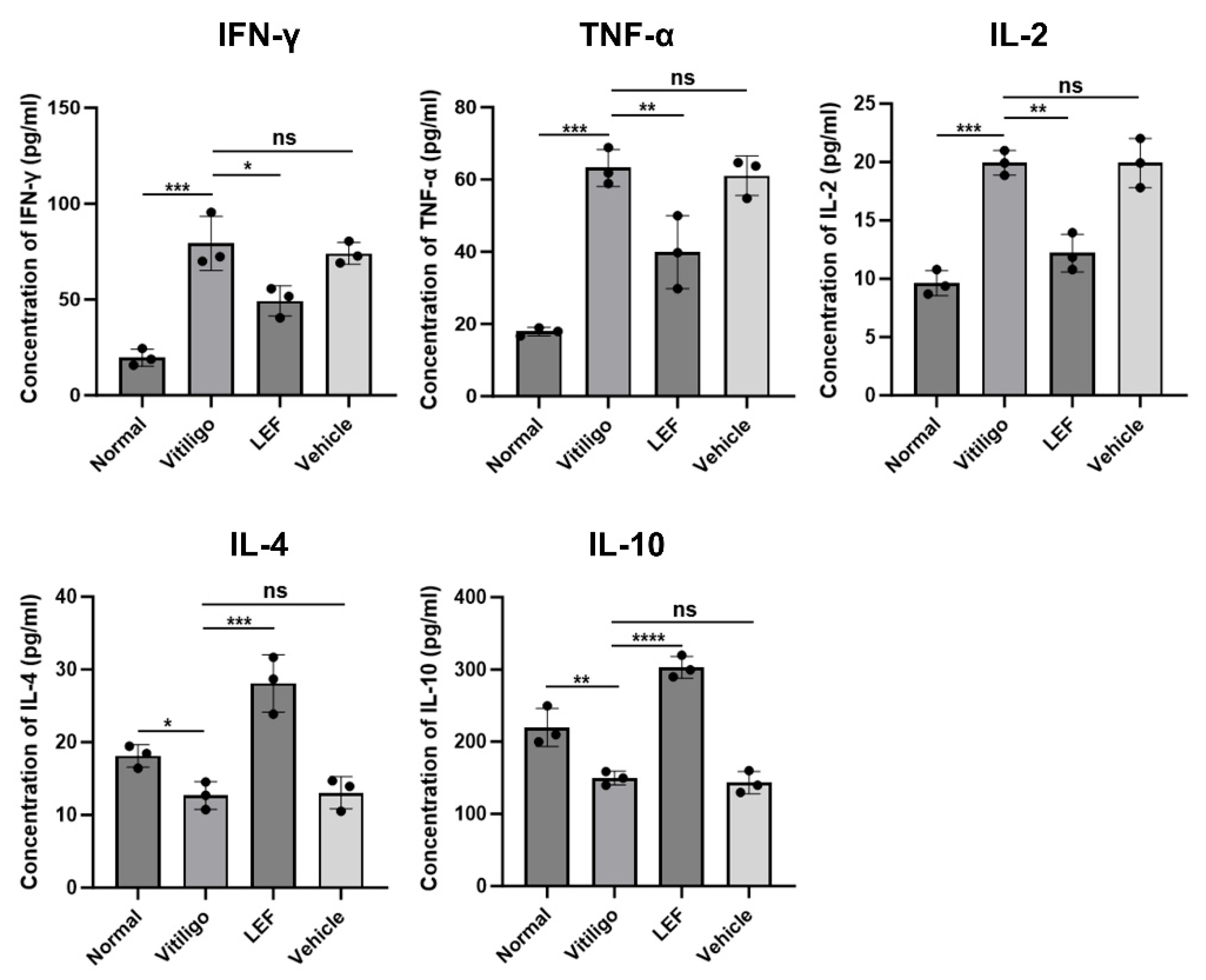
2.5. LEF Modulates Pro-Inflammatory and Anti-Inflammatory Cytokine Profiles
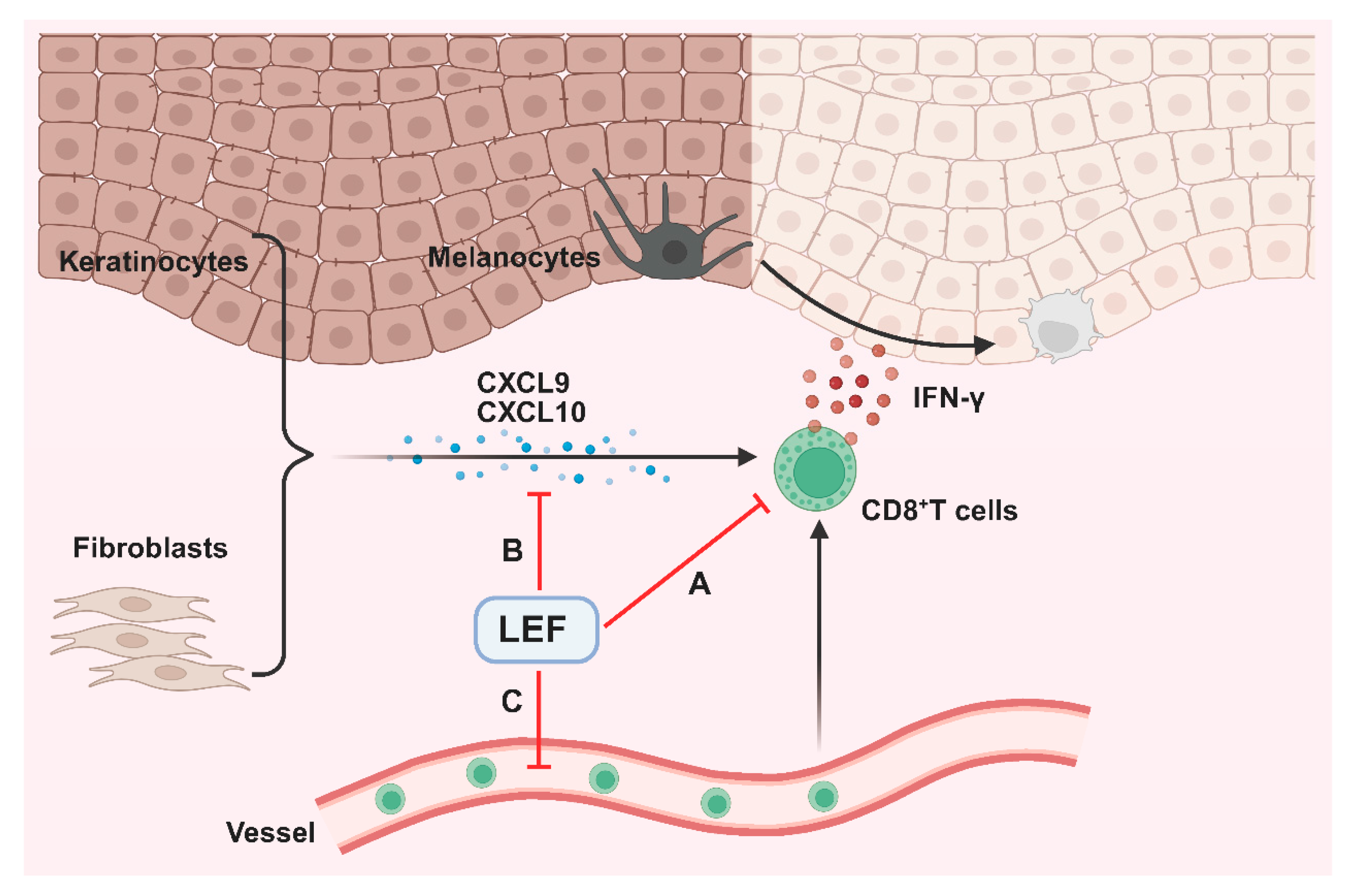
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Design
4.2. Establishment of a Vitiligo Mouse Model and LEF Treatment
4.3. Fontana Masson Staining
4.4. Immunohistochemical and Immunofluorescence Staining
4.5. Flow Cytometry
4.6. Enzyme-Linked Immunosorbent Assay (ELISA)
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LEF | Leflunomide |
| TRP2-180 | Tyrosine-related protein 2 -180 |
| IFN-γ | Interferon-γ |
| TNF-α | Tumor necrosis factor-α |
| CXCL9 | C-X-C motif chemokine ligand 9 |
| CXCL10 | C-X-C motif chemokine ligand 10 |
| CXCR3 | C-X-C chemokine receptor type 3 |
| PBMCs | Peripheral blood mononuclear cells |
| CTLs | Cytotoxic T lymphocytes |
| IL-2 | Interleukin-2 |
| IL-4 | Interleukin-4 |
| IL-10 | Interleukin-10 |
| ROS | Reactive oxygen species |
References
- Thakur, V.; Bishnoi, A.; Vinay, K.; Kumaran, S.M.; Parsad, D. Vitiligo: Translational Research and Effective Therapeutic Strategies. Pigment. Cell Melanoma Res. 2021, 34, 814–826. [Google Scholar] [CrossRef]
- Boniface, K.; Seneschal, J.; Picardo, M.; Taïeb, A. Vitiligo: Focus on Clinical Aspects, Immunopathogenesis, and Therapy. Clin. Rev. Allergy Immunol. 2018, 54, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-W.; Tan, Y.; Chen, T.; Liu, W.; Qian, Y.-T.; Ma, D.-L. Post-Traumatic Stress in Vitiligo Patients: A Neglected but Real-Existing Psychological Impairment. Clin. Cosmet. Investig. Dermatol. 2022, 15, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, W.; Chen, J.; Li, C.; Li, S. Management of the Refractory Vitiligo Patient: Current Therapeutic Strategies and Future Options. Front. Immunol. 2023, 14, 1294919. [Google Scholar] [CrossRef]
- Bergqvist, C.; Ezzedine, K. Vitiligo: A Review. Dermatology 2020, 236, 571–592. [Google Scholar] [CrossRef]
- Chen, J.; Li, S.; Li, C. Mechanisms of Melanocyte Death in Vitiligo. Med. Res. Rev. 2021, 41, 1138–1166. [Google Scholar] [CrossRef] [PubMed]
- Frisoli, M.L.; Essien, K.; Harris, J.E. Vitiligo: Mechanisms of Pathogenesis and Treatment. Annu. Rev. Immunol. 2020, 38, 621–648. [Google Scholar] [CrossRef]
- Harris, J.E.; Harris, T.H.; Weninger, W.; John Wherry, E.; Hunter, C.A.; Turka, L.A. A Mouse Model of Vitiligo with Focused Epidermal Depigmentation Requires IFN-γ for Autoreactive CD8+ T-Cell Accumulation in the Skin. J. Investig. Dermatol. 2012, 132, 1869–1876. [Google Scholar] [CrossRef]
- Speeckaert, R.; Belpaire, A.; Speeckaert, M.; van Geel, N. The Delicate Relation between Melanocytes and Skin Immunity: A Game of Hide and Seek. Pigment. Cell Melanoma Res. 2022, 35, 392–407. [Google Scholar] [CrossRef]
- Chang, Y.; Kang, P.; Cui, T.; Guo, W.; Zhang, W.; Du, P.; Yi, X.; Guo, S.; Gao, T.; Li, C.; et al. Pharmacological Inhibition of Demethylzeylasteral on JAK-STAT Signaling Ameliorates Vitiligo. J. Transl. Med. 2023, 21, 434. [Google Scholar] [CrossRef]
- Basak, P.Y.; Adiloglu, A.K.; Ceyhan, A.M.; Tas, T.; Akkaya, V.B. The Role of Helper and Regulatory T Cells in the Pathogenesis of Vitiligo. J. Am. Acad. Dermatol. 2009, 60, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Jafarzadeh, A.; Behrangi, E.; Khosravi, M.; Falakeh, S.; Amiri, J.K.; Goodarzi, A. Emerging Treatments for Dermatologic Diseases in Infants, Children, and Adolescents: A Systematic Review of Clinical Trials on Biologics and Small Molecule Inhibitors. Inflammopharmacology 2025, 33, 1617–1672. [Google Scholar] [CrossRef] [PubMed]
- Czarnowicki, T.; He, H.; Leonard, A.; Kim, H.J.; Kameyama, N.; Pavel, A.B.; Li, R.; Estrada, Y.; Wen, H.-C.; Kimmel, G.W.; et al. Blood Endotyping Distinguishes the Profile of Vitiligo from That of Other Inflammatory and Autoimmune Skin Diseases. J. Allergy Clin. Immunol. 2019, 143, 2095–2107. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Wang, W.; Lei, L.; Feng, T.; Hu, Y.; Liu, P.; Li, Y.; Sheng, R.; Zhang, Y.; Li, S.; et al. Antirheumatic Drug Leflunomide Attenuates Atherosclerosis by Regulating Lipid Metabolism and Endothelial Dysfunction via DHODH/AMPK Signaling Pathway. Int. J. Biol. Sci. 2024, 20, 3725–3741. [Google Scholar] [CrossRef]
- Awad, S.S. Leflunomide Is a Possible Deactivator for Vitiligo, a Pilot Study. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 1173. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Z.; Li, D.; He, X.; Xiang, L.; Li, B.; Zhang, C. The Emerging Role of Regulatory T Cells in the Immunopathogenesis of Vitiligo and Implications for Treatment. Br. J. Dermatol. 2024, 192, 796–806. [Google Scholar] [CrossRef]
- Levy, R.A.; de Jesús, G.R.; de Jesús, N.R.; Klumb, E.M. Critical Review of the Current Recommendations for the Treatment of Systemic Inflammatory Rheumatic Diseases during Pregnancy and Lactation. Autoimmun. Rev. 2016, 15, 955–963. [Google Scholar] [CrossRef]
- Pfaller, B.; Pupco, A.; Leibson, T.; Aletaha, D.; Ito, S. A Critical Review of the Reproductive Safety of Leflunomide. Clin. Rheumatol. 2020, 39, 607–612. [Google Scholar] [CrossRef]
- Jin, Y.; Birlea, S.A.; Fain, P.R.; Gowan, K.; Riccardi, S.L.; Holland, P.J.; Mailloux, C.M.; Sufit, A.J.D.; Hutton, S.M.; Amadi-Myers, A.; et al. Variant of TYR and Autoimmunity Susceptibility Loci in Generalized Vitiligo. N. Engl. J. Med. 2010, 362, 1686–1697. [Google Scholar] [CrossRef]
- Męcińska-Jundziłł, K.; Tadrowski, T.; Jundziłł, A.; Witmanowski, H.; Czajkowki, R. Evaluation of Polymorphisms and Expression of PTPN22, NLRP1 and TYR Genes in Vitiligo Patients. Postep. Dermatol. Alergol. 2023, 40, 225–233. [Google Scholar] [CrossRef]
- Jin, Y.; Mailloux, C.M.; Gowan, K.; Riccardi, S.L.; LaBerge, G.; Bennett, D.C.; Fain, P.R.; Spritz, R.A. NALP1 in Vitiligo-Associated Multiple Autoimmune Disease. N. Engl. J. Med. 2007, 356, 1216–1225. [Google Scholar] [CrossRef] [PubMed]
- Papaccio, F.; Ottaviani, M.; Truglio, M.; D’Arino, A.; Caputo, S.; Pacifico, A.; Iacovelli, P.; Di Nardo, A.; Picardo, M.; Bellei, B. Markers of Metabolic Abnormalities in Vitiligo Patients. Int. J. Mol. Sci. 2024, 25, 10201. [Google Scholar] [CrossRef]
- Białczyk, A.; Kamińska, B.; Czajkowski, R. The Association between Metabolic Syndrome and Vitiligo: A Systematic Review. Postep. Dermatol. Alergol. 2025, 42, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K. Networks of CD8+ T Cell Response Activation in Melanoma and Vitiligo. Front. Immunol. 2022, 13, 866703. [Google Scholar] [CrossRef] [PubMed]
- You, S.; Cho, Y.-H.; Byun, J.-S.; Shin, E.-C. Melanocyte-Specific CD8+ T Cells Are Associated with Epidermal Depigmentation in a Novel Mouse Model of Vitiligo. Clin. Exp. Immunol. 2013, 174, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Le, Y.; Geng, M.-M.; Dong, B.-Q.; Luo, L.-F.; Jiang, S.; Le Poole, I.C.; Lei, T.-C. Increased Splicing of CXCR3 Isoform B (CXCR3B) by Impaired NRF2 Signaling Leads to Melanocyte Apoptosis in Active Vitiligo. Free Radic. Biol. Med. 2024, 225, 687–698. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, D.; Hu, Y.; Jiang, K.; Huang, H.; Du, Y.; Wu, W.; Wang, J.; Sui, J.; Wang, W.; et al. Anatomically Distinct Fibroblast Subsets Determine Skin Autoimmune Patterns. Nature 2022, 601, 118–124. [Google Scholar] [CrossRef]
- Li, C.; Wang, W.; Shao, J.; Zhou, S.; Ji, X.; Xi, Y.; Xu, Q.; Huang, Y.; Wang, J.; Wan, Y.; et al. Biomimetic Polydopamine Loaded with Janus Kinase Inhibitor for Synergistic Vitiligo Therapy via Hydrogel Microneedles. J. Nanobiotechnol. 2025, 23, 63. [Google Scholar] [CrossRef]
- Rashighi, M.; Agarwal, P.; Richmond, J.M.; Harris, T.H.; Dresser, K.; Su, M.-W.; Zhou, Y.; Deng, A.; Hunter, C.A.; Luster, A.D.; et al. CXCL10 Is Critical for the Progression and Maintenance of Depigmentation in a Mouse Model of Vitiligo. Sci. Transl. Med. 2014, 6, 223ra23. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Y.; Le, Q.; Tong, J.; Wang, H. The IFN-γ-CXCL9/CXCL10-CXCR3 Axis in Vitiligo: Pathological Mechanism and Treatment. Eur. J. Immunol. 2024, 54, e2250281. [Google Scholar] [CrossRef]
- Giri, P.S.; Dwivedi, M.; Begum, R. Decreased Suppression of CD8+ and CD4+ T Cells by Peripheral Regulatory T Cells in Generalized Vitiligo Due to Reduced NFATC1 and FOXP3 Proteins. Exp. Dermatol. 2020, 29, 759–775. [Google Scholar] [CrossRef] [PubMed]
- Henning, S.W.; Fernandez, M.F.; Mahon, J.P.; Duff, R.; Azarafrooz, F.; Guevara-Patiño, J.A.; Rademaker, A.W.; Salzman, A.L.; Le Poole, I.C. HSP70iQ435A-Encoding DNA Repigments Vitiligo Lesions in Sinclair Swine. J. Investig. Dermatol. 2018, 138, 2531–2539. [Google Scholar] [CrossRef]
- Moretti, S.; Spallanzani, A.; Amato, L.; Hautmann, G.; Gallerani, I.; Fabiani, M.; Fabbri, P. New Insights into the Pathogenesis of Vitiligo: Imbalance of Epidermal Cytokines at Sites of Lesions. Pigment. Cell Res. 2002, 15, 87–92. [Google Scholar] [CrossRef]
- Grimes, P.E.; Morris, R.; Avaniss-Aghajani, E.; Soriano, T.; Meraz, M.; Metzger, A. Topical Tacrolimus Therapy for Vitiligo: Therapeutic Responses and Skin Messenger RNA Expression of Proinflammatory Cytokines. J. Am. Acad. Dermatol. 2004, 51, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewska, K.; Kozłowska, M.; Kaszuba, A.; Lesiak, A.; Narbutt, J.; Zalewska-Janowska, A. Increased Serum Levels of IFN-γ, IL-1β, and IL-6 in Patients with Alopecia Areata and Nonsegmental Vitiligo. Oxid. Med. Cell. Longev. 2020, 2020, 5693572. [Google Scholar] [CrossRef] [PubMed]
- Desai, K.; Kumar, H.K.; Naveen, S.; Somanna, P. Vitiligo: Correlation with Cytokine Profiles and Its Role in Novel Therapeutic Strategies: A Case-Control Study. Indian Dermatol. Online J. 2023, 14, 361–365. [Google Scholar]
- Ranges, G.E.; Figari, I.S.; Espevik, T.; Palladino, M.A. Inhibition of Cytotoxic T Cell Development by Transforming Growth Factor Beta and Reversal by Recombinant Tumor Necrosis Factor Alpha. J. Exp. Med. 1987, 166, 991–998. [Google Scholar] [CrossRef]
- Sushama, S.; Dixit, N.; Gautam, R.K.; Arora, P.; Khurana, A.; Anubhuti, A. Cytokine Profile (IL-2, IL-6, IL-17, IL-22, and TNF-α) in Vitiligo-New Insight into Pathogenesis of Disease. J. Cosmet. Dermatol. 2019, 18, 337–341. [Google Scholar] [CrossRef]
- Galadari, I. Serum Levels of the Soluble Interleukin-2 Receptor in Vitiligo Patients in UAE. Eur. Ann. Allergy Clin. Immunol. 2005, 37, 109–111. [Google Scholar]
- Zailaie, M.Z. Aspirin Reduces Serum Anti-Melanocyte Antibodies and Soluble Interleukin-2 Receptors in Vitiligo Patients. Saudi Med. J. 2005, 26, 1085–1091. [Google Scholar]
- Singh, S.; Singh, U.; Pandey, S.S. Serum Concentration of IL-6, IL-2, TNF-α, and IFNγ in Vitiligo Patients. Indian J. Dermatol. 2012, 57, 12–14. [Google Scholar] [CrossRef] [PubMed]
- Nouri-Koupaee, A.; Mansouri, P.; Jahanbini, H.; Sanati, M.H.; Jadali, Z. Differential Expression of mRNA for T-Bet and GATA-3 Transcription Factors in Peripheral Blood Mononuclear Cells of Patients with Vitiligo. Clin. Exp. Dermatol. 2015, 40, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Yeo, U.C.; Yang, Y.S.; Park, K.B.; Sung, H.T.; Jung, S.Y.; Lee, E.S.; Shin, M.H. Serum Concentration of the Soluble Interleukin-2 Receptor in Vitiligo Patients. J. Dermatol. Sci. 1999, 19, 182–188. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miao, F.; Li, X.; Zhao, L.; Zhang, S.; Geng, M.; Ye, C.; Shi, Y.; Lei, T. Leflunomide-Mediated Immunomodulation Inhibits Lesion Progression in a Vitiligo Mouse Model. Int. J. Mol. Sci. 2025, 26, 6787. https://doi.org/10.3390/ijms26146787
Miao F, Li X, Zhao L, Zhang S, Geng M, Ye C, Shi Y, Lei T. Leflunomide-Mediated Immunomodulation Inhibits Lesion Progression in a Vitiligo Mouse Model. International Journal of Molecular Sciences. 2025; 26(14):6787. https://doi.org/10.3390/ijms26146787
Chicago/Turabian StyleMiao, Fang, Xiaohui Li, Liang Zhao, Shijiao Zhang, Mengmeng Geng, Chuhuan Ye, Ying Shi, and Tiechi Lei. 2025. "Leflunomide-Mediated Immunomodulation Inhibits Lesion Progression in a Vitiligo Mouse Model" International Journal of Molecular Sciences 26, no. 14: 6787. https://doi.org/10.3390/ijms26146787
APA StyleMiao, F., Li, X., Zhao, L., Zhang, S., Geng, M., Ye, C., Shi, Y., & Lei, T. (2025). Leflunomide-Mediated Immunomodulation Inhibits Lesion Progression in a Vitiligo Mouse Model. International Journal of Molecular Sciences, 26(14), 6787. https://doi.org/10.3390/ijms26146787





