Conserved Yet Divergent Smc5/6 Complex Degradation by Mammalian Hepatitis B Virus X Proteins
Abstract
1. Introduction
2. Results
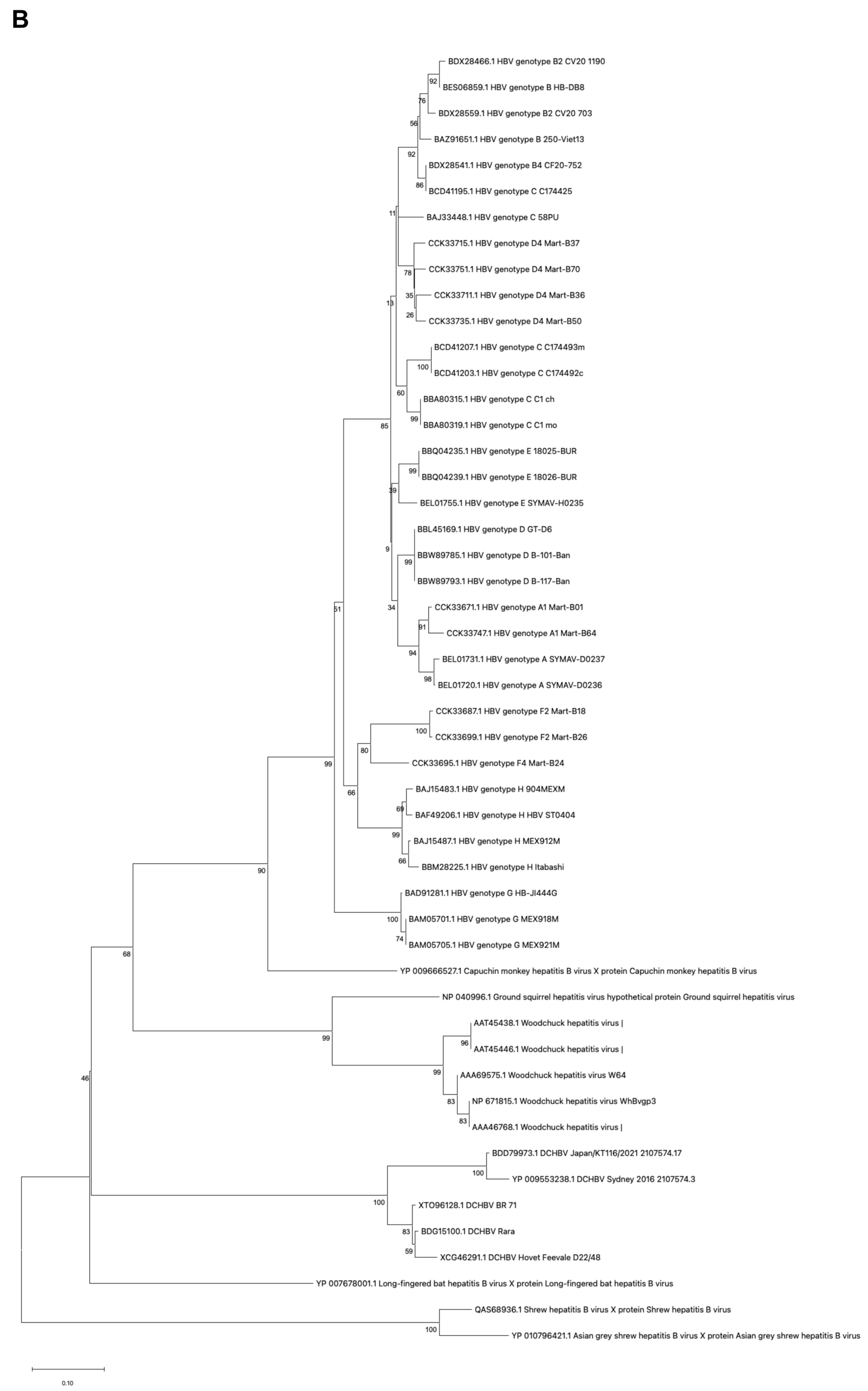
2.1. Genetic Similarity Between Mammalian Hepatitis B Virus X Proteins
2.2. Similarities Between Smc6 Proteins Among Mammals
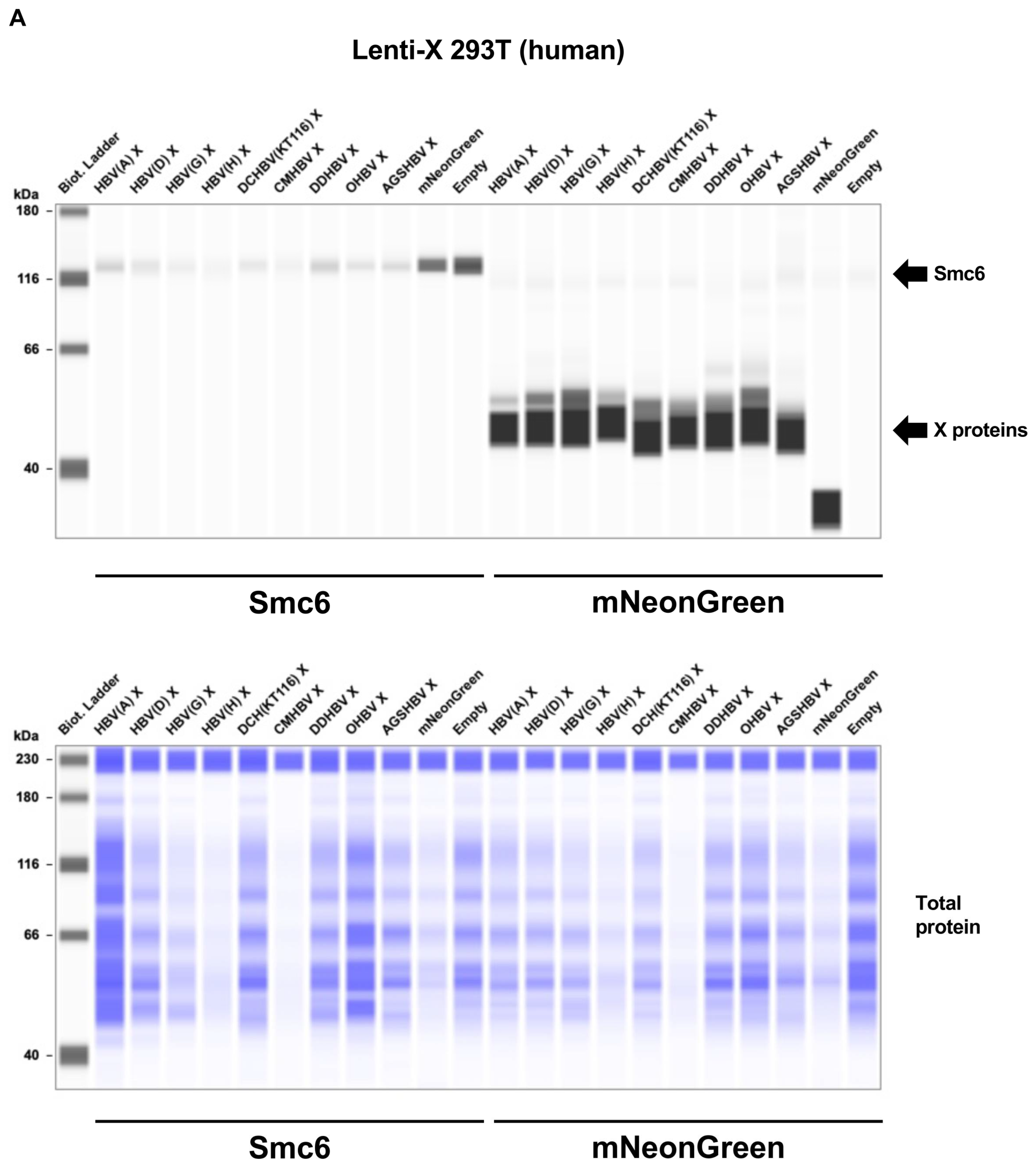
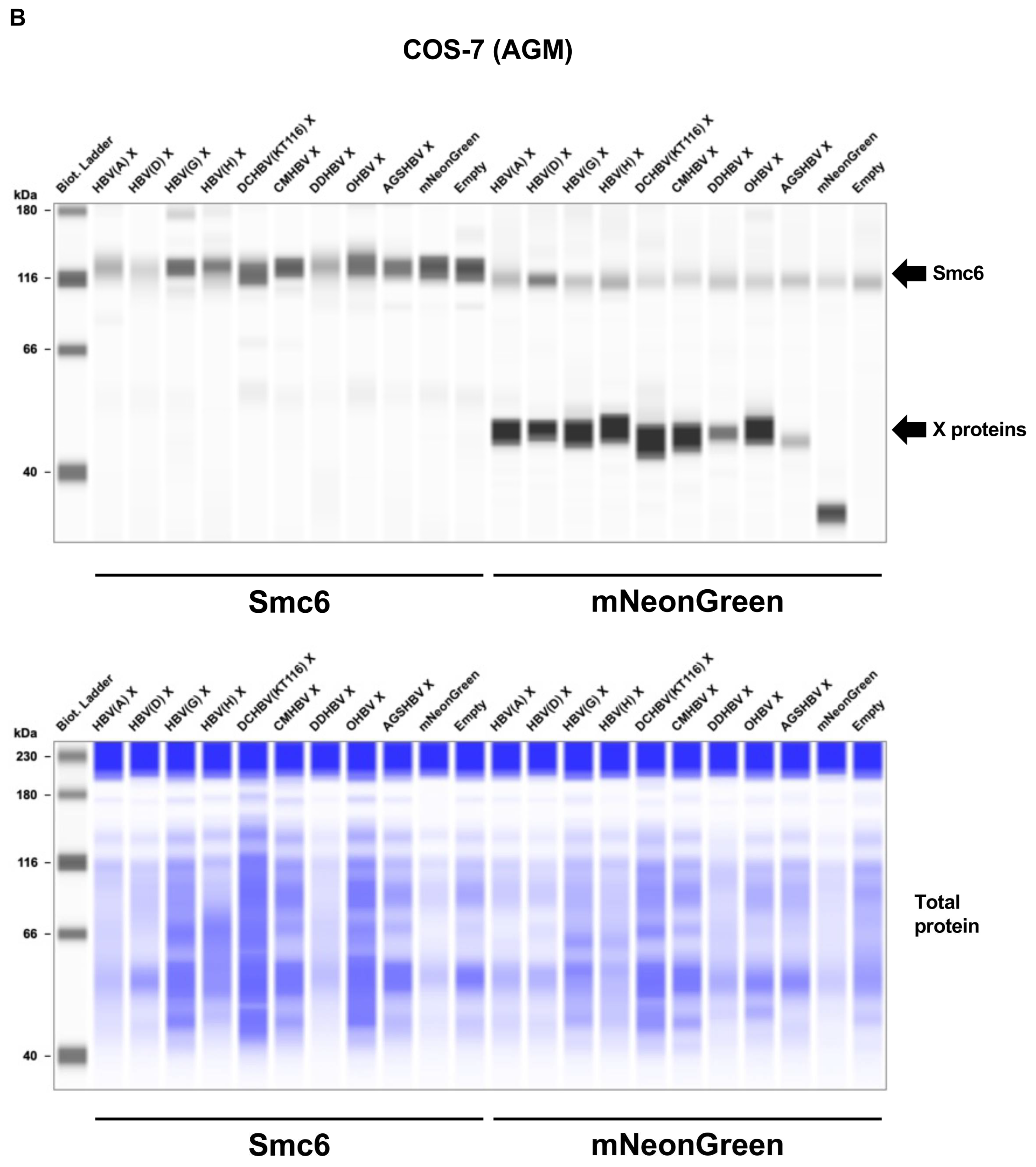
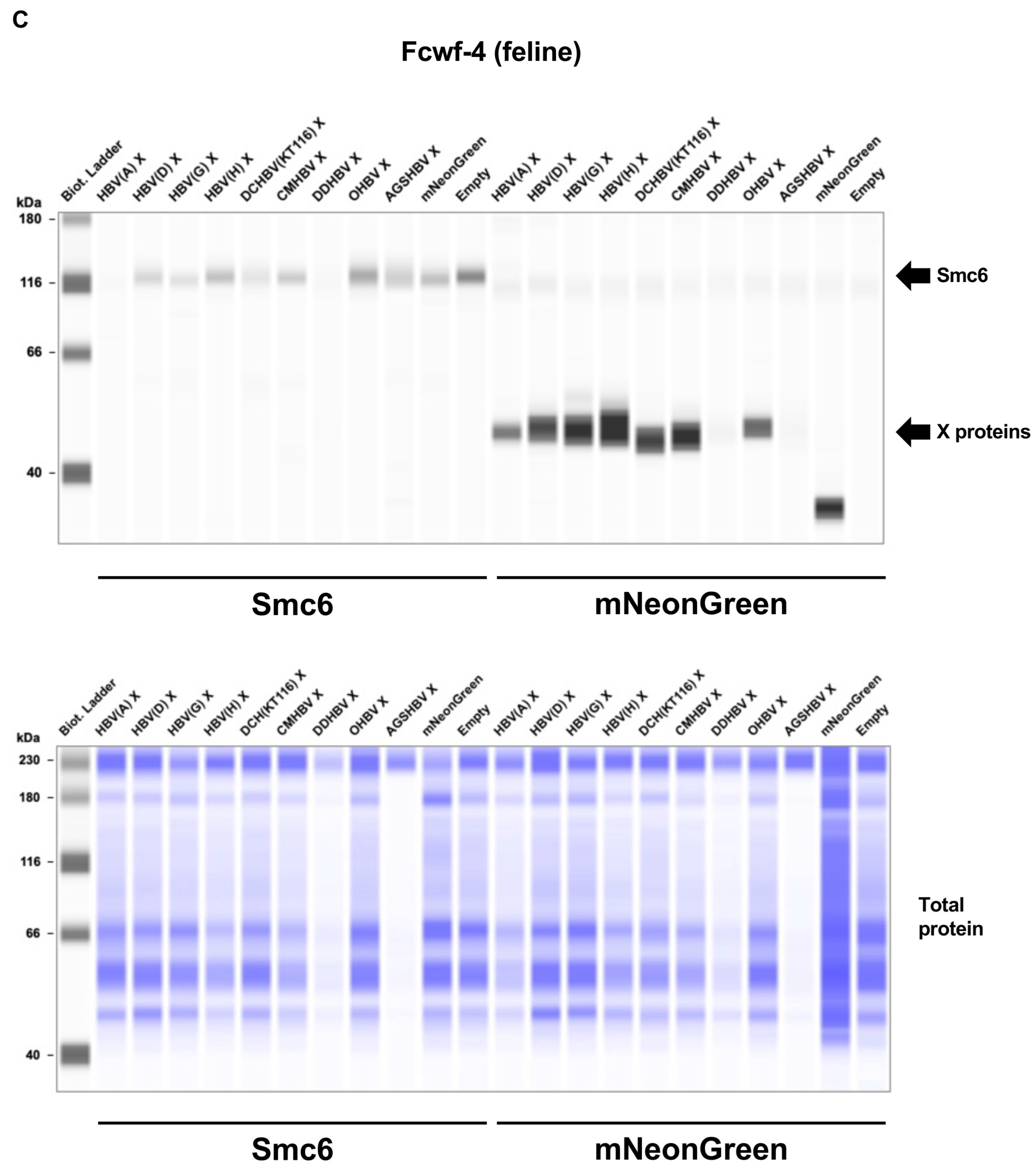
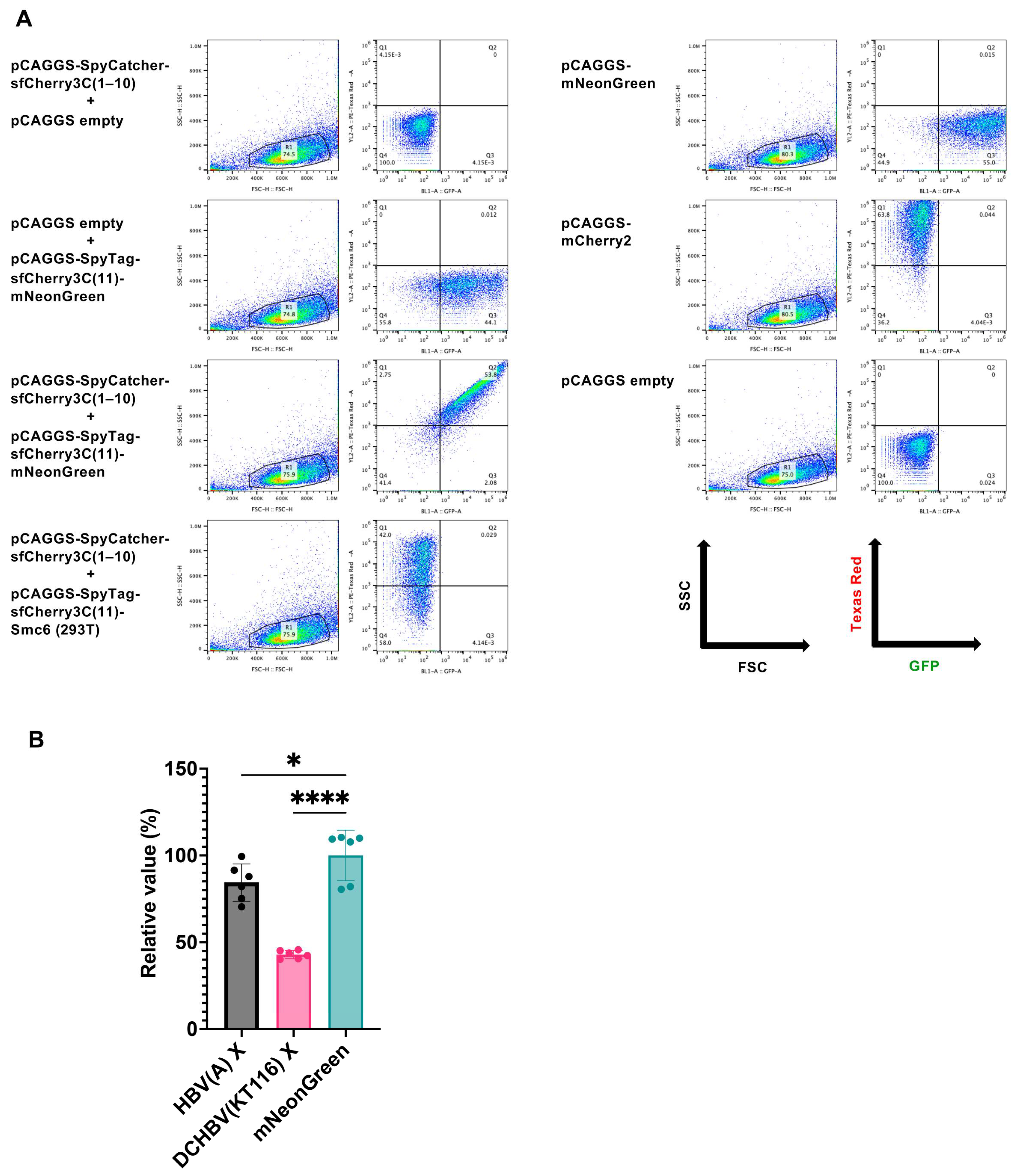
2.3. Mammalian Hepatitis B Virus X Proteins Exhibit Differential Smc5/6 Degradation Activity Depending on the Host Species
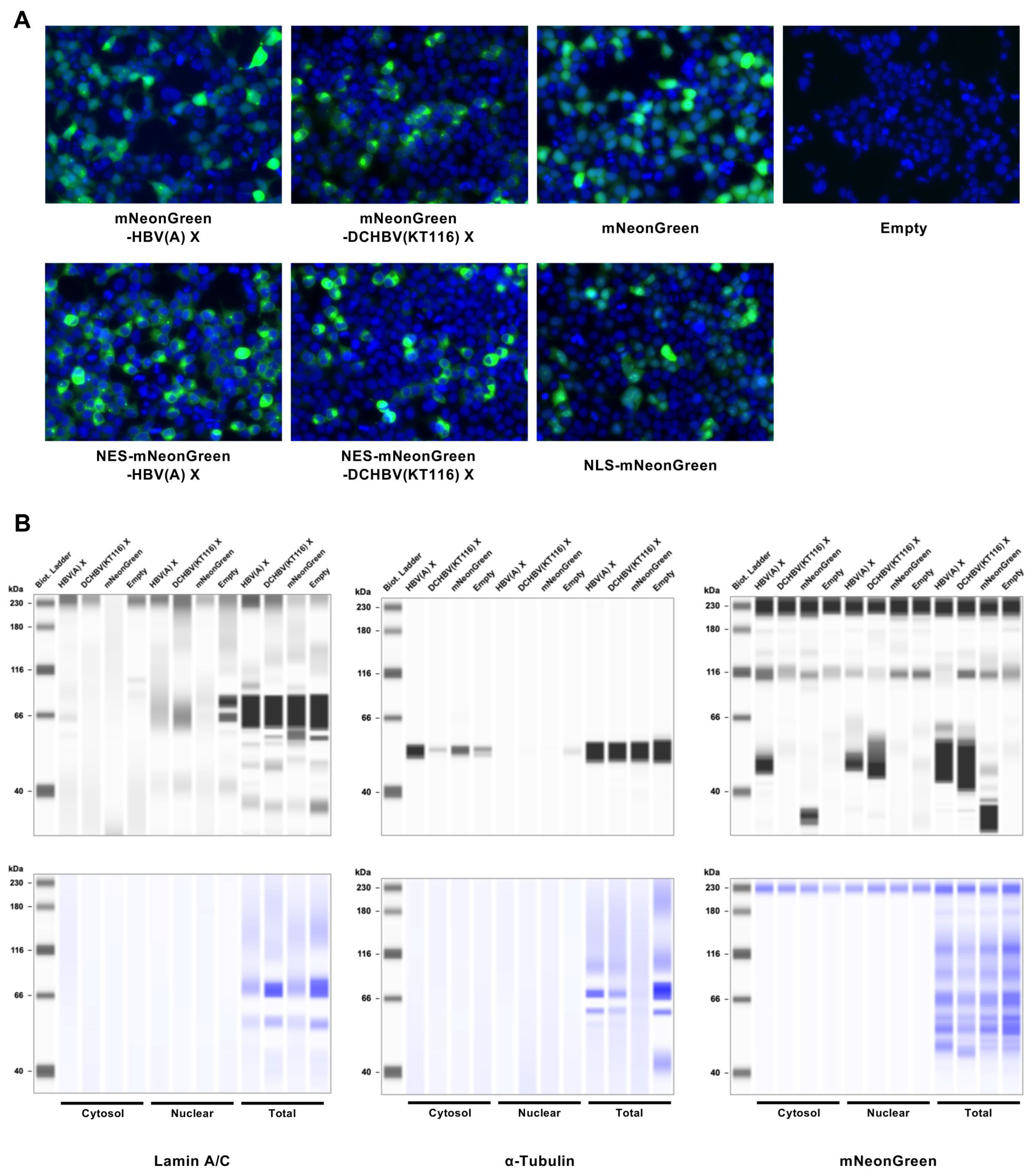
2.4. DCHBV(KT116) X Is More Predominantly Localized in the Nucleus than HBV(A) X
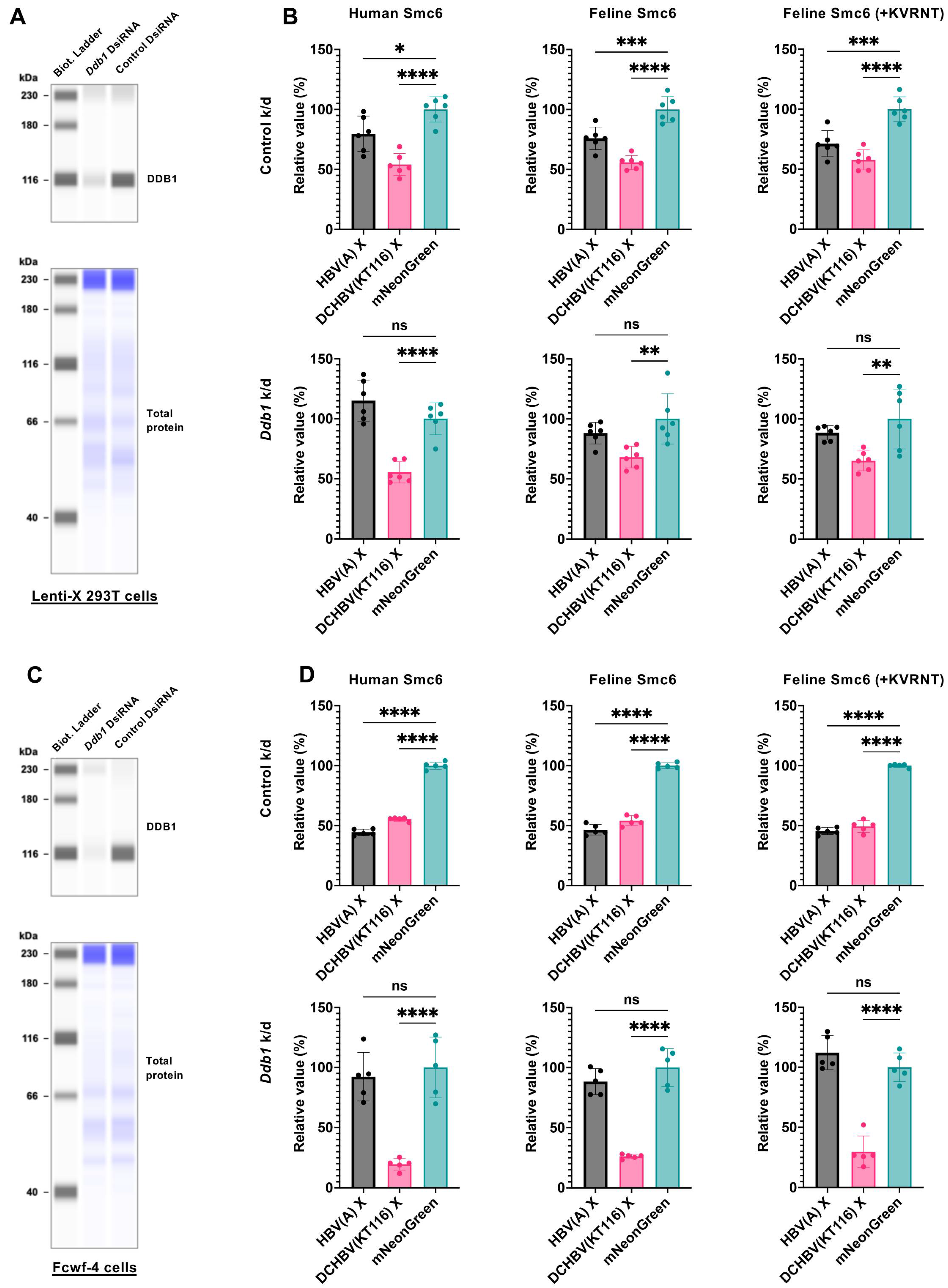
2.5. DCHBV(KT116) X Degrades the Smc6 Complex Independently of DDB1
3. Discussion
4. Materials and Methods
4.1. Alignment of the Mammalian Hepatitis B Virus X Proteins and Phylogenetic Analysis
4.2. Alignment of the Mammalian Smc6 Proteins and Phylogenetic Analysis
4.3. Calculation of the Identity of Smc6 Among Animal Species
4.4. Cell Culture
4.5. Plasmids
4.6. Smc5/6 Complex Degradation Assay
4.7. Western Blotting
4.8. Smc6 Degradation Assay Using the SpyTag/SpyCatcher System
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nguyen, M.H.; Wong, G.; Gane, E.; Kao, J.-H.; Dusheiko, G. Hepatitis B Virus: Advances in Prevention, Diagnosis, and Therapy. Clin. Microbiol. Rev. 2020, 33, e00046-19. [Google Scholar] [CrossRef] [PubMed]
- Magnius, L.; Mason, W.S.; Taylor, J.; Kann, M.; Glebe, D.; Dény, P.; Sureau, C.; Norder, H.; Ictv Report Consortium. ICTV Virus Taxonomy Profile: Hepadnaviridae. J. Gen. Virol. 2020, 101, 571–572. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Chen, P.; Zhao, F.; Nassal, M.; Hu, K. Evidence for Multiple Distinct Interactions between Hepatitis B Virus P Protein and Its Cognate RNA Encapsidation Signal during Initiation of Reverse Transcription. PLoS ONE 2013, 8, e72798. [Google Scholar] [CrossRef]
- Murakami, S. Hepatitis B Virus X Protein: Structure, Function and Biology. Intervirology 1999, 42, 81–99. [Google Scholar] [CrossRef] [PubMed]
- Lucifora, J.; Arzberger, S.; Durantel, D.; Belloni, L.; Strubin, M.; Levrero, M.; Zoulim, F.; Hantz, O.; Protzer, U. Hepatitis B Virus X Protein Is Essential to Initiate and Maintain Virus Replication after Infection. J. Hepatol. 2011, 55, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Arbuthnot, P.; Capovilla, A.; Kew, M. Putative Role of Hepatitis B Virus X Protein in Hepatocarcinogenesis: Effects on Apoptosis, DNA Repair, Mitogen-Activated Protein Kinase and JAK/STAT Pathways. J. Gastroenterol. Hepatol. 2000, 15, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Diao, J.; Garces, R.; Richardson, C.D. X Protein of Hepatitis B Virus Modulates Cytokine and Growth Factor Related Signal Transduction Pathways during the Course of Viral Infections and Hepatocarcinogenesis. Cytokine Growth Factor Rev. 2001, 12, 189–205. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.M.; Xu, Y.; Li, F.; Nio, K.; Reszka-Blanco, N.; Li, X.; Wu, Y.; Yu, Y.; Xiong, Y.; Su, L. Hepatitis B Virus X Protein Promotes Degradation of SMC5/6 to Enhance HBV Replication. Cell Rep. 2016, 16, 2846–2854. [Google Scholar] [CrossRef] [PubMed]
- Decorsière, A.; Mueller, H.; van Breugel, P.C.; Abdul, F.; Gerossier, L.; Beran, R.K.; Livingston, C.M.; Niu, C.; Fletcher, S.P.; Hantz, O.; et al. Hepatitis B Virus X Protein Identifies the Smc5/6 Complex as a Host Restriction Factor. Nature 2016, 531, 386–389. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Livingston, C.M.; Li, L.; Beran, R.K.; Daffis, S.; Ramakrishnan, D.; Burdette, D.; Peiser, L.; Salas, E.; Ramos, H.; et al. The Smc5/6 Complex Restricts HBV When Localized to ND10 without Inducing an Innate Immune Response and Is Counteracted by the HBV X Protein Shortly after Infection. PLoS ONE 2017, 12, e0169648. [Google Scholar] [CrossRef] [PubMed]
- Abdul, F.; Filleton, F.; Gerossier, L.; Paturel, A.; Hall, J.; Strubin, M.; Etienne, L. Smc5/6 Antagonism by HBx Is an Evolutionarily Conserved Function of Hepatitis B Virus Infection in Mammals. J. Virol. 2018, 92, e00769-18. [Google Scholar] [CrossRef] [PubMed]
- Aghazadeh, M.; Shi, M.; Barrs, V.R.; McLuckie, A.J.; Lindsay, S.A.; Jameson, B.; Hampson, B.; Holmes, E.C.; Beatty, J.A. A Novel Hepadnavirus Identified in an Immunocompromised Domestic Cat in Australia. Viruses 2018, 10, 269. [Google Scholar] [CrossRef] [PubMed]
- Pesavento, P.A.; Jackson, K.; Scase, T.; Tse, T.; Hampson, B.; Munday, J.S.; Barrs, V.R.; Beatty, J.A. A Novel Hepadnavirus Is Associated with Chronic Hepatitis and Hepatocellular Carcinoma in Cats. Viruses 2019, 11, 969. [Google Scholar] [CrossRef] [PubMed]
- Lanave, G.; Capozza, P.; Diakoudi, G.; Catella, C.; Catucci, L.; Ghergo, P.; Stasi, F.; Barrs, V.; Beatty, J.; Decaro, N.; et al. Identification of Hepadnavirus in the Sera of Cats. Sci. Rep. 2019, 9, 10668. [Google Scholar] [CrossRef] [PubMed]
- Jeanes, E.C.; Wegg, M.L.; Mitchell, J.A.; Priestnall, S.L.; Fleming, L.; Dawson, C. Comparison of the Prevalence of Domestic Cat Hepadnavirus in a Population of Cats with Uveitis and in a Healthy Blood Donor Cat Population in the United Kingdom. Vet. Ophthalmol. 2022, 25, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Anpuanandam, K.; Selvarajah, G.T.; Choy, M.M.K.; Ng, S.W.; Kumar, K.; Ali, R.M.; Rajendran, S.K.; Ho, K.L.; Tan, W.S. Molecular Detection and Characterisation of Domestic Cat Hepadnavirus (DCH) from Blood and Liver Tissues of Cats in Malaysia. BMC Vet. Res. 2021, 17, 9. [Google Scholar] [CrossRef] [PubMed]
- Piewbang, C.; Wardhani, S.W.; Chaiyasak, S.; Yostawonkul, J.; Chai-In, P.; Boonrungsiman, S.; Kasantikul, T.; Techangamsuwan, S. Insights into the Genetic Diversity, Recombination, and Systemic Infections with Evidence of Intracellular Maturation of Hepadnavirus in Cats. PLoS ONE 2020, 15, e0241212. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.B.I.; Chen, J.-Y.; Villanueva, B.H.A.; Lu, Z.-Y.; Hsing, H.-Z.; Montecillo, A.D.; Shofa, M.; Minh, H.; Chuang, J.-P.; Huang, H.-Y.; et al. Genetic Diversity of Domestic Cat Hepadnavirus in Southern Taiwan. Viruses 2023, 15, 2128. [Google Scholar] [CrossRef] [PubMed]
- Korkulu, E.; Şenlik, E.İ.; Adıgüzel, E.; Artut, F.G.; Çetinaslan, H.D.; Erdem-Şahinkesen, E.; Oğuzoğlu, T.Ç. Status Quo of Feline Leukaemia Virus Infection in Turkish Cats and Their Antigenic Prevalence. Animals 2024, 14, 385. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Kaneko, Y.; Shibanai, A.; Yamamoto, S.; Katagiri, A.; Osuga, T.; Inoue, Y.; Kuroda, K.; Tanabe, M.; Okabayashi, T.; et al. Identification of Domestic Cat Hepadnavirus from a Cat Blood Sample in Japan. J. Vet. Med. Sci. 2022, 84, 648–652. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, H.; Ito, G.; Goto-Koshino, Y.; Sakamoto, M.; Nishimura, R.; Momoi, Y. Detection of Domestic Cat Hepadnavirus by Next-Generation Sequencing and Epidemiological Survey in Japan. J. Vet. Med. Sci. 2023, 85, 642–646. [Google Scholar] [CrossRef] [PubMed]
- Beatty, J.A.; Choi, Y.R.; Nekouei, O.; Woodhouse, F.M.; Gray, J.J.; Hofmann-Lehmann, R.; Barrs, V.R. Epidemiology of Pathogenic Retroviruses and Domestic Cat Hepadnavirus in Community and Client-Owned Cats in Hong Kong. Viruses 2024, 16, 167. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.R.; Iturriaga, M.P.; Nekouei, O.; Tu, T.; Van Brussel, K.; Barrs, V.R.; Beatty, J.A. Domestic Cat Hepadnavirus and Pathogenic Retroviruses; A Sero-Molecular Survey of Cats in Santiago, Chile. Viruses 2023, 16, 46. [Google Scholar] [CrossRef] [PubMed]
- Tessmann, A.; Sumienski, J.; Sita, A.; Mallmann, L.; Birlem, G.E.; da Silva Nunes, N.J.; Lupion, C.G.; Eckert, J.S.; Demoliner, M.; Gularte, J.S.; et al. Domestic Cat Hepadnavirus Genotype B Is Present in Southern Brazil. Virus Genes 2024, 61, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Shofa, M.; Ohkawa, A.; Kaneko, Y.; Saito, A. Conserved Use of the Sodium/Bile Acid Cotransporter (NTCP) as an Entry Receptor by Hepatitis B Virus and Domestic Cat Hepadnavirus. Antivir. Res. 2023, 217, 105695. [Google Scholar] [CrossRef] [PubMed]
- Hoare, J.; Henkler, F.; Dowling, J.J.; Errington, W.; Goldin, R.D.; Fish, D.; McGarvey, M.J. Subcellular Localisation of the X Protein in HBV Infected Hepatocytes. J. Med. Virol. 2001, 64, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Cha, M.-Y.; Ryu, D.-K.; Jung, H.-S.; Chang, H.-E.; Ryu, W.-S. Stimulation of Hepatitis B Virus Genome Replication by HBx Is Linked to Both Nuclear and Cytoplasmic HBx Expression. J. Gen. Virol. 2009, 90, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Henkler, F.; Hoare, J.; Waseem, N.; Goldin, R.D.; McGarvey, M.J.; Koshy, R.; King, I.A. Intracellular Localization of the Hepatitis B Virus HBx Protein. J. Gen. Virol. 2001, 82, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wu, T.; Zhang, B.; Liu, S.; Song, W.; Qiao, J.; Ruan, H. Types of Nuclear Localization Signals and Mechanisms of Protein Import into the Nucleus. Cell Commun. Signal. 2021, 19, 60. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Farmer, A.; Collett, G.; Grishin, N.V.; Chook, Y.M. Sequence and Structural Analyses of Nuclear Export Signals in the NESdb Database. Mol. Biol. Cell 2012, 23, 3677–3693. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Varshney, A.; Coto Villa, D.; Modavi, C.; Kohler, J.; Farah, F.; Zhou, S.; Ali, N.; Müller, J.D.; Van Hoven, M.K.; et al. Bright Split Red Fluorescent Proteins for the Visualization of Endogenous Proteins and Synapses. Commun. Biol. 2019, 2, 344. [Google Scholar] [CrossRef] [PubMed]
- McClain, S.L.; Clippinger, A.J.; Lizzano, R.; Bouchard, M.J. Hepatitis B Virus Replication Is Associated with an HBx-Dependent Mitochondrion-Regulated Increase in Cytosolic Calcium Levels. J. Virol. 2007, 81, 12061–12065. [Google Scholar] [CrossRef] [PubMed]
- Clippinger, A.J.; Bouchard, M.J. Hepatitis B Virus HBx Protein Localizes to Mitochondria in Primary Rat Hepatocytes and Modulates Mitochondrial Membrane Potential. J. Virol. 2008, 82, 6798–6811. [Google Scholar] [CrossRef] [PubMed]
- Keasler, V.V.; Hodgson, A.J.; Madden, C.R.; Slagle, B.L. Hepatitis B Virus HBx Protein Localized to the Nucleus Restores HBx-Deficient Virus Replication in HepG2 Cells and in Vivo in Hydrodynamically-Injected Mice. Virology 2009, 390, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Nag, A.; Datta, A.; Yoo, K.; Bhattacharyya, D.; Chakrabortty, A.; Wang, X.; Slagle, B.L.; Costa, R.H.; Raychaudhuri, P. DDB2 Induces Nuclear Accumulation of the Hepatitis B Virus X Protein Independently of Binding to DDB1. J. Virol. 2001, 75, 10383–10392. [Google Scholar] [CrossRef] [PubMed]
- Sitterlin, D.; Bergametti, F.; Transy, C. UVDDB P127-Binding Modulates Activities and Intracellular Distribution of Hepatitis B Virus X Protein. Oncogene 2000, 19, 4417–4426. [Google Scholar] [CrossRef] [PubMed]
- Weil, R.; Sirma, H.; Giannini, C.; Kremsdorf, D.; Bessia, C.; Dargemont, C.; Bréchot, C.; Israël, A. Direct Association and Nuclear Import of the Hepatitis B Virus X Protein with the NF-kappaB Inhibitor IkappaBalpha. Mol. Cell. Biol. 1999, 19, 6345–6354. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Choonnasard, A.; Shofa, M.; Okabayashi, T.; Saito, A. Conserved Functions of Orthohepadnavirus X Proteins to Inhibit Type-I Interferon Signaling. Int. J. Mol. Sci. 2024, 25, 3753. [Google Scholar] [CrossRef] [PubMed]
- Sekiba, K.; Otsuka, M.; Ohno, M.; Yamagami, M.; Kishikawa, T.; Suzuki, T.; Ishibashi, R.; Seimiya, T.; Tanaka, E.; Koike, K. Inhibition of HBV Transcription From cccDNA With Nitazoxanide by Targeting the HBx-DDB1 Interaction. Cell Mol. Gastroenterol. Hepatol. 2019, 7, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Stecher, G.; Tamura, K.; Kumar, S. Molecular Evolutionary Genetics Analysis (MEGA) for macOS. Mol. Biol. Evol. 2020, 37, 1237–1239. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.T.; Taylor, W.R.; Thornton, J.M. The Rapid Generation of Mutation Data Matrices from Protein Sequences. Comput. Appl. Biosci. 1992, 8, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Niwa, H.; Yamamura, K.; Miyazaki, J. Efficient Selection for High-Expression Transfectants with a Novel Eukaryotic Vector. Gene 1991, 108, 193–199. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shofa, M.; Fukushima, Y.V.; Saito, A. Conserved Yet Divergent Smc5/6 Complex Degradation by Mammalian Hepatitis B Virus X Proteins. Int. J. Mol. Sci. 2025, 26, 6786. https://doi.org/10.3390/ijms26146786
Shofa M, Fukushima YV, Saito A. Conserved Yet Divergent Smc5/6 Complex Degradation by Mammalian Hepatitis B Virus X Proteins. International Journal of Molecular Sciences. 2025; 26(14):6786. https://doi.org/10.3390/ijms26146786
Chicago/Turabian StyleShofa, Maya, Yuri V Fukushima, and Akatsuki Saito. 2025. "Conserved Yet Divergent Smc5/6 Complex Degradation by Mammalian Hepatitis B Virus X Proteins" International Journal of Molecular Sciences 26, no. 14: 6786. https://doi.org/10.3390/ijms26146786
APA StyleShofa, M., Fukushima, Y. V., & Saito, A. (2025). Conserved Yet Divergent Smc5/6 Complex Degradation by Mammalian Hepatitis B Virus X Proteins. International Journal of Molecular Sciences, 26(14), 6786. https://doi.org/10.3390/ijms26146786




