Multistage Molecular Simulations, Design, Synthesis, and Anticonvulsant Evaluation of 2-(Isoindolin-2-yl) Esters of Aromatic Amino Acids Targeting GABAA Receptors via π-π Stacking
Abstract
1. Introduction
2. Results
2.1. In Silico Evaluation of Isoindoline Esters
2.2. Molecular Docking with the GABAA Receptor
2.3. Molecular Dynamics and Metadynamics Simulations
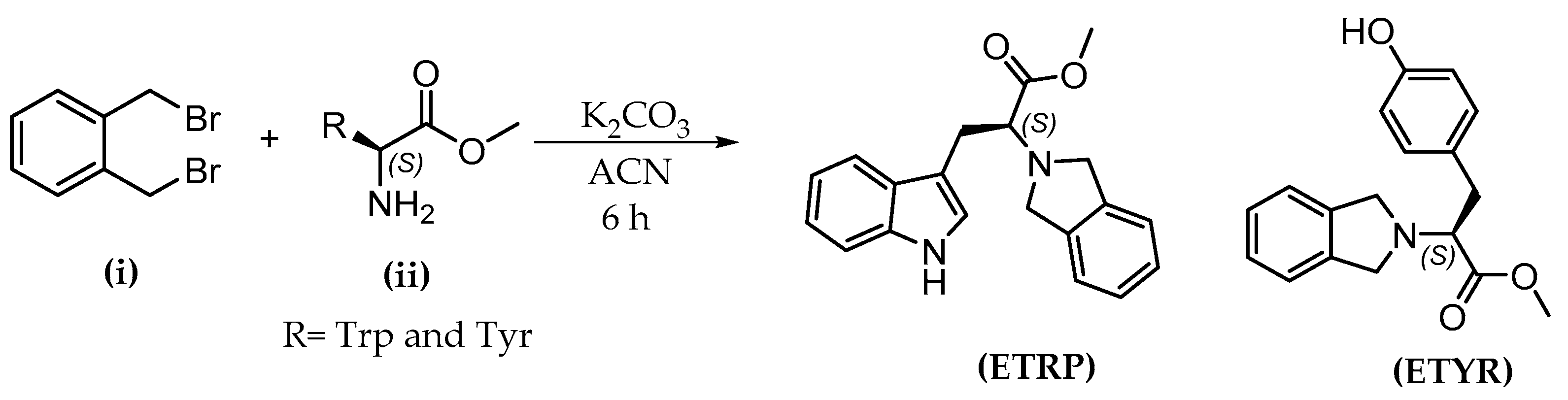
2.4. Synthetic Route and Characterization of Selected Esters
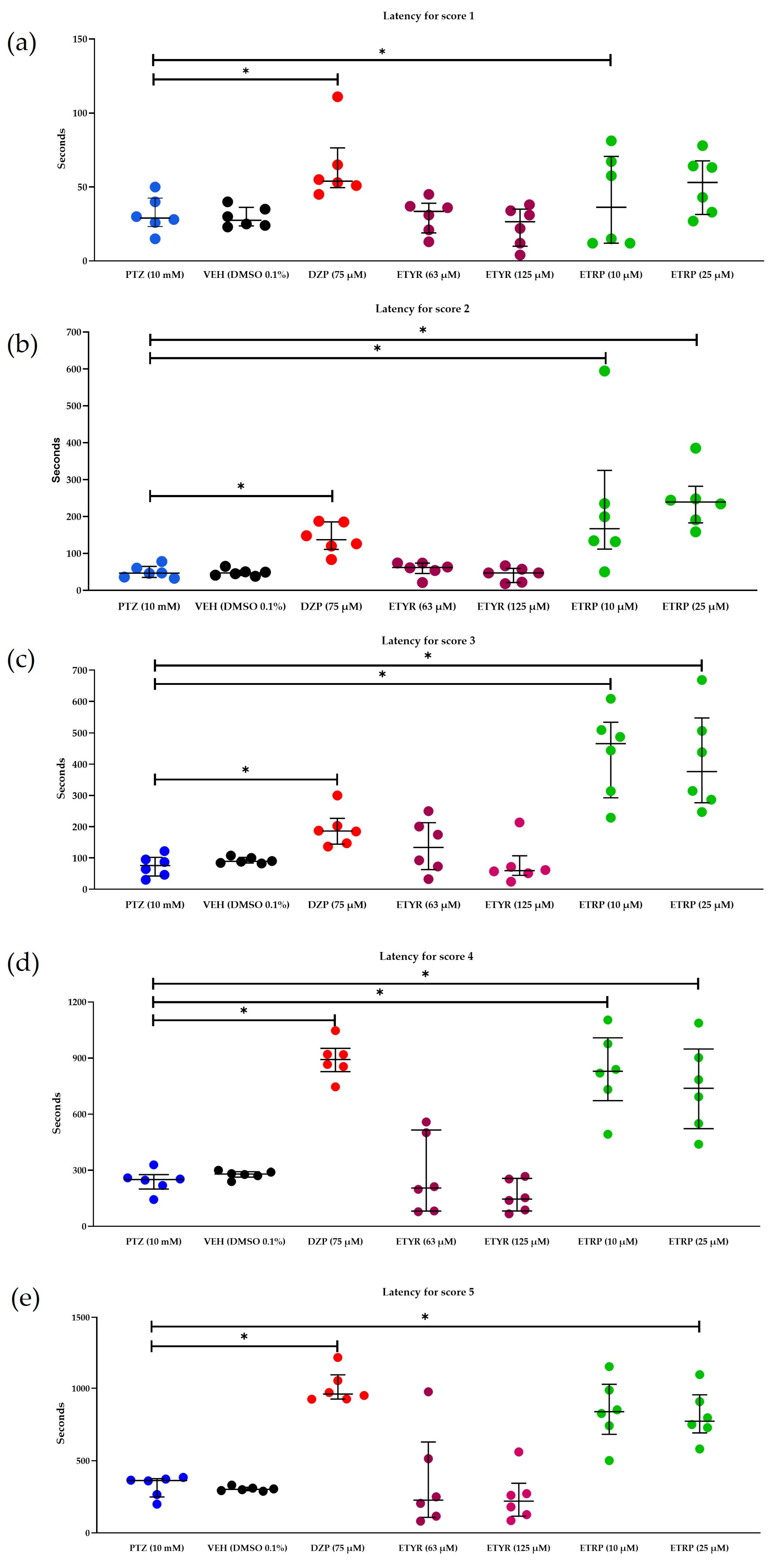
2.5. In Vivo Anticonvulsant Activity in Zebrafish
3. Discussion
4. Materials and Methods
4.1. Materials and Instrumentation
4.2. Computational Simulation Methods
4.3. Synthesis of 2-(Isoindolin-2-yl) Esters: General Procedure
4.4. Zebrafish PTZ-Induced Seizure Assay
4.5. Graphical and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Epilepsia. Available online: https://www.who.int/es/news-room/fact-sheets/detail/epilepsy (accessed on 20 December 2024).
- Chen, Z.; Brodie, M.J.; Ding, D.; Kwan, P. Editorial: Epidemiology of epilepsy and seizures. Front. Epidemiol. 2023, 3, 1273163. [Google Scholar] [CrossRef] [PubMed]
- Beniczky, S.; Trinka, E.; Wirrell, E.; Abdulla, F.; Al Baradie, R.; Alonso Vanegas, M.; Auvin, S.; Singh, M.B.; Blumenfeld, H.; Bogacz Fressola, A.; et al. Updated classification of epileptic seizures: Position paper of the International League Against Epilepsy. Epilepsia 2025, 66, 1804–1823. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Zhang, J.; Wang, J.; Sha, L.; Xia, Y.; Ortyl, T.C.; Tian, X.; Chen, L. Advances in Epilepsy: Mechanisms, Clinical Trials, and Drug Therapies. J. Med. Chem. 2023, 66, 4434–4467. [Google Scholar] [CrossRef]
- McCallan, N.; Davidson, S.; Ng, K.Y.; Biglarbeigi, P.; Finlay, D.; Lan, B.L.; McLaughlin, J. Epileptic multi-seizure type classification using electroencephalogram signals from the Temple University Hospital Seizure Corpus: A review. Expert Syst. Appl. 2023, 234, 121040. [Google Scholar] [CrossRef]
- Tomson, T.; Zelano, J.; Dang, Y.L.; Perucca, P. The pharmacological treatment of epilepsy in adults. Epileptic Disord. 2023, 25, 649–669. [Google Scholar] [CrossRef] [PubMed]
- Mesraoua, B.; Brigo, F.; Lattanzi, S.; Abou-Khalil, B.; Al Hail, H.; Asadi-Pooya, A.A. Drug-resistant epilepsy: Definition, pathophysiology, and management. J. Neurol. Sci. 2023, 452, 120766. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Z. An update for epilepsy research and antiepileptic drug development: Toward precise circuit therapy. Pharmacol. Ther. 2019, 201, 77–93. [Google Scholar] [CrossRef]
- Bryson, A.; Reid, C.; Petrou, S. Fundamental Neurochemistry Review: GABA(A) receptor neurotransmission and epilepsy: Principles, disease mechanisms and pharmacotherapy. J. Neurochem. 2023, 165, 6–28. [Google Scholar] [CrossRef]
- Perucca, E.; Bialer, M.; White, H.S. New GABA-Targeting Therapies for the Treatment of Seizures and Epilepsy: I. Role of GABA as a Modulator of Seizure Activity and Recently Approved Medications Acting on the GABA System. CNS Drugs 2023, 37, 755–779. [Google Scholar] [CrossRef]
- Masiulis, S.; Desai, R.; Uchanski, T.; Serna Martin, I.; Laverty, D.; Karia, D.; Malinauskas, T.; Zivanov, J.; Pardon, E.; Kotecha, A.; et al. GABA(A) receptor signalling mechanisms revealed by structural pharmacology. Nature 2019, 565, 454–459. [Google Scholar] [CrossRef]
- Laverty, D.; Desai, R.; Uchański, T.; Masiulis, S.; Stec, W.J.; Malinauskas, T.; Zivanov, J.; Pardon, E.; Steyaert, J.; Miller, K.W.; et al. Cryo-EM structure of the human α1β3γ2 GABAA receptor in a lipid bilayer. Nature 2019, 565, 516–520. [Google Scholar] [CrossRef] [PubMed]
- Dossi, E.; Huberfeld, G. GABAergic circuits drive focal seizures. Neurobiol. Dis. 2023, 180, 106102. [Google Scholar] [CrossRef]
- Zhao, P.; Ding, X.; Li, L.; Jiang, G. A review of cell-type specific circuit mechanisms underlying epilepsy. Acta Epileptol. 2024, 6, 18. [Google Scholar] [CrossRef]
- Kobayashi, K.; Endoh, F.; Ohmori, I.; Akiyama, T. Action of antiepileptic drugs on neurons. Brain Dev. 2020, 42, 2–5. [Google Scholar] [CrossRef]
- Nimgampalle, M.; Chakravarthy, H.; Sharma, S.; Shree, S.; Bhat, A.R.; Pradeepkiran, J.A.; Devanathan, V. Neurotransmitter systems in the etiology of major neurological disorders: Emerging insights and therapeutic implications. Ageing Res. Rev. 2023, 89, 101994. [Google Scholar] [CrossRef]
- Arora, I.; Mal, P.; Arora, P.; Paul, A.; Kumar, M. GABAergic implications in anxiety and related disorders. Biochem. Biophys. Res. Commun. 2024, 724, 150218. [Google Scholar] [CrossRef]
- Teleanu, R.I.; Niculescu, A.G.; Roza, E.; Vladacenco, O.; Grumezescu, A.M.; Teleanu, D.M. Neurotransmitters-Key Factors in Neurological and Neurodegenerative Disorders of the Central Nervous System. Int. J. Mol. Sci. 2022, 23, 5954. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Jaenisch, R.; Sur, M. The role of GABAergic signalling in neurodevelopmental disorders. Nat. Rev. Neurosci. 2021, 22, 290–307. [Google Scholar] [CrossRef] [PubMed]
- Maguire, J. Mechanisms of Psychiatric Comorbidities in Epilepsy. Curr. Top. Behav. Neurosci. 2022, 55, 107–144. [Google Scholar] [CrossRef]
- Sills, G.J.; Rogawski, M.A. Mechanisms of action of currently used antiseizure drugs. Neuropharmacology 2020, 168, 107966. [Google Scholar] [CrossRef]
- Jansen, M. An in-depth structural view of a GABAA brain receptor. Nature 2019, 565, 436–438. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Sridhar, A.; Teng, J.; Howard, R.J.; Lindahl, E.; Hibbs, R.E. Structural and dynamic mechanisms of GABA(A) receptor modulators with opposing activities. Nat. Commun. 2022, 13, 4582. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Noviello, C.M.; Teng, J.; Walsh, R.M., Jr.; Kim, J.J.; Hibbs, R.E. Structure of a human synaptic GABA(A) receptor. Nature 2018, 559, 67–72. [Google Scholar] [CrossRef]
- Maramai, S.; Benchekroun, M.; Ward, S.E.; Atack, J.R. Subtype Selective γ-Aminobutyric Acid Type A Receptor (GABAAR) Modulators Acting at the Benzodiazepine Binding Site: An Update. J. Med. Chem. 2020, 63, 3425–3446. [Google Scholar] [CrossRef]
- Golani, L.K.; Yeunus Mian, M.; Ahmed, T.; Pandey, K.P.; Mondal, P.; Sharmin, D.; Rezvanian, S.; Witkin, J.M.; Cook, J.M. Rationalizing the binding and alpha subtype selectivity of synthesized imidazodiazepines and benzodiazepines at GABAA receptors by using molecular docking studies. Bioorg. Med. Chem. Lett. 2022, 62, 128637. [Google Scholar] [CrossRef] [PubMed]
- Goldschen-Ohm, M.P. Benzodiazepine Modulation of GABA(A) Receptors: A Mechanistic Perspective. Biomolecules 2022, 12, 1784. [Google Scholar] [CrossRef]
- de Oliveira, M.; Viana, D.C.F.; Silva, A.A.; Pereira, M.C.; Duarte, F.S.; Pitta, M.G.R.; Pitta, I.R.; Pitta, M.G.R. Synthesis of novel thiazolidinic-phthalimide derivatives evaluated as new multi-target antiepileptic agents. Bioorganic Chem. 2022, 119, 105548. [Google Scholar] [CrossRef]
- Chelucci, R.C.; Chiquetto, R.; Chiba, D.E.; Scarim, C.B.; Chin, C.M.; Dos Santos, J.L. Isoindoline-1,3-dione Derivatives as Prototypes for Anticonvulsant Drug Discovery. Med. Chem. 2025, online ahead of print. [Google Scholar] [CrossRef]
- Jha, M.; Youssef, D.; Sheehy, H.; Jha, A. Synthesis and Pharmacology of Clinical Drugs Containing Isoindoline Heterocycle Core. Organics 2025, 6, 3. [Google Scholar] [CrossRef]
- Aronica, L.; Albano, G. Cyclization Reactions for the Synthesis of Phthalans and Isoindolines. Synthesis 2018, 50, 1209–1227. [Google Scholar] [CrossRef]
- Starosotnikov, A.M.; Bastrakov, M.A. Cycloaddition reactions in the synthesis of isoindolines (microreview). Chem. Heterocycl. Compd. 2018, 53, 1181–1183. [Google Scholar] [CrossRef]
- Fernandes, G.F.S.; Lopes, J.R.; Dos Santos, J.L.; Scarim, C.B. Phthalimide as a versatile pharmacophore scaffold: Unlocking its diverse biological activities. Drug Dev. Res. 2023, 84, 1346–1375. [Google Scholar] [CrossRef] [PubMed]
- Aliabadi, A.; Gholamine, B.; Karimi, T. Synthesis and antiseizure evaluation of isoindoline-1,3-dione derivatives in mice. Med. Chem. Res. 2014, 23, 2736–2743. [Google Scholar] [CrossRef]
- Abdel-Hafez, A.A.-M. Synthesis and anticonvulsant evaluation ofN-substituted-isoindolinedione derivatives. Arch. Pharmacal Res. 2004, 27, 495–501. [Google Scholar] [CrossRef]
- Iman, M.; Saadabadi, A.; Davood, A.; Shafaroodi, H.; Nikbakht, A.; Ansari, A.; Abedini, M. Docking, Synthesis and Anticonvulsant Activity of N-substituted Isoindoline-1,3-dione. Iran. J. Pharm. Res. 2017, 16, e125027. [Google Scholar] [CrossRef]
- Asadollahi, A.; Asadi, M.; Hosseini, F.S.; Ekhtiari, Z.; Biglar, M.; Amanlou, M. Synthesis, molecular docking, and antiepileptic activity of novel phthalimide derivatives bearing amino acid conjugated anilines. Res. Pharm. Sci. 2019, 14, 534–543. [Google Scholar] [CrossRef]
- Mancilla Percino, T.; Guzmán Ramírez, J.E.; Mera Jiménez, E.; Trejo Muñoz, C.R. Synthesis, characterization of novel isoindolinyl-and bis-isoindolinylphenylboronic anhydrides. Antiproliferative activity on glioblastoma cells and microglial cells assays of boron and isoindolines compounds. J. Organomet. Chem. 2019, 891, 35–43. [Google Scholar] [CrossRef]
- Mancilla Percino, T.; Hernández Rodríguez, M.; Mera Jiménez, E. Synthesis of New Isoindolines Derived from L-A-Amino Acids and their Selectivity on Cancer Cell Lines. ChemistrySelect 2024, 9, e20230429. [Google Scholar] [CrossRef]
- Campos-Rodriguez, C.; Trujillo-Ferrara, J.G.; Alvarez-Guerra, A.; Vargas, I.M.C.; Cuevas-Hernandez, R.I.; Andrade-Jorge, E.; Zamudio, S.; Juan, E.R. Neuropharmacological Screening of Chiral and Non-chiral Phthalimide- Containing Compounds in Mice: In vivo and in silico Experiments. Med. Chem. 2019, 15, 102–118. [Google Scholar] [CrossRef]
- Campos-Rodriguez, C.; Fredrick, E.; Ramirez-San Juan, E.; Olsson, R. Enantiomeric N-substituted phthalimides with excitatory amino acids protect zebrafish larvae against PTZ-induced seizures. Eur. J. Pharmacol. 2020, 888, 173489. [Google Scholar] [CrossRef]
- Özbek, O.; Gürdere, M.B. A review on the synthesis and applications of molecules as anticonvulsant drug agent candidates. Med. Chem. Res. 2020, 29, 1553–1578. [Google Scholar] [CrossRef]
- Ahuja, P.; Husain, A.; Siddiqui, N. Essential aminoacid incorporated GABA–phthalimide derivatives: Synthesis and anticonvulsant evaluation. Med. Chem. Res. 2014, 23, 4085–4098. [Google Scholar] [CrossRef]
- Banerjee, P.S.; Sharma, P.K. New antiepileptic agents: Structure–activity relationships. Med. Chem. Res. 2011, 21, 1491–1508. [Google Scholar] [CrossRef]
- Hollingsworth, S.A.; Dror, R.O. Molecular dynamics simulation for all. Neuron 2018, 99, 1129–1143. [Google Scholar] [CrossRef]
- De Vivo, M.; Masetti, M.; Bottegoni, G.; Cavalli, A. Role of Molecular Dynamics and Related Methods in Drug Discovery. J. Med. Chem. 2016, 59, 4035–4061. [Google Scholar] [CrossRef] [PubMed]
- Barducci, A.; Bonomi, M.; Parrinello, M. Metadynamics. WIREs Comput. Mol. Sci. 2011, 1, 826–843. [Google Scholar] [CrossRef]
- Cavalli, A.; Spitaleri, A.; Saladino, G.; Gervasio, F.L. Investigating drug-target association and dissociation mechanisms using metadynamics-based algorithms. Acc. Chem. Res. 2015, 48, 277–285. [Google Scholar] [CrossRef] [PubMed]
- D’Amora, M.; Galgani, A.; Marchese, M.; Tantussi, F.; Faraguna, U.; De Angelis, F.; Giorgi, F.S. Zebrafish as an Innovative Tool for Epilepsy Modeling: State of the Art and Potential Future Directions. Int. J. Mol. Sci. 2023, 24, 7702. [Google Scholar] [CrossRef]
- Lopez-Rosas, C.A.; Gonzalez-Perianez, S.; Pawar, T.J.; Zurutuza-Lormendez, J.I.; Ramos-Morales, F.R.; Olivares-Romero, J.L.; Saavedra Velez, M.V.; Hernandez-Rosas, F. Anticonvulsant Potential and Toxicological Profile of Verbesina persicifolia Leaf Extracts: Evaluation in Zebrafish Seizure and Artemia salina Toxicity Models. Plants 2025, 14, 1078. [Google Scholar] [CrossRef]
- Samokhina, E.; Samokhin, A. Neuropathological profile of the pentylenetetrazol (PTZ) kindling model. Int. J. Neurosci. 2018, 128, 1086–1096. [Google Scholar] [CrossRef]
- Murugan, R.; Ramya Ranjan Nayak, S.P.; Haridevamuthu, B.; Priya, D.; Chitra, V.; Almutairi, B.O.; Arokiyaraj, S.; Saravanan, M.; Kathiravan, M.K.; Arockiaraj, J. Neuroprotective potential of pyrazole benzenesulfonamide derivative T1 in targeted intervention against PTZ-induced epilepsy-like condition in in vivo zebrafish model. Int. Immunopharmacol. 2024, 131, 111859. [Google Scholar] [CrossRef] [PubMed]
- Ciubotaru, A.D.; Leferman, C.-E.; Ignat, B.-E.; Knieling, A.; Esanu, I.M.; Salaru, D.L.; Foia, L.G.; Minea, B.; Hritcu, L.D.; Dimitriu, C.D.; et al. Behavioral and Biochemical Insights into the Therapeutic Potential of Mitocurcumin in a Zebrafish–Pentylenetetrazole (PTZ) Epilepsy Model. Pharmaceuticals 2025, 18, 382. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Gharpure, A.; Teng, J.; Zhuang, Y.; Howard, R.J.; Zhu, S.; Noviello, C.M.; Walsh, R.M., Jr.; Lindahl, E.; Hibbs, R.E. Shared structural mechanisms of general anaesthetics and benzodiazepines. Nature 2020, 585, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Sadamitsu, K.; Shigemitsu, L.; Suzuki, M.; Ito, D.; Kashima, M.; Hirata, H. Characterization of zebrafish GABA(A) receptor subunits. Sci. Rep. 2021, 11, 6242. [Google Scholar] [CrossRef]
- Monesson-Olson, B.; McClain, J.J.; Case, A.E.; Dorman, H.E.; Turkewitz, D.R.; Steiner, A.B.; Downes, G.B. Expression of the eight GABAA receptor alpha subunits in the developing zebrafish central nervous system. PLoS ONE 2018, 13, e0196083. [Google Scholar] [CrossRef]
- Chitolina, R.; Reis, C.G.; Stahlhofer-Buss, T.; Linazzi, A.; Benvenutti, R.; Marcon, M.; Herrmann, A.P.; Piato, A. Effects of N-acetylcysteine and acetyl-l-carnitine on acute PTZ-induced seizures in larval and adult zebrafish. Pharmacol. Rep. 2023, 75, 1544–1555. [Google Scholar] [CrossRef]
- Mussulini, B.H.; Leite, C.E.; Zenki, K.C.; Moro, L.; Baggio, S.; Rico, E.P.; Rosemberg, D.B.; Dias, R.D.; Souza, T.M.; Calcagnotto, M.E.; et al. Seizures induced by pentylenetetrazole in the adult zebrafish: A detailed behavioral characterization. PLoS ONE 2013, 8, e54515. [Google Scholar] [CrossRef]
- Fontana, B.D.; Ziani, P.R.; Canzian, J.; Mezzomo, N.J.; Muller, T.E.; Dos Santos, M.M.; Loro, V.L.; Barbosa, N.V.; Mello, C.F.; Rosemberg, D.B. Taurine Protects from Pentylenetetrazole-Induced Behavioral and Neurochemical Changes in Zebrafish. Mol. Neurobiol. 2019, 56, 583–594. [Google Scholar] [CrossRef]
- Verma, R.; Raj Choudhary, P.; Kumar Nirmal, N.; Syed, F.; Verma, R. Neurotransmitter systems in zebrafish model as a target for neurobehavioural studies. Mater. Today Proc. 2022, 69, 1565–1580. [Google Scholar] [CrossRef]
- Horzmann, K.A.; Freeman, J.L. Zebrafish Get Connected: Investigating Neurotransmission Targets and Alterations in Chemical Toxicity. Toxics 2016, 4, 19. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision A.1; Gaussian, Inc.: Pittsburgh, PA, USA, 2009. [Google Scholar]
- Xiong, G.; Wu, Z.; Yi, J.; Fu, L.; Yang, Z.; Hsieh, C.; Yin, M.; Zeng, X.; Wu, C.; Lu, A.; et al. ADMETlab 2.0: An integrated online platform for accurate and comprehensive predictions of ADMET properties. Nucleic Acids Res. 2021, 49, W5–W14. [Google Scholar] [CrossRef]
- Dong, J.; Wang, N.N.; Yao, Z.J.; Zhang, L.; Cheng, Y.; Ouyang, D.; Lu, A.P.; Cao, D.S. ADMETlab: A platform for systematic ADMET evaluation based on a comprehensively collected ADMET database. J. Cheminform. 2018, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Rivel, T.; Ramseyer, C.; Yesylevskyy, S. The asymmetry of plasma membranes and their cholesterol content influence the uptake of cisplatin. Sci. Rep. 2019, 9, 5627. [Google Scholar] [CrossRef] [PubMed]
- Barducci, A.; Bussi, G.; Parrinello, M. Well-tempered metadynamics: A smoothly converging and tunable free-energy method. Phys. Rev. Lett. 2008, 100, 020603. [Google Scholar] [CrossRef]
- Tribello, G.A.; Bonomi, M.; Branduardi, D.; Camilloni, C.; Bussi, G. PLUMED 2: New feathers for an old bird. Comput. Phys. Commun. 2014, 185, 604–613. [Google Scholar] [CrossRef]
- Pereira, A.C.M.; Cunha, A.A.; Carvalho, H.d.O.; Ferreira, I.M.; Carvalho, J.C.T. Oral diazepam suppresses pentylenetetrazole-induced seizure-like behavior in adult zebrafish: A tool for nonclinical studies. J. Appl. Pharm. Sci. 2023, 13, 132–139. [Google Scholar] [CrossRef]
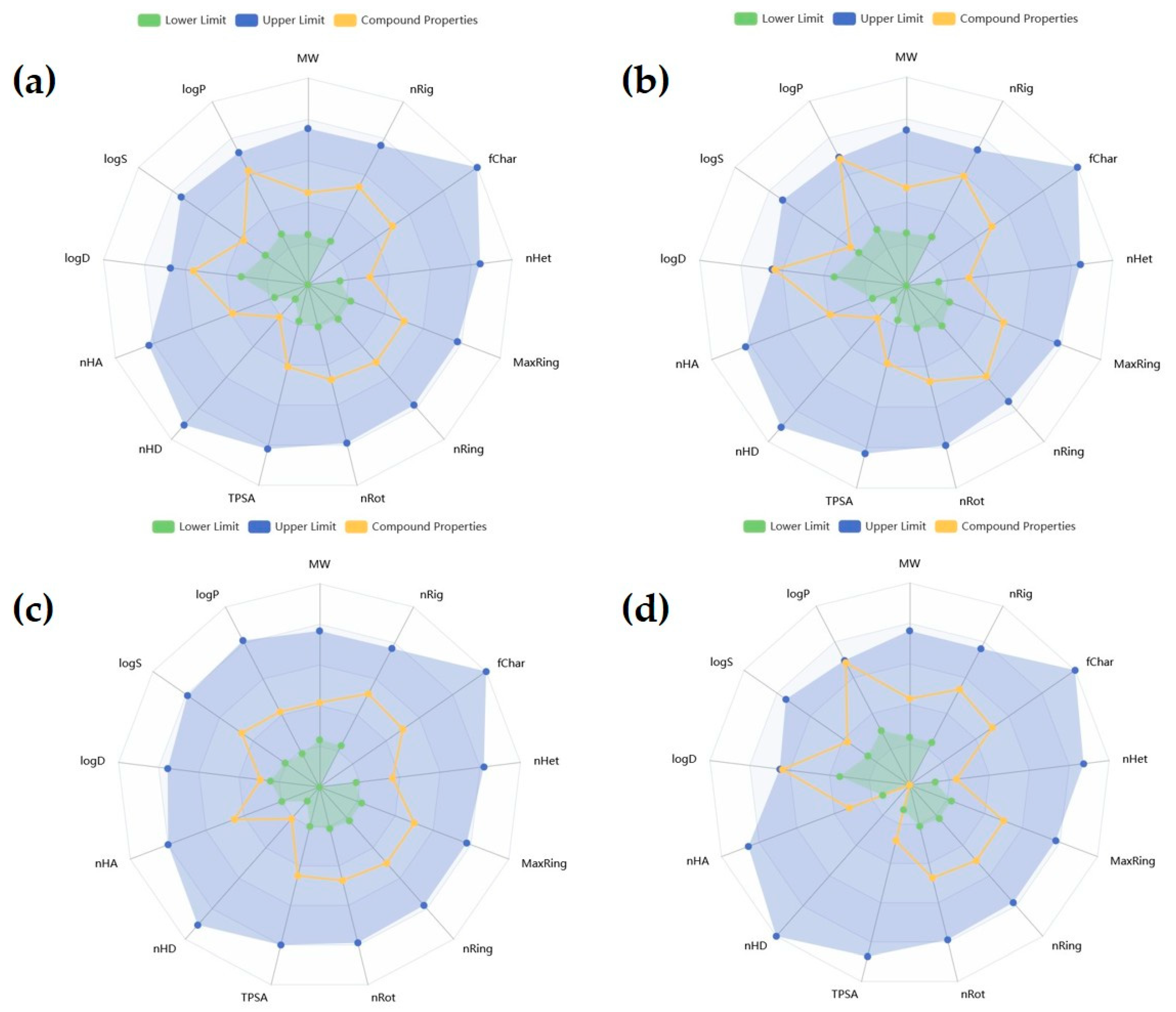
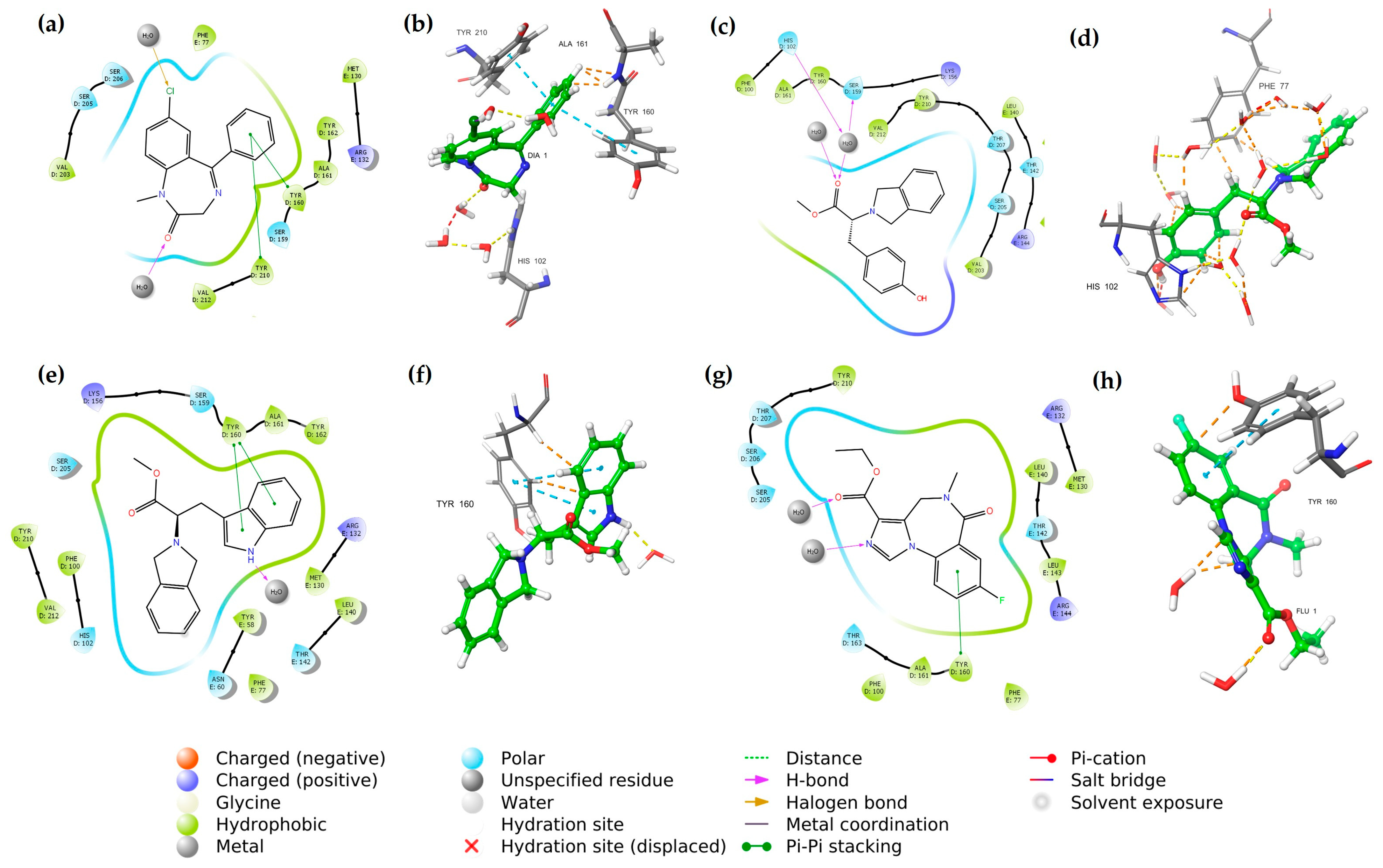

| Entry | Compd. | MW a (Da) | Log P b | TPSA c (Å2) | HBD d | HBA e | BBB Permeable | GI Absorption f | hERG Inhibition g | Ames Test h |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Dzp | 284.74 | 2.99 | 32.670 | 2 | 0 | YES | −4.265 | 0.464 | 0.2 |
| 2 | ETYR | 297.35 | 2.49 | 49.77 | 4 | 1 | YES | −4.865 | 0.386 | 0.513 |
| 3 | ETRP | 320.39 | 2.78 | 45.33 | 3 | 1 | YES | −4.786 | 0.33 | 0.593 |
| 4 | EHIS | 271.31 | 1.02 | 58.22 | 4 | 1 | YES | −5.365 | 0.146 | 0.496 |
| 5 | EPHE | 281.35 | 2.78 | 29.54 | 3 | 0 | YES | −4.641 | 0.416 | 0.52 |
| Entry | Compd. | Docking Score (kcal/mol) | Key Residue Involved | Dominant Interactions |
|---|---|---|---|---|
| 1 | Dzp | −9.3 | Tyr160 (α1), Tyr210 (α1), Phe100 (α1), Phe77 (γ2), Tyr58 (γ2), Val203 (α1) | π-π stacking |
| 2 | ETYR | −10.0 | Tyr160 (α1), Arg210 (α1), Phe77 (γ2), Ser205 (α1), His102 (α1) | π-π stacking, H-bond |
| 3 | ETRP | −9.8 | Phe77 (γ2), Tyr58 (γ2), Ser205 (α1), His102 (α1), Phe100 (α1), Tyr160 (α1), Tyr210 (α1) | π-π stacking, H-bond |
| 4 | EHIS | −9.3 | Asp56 (γ2), Ala79 (γ2), Phe77 (γ2), Met130 (γ2), Tyr160 (α1), Tyr210 (α1), Ser205 (α1), Tyr58 (γ2), Ser206 (α1) | π-π stacking |
| 5 | EPHE | −9.0 | Tyr210 (α1), Tyr160 (α1), Phe77 (γ2), Tyr58 (γ2) | π-π stacking, polar contacts |
| Entry | Compd. | Bound State * | Unbound State * | ΔG Bind * | ΔΔG * |
|---|---|---|---|---|---|
| 1 | DZP | –737.24 ± 1.53 | –59.37 ± 2.99 | –77.87 ± 2.19 | 0 |
| 2 | FLU | –948.45 ± 0.27 | –677.51 ± 0.95 | –270.94 ± 1.24 | –193.07 |
| 3 | ETYR | –628.46 ± 2.44 | –567.49 ± 4.83 | –60.97 ± 2.38 | 16.9 |
| 5 | ETRP | –705.35 ± 2.96 | –537.3 ± 4.55 | –168.05 ± 1.18 | –90.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Periañez, S.; Hernández-Rosas, F.; López-Rosas, C.A.; Ramos-Morales, F.R.; Zurutuza-Lorméndez, J.I.; García-Rodríguez, R.V.; Olivares-Romero, J.L.; Ramos-Hernández, R.R.; Bravo-Espinoza, I.; Vidal-Limon, A.; et al. Multistage Molecular Simulations, Design, Synthesis, and Anticonvulsant Evaluation of 2-(Isoindolin-2-yl) Esters of Aromatic Amino Acids Targeting GABAA Receptors via π-π Stacking. Int. J. Mol. Sci. 2025, 26, 6780. https://doi.org/10.3390/ijms26146780
González-Periañez S, Hernández-Rosas F, López-Rosas CA, Ramos-Morales FR, Zurutuza-Lorméndez JI, García-Rodríguez RV, Olivares-Romero JL, Ramos-Hernández RR, Bravo-Espinoza I, Vidal-Limon A, et al. Multistage Molecular Simulations, Design, Synthesis, and Anticonvulsant Evaluation of 2-(Isoindolin-2-yl) Esters of Aromatic Amino Acids Targeting GABAA Receptors via π-π Stacking. International Journal of Molecular Sciences. 2025; 26(14):6780. https://doi.org/10.3390/ijms26146780
Chicago/Turabian StyleGonzález-Periañez, Santiago, Fabiola Hernández-Rosas, Carlos Alberto López-Rosas, Fernando Rafael Ramos-Morales, Jorge Iván Zurutuza-Lorméndez, Rosa Virginia García-Rodríguez, José Luís Olivares-Romero, Rodrigo Rafael Ramos-Hernández, Ivette Bravo-Espinoza, Abraham Vidal-Limon, and et al. 2025. "Multistage Molecular Simulations, Design, Synthesis, and Anticonvulsant Evaluation of 2-(Isoindolin-2-yl) Esters of Aromatic Amino Acids Targeting GABAA Receptors via π-π Stacking" International Journal of Molecular Sciences 26, no. 14: 6780. https://doi.org/10.3390/ijms26146780
APA StyleGonzález-Periañez, S., Hernández-Rosas, F., López-Rosas, C. A., Ramos-Morales, F. R., Zurutuza-Lorméndez, J. I., García-Rodríguez, R. V., Olivares-Romero, J. L., Ramos-Hernández, R. R., Bravo-Espinoza, I., Vidal-Limon, A., & Pawar, T. J. (2025). Multistage Molecular Simulations, Design, Synthesis, and Anticonvulsant Evaluation of 2-(Isoindolin-2-yl) Esters of Aromatic Amino Acids Targeting GABAA Receptors via π-π Stacking. International Journal of Molecular Sciences, 26(14), 6780. https://doi.org/10.3390/ijms26146780









