Molecular Insights into the Potential Cardiometabolic Effects of GLP-1 Receptor Analogs and DPP-4 Inhibitors
Abstract
1. Introduction
Mechanisms of Action and Clinical Indications of GLP-1 Receptor Agonists (GLP-1 RAs) and DPP-4 Inhibitors (DPP-4is)
| DPP-4i | GLP-1 RA | |
|---|---|---|
| Mechanism of action | Inhibition of the DDP-4 enzyme, which breaks down GLP-1, increasing the concentration of endogenous GIP and GLP-1 [56] | Direct activation of the GLP-1 receptor; modified to be resistant to DDP-4 [57] |
| Effect on body weight | No significant impact [58] | Weight reduction of approximately 1–4 kg [59] |
| HbA1c reduction | Smaller average reduction, moderate reduction of 0.4–1.1% [60] | Greater reduction of 0.6–1.9% [59] |
| Risk of hypoglycemia | Low [61] | Low [61] |
| Route of administration | Oral [62] | Subcutaneous, oral [62] |
| Dosing frequency | Once daily [63] | Ranges from once daily to once weekly [63] |
| Common side effects | Mild: headaches, upper respiratory tract infections [63] | Usually transient diarrhea, nausea, and vomiting [63] |
| Clinical indications | Patients with moderate hyperglycemia, without the need for weight loss, and who prefer oral therapy will benefit. | Patients who require tighter glycemic control, are at high cardiovascular risk, and are obese will benefit. |
2. Cardioprotective Properties of GLP-1 Analogs, Receptor Agonists, and DPP-4 Inhibitors
2.1. Major Adverse Cardiovascular Events
2.2. Cardiovascular Risk
2.3. Atherosclerosis and Atherothrombosis
2.3.1. Molecular Mechanisms Involved in GLP-1 RA and DPP-4i Effects in Atherosclerosis and Atherothrombosis
Atherosclerotic Plaque Markers
Extracellular Matrix Remodeling
Oxidative Stress and Endothelial Function
Apoptosis and Inflammation
Mitochondrial Function
| Source | Effect | Treatment | Model (Species, Intervention: Analyzed Tissues/Cell Line) |
|---|---|---|---|
| Preclinical in vivo studies | |||
| Mice with experimental arterial hypertension: blood, heart, and vascular tissue analysis. | liraglutide 30 µg/kg twice daily i.p. for a week. | ↓oxidative stress; ↓S-glutathionylation (a marker of eNOS uncoupling); ↑NO bioavailability. | [81] |
| Mice (apoE−/−, LDLr−/−) fed a Western diet: aorta and blood analysis. | liraglutide 1 mg/kg or semaglutide 4.0–60.0 μg/kg for 12–14 weeks (apoE−/−) or for 17 weeks (LDLr−/−). | ↓TNF-α, IFN-γ, osteopontin, Il-6, Il-1RN, CCL2, OPN; ↓SELE, VCAM1; ↓MMP3, MMp13; ↓Cd163; ↓ABCA1, PTGIS; ↓total cholesterol; ↓triglyceride; ↓multiple inflammatory pathways. | [82] |
| Mice and rats treated with LPS: cardiac tissue and blood analysis. | linagliptin 5 mg/kg/day or liraglutide 200 µg/kg/day orally or s.c. for 3 days prior to and 6 h post LPS treatment. | ↓Hb-NO levels and iNOS activity; ↓monocyte/macrophage infiltration; ↓expression of adhesion molecules VCAM-1, ICAM-1; ↓IL-6, MCP-1, and TNF-α; ↓aortic reactive oxygen species formation. | [88] |
| Mice, LDL receptor-deficient: blood and vascular tissue analysis. | Mice: liraglutide 300 mg/kg twice daily for 4 weeks. | In vivo: ↓LOX-1 expression in aortas; ↓plasma MDA; ↑ plasma SOD, NO; ↓plasma IL-1 beta, IL-6. | [89] |
| Rats fed a fructose-rich diet: analysis of the aorta and blood. | sitagliptin 5.0/10 mg/kg or exenatide 5/10 µg/kg for 4 weeks. | Exenatide: ↑plasma platelet-activating factor acetylhydrolase activity; ↓circulating ox-LDL and MCP-1; ↓PC/lyso-PC ratio; ↑apoB/apoA-I balance; ↓LDL oxidation; Sitagliptin: no change. | [95] |
| Mice with apoE knockout (apoE−/−): blood, heart, and aorta analysis. | Mice: liraglutide 300 µg/kg twice daily for 4 weeks. | ↓PAI-1 and VAM expression; ↑endothelial nitric oxide synthase (eNOS); ↓ICAM-1 in aortic endothelium. | [106] |
| Rat diabetes mellitus model: cardiac tissue and blood analysis. | Rats: vildagliptin 1 mg/kg or exenatide 1 nmol/kg for 12 weeks. | In vivo: ↑glucose uptake and microvascular barrier function. | [108] |
| Rat, euglycemic insulin clamp after 2 weeks of high-fat diet: aorta tissue analysis. | liraglutide 200 μg/kg s.c. twice daily for 2 weeks. | ↑ Nrf2 nuclear translocation; ↑AMPK phosphorylation; ↑muscle insulin sensitivity; ↑insulin-mediated muscle microvascular perfusion; ↓perivascular macrophage accumulation; ↓oxidative stress and inflammation. | [109] |
| Mice, apolipoprotein E–E-deficient: heart and aorta tissue and macrophage analysis. | Mice: Ex-4 300 pmol/kg/day or 24 pmol/kg/day. | ↓TNF-α and MCP-1 levels; ↓nuclear translocation of p65; ↓NF-κB; ↓expression of CD11b. | [123] |
| Mice, wire-mediated endovascular injury: femoral artery analysis. | Mice: Ex-4 24 nmol/kg/day for 4 weeks. | ↓SMC proliferation via the cAMP/PKA pathway; ↓TNF-α production by peritoneal macrophages in response to inflammatory stimuli. | [124] |
| Rat diabetes model: aorta tissue and blood analysis. | sitagliptin 30 mg/kg/day or exenatide 3 μg/kg/12 h for 12 weeks. | ↑NO level in serum; ↓ET-1 level; ↓inflammatory cytokines levels; ↓oxidative stress; ↓expression level in AGE/RAGE-induced RhoA/ROCK/NF-κB/IκBα signaling pathways; ↑AMPK activation; ↓RAGE, ROCK-2, ET-1, iNOS, p-NF-κB, NF-κB, p-IκBα, and IL-6 expression restoring GLP-1R, p-eNOS, and p-AMPK expression. | [113] |
| Experimental in vitro studies | |||
| HUVECs (ox-LDL-challenged human umbilical vein endothelial cells). | HUVECs: 1000 nM liraglutide for 1 h. | ↓reactive oxygen species production; ↓apoptosis; ↓ICAM-1, VCAM-1, LOX-1, NOX4, and NF-κB expression. | [89] |
| Human umbilical vein endothelial cell line (C11-STH20). | C11-STH20: liraglutide 100 nM for 16 h. | ↓TNF-α-mediated NF-κB induction, independent of PKA signaling. | [106] |
| Microvascular endothelial cells (CMECs). | CMECs: high glucose medium, GLP-1 (10−10, 10−9, 10−8, 10−7 mol/L). | ↓reactive oxygen species production; ↓apoptotic index; ↓levels of NADPH oxidases; ↑cAMP/PKA; ↓Rho expression. | [108] |
| Mouse macrophage cell line RAW264 and mouse preadipocyte cell line 3T3-L1s. | Ex-4 2.5 nM. | ↓TNF-β, IL-6, and IL-1β secretion; ↓activation of NF-κB in macrophages. | [116] |
| Rat vascular smooth muscle cells (VSMCs). | liraglutide 100 nM for 1h. | ↓apoptosis; ↑phosphorylation of protein kinase B (Akt) and ERK1/2. | [121] |
| THP-1 cell line. | THP-1: Ex-4 0.03, 0.3, and 3 nmol/L for 1 h. | ↓TNF-α and MCP-1 levels; ↓nuclear translocation of p65; ↓NF-κB; ↓expression of CD11b. | [123] |
| Rat aortic SMCs. | rat aortic SMCs: Ex-4 5 nmol/L, 60 min. | ↓SMC proliferation via the cAMP/PKA pathway. | [124] |
| Human primary endothelial cells, coronary artery endothelial cells, and aorta endothelial cells. | exenatide 1 and 10 nmol/L for 24 h. | ↓caspase 3/7 activation; ↓NF-kB activation; ↓GFP expression; ↓VCAM-1 and ICAM-1 expression; ↑TIMP-1 and TIMP-2. | [93] |
| Human aortic endothelial cells (HAECs), ox-LDL-treated. | dulaglutide 50 and 100 nM for 24 h. | ↓ox-LDL-induced oxidative stress and mitochondrial dysfunction; ↓secretion of IL-1β, IL-6, MCP-1, and HMG-1; ↓cell viability and release of LDH; ↓attachment of THP-1 to HAECs by inhibiting VCAM-1, E-selectin; ↑expression of KLF2 through inhibiting the activation of p53. | [125] |
| Human studies | |||
| Human women with PCOS: blood analysis. | liraglutide in increasing doses (0.6–1.8 mg) s.c. for six months. | ↓HOMA-IR; ↓triglyceride; ↓hsCRP; ↓urinary isoprostanes; ↓sP-selectin, sICAM, and sVCAM; ↓platelet P-selectin expression. | [79] |
| Humans with type 2 diabetes: blood and serum analysis. | GLP-1 RA treatment for one year. | ↓ROS production; ↑mitochondrial membrane potential, oxygen consumption; ↓MPO; ↓ICAM-1, VCAM-1, IL-6, TNF-α, and IL-12; ↑IL-10; ↓CIMT. | [80] |
| Humans with type 2 diabetes: blood and serum analysis. | liraglutide 0.6 mg once daily for 2 weeks and increasing to 1.2 mg once daily. | ↓TNF-α; ↓IL-1β; ↓IL-6; ↑adipokine adiponectin. | [111] |
| Humans with type 2 diabetes: blood, serum, and urine analysis. | liraglutide 1.2–1.8 mg/day for 26 weeks. | ↓IL-6 ↓albumin/creatinine ratio in urine. | [117] |
| Humans with type 2 diabetes and albuminuria: blood and serum analysis. | liraglutide 1.8 mg/day for 12 weeks. | ↓TNF-α level; ↓MR-proADM level; ↓MR-proANP level. | [118] |
| Humans with type 2 diabetes and obesity: blood and serum analysis. | exenatide 10 μg s.c. twice daily for 12 weeks. | ↓blood glucose; ↓HbA1c; ↓free fatty acids; ↓reactive oxygen species generation; ↓NF-κB; ↓mRNA expression of TNF-α, IL-1β, JNK-1, TLR-2, TLR-4, and SOCS-3 in mononuclear cells; ↓monocyte chemoattractant protein-1; ↓MMP9; ↓serum amyloid A; ↓IL-6. | [119] |
| Humans with type 2 diabetes: blood and serum analysis. | sitagliptin 100 mg/day for 12 weeks. | ↓HbA1c; ↑glucagon-like peptide-1 concentrations; ↓expression of CD26, TNF-α, TLR-4, TLR-2, c-Jun N-terminal kinase-1, and inhibitory-κB kinase (IKKβ) (proinflammatory kinases) in mononuclear cells; ↓expression of TLR-2, IKKβ, CCR-2, and CD26 and NF-κB; ↓expression of JNK-1, IKKβ, and TLR-4 and plasma concentrations of C-reactive protein, IL-6, and free fatty acids. | [120] |
2.4. Cardiac Arrhythmias
2.4.1. Molecular Mechanisms Involved in GLP-1 RA and DPP-4i Effects in Cardiac Arrhythmias
Cardiac Remodeling and Fibrosis
| Source | Effect | Treatment | Model (Species, Intervention: Analyzed Tissues) |
|---|---|---|---|
| Preclinical in vivo studies | |||
| Mice, angiotensin II-induced proliferation model (atrial fibrillation model): atrial fibroblast analysis. | Liraglutide 10, 50, or 100 nmol/L. | ↓AngII-induced increase in the expression level of miR-21; ↑expression of PTEN (a target of miR-21); ↓phosphoinositide 3-kinase (PI3K)/AKT signaling pathway; ↓angiotensin II-induced proliferation, migration, and invasion of fibroblasts. | [132] |
| Rats fed a fructose-rich diet: analysis of fibrosis, aorta, and blood. | Sitagliptin 5.0/10 mg/kg or exenatide 5/10 µg/kg for 4 weeks. | Exenatide: ↓ADMA (plasma); ↑NO and PRMT; ↓TGF-ß1 and α-SMA; ↓COL1A1 expression; Sitagliptin: ↑NO; ↓circulating SDMA; ↑renal DDAH activity; ↓myocardial DDAH activity; Exenatide and sitagliptin: ↓immunoexpression of Smad2/3/P proteins; positively modulated cardiac fibrotic remodeling. | [134] |
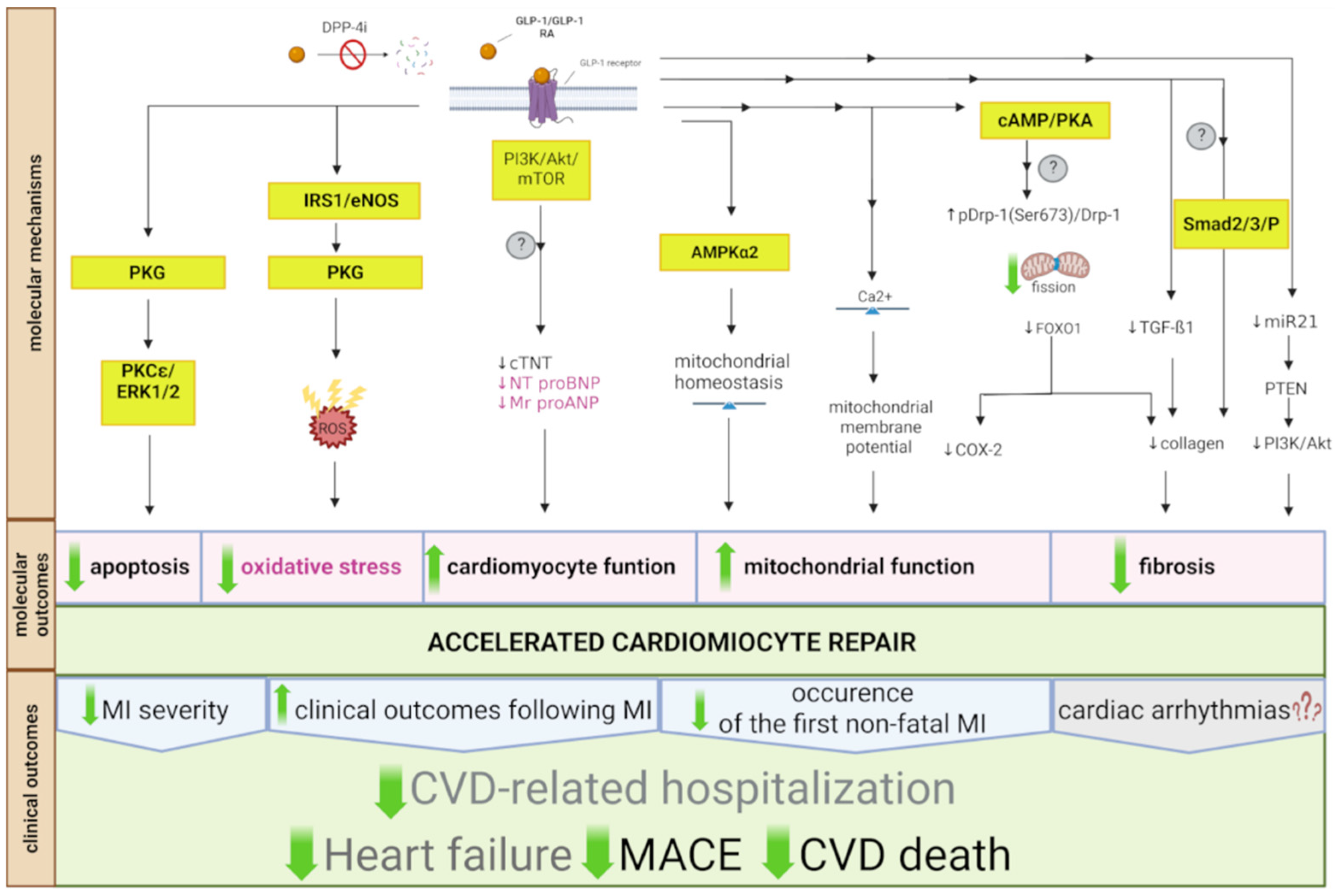
2.5. Myocardial Infarction and Heart Failure
2.5.1. Molecular Mechanisms Involved in GLP-1 RA and DPP-4i Effects in Myocardial Infarction and Heart Failure
Natriuretic Peptides and Extracellular Matrix Remodeling
Mitochondrial Function
Myocardial Damage
| Model (Species, Intervention: Analyzed Tissues) | Treatment | Effect | Source |
|---|---|---|---|
| Preclinical in vivo studies | |||
| Mice, ischemia/reperfusion (MI) model: cardiac tissue analysis. | 5 nmol exenatide during reperfusion. | ↑cGMP/NO release; ↑glucose uptake; ↑cAMP and cGMP release. | [24] |
| Rats, ischemia/reperfusion injury (MI): cardiac tissue analysis. | semaglutide 0.3 mg/kg, 30 min prior to ischemia/reperfusion injury. | ↑GLP-1R expression; ↑PKG/PKCε/ERK1/2 pathway; ↓cardiomyocyte apoptosis; ↓hs-cTNT levels; ↑NT-proBNP levels. | [165] |
| Rats, isolated atrial perfusion model: atrial tissue analysis. Mice, DPP-4 knockout (DPP-4−/−): cardiac tissue and blood analysis. | Mice: liraglutide 30 μg/kg/day for 14 days; Rats, ex vivo: liraglutide 3.2 nmol/L. | ↓ANP secretion; ↑expression of GLP-1R and PI3K/AKT/mTOR. | [168] |
| Rats, MI model: blood and cardiac tissue analysis. | sitagliptin 300 mg/kg/day via oral tube for 6 weeks. | ↓collagen 1 abundance. | [171] |
| Rats, MI model: cardiac tissue analysis. | pretreatment with vildagliptin 15 mg/kg/day for 2 days or 3 weeks after coronary artery ligation. | No reduction in ANP and BNP mRNA levels. | [172] |
| Mice, high-fat diet/streptozotocin-induced type 2 diabetes model: cardiac tissue and blood analysis. | dulaglutide 0.6 mg/kg/week s.c. for 8 weeks. | ↑AMPKα2 signaling; ↓mitochondrial fragmentation in cardiomyocytes; ↓insulin resistance; ↑ glucose tolerance; ↓hyperlipidemia; ↑fatty acid use in the myocardium; ↑phosphorylated (Ser 637) Drp-1 while ↓total Drp-1 protein levels. | [175] |
| Rats, aged (24 months) and adult (6 months): freshly isolated ventricular cardiomyocytes. | Adult rats: liraglutide 200 µg/kg/day i.p. for 6 weeks; Aged rats: liraglutide 300 µg/kg/day i.p. for 4 weeks. | ↑blood pressure; ↓oxidative stress; ↑Na+/Ca2+ exchanger currents; ↓SGLT2; ↑IRS1 expression; ↓protein kinase G (PKG) activity; restored normal mitochondrial membrane potential; improved the pNOS3/NOS3 ratio. | [179] |
| Pigs after global warm ischemia/reperfusion (MI): cardiac tissue analysis. | exenatide 5 nmol during reperfusion. | ↑myocardial oxygen consumption; ↑activated eNOS; ↓ troponin-I; ↓endothelial damage; ↓lactate. | [180] |
| Human studies | |||
| Humans with heart failure with reduced ejection fraction, with/without type 2 diabetes: blood analysis. | liraglutide 1.8 mg/day for 24 weeks | ↓NT-proBNP and MR-proANP levels; no effect on MR-proADM and copeptin. | [169] |
| Humans with type 2 diabetes mellitus: blood analysis. | liraglutide 1.8 mg/day s.c. for 6 months | ↓malondialdehyde (oxidative stress indicator); ↓NT-proBNP. | [170] |
| Human, post-acute MI: blood analysis. | vildagliptin 50 mg twice a day for 6 months | ↓BNP levels; ↓HbA1c levels. | [171] |
3. Conclusions
4. Materials and Methods
4.1. Search Strategy
4.2. Eligibility Criteria
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADA | American Diabetes Association |
| Akt | Protein kinase B |
| AMP | Adenosine monophosphate |
| AMPK | AMP-activated protein kinase |
| AMPKα2 | AMP-activated protein kinase alpha 2 |
| AngII | Angiotensin II |
| ANP | Atrial natriuretic peptide |
| apoA-I | Apolipoprotein AI |
| apoB | Apolipoprotein B |
| apoE | Apolipoprotein E |
| ASCVD | Atherosclerotic cardiovascular disease |
| ATP | Adenosine triphosphate |
| BH4 | Tetrahydrobipterin |
| BMI | Body mass index |
| BNP | Brain natriuretic peptide |
| CAD | Coronary artery disease |
| cAMP | Cyclic adenosine monophosphate |
| CD | Cerebrovascular disease |
| cGMP | Cyclic guanosine monophosphate |
| CHF | Congestive heart failure |
| CMECs | Cardiac microvascular endothelial cells |
| COX-2 | Cyclooxygenase-2 |
| CVD | Cardiovascular disease |
| DBP | Diastolic blood pressure |
| DCD | Donation after circulatory death |
| DM | Diabetes mellitus |
| DNA | Deoxyribonucleic acid |
| DPP-4 | Dipeptidyl peptidase-4 |
| DPP-4i | Dipeptidyl peptidase-4 inhibitor |
| Drp-1 | Dynamin-related protein-1 |
| ECM | Extracellular matrix |
| eGFR | Estimated glomerular filtration rate |
| eNOS | Endothelial nitric oxide synthase |
| ERK1/2 | Extracellular signal-regulated kinase 1/2 |
| ET-1 | Endothelin-1 |
| Ex-4 | Exendin-4 |
| FBG | Fasting blood glucose |
| FoxO1 | Forkhead box protein O1 |
| GIP | Gastric inhibitory polypeptide |
| GLP-1 RA | Glucagon-like peptide-1 receptor agonist |
| GLP-1R | Glucagon-like peptide-1 receptor |
| GTP | Guanosine triphosphate |
| GPx | Glutathione peroxidase |
| HAECs | Human aortic endothelial cells |
| HbA1c | Hemoglobin A1c |
| HF | Heart failure |
| HFrEF | Heart failure with reduced ejection fraction |
| I/R | Ischemia/reperfusion |
| ICAM-1 | Intercellular adhesion molecule 1 |
| IL-1β | Interleukin-1 beta |
| IL-6 | Interleukin 6 |
| iNOS | Inducible nitric oxide synthase |
| IRS1 | Insulin receptor substrate-1 |
| IκBα | Inhibitor of kappa B alpha |
| JNK-1 | c-Jun N-terminal kinase 1 |
| KLF2 | Krüppel-like factor 2 |
| LDL | Low-density lipoprotein |
| LDL-C | Low-density lipoprotein cholesterol |
| LOX-1 | Lectin-like oxidized low-density lipoprotein receptor 1 |
| LPS | Lipopolysaccharide |
| LVEF | Left ventricular ejection fraction |
| lyso-PC | Lysophosphatidylcholine |
| MACE | Major adverse cardiovascular event |
| MCP-1 | Monocyte chemoattractant protein 1 |
| MI | Myocardial infarction |
| miR-21/PTEN/PI3K | microRNA-21/phosphatase and tensin homolog/phosphoinositide 3-kinase |
| MMP | Matrix metalloproteinase |
| MNCs | Mononuclear cells |
| mRNA | Messenger ribonucleic acid |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NO | Nitric oxide |
| NOS | Nitric oxide synthase |
| NOX4 | Nicotinamide adenine dinucleotide phosphate oxidase 4 |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| Ox-LDL | Oxidized low-density lipoprotein |
| PAD | Peripheral artery disease |
| PAF-AH | Plasma platelet-activating factor acetylhydrolase |
| p-AMPK | Phosphorylated AMP-activated protein kinase |
| PC | Phosphatidylcholine |
| PCOS | Polycystic ovary syndrome |
| p-eNOS | Phosphorylated endothelial nitric oxide synthase |
| PI3K | Phosphoinositide 3-kinase |
| p-IκBα | Phosphorylated inhibitor of kappa B alpha |
| PKA | Protein kinase A |
| PKG | Protein kinase G |
| PKG/PKCε/ERK1/2 | Protein kinase G/protein kinase C epsilon/extracellular signal-regulated |
| PMNs | Polymorphonuclear leukocytes |
| p-NF-κB | Phosphorylated nuclear factor kappa-light-chain-enhancer of activated B cells |
| pNOS3/NOS3 | Phosphorylated nitric oxide synthase 3/nitric oxide synthase 3 |
| RAGE | Receptors of advanced glycation end product |
| ROCK | Rho-associated coiled-coil containing protein kinase |
| ROCK-2 | Rho-associated coiled-coil containing protein kinase 2 |
| ROS | Reactive oxygen species |
| SBP | Systolic blood pressure |
| sGC | Soluble guanylyl cyclase |
| SGLT2i | Sodium–glucose cotransporter 2 inhibitor |
| sICAM | Soluble intercellular adhesion molecule |
| SMC | Smooth muscle cell |
| SOCS-3 | suppressor of cytokine signaling 3 |
| sVCAM | Soluble vascular cell adhesion molecule |
| T2DM | Type 2 diabetes mellitus |
| TC | Total cholesterol |
| TGF-β1 | Transforming growth factor beta 1 |
| TIMP | Tissue inhibitor of metalloproteinase |
| TLR2 | Toll-like receptor 2 |
| TLR4 | Toll-like receptor 4 |
| TNF | Tumor necrosis factor |
| VCAM-1 | Vascular cellular adhesion molecule-1 |
References
- Mensah, G.A.; Roth, G.A.; Fuster, V. The Global Burden of Cardiovascular Diseases and Risk Factors. J. Am. Coll. Cardiol. 2019, 74, 2529–2532. [Google Scholar] [CrossRef] [PubMed]
- Vos, T.; Lim, S.S.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A.; et al. Global Burden of 369 Diseases and Injuries in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R.; et al. Heart Disease and Stroke Statistics—2018 Update: A Report From the American Heart Association. Circulation 2018, 137, e67–e492. [Google Scholar] [CrossRef] [PubMed]
- Sattar, N.; McGuire, D.K.; Pavo, I.; Weerakkody, G.J.; Nishiyama, H.; Wiese, R.J.; Zoungas, S. Tirzepatide Cardiovascular Event Risk Assessment: A Pre-Specified Meta-Analysis. Nat. Med. 2022, 28, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.S.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Barone Gibbs, B.; Beaton, A.Z.; Boehme, A.K.; et al. 2024 Heart Disease and Stroke Statistics: A Report of US and Global Data from the American Heart Association. Circulation 2024, 149, E347–E913. [Google Scholar] [CrossRef] [PubMed]
- Bosco, E.; Hsueh, L.; McConeghy, K.W.; Gravenstein, S.; Saade, E. Major Adverse Cardiovascular Event Definitions Used in Observational Analysis of Administrative Databases: A Systematic Review. BMC Med. Res. Methodol. 2021, 21, 241. [Google Scholar] [CrossRef] [PubMed]
- Dubois-Deruy, E.; Peugnet, V.; Turkieh, A.; Pinet, F. Oxidative Stress in Cardiovascular Diseases. Antioxidants 2020, 9, 864. [Google Scholar] [CrossRef] [PubMed]
- Sverdlov, A.L.; Elezaby, A.; Qin, F.; Behring, J.B.; Luptak, I.; Calamaras, T.D.; Siwik, D.A.; Miller, E.J.; Liesa, M.; Shirihai, O.S.; et al. Mitochondrial Reactive Oxygen Species Mediate Cardiac Structural, Functional, and Mitochondrial Consequences of Diet-Induced Metabolic Heart Disease. J. Am. Heart Assoc. 2016, 5, e002555. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Mondragón, R.; Lozhkin, A.; Vendrov, A.E.; Runge, M.S.; Isom, L.L.; Madamanchi, N.R. NADPH Oxidases and Oxidative Stress in the Pathogenesis of Atrial Fibrillation. Antioxidants 2023, 12, 1833. [Google Scholar] [CrossRef] [PubMed]
- Senoner, T.; Dichtl, W. Oxidative Stress in Cardiovascular Diseases: Still a Therapeutic Target? Nutrients 2019, 11, 2090. [Google Scholar] [CrossRef] [PubMed]
- Pirillo, A.; Norata, G.D.; Catapano, A.L. LOX-1, OxLDL, and Atherosclerosis. Mediat. Inflamm. 2013, 2013, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Townsend, N.; Kazakiewicz, D.; Lucy Wright, F.; Timmis, A.; Huculeci, R.; Torbica, A.; Gale, C.P.; Achenbach, S.; Weidinger, F.; Vardas, P. Epidemiology of Cardiovascular Disease in Europe. Nat. Rev. Cardiol. 2022, 19, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Short, L.; La, V.T.; Patel, M.; Pai, R.G. Primary and Secondary Prevention of CAD: A Review. Int. J. Angiol. 2022, 31, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Baggio, L.L.; Yusta, B.; Mulvihill, E.E.; Cao, X.; Streutker, C.J.; Butany, J.; Cappola, T.P.; Margulies, K.B.; Drucker, D.J. GLP-1 Receptor Expression Within the Human Heart. Endocrinology 2018, 159, 1570–1584. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.J.; Philippe, J.; Mojsov, S.; Chick, W.L.; Habener, J.F. Glucagon-like Peptide I Stimulates Insulin Gene Expression and Increases Cyclic AMP Levels in a Rat Islet Cell Line. Proc. Natl. Acad. Sci. USA 1987, 84, 3434–3438. [Google Scholar] [CrossRef] [PubMed]
- Calanna, S.; Christensen, M.; Holst, J.J.; Laferrère, B.; Gluud, L.L.; Vilsbøll, T.; Knop, F.K. Secretion of Glucagon-like Peptide-1 in Patients with Type 2 Diabetes Mellitus: Systematic Review and Meta-Analyses of Clinical Studies. Diabetologia 2013, 56, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Lastya, A.; Saraswati, M.R.; Suastika, K. The Low Level of Glucagon-like Peptide-1 (Glp-1) Is a Risk Factor of Type 2 Diabetes Mellitus. BMC Res. Notes 2014, 7, 849. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, R.; Kim, M.-H.; Lee, S.-H.; Cho, J.-H.; Lee, J.M.; Jang, S.-A.; Kim, H.-S. Weight Loss and Side-Effects of Liraglutide and Lixisenatide in Obesity and Type 2 Diabetes Mellitus. Prim. Care Diabetes 2023, 17, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Wang, Y.; Hao, Q.; Vandvik, P.O.; Guyatt, G.; Li, J.; Chen, Z.; Xu, S.; Shen, Y.; Ge, L.; et al. Pharmacotherapy for Adults with Overweight and Obesity: A Systematic Review and Network Meta-Analysis of Randomised Controlled Trials. Lancet 2024, 403, e21–e31. [Google Scholar] [CrossRef] [PubMed]
- McLean, B.A.; Wong, C.K.; Kabir, M.G.; Drucker, D.J. Glucagon-like Peptide-1 Receptor Tie2+ Cells Are Essential for the Cardioprotective Actions of Liraglutide in Mice with Experimental Myocardial Infarction. Mol. Metab. 2022, 66, 101641. [Google Scholar] [CrossRef] [PubMed]
- Richards, P.; Parker, H.E.; Adriaenssens, A.E.; Hodgson, J.M.; Cork, S.C.; Trapp, S.; Gribble, F.M.; Reimann, F. Identification and Characterization of GLP-1 Receptor–Expressing Cells Using a New Transgenic Mouse Model. Diabetes 2014, 63, 1224–1233. [Google Scholar] [CrossRef] [PubMed]
- Lubberding, A.F.; Veedfald, S.; Achter, J.S.; Nissen, S.D.; Soattin, L.; Sorrentino, A.; Vega, E.T.; Linz, B.; Eggertsen, C.H.E.; Mulvey, J.; et al. Glucagon-like Peptide-1 Increases Heart Rate by a Direct Action on the Sinus Node. Cardiovasc. Res. 2024, 120, 1427–1441. [Google Scholar] [CrossRef] [PubMed]
- Ban, K.; Noyan-Ashraf, M.H.; Hoefer, J.; Bolz, S.-S.; Drucker, D.J.; Husain, M. Cardioprotective and Vasodilatory Actions of Glucagon-Like Peptide 1 Receptor Are Mediated Through Both Glucagon-Like Peptide 1 Receptor–Dependent and –Independent Pathways. Circulation 2008, 117, 2340–2350. [Google Scholar] [CrossRef] [PubMed]
- Pyke, C.; Heller, R.S.; Kirk, R.K.; Ørskov, C.; Reedtz-Runge, S.; Kaastrup, P.; Hvelplund, A.; Bardram, L.; Calatayud, D.; Knudsen, L.B. GLP-1 Receptor Localization in Monkey and Human Tissue: Novel Distribution Revealed with Extensively Validated Monoclonal Antibody. Endocrinology 2014, 155, 1280–1290. [Google Scholar] [CrossRef] [PubMed]
- Helmstädter, J.; Keppeler, K.; Küster, L.; Münzel, T.; Daiber, A.; Steven, S. Glucagon-like Peptide-1 (GLP-1) Receptor Agonists and Their Cardiovascular Benefits—The Role of the GLP-1 Receptor. Br. J. Pharmacol. 2022, 179, 659–676. [Google Scholar] [CrossRef] [PubMed]
- Nizari, S.; Basalay, M.; Chapman, P.; Korte, N.; Korsak, A.; Christie, I.N.; Theparambil, S.M.; Davidson, S.M.; Reimann, F.; Trapp, S.; et al. Glucagon-like Peptide-1 (GLP-1) Receptor Activation Dilates Cerebral Arterioles, Increases Cerebral Blood Flow, and Mediates Remote (Pre)Conditioning Neuroprotection against Ischaemic Stroke. Basic Res. Cardiol. 2021, 116, 32. [Google Scholar] [CrossRef] [PubMed]
- Pujadas, G.; Drucker, D.J. Vascular Biology of Glucagon Receptor Superfamily Peptides: Mechanistic and Clinical Relevance. Endocr. Rev. 2016, 37, 554–583. [Google Scholar] [CrossRef] [PubMed]
- 2025 Broad Institute of MIT and Harvard Gene Symbol Gencode ID Entrez Gene ID Location Gene Description GLP1R ENSG00000112164.6 2740 chr6:39048781-39091303:+ Glucagon Like Peptide 1 Receptor [Source:HGNC Symbol;Acc:HGNC:4324]. Available online: https://gtexportal.org/home/gene/GLP1R#geneExpression (accessed on 10 January 2025).
- Deacon, C.F. Circulation and Degradation of GIP and GLP-1. Horm. Metab. Res. 2004, 36, 761–765. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Quast, D.R.; Wefers, J.; Meier, J.J. GLP-1 Receptor Agonists in the Treatment of Type 2 Diabetes—State-of-the-Art. Mol. Metab. 2021, 46, 101102. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.-H.; Yu, T.; Lee, D.H. The Nonglycemic Actions of Dipeptidyl Peptidase-4 Inhibitors. Biomed. Res. Int. 2014, 2014, 368703. [Google Scholar] [CrossRef] [PubMed]
- Deacon, C.F. Physiology and Pharmacology of DPP-4 in Glucose Homeostasis and the Treatment of Type 2 Diabetes. Front. Endocrinol. 2019, 10, 80. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.J. Dipeptidyl Peptidase-4 Inhibition and the Treatment of Type 2 Diabetes: Preclinical Biology and Mechanisms of Action. Diabetes Care 2007, 30, 1335–1343. [Google Scholar] [CrossRef] [PubMed]
- Deacon, C.F. Dipeptidyl Peptidase-4 Inhibitors in the Treatment of Type 2 Diabetes: A Comparative Review. Diabetes Obes. Metab. 2011, 13, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Duez, H.; Cariou, B.; Staels, B. DPP-4 Inhibitors in the Treatment of Type 2 Diabetes. Biochem. Pharmacol. 2012, 83, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Scheen, A.J. DPP-4 Inhibitors in the Management of Type 2 Diabetes: A Critical Review of Head-to-Head Trials. Diabetes Metab. 2012, 38, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Shah, Z.; Kampfrath, T.; Deiuliis, J.A.; Zhong, J.; Pineda, C.; Ying, Z.; Xu, X.; Lu, B.; Moffatt-Bruce, S.; Durairaj, R.; et al. Long-Term Dipeptidyl-Peptidase 4 Inhibition Reduces Atherosclerosis and Inflammation via Effects on Monocyte Recruitment and Chemotaxis. Circulation 2011, 124, 2338–2349. [Google Scholar] [CrossRef] [PubMed]
- Dao, K.; Shechtman, S.; Weber-Schoendorfer, C.; Diav-Citrin, O.; Murad, R.H.; Berlin, M.; Hazan, A.; Richardson, J.L.; Eleftheriou, G.; Rousson, V.; et al. Use of GLP1 Receptor Agonists in Early Pregnancy and Reproductive Safety: A Multicentre, Observational, Prospective Cohort Study Based on the Databases of Six Teratology Information Services. BMJ Open 2024, 14, e083550. [Google Scholar] [CrossRef] [PubMed]
- Parkes, D.G.; Mace, K.F.; Trautmann, M.E. Discovery and Development of Exenatide: The First Antidiabetic Agent to Leverage the Multiple Benefits of the Incretin Hormone, GLP-1. Expert Opin. Drug Discov. 2013, 8, 219–244. [Google Scholar] [CrossRef] [PubMed]
- Zummo, F.P.; Cullen, K.S.; Honkanen-Scott, M.; Shaw, J.A.M.; Lovat, P.E.; Arden, C. Glucagon-Like Peptide 1 Protects Pancreatic β-Cells From Death by Increasing Autophagic Flux and Restoring Lysosomal Function. Diabetes 2017, 66, 1272–1285. [Google Scholar] [CrossRef] [PubMed]
- Lingvay, I.; Benamar, M.; Chen, L.; Fu, A.; Jódar, E.; Nishida, T.; Riveline, J.-P.; Yabe, D.; Zueger, T.; Réa, R. Once-Weekly IcoSema versus Once-Weekly Semaglutide in Adults with Type 2 Diabetes: The COMBINE 2 Randomised Clinical Trial. Diabetologia 2025, 68, 739–751. [Google Scholar] [CrossRef] [PubMed]
- Seetharaman, R. IcoSema’s Leap Forward: New Data from COMBINE 3 Paves the Way. J. Basic. Clin. Physiol. Pharmacol. 2024, 35, 323–324. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Cheng, Z.; Chen, J.; Zhang, X.; Liu, D.; Jiang, H.; Ma, G.; Wang, X.; Gan, S.; Sun, J.; et al. Efficacy and Safety of Mazdutide in Chinese Patients with Type 2 Diabetes: A Randomized, Double-Blind, Placebo-Controlled Phase 2 Trial. Diabetes Care 2024, 47, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Jiang, H.; Cheng, Z.; Qiu, W.; Liao, L.; Zhang, Y.; Li, X.; Pang, S.; Zhang, L.; Chen, L.; et al. A Phase 2 Randomised Controlled Trial of Mazdutide in Chinese Overweight Adults or Adults with Obesity. Nat. Commun. 2023, 14, 8289. [Google Scholar] [CrossRef] [PubMed]
- Frias, J.P.; Deenadayalan, S.; Erichsen, L.; Knop, F.K.; Lingvay, I.; Macura, S.; Mathieu, C.; Pedersen, S.D.; Davies, M. Efficacy and Safety of Co-Administered Once-Weekly Cagrilintide 2·4 Mg with Once-Weekly Semaglutide 2·4 Mg in Type 2 Diabetes: A Multicentre, Randomised, Double-Blind, Active-Controlled, Phase 2 Trial. Lancet 2023, 402, 720–730. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Zhang, A.; Li, D.; Wu, Y.; Wang, C.-Z.; Wan, J.-Y.; Yuan, C.-S. Comparative Effectiveness of GLP-1 Receptor Agonists on Glycaemic Control, Body Weight, and Lipid Profile for Type 2 Diabetes: Systematic Review and Network Meta-Analysis. BMJ 2024, 384, e076410. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.J.; Kaplan, L.M.; Frias, J.P.; Brouwers, B.; Wu, Q.; Thomas, M.K.; Harris, C.; Schloot, N.C.; Du, Y.; Mather, K.J.; et al. Triple Hormone Receptor Agonist Retatrutide for Metabolic Dysfunction-Associated Steatotic Liver Disease: A Randomized Phase 2a Trial. Nat. Med. 2024, 30, 2037–2048. [Google Scholar] [CrossRef] [PubMed]
- Le Roux, C.W.; Steen, O.; Lucas, K.J.; Startseva, E.; Unseld, A.; Hennige, A.M. Glucagon and GLP-1 Receptor Dual Agonist Survodutide for Obesity: A Randomised, Double-Blind, Placebo-Controlled, Dose-Finding Phase 2 Trial. Lancet Diabetes Endocrinol. 2024, 12, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Xiao, H.; Yang, K.; Li, J.; Ye, S.; Liu, Y.; Jing, S.; Lin, Y.; Yang, Y.; Huang, L.; et al. Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of TG103 Injection in Participants Who Are Overweight or Obese: A Randomized, Double-Blind, Placebo-Controlled, Multiple-Dose Phase 1b Study. BMC Med. 2024, 22, 209. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, L.L.; Young, A.A.; Parkes, D.G. Pharmacology of Exenatide (Synthetic Exendin-4): A Potential Therapeutic for Improved Glycemic Control of Type 2 Diabetes. Regul. Pept. 2004, 117, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.-J.; Shin, H.-J.; Jeong, E.-A.; An, H.-S.; Lee, J.-Y.; Jang, H.-M.; Kim, K.-E.; Lee, J.; Shin, M.-C.; Roh, G.-S. Exendin-4 Pretreatment Attenuates Kainic Acid-Induced Hippocampal Neuronal Death. Cells 2021, 10, 2527. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, H.; Ahmadi, M.; Aslani, S.; Saberianpour, S.; Rahbarghazi, R. Exendin-4 as a Versatile Therapeutic Agent for the Amelioration of Diabetic Changes. Adv. Pharm. Bull. 2021, 12, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V. Glucagon-like Peptide-1 Analogues: An Overview. Indian. J. Endocrinol. Metab. 2013, 17, 413. [Google Scholar] [CrossRef] [PubMed]
- Górriz, J.L.; Soler, M.J.; Navarro-González, J.F.; García-Carro, C.; Puchades, M.J.; D’Marco, L.; Martínez Castelao, A.; Fernández-Fernández, B.; Ortiz, A.; Górriz-Zambrano, C.; et al. GLP-1 Receptor Agonists and Diabetic Kidney Disease: A Call of Attention to Nephrologists. J. Clin. Med. 2020, 9, 947. [Google Scholar] [CrossRef] [PubMed]
- Kasina, S.V.S.K.; Baradhi, K.M. Dipeptidyl Peptidase IV (DPP IV) Inhibitors. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Neumiller, J.J. Differential Chemistry (Structure), Mechanism of Action, and Pharmacology of GLP-1 Receptor Agonists and DPP-4 Inhibitors. J. Am. Pharm. Assoc. 2009, 49 (Suppl. S1), S16–S29. [Google Scholar] [CrossRef] [PubMed]
- Deacon, C.F.; Mannucci, E.; Ahrén, B. Glycaemic Efficacy of Glucagon-like Peptide-1 Receptor Agonists and Dipeptidyl Peptidase-4 Inhibitors as Add-on Therapy to Metformin in Subjects with Type 2 Diabetes-a Review and Meta Analysis. Diabetes Obes. Metab. 2012, 14, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Tran, S.; Retnakaran, R.; Zinman, B.; Kramer, C.K. Efficacy of Glucagon-like Peptide-1 Receptor Agonists Compared to Dipeptidyl Peptidase-4 Inhibitors for the Management of Type 2 Diabetes: A Meta-Analysis of Randomized Clinical Trials. Diabetes Obes. Metab. 2018, 20 (Suppl. S1), 68–76. [Google Scholar] [CrossRef] [PubMed]
- Morales, J. The Pharmacologic Basis for Clinical Differences among GLP-1 Receptor Agonists and DPP-4 Inhibitors. Postgrad. Med. 2011, 123, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Farngren, J.; Ahrén, B. Incretin-Based Medications (GLP-1 Receptor Agonists, DPP-4 Inhibitors) as a Means to Avoid Hypoglycaemic Episodes. Metabolism 2019, 99, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Scheen, A.J. GLP-1 Receptor Agonists or DPP-4 Inhibitors: How to Guide the Clinician? Ann. Endocrinol. 2013, 74, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.P.; Pratley, R.E. GLP-1 Analogs and DPP-4 Inhibitors in Type 2 Diabetes Therapy: Review of Head-to-Head Clinical Trials. Front. Endocrinol. 2020, 11, 178. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiang, L.; Wang, J.; Wang, T.; Chien, C.; Huang, W.; Fu, X.; Xiao, Y.; Fu, Q.; Wang, S.; et al. Network Meta-Analysis on the Effects of Finerenone versus SGLT2 Inhibitors and GLP-1 Receptor Agonists on Cardiovascular and Renal Outcomes in Patients with Type 2 Diabetes Mellitus and Chronic Kidney Disease. Cardiovasc. Diabetol. 2022, 21, 232. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.S.-H.; Lee, J.-K.; Hung, C.-S.; Chen, W.-J. The Efficacy and Safety of Novel Classes of Glucose-Lowering Drugs for Cardiovascular Outcomes: A Network Meta-Analysis of Randomised Clinical Trials. Diabetologia 2021, 64, 2676–2686. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.; Kozlovski, P.; Paldánius, P.M.; Foley, J.E.; Bhosekar, V.; Serban, C.; Avogaro, A. Factors That May Account for Cardiovascular Risk Reduction with a Dipeptidyl Peptidase-4 Inhibitor, Vildagliptin, in Young Patients with Type 2 Diabetes Mellitus. Diabetes Ther. 2018, 9, 27–36. [Google Scholar] [CrossRef] [PubMed]
- McInnes, G.; Evans, M.; Del Prato, S.; Stumvoll, M.; Schweizer, A.; Lukashevich, V.; Shao, Q.; Kothny, W. Cardiovascular and Heart Failure Safety Profile of Vildagliptin: A Meta-analysis of 17 000 Patients. Diabetes Obes. Metab. 2015, 17, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Sohn, M.; Dietrich, J.W.; Nauck, M.A.; Lim, S. Characteristics Predicting the Efficacy of SGLT-2 Inhibitors versus GLP-1 Receptor Agonists on Major Adverse Cardiovascular Events in Type 2 Diabetes Mellitus: A Meta-Analysis Study. Cardiovasc. Diabetol. 2023, 22, 153. [Google Scholar] [CrossRef] [PubMed]
- Baviera, M.; Foresta, A.; Colacioppo, P.; Macaluso, G.; Roncaglioni, M.C.; Tettamanti, M.; Fortino, I.; Genovese, S.; Caruso, I.; Giorgino, F. Effectiveness and Safety of GLP-1 Receptor Agonists versus SGLT-2 Inhibitors in Type 2 Diabetes: An Italian Cohort Study. Cardiovasc. Diabetol. 2022, 21, 162. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee. 10. Cardiovascular Disease and Risk Management: Standards of Care in Diabetes-2025. Diabetes Care 2025, 48, S207–S238. [Google Scholar] [CrossRef] [PubMed]
- Kan, M.; Fu, H.; Xu, Y.; Yue, Z.; Du, B.; Chen, Q.; Wang, X.; Yu, S.; Zhang, Z. Effects of Once-weekly Glucagon-like Peptide-1 Receptor Agonists on Type 2 Diabetes Mellitus Complicated with Coronary Artery Disease: Potential Role of the Renin-angiotensin System. Diabetes Obes. Metab. 2023, 25, 3223–3234. [Google Scholar] [CrossRef] [PubMed]
- Gerstein, H.C.; Colhoun, H.M.; Dagenais, G.R.; Diaz, R.; Lakshmanan, M.; Pais, P.; Probstfield, J.; Riesmeyer, J.S.; Riddle, M.C.; Rydén, L.; et al. Dulaglutide and Cardiovascular Outcomes in Type 2 Diabetes (REWIND): A Double-Blind, Randomised Placebo-Controlled Trial. Lancet 2019, 394, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Riley, D.R.; Essa, H.; Austin, P.; Preston, F.; Kargbo, I.; Ibarburu, G.H.; Ghuman, R.; Cuthbertson, D.J.; Lip, G.Y.H.; Alam, U. All-cause Mortality and Cardiovascular Outcomes with SODIUM-GLUCOSE Co-transporter 2 Inhibitors, Glucagon-like Peptide-1 Receptor Agonists and with Combination Therapy in People with Type 2 Diabetes. Diabetes Obes. Metab. 2023, 25, 2897–2909. [Google Scholar] [CrossRef] [PubMed]
- Ansari, H.U.H.; Qazi, S.U.; Sajid, F.; Altaf, Z.; Ghazanfar, S.; Naveed, N.; Ashfaq, A.S.; Siddiqui, A.H.; Iqbal, H.; Qazi, S. Efficacy and Safety of Glucagon-Like Peptide-1 Receptor Agonists on Body Weight and Cardiometabolic Parameters in Individuals with Obesity and without Diabetes: A Systematic Review and Meta-Analysis. Endocr. Pract. 2024, 30, 160–171. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Almeida, G.; Nienkötter, T.F.; Balieiro, C.C.A.; Pasqualotto, E.; Cintra, J.B.; Carvalho, H.C.P.; Silva, A.L.S.; Kabariti, J.C.; Minucci, B.S.; Bertoli, E.D.; et al. Cardiovascular Benefits of GLP-1 Receptor Agonists in Patients Living with Obesity or Overweight: A Meta-Analysis of Randomized Controlled Trials. Am. J. Cardiovasc. Drugs 2024, 24, 509–521. [Google Scholar] [CrossRef] [PubMed]
- Grover, S.P.; Mackman, N. Tissue Factor in Atherosclerosis and Atherothrombosis. Atherosclerosis 2020, 307, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Viles-Gonzalez, J.F.; Fuster, V.; Badimon, J.J. Atherothrombosis: A Widespread Disease with Unpredictable and Life-Threatening Consequences. Eur. Heart J. 2004, 25, 1197–1207. [Google Scholar] [CrossRef] [PubMed]
- Jebari-Benslaiman, S.; Galicia-García, U.; Larrea-Sebal, A.; Olaetxea, J.R.; Alloza, I.; Vandenbroeck, K.; Benito-Vicente, A.; Martín, C. Pathophysiology of Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 3346. [Google Scholar] [CrossRef] [PubMed]
- Kahal, H.; Aburima, A.; Ungvari, T.; Rigby, A.S.; Coady, A.M.; Vince, R.V.; Ajjan, R.A.; Kilpatrick, E.S.; Naseem, K.M.; Atkin, S.L. The Effects of Treatment with Liraglutide on Atherothrombotic Risk in Obese Young Women with Polycystic Ovary Syndrome and Controls. BMC Endocr. Disord. 2015, 15, 14. [Google Scholar] [CrossRef] [PubMed]
- Luna-Marco, C.; De Marañon, A.M.; Hermo-Argibay, A.; Rodriguez-Hernandez, Y.; Hermenejildo, J.; Fernandez-Reyes, M.; Apostolova, N.; Vila, J.; Sola, E.; Morillas, C.; et al. Effects of GLP-1 Receptor Agonists on Mitochondrial Function, Inflammatory Markers and Leukocyte-Endothelium Interactions in Type 2 Diabetes. Redox Biol. 2023, 66, 102849. [Google Scholar] [CrossRef] [PubMed]
- Helmstädter, J.; Frenis, K.; Filippou, K.; Grill, A.; Dib, M.; Kalinovic, S.; Pawelke, F.; Kus, K.; Kröller-Schön, S.; Oelze, M.; et al. Endothelial GLP-1 (Glucagon-Like Peptide-1) Receptor Mediates Cardiovascular Protection by Liraglutide In Mice with Experimental Arterial Hypertension. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Rakipovski, G.; Rolin, B.; Nøhr, J.; Klewe, I.; Frederiksen, K.S.; Augustin, R.; Hecksher-Sørensen, J.; Ingvorsen, C.; Polex-Wolf, J.; Knudsen, L.B. The GLP-1 Analogs Liraglutide and Semaglutide Reduce Atherosclerosis in ApoE−/− and LDLr−/− Mice by a Mechanism That Includes Inflammatory Pathways. JACC Basic. Transl. Sci. 2018, 3, 844–857. [Google Scholar] [CrossRef] [PubMed]
- Violi, F.; Cammisotto, V.; Bartimoccia, S.; Pignatelli, P.; Carnevale, R.; Nocella, C. Gut-Derived Low-Grade Endotoxaemia, Atherothrombosis and Cardiovascular Disease. Nat. Rev. Cardiol. 2023, 20, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Possemiers, S.; Van de Wiele, T.; Guiot, Y.; Everard, A.; Rottier, O.; Geurts, L.; Naslain, D.; Neyrinck, A.; Lambert, D.M.; et al. Changes in Gut Microbiota Control Inflammation in Obese Mice through a Mechanism Involving GLP-2-Driven Improvement of Gut Permeability. Gut 2009, 58, 1091–1103. [Google Scholar] [CrossRef] [PubMed]
- Boutagy, N.E.; McMillan, R.P.; Frisard, M.I.; Hulver, M.W. Metabolic Endotoxemia with Obesity: Is It Real and Is It Relevant? Biochimie 2016, 124, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Zawieja, S.D.; Wang, W.; Lee, Y.; Wang, Y.J.; von der Weid, P.-Y.; Zawieja, D.C.; Muthuchamy, M. Lipopolysaccharide Modulates Neutrophil Recruitment and Macrophage Polarization on Lymphatic Vessels and Impairs Lymphatic Function in Rat Mesentery. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H2042–H2057. [Google Scholar] [CrossRef] [PubMed]
- Athapaththu, A.M.G.K.; Lee, K.T.; Kavinda, M.H.D.; Lee, S.; Kang, S.; Lee, M.-H.; Kang, C.-H.; Choi, Y.H.; Kim, G.-Y. Pinostrobin Ameliorates Lipopolysaccharide (LPS)-Induced Inflammation and Endotoxemia by Inhibiting LPS Binding to the TLR4/MD2 Complex. Biomed. Pharmacother. 2022, 156, 113874. [Google Scholar] [CrossRef] [PubMed]
- Steven, S.; Hausding, M.; Kröller-Schön, S.; Mader, M.; Mikhed, Y.; Stamm, P.; Zinßius, E.; Pfeffer, A.; Welschof, P.; Agdauletova, S.; et al. Gliptin and GLP-1 Analog Treatment Improves Survival and Vascular Inflammation/Dysfunction in Animals with Lipopolysaccharide-induced Endotoxemia. Basic Res. Cardiol. 2015, 110, 6. [Google Scholar] [CrossRef] [PubMed]
- Ying, W.; Meiyan, S.; Wen, C.; Kaizu, X.; Meifang, W.; Liming, L. Liraglutide Ameliorates Oxidized LDL-Induced Endothelial Dysfunction by GLP-1R-Dependent Downregulation of LOX-1-Mediated Oxidative Stress and Inflammation. Redox Rep. 2023, 28, 2218684. [Google Scholar] [CrossRef] [PubMed]
- Terasaki, M.; Yashima, H.; Mori, Y.; Saito, T.; Matsui, T.; Hiromura, M.; Kushima, H.; Osaka, N.; Ohara, M.; Fukui, T.; et al. A Dipeptidyl Peptidase-4 Inhibitor Inhibits Foam Cell Formation of Macrophages in Type 1 Diabetes via Suppression of CD36 and ACAT-1 Expression. Int. J. Mol. Sci. 2020, 21, 4811. [Google Scholar] [CrossRef] [PubMed]
- Herman, A.B.; Tsitsipatis, D.; Anerillas, C.; Mazan-Mamczarz, K.; Carr, A.E.; Gregg, J.M.; Wang, M.; Zhang, J.; Michel, M.; Henry-Smith, C.A.; et al. DPP4 Inhibition Impairs Senohemostasis to Improve Plaque Stability in Atherosclerotic Mice. J. Clin. Investig. 2023, 133, e165933. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Kim, S.H.; Monticone, R.E.; Lakatta, E.G. Matrix Metalloproteinases Promote Arterial Remodeling in Aging, Hypertension, and Atherosclerosis. Hypertension 2015, 65, 698–703. [Google Scholar] [CrossRef] [PubMed]
- Garczorz, W.; Gallego-Colon, E.; Kosowska, A.; Kłych-Ratuszny, A.; Woźniak, M.; Marcol, W.; Niesner, K.J.; Francuz, T. Exenatide Exhibits Anti-inflammatory Properties and Modulates Endothelial Response to Tumor Necrosis Factor A-mediated Activation. Cardiovasc. Ther. 2018, 36, e12317. [Google Scholar] [CrossRef] [PubMed]
- Maiolino, G.; Rossitto, G.; Caielli, P.; Bisogni, V.; Rossi, G.P.; Calò, L.A. The Role of Oxidized Low-Density Lipoproteins in Atherosclerosis: The Myths and the Facts. Mediat. Inflamm. 2013, 2013, 714653. [Google Scholar] [CrossRef] [PubMed]
- Wójcicka, G.; Zaręba, M.; Warpas, A.; Jamroz-Wiśniewska, A.; Rusek, M.; Czechowska, G.; Bełtowski, J. The Effect of Exenatide (a GLP-1 Analog) and Sitagliptin (a DPP-4 Inhibitor) on Plasma Platelet-Activating Factor Acetylhydrolase (PAF-AH) Activity and Concentration in Normal and Fructose-Fed Rats. Eur. J. Pharmacol. 2019, 850, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Pignatelli, P.; Baratta, F.; Buzzetti, R.; D’Amico, A.; Castellani, V.; Bartimoccia, S.; Siena, A.; D’Onofrio, L.; Maddaloni, E.; Pingitore, A.; et al. The Sodium-Glucose Co-Transporter-2 (SGLT2) Inhibitors Reduce Platelet Activation and Thrombus Formation by Lowering NOX2-Related Oxidative Stress: A Pilot Study. Antioxidants 2022, 11, 1878. [Google Scholar] [CrossRef] [PubMed]
- Oeseburg, H.; de Boer, R.A.; Buikema, H.; van der Harst, P.; van Gilst, W.H.; Silljé, H.H.W. Glucagon-like Peptide 1 Prevents Reactive Oxygen Species-Induced Endothelial Cell Senescence through the Activation of Protein Kinase A. Arter. Thromb. Vasc. Biol. 2010, 30, 1407–1414. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A.; Novials, A.; Ortega, E.; Canivell, S.; La Sala, L.; Pujadas, G.; Esposito, K.; Giugliano, D.; Genovese, S. Glucagon-like Peptide 1 Reduces Endothelial Dysfunction, Inflammation, and Oxidative Stress Induced by Both Hyperglycemia and Hypoglycemia in Type 1 Diabetes. Diabetes Care 2013, 36, 2346–2350. [Google Scholar] [CrossRef] [PubMed]
- Dave, C.V.; Kim, S.C.; Goldfine, A.B.; Glynn, R.J.; Tong, A.; Patorno, E. Risk of Cardiovascular Outcomes in Patients with Type 2 Diabetes After Addition of SGLT2 Inhibitors Versus Sulfonylureas to Baseline GLP-1RA Therapy. Circulation 2021, 143, 770–779. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.M.Y.; Kristensen, S.L.; Gerstein, H.C.; McMurray, J.J.V.; Sattar, N. Cardiovascular and Mortality Outcomes with GLP-1 Receptor Agonists in Patients with Type 2 Diabetes: A Meta-Analysis with the FREEDOM Cardiovascular Outcomes Trial. Diabetes Metab. Syndr. 2022, 16, 102382. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; She, M.; Xu, M.; Chen, H.; Li, J.; Chen, X.; Zheng, D.; Liu, J.; Chen, S.; Zhu, J.; et al. GLP-1 Treatment Protects Endothelial Cells from Oxidative Stress-Induced Autophagy and Endothelial Dysfunction. Int. J. Biol. Sci. 2018, 14, 1696–1708. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Yang, L.; Wang, S.; Liu, Y.; Yue, L.; Chen, S. Semaglutide Ameliorates Obesity-Induced Cardiac Inflammation and Oxidative Stress Mediated via Reduction of Neutrophil Cxcl2, S100a8, and S100a9 Expression. Mol. Cell Biochem. 2024, 479, 1133–1147. [Google Scholar] [CrossRef] [PubMed]
- Bułdak, Ł.; Łabuzek, K.; Bułdak, R.J.; Machnik, G.; Bołdys, A.; Okopień, B. Exenatide (a GLP-1 Agonist) Improves the Antioxidative Potential of in Vitro Cultured Human Monocytes/Macrophages. Naunyn Schmiedebergs Arch. Pharmacol. 2015, 388, 905–919. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Lu, D.; Tian, J.; Yu, Y.; Zhang, Q.; Zhang, L.; Chang, D. The Protective Effects of GLP-1 Receptor Agonist Lixisenatide on Oxygen-Glucose Deprivation/Reperfusion (OGD/R)-Induced Deregulation of Endothelial Tube Formation. RSC Adv. 2020, 10, 10245–10253. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.K.; Lau, C.W.; Tian, X.; Huang, Y. Abstract 18087: GLP-1 Receptor Agonist Exenatide Attenuates Endothelial Dysfunction by Reducing Oxidative Stress in Hyperhomocysteinemia via AMPK/eNOS Pathway. Circulation 2017, 136, A18087. [Google Scholar] [CrossRef]
- Gaspari, T.; HongBin, L.; Welungoda, I.; Yunshan, H.; Widdop, R.E.; Knudsen, L.B.; Simpson, R.W.; Dear, A.E. A GLP-1 Receptor Agonist Liraglutide Inhibits Endothelial Cell Dysfunction and Vascular Adhesion Molecule Expression in an ApoE-/- Mouse Model. Diabetes Vasc. Dis. Res. 2011, 8, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-M.; Huang, A.; Kaley, G.; Sun, D. eNOS Uncoupling and Endothelial Dysfunction in Aged Vessels. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1829–H1836. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Luo, P.; Wang, Y.; Li, W.; Wang, C.; Sun, D.; Zhang, R.; Su, T.; Ma, X.; Zeng, C.; et al. Glucagon-Like Peptide-1 Protects Against Cardiac Microvascular Injury in Diabetes via a cAMP/PKA/Rho-Dependent Mechanism. Diabetes 2013, 62, 1697–1708. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Aylor, K.W.; Liu, Z. Liraglutide and Exercise Synergistically Attenuate Vascular Inflammation and Enhance Metabolic Insulin Action in Early Diet-Induced Obesity. Diabetes 2023, 72, 918–931. [Google Scholar] [CrossRef] [PubMed]
- Münzel, T.; Daiber, A.; Ullrich, V.; Mülsch, A. Vascular Consequences of Endothelial Nitric Oxide Synthase Uncoupling for the Activity and Expression of the Soluble Guanylyl Cyclase and the cGMP-Dependent Protein Kinase. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Hogan, A.E.; Gaoatswe, G.; Lynch, L.; Corrigan, M.A.; Woods, C.; O’Connell, J.; O’Shea, D. Glucagon-like Peptide 1 Analogue Therapy Directly Modulates Innate Immune-Mediated Inflammation in Individuals with Type 2 Diabetes Mellitus. Diabetologia 2014, 57, 781–784. [Google Scholar] [CrossRef] [PubMed]
- Dabravolski, S.A.; Sukhorukov, V.N.; Kalmykov, V.A.; Grechko, A.V.; Shakhpazyan, N.K.; Orekhov, A.N. The Role of KLF2 in the Regulation of Atherosclerosis Development and Potential Use of KLF2-Targeted Therapy. Biomedicines 2022, 10, 254. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Zhang, Q.; Tang, H.; Wang, C.; Su, H.; Zhou, Q.; Wei, W.; Zhu, H.; Wang, Y. Effects of Glucagon-like Peptide-1 on Advanced Glycation Endproduct-Induced Aortic Endothelial Dysfunction in Streptozotocin-Induced Diabetic Rats: Possible Roles of Rho Kinase- and AMP Kinase-Mediated Nuclear Factor κB Signaling Pathways. Endocrine 2016, 53, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhang, H.; Schmidt, A.M.; Zhang, C. AGE/RAGE Produces Endothelial Dysfunction in Coronary Arterioles in Type 2 Diabetic Mice. Am. J. Physiol.-Heart Circ. Physiol. 2008, 295, H491–H498. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yu, J.; Fu, M.; Dong, R.; Yang, Y.; Luo, J.; Hu, S.; Li, W.; Xu, X.; Tu, L. Dipeptidyl Peptidase-4 Inhibition Improves Endothelial Senescence by Activating AMPK/SIRT1/Nrf2 Signaling Pathway. Biochem. Pharmacol. 2020, 177, 113951. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Huang, T.; Chen, A.; Chen, X.; Wang, L.; Shen, F.; Gu, X. Glucagon-like Peptide 1 Improves Insulin Resistance in Vitro through Anti-Inflammation of Macrophages. Braz. J. Med. Biol. Res. 2016, 49, e5826. [Google Scholar] [CrossRef] [PubMed]
- Brock, C.; Hansen, C.S.; Karmisholt, J.; Møller, H.J.; Juhl, A.; Farmer, A.D.; Drewes, A.M.; Riahi, S.; Lervang, H.H.; Jakobsen, P.E.; et al. Liraglutide Treatment Reduced Interleukin-6 in Adults with Type 1 Diabetes but Did Not Improve Established Autonomic or Polyneuropathy. Br. J. Clin. Pharmacol. 2019, 85, 2512–2523. [Google Scholar] [CrossRef] [PubMed]
- Von Scholten, B.J.; Persson, F.; Rosenlund, S.; Eugen-Olsen, J.; Pielak, T.; Faber, J.; Hansen, T.W.; Rossing, P. Effects of Liraglutide on Cardiovascular Risk Biomarkers in Patients with Type 2 Diabetes and Albuminuria: A Sub-analysis of a Randomized, Placebo-controlled, Double-blind, Crossover Trial. Diabetes Obes. Metab. 2017, 19, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, A.; Ghanim, H.; Vora, M.; Sia, C.L.; Korzeniewski, K.; Dhindsa, S.; Makdissi, A.; Dandona, P. Exenatide Exerts a Potent Antiinflammatory Effect. J. Clin. Endocrinol. Metab. 2012, 97, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Makdissi, A.; Ghanim, H.; Vora, M.; Green, K.; Abuaysheh, S.; Chaudhuri, A.; Dhindsa, S.; Dandona, P. Sitagliptin Exerts an Antinflammatory Action. J. Clin. Endocrinol. Metab. 2012, 97, 3333–3341. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Ji, Y.; Jiang, X.; Zhou, L.; Xu, Y.; Li, Y.; Jiang, W.; Meng, P.; Liu, X. Liraglutide Attenuates High Glucose-Induced Abnormal Cell Migration, Proliferation, and Apoptosis of Vascular Smooth Muscle Cells by Activating the GLP-1 Receptor, and Inhibiting ERK1/2 and PI3K/Akt Signaling Pathways. Cardiovasc. Diabetol. 2015, 14, 18. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, C.J.; Longenecker, J.Z.; Accornero, F. ERK1/2: An Integrator of Signals That Alters Cardiac Homeostasis and Growth. Biology 2021, 10, 346. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, M.; Mita, T.; Azuma, K.; Ebato, C.; Goto, H.; Nomiyama, T.; Fujitani, Y.; Hirose, T.; Kawamori, R.; Watada, H. Inhibition of Monocyte Adhesion to Endothelial Cells and Attenuation of Atherosclerotic Lesion by a Glucagon-like Peptide-1 Receptor Agonist, Exendin-4. Diabetes 2010, 59, 1030–1037. [Google Scholar] [CrossRef] [PubMed]
- Hirata, Y.; Kurobe, H.; Nishio, C.; Tanaka, K.; Fukuda, D.; Uematsu, E.; Nishimoto, S.; Soeki, T.; Harada, N.; Sakaue, H.; et al. Exendin-4, a Glucagon-like Peptide-1 Receptor Agonist, Attenuates Neointimal Hyperplasia after Vascular Injury. Eur. J. Pharmacol. 2013, 699, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Zhu, F.; Zheng, H.; Zhou, Z.; Miao, P.; Zhao, L.; Mao, Z. Glucagon-like Peptide-1 Receptor Agonist Dulaglutide Prevents Ox-LDL-Induced Adhesion of Monocytes to Human Endothelial Cells: An Implication in the Treatment of Atherosclerosis. Mol. Immunol. 2019, 116, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Boulmpou, A.; Patoulias, D.; Papadopoulos, C.E.; Teperikidis, E.; Doumas, M.; Vassilikos, V. Meta-Analysis of Cardiovascular Outcome Trials Assessing the Impact of Glucagon-like Peptide-1 Receptor Agonists on Major Cardiac Arrhythmias. Acta Cardiol. 2023, 78, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.-H.; Chao, T.-F.; Chen, S.-W.; Lee, H.-F.; Li, P.-R.; Chen, W.-M.; Yeh, Y.-H.; Kuo, C.-T.; See, L.-C.; Lip, G.Y.H. The Risk of Incident Atrial Fibrillation in Patients with Type 2 Diabetes Treated with Sodium Glucose Cotransporter-2 Inhibitors, Glucagon-like Peptide-1 Receptor Agonists, and Dipeptidyl Peptidase-4 Inhibitors: A Nationwide Cohort Study. Cardiovasc. Diabetol. 2022, 21, 118. [Google Scholar] [CrossRef] [PubMed]
- Lui, D.T.W.; Tang, E.H.M.; Wu, T.; Au, I.C.H.; Lee, C.H.; Woo, Y.C.; Tan, K.C.B.; Wong, C.K.H. Risks of Stroke, Its Subtypes and Atrial Fibrillation Associated with Glucagon-like Peptide 1 Receptor Agonists versus Sodium-Glucose Cotransporter 2 Inhibitors: A Real-World Population-Based Cohort Study in Hong Kong. Cardiovasc. Diabetol. 2023, 22, 40. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Chen, K.; Zhao, Y.; Xia, S. Effects of Liraglutide on Left Ventricular Function: A Meta-Analysis of Randomized, Placebo-Controlled Trials. Int. J. Endocrinol. 2021, 2021, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Thotamgari, S.R.; Grewal, U.S.; Sheth, A.R.; Babbili, A.; Dominic, P. Can Glucagon-like Peptide-1 Receptor Agonists and Dipeptidyl Peptidase-4 Inhibitors Help in Mitigating the Risk of Atrial Fibrillation in Patients with Diabetes? Cardiovasc. Endocrinol. Metab. 2022, 11, e0265. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Chen, X.; Ren, Q.; Yue, L.; Niu, S.; Li, Z.; Zhu, R.; Chen, X.; Jia, Z.; Zhen, R.; et al. Single-Cell Transcriptome Reveals Effects of Semaglutide on Non-Cardiomyocytes of Obese Mice. Biochem. Biophys. Res. Commun. 2022, 622, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Guo, R.; Ma, X.; Wang, Y.; Zhang, Q.; Zheng, N.; Zhang, J.; Li, C. Liraglutide Inhibits AngII-Induced Cardiac Fibroblast Proliferation and ECM Deposition through Regulating miR-21/PTEN/PI3K Pathway. Cell Tissue Bank. 2023, 24, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Surina, S.; Fontanella, R.A.; Scisciola, L.; Marfella, R.; Paolisso, G.; Barbieri, M. miR-21 in Human Cardiomyopathies. Front. Cardiovasc. Med. 2021, 8, 767064. [Google Scholar] [CrossRef] [PubMed]
- Wójcicka, G.; Pradiuch, A.; Fornal, E.; Stachniuk, A.; Korolczuk, A.; Marzec-Kotarska, B.; Nikolaichuk, H.; Czechowska, G.; Kozub, A.; Trzpil, A.; et al. The effect of exenatide (a GLP-1 analogue) and sitagliptin (a DPP-4 inhibitor) on asymmetric dimethylarginine (ADMA) metabolism and selected biomarkers of cardiac fibrosis in rats with fructose-induced metabolic syndrome. Biochem. Pharmacol. 2023, 214, 115637. [Google Scholar] [CrossRef] [PubMed]
- Heeringa, J.; Van Der Kuip, D.A.M.; Hofman, A.; Kors, J.A.; Van Herpen, G.; Stricker, B.H.C.; Stijnen, T.; Lip, G.Y.H.; Witteman, J.C.M. Prevalence, Incidence and Lifetime Risk of Atrial Fibrillation: The Rotterdam Study. Eur. Heart J. 2006, 27, 949–953. [Google Scholar] [CrossRef] [PubMed]
- Wattigney, W.A.; Mensah, G.A.; Croft, J.B. Increasing Trends in Hospitalization for Atrial Fibrillation in the United States, 1985 Through 1999: Implications for Primary Prevention. Circulation 2003, 108, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Polyakova, V.; Miyagawa, S.; Szalay, Z.; Risteli, J.; Kostin, S. Atrial Extracellular Matrix Remodelling in Patients with Atrial Fibrillation. J. Cell. Mol. Med. 2008, 12, 189–208. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Sanchis-Gomar, F.; Cervellin, G. Global Epidemiology of Atrial Fibrillation: An Increasing Epidemic and Public Health Challenge. Int. J. Stroke 2021, 16, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Bodin, A.; Bisson, A.; Gaborit, C.; Herbert, J.; Clementy, N.; Babuty, D.; Lip, G.Y.H.; Fauchier, L. Ischemic Stroke in Patients with Sinus Node Disease, Atrial Fibrillation, and Other Cardiac Conditions. Stroke 2020, 51, 1674–1681. [Google Scholar] [CrossRef] [PubMed]
- Palazzuoli, A.; Iacoviello, M. Diabetes Leading to Heart Failure and Heart Failure Leading to Diabetes: Epidemiological and Clinical Evidence. Heart Fail. Rev. 2022, 28, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.-P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef] [PubMed]
- Oh, G.C.; Cho, H.-J. Blood Pressure and Heart Failure. Clin. Hypertens. 2020, 26, 1. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Fonarow, G.C.; McGuire, D.K.; Hernandez, A.F.; Vaduganathan, M.; Rosenstock, J.; Handelsman, Y.; Verma, S.; Anker, S.D.; McMurray, J.J.V.; et al. Glucagon-Like Peptide 1 Receptor Agonists and Heart Failure: The Need for Further Evidence Generation and Practice Guidelines Optimization. Circulation 2020, 142, 1205–1218. [Google Scholar] [CrossRef] [PubMed]
- Kreiner, F.F.; Hovingh, G.K.K.; Von Scholten, B.J. The Potential of Glucagon-like Peptide-1 Receptor Agonists in Heart Failure. Front. Physiol. 2022, 13, 983961. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.P.; Sharma, A.; Butler, J.; Packer, M.; Zannad, F.; Vasques-Nóvoa, F.; Leite-Moreira, A.; Neves, J.S. Glucagon-Like Peptide-1 Receptor Agonists Across the Spectrum of Heart Failure. J. Clin. Endocrinol. Metab. 2023, 109, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Lincoff, A.M.; Brown-Frandsen, K.; Colhoun, H.M.; Deanfield, J.; Emerson, S.S.; Esbjerg, S.; Hardt-Lindberg, S.; Hovingh, G.K.; Kahn, S.E.; Kushner, R.F.; et al. Semaglutide and Cardiovascular Outcomes in Obesity without Diabetes. N. Engl. J. Med. 2023, 389, 2221–2232. [Google Scholar] [CrossRef] [PubMed]
- Monami, M.; Zannoni, S.; Pala, L.; Silverii, A.; Andreozzi, F.; Sesti, G.; Mannucci, E. Effects of Glucagon-like Peptide-1 Receptor Agonists on Mortality and Cardiovascular Events: A Comprehensive Meta-Analysis of Randomized Controlled Trials. Int. J. Cardiol. 2017, 240, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Scirica, B.M.; Bhatt, D.L.; Braunwald, E.; Steg, P.G.; Davidson, J.; Hirshberg, B.; Ohman, P.; Frederich, R.; Wiviott, S.D.; Hoffman, E.B.; et al. Saxagliptin and Cardiovascular Outcomes in Patients with Type 2 Diabetes Mellitus. N. Engl. J. Med. 2013, 369, 1317–1326. [Google Scholar] [CrossRef] [PubMed]
- Zannad, F.; Cannon, C.P.; Cushman, W.C.; Bakris, G.L.; Menon, V.; Perez, A.T.; Fleck, P.R.; Mehta, C.R.; Kupfer, S.; Wilson, C.; et al. Heart Failure and Mortality Outcomes in Patients with Type 2 Diabetes Taking Alogliptin versus Placebo in EXAMINE: A Multicentre, Randomised, Double-Blind Trial. Lancet 2015, 385, 2067–2076. [Google Scholar] [CrossRef] [PubMed]
- White, W.B.; Cannon, C.P.; Heller, S.R.; Nissen, S.E.; Bergenstal, R.M.; Bakris, G.L.; Perez, A.T.; Fleck, P.R.; Mehta, C.R.; Kupfer, S.; et al. Alogliptin after Acute Coronary Syndrome in Patients with Type 2 Diabetes. N. Engl. J. Med. 2013, 369, 1327–1335. [Google Scholar] [CrossRef] [PubMed]
- The U.S. Food and Drug Administration FDA Drug Safety Communication: FDA Adds Warnings about Heart Failure Risk to Labels of Type 2 Diabetes Medicines Containing Saxagliptin and Alogliptin. Available online: https://www.fda.gov/about-fda/fda-organization (accessed on 10 January 2025).
- Williams, R.; De Vries, F.; Kothny, W.; Serban, C.; Lopez-Leon, S.; Chu, C.; Schlienger, R. Cardiovascular Safety of Vildagliptin in Patients with Type 2 Diabetes: A European Multi-database, Non-interventional Post-authorization Safety Study. Diabetes Obes. Metab. 2017, 19, 1473–1478. [Google Scholar] [CrossRef] [PubMed]
- Rosenstock, J.; Kahn, S.E.; Johansen, O.E.; Zinman, B.; Espeland, M.A.; Woerle, H.J.; Pfarr, E.; Keller, A.; Mattheus, M.; Baanstra, D.; et al. Effect of Linagliptin vs Glimepiride on Major Adverse Cardiovascular Outcomes in Patients with Type 2 Diabetes: The CAROLINA Randomized Clinical Trial. JAMA 2019, 322, 1155. [Google Scholar] [CrossRef] [PubMed]
- Rosenstock, J.; Perkovic, V.; Johansen, O.E.; Cooper, M.E.; Kahn, S.E.; Marx, N.; Alexander, J.H.; Pencina, M.; Toto, R.D.; Wanner, C.; et al. Effect of Linagliptin vs Placebo on Major Cardiovascular Events in Adults with Type 2 Diabetes and High Cardiovascular and Renal Risk: The CARMELINA Randomized Clinical Trial. JAMA 2019, 321, 69. [Google Scholar] [CrossRef] [PubMed]
- Green, J.B.; Bethel, M.A.; Armstrong, P.W.; Buse, J.B.; Engel, S.S.; Garg, J.; Josse, R.; Kaufman, K.D.; Koglin, J.; Korn, S.; et al. Effect of Sitagliptin on Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Neuen, B.L.; Heerspink, H.J.L.; Vart, P.; Claggett, B.L.; Fletcher, R.A.; Arnott, C.; De Oliveira Costa, J.; Falster, M.O.; Pearson, S.-A.; Mahaffey, K.W.; et al. Estimated Lifetime Cardiovascular, Kidney, and Mortality Benefits of Combination Treatment with SGLT2 Inhibitors, GLP-1 Receptor Agonists, and Nonsteroidal MRA Compared with Conventional Care in Patients with Type 2 Diabetes and Albuminuria. Circulation 2024, 149, 450–462. [Google Scholar] [CrossRef] [PubMed]
- Brønden, A.; Christensen, M.B.; Glintborg, D.; Snorgaard, O.; Kofoed-Enevoldsen, A.; Madsen, G.K.; Toft, K.; Kristensen, J.K.; Højlund, K.; Hansen, T.K.; et al. Effects of DPP-4 Inhibitors, GLP-1 Receptor Agonists, SGLT-2 Inhibitors and Sulphonylureas on Mortality, Cardiovascular and Renal Outcomes in Type 2 Diabetes: A Network Meta-analyses-driven Approach. Diabet. Med. 2023, 40, e15157. [Google Scholar] [CrossRef] [PubMed]
- van Poppel, P.C.M.; Netea, M.G.; Smits, P.; Tack, C.J. Vildagliptin Improves Endothelium-Dependent Vasodilatation in Type 2 Diabetes. Diabetes Care 2011, 34, 2072–2077. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McMurray, J.J.V.; Ponikowski, P.; Bolli, G.B.; Lukashevich, V.; Kozlovski, P.; Kothny, W.; Lewsey, J.D.; Krum, H. Effects of Vildagliptin on Ventricular Function in Patients with Type 2 Diabetes Mellitus and Heart Failure. JACC Heart Fail. 2018, 6, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.J.; Byiers, S.; Carr, D.; Maldonado, M.; Warner, B.A. Dipeptidyl Peptidase-IV Inhibitor Use Associated with Increased Risk of ACE Inhibitor-Associated Angioedema. Hypertension 2009, 54, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Lyu, B.; Grams, M.E.; Chang, A.; Inker, L.A.; Coresh, J.; Shin, J.-I. Sodium-Glucose Cotransporter 2 Inhibitors, Glucagon-Like Peptide-1 Receptor Agonists, and Dipeptidyl Peptidase-4 Inhibitors, and Risk of Hospitalization. Am. J. Cardiol. 2022, 165, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Giugliano, D.; Longo, M.; Signoriello, S.; Maiorino, M.I.; Solerte, B.; Chiodini, P.; Esposito, K. The Effect of DPP-4 Inhibitors, GLP-1 Receptor Agonists and SGLT-2 Inhibitors on Cardiorenal Outcomes: A Network Meta-Analysis of 23 CVOTs. Cardiovasc. Diabetol. 2022, 21, 42. [Google Scholar] [CrossRef] [PubMed]
- Kutz, A.; Kim, D.H.; Wexler, D.J.; Liu, J.; Schneeweiss, S.; Glynn, R.J.; Patorno, E. Comparative Cardiovascular Effectiveness and Safety of SGLT-2 Inhibitors, GLP-1 Receptor Agonists, and DPP-4 Inhibitors According to Frailty in Type 2 Diabetes. Diabetes Care 2023, 46, 2004–2014. [Google Scholar] [CrossRef] [PubMed]
- Goldman, A.; Fishman, B.; Twig, G.; Raschi, E.; Cukierman-Yaffe, T.; Moshkovits, Y.; Pomerantz, A.; Ben-Zvi, I.; Dankner, R.; Maor, E. The Real-World Safety Profile of Sodium-Glucose Co-Transporter-2 Inhibitors among Older Adults (≥75 Years): A Retrospective, Pharmacovigilance Study. Cardiovasc. Diabetol. 2023, 22, 16. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Luo, Y.; Wen, Y.; Wang, D.; Li, J.; Fan, Z. Semaglutide Inhibits Ischemia/Reperfusion-Induced Cardiomyocyte Apoptosis through Activating PKG/PKCε/ERK1/2 Pathway. Biochem. Biophys. Res. Commun. 2023, 647, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kinnunen, P.; Vuolteenaho, O.; Ruskoaho, H. Mechanisms of Atrial and Brain Natriuretic Peptide Release from Rat Ventricular Myocardium: Effect of Stretching. Endocrinology 1993, 132, 1961–1970. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, K. The Natriuretic Peptide System in Heart Failure: Diagnostic and Therapeutic Implications. Pharmacol. Ther. 2021, 227, 107863. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Zuo, Y.; Cui, X.; Zhang, M.; Jin, H.; Hong, L. Effects of Liraglutide on ANP Secretion and Cardiac Dynamics. Endocr. Connect. 2023, 12, e230176. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, R.; Jorsal, A.; Tougaard, R.S.; Rasmussen, J.J.; Schou, M.; Videbæk, L.; Gustafsson, I.; Faber, J.; Flyvbjerg, A.; Wiggers, H.; et al. The Impact of the Glucagon-like Peptide-1 Receptor Agonist Liraglutide on Natriuretic Peptides in Heart Failure Patients with Reduced Ejection Fraction with and without Type 2 Diabetes. Diabetes Obes. Metab. 2020, 22, 2141–2150. [Google Scholar] [CrossRef] [PubMed]
- Lambadiari, V.; Pavlidis, G.; Kousathana, F.; Varoudi, M.; Vlastos, D.; Maratou, E.; Georgiou, D.; Andreadou, I.; Parissis, J.; Triantafyllidi, H.; et al. Effects of 6-Month Treatment with the Glucagon like Peptide-1 Analogue Liraglutide on Arterial Stiffness, Left Ventricular Myocardial Deformation and Oxidative Stress in Subjects with Newly Diagnosed Type 2 Diabetes. Cardiovasc. Diabetol. 2018, 17, 8. [Google Scholar] [CrossRef] [PubMed]
- Connelly, K.A.; Zhang, Y.; Advani, A.; Advani, S.L.; Thai, K.; Yuen, D.A.; Gilbert, R.E. DPP-4 Inhibition Attenuates Cardiac Dysfunction and Adverse Remodeling Following Myocardial Infarction in Rats with Experimental Diabetes. Cardiovasc. Ther. 2013, 31, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Silljé, H.H.; Meissner, M.; Van Gilst, W.H.; De Boer, R.A. Early and Late Effects of the DPP-4 Inhibitor Vildagliptin in a Rat Model of Post-Myocardial Infarction Heart Failure. Cardiovasc. Diabetol. 2011, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- Nishikido, T.; Oyama, J.; Ohira, H.; Node, K. The Effects and Safety of Vildagliptin on Cardiac Function after Acute Myocardial Infarction. Int. J. Cardiol. 2015, 188, 13–15. [Google Scholar] [CrossRef] [PubMed]
- Bottomley, P.A.; Panjrath, G.S.; Lai, S.; Hirsch, G.A.; Wu, K.; Najjar, S.S.; Steinberg, A.; Gerstenblith, G.; Weiss, R.G. Metabolic Rates of ATP Transfer Through Creatine Kinase (CK Flux) Predict Clinical Heart Failure Events and Death. Sci. Transl. Med. 2013, 5, 215re3. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Zhang, M.; Shi, W.; Xing, Y.; Huang, Y.; Fang, W.; Liu, S.; Chen, M.; Zhang, T.; Chen, S.; et al. Long-Term Activation of Glucagon-like Peptide-1 Receptor by Dulaglutide Prevents Diabetic Heart Failure and Metabolic Remodeling in Type 2 Diabetes. J. Am. Heart Assoc. 2022, 11, e026728. [Google Scholar] [CrossRef] [PubMed]
- Jahani-Asl, A.; Slack, R.S. The Phosphorylation State of Drp1 Determines Cell Fate. EMBO Rep. 2007, 8, 912–913. [Google Scholar] [CrossRef] [PubMed]
- Tseng, H.-C.; Lin, C.-C.; Hsiao, L.-D.; Yang, C.-M. Lysophosphatidylcholine-Induced Mitochondrial Fission Contributes to Collagen Production in Human Cardiac Fibroblasts. J. Lipid Res. 2019, 60, 1573–1589. [Google Scholar] [CrossRef] [PubMed]
- Hasan, P.; Saotome, M.; Ikoma, T.; Iguchi, K.; Kawasaki, H.; Iwashita, T.; Hayashi, H.; Maekawa, Y. Mitochondrial Fission Protein, Dynamin-Related Protein 1, Contributes to the Promotion of Hypertensive Cardiac Hypertrophy and Fibrosis in Dahl-Salt Sensitive Rats. J. Mol. Cell. Cardiol. 2018, 121, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Durak, A.; Turan, B. Liraglutide Provides Cardioprotection through the Recovery of Mitochondrial Dysfunction and Oxidative Stress in Aging Hearts. J. Physiol. Biochem. 2023, 79, 297–311. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, S.; Siraj, M.A.; Chen, W.; Wang, J.; Parker, M.; Nagy, A.; Steve Fan, C.-P.; Runeckles, K.; Li, J.; Kobayashi, J.; et al. Cardioprotective Actions of a Glucagon-like Peptide-1 Receptor Agonist on Hearts Donated After Circulatory Death. J. Am. Heart Assoc. 2023, 12, e027163. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention National Center for Health Statistics Mortality Data on CDC WONDER. Available online: https://wonder.cdc.gov/mcd.html (accessed on 10 January 2025).
- Sharifi-Rad, J.; Rodrigues, C.F.; Sharopov, F.; Docea, A.O.; Can Karaca, A.; Sharifi-Rad, M.; Kahveci Karıncaoglu, D.; Gülseren, G.; Şenol, E.; Demircan, E.; et al. Diet, Lifestyle and Cardiovascular Diseases: Linking Pathophysiology to Cardioprotective Effects of Natural Bioactive Compounds. Int. J. Env. Res. Public. Health 2020, 17, 2326. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Zong, Y.; Ma, Y.; Tian, Y.; Pang, Y.; Zhang, C.; Gao, J. Glucagon-like Peptide-1 Receptor: Mechanisms and Advances in Therapy. Sig Transduct. Target. Ther. 2024, 9, 234. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, n71, 71. [Google Scholar] [CrossRef] [PubMed]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.; Thomas, J. Updated Guidance for Trusted Systematic Reviews: A New Edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 10, ED000142. [Google Scholar] [CrossRef] [PubMed]


| DPP-4is | GLP-1 Ras | |
|---|---|---|
| MACE | No significant MACE reduction; possible beneficial effects in patients younger than 65 years | Proven MACE reduction |
| Atherosclerosis and atherothrombosis |
= lower cardiovascular risk. However, fewer studies with proven molecular effects. |
|
| Cardiac arrhythmias |
Important: might be associated with the highest proportion of AF events in diabetic patients. |
Could potentially be an alternative or adjunctive treatment for cardiac fibrosis-related conditions. |
| Myocardial infarction and heart failure |
|
Lower risk of hospitalization due to cardiovascular disease in T2DM patients; recommended for lowering the risk of MI. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Król, M.; Kupnicka, P.; Żychowska, J.; Kapczuk, P.; Szućko-Kociuba, I.; Prajwos, E.; Chlubek, D. Molecular Insights into the Potential Cardiometabolic Effects of GLP-1 Receptor Analogs and DPP-4 Inhibitors. Int. J. Mol. Sci. 2025, 26, 6777. https://doi.org/10.3390/ijms26146777
Król M, Kupnicka P, Żychowska J, Kapczuk P, Szućko-Kociuba I, Prajwos E, Chlubek D. Molecular Insights into the Potential Cardiometabolic Effects of GLP-1 Receptor Analogs and DPP-4 Inhibitors. International Journal of Molecular Sciences. 2025; 26(14):6777. https://doi.org/10.3390/ijms26146777
Chicago/Turabian StyleKról, Małgorzata, Patrycja Kupnicka, Justyna Żychowska, Patrycja Kapczuk, Izabela Szućko-Kociuba, Eryk Prajwos, and Dariusz Chlubek. 2025. "Molecular Insights into the Potential Cardiometabolic Effects of GLP-1 Receptor Analogs and DPP-4 Inhibitors" International Journal of Molecular Sciences 26, no. 14: 6777. https://doi.org/10.3390/ijms26146777
APA StyleKról, M., Kupnicka, P., Żychowska, J., Kapczuk, P., Szućko-Kociuba, I., Prajwos, E., & Chlubek, D. (2025). Molecular Insights into the Potential Cardiometabolic Effects of GLP-1 Receptor Analogs and DPP-4 Inhibitors. International Journal of Molecular Sciences, 26(14), 6777. https://doi.org/10.3390/ijms26146777





