Oxidative Stress and Psychiatric Symptoms in Wilson’s Disease
Abstract
1. Introduction
2. Results
2.1. Genetic Studies
2.1.1. Antioxidant Gene Polymorphisms and the Prevalence of Psychiatric Symptoms in WD Patients
2.1.2. Antioxidant Gene Polymorphisms and Age at Onset of Psychiatric Symptoms in Patients with WD
2.2. Biochemical Studies—Copper Metabolism, Oxidative Damage, and Antioxidant Parameters in WD Patients with and Without Psychiatric Symptoms
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Genetic Studies
Genotyping of Single Nucleotide Polymorphisms of the Glutathione Peroxidase 1 (GPX1), Catalase (CAT), and Manganese Superoxide Dismutase (SOD2) Genes
4.3. Biochemical Studies
4.3.1. Analysis of Copper Metabolism
4.3.2. Analysis of Selected Peripheral Markers of Oxidative Damage to DNA, Lipids, and Proteins
4.3.3. Analysis of Systemic Antioxidant Capacity and Selected Small and Large Antioxidant Molecules
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 8-iso-PGF2α | Prostaglandin F2α 8-epimer |
| 8-OHdG | 8-hydroxy-2′deoxyguanosine |
| ATP7B | ATP-ase 7B |
| Cat | Catalase |
| CNS | Central Nervous System |
| Cu | Copper |
| DNA | Deoxyribonucleic Acid |
| GPx | Glutathione Peroxidase |
| GSH | Glutathione |
| HWE | Hardy–Weinberg equilibrium |
| IQR | Interquartile Range |
| MnSOD | Manganese Superoxide Dismutase |
| OMIM | Online Mendelian Inheritance in Man |
| ROS | Reactive Oxygen Species |
| SNP | Single Nucleotide Polymorphism |
References
- Steinberg, H.; Sternlieb, I. Wilson’s disease. In Major Problems in Internal Medicine; Saunders, W.B., Ed.; Wiley: Hoboken, NJ, USA, 1984; Volume XXIII. [Google Scholar] [CrossRef]
- Lucena-Valera, A.; Ruz-Zafra, P.; Ampuero, J. Wilson’s disease: Overview. Enfermedad de Wilson. Med. Clin. 2023, 160, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Bull, P.C.; Thomas, G.R.; Rommens, J.M.; Forbes, J.R.; Cox, D.W. The Wilson disease gene is a putative copper transporting P-type ATPase similar to the Menkes gene. Nat. Genet. 1993, 5, 327–337, Erratum in Nat. Genet. 1994, 6, 214. [Google Scholar] [CrossRef] [PubMed]
- Brewer, G.J. Wilson’s Disease: A Clinician’s Guide to Recognition, Diagnosis, and Management; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001. [Google Scholar]
- Ala, A.; Walker, A.P.; Ashkan, K.; Dooley, J.S.; Schilsky, M.L. Wilson’s disease. Lancet 2007, 369, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Gromadzka, G.; Grycan, M.; Przybyłkowski, A.M. Monitoring of Copper in Wilson Disease. Diagnostics 2023, 13, 1830. [Google Scholar] [CrossRef] [PubMed]
- Członkowska, A.; Litwin, T.; Dusek, P.; Ferenci, P.; Lutsenko, S.; Medici, V.; Rybakowski, J.K.; Weiss, K.H.; Schilsky, M.L. Wilson Disease. Nat. Rev. Dis. Primers 2018, 4, 21. [Google Scholar] [CrossRef] [PubMed]
- Akil, M.; Brewer, G.J. Psychiatric and behavioral abnormalities in Wilson’s disease. Adv. Neurol. 1995, 65, 171–178. [Google Scholar] [PubMed]
- Hesse, S.; Barthel, H.; Hermann, W.; Murai, T.; Kluge, R.; Wagner, A.; Sabri, O.; Eggers, B. Regional serotonin transporter availability and depression are correlated in Wilson’s disease. J. Neural. Transm. 2003, 110, 923–933. [Google Scholar] [CrossRef] [PubMed]
- Zimbrean, P.C.; Schilsky, M.L. Psychiatric aspects of Wilson disease: A review. Gen. Hosp. Psychiatry 2014, 36, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Shanmugiah, A.; Sinha, S.; Taly, A.B.; Prashanth, L.K.; Tomar, M.; Arunodaya, G.R.; Reddy, J.Y.; Khanna, S. Psychiatric manifestations in Wilson’s disease: A crosssectional analysis. J. Neuropsychiatry Clin. Neurosci. 2008, 20, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Dening, T.R.; Berrios, G.E. Wilson’s disease: A longitudinal study of psychiatric symptoms. Biol. Psychiatry 1990, 28, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, K.; Sinha, S.; Taly, A.B.; Prashanth, L.K.; Arunodaya, G.R.; Janardhana Reddy, Y.C.; Khanna, S. Dominant psychiatric manifestations in Wilson’s disease:a diagnostic and therapeutic challenge. J. Neurol. Sci. 2008, 266, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Carta, M.G.; Sorbello, O.; Moro, M.F.; Bhat, K.M.; Demelia, E.; Serra, A.; Mura, G.; Sancassiani, F.; Piga, M.; Demelia, L. Bipolar disorders and Wilson’s disease. BMC Psychiatry 2012, 12, 52. [Google Scholar] [CrossRef] [PubMed]
- Roberts, E.A.; Longo, D.L.; Schilsky, M.L. Current and emerging issues in Wilson’s disease. N. Engl. J. Med. 2023, 389, 922–938. [Google Scholar] [CrossRef] [PubMed]
- Brewer, G.J.; Yuzbasiyan-Gurkan, V. Wilson disease. Medicine 1992, 71, 139–164. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, M.K.; Avasthi, A.; Sahoo, M.; Modi, M.; Biswas, P. Psychiatric manifestations of Wilson’s disease and treatment with electroconvulsive therapy. Indian J. Psychiatry 2010, 52, 66–68. [Google Scholar] [CrossRef] [PubMed]
- Dening, T.R.; Berrios, G.E. Wilson’s disease: Psychiatric symptoms in 195 cases. Arch. Gen. Psychiatry 1989, 46, 1126–1134. [Google Scholar] [CrossRef] [PubMed]
- Oder, W.; Grimm, G.; Kollegger, H.; Ferenci, P.; Schneider, B.; Deecke, L. Neurological and neuropsychiatric spectrum of Wilson’s disease: A prospective study of 45 cases. J. Neurol. 1991, 238, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Frota, N.A.F.; Caramelli, P.; Barbosa, E.R. Cognitive impairment in Wilson’s disease. Dement. Neuropsychol. 2009, 3, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Rathbun, J.K. Neuropsychological aspects of Wilson’s disease. Int. J. Neurosci. 1996, 85, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Akil, M.; Schwartz, J.A.; Dutchak, D.; Yuzbasiyan-Gurkan, V.; Brewer, G.J. The psychiatric presentations of Wilson’s disease. J. Neuropsychiatry Clin. Neurosci. 1991, 3, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Gromadzka, G.; Antos, A.; Sorysz, Z.; Litwin, T. Psychiatric Symptoms in Wilson’s Disease-Consequence of ATP7B Gene Mutations or Just Coincidence?—Possible Causal Cascades and Molecular Pathways. Int. J. Mol. Sci. 2024, 25, 12354. [Google Scholar] [CrossRef] [PubMed]
- Walshe, J.M.; Yealland, M. Wilson’s disease: The problem of delayed diagnosis. J. Neurol. Neurosurg. Psychiatry 1992, 55, 692–696. [Google Scholar] [CrossRef] [PubMed]
- Åberg, F.; Shang, Y.; Strandberg, R.; Wester, A.; Widman, L.; Hagström, H. Four-fold increased mortality rate in patients with Wilson’s disease: A population-based cohort study of 151 patients. United Eur. Gastroenterol. J. 2023, 11, 852–860. [Google Scholar] [CrossRef] [PubMed]
- Antos, A.; Członkowska, A.; Smolinski, L.; Bembenek, J.; Przybyłkowski, A.; Skowrońska, M.; Kurkowska-Jastrzębska, I.; Litwin, T. Early neurological deterioration in Wilson’s disease: A systematic literature review and meta-analysis. Neurol. Sci. 2023, 44, 3443–3455. [Google Scholar] [CrossRef] [PubMed]
- Kalita, J.; Kumar, V.; Misra, U.K.; Parashar, V.; Ranjan, A. Adjunctive antioxidant therapy in neurologic Wilson’s disease improves the outcomes. J. Mol. Neurosci. 2020, 70, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Ercal, N.; Gurer-Orhan, H.; Aykin-Burns, N. Toxic metals and oxidative stress part I: Mechanisms involved in metal-induced oxidative damage. Curr. Top. Med. Chem. 2001, 1, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Gromadzka, G.; Tarnacka, B.; Flaga, A.; Adamczyk, A. Copper Dyshomeostasis in Neurodegenerative Diseases-Therapeutic Implications. Int. J. Mol. Sci. 2020, 21, 9259. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.; Laurie, C.; Mosley, R.L.; Gendelman, H.E. Oxidative stress and the pathogenesis of neurodegenerative disorders. Int. Rev. Neurobiol. 2007, 82, 297–325. [Google Scholar] [CrossRef] [PubMed]
- Jelinek, M.; Jurajda, M.; Duris, K. Oxidative Stress in the Brain: Basic Concepts and Treatment Strategies in Stroke. Antioxidants 2021, 10, 1886. [Google Scholar] [CrossRef] [PubMed]
- Khairova, R.; Pawar, R.; Salvadore, G.; Juruena, M.F.; De Sousa, R.T.; Soeiro-de-Souza, M.G.; Salvador, M.; Zarate, C.A.; Gattaz, W.F.; Machado-Vieira, R. Effects of lithium on oxidative stress parameters in healthy subjects. Mol. Med. Rep. 2012, 5, 680–682. [Google Scholar] [CrossRef] [PubMed]
- Machado-Vieira, R.; Andreazza, A.C.; Viale, C.I.; Zanatto, V.; Cereser, V., Jr.; da Silva Vargas, R.; Kapczinski, F.; Portela, L.V.; Souza, D.O.; Salvador, M.; et al. Oxidative stress parameters in unmedicated and treated bipolar subjects during initial manic episode: A possible role for lithium antioxidant effects. Neurosci. Lett. 2007, 421, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Zarate, C.A.; Singh, J., Jr.; Manji, H.K. Cellular plasticity cascades: Targets for the development of novel therapeutics for bipolar disorder. Biol. Psychiatry 2006, 59, 1006–1020. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Dong, T.; Wei, T.; Wu, H.; Yang, Y.; Ding, Y.; Li, C.; Yang, W. Large-scale networks changes in Wilson’s disease associated with neuropsychiatric impairments: A resting-state functional magnetic resonance imaging study. BMC Psychiatry 2023, 23, 805. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Pu, C.; Zhang, Y.; Sun, Y.; Liao, Y.; Kang, Z.; Feng, X.; Yue, W. Oxidative Stress and Psychiatric Disorders: Evidence from the Bidirectional Mendelian Randomization Study. Antioxidants 2022, 11, 1386. [Google Scholar] [CrossRef] [PubMed]
- Ng, F.; Berk, M.; Dean, O.; Bush, A.I. Oxidative stress in psychiatric disorders: Evidence base and therapeutic implications. Int. J. Neuropsychopharmacol. 2008, 11, 851–876. [Google Scholar] [CrossRef] [PubMed]
- Cecerska-Heryć, E.; Polikowska, A.; Serwin, N.; Roszak, M.; Grygorcewicz, B.; Heryć, R.; Michalczyk, A.; Dołęgowska, B. Importance of oxidative stress in the pathogenesis, diagnosis, and monitoring of patients with neuropsychiatric disorders, a review. Neurochem. Int. 2022, 153, 105269. [Google Scholar] [CrossRef] [PubMed]
- Ravn-Haren, G.; Olsen, A.; Tjønneland, A.; Dragsted, L.O.; Nexø, B.A.; Wallin, H.; Overvad, K.; Raaschou-Nielsen, O.; Vogel, U. Associations between GPX1 Pro198Leu polymorphism, erythrocyte GPX activity, alcohol consumption and breast cancer risk in a prospective cohort study. Carcinogenesis 2006, 27, 820–825. [Google Scholar] [CrossRef] [PubMed]
- Bastaki, S.; Huen, K.; Manzanillo, P.; Chande, N.; Chowdhury, Z.; Lipsett, M.; Eskenazi, B.; Holland, N. Genetic susceptibility to oxidative stress and childhood asthma. Pediatr. Allergy Immunol. 2006, 17, 490–497. [Google Scholar] [CrossRef]
- Ravn-Haren, G.; Olsen, A.; Tjønneland, A.; Dragsted, L.O.; Nexo, B.A.; Wallin, H.; Overvad, K.; Raaschou-Nielsen, O.; Vogel, U. Associations between GPX1 Pro198Leu polymorphism, interaction with antioxidant intake and breast cancer risk: A Danish cohort study. Cancer Lett. 2006, 239, 190–197. [Google Scholar] [CrossRef]
- Lubos, E.; Loscalzo, J.; Handy, D.E. Glutathione peroxidase-1 in health and disease: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal 2011, 15, 1957–1997. [Google Scholar] [CrossRef] [PubMed]
- Chhetri, G.; Kalita, J.; Misra, U.K. Oxidative stress in Wilson’s disease and its relationship with mutational status and clinical severity. Park. Relat. Disord. 2021, 84, 17–21. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, H.; Zhou, J.; Shao, Q. Glutathione Peroxidase GPX1 and Its Dichotomous Roles in Cancer. Cancers 2022, 14, 2560. [Google Scholar] [CrossRef] [PubMed]
- Dringen, R. Metabolism and functions of glutathione in brain. Prog. Neurobiol. 2000, 62, 649–671. [Google Scholar] [CrossRef] [PubMed]
- Shimoda-Matsubayashi, S.; Matsumine, H.; Kobayashi, T.; Nakagawa-Hattori, Y.; Shimizu, Y.; Mizuno, Y. Structural dimorphism in the mitochondrial targeting sequence in the human manganese superoxide dismutase gene. A predictive evidence for conformational change to influence mitochondrial transport and a study of allelic association in Parkinson’s disease. Biochem. Biophys. Res. Commun. 1996, 226, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Sutton, A.; Khoury, H.; Prip-Buus, C.; Cepanec, C.; Pessayre, D.; Degoul, F. The Ala16Val genetic dimorphism modulates the import of human manganese superoxide dismutase into rat liver mitochondria. Pharmacogenetics 2003, 13, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Sutton, A.; Imbert, A.; Igoudjil, A.; Descatoire, V.; Cazanave, S.; Pessayre, D.; Degoul, F. The manganese superoxide dismutase Ala16Val dimorphism modulates both mitochondrial import and mRNA stability. Pharmacogenet. Genom. 2005, 15, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Broz, M.; Furlan, V.; Lešnik, S.; Jukič, M.; Bren, U. The Effect of the Ala16Val Mutation on the Secondary Structure of the Manganese Superoxide Dismutase Mitochondrial Targeting Sequence. Antioxidants 2022, 11, 2348. [Google Scholar] [CrossRef] [PubMed]
- Holley, A.K.; Bakthavatchalu, V.; Velez-Roman, J.M.; St Clair, D.K. Manganese superoxide dismutase: Guardian of the powerhouse. Int. J. Mol. Sci. 2011, 12, 7114–7162. [Google Scholar] [CrossRef] [PubMed]
- Akyol, O.; Yanik, M.; Elyas, H.; Namli, M.; Canatan, H.; Akin, H.; Yuce, H.; Yilmaz, H.R.; Tutkun, H.; Sogut, S.; et al. Association between Ala-9Val polymorphism of Mn-SOD gene and schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2005, 29, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Chowdari, K.V.; Bamne, M.N.; Nimgaonkar, V.L. Genetic association studies of antioxidant pathway genes and schizophrenia. Antioxid. Redox Signal. 2011, 15, 2037–2045. [Google Scholar] [CrossRef] [PubMed]
- Pae, C.U.; Kim, T.S.; Patkar, A.A.; Kim, J.J.; Lee, C.U.; Lee, S.J.; Jun, T.Y.; Lee, C.; Paik, I.H. Manganese superoxide dismutase (MnSOD: Ala-9Val) gene polymorphism may not be associated with schizophrenia and tardive dyskinesia. Psychiatry Res. 2007, 15, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Chen, C.K.; Sekine, Y.; Iwata, Y.; Anitha, A.; Loh, E.W.; Takei, N.; Suzuki, A.; Kawai, M.; Takebayashi, K.; et al. Association analysis of SOD2 variants with methamphetamine psychosis in Japanese and Taiwanese populations. Hum. Genet. 2006, 120, 243–352. [Google Scholar] [CrossRef] [PubMed]
- Forsberg, L.; Lyrenäs, L.; de Faire, U.; Morgenstern, R. A common functional C-T substitution polymorphism in the promoter region of the human catalase gene influences transcription factor binding, reporter gene transcription and is correlated to blood catalase levels. Free Radic. Biol. Med. 2001, 30, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Oxidative stress and neurodegeneration: Where are we now? J. Neurochem. 2006, 97, 1634–1658. [Google Scholar] [CrossRef] [PubMed]
- Gsell, W.; Conrad, R.; Hickethier, M.; Sofic, E.; Frölich, L.; Wichart, I.; Jellinger, K.; Moll, G.; Ransmayr, G.; Beckmann, H.; et al. Decreased catalase activity but unchanged superoxide dismutase activity in brains of patients with dementia of Alzheimer type. J. Neurochem. 1995, 64, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.M.; Zhu, R.R.; Tian, Y.; Uludag, K.; Chen, J.J.; Zhou, H.X.; Wang, L.; Kosten, T.R.; Zhang, X.Y. Association between MnSOD Activity and Cognitive Impairment in Unmedicated First-Episode Schizophrenia: Regulated by MnSOD Ala-9Val Gene Polymorphism. Antioxidants 2022, 11, 1981. [Google Scholar] [CrossRef] [PubMed]
- Milne, G.L.; Musiek, E.S.; Morrow, J.D. F2-isoprostanes as markers of oxidative stress in vivo: An overview. Biomarkers 2005, 10 (Suppl. 1), S10–S23. [Google Scholar] [CrossRef] [PubMed]
- Shichiri, M. The role of lipid peroxidation in neurological disorders. J. Clin. Biochem. Nutr. 2014, 54, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Mahadik, S.P.; Evans, D.; Lal, H. Oxidative stress and role of antioxidant and omega-3 essential fatty acid supplementation in schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2001, 25, 463–493. [Google Scholar] [CrossRef] [PubMed]
- Wood, S.J.; Yücel, M.; Pantelis, C.; Berk, M. Neurobiology of schizophrenia spectrum disorders: The role of oxidative stress. Ann. Acad. Med. Singap. 2009, 38, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Mariani, E.; Polidori, M.C.; Cherubini, A.; Mecocci, P. Oxidative stress in brain aging, neurodegenerative and vascular diseases: An overview. J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 2005, 827, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Markers of oxidative stress in erythrocytes and plasma during aging in humans. Oxid. Med. Cell. Longev. 2010, 3, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Pillai, A.; Parikh, V.; Terry, A.V., Jr.; Mahadik, S.P. Long-term antipsychotic treatments and crossover studies in rats: Differential effects of typical and atypical agents on the expression of antioxidant enzymes and membrane lipid peroxidation in rat brain. J. Psychiatr. Res. 2007, 41, 372–386. [Google Scholar] [CrossRef] [PubMed]
- Cai, N.; Verhulst, B.; Andreassen, O.A.; Buitelaar, J.; Edenberg, H.J.; Hettema, J.M.; Gandal, M.; Grotzinger, A.; Jonas, K.; Lee, P.; et al. Assessment and ascertainment in psychiatric molecular genetics: Challenges and opportunities for cross-disorder research. Mol. Psychiatry 2025, 30, 1627–1638, Erratum in Mol. Psychiatry 2025, 30, 1715. https://doi.org/10.1038/s41380-025-02914-4. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver Disease. EASL clinical practice guidelines: Wilson’s disease. J. Hepatol. 2012, 56, 671–685. [Google Scholar] [CrossRef] [PubMed]
- Socha, P.; Janczyk, W.; Dhawan, A.; Baumann, U.; Vajro, P.; Fischler, B.; Hadzic, N. Wilson’s Disease in Children: A Position Paper by the Hepatology Committee of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition. J. Pediatr. Gastroenterol. Nutr. 2025, 71, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Schilsky, M.L.; Roberts, E.A.; Bronstein, J.M.; Dhawan, A.; Hamilton, J.P.; Rivard, A.M.; Washington, M.K.; Weiss, K.H. A Multidisciplinary Approach to the Diagnosis and Management of Wilson Disease: 2023 Practice Guidance from the American Association for the Study of Liver Diseases. Hepatology 2023, 77, 1164–1192. [Google Scholar] [CrossRef]
- Piotrowski, P.; Gondek, T.; Rymaszewska, J.; Beszłej, J.; Czachowski, S.; Parmentier, H.; Kurpas, D. Guidelines of the Polish Psychiatric Association—Wroclaw Division, the Polish Society of Family Medicine and the College of Family Physicians in Poland for diagnosis and treatment of depressive disorders in primary health care. Fam. Med. Prim. Care Rev. 2017, 3, 335–346. [Google Scholar] [CrossRef]
- Sheehan, D.V.; Lecrubier, Y.; Sheehan, K.H.; Amorim, P.; Janavs, J.; Weiller, E.; Hergueta, T.; Baker, R.; Dunbar, G.C. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 1998, 59 (Suppl. S20), 22–33, quiz 34–57. [Google Scholar] [PubMed]
- ICD-10: International Statistical Classification of Diseases and Related Health Problems: Tenth Revision, 2nd ed.; World Health Organization: Geneva, Switzerland, 2004; Available online: https://iris.who.int/handle/10665/42980 (accessed on 15 February 2025).
- Xiong, Y.M.; Mo, X.Y.; Zou, X.Z.; Song, R.X.; Sun, W.Y.; Lu, W.; Chen, Q.; Yu, Y.X.; Zang, W.J. Association study between polymorphisms in selenoprotein genes and susceptibility to Kashin-Beck disease. Osteoarthr. Cartil. 2010, 18, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Suzen, H.S.; Gucyener, E.; Sakalli, O.; Uckun, Z.; Kose, G.; Ustel, D.; Duydu, Y. CAT C-262T and GPX1 Pro198Leu polymorphisms in a Turkish population. Mol. Biol. Rep. 2010, 37, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Choi, M.G.; Kim, D.S.; Kim, T.W. Manganese superoxide dismutase gene polymorphism V16A is associated with stages of albuminuria in Korean type 2 diabetic patients. Metabolism 2006, 55, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ravin, H.A. An Improved Colorimetric Enzymatic Assay of Ceruloplasmin. J. Lab. Clin. Med. 1961, 58, 161–168. [Google Scholar] [PubMed]
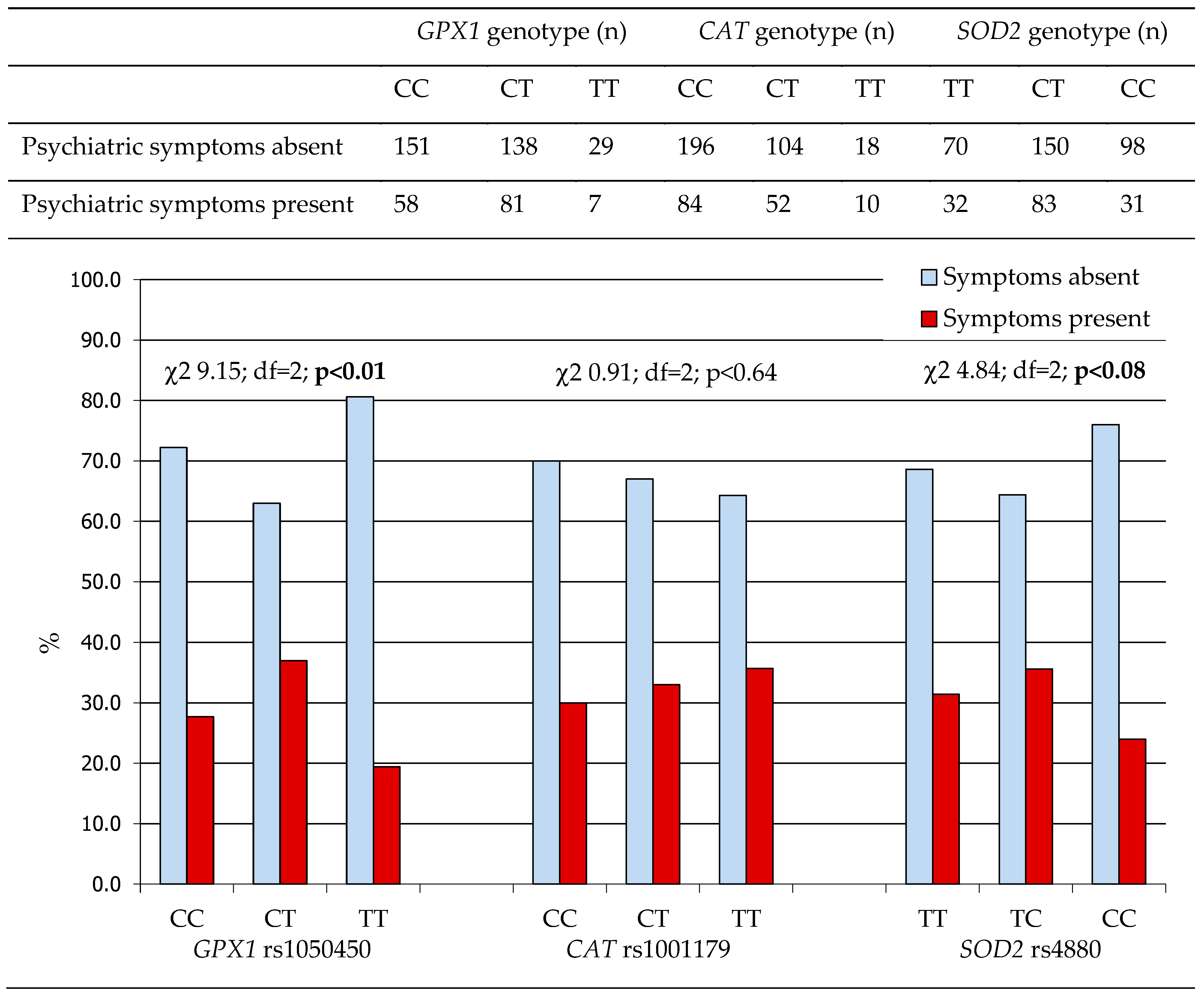
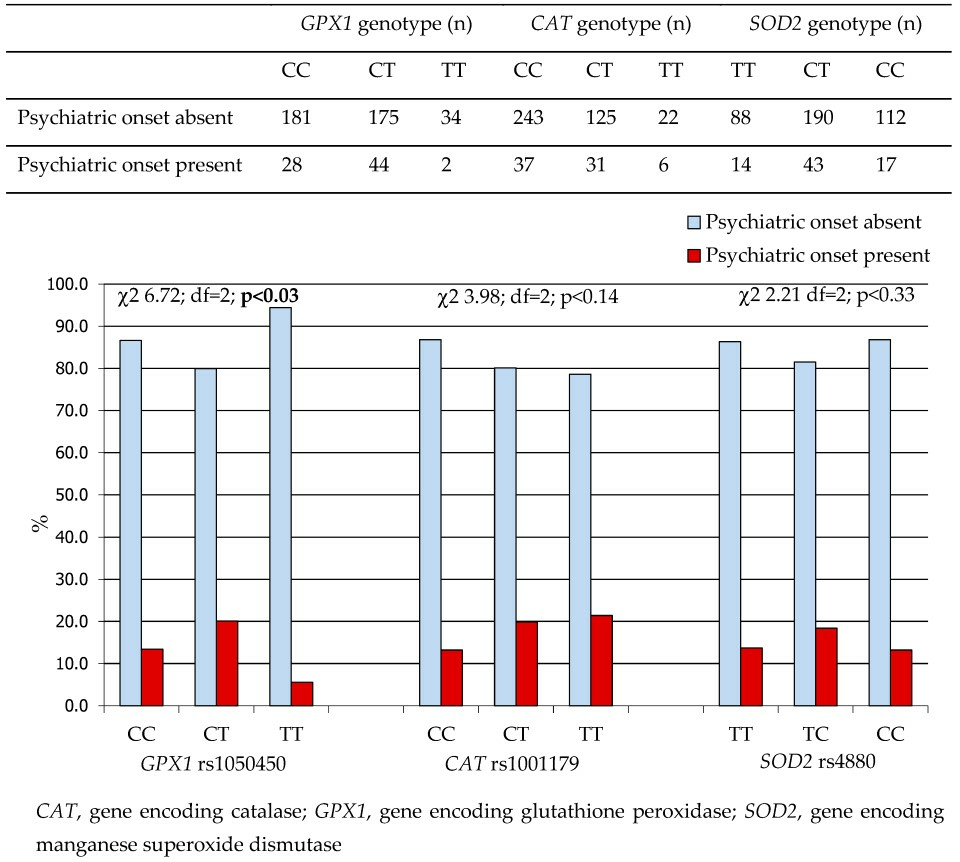
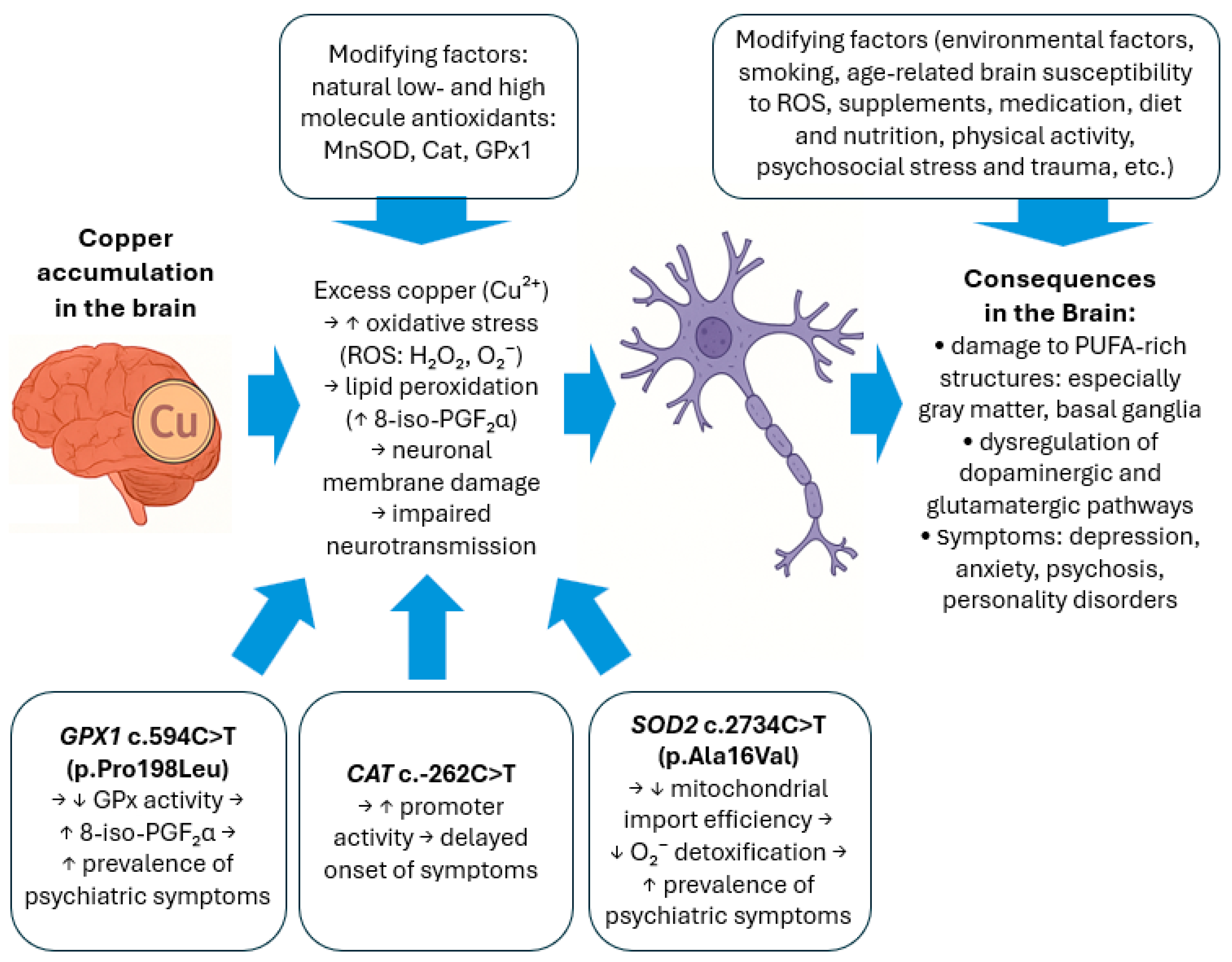
| Characteristics | |||
|---|---|---|---|
| Sex, female/male, N (%) | 254 (54.7)/210 (45.2) | ||
| Age in years at onset of WD, median (IQR) (N = 420) a | 23.9 (13) | ||
| The presence of psychiatric symptoms, n (%) (N = 464) | 157 (33.8) | ||
| The type of psychiatric symptoms b (N = 464) | |||
| behavioral or personality disorders | 39 (8.4) | ||
| mild cognitive impairment | 40 (8.6) | ||
| psychotic disorder | 7 (1.5) | ||
| mood disorder | 91 (19.6) | ||
| others (apathy, sleep disturbances, irritability and emotional lability, impulsivity, anxiety disorders, obsessive– compulsive symptoms, attention deficits) | 15 (3.2) | ||
| Age in years at manifestation of psychiatric symptoms, median (IQR) (N = 125) a | 27.0 (17.0) | ||
| The presence of psychiatric symptoms at WD onset, N (%) (N = 464) | 74 (15.9) | ||
| GPX1 rs1050450 (c.593 C>T [p.Pro198Leu]) genotype, N (%) c | CC (wt) | 209 (45.0) | HWE χ2 4.31; df 2; p < 0.04 |
| CT (het) | 219 (47.2) | ||
| TT (var) | 36 (7.8) | ||
| CAT rs1001179 (c.-262C>T) genotype, N (%) c | CC (wt) | 280 (60.3) | HWE χ2 0.99; df 2; p < 0.32 |
| CT (het) | 156 (33.6) | ||
| TT (var) | 28 (6.1) | ||
| SOD2 rs4880 (c.-9 T>C [p.Val16Ala]) genotype, N (%) c | TT (wt) | 102 (22.0) | HWE χ2 0.02; df 2; p < 0.87 |
| TC (het) | 233 (50.2) | ||
| CC (var) | 129 (27.8) | ||
| (a) GPX1 rs1050450. | |||||
| Psychiatric Symptoms at WD Onset | GPX1 rs1050450 genotype; n (%) | ||||
| CC | CT | TT | statistics | ||
| Mood disorder | absent | 173 (82.8) | 171 (78.1) | 29 (80.6) | χ2 1.49; df 2 p < 0.47 |
| present | 36 (17.2) | 48 (21.9) | 7 (19.4) | ||
| Cognitive impairment | absent | 194 (92.3) | 195 (89.0) | 35 (97.2) | χ2 3.63; df 2 p < 0.16 |
| present | 15 (7.2) | 24 (11.0) | 1 (2.8) | ||
| Personality disorders | absent | 193 (92.3) | 197 (89.9) | 35 (97.2) | χ2 2.40; df 2 p < 0.30 |
| present | 16 (7.7) | 22 (10.8) | 1 (2.8) | ||
| Psychosis | absent | 209 (100.0) | 212 (96.8) | 36 (100.0) | χ2 7.95; df 2 p < 0.02 |
| present | 0 (0.0) | 7 (3.2) | 0 (0.0) | ||
| Others | absent | 202 (96.0) | 212 (96.8) | 35 (97.2) | χ2 0.33; df 2 p < 0.98 |
| present | 7 (3.3) | 7 (3.2) | 1 (2.8) | ||
| (b) CAT rs1001179. | |||||
| Psychiatric Symptoms at WD onset | CAT rs1001179 genotype; n (%) | ||||
| CC | CT | TT | statistics | ||
| Mood disorder | absent | 232 (82.9) | 118 (75.6) | 23 (82.1) | χ2 3.37; df 2 p < 0.18 |
| present | 48 (17.1) | 38 (24.4) | 5 (17.9) | ||
| Cognitive impairment | absent | 261 (93.2) | 138 (88.5) | 25 (89.3) | χ2 3.04; df 2 p < 0.21 |
| present | 19 (6.8) | 18 (11.5) | 3 (10.7) | ||
| Personality disorders | absent | 253 (90.4) | 147 (94.2) | 25 (89.3) | χ2 2.16; df 2 p < 0.34 |
| present | 27 (9.6) | 9 (5.8) | 3 (10.7) | ||
| Psychosis | absent | 278 (99.3) | 153 (98.1) | 26 (92.9) | χ2 7.35; df 2 p < 0.02 |
| present | 2 (0.7) | 3 (1.9) | 2 (7.1) | ||
| Others | absent | 269 (96.1) | 152 (97.4) | 28 (100.0) | χ2 1.59; df 2 p < 0.45 |
| present | 11 (3.9) | 4 (2.6) | 0 (0.0) | ||
| (c) SOD2 rs4880. | |||||
| Psychiatric Symptoms at WD onset | SOD2 rs4880 genotype; n (%) | ||||
| TT | CT | CC | statistics | ||
| Mood disorder | absent | 82 (80.4) | 180 (77.2) | 111 (86.0) | χ2 4.07; df 2 p < 0.13 |
| present | 20 (19.6) | 53 (22.7) | 18 (13.9) | ||
| Cognitive impairment | absent | 94 (92.2) | 209 (89.7) | 121 (93.8) | χ2 1.87; df 2 p < 0.39 |
| present | 8 (7.8) | 24 (10.3) | 8 (6.2) | ||
| Personality disorders | absent | 93 (91.2) | 217 (93.1) | 115 (89.1) | χ2 1.75; df 2 p < 0.41 |
| present | 9 (8.8) | 16 (6.9) | 14 (10.5) | ||
| Psychosis | absent | 99 (97.1) | 230 (98.7) | 128 (99.2) | χ2 1.95; df 2 p < 0.37 |
| present | 3 (2.9) | 3 (1.3) | 1 (0.8) | ||
| Others | absent | 95 (93.1) | 228 (97.8) | 126 (97.7) | χ2 5.51; df 2 p < 0.06 |
| present | 7 (6.9) | 5 (2.1) | 3 (2.3) | ||
| Age at Manifestation of Psychiatric Symptoms, Years, Median (IQR) | ||||||
| GPX1 rs1050450 genotype | p | GPX1 rs1050450 allele T status | p | |||
| CC | CT | TT | T (−) | T (+) | ||
| 27.0 (16.0) [n = 50] | 27.0 (18.0) [n = 71] | 25.0 (23.0) [n = 4] | ns | 27.0 (16.0) [n = 50] | 27.0 (19.0) [n = 75] | ns |
| CAT rs1001179 genotype | CAT rs1001179 allele C status | |||||
| CC | CT | TT | C (−) | C (+) | ||
| 27.5 (15.0) [n = 72] | 25.0 (17.0) [n = 45] | 33.5 (16.0) [n = 8] | CC vs. CT < 0.02 CT vs. TT < 0.06 | 33.5 (16.0) [n = 8] | 27.0 (17.0) [n = 117] | <0.07 |
| SOD2 rs1050450 genotype | SOD2 rs1050450 allele T status | |||||
| TT | CT | CC | T (−) | T (+) | ||
| 31.0 (16.0) [n = 29] | 26.0 (17.5) [n = 68] | 28.0 (16.5) [n = 28] | ns | 28.0 (19.0) [n = 28] | 27.0 (17.0) [n = 97] | ns |
| Psychiatric Symptoms Before Decoppering Treatment | Psychiatric Symptoms at WD Onset | |||||
| No | Yes | p | No | Yes | p | |
| N = 22 | N = 11 | N = 28 | N = 5 | |||
| Copper metabolism parameters, ƒ median (IQR) | ||||||
| Serum copper, µg/dL | 69.5 (12.0) | 58.0 (49.0) | <0.04 | 68.0 (17.5) | 36.0 (49.0) | <0.021 |
| Serum ceruloplasmin, mg/dL | 17.2 (4.5) | 2.5 (31.4) | <0.0006 | 16.6 (7.5) | 2.1 (13.2) | <0.032 |
| Oxidative damage parameters, median (IQR) | ||||||
| 8-iso-PGF2α, pg/mL, median (IQR) | 1149.5 (521.0) | 1830.0 (1435.0) | <0.001 | 1286.0 (845.0) | 1696.0 (375.0) | ns |
| OHdG, pg/mL, median (IQR) | 10,280.0 (12,323.0) | 5751.0 (5695.5) | ns | 9080.0 (10,480.0) | 9490.0 (40,497.5) | ns |
| Antioxidant capacity parameters, median (IQR) | ||||||
| Total antioxidant capacity, median (IQR) | 647.0 (194.0) | 654.0 (93.0) | ns | 657.5 (209.0) | 652.5 (147.5) | ns |
| Serum glutathione, µmol/L, median (IQR) | 0.0 (0.7) | 0.8 (1.3) | ns | 0.0 (0.9) | 0.4 (1.0) | ns |
| Catalase, nmol/min/mL, median (IQR) | 100.0 (87.5) | 135.0 (50.0) | ns | 101.0 (80.0) | 112.5 (69.0) | ns |
| Glutathione peroxidase, nmol/min/mL, median (IQR) | 90.4 (24.8) | 74.7 (40.8) | <0.056 | 88.9 (29.3) | 59.3 (35.8) | <0.06 |
| MnSOD, units/mL, median (IQR) | 13.2 (5.0) | 13.9 (3.0) | ns | 13.3 (4.3) | 13.9 (2.6) | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gromadzka, G.; Karpińska, A.; Szafrański, T.K.; Litwin, T. Oxidative Stress and Psychiatric Symptoms in Wilson’s Disease. Int. J. Mol. Sci. 2025, 26, 6774. https://doi.org/10.3390/ijms26146774
Gromadzka G, Karpińska A, Szafrański TK, Litwin T. Oxidative Stress and Psychiatric Symptoms in Wilson’s Disease. International Journal of Molecular Sciences. 2025; 26(14):6774. https://doi.org/10.3390/ijms26146774
Chicago/Turabian StyleGromadzka, Grażyna, Agata Karpińska, Tomasz Krzysztof Szafrański, and Tomasz Litwin. 2025. "Oxidative Stress and Psychiatric Symptoms in Wilson’s Disease" International Journal of Molecular Sciences 26, no. 14: 6774. https://doi.org/10.3390/ijms26146774
APA StyleGromadzka, G., Karpińska, A., Szafrański, T. K., & Litwin, T. (2025). Oxidative Stress and Psychiatric Symptoms in Wilson’s Disease. International Journal of Molecular Sciences, 26(14), 6774. https://doi.org/10.3390/ijms26146774







