C-Terminal Analogues of Camostat Retain TMPRSS2 Protease Inhibition: New Synthetic Directions for Antiviral Repurposing of Guanidinium-Based Drugs in Respiratory Infections
Abstract
1. Introduction
1.1. Strategy of Functionality Variance
1.2. In Vitro Bioassay
1.3. Organic Synthesis Considerations
2. Results and Discussion
2.1. In Silico Docking
2.2. Synthesis
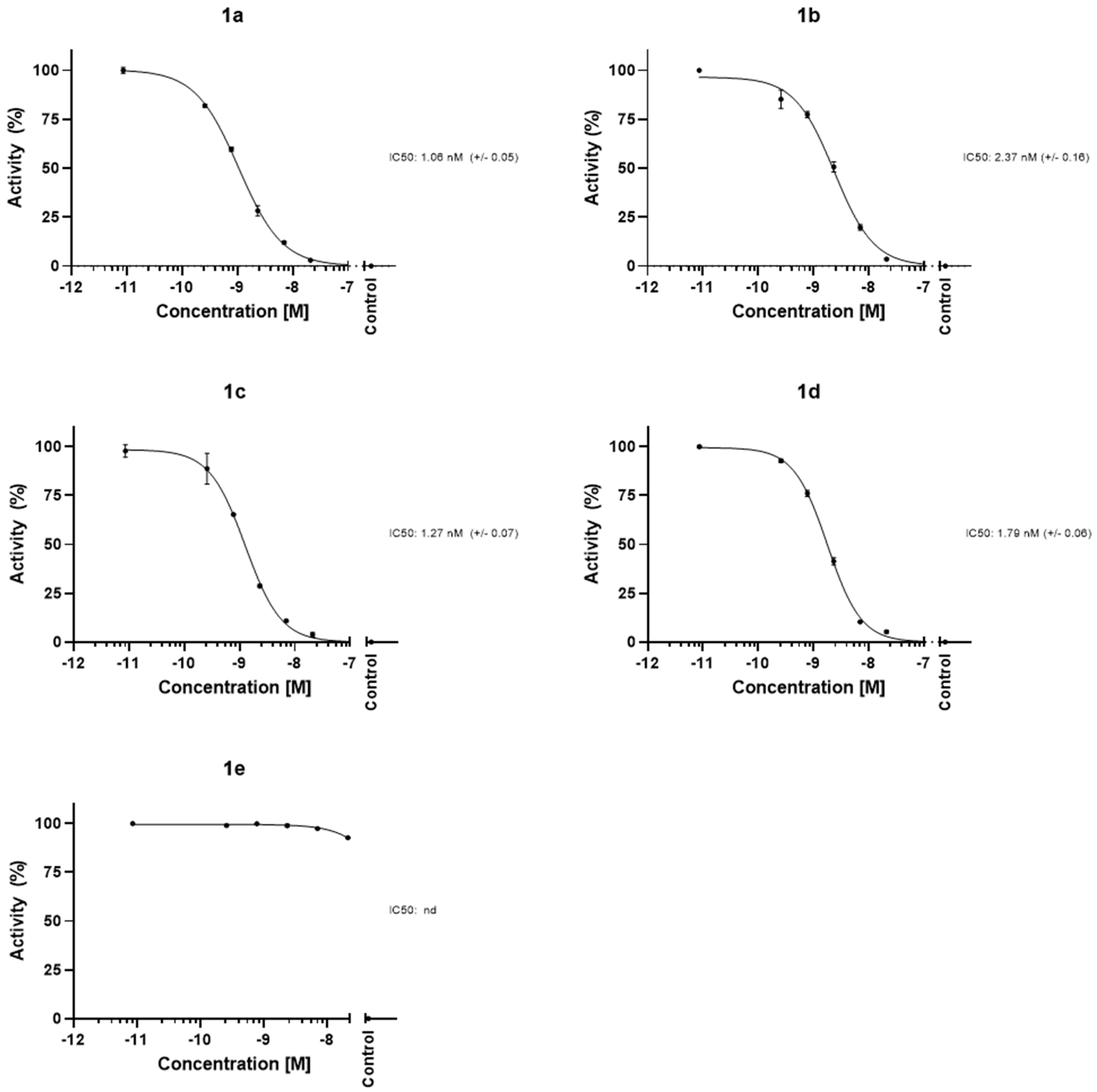
2.3. Protease Inhibition Assays
3. Materials and Methods
3.1. Molecular Docking
3.2. Organic Synthesis Procedures
3.3. Protease Inhibition Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BE | Binding energy |
| BOC | tert-Butyloxycarbonyl |
| DCM | Dichloromethane |
| DIPEA | N,N-Diisopropylethylamine |
| DMAP | 4-(Dimethylamino)pyridine |
| DMF | N,N-Dimethylformamide |
| DMSO | Dimethyl sulfoxide |
| EDCI | 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride |
| GBPA | 4-(Guanidinobenzoyloxy)phenylacetic acid |
| SAR | Structure–activity relationship |
| SEM | Standard error on the mean |
| TFA | Trifluoroacetic acid/trifluoroacetate |
| THF | Tetrahydrofuran |
| TLC | Thin-layer chromatography |
| TMPRSS2 | Transmembrane serine protease 2 |
References
- Ciotti, M.; Ciccozzi, M.; Terrinoni, A.; Jiang, W.C.; Wang, C.B.; Bernardini, S. The COVID-19 pandemic. Crit. Rev. Clin. Lab. Sci. 2020, 57, 365–388. [Google Scholar] [CrossRef] [PubMed]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Wettstein, L.; Kirchhoff, F.; Münch, J. The transmembrane protease TMPRSS2 as a therapeutic target for COVID-19 treatment. Int. J. Mol. Sci. 2022, 23, 1351. [Google Scholar] [CrossRef]
- Hempel, T.; Raich, L.; Olsson, S.; Azouz, N.P.; Klingler, A.M.; Hoffmann, M.; Pöhlmann, S.; Rothenberg, M.E.; Noé, F. Molecular mechanism of inhibiting the SARS-CoV-2 cell entry facilitator TMPRSS2 with camostat and nafamostat. Chem. Sci. 2021, 12, 983–992. [Google Scholar] [CrossRef] [PubMed]
- Barros de Lima, G.; Nencioni, E.; Thimoteo, F.; Perea, C.; Pinto, R.F.A.; Sasaki, S.D. TMPRSS2 as a key player in viral pathogenesis: Influenza and coronaviruses. Biomolecules 2025, 15, 75. [Google Scholar] [CrossRef]
- Mohammadi, K.; Faramarzi, S.; Yaribash, S.; Valizadeh, Z.; Rajabi, E.; Ghavam, M.; Samiee, R.; Karim, B.; Salehi, M.; Seifi, A.; et al. Human metapneumovirus (hMPV) in 2025: Emerging trends and insights from community and hospital-based respiratory panel analyse—A comprehensive review. Virol. J. 2025, 22, 150. [Google Scholar] [CrossRef]
- Laporte, M.; Naesens, L. Airway proteases: An emerging drug target for influenza and other respiratory virus infections. Curr. Opin. Virol. 2017, 24, 16–24. [Google Scholar] [CrossRef]
- Esumi, M.; Ishibashi, M.; Yamaguchi, H.; Nakajima, S.; Tai, Y.; Kikuta, S.; Sugitani, M.; Takayama, T.; Tahara, M.; Takeda, M.; et al. Transmembrane serine protease TMPRSS2 activates hepatitis C virus infection. Hepatology 2015, 61, 437–446. [Google Scholar] [CrossRef]
- Mahoney, M.; Damalanka, V.C.; Tartell, M.A.; Chung, D.H.; Lourenço, A.L.; Pwee, D.; Bridwell, A.E.M.; Hoffmann, M.; Voss, J.; Karmakar, P.; et al. A novel class of TMPRSS2 inhibitors potently block SARS-CoV-2 and MERS-CoV viral entry and protect human epithelial lung cells. Proc. Natl. Acad. Sci. USA 2021, 118, e2108728118. [Google Scholar] [CrossRef]
- Yamamoto, M.; Kiso, M.; Sakai-Tagawa, Y.; Iwatsuki-Horimoto, K.; Imai, M.; Takeda, M.; Kinoshita, N.; Ohmagari, N.; Gohda, J.; Semba, K.; et al. The anticoagulant nafamostat potently inhibits SARS-CoV-2 S protein-mediated fusion in a cell fusion assay system and viral infection in vitro in a cell-type-dependent manner. Viruses 2020, 12, 629. [Google Scholar] [CrossRef]
- Kitagawa, J.; Arai, H.; Iida, H.; Mukai, J.; Furukawa, K.; Ohtsu, S.; Nakade, S.; Hikima, T.; Haranaka, M.; Uemura, N. A phase I study of high dose camostat mesylate in healthy adults provides a rationale to repurpose the TMPRSS2 inhibitor for the treatment of COVID-19. Clin. Transl. Sci. 2021, 14, 1967–1976. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Mitre, M.P.; Morpeth, S.C.; Venkatesh, B.; Hills, T.E.; Davis, J.; Mahar, R.K.; McPhee, G.; Jones, M.; Totterdell, J.; Tong, S.Y.; et al. TMPRSS2 inhibitors for the treatment of COVID-19 in adults: A systematic review and meta-analysis of randomized clinical trials of nafamostat and camostat mesylate. Clin. Microbiol. Infect. 2024, 30, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Fraser, B.J.; Beldar, S.; Seitova, A.; Hutchinson, A.; Mannar, D.; Li, Y.; Kwon, D.; Tan, R.; Wilson, R.P.; Leopold, K.; et al. Structure and activity of human TMPRSS2 protease implicated in SARS-CoV-2 activation. Nat. Chem. Biol. 2022, 18, 963–971. [Google Scholar] [CrossRef]
- Fujimoto, K.J.; Hobbs, D.C.F.; Umeda, M.; Nagata, A.; Yamaguchi, R.; Sato, Y.; Sato, A.; Ohmatsu, K.; Ooi, T.; Yanai, T.; et al. In silico analysis and synthesis of nafamostat derivatives and evaluation of their anti-SARS-CoV-2 activity. Viruses 2022, 14, 389. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020, 181, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Rabi, S.A.; Michaud, W.A.; Becerra, D.; Gilpin, S.E.; Mino-Kenudson, M.; Ott, H.C. Protease inhibitor camostat mesyalte blocks wild type SARS-CoV-2 and D614G viral entry in human engineered miniature lungs. Biomaterials 2022, 285, 121509. [Google Scholar] [CrossRef]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef]
- Iwata-Yoshikawa, N.; Kakizaki, M.; Shiwa-Sudo, N.; Okura, T.; Tahara, M.; Fukushi, S.; Maeda, K.; Kawase, M.; Asanuma, H.; Tomita, Y.; et al. Essential role of TMPRSS2 in SARS-CoV-2 infection in murine airways. Nat. Commun. 2022, 13, 6100. [Google Scholar] [CrossRef]
- Rossi, Á.D.; de Araújo, J.L.F.; de Almeida, T.B.; Ribeiro-Alves, M.; de Almeida Velozo, C.; Almeida, J.M.D.; de Carvalho Leitão, I.; Ferreira, S.N.; da Silva Oliveira, J.; Alves, H.J.; et al. Association between ACE2 and TMPRSS2 nasopharyngeal expression and COVID-19 respiratory distress. Sci. Rep. 2021, 11, 9658. [Google Scholar] [CrossRef]
- Rokni, M.; Heidari Nia, M.; Sarhadi, M.; Mirinejad, S.; Sargazi, S.; Moudi, M.; Saravani, R.; Rahdar, S.; Kargar, M. Association of TMPRSS2 gene polymorphisms with COVID-19 severity and mortality: A case-control study with computational analyses. Appl. Biochem. Biotechnol. 2022, 8, 3507–3526. [Google Scholar] [CrossRef]
- Sharma, T.; Baig, M.H.; Khan, M.I.; Alotaibi, S.S.; Alorabi, M.; Dong, J.J. Computational screening of camostat and related compounds against human TMPRSS2: A potential treatment of COVID-19. Saudi Pharm. J. 2022, 3, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Winkler, D.A. Computational repurposing of drugs for viral diseases and current and future pandemics. J. Math. Chem. 2024, 62, 2844–2879. [Google Scholar] [CrossRef]
- Hamdy, R.; Fayed, B.; Mostafa, A.; Shama, N.M.A.; Mahmoud, S.H.; Mehta, C.H.; Nayak, Y.; Soliman, S.S.M. Iterated virtual screening-assisted antiviral and enzyme inhibition assays reveal the discovery of novel promising anti-SARS-CoV-2 with dual activity. Int. J. Mol. Sci. 2021, 22, 9057. [Google Scholar] [CrossRef]
- Rahman, N.; Basharat, Z.; Yousuf, M.; Castaldo, G.; Rastrelli, L.; Khan, H. Virtual screening of natural products against type II transmembrane serine protease (TMPRSS2), the priming agent of coronavirus 2 (SARS-CoV-2). Molecules 2020, 25, 2271. [Google Scholar] [CrossRef] [PubMed]
- Salleh, M.Z.; Deris, Z.Z. In Silico molecular characterization of human TMPRSS2 protease polymorphic variants and associated SARS-CoV-2 Susceptibility. Life 2022, 12, 231. [Google Scholar] [CrossRef] [PubMed]
- Escalante, D.E.; Ferguson, D.M. Structural modeling and analysis of the SARS-CoV-2 cell entry inhibitor camostat bound to the trypsin-like protease TMPRSS2. Med. Chem. Res. 2021, 30, 399–409. [Google Scholar] [CrossRef]
- Peiffer, A.L.; Garlick, J.M.; Wu, Y.; Soellner, M.B.; Brooks, C.L.; Mapp, A.K. TMPRSS2 inhibitor discovery facilitated through an in silico and biochemical screening platform. ACS Med. Chem. Lett. 2023, 14, 860–866. [Google Scholar] [CrossRef]
- Hoffmann, M.; Hofmann-Winkler, H.; Smith, J.C.; Krüger, N.; Arora, P.; Sørensen, L.K.; Søgaard, O.S.; Hasselstrøm, J.B.; Winkler, M.; Hempel, T.; et al. Camostat mesylate inhibits SARS-CoV-2 activation by TMPRSS2-related proteases and its metabolite GBPA exerts antiviral activity. eBioMedicine 2021, 65, 103255. [Google Scholar] [CrossRef]
- Bestle, D.; Heindl, M.R.; Limburg, H.; Pilgram, O.; Moulton, H.; Stein, D.A.; Hardes, K.; Eickmann, M.; Dolnik, O.; Rohde, C.; et al. TMPRSS2 and furin are both essential for proteolytic activation of SARS-CoV-2 in human airway cells. Life Sci. Alliance 2020, 3, e202000786. [Google Scholar] [CrossRef]
- Pérez-Vargas, J.; Lemieux, G.; Thompson, C.A.H.; Désilets, A.; Ennis, S.; Gao, G.; Gordon, D.G.; Schulz, A.L.; Niikura, M.; Nabi, I.R.; et al. Nanomolar anti-SARS-CoV-2 Omicron activity of the host-directed TMPRSS2 inhibitor N-0385 and synergistic action with direct-acting antivirals. Antivir. Res. 2024, 225, 105869. [Google Scholar] [CrossRef]
- Riches, A.G.; Cablewski, T.; Glattauer, V.; Thissen, H.; Meagher, L. Scalable synthesis of an integrin-binding peptide mimetic for biomedical applications. Tetrahedron 2012, 68, 9448–9455. [Google Scholar] [CrossRef]
- Cai, L.; Han, Y.; Ren, S.; Huang, L. Dication C(R1)–N(R2)2 synthons and their use in the synthesis of formamidines, amidines, and α-aminonitriles. Tetrahedron 2000, 56, 8253–8262. [Google Scholar] [CrossRef]
- Vasalatiy, O.; Zhao, P.; Woods, M.; Marconescu, A.; Castillo-Muzquiz, A.; Thorpe, P.; Kiefer, G.E.; Sherry, A.D. Strategies for labeling proteins with PARACEST agents. Bioorg. Med. Chem. 2011, 19, 1106–1114. [Google Scholar] [CrossRef]
- Fujii, S.U.Y.; Watanabe, T.; Kayama, N.; Ono Pharmaceutical Co., Ltd. Guanidinobenzoic Acid Derivatives. U.S. Patent US4021472A, 3 May 1977. [Google Scholar]
- Pászti-Gere, E.; Czimmermann, E.; Ujhelyi, G.; Balla, P.; Maiwald, A.; Steinmetzer, T. In vitro characterization of TMPRSS2 inhibition in IPEC-J2 cells. J. Enzym. Inhib. Med. Chem. 2016, 31, 123–129. [Google Scholar] [CrossRef][Green Version]
- Tocris Bioscience (Company Website). Available online: https://www.tocris.com/products/i-432_7292 (accessed on 12 June 2025).
- De Lassauniere, P.E.C.; Auvin, S.; Bigg, D.; Auguet, M.; Harnett, J. Derivatives of 2-(Iminomethyl)amino-phenyl, Their Preparation, Their Use as Medicaments and the Pharmaceutical Compositions Containing Them. U.S. Patent US6809088B2, 26 October 2004. [Google Scholar]
- Kawasaki, Y.; Freire, E. Finding a better path to drug selectivity. Drug Discov. Today 2011, 16, 985–990. [Google Scholar] [CrossRef] [PubMed]
- Kiss, R.; Sandor, M.; Szalai, F.A. http://mcule.com: A public web service for drug discovery. J. Cheminform. 2012, 4 (Suppl. S1), P17. [Google Scholar] [CrossRef]
- Roviello, V.; Scognamiglio, P.L.; Caruso, U.; Vicidomini, C.; Roviello, G.N. Evaluating in silico the potential health and environmental benefits of houseplant volatile organic compounds for an emerging ‘indoor forest bathing’ approach. Int. J. Environ. Res. Public Health 2021, 19, 273. [Google Scholar] [CrossRef]
- Potemkin, V.; Potemkin, A.; Grishina, M. Internet resources for drug discovery and design. Curr. Top. Med. Chem. 2018, 18, 1955–1975. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Sargsyan, T.; Stepanyan, L.; Panosyan, H.; Hakobyan, H.; Israyelyan, M.; Tsaturyan, A.; Hovhannisyan, N.; Vicidomini, C.; Mkrtchyan, A.; Saghyan, A.; et al. Synthesis and Antifungal Activity of Fmoc-Protected 1,2,4-Triazolyl-α-Amino Acids and Their Dipeptides Against Aspergillus Species. Biomolecules 2025, 15, 61. [Google Scholar] [CrossRef]
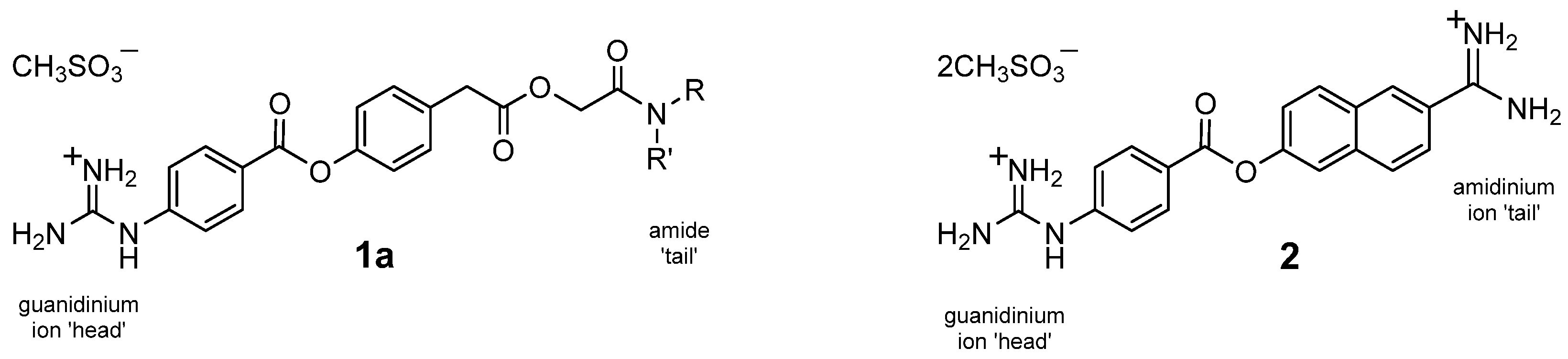

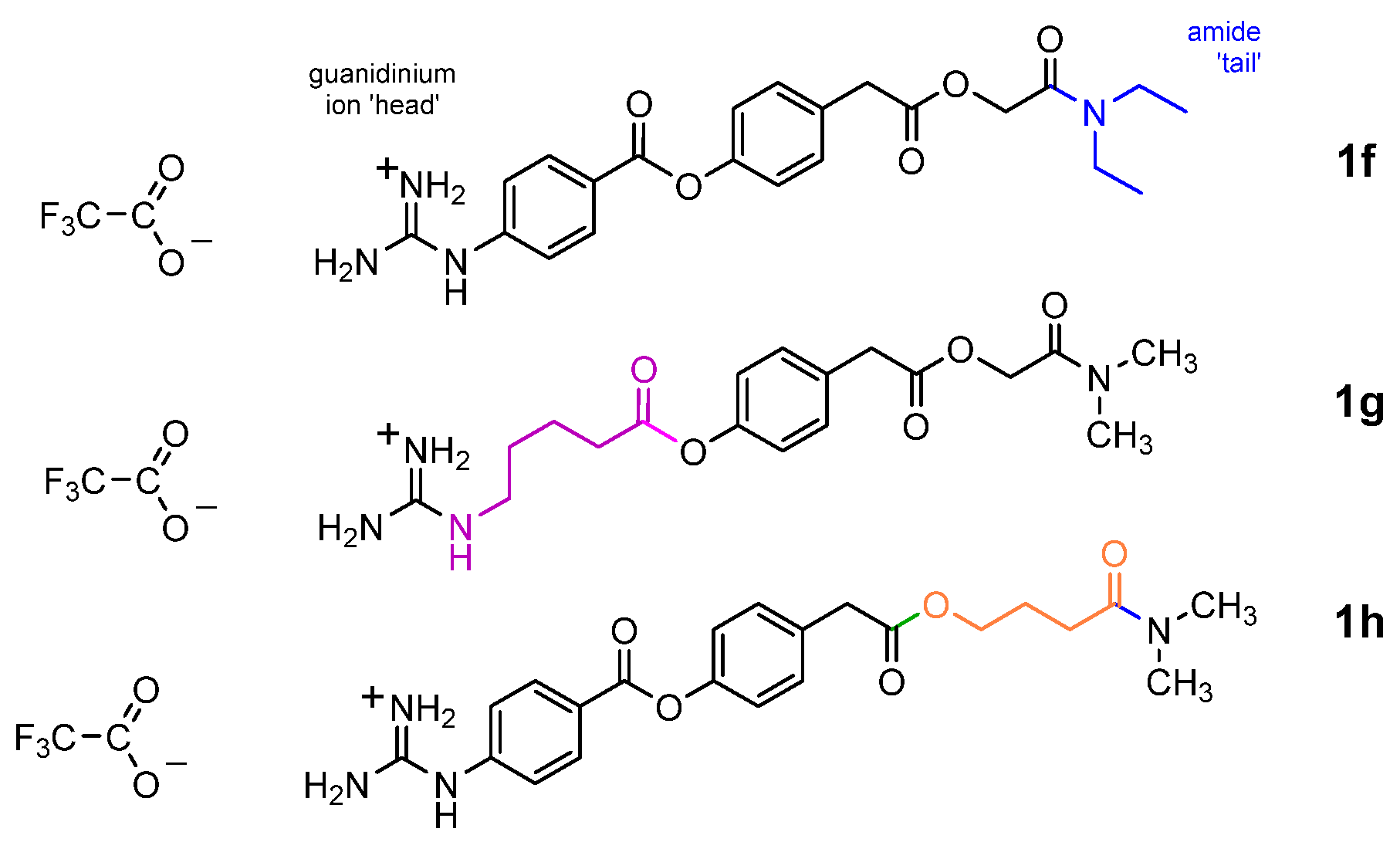
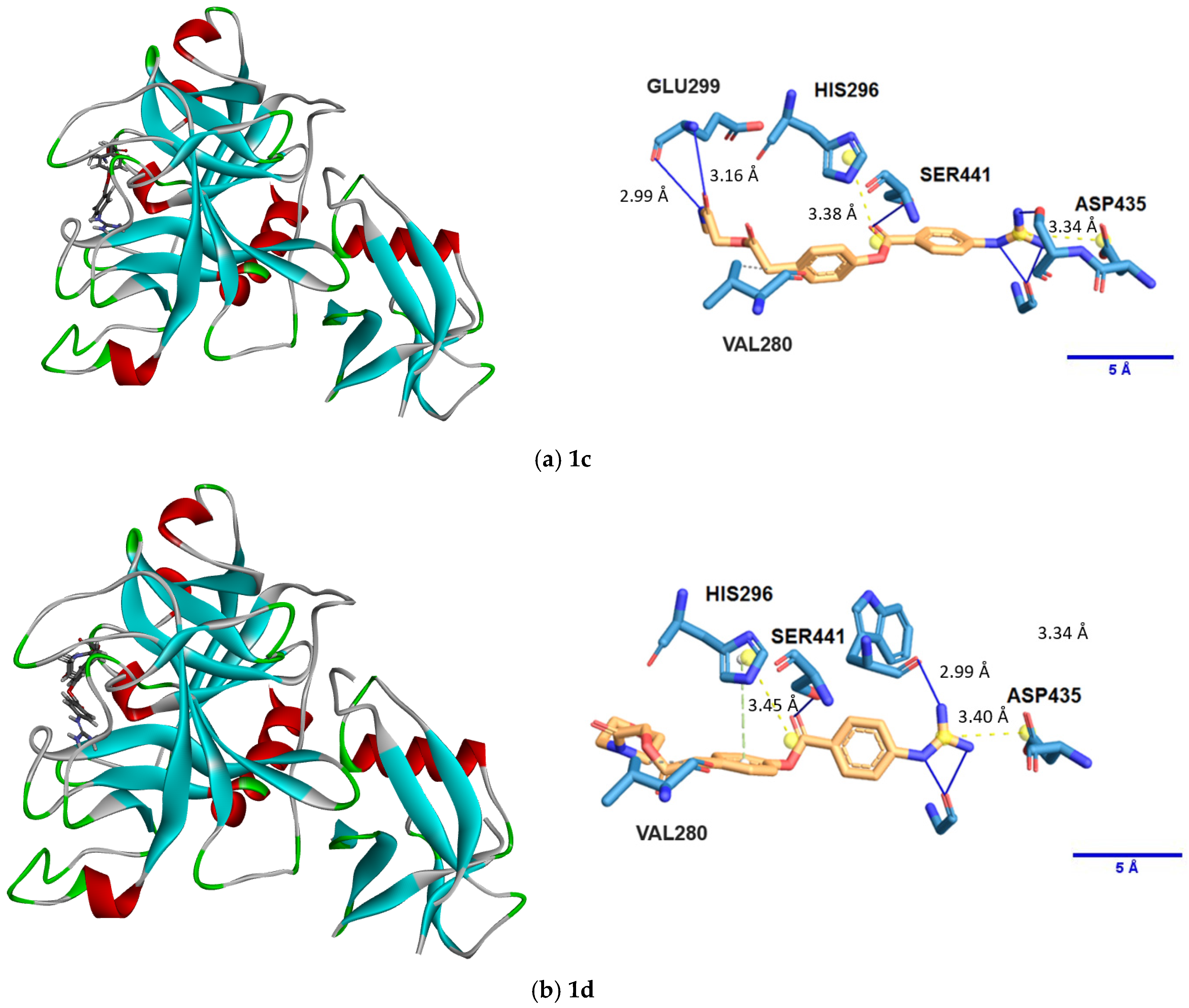
| Compound | Top Pose (kcal/mol) | Top Three Poses (ave.) (kcal/mol) | st. dev. (kcal/mol) |
|---|---|---|---|
| 1a/b | −6.6 | −6.4 | ±0.2 |
| 1c | −7.0 | −6.7 | ±0.3 |
| 1d | −7.0 | −6.6 | ±0.3 |
| 1e | −6.8 | −6.7 | ±0.1 |
| 1f | −6.0 | −5.9 | ±0.2 |
| 1g | −5.7 | −5.6 | ±0.1 |
| 1h | −6.3 | −6.1 | ±0.3 |
| Compound | IC50 (nM) | SEM |
|---|---|---|
| 1a | 1.1 | ±0.1 |
| 1b | 2.4 | ±0.2 |
| 1c | 1.3 | ±0.1 |
| 1d | 1.8 | ±0.1 |
| 1e | nd | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrara, B.T.; Thompson, E.P.; Roviello, G.N.; Gale, T.F. C-Terminal Analogues of Camostat Retain TMPRSS2 Protease Inhibition: New Synthetic Directions for Antiviral Repurposing of Guanidinium-Based Drugs in Respiratory Infections. Int. J. Mol. Sci. 2025, 26, 6761. https://doi.org/10.3390/ijms26146761
Ferrara BT, Thompson EP, Roviello GN, Gale TF. C-Terminal Analogues of Camostat Retain TMPRSS2 Protease Inhibition: New Synthetic Directions for Antiviral Repurposing of Guanidinium-Based Drugs in Respiratory Infections. International Journal of Molecular Sciences. 2025; 26(14):6761. https://doi.org/10.3390/ijms26146761
Chicago/Turabian StyleFerrara, Bill T., Elinor P. Thompson, Giovanni N. Roviello, and Thomas F. Gale. 2025. "C-Terminal Analogues of Camostat Retain TMPRSS2 Protease Inhibition: New Synthetic Directions for Antiviral Repurposing of Guanidinium-Based Drugs in Respiratory Infections" International Journal of Molecular Sciences 26, no. 14: 6761. https://doi.org/10.3390/ijms26146761
APA StyleFerrara, B. T., Thompson, E. P., Roviello, G. N., & Gale, T. F. (2025). C-Terminal Analogues of Camostat Retain TMPRSS2 Protease Inhibition: New Synthetic Directions for Antiviral Repurposing of Guanidinium-Based Drugs in Respiratory Infections. International Journal of Molecular Sciences, 26(14), 6761. https://doi.org/10.3390/ijms26146761







