Rubus caesius L. (European Dewberry) Extracts as a Novel Therapeutic Strategy Against MRSA Strains
Abstract
1. Introduction
2. Results
2.1. Phytochemical Analysis of R. caesius Extracts
2.2. Determination of Minimum Inhibitory Concentrations (MIC) and Minimum Bactericidal Concentrations (MBC)
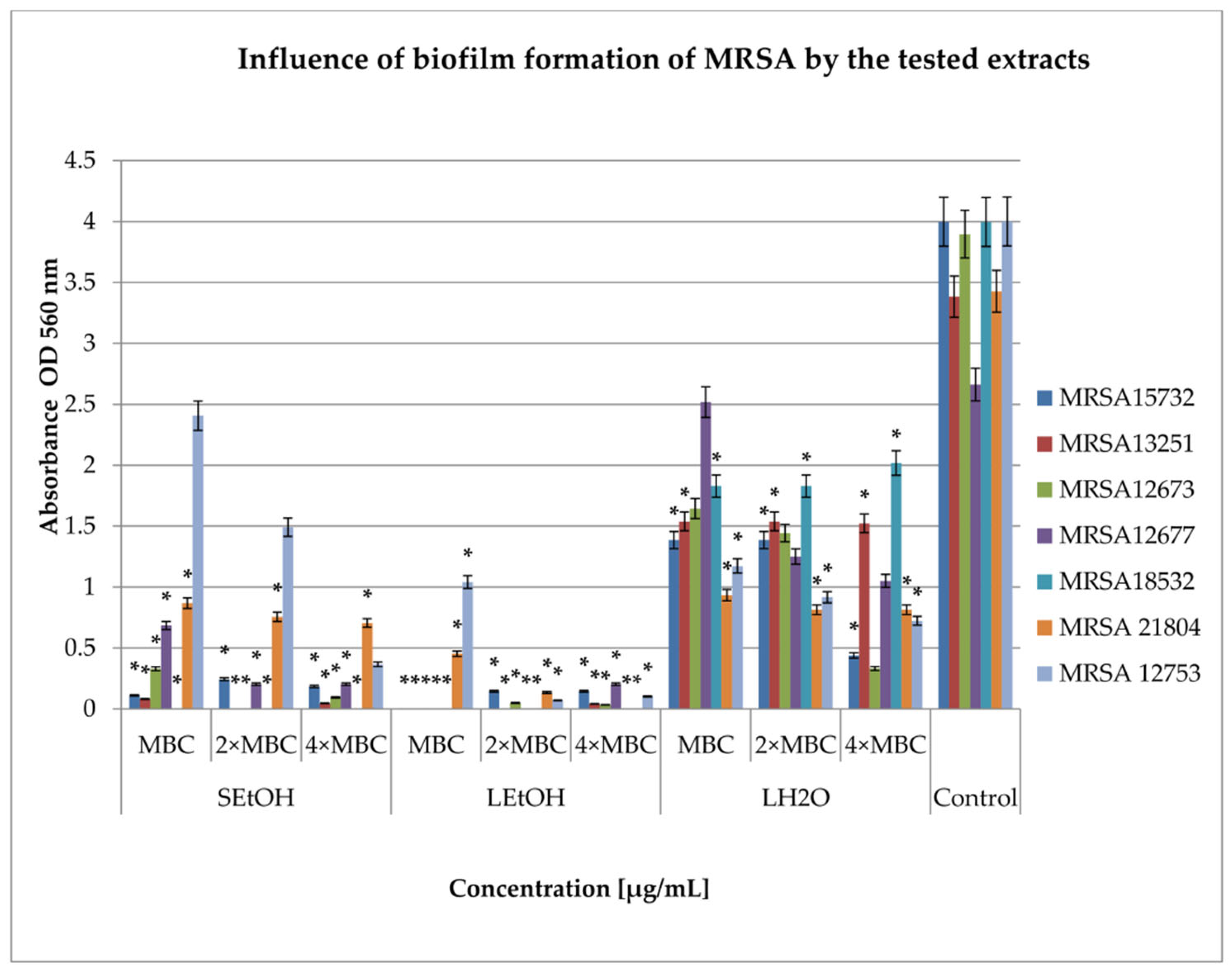
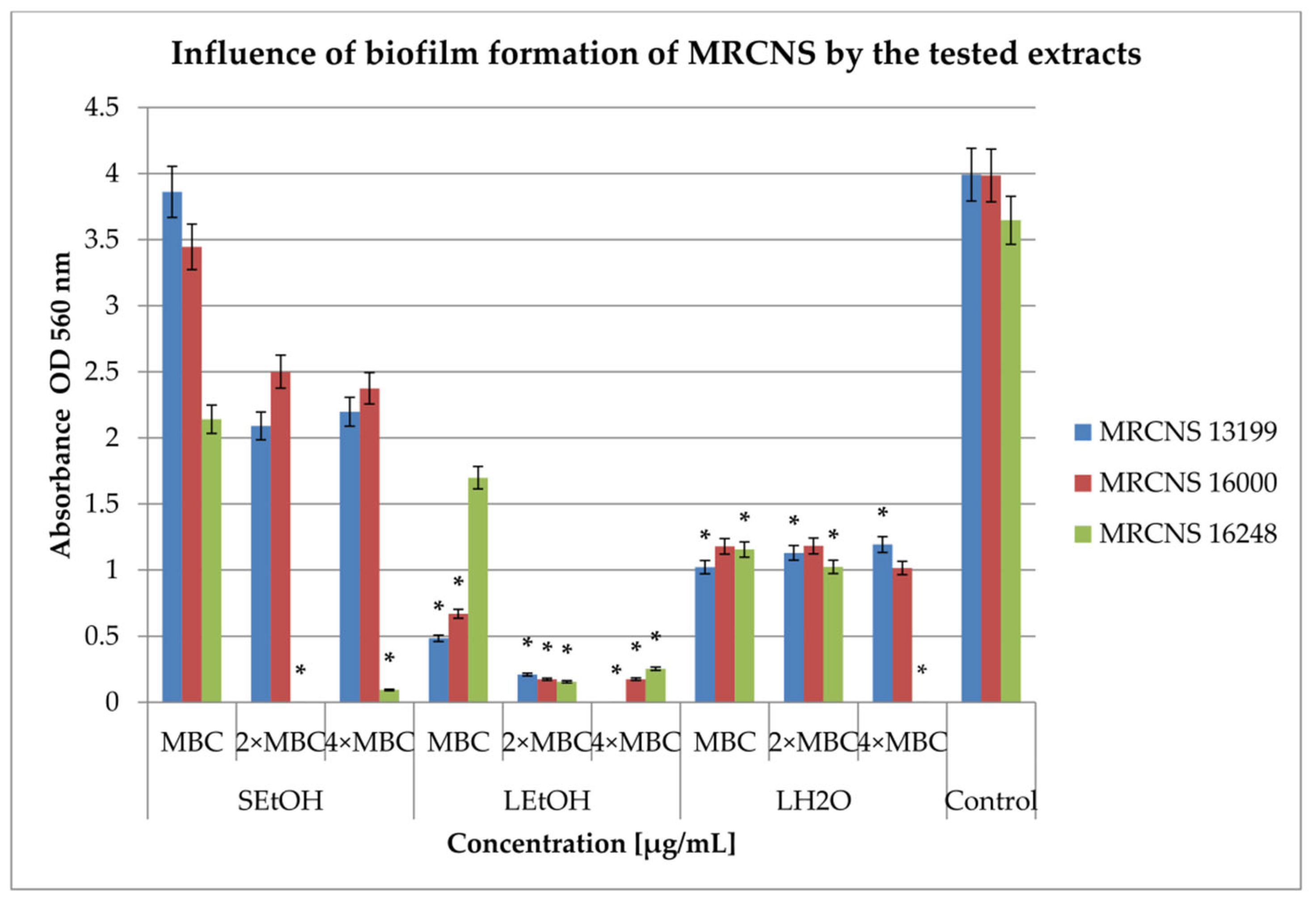
2.3. Inhibition of Biofilm Formation
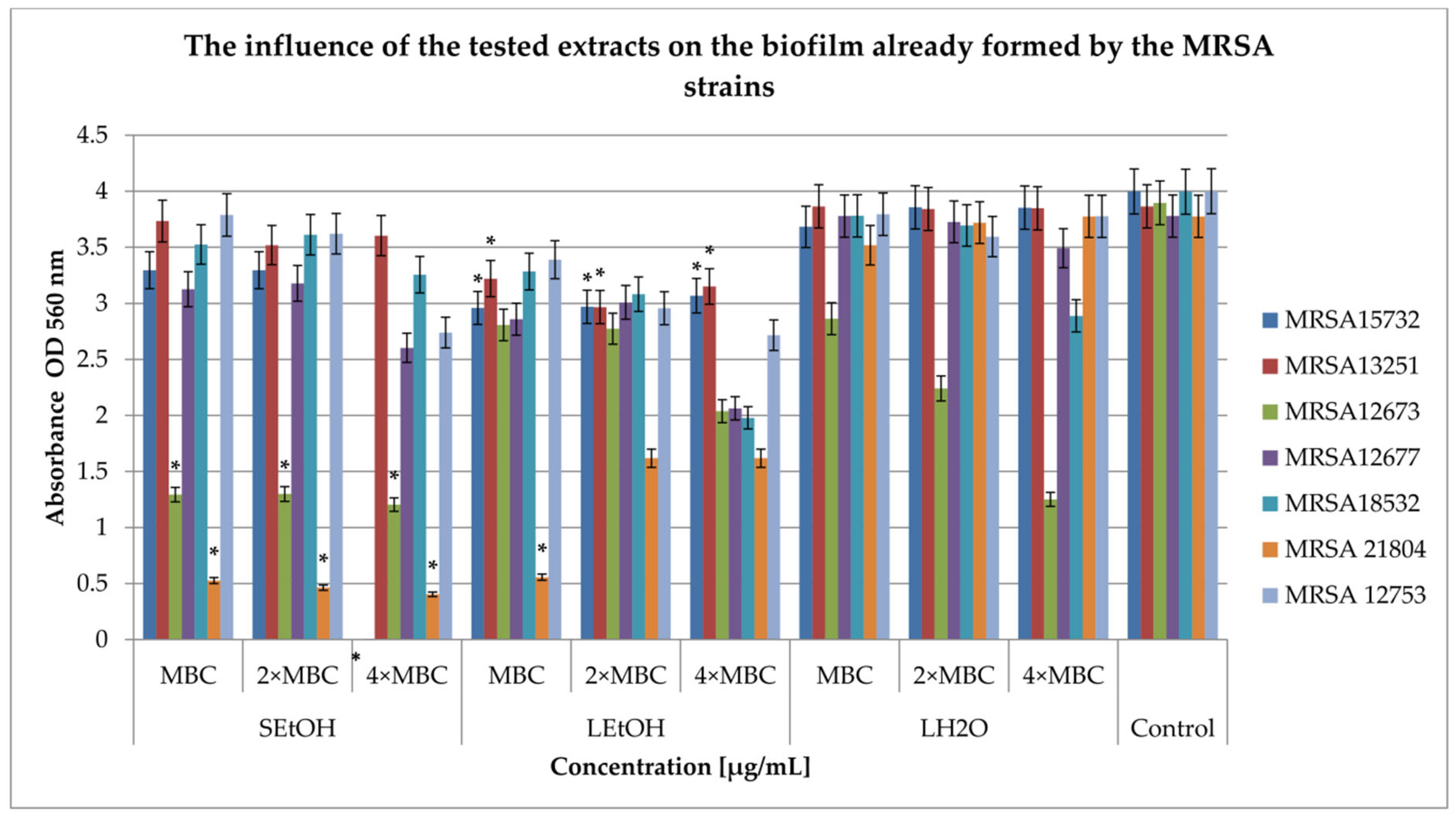
2.4. Influence on the Biofilm Formed
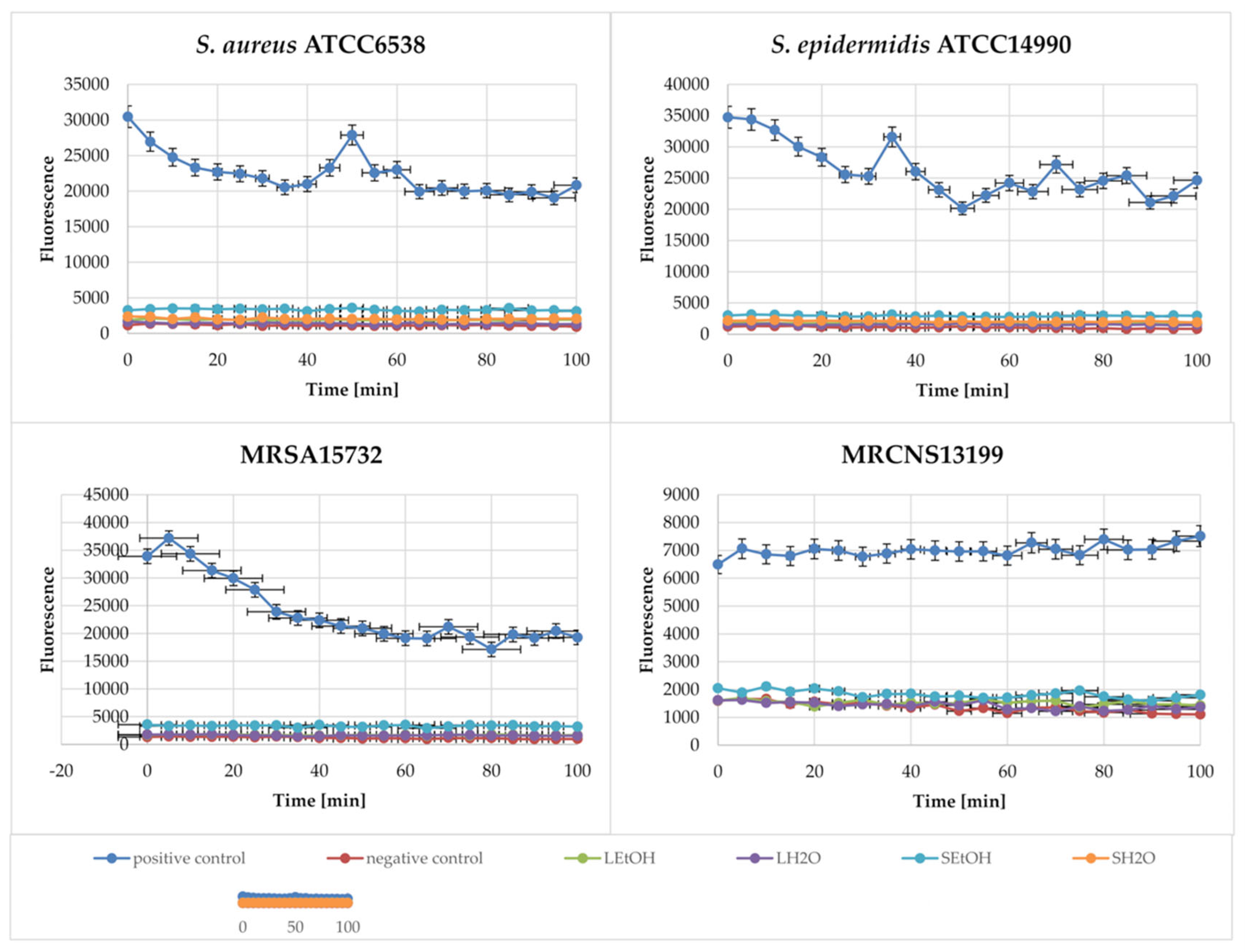
2.5. Membrane Depolarization Assay
2.6. Membrane Integrity Assay
2.7. Extract–Antibiotic Synergy Against MRSA
3. Discussion
4. Materials and Methods
4.1. Materials
4.1.1. Extract Preparation
4.1.2. Bacteria
4.1.3. Materials and Growing Conditions
4.2. Determination of Minimum Inhibitory Concentrations and Minimum Bactericidal Concentrations
4.3. Influence of Extracts on Biofilm Formation and on Pre-Formed Biofilm
4.4. 3,3-dipropylthiacarbocyanine (DiSC3(5) Assays
4.5. N-phenyl-1-naphthylamine (NPN) Assays
4.6. Checkerboard Arrays for Planktonic Bacteria (Fractional Inhibitory Concentration Index)
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MRSA | methicillin-resistant Staphylococcus aureus |
| MRCNS | methicyllin-resistant coagulase-negative Staphylococcus |
| LH2O | aqueous extract of dewberry leaves |
| LEtOH | ethanolic extract of dewberry leaves |
| SH2O | aqueous extract of dewberry stems |
| SEtOH | ethanolic extract of dewberry stems |
| ESKAPE | is an acronym comprising the scientific names of six highly virulent and antibiotic-resistant bacterial pathogens including: Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, |
| MIC | minimum inhibitory concentrations |
| MBC | minimum bactericidal concentrations |
| FICI | Fractional Inhibitor Concentration Index |
| ATCC | American Type Culture Collection |
| PCM | Polish Collection of Microorganisms |
| CFU | colony-forming unit |
References
- Baran, A.; Kwiatkowska, A.; Potocki, L. Antibiotics and Bacterial Resistance—A Short Story of an Endless Arms Race. Int. J. Mol. Sci. 2023, 24, 5777. [Google Scholar] [CrossRef] [PubMed]
- Gajdács, M.; Albericio, F. Antibiotic Resistance: From the Bench to Patients. Antibiotics 2019, 8, 129. [Google Scholar] [CrossRef] [PubMed]
- Machado, T.S.; Pinheiro, F.R.; Andre, L.S.P.; Pereira, R.F.A.; Correa, R.F.; de Mello, G.C.; Ribeiro, T.A.N.; Penna, B.; Sachs, D.; Aguiar-Alves, F. Virulence Factors Found in Nasal Colonization and Infection of Methicillin-Resistant Staphylococcus aureus (MRSA) Isolates and Their Ability to Form a Biofilm. Toxins 2021, 13, 14. [Google Scholar] [CrossRef] [PubMed]
- Derakhshan, S.; Navidinia, M.; Haghi, F. Antibiotic susceptibility of humanassociated Staphylococcus aureus and its relation to agr typing, virulence genes, and biofilm formation. Infect. Dis. 2021, 21, 627. [Google Scholar] [CrossRef]
- Mone, N.S.; Kamble, E.E.; Pardesi, K.R.; Satpute, S.K. Antibacterial and Antibiofilm Potency of Menadione Against Multidrug-Resistant S. aureus. Curr. Microbiol. 2022, 79, 282. [Google Scholar] [CrossRef]
- Kot, B.; Wierzchowska, K.; Piechota, M.; Grużewska, A. Antimicrobial resistance patterns in methicillin-resistant Staphylococcus aureus from patients hospitalized during 2015–2017 in hospitals in Poland. Med. Princ. Pract. 2020, 29, 61–68. [Google Scholar] [CrossRef]
- Hall, C.W.; Mah, T.F. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol. Rev. 2017, 41, 276–301. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Yan, S.; Zhou, C.; Liu, Q.; Zhu, H.; Wen, Z. Total flavonoids from Potentilla kleiniana Wight et Arn inhibits biofilm formation and virulence factors production in methicillin-resistant Staphylococcus aureus (MRSA). J. Ethnopharmacol. 2021, 279, 114383. [Google Scholar] [CrossRef]
- Paul, P.; Chakraborty, P.; Chatterjee, A.; Sarker, R.K.; Dastidar, D.G.; Kundu, T.; Sarkar, N.; Das, A.; Tribedi, P. 1,4-Naphthoquinone accumulates reactive oxygen species in S. aureus: A promising approach towards effective management of biofilm threat. Arch. Microbiol. 2021, 203, 1183–1193. [Google Scholar] [CrossRef]
- Salaheen, S.; Peng, M.; Joo, J.; Teramoto, H.; Biswas, D. Eradication and Sensitization of Methicillin Resistant Staphylococcus aureus to Methicillin with Bioactive Extracts of Berry Pomace. Front. Microbiol. 2017, 8, 253. [Google Scholar] [CrossRef]
- Welia, A.M.; Al-Saadi, H.S.; Al-Fudhaili, R.S.; Hossain, A.; Putit, Z.B.; Jasim, M.K. Cytotoxic and antimicrobial potential of different leaves extracts of R. fruticosus used traditionally to treat diabetes. Toxicol. Rep. 2020, 7, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Aguilera-Correa, J.J.; Nohynek, L.; Alakomi, H.-L.; Esteban, J.; Oksman-Caldentey, K.-M.; Puupponen-Pimiä, R.; Kinnari, T.J.; Perez-Tanoira, R. Reduction of methicillin-resistant Staphylococcus aureus biofilm growth and development using arctic berry extracts. Front. Cell. Infect. Microbiol. 2023, 13, 1176755. [Google Scholar] [CrossRef] [PubMed]
- Saeed, A. Antibacterial effect of Rubus chingii flower extract against multidrug-resistant bacteria. Bioinformation 2022, 18, 938–942. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, J.; Wang, L.; Cao, J.; Xu, L. Chemical composition and biological activities of the essential oil from Rubus pungens var. oldhamii. Nat. Prod. Res. 2016, 31, 1454–1458. [Google Scholar] [CrossRef]
- Krauze-Baranowska, M.; Głód, D.; Kula, M.; Majdan, M.; Hałasa, R.; Matkowski, M.; Kozłowska, W.; Kawiak, A. Chemical composition and biological activity of Rubus idaeus shoots—A traditional herbal remedy of Eastern Europe. BMC Complement. Altern. Med. 2014, 14, 480. [Google Scholar] [CrossRef]
- Krauze-Baranowska, M.; Majdan, M.; Hałasa, R.; Głod, D.; Kula, M.; Feckac, I.; Orzeł, A. The antimicrobial activity of fruits from some cultivar varieties of Rubus idaeus and Rubus occidentalis. Food Funct. 2014, 5, 2536. [Google Scholar] [CrossRef]
- Hering, A.; Stefanowicz-Hajduk, J.; Hałasa, R.; Olech, M.; Nowak, R.; Kosinski, P.; Ochocka, J.R. Polyphenolic Characterization, Antioxidant, Antihyaluronidase and Antimicrobial Activity of Young Leaves and Stem Extracts from Rubus caesius L. Molecules 2022, 27, 6181. [Google Scholar] [CrossRef]
- Oulahal, N.; Degraeve, P. Phenolic-Rich Plant Extracts With Antimicrobial Activity: An Alternative to Food Preservatives and Biocides? Front. Microbiol. 2022, 12, 753518. [Google Scholar] [CrossRef]
- Benfield, A.H.; Henriques, S.T. Mode-of-Action of Antimicrobial Peptides: Membrane Disruption vs. Intracellular Mechanisms. Front. Med. Technol. 2020, 2, 610997. [Google Scholar] [CrossRef]
- Grabek-Lejko, D.; Miłek, M.; Sidor, E.; Puchalski, C.; Dzugan, M. Antiviral and Antibacterial Effect of Honey Enriched with Rubus spp. as a Functional Food with Enhanced Antioxidant Properties. Molecules 2022, 27, 4859. [Google Scholar] [CrossRef]
- De Santis, D.; Carbone, K.; Garzoli, S.; Laghezza Masci, V.; Turchetti, G. Bioactivity and Chemical Profile of Rubus idaeus L. Leaves Steam-Distillation Extract. Foods 2022, 11, 1455. [Google Scholar] [CrossRef] [PubMed]
- Krzepiłko, A.; Prażak, R.; Święciło, A. Chemical Composition, Antioxidant and Antimicrobial Activity of Raspberry, Blackberry and Raspberry-Blackberry Hybrid Leaf Buds. Molecules 2021, 26, 327. [Google Scholar] [CrossRef] [PubMed]
- Grabek-Lejko, D.; Wójtowicz, K. Comparison of antibacterial and antioxidant properties of fruits and leaves of blackberry (Rubus plicatus) and raspberry (Rubus idaeus). J. Microbiol. Biotechnol. Food Sci. 2014, 3, 514–518. [Google Scholar]
- Quave, C.L.; Estevez-Carmona, M.; Compadre, C.M.; Hobby, G.; Hendrickson, H.P.; Beenken, K.E.; Smeltzer, M.S. Ellagic acid derivatives from Rubus ulmifolius inhibit Staphylococcus aureus biofilm formation and improve response to antibiotics. PLoS ONE 2012, 7, 28737. [Google Scholar] [CrossRef]
- Quavea, C.L.; Plano, L.R.W.; Pantuso, T.; Bennett, B.C. Effects of extracts from Italian medicinal plants on planktonic growth, biofilm formation and adherence of methicillin-resistant Staphylococcus aureus. J. Ethnopharmacol. 2008, 118, 418–428. [Google Scholar] [CrossRef]
- Reygaert, W.C. The antimicrobial possibilities of green tea. Front. Microbiol. 2014, 5, 434. [Google Scholar] [CrossRef]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef]
- Sanver, D.; Murray, B.S.; Sadeghpour, A.; Rappolt, M.; Nelson, A.L. Experimental modeling of flavonoid-biomembrane interactions. Langmuir 2016, 32, 13234–13243. [Google Scholar] [CrossRef]
- Zhang, L.; Kong, Y.; Wu, D.; Zhang, H.; Wu, J.; Chen, J.; Ding, J.; Hu, L.; Jiang, H.; Shen, X. Three flavonoids targeting the beta-hydroxyacyl-acyl carrier protein dehydratase from Helicobacter pylori: Crystal structure characterization with enzymatic inhibition assay. Protein Sci. 2008, 17, 1971–1978. [Google Scholar] [CrossRef]
- Wu, D.; Kong, Y.; Han, C.; Chen, J.; Hu, L.; Jiang, H.; Shen, X. DAlanine: D-alanine ligase as a new target for the flavonoids quercetin and apigenin. Int. J. Antimicrob. Agents 2008, 32, 421–426. [Google Scholar] [CrossRef]
- Suriyanarayanan, B.; Shanmugam, K.; Santhosh, R. Synthetic quercetin inhibits mycobacterial growth possibly by interacting with DNA gyrase. Rom. Biotechnol. Lett. 2013, 18, 1587–1593. [Google Scholar]
- Chinnam, N.; Dadi, P.K.; Sabri, S.A.; Ahmad, M.; Kabir, M.A.; Ahmad, Z. Dietary bioflavonoids inhibit Escherichia coli ATP synthase in a differential manner. Int. J. Biol. Macromol. 2010, 46, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Raheem, N.; Straus, S.K. Mechanisms of Action for Antimicrobial Peptides With Antibacterial and Antibiofilm Functions. Front. Microbiol. 2019, 10, 2866. [Google Scholar] [CrossRef] [PubMed]
- Vikram, A.; Jayaprakasha, G.K.; Jesudhasan, P.R.; Pillai, S.D.; Patil, B.S. Suppression of bacterial cell–cell signalling, biofilm formation and type III secretion system by citrus flavonoids. J. Appl. Microbiol. 2010, 109, 515–527. [Google Scholar] [CrossRef]
- Ouyang, J.; Sun, F.; Feng, W.; Sun, Y.; Qiu, X.; Xiong, L.; Liu, Y.; Chen, Y. Quercetin is an effective inhibitor of quorum sensing, biofilm formation and virulence factors in Pseudomonas aeruginosa. J. Appl. Microbiol. 2016, 120, 966–974. [Google Scholar] [CrossRef]
- Symeonidis, A.; Marangos, M. Iron and microbial growth. In Insight and Control of Infectious Disease in Global Scenario; Priti, D.R., Ed.; InTechOpen: London, UK, 2012. [Google Scholar]
- Fontaine, B.M.; Nelson, K.; Lyles, J.T.; Jariwala, P.B.; García-Rodriguez, J.M.; Quave, C.L.; Weinert, E.E. Identification of Ellagic Acid Rhamnoside as a Bioactive Component of a Complex Botanical Extract with Anti-biofilm Activity. Front. Microbiol. 2017, 8, 496. [Google Scholar] [CrossRef]
- Cushnie, T.P.; Taylor, P.W.; Nagaoka, Y.; Uesato, S.; Hara, Y.; Lamb, A.J. Investigation of the antibacterial activity of 3-Ooctanoyl-(-)- epicatechin. J. Appl. Microbiol. 2008, 105, 1461–1469. [Google Scholar] [CrossRef]
- Hałasa, R.; Mizerska, U.; Kula, M.; Krauze-Baranowska, M. Screening Tests for the Interaction of Rubus idaeus and Rubus occidentalis Extracts with Antibiotics against Gram-Positive and Gram-Negative Human Pathogens. Antibiotics 2024, 13, 653. [Google Scholar] [CrossRef]
- Savedra, M.J.; Borges, A.; Dias, C.; Aires, A.; Bennett, R.N.; Rosa, E.S.; Simões, M. Antimicrobial activity of phenolics and glucosinolate hydrolysis products and their synergy with streptomycin against pathogenic bacteria. Med. Chem. 2010, 6, 174–183. [Google Scholar] [CrossRef]
- Wang, S.; Yao, J.; Zhou, B.; Yang, J.; Chaudry, M.T.; Wang, M.; Fenglin, X.; Li, Y.; Yin, W. Bacteriostatic effect of quercetin as an antibiotic alternative in vivo and its antibacterial mechanism in vitro. J. Food Prot. 2018, 81, 68–78. [Google Scholar] [CrossRef]
- Miklasińska-Majdanik, M.; Kępa, M.; Wojtyczka, R.D.; Idzik, D.; Wąsik, T.J. Phenolic Compounds Diminish Antibiotic Resistance of Staphylococcus aureus Clinical Strains. Int. J. Environ. Res. Public Health 2018, 15, 2321. [Google Scholar] [CrossRef] [PubMed]
- Marni, S.; D’Addariob, C.; Colacevichc, A.; Focardi, S.; Borghinic, F.; Santuccid, A.; Figurae, N.; Rossi, C. Antimicrobial activity against Helicobacter pylori strains and antioxidant properties of blackberry leaves (Rubus ulmifolius) and isolated compounds. Int. J. Antimicrob. Agents 2009, 34, 50–59. [Google Scholar] [CrossRef]
- Kepa, M.; Miklasinska-Majdanik, M.; Wojtyczka, R.D.; Idzik, D.; Korzeniowski, K.; Smolen-Dzirba, J.; Wasik, T.J. Antimicrobial Potential of Caffeic Acid against Staphylococcus aureus ClinicalStrains. Biomed. Res. Int. 2018, 2018, 7413504. [Google Scholar] [CrossRef] [PubMed]
- Siriwong, S.; Thumanu, K.; Hengpratom, T.; Eumkeb, G. Synergy and mode of action of ceftazidime plus quercetin or luteolin on Streptococcus pyogenes. Evid. Based Complement. Alternat. Med. 2015, 2015, 759459. [Google Scholar] [CrossRef]
- Zhao, W.H.; Hu, Z.Q.; Okubo, S.; Hara, Y.; Shimamura, T. Mechanism of synergy between epigallocatechin gallate and beta-lactams against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2001, 45, 1737–1742. [Google Scholar] [CrossRef]
- Zhao, W.H.; Hu, Z.Q.; Okubo, S.; Hara, Y.; Shimamura, T. Inhibition of penicillinase by epigallocatechin gallate resulting in restoration of antibacterial activity of penicillin against penicillinase-producing Staphylococcus aureus. Antimicrob. Agents Chemother. 2002, 46, 2266–2268. [Google Scholar] [CrossRef]
- Novy, P.; Rondevaldova, J.; Kourimska, L.; Kokoska, L. Synergistic interactions of epigallocatechin gallate and oxytetracycline against various drug resistant Staphylococcus aureus strains in vitro. Phytomedicine 2013, 20, 432–435. [Google Scholar] [CrossRef]
- Hu, Z.Q.; Zhao, W.H.; Asano, N.; Yoda, Y.; Hara, Y.; Shimamura, T. Epigallocatechin gallate synergistically enhances the activity of carbapenems against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2002, 46, 558–560. [Google Scholar] [CrossRef]
- Bułakowska, A.; Sławiński, J.; Hałasa, R.; Hering, A.; Gucwa, M.; Ochocka, J.R.; Stefanowicz-Hajduk, J. An In Vitro Antimicrobial, Anticancer and Antioxidant Activity of N–[(2–Arylmethylthio)phenylsulfonyl] cinnamamide Derivatives. Molecules 2023, 28, 3087. [Google Scholar] [CrossRef]
- Cheng, M.; Huang, J.X.; Ramu, S.; Butler, M.S.; Cooper, M.A. Ramoplanin at Bactericidal Concentrations Induces Bacterial Membrane Depolarization in Staphylococcus aureus. Antimicrob. Agents Chemother. 2014, 58, 6819–6827. [Google Scholar] [CrossRef]
- Cheng, J.T.; Hale, J.D.; Elliot, M.; Hancock, R.E.; Straus, S.K. Effect of membrane composition on antimicrobial peptides aurein 2.2 and 2.3 from Australian southern bell frogs. Biophys. J. 2009, 96, 552–565. [Google Scholar] [CrossRef] [PubMed]
- Ombredane, A.S.; Martins, N.O.; de Souza, G.M.V.; Araujo, V.H.S.; Szlachetka, Í.O.; da Silva, S.W.; da Rocha, M.C.O.; Oliveira, A.S.d.; Holanda, C.A.; Romeiro, L.A.S.; et al. Combinatory Effect of Pequi Oil (Caryocar brasiliense)-Based Nanoemulsions Associated to Docetaxel and Anacardic Acid (Anacardium occidentale) in Triple Negative Breast Cancer Cells In Vitro. Pharmaceutics 2024, 16, 1170. [Google Scholar] [CrossRef] [PubMed]
- Jacob, B.; Kim, Y.; Hyun, J.K.; Park, I.S.; Bang, J.K.; Shin, S.Y. Bacterial killing mechanism of sheep myeloid antimicrobial peptide-18 (SMAP-18) and its Trp-substituted analog with improved cell selectivity and reduced mammalian cell toxicity. Amino Acids 2014, 46, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Turecka, K.; Chylewska, A.; Kawiak, A.; Waleron, K.F. Antifungal Activity and Mechanism of Action of the Co(III) Coordination Complexes With Diamine Chelate Ligands Against Reference and Clinical Strains of Candida spp. Front. Microbiol. 2018, 9, 1594. [Google Scholar] [CrossRef]

| Bacteria | Gramicidin | Extracts [mg/mL] | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SEtOH | SH2O | LEtOH | LH2O | |||||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| S. AUREUS ATCC6538 | 0.03125 ±0.15 | >0.125 | 0.16 ±0.40 | 10 ±0.58 | 0.16 ±0.40 | 5 ±0.32 | 0.16 ±0.40 | 10 ±0.55 | 0.16 ±0.20 | 5 ±0.54 |
| S. EPIDERMIDIS ATCC14990 | 0.00196 ±0.20 | 0.125 ±0.50 | 0.625 ±0.40 | 5 ±0.52 | 0.04 ±0.15 | >10 | 0.625 ±0.50 | 5 ±0.49 | 10 ±0.54 | 10 ±0.65 |
| S. AUREUS ATCC43300 MRSA | 0.125 ±0.14 | >0.125 | 0.625 ±0.40 | 5 ±0.38 | 0.3125 ±0.20 | 10 ±0.10 | 0.625 ±0.20 | 5 ±0.38 | 0.16 ±0.42 | 5 ±0.12 |
| MRSA 12673 | 0.03125 ±0.22 | >0.125 | 0.39 ±0.10 | 1.56 ±0.40 | 1.56 ±0.50 | >12.5 | 0.78 ±0.50 | 12.5 ±0.54 | 0.39 ±0.10 | 3.125 ±0.40 |
| MRSA 15732 | 0.06250 ±0.24 | 0.125 ±0.50 | 1.56 ±0.60 | 12.5 ±0.65 | 0.78 ±0.10 | >12.5 | 0.78 ±0.40 | 6.25 ±0.40 | 0.78 ±0.30 | 12.5 ±0.40 |
| MRSA 12677 | >0.250 | >0.125 | 0.39 ±0.50 | 6.25 ±0.52 | 3.125 ±0.51 | >12.5 | 1.56 ±0.64 | 6.25 ±0.43 | 0.78 ±0.25 | 6.25 ±0.55 |
| MRSA 12753 | 0.0625 ±0.17 | >0.125 | 0.78 ±0.52 | 0.78 ±0.48 | 0.78 ±0.35 | >12.5 | 1.56 ±0.52 | 1.56 ±0.12 | 1.56 ±0.56 | 3.125 ±0.18 |
| MRSA 13251 | 0.01563 ±0.20 | 0.125 | 0.39 ±0.25 | 1.56 ±0.44 | 3.125 ±0.52 | >12.5 | 0.78 ±0.45 | 3.125 ±0.52 | 0.78 ±0.56 | 1.56 ±0.23 |
| MRSA 18532 | 0.03125 ±0.18 | >0.125 | 3.125 ±0.45 | 3.125 ±0.58 | 12.5 ±0.56 | 12.5 ±0.10 | 0.78 ±0.61 | 1.56 ±0.48 | 0.78 ±0.43 | 1.56 ±0.65 |
| MRSA 21804 | 0.03125 ±0.21 | >0.125 | 0.39 ±0.18 | 0.78 ±0.41 | >12.5 | >12.5 | 0.78 ±0.65 | 1.56 ±0.50 | 0.78 ±0.68 | 1.56 ±0.58 |
| MRSE 13199 | 0.00024 ±0.12 | 0.01563 ±0.18 | 0.78 ±0.28 | 0.78 ±0.65 | 0.78 ±0.24 | >12.5 | 1.56 ±0.54 | 3.125 ±0.22 | 0.78 ±0.12 | 0.78 ±0.48 |
| MRCNS 16000 | >0.250 | >0.125 | 0.39 ±0.65 | 0.78 ±0.54 | 1.56 ±0.35 | >12.5 | 0.78 ±0.58 | 1.56 ±0.32 | 0.78 ±0.25 | 1.56 ±0.56 |
| MRCNS 16248 | 0.00098 ±0.21 | 0.0625 ±0.22 | 0.78 ±0.56 | 1.56 ±0.54 | >12.5 | >12.5 | 1.56 ±0.23 | 1.56 ±0.45 | 3.125 ±0.21 | 6.25 ±0.25 |
| S. aureus ATCC6538 | S. epidermidis ATCC14990 | MRSA15732 | MRCNS13199 | |
|---|---|---|---|---|
| SEtOH | 298.58 ± 0.15 | 113.75 ± 0.21 | 62.24 ± 0.21 | 141.43 ± 0.10 |
| SH2O | −1.85 ± 0.10 | −6.90 ± 0.11 | −0.78 ± 0.23 | −33.93 ± 0.12 |
| LEtOH | 165.03 ± 0.12 | 43.97 ± 0.34 | 73.00 ± 0.12 | −11.28 ± 0.23 |
| LH2O | −0.38 ± 0.23 | −12.66 ± 0.42 | −14.65 ± 0.10 | −59.63 ± 0.25 |
| Strains | Antibiotic | MIC Antibiotic [mg/mL] | R. caesius Extract | MIC Extract [mg/mL] | FICI | Outcome | ||
|---|---|---|---|---|---|---|---|---|
| Alone | Comb. | Alone | Comb. | |||||
| MRSA12673 | amikacin | 0.003906 | 0.001953 | SEtOH | 1.563 | 0.781 | 1.000 | additivity |
| 0.015625 | SH2O | 3.125 | 3.125 | 4.995 | antagonism | |||
| 0.000490 | LEtOH | 6.250 | 0.781 | 0.250 | synergy | |||
| 0.015625 | LH2O | 3.125 | 1.563 | 4.495 | antagonism | |||
| cefoxitin | 0.250000 | 0.015625 | SEtOH | 1.563 | 0.390 | 0.281 | synergy | |
| 0.500000 | SH2O | 6.250 | 6.250 | 2.000 | additivity | |||
| 0.015625 | LEtOH | 3.125 | 0.781 | 0.313 | synergy | |||
| 0.015625 | LH2O | 0.390 | 0.195 | 0.563 | semi-synergy | |||
| MRSA15732 | amikacin | 0.003906 | 0.001953 | SEtOH | 1.563 | 0.781 | 1.000 | additivity |
| 0.062500 | SH2O | 12.500 | 12.500 | 17.000 | antagonism | |||
| 0.001953 | LEtOH | 6.250 | 1.563 | 0.750 | semi-synergy | |||
| 0.031250 | LH2O | 1.563 | 1.563 | 9.000 | antagonism | |||
| cefoxitin | 0.250000 | 0.062500 | SEtOH | 1.563 | 0.781 | 0.750 | semi-synergy | |
| 0.125000 | SH2O | 6.250 | 3.125 | 1.000 | additivity | |||
| 0.031250 | LEtOH | 6.250 | 0.781 | 0.250 | synergy | |||
| 0.031250 | LH2O | 3.125 | 0.391 | 0.250 | synergy | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivashchanka, Y.; Hering, A.; Kastsevich, A.; Stefanowicz-Hajduk, J.; Hałasa, R. Rubus caesius L. (European Dewberry) Extracts as a Novel Therapeutic Strategy Against MRSA Strains. Int. J. Mol. Sci. 2025, 26, 6754. https://doi.org/10.3390/ijms26146754
Ivashchanka Y, Hering A, Kastsevich A, Stefanowicz-Hajduk J, Hałasa R. Rubus caesius L. (European Dewberry) Extracts as a Novel Therapeutic Strategy Against MRSA Strains. International Journal of Molecular Sciences. 2025; 26(14):6754. https://doi.org/10.3390/ijms26146754
Chicago/Turabian StyleIvashchanka, Yahor, Anna Hering, Alina Kastsevich, Justyna Stefanowicz-Hajduk, and Rafał Hałasa. 2025. "Rubus caesius L. (European Dewberry) Extracts as a Novel Therapeutic Strategy Against MRSA Strains" International Journal of Molecular Sciences 26, no. 14: 6754. https://doi.org/10.3390/ijms26146754
APA StyleIvashchanka, Y., Hering, A., Kastsevich, A., Stefanowicz-Hajduk, J., & Hałasa, R. (2025). Rubus caesius L. (European Dewberry) Extracts as a Novel Therapeutic Strategy Against MRSA Strains. International Journal of Molecular Sciences, 26(14), 6754. https://doi.org/10.3390/ijms26146754









