Microbiome-Based Products: Therapeutic Potential for Inflammatory Skin Diseases
Abstract
1. Introduction
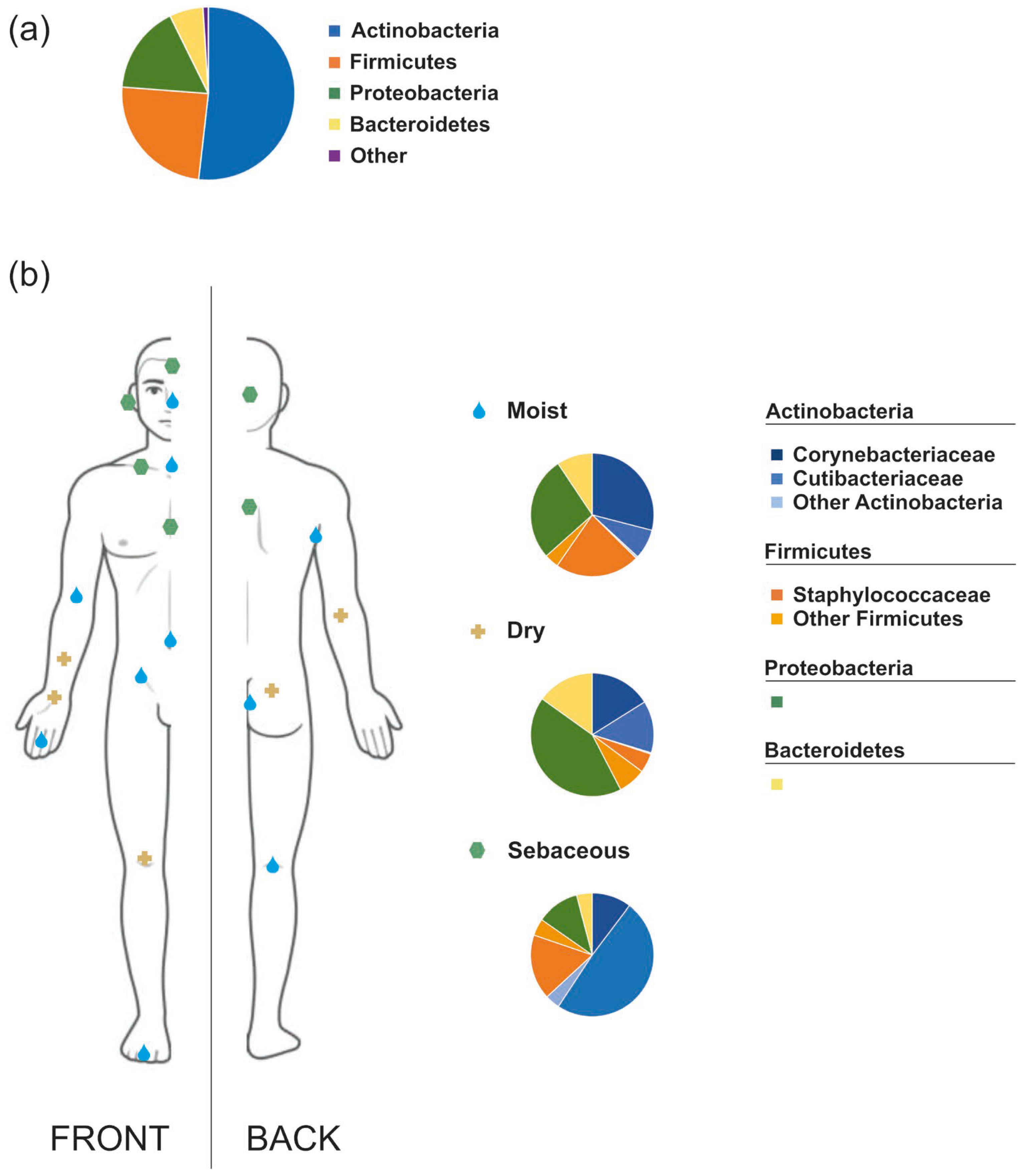
2. The Human Skin Microbiota
3. Skin Microbiota Dysbiosis and Inflammatory Skin Diseases
3.1. Atopic Dermatitis
3.2. Seborrheic Dermatitis
3.3. Acne Vulgaris
3.4. Psoriasis
3.5. Rosacea
4. Microbiome-Based Products and Skin Health—Treatment Approaches
4.1. Topical MBPs
4.2. Oral MBPs
4.3. Regulatory Landscape
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AD | Atopic dermatitis |
| AMP | Antimicrobial peptides |
| CFU | Colony forming units |
| FDA | Food and Drug Administration |
| GRAS | Generally Recognized as Safe |
| ISDs | Inflammatory skin diseases |
| LBP | Live biotherapeutic product |
| MBP | Microbiome-based product |
| SCORAD | Scoring atopic dermatitis |
| SD | Seborrheic dermatitis |
References
- Mousa, W.K.; Chehadeh, F.; Husband, S. Recent Advances in Understanding the Structure and Function of the Human Microbiome. Front. Microbiol. 2022, 13, 825338. [Google Scholar] [CrossRef]
- De Almeida, C.V.; Antiga, E.; Lulli, M. Oral and Topical Probiotics and Postbiotics in Skincare and Dermatological Therapy: A Concise Review. Microorganisms 2023, 11, 1420. [Google Scholar] [CrossRef] [PubMed]
- Hrestak, D.; Matijašić, M.; Paljetak, H.Č.; Drvar, D.L.; Hadžavdić, S.L.; Perić, M. Skin Microbiota in Atopic Dermatitis. Int. J. Mol. Sci. 2022, 23, 3503. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Cruz, S.; Orozco-Covarrubias, L.; Sáez-de-Ocariz, M. The Human Skin Microbiome in Selected Cutaneous Diseases. Front. Cell Infect. Microbiol. 2022, 12, 834135. [Google Scholar] [CrossRef] [PubMed]
- Sanford, J.A.; Gallo, R.L. Functions of the Skin Microbiota in Health and Disease. Semin. Immunol. 2013, 25, 370–377. [Google Scholar] [CrossRef]
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The Human Skin Microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, M. Skin Barrier Function and the Microbiome. Int. J. Mol. Sci. 2022, 23, 13071. [Google Scholar] [CrossRef]
- Lee, E.S.; Song, E.J.; Nam, Y.-D.; Lee, S.Y. Probiotics in Human Health and Disease: From Nutribiotics to Pharmabiotics. J. Microbiol. 2018, 56, 773–782. [Google Scholar] [CrossRef]
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.C.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome Definition Re-Visited: Old Concepts and New Challenges. Microbiome 2020, 8, 103. [Google Scholar] [CrossRef]
- Nguyen, U.T.; Kalan, L.R. Forgotten Fungi: The Importance of the Skin Mycobiome. Curr. Opin. Microbiol. 2022, 70, 102235. [Google Scholar] [CrossRef]
- Modi, S.R.; Lee, H.H.; Spina, C.S.; Collins, J.J. Antibiotic Treatment Expands the Resistance Reservoir and Ecological Network of the Phage Metagenome. Nature 2013, 499, 219. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Scott, K.P.; Rastall, R.A.; Tuohy, K.M.; Hotchkiss, A.; Dubert-Ferrandon, A.; Gareau, M.; Murphy, E.F.; Saulnier, D.; Loh, G.; et al. Dietary Prebiotics: Current Status and New Definition. Food Sci. Technol. Bull. Funct. Foods 2010, 7, 1–19. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.; Cordaillat-Simmons, M.; Pot, B.; Druart, C. The Regulatory Framework for Microbiome-Based Therapies: Insights into European Regulatory Developments. NPJ Biofilms Microbiomes 2025, 11, 53. [Google Scholar] [CrossRef]
- McLoughlin, I.J.; Wright, E.M.; Tagg, J.R.; Jain, R.; Hale, J.D.F. Skin Microbiome—The Next Frontier for Probiotic Intervention. Probiotics Antimicrob. Proteins 2021, 14, 630–647. [Google Scholar] [CrossRef]
- Iwase, T.; Uehara, Y.; Shinji, H.; Tajima, A.; Seo, H.; Takada, K.; Agata, T.; Mizunoe, Y. Staphylococcus Epidermidis Esp Inhibits Staphylococcus Aureus Biofilm Formation and Nasal Colonization. Nature 2010, 465, 346–349. [Google Scholar] [CrossRef]
- Belkaid, Y.; Serge, J.A. Dialogue between Skin Microbiota and Immunity. Science 2014, 346, 954–959. [Google Scholar] [CrossRef]
- Skowron, K.; Bauza-Kaszewska, J.; Kraszewska, Z.; Wiktorczyk-Kapischke, N.; Grudlewska-Buda, K.; Kwiecińska-Piróg, J.; Wałecka-Zacharska, E.; Radtke, L.; Gospodarek-Komkowska, E. Human Skin Microbiome: Impact of Intrinsic and Extrinsic Factors on Skin Microbiota. Microorganisms 2021, 9, 543. [Google Scholar] [CrossRef]
- Saheb Kashaf, S.; Proctor, D.M.; Deming, C.; Saary, P.; Hölzer, M.; Mullikin, J.; Thomas, J.; Young, A.; Bouffard, G.; Barnabas, B.; et al. Integrating Cultivation and Metagenomics for a Multi-Kingdom View of Skin Microbiome Diversity and Functions. Nat. Microbiol. 2022, 7, 169–179. [Google Scholar] [CrossRef]
- Grice, E.A. The Intersection of Microbiome and Host at the Skin Interface: Genomic- and Metagenomic-Based Insights. Genome Res. 2015, 25, 1514–1520. [Google Scholar] [CrossRef] [PubMed]
- Tavaria, F.K. Topical Use of Probiotics: The Natural Balance. Porto Biomed. J. 2017, 2, 69–70. [Google Scholar] [CrossRef] [PubMed]
- Niemeyer-van der Kolk, T.; van der Wall, H.E.C.; Balmforth, C.; Van Doorn, M.B.A.; Rissmann, R. A Systematic Literature Review of the Human Skin Microbiome as Biomarker for Dermatological Drug Development. Br. J. Clin. Pharmacol. 2018, 84, 2178–2193. [Google Scholar] [CrossRef]
- Grice, E.A.; Segre, J.A. The Skin Microbiome. Nat. Rev. Microbiol. 2011, 9, 244–253. [Google Scholar] [CrossRef]
- Dréno, B.; Araviiskaia, E.; Berardesca, E.; Gontijo, G.; Sanchez Viera, M.; Xiang, L.F.; Martin, R.; Bieber, T. Microbiome in Healthy Skin, Update for Dermatologists. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 2038–2047. [Google Scholar] [CrossRef]
- Rafat, Z.; Hashemi, S.J.; Ahamdikia, K.; Daie Ghazvini, R.; Bazvandi, F. Study of Skin and Nail Candida Species as a Normal Flora Based on Age Groups in Healthy Persons in Tehran-Iran. J. Mycol. Med. 2017, 27, 501–505. [Google Scholar] [CrossRef]
- Zhang, E.; Tanaka, T.; Tajima, M.; Tsuboi, R.; Nishikawa, A.; Sugita, T. Characterization of the Skin Fungal Microbiota in Patients with Atopic Dermatitis and in Healthy Subjects. Microbiol. Immunol. 2011, 55, 625–632. [Google Scholar] [CrossRef]
- Oh, J.; Byrd, A.L.; Park, M.; Kong, H.H.; Segre, J.A. Temporal Stability of the Human Skin Microbiome. Cell 2016, 165, 854–866. [Google Scholar] [CrossRef]
- Boxberger, M.; Cenizo, V.; Cassir, N.; La Scola, B. Challenges in Exploring and Manipulating the Human Skin Microbiome. Microbiome 2021, 9, 125. [Google Scholar] [CrossRef]
- Wielscher, M.; Pfisterer, K.; Samardzic, D.; Balsini, P.; Bangert, C.; Jäger, K.; Buchberger, M.; Selitsch, B.; Pjevac, P.; Willinger, B.; et al. The Phageome in Normal and Inflamed Human Skin. Sci. Adv. 2023, 9, eadg4015. [Google Scholar] [CrossRef]
- Forton, F.M.N.; De Maertelaer, V. Which Factors Influence Demodex Proliferation? A Retrospective Pilot Study Highlighting a Possible Role of Subtle Immune Variations and Sebaceous Gland Status. J. Dermatol. 2021, 48, 1210–1220. [Google Scholar] [CrossRef] [PubMed]
- Grice, E.A.; Kong, H.H.; Conlan, S.; Deming, C.B.; Davis, J.; Young, A.C.; Bouffard, G.G.; Blakesley, R.W.; Murray, P.R.; Green, E.D.; et al. Topographical and Temporal Diversity of the Human Skin Microbiome. Science 2009, 324, 1190–1192. [Google Scholar] [CrossRef] [PubMed]
- Timm, C.M.; Loomis, K.; Stone, W.; Mehoke, T.; Brensinger, B.; Pellicore, M.; Staniczenko, P.P.A.; Charles, C.; Nayak, S.; Karig, D.K. Isolation and Characterization of Diverse Microbial Representatives from the Human Skin Microbiome. Microbiome 2020, 8, 58. [Google Scholar] [CrossRef]
- Sanmiguel, A.; Grice, E.A. Interactions between Host Factors and the Skin Microbiome. Cell. Mol. Life Sci. 2015, 72, 1499–1515. [Google Scholar] [CrossRef]
- Zhang, X.E.; Zheng, P.; Ye, S.Z.; Ma, X.; Liu, E.; Pang, Y.-B.; He, Q.Y.; Zhang, Y.X.; Li, W.Q.; Zeng, J.H.; et al. Microbiome: Role in Inflammatory Skin Diseases. J. Inflamm. Res. 2024, 17, 1057–1082. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuji, T.; Chen, T.H.; Two, A.M.; Chun, K.A.; Narala, S.; Geha, R.S.; Hata, T.R.; Gallo, R.L. Staphylococcus Aureus Exploits Epidermal Barrier Defects in Atopic Dermatitis to Trigger Cytokine Expression. J. Investig. Dermatol. 2016, 136, 2192–2200. [Google Scholar] [CrossRef]
- Kong, H.H.; Oh, J.; Deming, C.; Conlan, S.; Grice, E.A.; Beatson, M.A.; Nomicos, E.; Polley, E.C.; Komarow, H.D.; Mullikin, J.; et al. Temporal Shifts in the Skin Microbiome Associated with Disease Flares and Treatment in Children with Atopic Dermatitis. Genome Res. 2012, 22, 850–859. [Google Scholar] [CrossRef]
- Brodská, P.; Panzner, P.; Pizinger, K.; Schmid-Grendelmeier, P. IgE-Mediated Sensitization to Malassezia in Atopic Dermatitis: More Common in Male Patients and in Head and Neck Type. Dermatitis 2014, 25, 120–126. [Google Scholar] [CrossRef]
- Watanabe, S.; Kano, R.; Sato, H.; Nakamura, Y.; Hasegawa, A. The Effects of Malassezia Yeasts on Cytokine Production by Human Keratinocytes. J. Investig. Dermatol. 2001, 116, 769–773. [Google Scholar] [CrossRef]
- Dityen, K.; Soonthornchai, W.; Kueanjinda, P.; Kullapanich, C.; Tunsakul, N.; Somboonna, N.; Wongpiyabovorn, J. Analysis of Cutaneous Bacterial Microbiota of Thai Patients with Seborrheic Dermatitis. Exp. Dermatol. 2022, 31, 1949–1955. [Google Scholar] [CrossRef]
- Tanaka, A.; Cho, O.; Saito, C.; Saito, M.; Tsuboi, R.; Sugita, T. Comprehensive Pyrosequencing Analysis of the Bacterial Microbiota of the Skin of Patients with Seborrheic Dermatitis. Microbiol. Immunol. 2016, 60, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Dreno, B.; Martin, R.; Moyal, D.; Henley, J.B.; Khammari, A.; Seité, S. Skin Microbiome and Acne Vulgaris: Staphylococcus, a New Actor in Acne. Exp. Dermatol. 2017, 26, 798–803. [Google Scholar] [CrossRef] [PubMed]
- Dagnelie, M.A.; Corvec, S.; Saint-Jean, M.; Nguyen, J.M.; Khammari, A.; Dréno, B. Cutibacterium Acnes Phylotypes Diversity Loss: A Trigger for Skin Inflammatory Process. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 2340–2348. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yan, R.; Zhong, Q.; Ngo, S.; Bangayan, N.J.; Nguyen, L.; Lui, T.; Liu, M.; Erfe, M.C.; Craft, N.; et al. The Diversity and Host Interactions of Propionibacterium Acnes Bacteriophages on Human Skin. ISME J. 2015, 9, 2078–2093. [Google Scholar] [CrossRef]
- Barnard, E.; Shi, B.; Kang, D.; Craft, N.; Li, H. The Balance of Metagenomic Elements Shapes the Skin Microbiome in Acne and Health. Sci. Rep. 2016, 6, 39491. [Google Scholar] [CrossRef]
- Olejniczak-Staruch, I.; Ciążyńska, M.; Sobolewska-Sztychny, D.; Narbutt, J.; Skibińska, M.; Lesiak, A. Alterations of the Skin and Gut Microbiome in Psoriasis and Psoriatic Arthritis. Int. J. Mol. Sci. 2021, 22, 3998. [Google Scholar] [CrossRef]
- Chang, H.W.; Yan, D.; Singh, R.; Liu, J.; Lu, X.; Ucmak, D.; Lee, K.; Afifi, L.; Fadrosh, D.; Leech, J.; et al. Alteration of the Cutaneous Microbiome in Psoriasis and Potential Role in Th17 Polarization. Microbiome 2018, 6, 154. [Google Scholar] [CrossRef]
- Rudramurthy, S.M.; Honnavar, P.; Chakrabarti, A.; Dogra, S.; Singh, P.; Handa, S. Association of Malassezia Species with Psoriatic Lesions. Mycoses 2014, 57, 483–488. [Google Scholar] [CrossRef]
- Yousefi, A.; Karbalaei, M.; Keikha, M. Impact of Streptococcus Pyogenes Infection in Susceptibility to Psoriasis: A Systematic Review and Meta-Analysis. World J. Metaanal 2021, 9, 309–316. [Google Scholar] [CrossRef]
- Wang, H.; Chan, H.H.; Ni, M.Y.; Lam, W.W.; Chan, W.M.M.; Pang, H. Bacteriophage of the Skin Microbiome in Patients with Psoriasis and Healthy Family Controls. J. Investig. Dermatol. 2020, 140, 182–190.e5. [Google Scholar] [CrossRef]
- Holmes, A.D. Potential Role of Microorganisms in the Pathogenesis of Rosacea. J. Am. Acad. Dermatol. 2013, 69, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Tatu, A.L.; Clatici, V.; Cristea, V. Isolation of Bacillus Simplex Strain from Demodex Folliculorum and Observations about Demodicosis Spinulosa. Clin. Exp. Dermatol. 2016, 41, 818–820. [Google Scholar] [CrossRef] [PubMed]
- Tutka, K.; Żychowska, M.; Reich, A. Diversity and Composition of the Skin, Blood and Gut Microbiome in Rosacea—A Systematic Review of the Literature. Microorganisms 2020, 8, 1756. [Google Scholar] [CrossRef]
- Zhu, W.; Hamblin, M.R.; Wen, X. Role of the Skin Microbiota and Intestinal Microbiome in Rosacea. Front. Microbiol. 2023, 14, 1108661. [Google Scholar] [CrossRef]
- Rainer, B.M.; Thompson, K.G.; Antonescu, C.; Florea, L.; Mongodin, E.F.; Bui, J.; Fischer, A.H.; Pasieka, H.B.; Garza, L.A.; Kang, S.; et al. Characterization and Analysis of the Skin Microbiota in Rosacea: A Case–Control Study. Am. J. Clin. Dermatol. 2020, 21, 139–147. [Google Scholar] [CrossRef]
- Mahmud, M.R.; Akter, S.; Tamanna, S.K.; Mazumder, L.; Esti, I.Z.; Banerjee, S.; Akter, S.; Hasan, M.R.; Acharjee, M.; Hossain, M.S.; et al. Impact of Gut Microbiome on Skin Health: Gut-Skin Axis Observed through the Lenses of Therapeutics and Skin Diseases. Gut Microbes 2022, 14, 2096995. [Google Scholar] [CrossRef]
- Salem, I.; Ramser, A.; Isham, N.; Ghannoum, M.A. The Gut Microbiome as a Major Regulator of the Gut-Skin Axis. Front. Microbiol. 2018, 9, 382698. [Google Scholar] [CrossRef]
- De Pessemier, B.; Grine, L.; Debaere, M.; Maes, A.; Paetzold, B.; Callewaert, C. Gut–Skin Axis: Current Knowledge of the In-terrelationship between Microbial Dysbiosis and Skin Conditions. Microorganisms 2021, 9, 353. [Google Scholar] [CrossRef]
- Slominski, R.M.; Raman, C.; Jetten, A.M.; Slominski, A.T. Neuro–Immuno–Endocrinology of the Skin: How Environment Regulates Body Homeostasis. Nat. Rev. Endocrinol. 2025, 21, 495–509. [Google Scholar] [CrossRef]
- Tian, J.; Zhang, D.; Yang, Y.; Huang, Y.; Wang, L.; Yao, X.; Lu, Q. Global Epidemiology of Atopic Dermatitis: A Comprehensive Systematic Analysis and Modelling Study. Br. J. Dermatol. 2023, 190, 55–61. [Google Scholar] [CrossRef]
- Stefanovic, N.; Irvine, A.D. Filaggrin and beyond: New Insights into the Skin Barrier in Atopic Dermatitis and Allergic Diseases, from Genetics to Therapeutic Perspectives. Ann. Allergy Asthma Immunol. 2024, 132, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Schuler, C.F.; Tsoi, L.C.; Billi, A.C.; Harms, P.W.; Weidinger, S.; Gudjonsson, J.E. Genetic and Immunological Pathogenesis of Atopic Dermatitis. J. Investig. Dermatol. 2024, 144, 954–968. [Google Scholar] [CrossRef] [PubMed]
- Towell, A.M.; Feuillie, C.; Vitry, P.; da Costa, T.M.; Mathelié-Guinlet, M.; Kezic, S.; Fleury, O.M.; McAleer, M.A.; Dufrêne, Y.F.; Irvine, A.D.; et al. Staphylococcus Aureus Binds to the N-Terminal Region of Corneodesmosin to Adhere to the Stratum Corneum in Atopic Dermatitis. Proc. Natl. Acad. Sci. USA 2021, 118, e2014444118. [Google Scholar] [CrossRef] [PubMed]
- Geoghegan, J.A.; Irvine, A.D.; Foster, T.J. Staphylococcus Aureus and Atopic Dermatitis: A Complex and Evolving Relationship. Trends Microbiol. 2018, 26, 484–497. [Google Scholar] [CrossRef]
- Tauber, M.; Balica, S.; Hsu, C.Y.; Jean-Decoster, C.; Lauze, C.; Redoules, D.; Viodé, C.; Schmitt, A.M.; Serre, G.; Simon, M.; et al. Staphylococcus Aureus Density on Lesional and Nonlesional Skin Is Strongly Associated with Disease Severity in Atopic Dermatitis. J. Allergy Clin. Immunol. 2016, 137, 1272–1274.e3. [Google Scholar] [CrossRef]
- Nakatsuji, T.; Chen, T.H.; Narala, S.; Chun, K.A.; Two, A.M.; Yun, T.; Shafiq, F.; Kotol, P.F.; Bouslimani, A.; Melnik, A.V.; et al. Antimicrobials from Human Skin Commensal Bacteria Protect against Staphylococcus Aureus and Are Deficient in Atopic Dermatitis. Sci. Transl. Med. 2017, 9, eaah4680. [Google Scholar] [CrossRef]
- Kennedy, E.A.; Connolly, J.; Hourihane, J.O.B.; Fallon, P.G.; McLean, W.H.I.; Murray, D.; Jo, J.H.; Segre, J.A.; Kong, H.H.; Irvine, A.D. Skin Microbiome before Development of Atopic Dermatitis: Early Colonization with Commensal Staphylococci at 2 Months Is Associated with a Lower Risk of Atopic Dermatitis at 1 Year. J. Allergy Clin. Immunol. 2017, 139, 166–172. [Google Scholar] [CrossRef]
- Meylan, P.; Lang, C.; Mermoud, S.; Johannsen, A.; Norrenberg, S.; Hohl, D.; Vial, Y.; Prod’hom, G.; Greub, G.; Kypriotou, M.; et al. Skin Colonization by Staphylococcus Aureus Precedes the Clinical Diagnosis of Atopic Dermatitis in Infancy. J. Investig. Dermatol. 2017, 137, 2497–2504. [Google Scholar] [CrossRef]
- Cau, L.; Williams, M.R.; Butcher, A.M.; Nakatsuji, T.; Kavanaugh, J.S.; Cheng, J.Y.; Shafiq, F.; Higbee, K.; Hata, T.R.; Horswill, A.R.; et al. Staphylococcus Epidermidis Protease EcpA Can Be a Deleterious Component of the Skin Microbiome in Atopic Dermatitis. J. Allergy Clin. Immunol. 2021, 147, 955–966.e16. [Google Scholar] [CrossRef]
- Kim, J.E.; Kim, H.S. Microbiome of the Skin and Gut in Atopic Dermatitis (Ad): Understanding the Pathophysiology and Finding Novel Management Strategies. J. Clin. Med. 2019, 8, 444. [Google Scholar] [CrossRef]
- Nowicka, D.; Nawrot, U. Contribution of Malassezia Spp. to the Development of Atopic Dermatitis. Mycoses 2019, 62, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Borda, L.J.; Wikramanayake, T.C. Seborrheic Dermatitis and Dandruff: A Comprehensive Review. J. Clin. Investig. Dermatol. 2015, 3, 10–13188. [Google Scholar] [CrossRef]
- Goldust, M.; Ranjkesh, M.R.; Amirinia, M.; Golforoushan, F.; Rezaee, E.; Rezazadeh Saatlou, M.A. Diagnosis and Treatment of Seborrheic Dermatitis. Am. Fam. Physician 2015, 91, 185–190. [Google Scholar] [CrossRef]
- Lin, Q.; Panchamukhi, A.; Li, P.; Shan, W.; Zhou, H.; Hou, L.; Chen, W. Malassezia and Staphylococcus Dominate Scalp Microbiome for Seborrheic Dermatitis. Bioprocess. Biosyst. Eng. 2020, 44, 965–975. [Google Scholar] [CrossRef]
- Chen, G.; Chen, Z.-m.; Fan, X.-y.; Jin, Y.-l.; Li, X.; Wu, S.-r.; Ge, W.-w.; Lv, C.-h.; Wang, Y.-k. Gut-Brain-Skin Axis in Psoriasis: A Review. Dermatol. Ther. 2021, 11, 25–38. [Google Scholar] [CrossRef]
- Adalsteinsson, J.A.; Kaushik, S.; Muzumdar, S.; Guttman, E.; Ungar, J. An Update on the Microbiology, Immunology and Genetics of Seborrheic Dermatitis. Exp. Dermatol. 2020, 29, 481–489. [Google Scholar] [CrossRef]
- Schwartz, J.R.; Mesenger, A.G.; Tosti, A.; Todd, G.; Hordinsky, M.; Hay, R.J.; Wang, X.; Zachariae, C.; Ker, K.M.; Henry, J.P.; et al. A Comprehensive Pathophysiology of Dandruff and Seborrheic Dermatitis–Towards a More Precise Definition of Scalp Health. Acta Derm. Venereol. 2013, 93, 131–137. [Google Scholar] [CrossRef]
- Wikramanayake, T.C.; Borda, L.J.; Miteva, M.; Paus, R. Seborrheic Dermatitis-Looking beyond Malassezia. Exp. Dermatol. 2019, 28, 991–1001. [Google Scholar] [CrossRef]
- Park, M.; Cho, Y.J.; Kim, D.; Yang, C.S.; Lee, S.M.; Dawson, T.L.; Nakamizo, S.; Kabashima, K.; Lee, Y.W.; Jung, W.H. A Novel Virus Alters Gene Expression and Vacuolar Morphology in Malassezia Cells and Induces a TLR3-Mediated Inflammatory Immune Response. mBio 2020, 11, e01521-20. [Google Scholar] [CrossRef]
- Tamer, F.; Kekilli, M. Exploring the Therapeutic Potential of Topical Probiotics in Dermatological Diseases: A Comprehensive Review of Clinical Studies. J. Ger. Soc. Dermatol. 2024, 22, 1195–1204. [Google Scholar] [CrossRef]
- Park, T.; Kim, H.J.; Myeong, N.R.; Lee, H.G.; Kwack, I.; Lee, J.; Kim, B.J.; Sul, W.J.; An, S. Collapse of Human Scalp Microbiome Network in Dandruff and Seborrhoeic Dermatitis. Exp. Dermatol. 2017, 26, 835–838. [Google Scholar] [CrossRef]
- Tao, R.; Li, R.; Wang, R. Skin Microbiome Alterations in Seborrheic Dermatitis and Dandruff: A Systematic Review. Exp. Dermatol. 2021, 30, 1546–1553. [Google Scholar] [CrossRef]
- Moradi Tuchayi, S.; Makrantonaki, E.; Ganceviciene, R.; Dessinioti, C.; Feldman, S.R.; Zouboulis, C.C. Acne Vulgaris. Nat. Rev. Dis. Prim. 2015, 1, 15029. [Google Scholar] [CrossRef] [PubMed]
- Fitz-Gibbon, S.; Tomida, S.; Chiu, B.H.; Nguyen, L.; Du, C.; Liu, M.; Elashoff, D.; Erfe, M.C.; Loncaric, A.; Kim, J.; et al. Propionibacterium Acnes Strain Populations in the Human Skin Microbiome Associated with Acne. J. Investig. Dermatol. 2013, 133, 2152–2160. [Google Scholar] [CrossRef] [PubMed]
- Dréno, B.; Dagnelie, M.A.; Khammari, A.; Corvec, S. The Skin Microbiome: A New Actor in Inflammatory Acne. Am. J. Clin. Dermatol. 2020, 21, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Shi, B.; Erfe, M.C.; Craft, N.; Li, H. Vitamin B12 Modulates the Transcriptome of the Skin Microbiota in Acne Pathogenesis. Sci. Transl. Med. 2015, 7, 293ra103. [Google Scholar] [CrossRef]
- Ahle, C.M.; Stødkilde, K.; Poehlein, A.; Bömeke, M.; Streit, W.R.; Wenck, H.; Reuter, J.H.; Hüpeden, J.; Brüggemann, H. Interference and Co-Existence of Staphylococci and Cutibacterium Acnes within the Healthy Human Skin Microbiome. Commun. Biol. 2022, 5, 293. [Google Scholar] [CrossRef]
- Skabytska, Y.; Biedermann, T. Staphylococcus Epidermidis Sets Things Right Again. J. Investig. Dermatol. 2016, 136, 559–560. [Google Scholar] [CrossRef]
- Wang, Y.; Kuo, S.; Shu, M.; Yu, J.; Huang, S.; Dai, A.; Two, A.; Gallo, R.L.; Huang, C.M. Staphylococcus Epidermidis in the Human Skin Microbiome Mediates Fermentation to Inhibit the Growth of Propionibacterium Acnes: Implications of Probiotics in Acne Vulgaris. Appl. Microbiol. Biotechnol. 2014, 98, 411–424. [Google Scholar] [CrossRef]
- Afonina, I.S.; Van Nuffel, E.; Beyaert, R. Immune Responses and Therapeutic Options in Psoriasis. Cell Mol. Life Sci. 2021, 78, 2709–2727. [Google Scholar] [CrossRef]
- Fyhrquist, N.; Muirhead, G.; Prast-Nielsen, S.; Jeanmougin, M.; Olah, P.; Skoog, T.; Jules-Clement, G.; Feld, M.; Barrientos-Somarribas, M.; Sinkko, H.; et al. Microbe-Host Interplay in Atopic Dermatitis and Psoriasis. Nat. Commun. 2019, 10, 4703. [Google Scholar] [CrossRef] [PubMed]
- Tett, A.; Pasolli, E.; Farina, S.; Truong, D.T.; Asnicar, F.; Zolfo, M.; Beghini, F.; Armanini, F.; Jousson, O.; De Sanctis, V.; et al. Unexplored Diversity and Strain-Level Structure of the Skin Microbiome Associated with Psoriasis. NPJ Biofilms Microbiomes 2017, 3, 14. [Google Scholar] [CrossRef] [PubMed]
- Tomi, N.S.; Kränke, B.; Aberer, E. Staphylococcal Toxins in Patients with Psoriasis, Atopic Dermatitis, and Erythroderma, and in Healthy Control Subjects. J. Am. Acad. Dermatol. 2005, 53, 67–72. [Google Scholar] [CrossRef]
- De Jesús-Gil, C.; Ruiz-Romeu, E.; Ferran, M.; Chiriac, A.; Deza, G.; Hóllo, P.; Celada, A.; Pujol, R.M.; Santamaria-Babí, L.F. CLA+ T Cell Response to Microbes in Psoriasis. Front. Immunol. 2018, 9, 1488. [Google Scholar] [CrossRef]
- Ruiz-Romeu, E.; Ferran, M.; Sagristà, M.; Gómez, J.; Giménez-Arnau, A.; Herszenyi, K.; Hóllo, P.; Celada, A.; Pujol, R.; Santamaria-Babí, L.F. Streptococcus Pyogenes-Induced Cutaneous Lymphocyte Antigen-Positive T Cell-Dependent Epidermal Cell Activation Triggers TH17 Responses in Patients with Guttate Psoriasis. J. Allergy Clin. Immunol. 2016, 138, 491–499.e6. [Google Scholar] [CrossRef]
- Fry, L.; Baker, B.S.; Powles, A.V.; Fahlen, A.; Engstrand, L. Is Chronic Plaque Psoriasis Triggered by Microbiota in the Skin? Br. J. Dermatol. 2013, 169, 47–52. [Google Scholar] [CrossRef]
- Pietrzak, A.; Grywalska, E.; Socha, M.; Roliński, J.; Franciszkiewicz-Pietrzak, K.; Rudnicka, L.; Rudzki, M.; Krasowska, D. Prevalence and Possible Role of Candida Species in Patients with Psoriasis: A Systematic Review and Meta-Analysis. Mediat. Inflamm. 2018, 2018, 9602362. [Google Scholar] [CrossRef]
- Navarro-López, V.; Martínez-Andrés, A.; Ramírez-Boscà, A.; Ruzafa-Costas, B.; Núñez-Delegido, E.; Carrión-Gutiérrez, M.A.; Prieto-Merino, D.; Codoñer-Cortés, F.; Ramón-Vidal, D.; Genovés-Martínez, S.; et al. Efficacy and Safety of Oral Administration of a Mixture of Probiotic Strains in Patients with Psoriasis: A Randomized Controlled Clinical Trial. Acta Derm. Venereol. 2019, 99, 1078–1084. [Google Scholar] [CrossRef]
- Ramírez-Boscá, A.; Navarro-López, V.; Martínez-Andrés, A.; Such, J.; Francés, R.; De La Parte, J.; Asín-Llorca, M. Identification of Bacterial DNA in the Peripheral Blood of Patients with Active Psoriasis. JAMA Dermatol. 2015, 151, 670–671. [Google Scholar] [CrossRef]
- Barakji, Y.A.; Rønnstad, A.T.M.; Christensen, M.O.; Zachariae, C.; Wienholtz, N.K.F.; Halling, A.S.; Maul, J.T.; Thomsen, S.F.; Egeberg, A.; Thyssen, J.P. Assessment of Frequency of Rosacea Subtypes in Patients with Rosacea: A Systematic Review and Meta-Analysis. JAMA Dermatol. 2022, 158, 617–625. [Google Scholar] [CrossRef]
- Two, A.M.; Wu, W.; Gallo, R.L.; Hata, T.R. Rosacea: Part I. Introduction, Categorization, Histology, Pathogenesis, and Risk Factors. J. Am. Acad. Dermatol. 2015, 72, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Casas, C.; Paul, C.; Lahfa, M.; Livideanu, B.; Lie Lejeune, O.; Alvarez-Georges, S.; Saint-Martory, C.; Degouy, A.; Rie Mengeaud, V.; Ginisty, H.; et al. Quantification of Demodex Folliculorum by PCR in Rosacea and Its Relationship to Skin Innate Immune Activation. Exp. Dermatol. 2012, 21, 906–910. [Google Scholar] [CrossRef] [PubMed]
- Lacey, N.; Russell-Hallinan, A.; Zouboulis, C.C.; Powell, F.C. Demodex Mites Modulate Sebocyte Immune Reaction: Possible Role in the Pathogenesis of Rosacea. Br. J. Dermatol. 2018, 179, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Lacey, N.; Delaney, S.; Kavanagh, K.; Powell, F.C. Mite-related Bacterial Antigens Stimulate Inflammatory Cells in Rosacea. Br. J. Dermatol. 2007, 157, 474–481. [Google Scholar] [CrossRef]
- McMahon, F.; Banville, N.; Bergin, D.A.; Smedman, C.; Paulie, S.; Reeves, E.; Kavanagh, K. Activation of Neutrophils via IP3 Pathway Following Exposure to Demodex-Associated Bacterial Proteins. Inflammation 2016, 39, 425–433. [Google Scholar] [CrossRef]
- Mylonas, A.; Hawerkamp, H.C.; Wang, Y.; Chen, J.; Messina, F.; Demaria, O.; Meller, S.; Homey, B.; Di Domizio, J.; Mazzolai, L.; et al. Type I IFNs Link Skin-Associated Dysbiotic Commensal Bacteria to Pathogenic Inflammation and Angiogenesis in Rosacea. JCI Insight 2023, 8, e151846. [Google Scholar] [CrossRef]
- Dahl, M.V.; Ross, A.J.; Schlievert, P.M. Temperature Regulates Bacterial Protein Production: Possible Role in Rosacea. J. Am. Acad. Dermatol. 2004, 50, 266–272. [Google Scholar] [CrossRef]
- Whitfeld, M.; Gunasingam, N.; Leow, L.J.; Shirato, K.; Preda, V. Staphylococcus Epidermidis: A Possible Role in the Pustules of Rosacea. J. Am. Acad. Dermatol. 2011, 64, 49–52. [Google Scholar] [CrossRef]
- Clanner-Engelshofen, B.M.; French, L.E.; Reinholz, M. Corynebacterium Kroppenstedtii Subsp. Demodicis Is the Endobacterium of Demodex Folliculorum. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1043–1049. [Google Scholar] [CrossRef]
- Cordaillat-Simmons, M.; Rouanet, A.; Pot, B. Live Biotherapeutic Products: The Importance of a Defined Regulatory Framework. Exp. Mol. Med. 2020, 52, 1397–1406. [Google Scholar] [CrossRef]
- FDA. Early Clinical Trials with Live Biotherapeutic Products: Chemistry, Manufacturing, and Control Information; Guidance for Industry; Food and Drug Administration: Muntinlupa, Philippines, 2016. [Google Scholar]
- Yu, Y.; Dunaway, S.; Champer, J.; Kim, J.; Alikhan, A. Changing Our Microbiome: Probiotics in Dermatology. Br. J. Dermatol. 2020, 182, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Habeebuddin, M.; Karnati, R.K.; Shiroorkar, P.N.; Nagaraja, S.; Asdaq, S.M.B.; Anwer, M.K.; Fattepur, S. Topical Probiotics: More Than a Skin Deep. Pharmaceutics 2022, 14, 557. [Google Scholar] [CrossRef] [PubMed]
- Gowda, V.; Sarkar, R.; Verma, D.; Das, A. Probiotics in Dermatology: An Evidence-Based Approach. Indian Dermatol. Online J. 2024, 15, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.C.; Martins, S.M.d.S.B., Jr.; Belo, J.V.T.; Lemos, M.V.C.; Lima, C.E.d.M.C.; Silva, C.D.d.; Zagmignan, A.; Nascimento da Silva, L.C. Global Trends and Scientific Impact of Topical Probiotics in Dermatological Treatment and Skincare. Microorganisms 2024, 12, 2010. [Google Scholar] [CrossRef]
- Guttman-Yassky, E.; Renert-Yuval, Y.; Brunner, P.M. Atopic Dermatitis. Lancet 2025, 405, 583–596. [Google Scholar] [CrossRef]
- França, K.; Frost, P.; Ther, D. Topical Probiotics in Dermatological Therapy and Skincare: A Concise Review. Dermatol. Ther. 2021, 11, 71–77. [Google Scholar] [CrossRef]
- Gao, T.; Wang, X.; Li, Y.; Ren, F. The Role of Probiotics in Skin Health and Related Gut–Skin Axis: A Review. Nutrients 2023, 15, 3123. [Google Scholar] [CrossRef]
- Knackstedt, R.; Knackstedt, T.; Gatherwright, J. The Role of Topical Probiotics in Skin Conditions: A Systematic Review of Animal and Human Studies and Implications for Future Therapies. Exp. Dermatol. 2020, 29, 15–21. [Google Scholar] [CrossRef]
- Lee, G.R.; Maarouf, M.; Hendricks, A.J.; Lee, D.E.; Shi, V.Y. Topical Probiotics: The Unknowns behind Their Rising Popularity. Dermatol. Online J. 2019, 25, 5. [Google Scholar] [CrossRef]
- Di Marzio, L.; Centi, C.; Cinque, B.; Masci, S.; Giuliani, M.; Arcieri, A.; Zicari, L.; De Simone, C.; Cifone, M.G. Effect of the Lactic Acid Bacterium Streptococcus Thermophilus on Stratum Corneum Ceramide Levels and Signs and Symptoms of Atopic Dermatitis Patients. Exp. Dermatol. 2003, 12, 615–620. [Google Scholar] [CrossRef]
- Park, S.B.; Im, M.; Lee, Y.; Lee, J.H.; Lim, J.; Park, Y.H.; Seo, Y.J. Effect of Emollients Containing Vegetable-Derived Lactobacillus in the Treatment of Atopic Dermatitis Symptoms: Split-Body Clinical Trial. Ann. Dermatol. 2014, 26, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Blanchet-Réthoré, S.; Bourdès, V.; Mercenier, A.; Haddar, C.H.; Verhoeven, P.O.; Andres, P. Effect of a Lotion Containing the Heat-Treated Probiotic Strain Lactobacillus Johnsonii NCC 533 on Staphylococcus Aureus Colonization in Atopic Dermatitis. Clin. Cosmet. Investig. Dermatol. 2017, 10, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Butler, É.; Lundqvist, C.; Axelsson, J. Lactobacillus Reuteri DSM 17938 as a Novel Topical Cosmetic Ingredient: A Proof of Concept Clinical Study in Adults with Atopic Dermatitis. Microorganisms 2020, 8, 1026. [Google Scholar] [CrossRef]
- Myles, I.A.; Castillo, C.R.; Barbian, K.D.; Kanakabandi, K.; Virtaneva, K.; Fitzmeyer, E.; Paneru, M.; Otaizo-Carrasquero, F.; Myers, T.G.; Markowitz, T.E.; et al. Therapeutic Responses to Roseomonas Mucosa in Atopic Dermatitis May Involve Lipid-Mediated TNF-Related Epithelial Repair. Sci. Transl. Med. 2020, 12, eaaz8631. [Google Scholar] [CrossRef]
- Liu, X.; Qin, Y.; Dong, L.; Han, Z.; Liu, T.; Tang, Y.; Yu, Y.; Ye, J.; Tao, J.; Zeng, X.; et al. Living Symbiotic Bacteria-Involved Skin Dressing to Combat Indigenous Pathogens for Microbiome-Based Biotherapy toward Atopic Dermatitis. Bioact. Mater. 2023, 21, 253–266. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Lio, P.A.; Simpson, E.L.; Li, C.; Brownell, D.R.; Gryllos, I.; Ng-Cashin, J.; Krueger, T.; Swaidan, V.R.; Bliss, R.L.; et al. Efficacy and Safety of Topically Applied Therapeutic Ammonia Oxidising Bacteria in Adults with Mild-to-Moderate Atopic Dermatitis and Moderate-to-Severe Pruritus: A Randomised, Double-Blind, Placebo-Controlled, Dose-Ranging, Phase 2b Trial. eClinicalMedicine 2023, 60, 102002. [Google Scholar] [CrossRef]
- Guéniche, A.; Cathelineau, A.; Bastien, P.; Esdaile, J.; Martin, R.; Queille Roussel, C.; Breton, L. Vitreoscilla Filiformis Biomass Improves Seborrheic Dermatitis. J. Eur. Acad. Dermatol. Venereol. 2008, 22, 1014–1015. [Google Scholar] [CrossRef]
- Gueniche, A.; Knaudt, B.; Schuck, E.; Volz, T.; Bastien, P.; Martin, R.; Röcken, M.; Breton, L.; Biedermann, T. Effects of Nonpathogenic Gram-Negative Bacterium Vitreoscilla Filiformis Lysate on Atopic Dermatitis: A Prospective, Randomized, Double-Blind, Placebo-Controlled Clinical Study. Br. J. Dermatol. 2008, 159, 1357–1363. [Google Scholar] [CrossRef]
- Gueniche, A.; Liboutet, M.; Cheilian, S.; Fagot, D.; Juchaux, F.; Breton, L. Vitreoscilla Filiformis Extract for Topical Skin Care: A Review. Front. Cell Infect. Microbiol. 2021, 11, 747663. [Google Scholar] [CrossRef]
- Fithian, E.; Thivalapill, N.; Kosner, J.; Necheles, J.; Bilaver, L. Natural Topical Treatment Contributes to a Reduction of Dry Scalp Symptoms in Children. Clin. Cosmet. Investig. Dermatol. 2023, 16, 2757–2762. [Google Scholar] [CrossRef]
- Truglio, M.; Sivori, F.; Cavallo, I.; Abril, E.; Licursi, V.; Fabrizio, G.; Cardinali, G.; Pignatti, M.; Toma, L.; Valensise, F.; et al. Modulating the Skin Mycobiome-Bacteriome and Treating Seborrheic Dermatitis with a Probiotic-Enriched Oily Suspension. Sci. Rep. 2024, 14, 2722. [Google Scholar] [CrossRef] [PubMed]
- Shimamori, Y.; Mitsunaka, S.; Yamashita, H.; Suzuki, T.; Kitao, T.; Kubori, T.; Nagai, H.; Takeda, S.; Ando, H. Staphylococcal Phage in Combination with Staphylococcus Epidermidis as a Potential Treatment for Staphylococcus Aureus-Associated Atopic Dermatitis and Suppressor of Phage-Resistant Mutants. Viruses 2021, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Geng, H.; Yang, X.; Zou, C.; Zhang, W.; Xiang, J.; Yang, K.; Shu, Y.; Luan, G.; Jia, X.; Lu, M. Isolation of the Novel Phage SAP71 and Its Potential Use against Staphylococcus Aureus in an Atopic Dermatitis Mouse Model. Virus Genes 2024, 60, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Lebeer, S.; Oerlemans, E.F.M.; Claes, I.; Henkens, T.; Delanghe, L.; Wuyts, S.; Spacova, I.; van den Broek, M.F.L.; Tuyaerts, I.; Wittouck, S.; et al. Selective Targeting of Skin Pathobionts and Inflammation with Topically Applied Lactobacilli. Cell Rep. Med. 2022, 3, 100521. [Google Scholar] [CrossRef]
- Podrini, C.; Schramm, L.; Marianantoni, G.; Apolinarska, J.; McGuckin, C.; Forraz, N.; Milet, C.; Desroches, A.L.; Payen, P.; D’Aguanno, M.; et al. Topical Administration of Lactiplantibacillus Plantarum (SkinDuoTM) Serum Improves Anti-Acne Properties. Microorganisms 2023, 11, 417. [Google Scholar] [CrossRef]
- Cui, H.; Guo, C.; Wang, Q.; Feng, C.; Duan, Z. A Pilot Study on the Efficacy of Topical Lotion Containing Anti-Acne Postbiotic in Subjects with Mild -to -Moderate Acne. Front. Med. 2022, 9, 1064460. [Google Scholar] [CrossRef]
- Cui, H.; Feng, C.; Guo, C.; Duan, Z. Development of Novel Topical Anti-Acne Cream Containing Postbiotics for Mild-to-Moderate Acne. Indian. J. Dermatol. 2022, 67, 667–673. [Google Scholar] [CrossRef]
- Majeed, M.; Majeed, S.; Nagabhushanam, K.; Mundkur, L.; Rajalakshmi, H.R.; Shah, K.; Beede, K. Novel Topical Application of a Postbiotic, Lactosporin®, in Mild to Moderate Acne: A Randomized, Comparative Clinical Study to Evaluate Its Efficacy, Tolerability and Safety. Cosmetics 2020, 7, 70. [Google Scholar] [CrossRef]
- Kang, B.S.; Seo, J.-G.; Lee, G.-S.; Kim, J.-H.; Kim, S.Y.; Han, Y.W.; Kang, H.; Kim, H.O.; Rhee, J.H.; Chung, M.-J.; et al. Antimicrobial Activity of Enterocins from Enterococcus Faecalis SL-5 against Propionibacterium Acnes, the Causative Agent in Acne Vulgaris, and Its Therapeutic Effect. J. Microbiol. 2009, 47, 101–109. [Google Scholar] [CrossRef]
- Kim, M.J.; Eun, D.H.; Kim, S.M.; Kim, J.; Lee, W.J. Efficacy of Bacteriophages in Propionibacterium Acnes-Induced Inflammation in Mice. Ann. Dermatol. 2019, 31, 22–28. [Google Scholar] [CrossRef]
- Rimon, A.; Rakov, C.; Lerer, V.; Sheffer-Levi, S.; Oren, S.A.; Shlomov, T.; Shasha, L.; Lubin, R.; Zubeidat, K.; Jaber, N.; et al. Topical Phage Therapy in a Mouse Model of Cutibacterium Acnes-Induced Acne-like Lesions. Nat. Commun. 2023, 14, 1005. [Google Scholar] [CrossRef] [PubMed]
- Lam, H.Y.P.; Lai, M.J.; Chen, T.Y.; Wu, W.J.; Peng, S.Y.; Chang, K.C. Therapeutic Effect of a Newly Isolated Lytic Bacteriophage against Multi-Drug-Resistant Cutibacterium Acnes Infection in Mice. Int. J. Mol. Sci. 2021, 22, 7031. [Google Scholar] [CrossRef] [PubMed]
- Golembo, M.; Puttagunta, S.; Rappo, U.; Weinstock, E.; Engelstein, R.; Gahali-Sass, I.; Moses, A.; Kario, E.; Ben-Dor Cohen, E.; Nicenboim, J.; et al. Development of a Topical Bacteriophage Gel Targeting Cutibacterium Acnes for Acne Prone Skin and Results of a Phase 1 Cosmetic Randomized Clinical Trial. Ski. Health Dis. 2022, 2, e93. [Google Scholar] [CrossRef] [PubMed]
- Rather, I.A.; Bajpai, V.K.; Huh, Y.S.; Han, Y.K.; Bhat, E.A.; Lim, J.; Paek, W.K.; Park, Y.H. Probiotic Lactobacillus Sakei ProBio-65 Extract Ameliorates the Severity of Imiquimod Induced Psoriasis-like Skin Inflammation in a Mouse Model. Front. Microbiol. 2018, 9, 1021. [Google Scholar] [CrossRef]
- Zapi-Colín, L.A.; Gutiérrez-González, G.; Rodríguez-Martínez, S.; Cancino-Diaz, J.C.; Méndez-Tenorio, A.; Pérez-Tapia, S.M.; Gómez-Chávez, F.; Cedillo-Peláez, C.; Cancino-Diaz, M.E. A Peptide Derived from Phage-Display Limits Psoriasis-like Lesions in Mice. Heliyon 2020, 6, e04162. [Google Scholar] [CrossRef]
- Waghralkar, R.; Jhunjhunwala, B. Observational Case Studies of the Effect of Phage Laden Ganga Water on Psoriasis. IP Indian J. Clin. Exp. Dermatol. 2021, 7, 186–190. [Google Scholar] [CrossRef]
- Jain, R.; Voss, A.L.; Tagg, J.R.; Hale, J.D.F. Evaluation of the Preliminary Safety, Tolerability and Colonisation Efficacy of Topical Probiotic Formulations Containing Micrococcus Luteus Q24 in Healthy Human Adults. Cosmetics 2022, 9, 121. [Google Scholar] [CrossRef]
- Jain, R.; Voss, A.L.; Del Rosario, J.; Hale, J.D.F. Efficacy of a Topical Live Probiotic in Improving Skin Health. Int. J. Cosmet. Sci. 2025, 47, 488–496. [Google Scholar] [CrossRef]
- Szántó, M.; Dózsa, A.; Antal, D.; Szabó, K.; Kemény, L.; Bai, P. Targeting the Gut-Skin Axis—Probiotics as New Tools for Skin Disorder Management? Exp. Dermatol. 2019, 28, 1210–1218. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, Z.; Ma, Y.; Miao, L.; Zhao, K.; Wang, D.; Wang, M.; Ruan, H.; Xu, F.; Zhou, Q.; et al. Fecal Microbiota Transplantation Affects the Recovery of AD-Skin Lesions and Enhances Gut Microbiota Homeostasis. Int. Immunopharmacol. 2023, 118, 110005. [Google Scholar] [CrossRef]
- Kim, S.; Han, S.Y.; Lee, J.; Kim, N.R.; Lee, B.R.; Kim, H.; Kwon, M.; Ahn, K.; Noh, Y.; Kim, S.J.; et al. Bifidobacterium Longum and Galactooligosaccharide Improve Skin Barrier Dysfunction and Atopic Dermatitis-like Skin. Allergy Asthma Immunol. Res. 2022, 14, 549–564. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.F.; Shih, T.W.; Lee, C.L.; Pan, T.M. The Beneficial Role of Lactobacillus Paracasei Subsp. Paracasei NTU 101 in the Prevention of Atopic Dermatitis. Curr. Issues Mol. Biol. 2024, 46, 2236–2250. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Yoon, J.M.; Kim, Y.H.; Jeong, D.G.; Park, S.; Kang, D.J. Therapeutic Effect of Tyndallized Lactobacillus Rhamnosus IDCC 3201 on Atopic Dermatitis Mediated by Down-Regulation of Immunoglobulin E in NC/Nga Mice. Microbiol. Immunol. 2016, 60, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.S.; Lim, S.K.; Jang, J.Y.; Lee, J.; Park, H.K.; Kim, N.; Yun, M.; Shin, M.Y.; Jo, H.E.; Oh, Y.J.; et al. Lactobacillus Sakei WIKIM30 Ameliorates Atopic Dermatitis-like Skin Lesions by Inducing Regulatory T Cells and Altering Gut Microbiota Structure in Mice. Front. Immunol. 2018, 9, 1905. [Google Scholar] [CrossRef]
- Suzuki, T.; Nishiyama, K.; Kawata, K.; Sugimoto, K.; Isome, M.; Suzuki, S.; Nozawa, R.; Ichikawa, Y.; Watanabe, Y.; Suzutani, T. Effect of the Lactococcus Lactis 11/19-B1 Strain on Atopic Dermatitis in a Clinical Test and Mouse Model. Nutrients 2020, 12, 763. [Google Scholar] [CrossRef]
- Navarro-Lopez, V.; Ramirez-Bosca, A.; Ramon-Vidal, D.; Ruzafa-Costas, B.; Genoves-Martinez, S.; Chenoll-Cuadros, E.; Carrion-Gutierrez, M.; De La Parte, J.H.; Prieto-Merino, D.; Codoner-Cortes, F.M. Effect of Oral Administration of a Mixture of Probiotic Strains on SCORAD Index and Use of Topical Steroids in Young Patients with Moderate Atopic Dermatitis a Randomized Clinical Trial. JAMA Dermatol. 2018, 154, 37–43. [Google Scholar] [CrossRef]
- Wang, I.J.; Wang, J.Y. Children with Atopic Dermatitis Show Clinical Improvement after Lactobacillus Exposure. Clin. Exp. Allergy 2015, 45, 779–787. [Google Scholar] [CrossRef]
- Lee, Y.; Byeon, H.R.; Jang, S.Y.; Hong, M.G.; Kim, D.; Lee, D.; Shin, J.H.; Kim, Y.; Kang, S.G.; Seo, J.G. Oral Administration of Faecalibacterium Prausnitzii and Akkermansia Muciniphila Strains from Humans Improves Atopic Dermatitis Symptoms in DNCB Induced NC/Nga Mice. Sci. Rep. 2022, 12, 7324. [Google Scholar] [CrossRef]
- Lopes, E.G.; Moreira, D.A.; Gullón, P.; Gullón, B.; Cardelle-Cobas, A.; Tavaria, F.K. Topical Application of Probiotics in Skin: Adhesion, Antimicrobial and Antibiofilm in Vitro Assays. J. Appl. Microbiol. 2017, 122, 450–461. [Google Scholar] [CrossRef]
- Cosseau, C.; Devine, D.A.; Dullaghan, E.; Gardy, J.L.; Chikatamarla, A.; Gellatly, S.; Yu, L.L.; Pistolic, J.; Falsafi, R.; Tagg, J.; et al. The Commensal Streptococcus Salivarius K12 Downregulates the Innate Immune Responses of Human Epithelial Cells and Promotes Host-Microbe Homeostasis. Infect. Immun. 2008, 76, 4163–4175. [Google Scholar] [CrossRef]
- Lee, D.K.; Kim, M.J.; Ham, J.W.; An, H.M.; Cha, M.K.; Lee, S.W.; Park, C.I.; Shin, S.H.; Lee, K.O.; Kim, K.J.; et al. In Vitro Evaluation of Antibacterial Activities and Anti-Inflammatory Effects of Bifidobacterium Spp. Addressing Acne Vulgaris. Arch. Pharm. Res. 2012, 35, 1065–1071. [Google Scholar] [CrossRef]
- Al-Ghazzewi, F.H.; Tester, R.F. Effect of Konjac Glucomannan Hydrolysates and Probiotics on the Growth of the Skin Bacterium Propionibacterium Acnes in Vitro. Int. J. Cosmet. Sci. 2010, 32, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Deidda, F.; Amoruso, A.; Nicola, S.; Graziano, T.; Pane, M.; Mogna, L. New Approach in Acne Therapy A Specific Bacteriocin Activity and a Targeted Anti IL-8 Property in Just 1 Probiotic Strain, the L. Salivarius LS03. J. Clin. Gastroenterol. 2018, 52, S78–S81. [Google Scholar] [CrossRef] [PubMed]
- Espinoza-Monje, M.; Campos, J.; Alvarez Villamil, E.; Jerez, A.; Dentice Maidana, S.; Elean, M.; Salva, S.; Kitazawa, H.; Villena, J.; García-Cancino, A. Characterization of Weissella Viridescens Uco-Smc3 as a Potential Probiotic for the Skin: Its Beneficial Role in the Pathogenesis of Acne Vulgaris. Microorganisms 2021, 9, 1486. [Google Scholar] [CrossRef]
- Eguren, C.; Navarro-Blasco, A.; Corral-Forteza, M.; Reolid-Pérez, A.; Setó-Torrent, N.; García-Navarro, A.; Prieto-Merino, D.; Núñez-Delegido, E.; Sánchez-Pellicer, P.; Navarro-López, V. A Randomized Clinical Trial to Evaluate the Efficacy of an Oral Probiotic in Acne Vulgaris. Acta Derm. Venereol. 2024, 104, adv33206. [Google Scholar] [CrossRef]
- Rinaldi, F.; Marotta, L.; Mascolo, A.; Amoruso, A.; Pane, M.; Giuliani, G.; Pinto, D. Facial Acne: A Randomized, Double-Blind, Placebo-Controlled Study on the Clinical Efficacy of a Symbiotic Dietary Supplement. Dermatol. Ther. 2022, 12, 577–589. [Google Scholar] [CrossRef]
- Jung, G.W.; Tse, J.E.; Guiha, I.; Rao, J. Prospective, Randomized, Open-Label Trial Comparing the Safety, Efficacy, and Tolerability of an Acne Treatment Regimen with and without a Probiotic Supplement and Minocycline in Subjects with Mild to Moderate Acne. J. Cutan. Med. Surg. 2013, 17, 114–122. [Google Scholar] [CrossRef]
- Manzhalii, E.; Hornuss, D.; Stremmel, W. Intestinal-Borne Dermatoses Significantly Improved by Oral Application of Escherichia Coli Nissle 1917. World J. Gastroenterol. 2016, 22, 5415–5421. [Google Scholar] [CrossRef]
- Chen, Y.H.; Wu, C.S.; Chao, Y.H.; Lin, C.C.; Tsai, H.Y.; Li, Y.R.; Chen, Y.Z.; Tsai, W.H.; Chen, Y.K. Lactobacillus Pentosus GMNL-77 Inhibits Skin Lesions in Imiquimod-Induced Psoriasis-like Mice. J. Food Drug Anal. 2016, 25, 559–566. [Google Scholar] [CrossRef]
- Reygagne, P.; Bastien, P.; Couavoux, M.P.; Philippe, D.; Renouf, M.; Castiel-Higounenc, I.; Gueniche, A. The Positive Benefit of Lactobacillus Paracasei NCC2461 ST11 in Healthy Volunteers with Moderate to Severe Dandruff. Benef. Microbes 2017, 8, 671–680. [Google Scholar] [CrossRef]
- Fortuna, M.C.; Garelli, V.; Pranteda, G.; Romaniello, F.; Cardone, M.; Carlesimo, M.; Rossi, A. A Case of Scalp Rosacea Treated with Low Dose Doxycycline and Probiotic Therapy and Literature Review on Therapeutic Options. Dermatol. Ther. 2016, 29, 249–251. [Google Scholar] [CrossRef] [PubMed]
- Vinderola, G.; Druart, C.; Gosálbez, L.; Salminen, S.; Vinot, N.; Lebeer, S. Postbiotics in the Medical Field under the Perspective of the ISAPP Definition: Scientific, Regulatory, and Marketing Considerations. Front. Pharmacol. 2023, 14, 1239745. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EU) 2024/1938 of the European Parliament and of the Council of 13 June 2024 on Standards of Quality and Safety for Substances of Human Origin Intended for Human Application and Repealing Directives 2002/98/EC and 2004/23/EC (Text with EEA Relevance). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=OJ:L_202401938 (accessed on 13 June 2025).

| Term | Definition | Ref. |
|---|---|---|
| Microbiota | A community of microorganisms (bacteria, fungi, archaea and viruses) living within a specific environment (e.g., gut, oral, respiratory, and skin microbiota). | [9] |
| Microbiome | The collection of microorganisms, their genomes, structural elements and metabolites, and the specific environment in which they live. | [9] |
| Mycobiome | Total community of fungi and their genetic material present in a particular environment or host | [10] |
| Phageome | Total community of bacteriophages and their genetic material present in a particular environment or host | [11] |
| Probiotic | Live microorganisms that, when administered in adequate amounts, confer a health benefit on the host. | [12] |
| Prebiotic | Non-digestible food ingredients which selectively stimulate the growth and/or activity of microorganisms with a positive influence on the health of the host. | [13] |
| Postbiotic | Bioactive compounds produced by microbiota, their components and/or inanimate microorganisms which confer a health benefit to the host. | [14] |
| Microbiome-based product (MBP) | A wide range of products, from food to medicinal products, including food supplements, foods for special medical purposes, cosmetics or medical devices, based on complex living systems. | [15] |
| Disease | Skin Microbiota Alternations | Ref. | |
|---|---|---|---|
| Increase | Decrease | ||
| Atopic dermatitis | Staphylococcus spp. Staphylococcus aureus Malassezia restricta/ Malassezia globosa ratio Staphilococcus aureus phages | Cutibacterium spp. Streptococcus spp. Acinetobacter spp. Corynebacterium spp. Prevotella spp. | [30,36,37,38,39] |
| Seborrheic dermatitis | Staphylococcus spp. Staphylococcus aureus Pseudomonas spp. Micrococcus spp. Malassezia spp. Acinetobacter spp. Streptococcus spp. | Cutibacterium spp. Corynebacterium spp. | [40,41] |
| Acne | Staphylococcus spp. Cutibacterium acnes IA1 | Cutibacterium acnes phages | [42,43,44,45] |
| Psoriasis | Staphylococcus aureus Streptococcus pyogenes Corynebacterium kroppenstedtii Corynebacterium simulans Finegoldia spp. Malassezia spp. | Cutibacterium spp. Lactobacillus spp. Bacteriophages | [46,47,48,49,50] |
| Rosacea | Demodex folliculorum Bacillus oleronius Bacillus simplex Staphylococcus epidermidis Corynebacterium kroppenstedtii | Roseomonas mucosa | [51,52,53,54,55] |
| No. | Product/Brand | Microbial Component | Benefits Claimed |
|---|---|---|---|
| Live bacteria | |||
| 1 | Serum BLIS Q24 (Blis Technologies, New Zealand) | Micrococcus luteus Q24—strain isolated from skin of a healthy human adult | Balances the skin microbiome; reduces oiliness, acne, redness/rosacea, rough skin, wrinkles [148,149]. |
| 2 | BAK Serum for acne-prone skin (BAK Skincare, Denmark) | Lactobacillus plantarum LB356R and LB244R—isolated from fermented cabbage and beets | Balances the skin microbiome; restores skin barrier; reduces the formation of C. acnes biofilm. |
| 3 | SkinDuo Probiotic Topical Serum (The BioArte, Malta) | Lactobacillus plantarum | Balances the dysbiosis in hair follicles environment; reduces sebum production and inflammation [136]. |
| 4 | Esse Probiotic Serum (Esse Skincare, South Africa) | Lactobacillus spp. isolated from human gut | Complements natural microbial diversity; strengthens the skin barrier. |
| 5 | Squalane + Probiotic Gel Moisturizer (Biossance, USA) | Lactobacillus, Lactococcus, Leuconostoc, Pediococcus, Saccharomyces, Hansenula | Supports a healthy microbiome for balanced skin. Suitable for oily, acne-prone, or sensitive skin. |
| 6 | Youthful Serum (Gallinée, UK) | Lactobacillus crispatus + Lactobacillus ferment lysate | Improves skin hydration and balances skin microbiome. |
| Inanimate bacteria/extract/ferment lysate/cell-free supernatants | |||
| 1 | LactoSporin (Sabinsa Cosmetics, USA) | Cell-free supernatant of Bacillus coagulans and inactivated cells of Bacillus longum | Effective against mild-to-moderate acne and other seborrheic conditions [139]. |
| 2 | Probiotic Cream (NiKEL, Croatia) | Lactobacillus ferment | Regenerates damaged skin, reduces inflammation, and accelerates skin recovery. It is suitable for conditions like redness, flaking, and seborrheic dermatitis, and can complement psoriasis treatments. |
| 3 | The Probiotic Concentrate (Aurelia, UK) | Bifida ferment | Strengthens skin immune system and protect the skin barrier. Reduces skin irritation and inflammation. Balances skin microbiome. |
| 4 | Probiotic Concentrate (Columbia skincare, USA) | Lactococcus ferment lysate | Enhances the skin’s renewal process. |
| 5 | Lipikar Eczema Cream (La Roche Posay, France) | Vitreoscilla ferment | Visibly reduces signs of eczema and provides long-lasting relief. Helps relieve itching and irritation. |
| 6 | Advanced Génifique serum (Lancome, Australia) | Bifida ferment lysate | Strengthens skin barrier function. Balances skin microbiome. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rušanac, A.; Škibola, Z.; Matijašić, M.; Čipčić Paljetak, H.; Perić, M. Microbiome-Based Products: Therapeutic Potential for Inflammatory Skin Diseases. Int. J. Mol. Sci. 2025, 26, 6745. https://doi.org/10.3390/ijms26146745
Rušanac A, Škibola Z, Matijašić M, Čipčić Paljetak H, Perić M. Microbiome-Based Products: Therapeutic Potential for Inflammatory Skin Diseases. International Journal of Molecular Sciences. 2025; 26(14):6745. https://doi.org/10.3390/ijms26146745
Chicago/Turabian StyleRušanac, Anamarija, Zara Škibola, Mario Matijašić, Hana Čipčić Paljetak, and Mihaela Perić. 2025. "Microbiome-Based Products: Therapeutic Potential for Inflammatory Skin Diseases" International Journal of Molecular Sciences 26, no. 14: 6745. https://doi.org/10.3390/ijms26146745
APA StyleRušanac, A., Škibola, Z., Matijašić, M., Čipčić Paljetak, H., & Perić, M. (2025). Microbiome-Based Products: Therapeutic Potential for Inflammatory Skin Diseases. International Journal of Molecular Sciences, 26(14), 6745. https://doi.org/10.3390/ijms26146745






