The PAS-B Domain of BMAL1 Controls Proliferation, Cellular Energetics, and Inflammatory Response in Human Monocytic Cell Line THP-1
Abstract
1. Introduction
2. Results
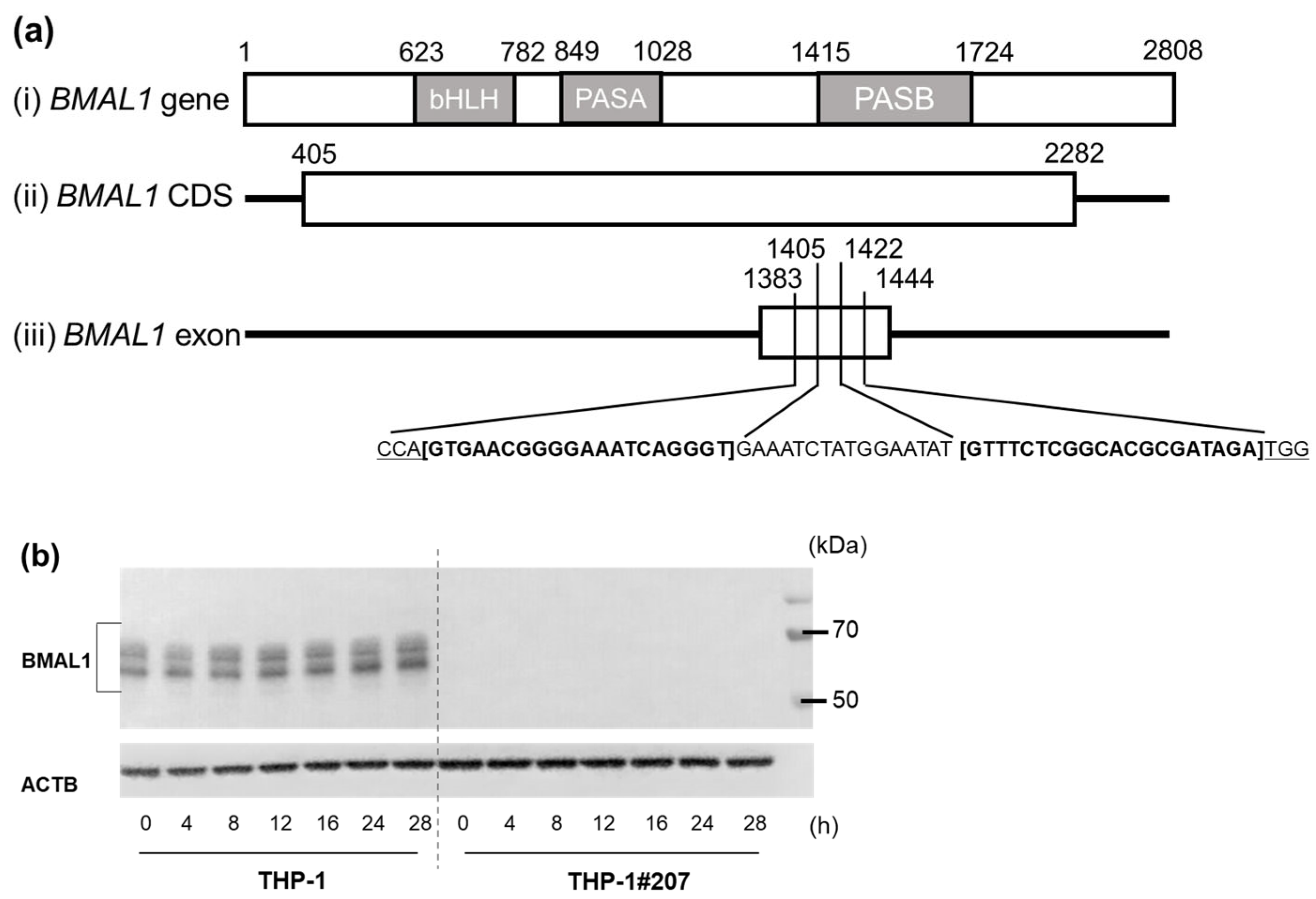
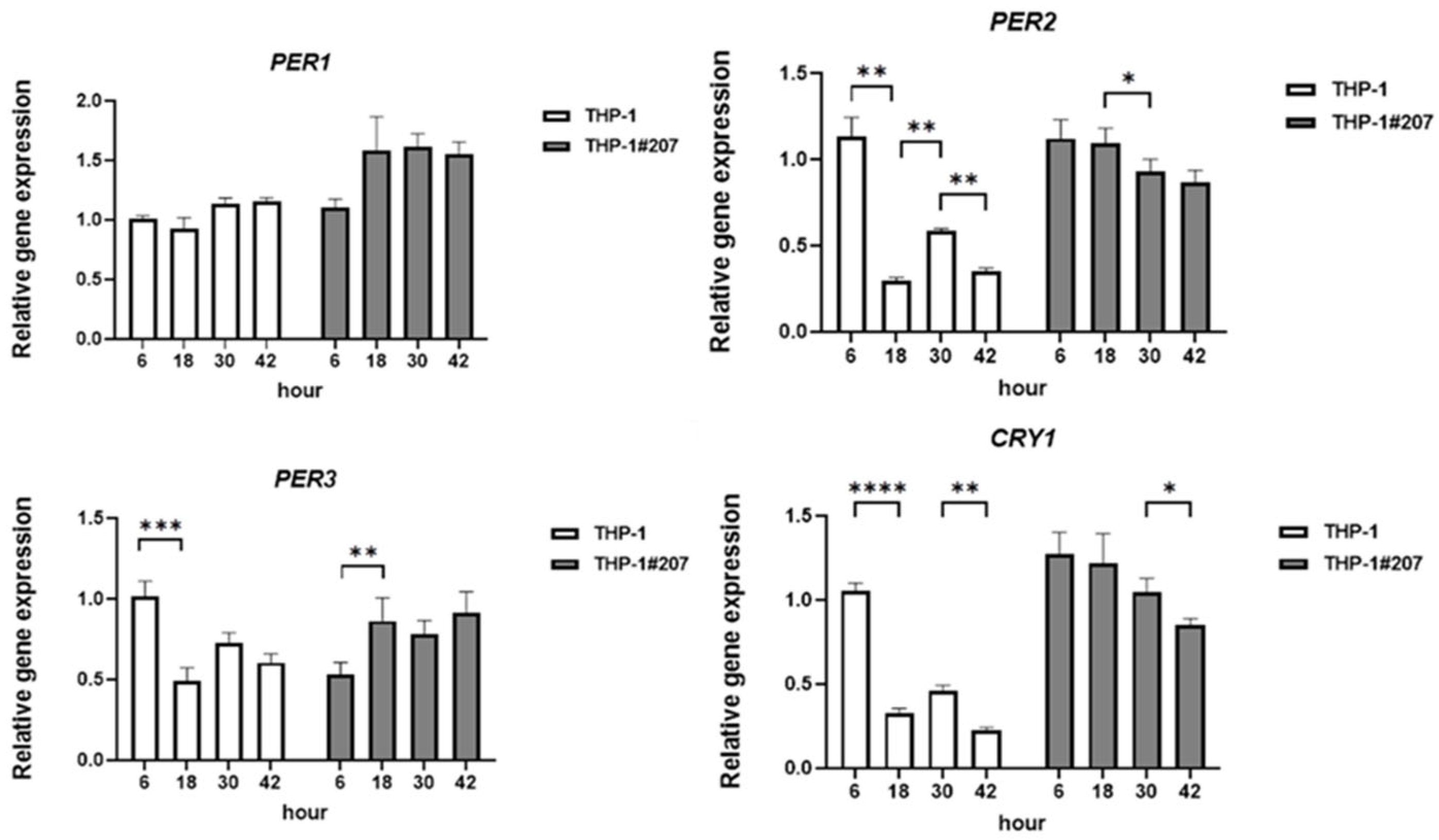
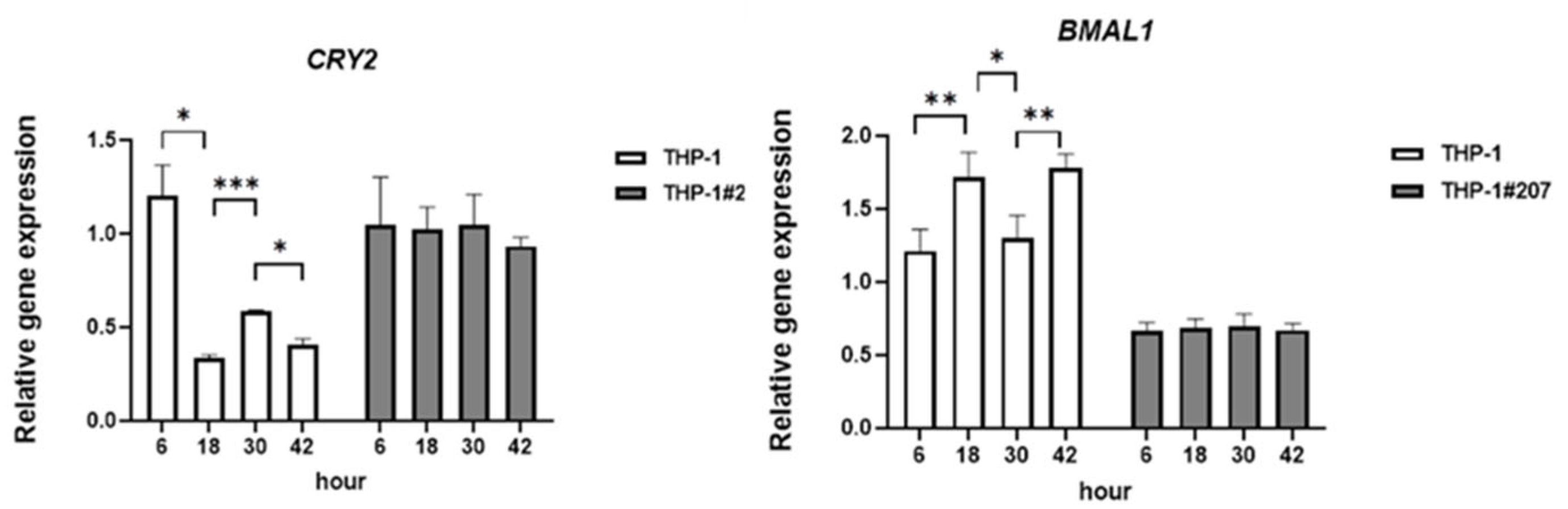
2.1. The BMAL1-PAS-B Domain Deletion Abrogates Circadian Expressions of Core Clock Genes in THP-1 Cells
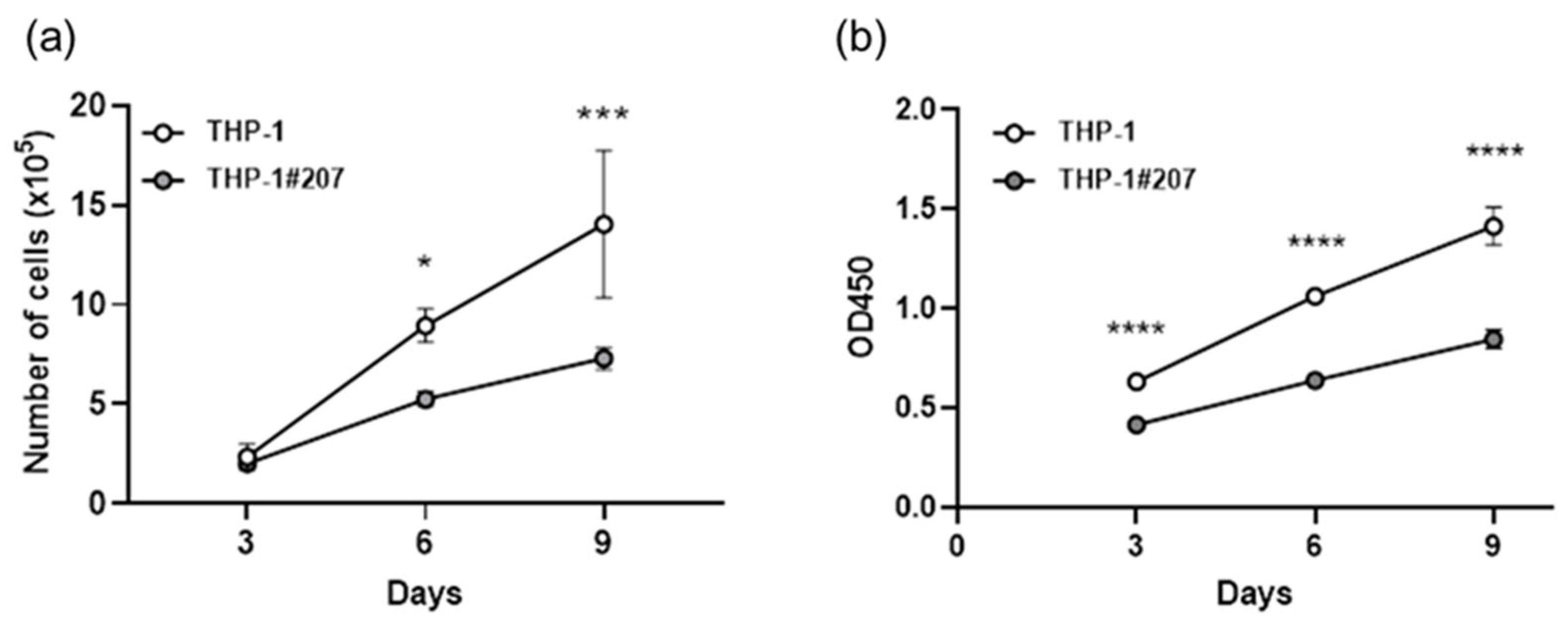
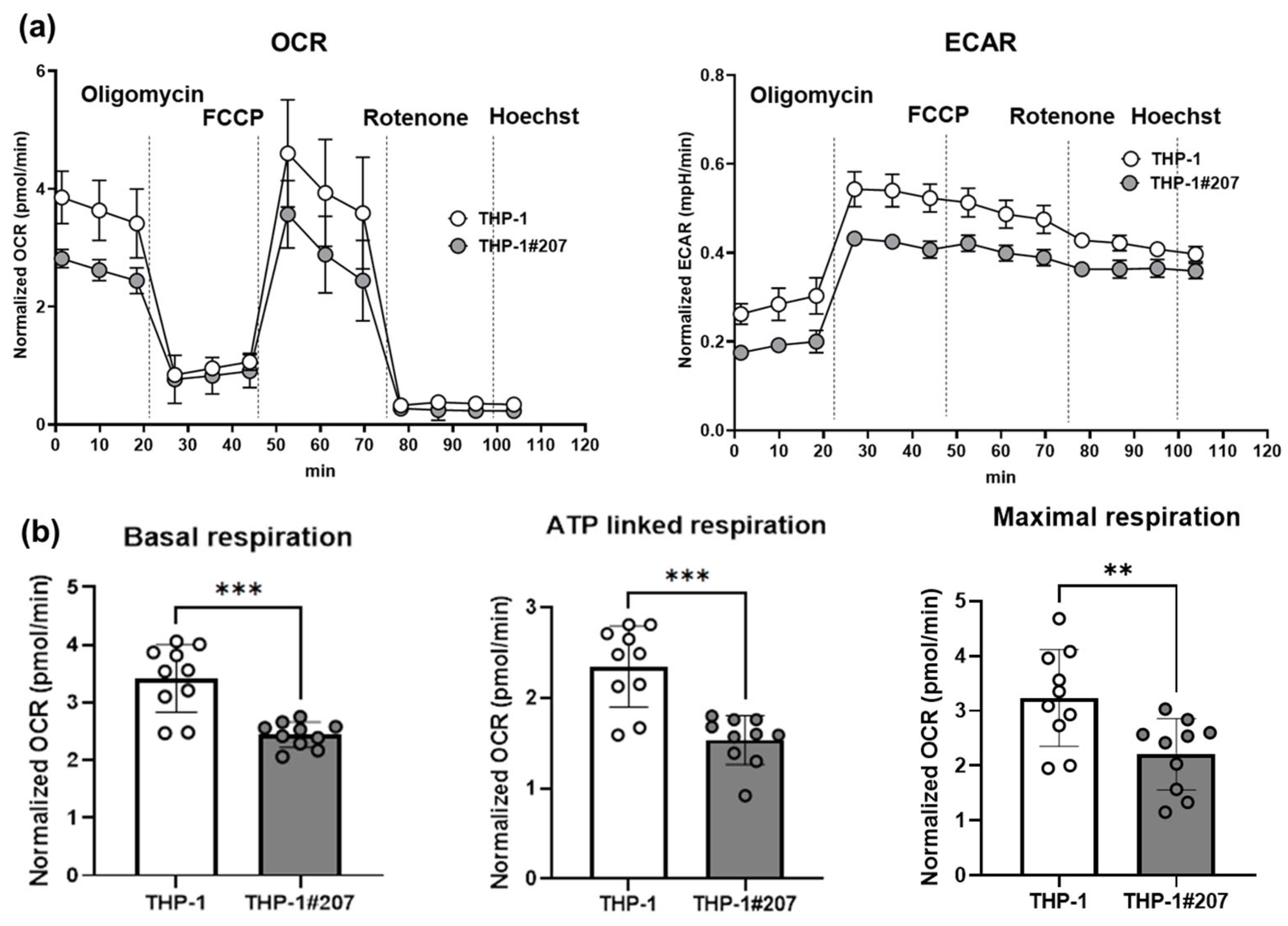
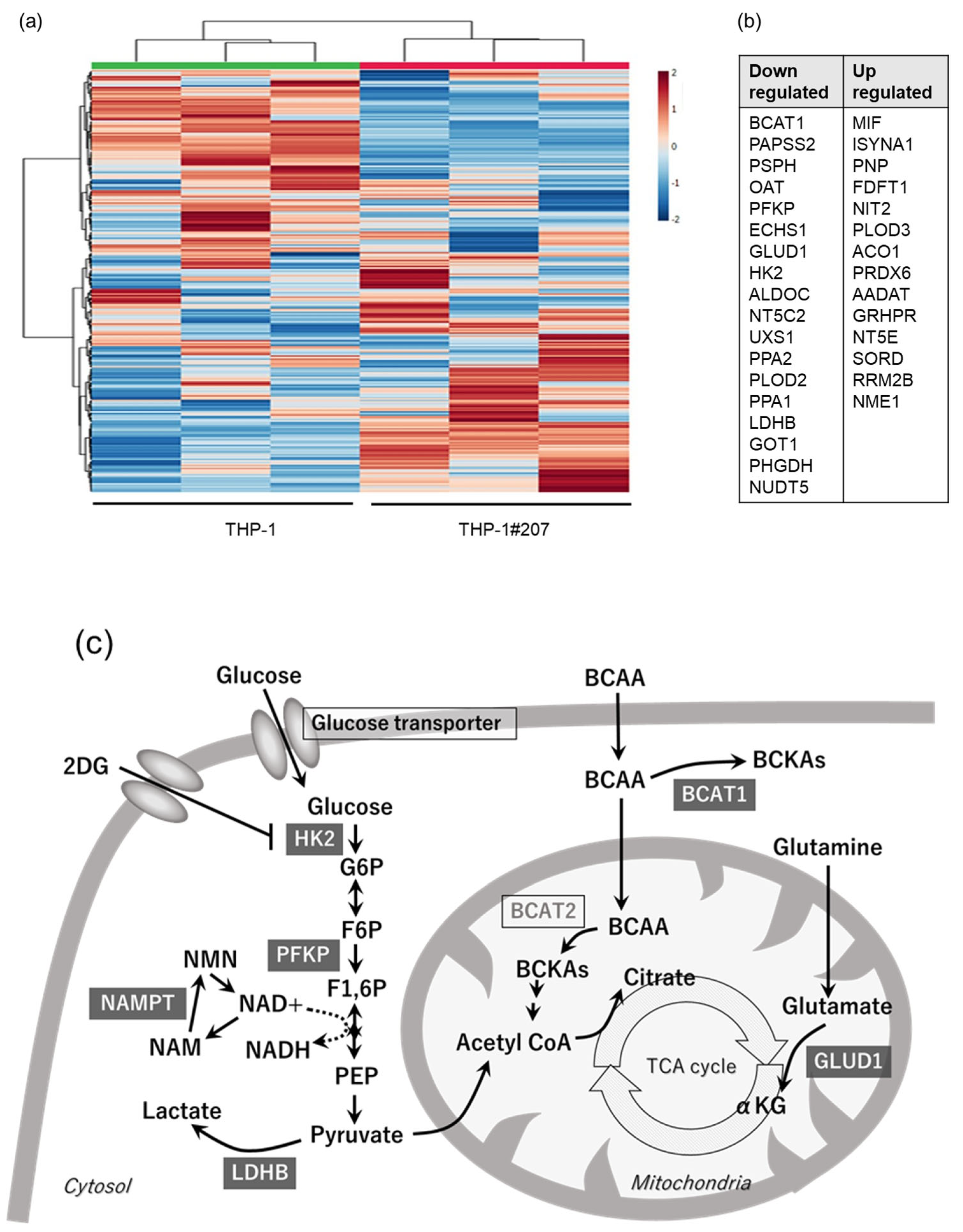
2.2. The BMAL1-PAS-B Domain Deletion Suppresses Proliferation in THP-1 Cells Associated with Low Activity of Glycolysis and Oxidative Phosphorylation
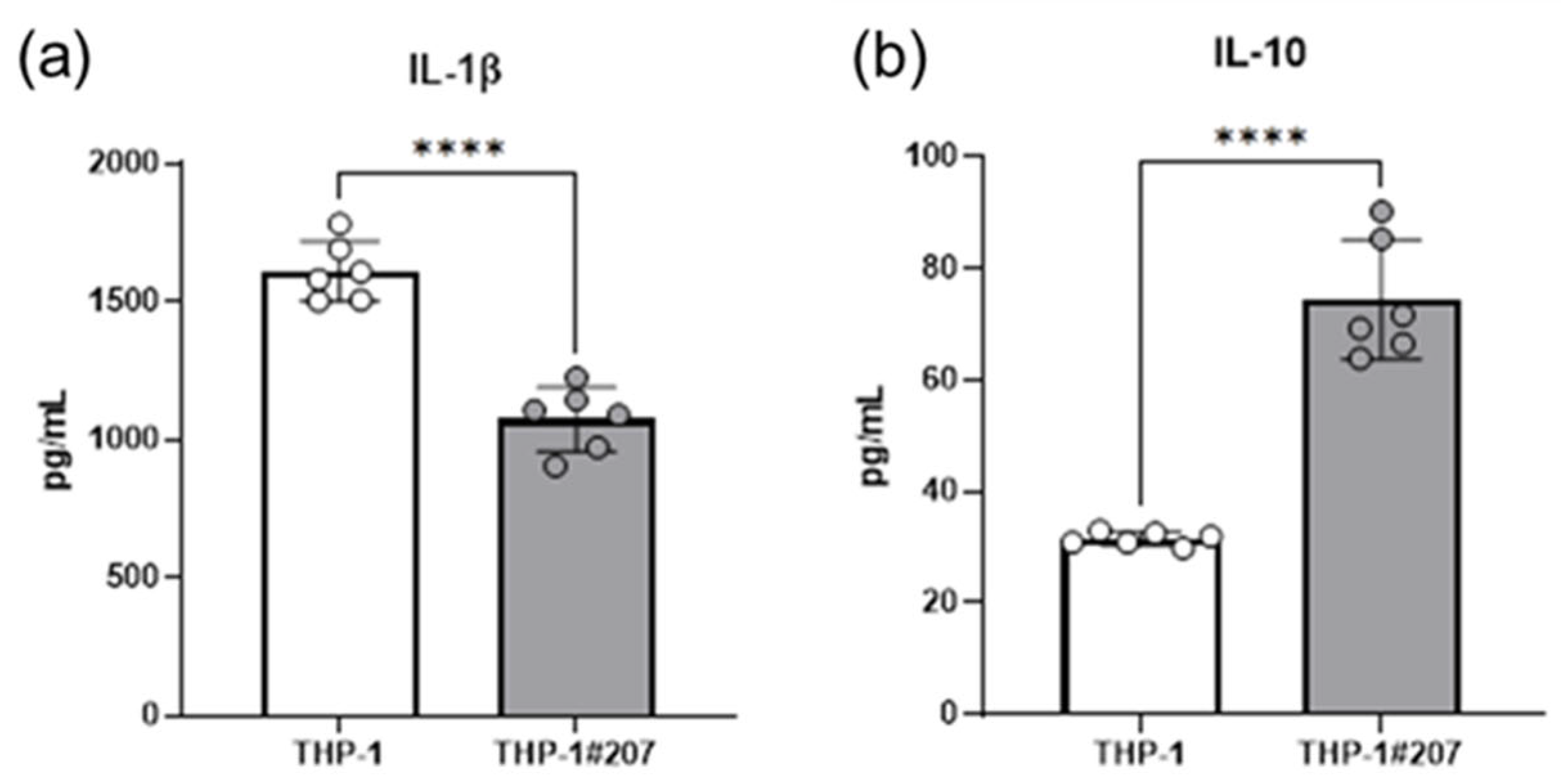
2.3. The BMAL1-PAS-B Domain Deletion Suppresses LPS-Induced IL-1b, but Increases IL-10 Production in THP-1 Cells
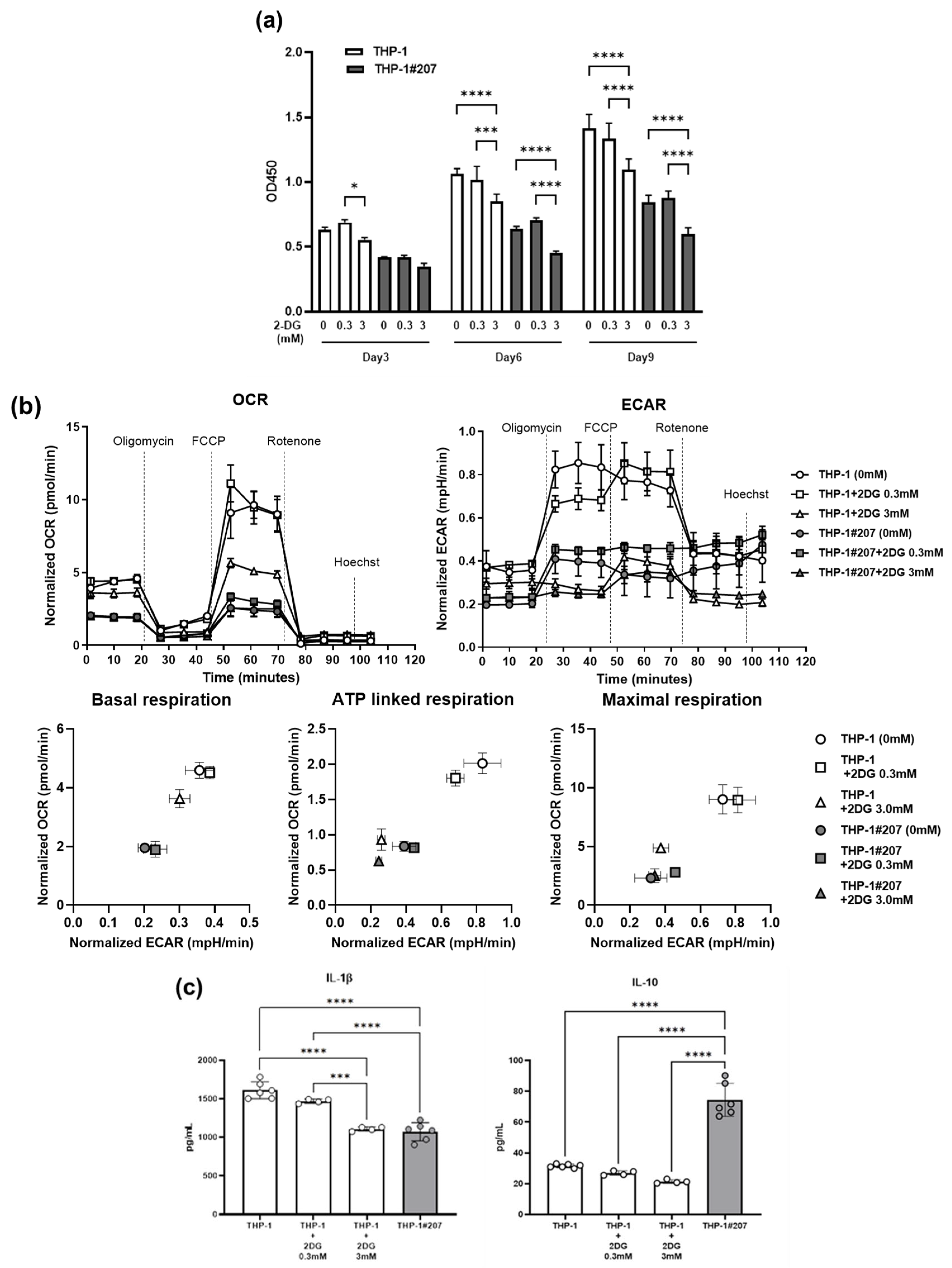
2.4. Inhibition of the Glycolytic Activity in THP-1 Cells Largely Recapitulates Phenotypes of THP-1#207 Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Establishment of BMAL1-PAS-B Domain-Deleted Cell Line THP-1#207 Cells
4.3. Quantitative Real-Time PCR (qPCR)
4.4. Western Blot
4.5. Synchronization
4.6. Cell Proliferation
4.7. Extracellular Flux Analysis
4.8. Enzyme-Linked Immunosorbent Assay (ELISA)
4.9. iMPAQT Proteomics
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Bmal1 | Brain muscle ARNT-like1 |
| bHLH | Basic helix–loop–helix (bHLH) |
| PAS | PER-ARNT-SIM |
| HK2 | hexokinase2 |
| 2-DG | 2-deoxy-D-glucose |
References
- Turek, F.W. Circadian clocks: Not your grandfather’s clock. Science 2016, 354, 992–993. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, J.S. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 2017, 18, 164–179. [Google Scholar] [CrossRef]
- Patke, A.; Young, M.W.; Axelrod, S. Molecular mechanisms and physiological importance of circadian rhythms. Nat. Rev. Mol. Cell Biol. 2020, 21, 67–84. [Google Scholar] [CrossRef] [PubMed]
- Ripperger, J.A.; Jud, C.; Albrecht, U. The daily rhythm of mice. FEBS Lett. 2011, 585, 1384–1392. [Google Scholar] [CrossRef]
- Huang, N.; Chelliah, Y.; Shan, Y.; Taylor, C.A.; Yoo, S.H.; Partch, C.; Green, C.B.; Zhang, H.; Takahashi, J.S. Crystal structure of the heterodimeric CLOCK:BMAL1 transcriptional activator complex. Science 2012, 337, 189–194. [Google Scholar] [CrossRef]
- Ruan, W.; Li, T.; Bang, I.H.; Lee, J.; Deng, W.; Ma, X.; Luo, C.; Du, F.; Yoo, S.H.; Kim, B.; et al. BMAL1-HIF2A heterodimer modulates circadian variations of myocardial injury. Nature 2025, 641, 1017–1028. [Google Scholar] [CrossRef] [PubMed]
- Pu, H.; Bailey, L.C.; Bauer, L.G.; Voronkov, M.; Baxter, M.; Huber, K.V.M.; Khorasanizadeh, S.; Ray, D.; Rastinejad, F. Pharmacological targeting of BMAL1 modulates circadian and immune pathways. Nat. Chem. Biol. 2025, 21, 736–745. [Google Scholar] [CrossRef]
- Wu, D.; Rastinejad, F. Structural characterization of mammalian bHLH-PAS transcription factors. Curr. Opin. Struct. Biol. 2017, 43, 1–9. [Google Scholar] [CrossRef]
- Sharma, D.; Partch, C.L. PAS Dimerization at the Nexus of the Mammalian Circadian Clock. J. Mol. Biol. 2024, 436, 168341. [Google Scholar] [CrossRef]
- Cermakian, N.; Lange, T.; Golombek, D.; Sarkar, D.; Nakao, A.; Shibata, S.; Mazzoccoli, G. Crosstalk between the circadian clock circuitry and the immune system. Chronobiol. Int. 2013, 30, 870–888. [Google Scholar] [CrossRef]
- Scheiermann, C.; Gibbs, J.; Ince, L.; Loudon, A. Clocking into immunity. Nat. Rev. Immunol. 2018, 18, 423–437. [Google Scholar] [CrossRef] [PubMed]
- Mok, H.; Ostendorf, E.; Ganninger, A.; Adler, A.J.; Hazan, G.; Haspel, J.A. Circadian immunity from bench to bedside: A practical guide. J. Clin. Investig. 2024, 134, e175706. [Google Scholar] [CrossRef] [PubMed]
- Timmons, G.A.; O’Siorain, J.R.; Kennedy, O.D.; Curtis, A.M.; Early, J.O. Innate Rhythms: Clocks at the Center of Monocyte and Macrophage Function. Front. Immunol. 2020, 11, 1743. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.D.; Fentress, S.J.; Qiu, Y.; Yun, K.; Cox, J.S.; Chawla, A. Circadian gene Bmal1 regulates diurnal oscillations of Ly6C(hi) inflammatory monocytes. Science 2013, 341, 1483–1488. [Google Scholar] [CrossRef]
- Huo, M.; Huang, Y.; Qu, D.; Zhang, H.; Wong, W.T.; Chawla, A.; Huang, Y.; Tian, X.Y. Myeloid Bmal1 deletion increases monocyte recruitment and worsens atherosclerosis. FASEB J. 2017, 31, 1097–1106. [Google Scholar] [CrossRef]
- Tsuchiya, S.; Yamabe, M.; Yamaguchi, Y.; Kobayashi, Y.; Konno, T.; Tada, K. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int. J. Cancer 1980, 26, 171–176. [Google Scholar] [CrossRef]
- Gauthier, T.; Chen, W. Modulation of Macrophage Immunometabolism: A New Approach to Fight Infections. Front. Immunol. 2022, 13, 780839. [Google Scholar] [CrossRef]
- Matsumoto, M.; Matsuzaki, F.; Oshikawa, K.; Goshima, N.; Mori, M.; Kawamura, Y.; Ogawa, K.; Fukuda, E.; Nakatsumi, H.; Natsume, T.; et al. A large-scale targeted proteomics assay resource based on an in vitro human proteome. Nat. Methods 2017, 14, 251–258. [Google Scholar] [CrossRef]
- Harfmann, B.D.; Schroder, E.A.; Kachman, M.T.; Hodge, B.A.; Zhang, X.; Esser, K.A. Muscle-specific loss of Bmal1 leads to disrupted tissue glucose metabolism and systemic glucose homeostasis. Skelet. Muscle 2016, 6, 12. [Google Scholar] [CrossRef]
- Wang, J.; Huang, Q.; Hu, X.; Zhang, S.; Jiang, Y.; Yao, G.; Hu, K.; Xu, X.; Liang, B.; Wu, Q.; et al. Disrupting Circadian Rhythm via the PER1-HK2 Axis Reverses Trastuzumab Resistance in Gastric Cancer. Cancer Res. 2022, 82, 1503–1517. [Google Scholar] [CrossRef]
- Timmons, G.A.; Carroll, R.G.; O’Siorain, J.R.; Cervantes-Silva, M.P.; Fagan, L.E.; Cox, S.L.; Palsson-McDermott, E.; Finlay, D.K.; Vincent, E.E.; Jones, N.; et al. The Circadian Clock Protein BMAL1 Acts as a Metabolic Sensor In Macrophages to Control the Production of Pro IL-1beta. Front. Immunol. 2021, 12, 700431. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, M.; Xu, L.; Cheng, J.; Shen, J.; Yang, T.; Zhang, L. Bmal1 Regulates Macrophage Polarize Through Glycolytic Pathway in Alcoholic Liver Disease. Front. Pharmacol. 2021, 12, 640521. [Google Scholar] [CrossRef] [PubMed]
- Alexander, R.K.; Liou, Y.H.; Knudsen, N.H.; Starost, K.A.; Xu, C.; Hyde, A.L.; Liu, S.; Jacobi, D.; Liao, N.S.; Lee, C.H. Bmal1 integrates mitochondrial metabolism and macrophage activation. eLife 2020, 9, e54090. [Google Scholar] [CrossRef] [PubMed]
- Mezhnina, V.; Ebeigbe, O.P.; Poe, A.; Kondratov, R.V. Circadian Control of Mitochondria in Reactive Oxygen Species Homeostasis. Antioxid. Redox Signal. 2022, 37, 647–663. [Google Scholar] [CrossRef] [PubMed]
- Jacobi, D.; Liu, S.; Burkewitz, K.; Kory, N.; Knudsen, N.H.; Alexander, R.K.; Unluturk, U.; Li, X.; Kong, X.; Hyde, A.L.; et al. Hepatic Bmal1 Regulates Rhythmic Mitochondrial Dynamics and Promotes Metabolic Fitness. Cell Metab. 2015, 22, 709–720. [Google Scholar] [CrossRef]
- Stoolman, J.S.; Grant, R.A.; Billingham, L.K.; Poor, T.A.; Weinberg, S.E.; Harding, M.C.; Lu, Z.; Miska, J.; Szibor, M.; Budinger, G.S.; et al. Mitochondria complex III-generated superoxide is essential for IL-10 secretion in macrophages. Sci. Adv. 2025, 11, eadu4369. [Google Scholar] [CrossRef]
- Zotta, A.; Toller-Kawahisa, J.; Palsson-McDermott, E.M.; O’Carroll, S.M.; Henry, Ó.C.; Day, E.A.; McGettrick, A.F.; Ward, R.W.; Ryan, D.G.; Watson, M.A.; et al. Mitochondrial respiratory complex III sustains IL-10 production in activated macrophages and promotes tumor-mediated immune evasion. Sci Adv. 2025, 11, eadq7307. [Google Scholar] [CrossRef]
- Schrader, L.A.; Ronnekleiv-Kelly, S.M.; Hogenesch, J.B.; Bradfield, C.A.; Malecki, K.M. Circadian disruption, clock genes, and metabolic health. J. Clin. Investig. 2024, 134, e170998. [Google Scholar] [CrossRef]
- Fortin, B.M.; Mahieu, A.L.; Fellows, R.C.; Kang, Y.; Lewis, A.N.; Ead, A.S.; Lamia, K.A.; Cao, Y.; Pannunzio, N.R.; Masri, S. The diverse roles of the circadian clock in cancer. Nat. Cancer 2025, 6, 753–767. [Google Scholar] [CrossRef]
- Naito, Y.; Hino, K.; Bono, H.; Ui-Tei, K. CRISPRdirect: Software for designing CRISPR/Cas guide RNA with reduced off-target sites. Bioinformatics 2015, 31, 1120–1123. [Google Scholar] [CrossRef]
- Balsalobre, A.; Marcacci, L.; Schibler, U. Multiple signaling pathways elicit circadian gene expression in cultured Rat-1 fibroblasts. Curr. Biol. 2000, 10, 1291–1294. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gozu, Y.; Hosoi, J.; Nagatomo, H.; Ishimaru, K.; Nakao, A. The PAS-B Domain of BMAL1 Controls Proliferation, Cellular Energetics, and Inflammatory Response in Human Monocytic Cell Line THP-1. Int. J. Mol. Sci. 2025, 26, 6737. https://doi.org/10.3390/ijms26146737
Gozu Y, Hosoi J, Nagatomo H, Ishimaru K, Nakao A. The PAS-B Domain of BMAL1 Controls Proliferation, Cellular Energetics, and Inflammatory Response in Human Monocytic Cell Line THP-1. International Journal of Molecular Sciences. 2025; 26(14):6737. https://doi.org/10.3390/ijms26146737
Chicago/Turabian StyleGozu, Yoko, Junichi Hosoi, Hiroaki Nagatomo, Kayako Ishimaru, and Atsuhito Nakao. 2025. "The PAS-B Domain of BMAL1 Controls Proliferation, Cellular Energetics, and Inflammatory Response in Human Monocytic Cell Line THP-1" International Journal of Molecular Sciences 26, no. 14: 6737. https://doi.org/10.3390/ijms26146737
APA StyleGozu, Y., Hosoi, J., Nagatomo, H., Ishimaru, K., & Nakao, A. (2025). The PAS-B Domain of BMAL1 Controls Proliferation, Cellular Energetics, and Inflammatory Response in Human Monocytic Cell Line THP-1. International Journal of Molecular Sciences, 26(14), 6737. https://doi.org/10.3390/ijms26146737






