Genotype–Phenotype Correlation of TNF-α (−238, rs361525) and Cystatin C for Early Detection of Sepsis-Associated AKI and Its Severity in Critically Ill Neonates
Abstract
1. Introduction
2. Results
2.1. Baseline Clinical and Demographic Characteristics
2.2. Serum Cystatin-C Levels
2.3. TNF-α (−238, rs361525) SNP Genotype Distribution
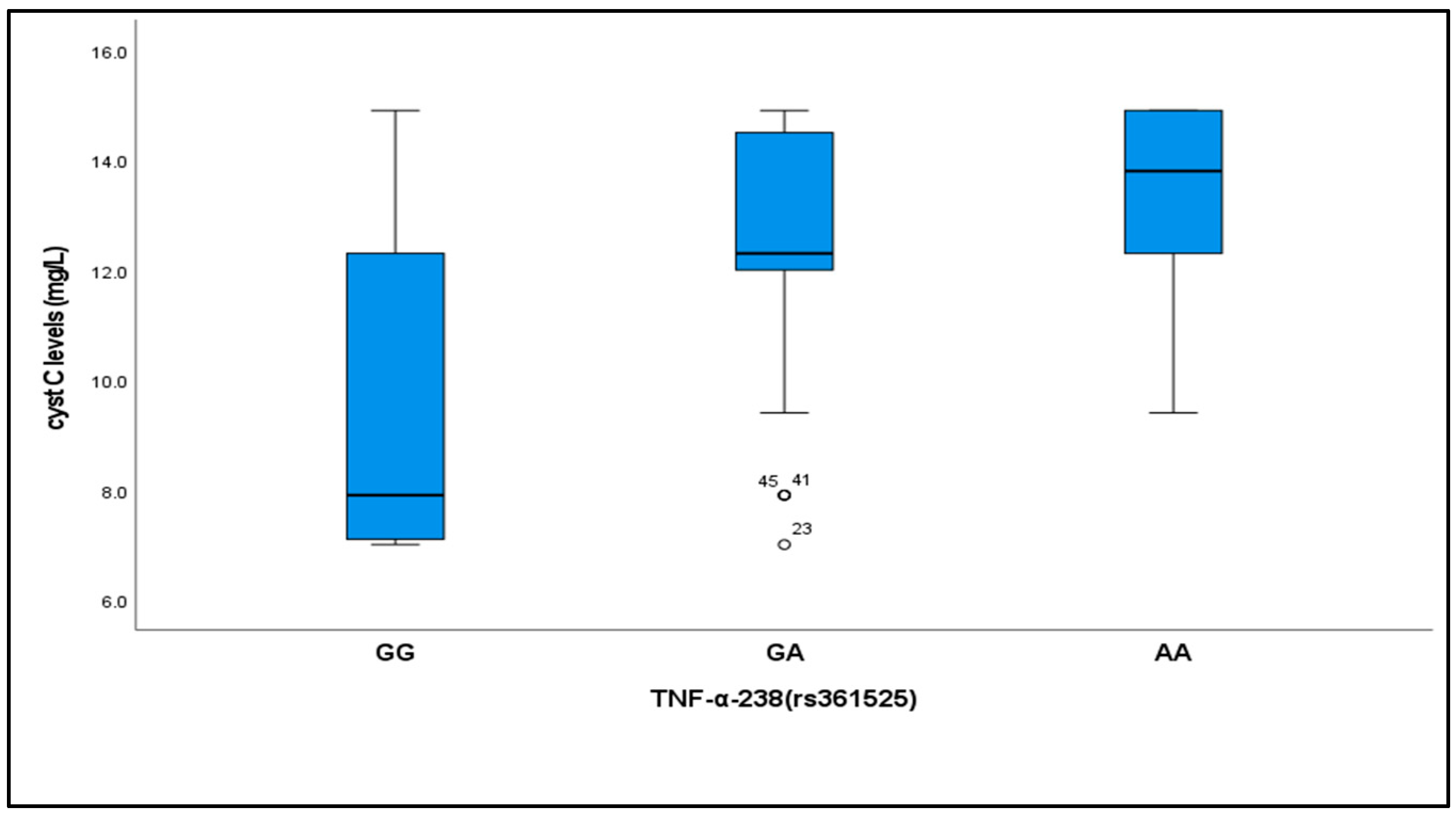
2.4. Relationship Between Cystatin C Levels and TNF-α (−238, rs361525) Genotypes
2.5. Serial Assessment of Renal Function Parameters
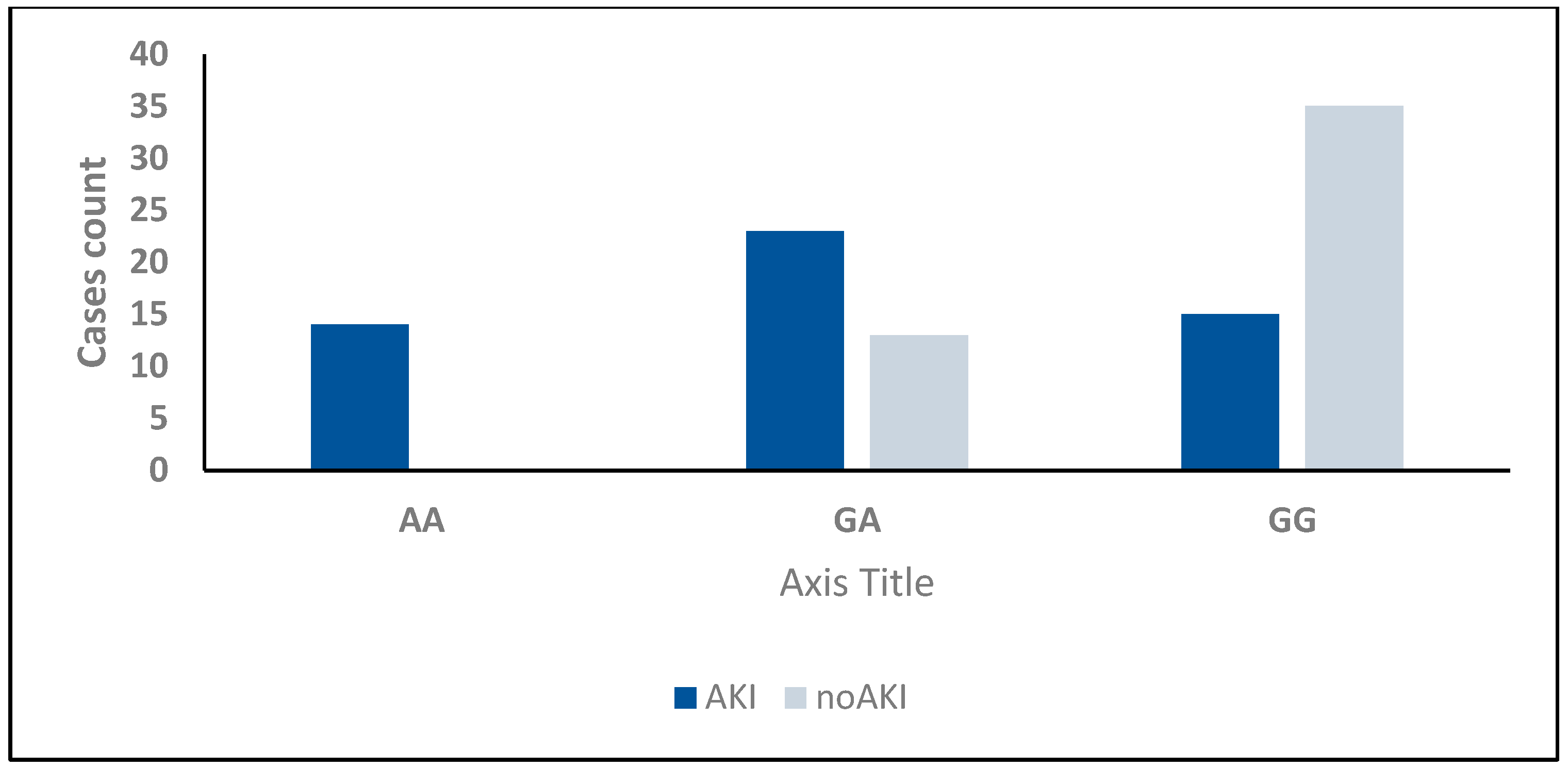
2.6. Development of Acute Kidney Injury (AKI)
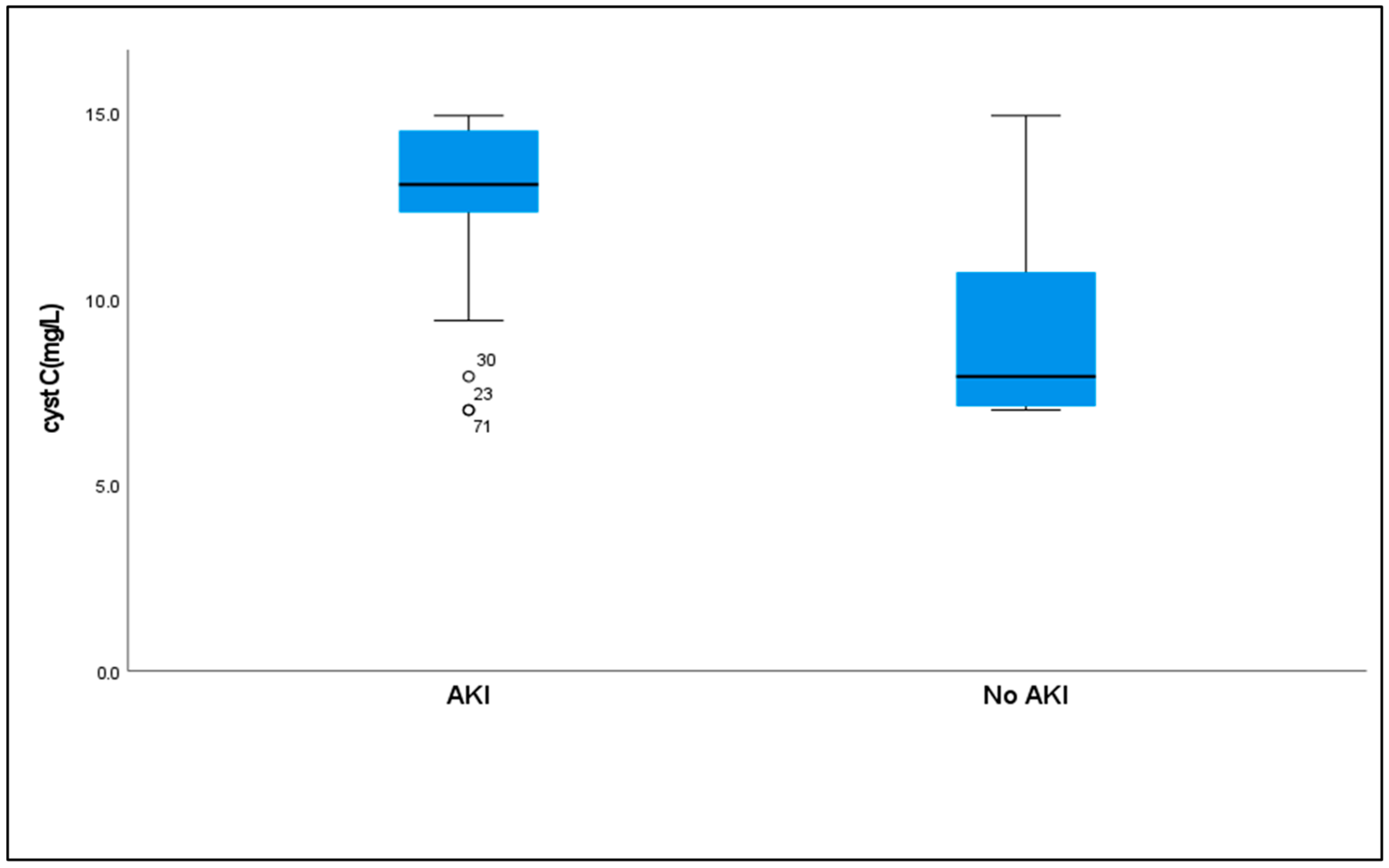
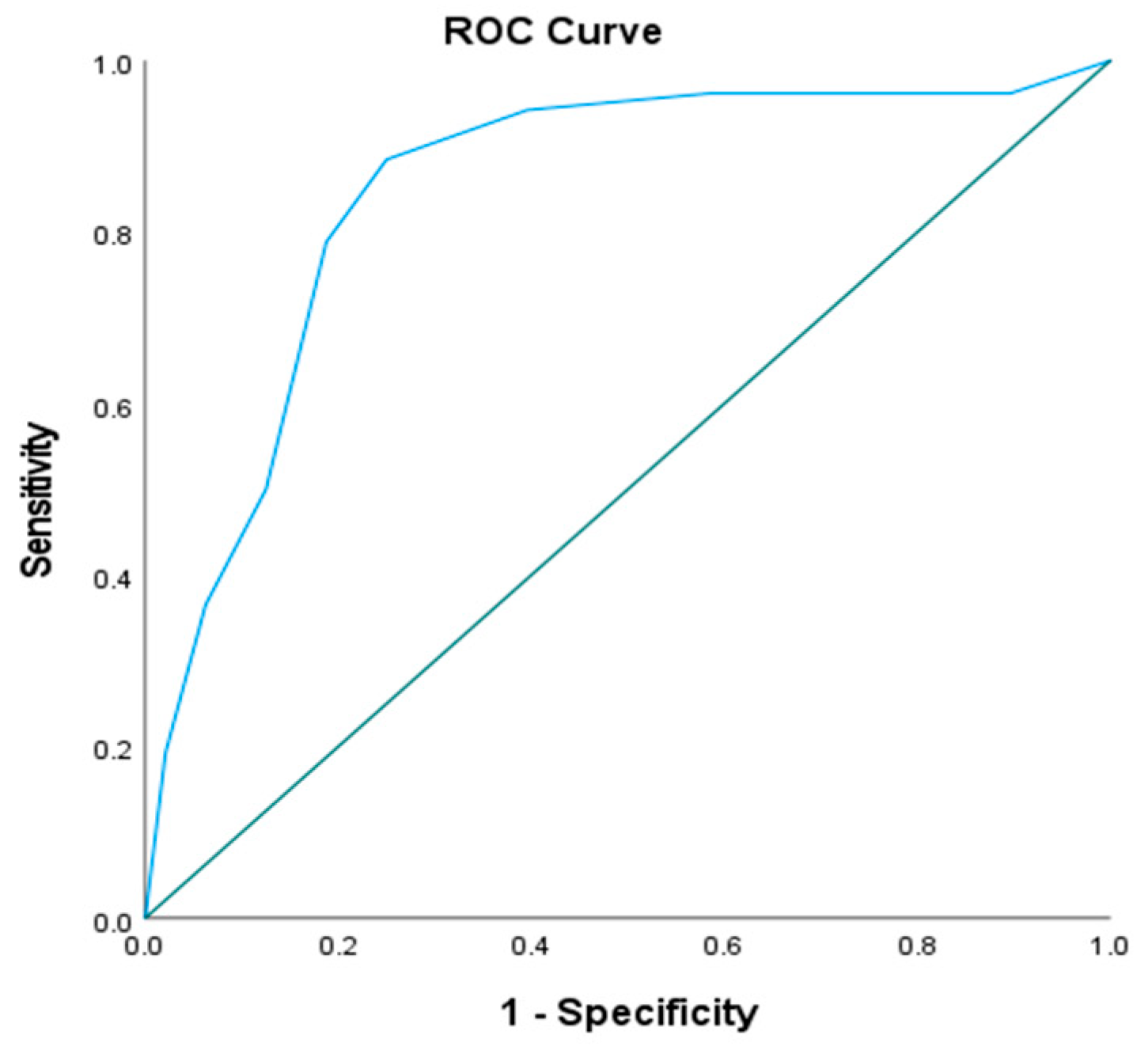
2.7. Serum Cystatin C Levels to AKI
2.8. Combined Diagnostic Value of TNF-α (−238, rs361525) Genotypes and Serum Cystatin C
2.9. Association Between AKI, TNF-α (−238, rs361525) Genotypes, and Cystatin C Levels
3. Discussion
Study Limitations
4. Methods
4.1. Study Design and Setting
4.2. Ethics Approval and Consent to Participate
4.3. Patient Selection and Classification
4.4. Data Collection
4.5. Blood Sample Collection
4.6. Biochemical Assessment
4.7. Cystatin C Measurement
4.8. Genotyping for TNF-α (−238, rs361525) SNP
4.8.1. DNA Extraction
4.8.2. Real-Time PCR (RT-PCR)
4.9. Diagnosis and Clinical Outcomes
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wenzel, R.P.; Pinsky, M.R.; Ulevitch, R.J.; Young, L. Current Understanding of Sepsis. Clin. Infect. Dis. 1996, 22, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Linde, A.; Mosier, D.; Blecha, F.; Melgarejo, T. Innate Immunity and Inflammation—New Frontiers in Comparative Cardiovascular Pathology. Cardiovasc. Res. 2007, 73, 26–36. [Google Scholar] [CrossRef]
- Feezor, R.J.; Oberholzer, C.; Baker, H.V.; Novick, D.; Rubinstein, M.; Moldawer, L.L.; Pribble, J.; Souza, S.; Dinarello, C.A.; Ertel, W.; et al. Molecular Characterization of the Acute Inflammatory Response to Infections with Gram-Negative versus Gram-Positive Bacteria. Infect. Immun. 2003, 71, 5803–5813. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, N.; Tokushige, K.; Yamaguchi, N.; Hasegawa, K.; Hashimoto, E.; Yamauchi, K.; Shiratori, K. Influence of TNF Gene Polymorphism in Patients with Acute and Fulminant Hepatitis. J. Gastroenterol. 2004, 39, 859–866. [Google Scholar] [CrossRef]
- Kothari, N.; Bogra, J.; Abbas, H.; Kohli, M.; Malik, A.; Kothari, D.; Srivastava, S.; Singh, P.K. Tumor Necrosis Factor Gene Polymorphism Results in High TNF Level in Sepsis and Septic Shock. Cytokine 2013, 61, 676–681. [Google Scholar] [CrossRef]
- Hillenbrand, A.; Knippschild, U.; Weiss, M.; Schrezenmeier, H.; Henne-Bruns, D.; Huber-Lang, M.; Wolf, A.M. Sepsis Induced Changes of Adipokines and Cytokines—Septic Patients Compared to Morbidly Obese Patients. BMC Surg. 2010, 10, 26. [Google Scholar] [CrossRef]
- Cartin-Ceba, R.; Haugen, E.N.; Iscimen, R.; Trillo-Alvarez, C.; Juncos, L.; Gajic, O. Evaluation of “Loss” and “End Stage Renal Disease” after Acute Kidney Injury Defined by the Risk, Injury, Failure, Loss and ESRD Classification in Critically Ill Patients. Intensive Care Med. 2009, 35, 2087–2095. [Google Scholar] [CrossRef] [PubMed]
- Plataki, M.; Kashani, K.; Cabello-Garza, J.; Maldonado, F.; Kashyap, R.; Kor, D.J.; Gajic, O.; Cartin-Ceba, R. Predictors of Acute Kidney Injury in Septic Shock Patients: An Observational Cohort Study. Clin. J. Am. Soc. Nephrol. 2011, 6, 1744–1751. [Google Scholar] [CrossRef]
- Oppert, M.; Engel, C.; Brunkhorst, F.M.; Bogatsch, H.; Reinhart, K.; Frei, U.; Eckardt, K.U.; Loeffler, M.; John, S. Acute Renal Failure in Patients with Severe Sepsis and Septic Shock—A Significant Independent Risk Factor for Mortality: Results from the German Prevalence Study. Nephrol. Dial. Transplant. 2008, 23, 904–909. [Google Scholar] [CrossRef]
- Pappachan, J.V.; Coulson, T.G.; Child, N.J.A.; Markham, D.J.; Nour, S.M.; Pulletz, M.C.K.; Rose-Zerilli, M.J.; De Courcey-Golder, K.; Barton, S.J.; Yang, I.A.; et al. Mortality in Adult Intensive Care Patients with Severe Systemic Inflammatory Response Syndromes Is Strongly Associated with the Hypo-Immune TNF -238A Polymorphism. Immunogenetics 2009, 61, 657–662. [Google Scholar] [CrossRef]
- Koyner, J.L.; Bennett, M.R.; Worcester, E.M.; Ma, Q.; Raman, J.; Jeevanandam, V.; Kasza, K.E.; O’Connor, M.F.; Konczal, D.J.; Trevino, S.; et al. Urinary Cystatin C as an Early Biomarker of Acute Kidney Injury Following Adult Cardiothoracic Surgery. Kidney Int. 2008, 74, 1059–1069. [Google Scholar] [CrossRef] [PubMed]
- Rossiter, A.; La, A.; Koyner, J.L.; Forni, L.G. New Biomarkers in Acute Kidney Injury. Crit. Rev. Clin. Lab. Sci. 2024, 61, 23–44. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Hu, X.; Dai, X.; Wang, S.; Bai, Z.; Chen, J.; Pan, J.; Li, X.; Wang, J.; Li, Y. Subclinical Acute Kidney Injury Is Associated with Adverse Outcomes in Critically Ill Neonates and Children. Crit. Care 2018, 22, 221. [Google Scholar] [CrossRef]
- Li, Y.; Fu, C.; Zhou, X.; Xiao, Z.; Zhu, X.; Jin, M.; Li, X.; Feng, X. Urine Interleukin-18 and Cystatin-C as Biomarkers of Acute Kidney Injury in Critically Ill Neonates. Pediatr. Nephrol. 2012, 27, 851–860. [Google Scholar] [CrossRef]
- Knight, E.L.; Verhave, J.C.; Spiegelman, D.; Hillege, H.L.; De Zeeuw, D.; Curhan, G.C.; De Jong, P.E. Factors Influencing Serum Cystatin C Levels Other than Renal Function and the Impact on Renal Function Measurement. Kidney Int. 2004, 65, 1416–1421. [Google Scholar] [CrossRef]
- Jacqz-Aigrain, E.; Zhao, W.; Sharland, M.; van den Anker, J.N. Use of Antibacterial Agents in the Neonate: 50 Years of Experience with Vancomycin Administration. Semin. Fetal Neonatal Med. 2013, 18, 28–34. [Google Scholar] [CrossRef]
- Lestner, J.M.; Hill, L.F.; Heath, P.T.; Sharland, M. Vancomycin Toxicity in Neonates: A Review of the Evidence. Curr. Opin. Infect. Dis. 2016, 29, 237–247. [Google Scholar] [CrossRef]
- Knoderer, C.A.; Gritzman, A.L.; Nichols, K.R.; Wilson, A.C. Late-Occurring Vancomycin-Associated Acute Kidney Injury in Children Receiving Prolonged Therapy. Ann. Pharmacother. 2015, 49, 1113–1119. [Google Scholar] [CrossRef] [PubMed]
- Bayley, J.P.; Ottenhoff, T.H.M.; Verweij, C.L. Is There a Future for TNF Promoter Polymorphisms? Genes Immun. 2004, 5, 315–329. [Google Scholar] [CrossRef]
- Bline, K.E.; Hall, M.W. Immune Function in Critically Ill Septic Children. Pathogens 2021, 10, 1239. [Google Scholar] [CrossRef]
- Shum, H.P.; Kong, H.H.Y.; Chan, K.C.; Yan, W.W.; Chan, T.M. Septic Acute Kidney Injury in Critically Ill Patients—A Single-Center Study on Its Incidence, Clinical Characteristics, and Outcome Predictors. Ren. Fail. 2016, 38, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Zarbock, A.; Koyner, J.L.; Gomez, H.; Pickkers, P.; Forni, L. Sepsis-Associated Acute Kidney Injury—Treatment Standard. Nephrol. Dial. Transplant. 2024, 39, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Zarbock, A.; Nadim, M.K.; Pickkers, P.; Gomez, H.; Bell, S.; Joannidis, M.; Kashani, K.; Koyner, J.L.; Pannu, N.; Meersch, M.; et al. Sepsis-Associated Acute Kidney Injury: Consensus Report of the 28th Acute Disease Quality Initiative Workgroup. Nat. Rev. Nephrol. 2023, 19, 401–417. [Google Scholar] [CrossRef]
- Angurana, S.K.; Bansal, A.; Muralidharan, J.; Aggarwal, R.; Singhi, S. Cytokine Levels in Critically Ill Children with Severe Sepsis and Their Relation with the Severity of Illness and Mortality. J. Intensive Care Med. 2021, 36, 576–583. [Google Scholar] [CrossRef]
- Phumeetham, S.; Chat-uthai, N.; Manavathongchai, M.; Viprakasit, V. Genetic Association Study of Tumor Necrosis Factor-Alpha with Sepsis and Septic Shock in Thai Pediatric Patients. J. Pediatr. (Rio J.) 2012, 88, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Hugo Montes, A.; Valle-Garay, E.; Martin, G.; Collazos, J.; Alvarez, V.; Meana, A.; Pérez-Is, L.; Carton, J.A.; Taboada, F.; Asensi, V. The TNF-α (–238 G/A) Polymorphism Could Protect against Development of Severe Sepsis. Innate Immun. 2021, 27, 409–420. [Google Scholar] [CrossRef]
- Li, L.; Zinger, J.; Sassen, S.D.T.; Juffermans, N.P.; Koch, B.C.P.; Endeman, H. The Relation between Inflammatory Biomarkers and Drug Pharmacokinetics in the Critically Ill Patients: A Scoping Review. Crit. Care 2024, 28, 103. [Google Scholar] [CrossRef]
- Babaeenezhad, E.; Dezfoulian, O.; Moradi Sarabi, M.; Ahmadvand, H. Monoterpene Linalool Restrains Gentamicin-Mediated Acute Kidney Injury in Rats by Subsiding Oxidative Stress, Apoptosis, and the NF-ΚB/INOS/TNF-α/IL-1β Pathway and Regulating TGF-β. Naunyn Schmiedebergs Arch. Pharmacol. 2024, 397, 5701–5714. [Google Scholar] [CrossRef]
- Tulassay, T.; Vásárhelyi, B. Birth Weight and Renal Function. Curr. Opin. Nephrol. Hypertens. 2002, 11, 347–352. [Google Scholar] [CrossRef]
- Shen, H.; Na, W.; Li, Y.; Qu, D. The Clinical Significance of Renal Resistance Index (RRI) and Renal Oxygen Saturation (RrSO2) in Critically Ill Children with AKI: A Prospective Cohort Study. BMC Pediatr. 2023, 23, 495. [Google Scholar] [CrossRef]
- Talat, F.; Alam, K.; Akhtar, K.; Ali, S.M. A Clinicopathological Study of Thrombocytopenia, Acute-Phase Reactants, and Blood Culture in Neonatal Sepsis. Pediatr. Respirol. Crit. Care Med. 2022, 6, 27–30. [Google Scholar] [CrossRef]
- da Silva Barbosa, J.; da Silva, G.B.; Meneses, G.C.; Martins, A.M.C.; De Francesco Daher, E.; Machado, R.P.G.; Lemes, R.P.G. Use of Non-Conventional Biomarkers in the Early Diagnosis of Acute Kidney Injury in Preterm Newborns with Sepsis. J. Bras. Nefrol. 2022, 44, 97–108. [Google Scholar] [CrossRef]
- da Silva Barbosa, J.; Meneses, G.C.; Castelo, L.R.; da Silva Júnior, G.B.; Costa Martins, A.M.; De Francesco Daher, E.; Sampaio, T.L.; de Oliveira Gomes, A.; Carvalho Dantas, S.M.; da Silva Rebouças, A.; et al. Urinary Cystatin-C and Urinary NGAL Associated with Sepsis Predicts Longer Hospital Stay in Premature Newborns. Biomark. Med. 2024, 18, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Al-Amodi, H.S.; Abdelsattar, S.; Kasemy, Z.A.; Bedair, H.M.; Elbarbary, H.S.; Kamel, H.F.M. Potential Value of TNF-α (–376 G/A) Polymorphism and Cystatin C (CysC) in the Diagnosis of Sepsis Associated Acute Kidney Injury (S-AKI) and Prediction of Mortality in Critically Ill Patients. Front. Mol. Biosci. 2021, 8, 751299. [Google Scholar] [CrossRef] [PubMed]
- Hashad, D.I.; Elsayed, E.T.; Helmy, T.A.; Elawady, S.M. Study of the Role of Tumor Necrosis Factor-α (–308G/A) and Interleukin-10 (–1082 G/A) Polymorphisms as Potential Risk Factors to Acute Kidney Injury in Patients with Severe Sepsis Using High-Resolution Melting Curve Analysis. Ren. Fail. 2017, 39, 77–82. [Google Scholar] [CrossRef]
- Xu, X.; Nie, S.; Xu, H.; Liu, B.; Weng, J.; Chen, C.; Liu, H.; Yang, Q.; Li, H.; Kong, Y.; et al. Detecting Neonatal AKI by Serum Cystatin C. J. Am. Soc. Nephrol. 2023, 34, 1253–1263. [Google Scholar] [CrossRef]
- Hidayati, E.L.; Utami, M.D.; Rohsiswatmo, R.; Tridjaja, B. Cystatin C Compared to Serum Creatinine as a Marker of Acute Kidney Injury in Critically Ill Neonates. Pediatr. Nephrol. 2021, 36, 181–186. [Google Scholar] [CrossRef]
- Nejat, M.; Pickering, J.W.; Walker, R.J.; Endre, Z.H. Rapid Detection of Acute Kidney Injury by Plasma Cystatin C in the Intensive Care Unit. Nephrol. Dial. Transplant. 2010, 25, 3283–3289. [Google Scholar] [CrossRef]
- Luna, S.A.; Bushra, F.; Sultana, J.; Sultana, N.; Mamun, A.-A.; Huque, S.S.; Jesmin, T.; Begum, A.; Roy, R.R. Role of Cystatin C for Early Detection of Acute Kidney Injury in Children. Paediatr. Nephrol. J. Bangladesh 2024, 9, 58–65. [Google Scholar] [CrossRef]
- Herget-Rosenthal, S.; Marggraf, G.; Hüsing, J.; Göring, F.; Pietruck, F.; Janssen, O.; Philipp, T.; Kribben, A. Early Detection of Acute Renal Failure by Serum Cystatin C. Kidney Int. 2004, 66, 1115–1122. [Google Scholar] [CrossRef]
- Zhang, Z.; Lu, B.; Sheng, X.; Jin, N. Cystatin C in Prediction of Acute Kidney Injury: A Systemic Review and Meta-Analysis. Am. J. Kidney Dis. 2011, 58, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Guo, S.; Wan, C.; Yang, T.; Zeng, N.; Wu, Y.; Chen, L.; Shen, Y.; Wen, F. Tumor Necrosis Factor-α -308 G/A Polymorphism and Risk of Sepsis, Septic Shock, and Mortality: An Updated Meta-Analysis. Oncotarget 2017, 8, 94910–94919. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cui, X.; Ning, L.; Wei, D. The Effects of Tumor Necrosis Factor-α (TNF-α) Rs1800629 and Rs361525 Polymorphisms on Sepsis Risk. Oncotarget 2017, 8, 111456–111469. [Google Scholar] [CrossRef]
- Van Doorn, K.J.; Spapen, H.; Geers, C.; Diltoer, M.; Shabana, W. Sepsis-Related Acute Kidney Injury: A Protective Effect of Drotrecogin Alfa (Activated) Treatment? Acta Anaesthesiol. Scand. 2008, 52, 1259–1264. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Wynn, J.L.; Wong, H.R. Pathophysiology and Treatment of Septic Shock in Neonates. Clin. Perinatol. 2010, 37, 439–479. [Google Scholar] [CrossRef]
- Matics, T.J.; Sanchez-Pinto, L.N. Adaptation and Validation of a Pediatric Sequential Organ Failure Assessment Score and Evaluation of the Sepsis-3 Definitions in Critically Ill Children. JAMA Pediatr. 2017, 171, e172352. [Google Scholar] [CrossRef]
- Khwaja, A. KDIGO Clinical Practice Guidelines for Acute Kidney Injury. Nephron Clin. Pract. 2012, 120, c179–c184. [Google Scholar] [CrossRef]
- Peerapornratana, S.; Manrique-Caballero, C.L.; Gómez, H.; Kellum, J.A. Acute Kidney Injury from Sepsis: Current Concepts, Epidemiology, Pathophysiology, Prevention and Treatment. Kidney Int. 2019, 96, 1083–1099. [Google Scholar] [CrossRef]
- Manrique-Caballero, C.L.; Del Rio-Pertuz, G.; Gomez, H. Sepsis-Associated Acute Kidney Injury. Crit. Care Clin. 2021, 37, 279–301. [Google Scholar] [CrossRef]
- Uhel, F.; Peters-Sengers, H.; Falahi, F.; Scicluna, B.P.; van Vught, L.A.; Bonten, M.J.; Cremer, O.L.; Schultz, M.J.; van der Poll, T. Mortality and Host Response Aberrations Associated with Transient and Persistent Acute Kidney Injury in Critically Ill Patients with Sepsis: A Prospective Cohort Study. Intensive Care Med. 2020, 46, 1576–1589. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, J.; Yadav, S.; Garrido-Maestu, A.; Azinheiro, S.; Trujillo, I.; Barros-Velázquez, J.; Prado, M. Evaluation of Simple Sequence Repeats (SSR) and Single Nucleotide Polymorphism (SNP)-Based Methods in Olive Varieties from the Northwest of Spain and Potential for Miniaturization. Food Chem. Mol. Sci. 2021, 3, 100038. [Google Scholar] [CrossRef] [PubMed]
- Graffelman, J.; Weir, B.S. On the Testing of Hardy–Weinberg Proportions and Equality of Allele Frequencies in Males and Females at Biallelic Genetic Markers. Genet. Epidemiol. 2018, 42, 34–48. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A Flexible Statistical Power Analysis Program for the Social, Behavioral, and Biomedical Sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Moons, K.G.M.; Altman, D.G.; Reitsma, J.B.; Ioannidis, J.P.A.; Macaskill, P.; Steyerberg, E.W.; Vickers, A.J.; Ransohoff, D.F.; Collins, G.S. Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD): Explanation and Elaboration. Ann. Intern. Med. 2015, 162, W1–W73. [Google Scholar] [CrossRef]
| Case (n = 100) | Control (n = 100) | Test of Sig. | p | |
|---|---|---|---|---|
| Age (Days) | ||||
| Median (IQR) | 7.0 (6.0–17.0) | 8.0 (6.0–48.0) | U = 4274.5 | 0.072 |
| Gestational Age | ||||
| Mean ± SD. | 37.64 ± 1.31 | 38.09 ± 1.44 | t = 2.311 | 0.022 * |
| Consanguinity | ||||
| Negative | 58 (58.0%) | 75 (75.0%) | χ2 = 6.486 | 0.011 * |
| Positive | 42 (42.0%) | 25 (25.0%) | ||
| Use of antenatal steroids | 46 (46.0%) | 42 (42.0%) | χ2 = 0.325 | 0.569 |
| Birth weight (kg) | ||||
| Mean ± SD. | 3.16 ± 0.29 | 3.21 ± 0.28 | t = 1.262 | 0.208 |
| Apgar score at 1st min | ||||
| Mean ± SD. | 6.36 ± 0.59 | 6.94 ± 0.93 | t = 5.253 | <0.001 * |
| Apgar score at 5 min | ||||
| Mean ± SD. | 7.37 ± 0.49 | 8.23 ± 0.75 | t = 9.626 | <0.001 * |
| Mean blood pressure (MBP) | ||||
| Mean ± SD. | 44.88 ± 3.16 | 45.13 ± 3.36 | t = 0.542 | 0.589 |
| Respiratory rate (RR) (c/m) | ||||
| Mean ± SD. | 66.76 ± 3.42 | 62.65 ± 7.45 | t = 5.011 | <0.001 * |
| Heart rate (HR) | ||||
| Mean ± SD. | 156.05 ± 6.58 | 155.78 ± 5.08 | t = 0.325 | 0.746 |
| Temperature °C | ||||
| Mean ± SD. | 37.83 ± 4.61 | 37.04 ± 0.20 | t = 1.707 | 0.091 |
| Hospital stay (days) | ||||
| Median (IQR) | 10 (10) | 5(2) | U = 643.50 | <0.001 * |
| Blood culture | ||||
| No growth | 31 (31.0%) | 100 (100.0%) | χ2 = 105.344 | <0.001 * |
| Growth | 69 (69.0%) | 0 (0.0) | ||
| Diagnosis by clinical examination | ||||
| Mild TTN | 0 (0.0%) | 40 (40.0%) | χ2 = 155.321 | <0.001 * |
| Neonatal jaundice | 0 (0.0%) | 34 (34.0%) | ||
| Neonatal jaundice (ABO) | 0 (0.0%) | 10 (10.0%) | ||
| Pneumonia | 63 (63.0%) | 0 (0.0%) | ||
| Severe TTN | 37 (37.0%) | 16 (16.0%) | ||
| Respiratory support | ||||
| No | 0 (0.0%) | 44 (44.0%) | χ2 = 146.563 | <0.001 * |
| CPAP | 45 (45.0%) | 19 (19.0%) | ||
| MV | 55 (55.0%) | 0 (0.0%) | ||
| Nasal cannula | 0 (0.0%) | 37 (37.0%) | ||
| Outcome | ||||
| Discharged | 90 (90.0%) | 100 (100.0%) | χ2 = 10.526 * | 0.001 * |
| Died | 10 (10.0%) | 0 (0.0) | ||
| AKI development Yes No | 52 (52.0%) 48 (48%) | - | - | - |
| Antibiotics intake Yes No | 51 (51.0%) 49 (49.0%) | - | - | - |
| Case (n = 100) | Control (n = 100) | Test of Sig | p | |
|---|---|---|---|---|
| PH Mean ± SD. | 7.35 ± 0.11 | 4.07 ± 3.63 | 1645.50 | <0.001 * |
| HCO3 (mmol/L) Mean ± SD. | 25.79 ± 4.04 | 11.27 ± 10.12 | 657.00 | <0.001 * |
| Na (mmol/L) | ||||
| Mean ± SD. | 137.49 ± 3.73 | 138.70 ± 2.47 | t = 2.707 | 0.007 * |
| K (mmol/L) | ||||
| Mean ± SD. | 4.37 ± 0.40 | 4.43 ± 0.38 | t = 1.065 | 0.288 |
| Ca (mg/dL) | ||||
| Mean ± SD. | 8.66 ± 0.50 | 8.73 ± 0.52 | t = 1.049 | 0.295 |
| RBS (mg/dL) | ||||
| Mean ± SD. | 69.70 ± 9.43 | 71.04 ± 9.31 | t = 1.011 | 0.313 |
| HB G/dL | ||||
| Mean ± SD. | 14.98 ± 1.74 | 15.47 ± 1.15 | t = 2.373 | 0.019 * |
| Platelets | ||||
| Mean ± SD. | 242.43 ± 35.92 | 230.78 ± 18.39 | t = 2.887 | 0.004 * |
| TLC | ||||
| Mean ± SD. | 17.44 ± 4.97 | 11.53 ± 1.61 | t = 11.30 | <0.001 * |
| ALT (U/L) | ||||
| Mean ± SD. | 31.60 ± 3.05 | 32.20 ± 3.49 | t = 1.295 | 0.197 |
| AST (U/L) | ||||
| Mean ± SD. | 33.51 ± 3.35 | 33.52 ± 3.10 | t = 0.022 | 0.983 |
| Urea (mg/dL) | ||||
| Mean ± SD. | 26.51 ± 3.87 | 26.32 ± 3.52 | t = 0.363 | 0.717 |
| Creatinine (mg/dL) | ||||
| Mean ± SD. | 0.75 ± 0.11 | 0.73 ± 0.11 | t = 1.260 | 0.209 |
| Lactate (mg/dL) | ||||
| Median (IQR) | 1.20 (0.89) | 0.90(0.57) | U = 3199 | <0.001 * |
| Cyst C (mg/L) | ||||
| Median (IQR) | 12.15 (5.9) | 6.0(3) | U = 949 | <0.001 * |
| CRP (mg/dL) Median (IQR) | 36.0 (60.0) | - | - | - |
| Case (n = 100) | Control (n = 100) | Test of Sig. | p | |
|---|---|---|---|---|
| TNF-α (−238, rs361525) | ||||
| GG | 50 (50.0%) | 67 (67.0%) | χ2 = 8.779 * | 0.012 * |
| GA | 36 (36.0%) | 29 (29.0%) | ||
| AA | 14 (14.0%) | 4 (4.0%) | ||
| HWp0 | 0.084 | 0.702 | ||
| Allele | ||||
| G | 136 (68.0%) | 163 (81.5%) | χ2 = 9.656 * | 0.002 * |
| A | 64 (32.0%) | 37 (18.5%) |
| TNF-α (−238, rs361525) | K | p Value | Sig. Bet. Groups | ||||
|---|---|---|---|---|---|---|---|
| GG (n = 55) | GA (n = 31) | AA (n = 14) | |||||
| Cystatin C (mg/L) Median (IQR) | 7.9 (5.2) | 12.39 (2.5) | 13.8 (2.7) | 31.8 | <0.001 ** | GG-GA | 0.001 ** |
| GG-AA | 0.001 ** | ||||||
| GA-AA | 0.32 | ||||||
| Case (n = 100) | Test of Sig | p | Wilcoxon Signed Ranks Test | |||
|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | ||||
| Creatinine (mg/dL) | ||||||
| Median (IQR) | 1.10 (0.5) | 1.50 (0.8) | 1.57 (1.8) | χ2 = 69.9 | <0.001 * | p ≤ 0.001 a |
| Urea (mg/dL) | ||||||
| Median (IQR) | 45.0 (30) | 92.0 (55.3) | 92.0 (55.3) | χ2 = 70.4 | <0.001 * | p ≤ 0.001 a |
| AKI (n = 52) | Non-AKI (n = 48) | Test of Sig. | p | |
|---|---|---|---|---|
| TNF-α (−238, rs361525) | ||||
| GG | 15 (28.8%) | 35 (72.9%) | χ2 = 24.657 | <0.001 * |
| GA | 23 (44.2%) | 13 (27.1%) | ||
| AA | 14 (26.9%) | 0 (0.0%) | ||
| HWp0 | 0.407 | 0.278 | ||
| Allele | ||||
| G | 53 (51.0%) | 83 (86.5%) | χ2 = 28.906 * | <0.001 * |
| A | 51 (49.0%) | 13 (3.5%) | ||
| Cyst C | ||||
| Median (IQR) | 13.05(2.2) | 7.90(4.3) | U = 380.50 * | <0.001 * |
| AUC | p | 95% CI | Cut Off | Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|---|---|---|---|
| Cyst C | 0.848 | <0.001 * | 0.767–0.928 | >9.4 | 88.46 | 75.0 | 79.3 | 85.7 |
| Non-AKI (n = 48) | AKI (n = 52) | Sensitivity | Specificity | PPV | NPV | Accuracy | |
|---|---|---|---|---|---|---|---|
| GA + AA | 13 (27.1%) | 37 (71.2%) | 71.15 | 72.92 | 74.0 | 70.0 | 72.0 |
| GA + AA & Cyst C (>9.4 mg/L) | 8 (16.7%) | 35 (67.3%) | 67.31 | 83.33 | 81.40 | 70.18 | 75.0 |
| AKI (n = 52) | Non-AKI (n = 48) | p | OR (LL–UL 95% CI) | |
|---|---|---|---|---|
| TNF-α (−238, rs361525) | ||||
| GG® | 15 (28.8%) | 35 (72.9%) | 1.000 | |
| GA | 23 (44.2%) | 13 (27.1%) | 0.002 ** | 4.128 (1.661–10.257) |
| AA | 14 (26.9%) | 0 (0.0%) | NA | NA |
| HWp | 0.407 | 0.278 | ||
| GA + AA | 37 (71.2%) | 13 (27.1%) | <0.001 ** | 6.641 (2.769–15.927) |
| Allele | ||||
| G | 53 (51.0%) | 83 (86.5%) | 1.000 | |
| A | 51 (49.0%) | 13 (3.5%) | <0.001 ** | 6.1437 (3.052 –12.368) |
| Cyst C (mg/L) | ||||
| Median (IQR) | 13.05 (2.2) | 7.90 (4.3) | <0.001 ** | 1.759(1.439–2.150) |
| Univariate | # Multivariate | |||
|---|---|---|---|---|
| p | OR (LL–UL 95% CI) | p | OR (LL–UL 95% CI) | |
| Age (months) | 0.163 | 1.006 (0.998–1.014) | ||
| Down score | 0.459 | 1.222 (0.719–2.078) | ||
| Platelets | 0.476 | 1.004 (0.993–1.015) | ||
| TLC | 0.448 | 1.032 (0.952–1.118) | ||
| Urea (mg/dL) | 0.057 | 0.900 (0.807–1.003) | ||
| Creatinine (mg/dL) | 0.246 | 7.927 (0.240–261.998) | ||
| Cyst C | <0.001 * | 1.759 (1.439–2.150) | <0.001 * | 1.627 (1.299–2.038) |
| Lactate | 0.002 * | 0.308 (0.146–0.647) | 0.586 | 0.763 (0.288–2.022) |
| CRP (mg/dL) | 0.274 | 1.007 (0.994–1.020) | ||
| TNF-α (−238, rs361525) (GA + AA) | <0.001 * | 6.641 (2.769–15.927) | 0.234 | 1.968 (0.645–6.004) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelsattar, S.; Al-Amodi, H.S.; Nazih, M.; Salem, E.H.M.; Mostafa, R.G.; Menshawy, S.S.; El-Banna, A.A.; Abdelgawad, B.M.; Nabih, O.S.; Mohsen, Y.; et al. Genotype–Phenotype Correlation of TNF-α (−238, rs361525) and Cystatin C for Early Detection of Sepsis-Associated AKI and Its Severity in Critically Ill Neonates. Int. J. Mol. Sci. 2025, 26, 6738. https://doi.org/10.3390/ijms26146738
Abdelsattar S, Al-Amodi HS, Nazih M, Salem EHM, Mostafa RG, Menshawy SS, El-Banna AA, Abdelgawad BM, Nabih OS, Mohsen Y, et al. Genotype–Phenotype Correlation of TNF-α (−238, rs361525) and Cystatin C for Early Detection of Sepsis-Associated AKI and Its Severity in Critically Ill Neonates. International Journal of Molecular Sciences. 2025; 26(14):6738. https://doi.org/10.3390/ijms26146738
Chicago/Turabian StyleAbdelsattar, Shimaa, Hiba S. Al-Amodi, Mahmoud Nazih, Eman H. M. Salem, Rasha G. Mostafa, Shymaa S. Menshawy, Amany A. El-Banna, Basma M. Abdelgawad, Omnia S. Nabih, Yasmin Mohsen, and et al. 2025. "Genotype–Phenotype Correlation of TNF-α (−238, rs361525) and Cystatin C for Early Detection of Sepsis-Associated AKI and Its Severity in Critically Ill Neonates" International Journal of Molecular Sciences 26, no. 14: 6738. https://doi.org/10.3390/ijms26146738
APA StyleAbdelsattar, S., Al-Amodi, H. S., Nazih, M., Salem, E. H. M., Mostafa, R. G., Menshawy, S. S., El-Banna, A. A., Abdelgawad, B. M., Nabih, O. S., Mohsen, Y., Abozeid, E., Abd El-Hamid, M. E.-S., Shoman, N. A., Ahmed, N. A., Nabil, M. M., & Mohamed, D. A.-W. (2025). Genotype–Phenotype Correlation of TNF-α (−238, rs361525) and Cystatin C for Early Detection of Sepsis-Associated AKI and Its Severity in Critically Ill Neonates. International Journal of Molecular Sciences, 26(14), 6738. https://doi.org/10.3390/ijms26146738





