Skeletal Muscle Pathology in Autosomal Recessive Cerebellar Ataxias: Insights from Marinesco–Sjögren Syndrome
Abstract
1. Introduction to ARCAs
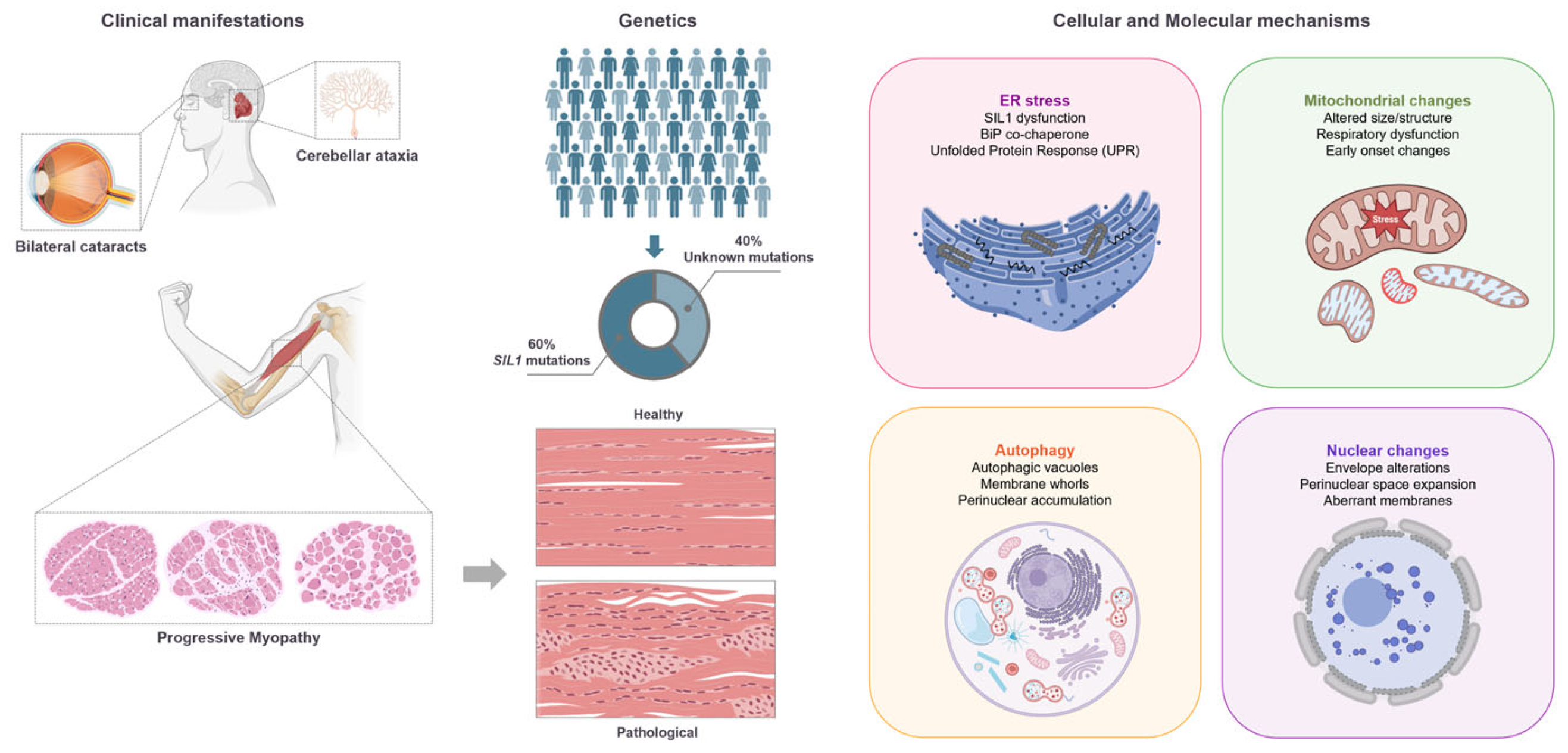
2. Overview of Marinesco–Sjögren Syndrome
2.1. Genetic and Molecular Basis of MSS
2.2. Skeletal Muscle Histopathology in MSS
2.3. Pathophysiological Mechanisms
2.4. Animal Models and Translational Insights of MSS
3. Comparative Analysis of MSS with Other ARCAs or Myopathies
ARCAs Animal Models
4. Clinical and Therapeutic Implications
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AAV | Adeno-associate virus |
| ADCAs | Autosomal dominant cerebellar ataxias |
| AOA1/2 | Oculomotor ataxia-apraxia type ½ |
| ARCAs | Autosomal recessive cerebellar ataxias |
| ATF6 | Activating transcription factor 6 |
| A-T | Ataxia-telangiectasia |
| AVED | Ataxia with vitamin E deficiency |
| BCL2 | BCL2 Apoptosis regulator |
| BiP/GRP78 | ER chaperone immunoglobulin binding protein |
| BniP-h (Atcay) | Caytaxin |
| CSA | Cross-sectional area |
| COX | Cytochrome c oxidase |
| DE | Differentially expressed (proteins) |
| DMF | Dimethyl fumarate |
| ER | Endoplasmic reticulum |
| FRDA | Friedreich’s ataxia |
| FXN | Frataxia |
| HSV | Herpes simplex virus |
| iPSCs | Induced pluripotent stem cells |
| IRE-1 | Inositol-requiring enzyme 1 |
| LC3 | Microtubule associated protein 1 light chain 3 Alpha (MAP1LC3A) |
| MCK | Muscle creatine kinase |
| MERRF | Myoclonus epilepsy with ragged-red fibers |
| MIRAS | Mitochondrial recessive ataxia syndrome |
| MRI | Magnetic resonance imaging |
| MSS | Marinesco–Sjögren syndrome |
| MT-CO2 | mtDNA variant in the cytochrome C oxidase II |
| mtDNA | Mitochondrial DNA |
| NEF | Nucleotide exchange factor |
| ORP150/GRP170 | 150 kDa Oxygen-regulated protein |
| PARP1 | Poly (ADP-Ribose) Polymerase 1 |
| PERK | ER kinase |
| PCr | Phosphocreatine recoveries |
| PCs | Purkinje cells |
| PKR | Protein kinase R |
| Polg | DNA polymerase gamma |
| p-eIF2α | Phospho-eukaryotic initiation factor 2α |
| SCAR | Autosomal recessive spinocerebellar ataxia |
| SIL1 | SIL1 nucleotide exchange factor |
| SPG7 | Spastic paraplegic 7 |
| SYNE1 | Spectron repeat containing nuclear envelope protein 1 |
| UPR | Unfolded protein response |
| WT | Wild-type |
References
- Klockgether, T. Ataxias. Park. Relat. Disord. 2007, 13, S391–S394. [Google Scholar] [CrossRef]
- Manto, M.; Marmolino, D. Cerebellar ataxias. Curr. Opin. Neurol. 2009, 22, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Marinesco, G. Nouvelle maladie familiale caracterisee par une cataracte congenitale et un arret du developpement somato-neuro-psychique. Encephale 1931, 26, 97–109. [Google Scholar]
- Sjogren, T. Hereditary congenital spinocerebellar ataxia accompanied by congenital cataract and oligophrenia; a genetic and clinical investigation. Confin. Neurol. 1950, 10, 293–308. [Google Scholar] [CrossRef] [PubMed]
- Alter, M.; Talbert, O.R.; Croffead, G. Cerebellar ataxia, congenital cataracts, and retarded somatic and mental maturation. Report of cases of Marinesco-Sjogren syndrome. Neurology 1962, 12, 836–847. [Google Scholar] [CrossRef]
- Arabia, G.; De Martino, A.; Moro, E. Chapter Four-Sex and gender differences in movement disorders: Parkinson’s disease, essential tremor, dystonia and chorea. In International Review of Neurobiology; Moro, E., Arabia, G., Tartaglia, M.C., Ferretti, M.T., Eds.; Academic Press: Cambridge, MA, USA, 2022; Volume 164, pp. 101–128. [Google Scholar]
- Bourque, M.; Dluzen, D.E.; Di Paolo, T. Neuroprotective actions of sex steroids in Parkinson’s disease. Front. Neuroendocrinol. 2009, 30, 142–157. [Google Scholar] [CrossRef]
- Zielonka, D.; Stawinska-Witoszynska, B. Gender Differences in Non-sex Linked Disorders: Insights From Huntington’s Disease. Front. Neurol. 2020, 11, 571. [Google Scholar] [CrossRef]
- Tsou, A.Y.; Paulsen, E.K.; Lagedrost, S.J.; Perlman, S.L.; Mathews, K.D.; Wilmot, G.R.; Ravina, B.; Koeppen, A.H.; Lynch, D.R. Mortality in Friedreich Ataxia. J. Neurol. Sci. 2011, 307, 46–49. [Google Scholar] [CrossRef]
- Ichhaporia, V.P.; Hendershot, L.M. Role of the HSP70 Co-Chaperone SIL1 in Health and Disease. Int. J. Mol. Sci. 2021, 22, 1564. [Google Scholar] [CrossRef]
- Todorov, A. Marinesco-Sjogren syndrome. 1st anatomo-clinical study. J. Genet. Hum. 1965, 14, 197–233. [Google Scholar]
- Sewry, C.A.; Voit, T.; Dubowitz, V. Myopathy with unique ultrastructural feature in Marinesco-Sjögren syndrome. Ann. Neurol. 1988, 24, 576–580. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, R.; Sallese, M. Review: Protein misfolding diseases-the rare case of Marinesco-Sjögren syndrome. Neuropathol. Appl. Neurobiol. 2020, 46, 323–343. [Google Scholar] [CrossRef] [PubMed]
- Hendershot, L.M. The ER function BiP is a master regulator of ER function. Mt. Sinai. J. Med. 2004, 71, 289–297. [Google Scholar] [PubMed]
- Shen, J.; Chen, X.; Hendershot, L.; Prywes, R. ER Stress Regulation of ATF6 Localization by Dissociation of BiP/GRP78 Binding and Unmasking of Golgi Localization Signals. Dev. Cell 2002, 3, 99–111. [Google Scholar] [CrossRef]
- Gardner, B.M.; Pincus, D.; Gotthardt, K.; Gallagher, C.M.; Walter, P. Endoplasmic Reticulum Stress Sensing in the Unfolded Protein Response. Cold Spring Harb. Perspect. Biol. 2013, 5, a013169. [Google Scholar] [CrossRef]
- Kashimada, A.; Hasegawa, S.; Isagai, T.; Uchiyama, T.; Matsuo, M.; Kawai, M.; Goto, M.; Morio, T.; Hayashi, Y.K.; Takagi, M. Targeting the enhanced ER stress response in Marinesco-Sjögren syndrome. J. Neurol. Sci. 2018, 385, 49–56. [Google Scholar] [CrossRef]
- Anttonen, A.-K.; Mahjneh, I.; Hämäläinen, R.H.; Lagier-Tourenne, C.; Kopra, O.; Waris, L.; Anttonen, M.; Joensuu, T.; Kalimo, H.; Paetau, A.; et al. The gene disrupted in Marinesco-Sjögren syndrome encodes SIL1, an HSPA5 cochaperone. Nat. Genet. 2005, 37, 1309–1311. [Google Scholar] [CrossRef]
- Senderek, J.; Krieger, M.; Stendel, C.; Bergmann, C.; Moser, M.; Breitbach-Faller, N.; Rudnik-Schöneborn, S.; Blaschek, A.; Wolf, N.I.; Harting, I.; et al. Mutations in SIL1 cause Marinesco-Sjögren syndrome, a cerebellar ataxia with cataract and myopathy. Nat. Genet. 2005, 37, 1312–1314. [Google Scholar] [CrossRef]
- Howes, J.; Shimizu, Y.; Feige, M.J.; Hendershot, L.M. C-terminal mutations destabilize SIL1/BAP and can cause Marinesco-Sjögren syndrome. J. Biol. Chem. 2012, 287, 8552–8560. [Google Scholar] [CrossRef]
- Mahloudji, M.; Amirhakimi, G.H.; Haghighi, P.; Khodadoust, A.A. Marinesco-Sjögren Syndrome Report of an Autopsy. Brain 1972, 95, 675–680. [Google Scholar] [CrossRef]
- Wang, L.; Valencak, T.G.; Shan, T. Fat infiltration in skeletal muscle: Influential triggers and regulatory mechanism. iScience 2024, 27, 109221. [Google Scholar] [CrossRef] [PubMed]
- Lagier-Tourenne, C.; Chaigne, D.; Gong, J.; Flori, J.; Mohr, M.; Ruh, D.; Christmann, D.; Flament, J.; Mandel, J.; Koenig, M.; et al. Linkage to 18qter differentiates two clinically overlapping syndromes: Congenital cataracts-facial dysmorphism-neuropathy (CCFDN) syndrome and Marinesco-Sjögren syndrome. J. Med. Genet. 2002, 39, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Mahjneh, I.; Anttonen, A.-K.; Somer, M.; Paetau, A.; Lehesjoki, A.-E.; Somer, H.; Udd, B. Myopathy is a prominent featurein Marinesco-Sjögren syndrome. J. Neurol. 2006, 253, 301–306. [Google Scholar] [CrossRef]
- Phan, V.; Cox, D.; Cipriani, S.; Spendiff, S.; Buchkremer, S.; O’Connor, E.; Horvath, R.; Goebel, H.H.; Hathazi, D.; Lochmüller, H.; et al. SIL1 deficiency causes degenerative changes of peripheral nerves and neuromuscular junctions in fish, mice and human. Neurobiol. Dis. 2019, 124, 218–229. [Google Scholar] [CrossRef]
- Roos, A.; Buchkremer, S.; Kollipara, L.; Labisch, T.; Gatz, C.; Zitzelsberger, M.; Brauers, E.; Nolte, K.; Schröder, J.M.; Kirschner, J.; et al. Myopathy in Marinesco–Sjögren syndrome links endoplasmic reticulum chaperone dysfunction to nuclear envelope pathology. Acta Neuropathol. 2014, 127, 761–777. [Google Scholar] [CrossRef]
- Superneau, D.W.; Wertelecki, W.; Zellweger, H.; Bastian, F. Myopathy in Marinesco-Sjogren syndrome. Eur. Neurol. 1987, 26, 8–16. [Google Scholar] [CrossRef]
- Suzuki, Y.; Murakami, N.; Goto, Y.; Orimo, S.; Komiyama, A.; Kuroiwa, Y.; Nonaka, I. Apoptotic nuclear degeneration in Marinesco-Sjögren syndrome. Acta Neuropathol. 1997, 94, 410–415. [Google Scholar] [CrossRef]
- Terracciano, A.; Renaldo, F.; Zanni, G.; D’Amico, A.; Pastore, A.; Barresi, S.; Valente, E.M.; Piemonte, F.; Tozzi, G.; Carrozzo, R.; et al. The use of muscle biopsy in the diagnosis of undefined ataxia with cerebellar atrophy in children. Eur. J. Paediatr. Neurol. 2012, 16, 248–256. [Google Scholar] [CrossRef][Green Version]
- Torbergsen, T.; Stålberg, E.; Aasly, J.; Lindal, S. Myopathy in Marinesco-Sjögren syndrome: An electrophysiological study. Acta Neurol. Scand. 1991, 84, 132–138. [Google Scholar] [CrossRef]
- Walker, P.D.; Blitzer, M.G.; Shapira, E. Marinesco-Sjögren syndrome. Neurology 1985, 35, 415. [Google Scholar] [CrossRef]
- Sasaki, K.; Suga, K.; Tsugawa, S.; Sakuma, K.; Tachi, N.; Chiba, S.; Imamura, S. Muscle pathology in Marinesco-Sjo¨gren syndrome: A unique ultrastructural feature. Brain Dev. 1996, 18, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Tachi, N.; Nagata, N.; Wakai, S.; Chiba, S. Congenital muscular dystrophy in Marinesco-Sjögren syndrome. Pediatr. Neurol. 1991, 7, 296–298. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Kitoh, H.; Awaya, A.; Nonaka, I. Rapid cataract formation in Marinesco-Sjögren syndrome. Pediatr. Neurol. 1993, 9, 407–408. [Google Scholar] [CrossRef] [PubMed]
- Komiyama, A.; Nonaka, I.; Hirayama, K. Muscle pathology in Marinesco-Sjögren syndrome. J. Neurol. Sci. 1989, 89, 103–113. [Google Scholar] [CrossRef]
- Zimmer, C.; Gosztonyi, G.; Cervos-Navarro, J.; von Moers, A.; Schröder, J.M. Neuropathy with lysosomal changes in Marinesco-Sjögren syndrome: Fine structural findings in skeletal muscle and conjunctiva. Neuropediatrics 1992, 23, 329–335. [Google Scholar] [CrossRef]
- Goto, Y.; Komiyama, A.; Tanabe, Y.; Katafuchi, Y.; Ohtaki, E.; Nonaka, I. Myopathy in Marinesco-Sjögren syndrome: An ultrastructural study. Acta Neuropathol. 1990, 80, 123–128. [Google Scholar] [CrossRef]
- Herva, R.; von Wendt, L.; von Wendt, G.; Saukkonen, A.L.; Leisti, J.; Dubowitz, V. A syndrome with juvenile cataract, cerebellar atrophy, mental retardation and myopathy. Neuropediatrics 1987, 18, 164–169. [Google Scholar] [CrossRef]
- Krieger, M.; Roos, A.; Stendel, C.; Claeys, K.G.; Sonmez, F.M.; Baudis, M.; Bauer, P.; Bornemann, A.; de Goede, C.; Dufke, A.; et al. SIL1 mutations and clinical spectrum in patients with Marinesco-Sjogren syndrome. Brain 2013, 136 Pt 12, 3634–3644. [Google Scholar] [CrossRef]
- Müller-Felber, W.; Zafiriou, D.; Scheck, R.; Pätzke, I.; Toepfer, M.; Pongratz, D.E.; Walther, U. Marinesco Sjögren Syndrome with Rhabdomyolysis. A New Subtype of the Disease. Neuropediatrics 2007, 29, 97–101. [Google Scholar] [CrossRef]
- Masiero, E.; Agatea, L.; Mammucari, C.; Blaauw, B.; Loro, E.; Komatsu, M.; Metzger, D.; Reggiani, C.; Schiaffino, S.; Sandri, M. Autophagy Is Required to Maintain Muscle Mass. Cell Metab. 2009, 10, 507–515. [Google Scholar] [CrossRef]
- Zhao, L.; Longo-Guess, C.; Harris, B.S.; Lee, J.-W.; Ackerman, S.L. Protein accumulation and neurodegeneration in the woozy mutant mouse is caused by disruption of SIL1, a cochaperone of BiP. Nat. Genet. 2005, 37, 974–979. [Google Scholar] [CrossRef] [PubMed]
- Buchkremer, S.; González Coraspe, J.A.; Weis, J.; Roos, A. Sil1-Mutant Mice Elucidate Chaperone Function in Neurological Disorders. J. Neuromuscul. Dis. 2016, 3, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Grande, V.; Ornaghi, F.; Comerio, L.; Restelli, E.; Masone, A.; Corbelli, A.; Tolomeo, D.; Capone, V.; Axten, J.M.; Laping, N.J.; et al. PERK inhibition delays neurodegeneration and improves motor function in a mouse model of Marinesco-Sjögren syndrome. Hum. Mol. Genet. 2018, 27, 2477–2489. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Onozato, T.; Wanajo, I.; Hayashi, M.; Takeda, H.; Fujimori, Y. Longitudinal analysis of motor symptoms and histopathology in woozy mice, a model of cerebellar ataxia. NeuroReport 2017, 28, 779. [Google Scholar] [CrossRef]
- Ichhaporia, V.P.; Kim, J.; Kavdia, K.; Vogel, P.; Horner, L.; Frase, S.; Hendershot, L.M. SIL1, the endoplasmic-reticulum-localized BiP co-chaperone, plays a crucial role in maintaining skeletal muscle proteostasis and physiology. Dis. Models Mech. 2018, 11, dmm.33043. [Google Scholar] [CrossRef]
- Kawahara, G.; Hayashi, Y.K. Characterization of Zebrafish Models of Marinesco-Sjögren Syndrome. PLoS ONE 2016, 11, e0165563. [Google Scholar] [CrossRef]
- Huynen, M.A.; Snel, B.; Bork, P.; Gibson, T.J. The phylogenetic distribution of frataxin indicates a role in iron-sulfur cluster protein assembly. Hum. Mol. Genet. 2001, 10, 2463–2468. [Google Scholar] [CrossRef]
- Pastore, A.; Puccio, H. Frataxin: A protein in search for a function. J. Neurochem. 2013, 126 (Suppl. S1), 43–52. [Google Scholar] [CrossRef]
- Clark, E.; Johnson, J.; Dong, Y.N.; Mercado-Ayon, E.; Warren, N.; Zhai, M.; McMillan, E.; Salovin, A.; Lin, H.; Lynch, D.R. Role of frataxin protein deficiency and metabolic dysfunction in Friedreich ataxia, an autosomal recessive mitochondrial disease. Neuronal. Signal. 2018, 2, NS20180060. [Google Scholar] [CrossRef]
- Ghorbani, M.; Pousset, F.; Tucker, A.; Swift, S.; Giunti, P.; Parkinson, M.; Gilbert, D.; Liu, X.; Payne, A. Analysis of Friedreich’s ataxia patient clinical data reveals importance of accurate GAA repeat determination in disease prognosis and gender differences in cardiac measures. Inform. Med. Unlocked 2019, 17, 100266. [Google Scholar] [CrossRef]
- Indelicato, E.; Faserl, K.; Amprosi, M.; Nachbauer, W.; Schneider, R.; Wanschitz, J.; Sarg, B.; Boesch, S. Skeletal muscle proteome analysis underpins multifaceted mitochondrial dysfunction in Friedreich’s ataxia. Front. Neurosci. 2023, 17, 1289027. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.L.; Blake, J.C.; Chamberlain, S.; Thomas, P.K.; Cooper, J.M.; Schapira, A.H.V. Clinical, biochemical and molecular genetic correlations in Friedreich’s ataxia. Hum. Mol. Genet. 2000, 9, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Vorgerd, M.; Schöls, L.; Hardt, C.; Ristow, M.; Epplen, J.T.; Zange, J. Mitochondrial impairment of human muscle in Friedreich ataxia in vivo. Neuromuscul. Disord. 2000, 10, 430–435. [Google Scholar] [CrossRef]
- Nachbauer, W.; Boesch, S.; Reindl, M.; Eigentler, A.; Hufler, K.; Poewe, W.; Lo¨scher, W.; Wanschitz, J. Skeletal Muscle Involvement in Friedreich Ataxia and Potential Effects of Recombinant Human Erythropoietin Administration on Muscle Regeneration and Neovascularization. J. Neuropathol. Exp. Neurol. 2012, 71, 708–715. [Google Scholar] [CrossRef]
- Nachbauer, W.; Boesch, S.; Schneider, R.; Eigentler, A.; Wanschitz, J.; Poewe, W.; Schocke, M. Bioenergetics of the Calf Muscle in Friedreich Ataxia Patients Measured by 31P-MRS Before and After Treatment with Recombinant Human Erythropoietin. PLoS ONE 2013, 8, e69229. [Google Scholar] [CrossRef]
- Gros-Louis, F.; Dupré, N.; Dion, P.; Fox, M.A.; Laurent, S.; Verreault, S.; Sanes, J.R.; Bouchard, J.-P.; Rouleau, G.A. Mutations in SYNE1 lead to a newly discovered form of autosomal recessive cerebellar ataxia. Nat. Genet. 2007, 39, 80–85. [Google Scholar] [CrossRef]
- King, S.J.; Nowak, K.; Suryavanshi, N.; Holt, I.; Shanahan, C.M.; Ridley, A.J. Nesprin-1 and nesprin-2 regulate endothelial cell shape and migration. Cytoskeleton 2014, 71, 423–434. [Google Scholar] [CrossRef]
- Baumann, M.; Steichen-Gersdorf, E.; Krabichler, B.; Petersen, B.-S.; Weber, U.; Schmidt, W.M.; Zschocke, J.; Müller, T.; Bittner, R.E.; Janecke, A.R. Homozygous SYNE1 mutation causes congenital onset of muscular weakness with distal arthrogryposis: A genotype–phenotype correlation. Eur. J. Hum. Genet. 2017, 25, 262–266. [Google Scholar] [CrossRef]
- Serag, M.; Plutino, M.; Charles, P.; Azulay, J.-P.; Chaussenot, A.; Paquis-Flucklinger, V.; Ait-El-Mkadem Saadi, S.; Rouzier, C. A Case Report of SYNE1 Deficiency-Mimicking Mitochondrial Disease and the Value of Pangenomic Investigations. Genes 2023, 14, 2154. [Google Scholar] [CrossRef]
- Lamperti, C.; Naini, A.; Hirano, M.; De Vivo, D.C.; Bertini, E.; Servidei, S.; Valeriani, M.; Lynch, D.; Banwell, B.; Berg, M.; et al. Cerebellar ataxia and coenzyme Q10 deficiency. Neurology 2003, 60, 1206–1208. [Google Scholar] [CrossRef]
- Bargiela, D.; Shanmugarajah, P.; Lo, C.; Blakely, E.L.; Taylor, R.W.; Horvath, R.; Wharton, S.; Chinnery, P.F.; Hadjivassiliou, M. Mitochondrial pathology in progressive cerebellar ataxia. Cerebellum Ataxias 2015, 2, 16. [Google Scholar] [CrossRef] [PubMed]
- Baty, K.; Farrugia, M.E.; Hopton, S.; Falkous, G.; Schaefer, A.M.; Stewart, W.; Willison, H.J.; Reilly, M.M.; Blakely, E.L.; Taylor, R.W.; et al. A novel MT-CO2 variant causing cerebellar ataxia and neuropathy: The role of muscle biopsy in diagnosis and defining pathogenicity. Neuromuscul. Disord. 2021, 31, 1186–1193. [Google Scholar] [CrossRef] [PubMed]
- Zierz, C.M.; Baty, K.; Blakely, E.L.; Hopton, S.; Falkous, G.; Schaefer, A.M.; Hadjivassiliou, M.; Sarrigiannis, P.G.; Ng, Y.S.; Taylor, R.W. A Novel Pathogenic Variant in MT-CO2 Causes an Isolated Mitochondrial Complex IV Deficiency and Late-Onset Cerebellar Ataxia. J. Clin. Med. 2019, 8, 789. [Google Scholar] [CrossRef]
- Pedroso, J.L.; de Rezende Pinto, W.B.V.; Barsottini, O.G.P.; Oliveira, A.S.B. Should we investigate mitochondrial disorders in progressive adult-onset undetermined ataxias? Cerebellum Ataxias 2020, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Casari, G.; Marconi, R. Spastic Paraplegia 7. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993–2025. [Google Scholar]
- McDermott, C.J.; Dayaratne, R.K.; Tomkins, J.; Lusher, M.E.; Lindsey, J.C.; Johnson, M.A.; Casari, G.; Turnbull, D.M.; Bushby, K.; Shaw, P.J. Paraplegin gene analysis in hereditary spastic paraparesis (HSP) pedigrees in northeast England. Neurology 2001, 56, 467–471. [Google Scholar] [CrossRef]
- van Gassen, K.L.I.; van der Heijden, C.D.C.C.; de Bot, S.T.; den Dunnen, W.F.A.; van den Berg, L.H.; Verschuuren-Bemelmans, C.C.; Kremer, H.P.H.; Veldink, J.H.; Kamsteeg, E.-J.; Scheffer, H.; et al. Genotype-phenotype correlations in spastic paraplegia type 7: A study in a large Dutch cohort. Brain 2012, 135, 2994–3004. [Google Scholar] [CrossRef]
- Casari, G.; De Fusco, M.; Ciarmatori, S.; Zeviani, M.; Mora, M.; Fernandez, P.; De Michele, G.; Filla, A.; Cocozza, S.; Marconi, R.; et al. Spastic paraplegia and OXPHOS impairment caused by mutations in paraplegin, a nuclear-encoded mitochondrial metalloprotease. Cell 1998, 93, 973–983. [Google Scholar] [CrossRef]
- Arnoldi, A.; Tonelli, A.; Crippa, F.; Villani, G.; Pacelli, C.; Sironi, M.; Pozzoli, U.; D’Angelo, M.G.; Meola, G.; Martinuzzi, A.; et al. A clinical, genetic, and biochemical characterization of SPG7 mutations in a large cohort of patients with hereditary spastic paraplegia. Hum. Mutat. 2008, 29, 522–531. [Google Scholar] [CrossRef]
- Wedding, I.M.; Koht, J.; Tran, G.T.; Misceo, D.; Selmer, K.K.; Holmgren, A.; Frengen, E.; Bindoff, L.; Tallaksen, C.M.E.; Tzoulis, C. Spastic Paraplegia Type 7 Is Associated with Multiple Mitochondrial DNA Deletions. PLoS ONE 2014, 9, e86340. [Google Scholar] [CrossRef]
- Wilkinson, P.A.; Crosby, A.H.; Turner, C.; Bradley, L.J.; Ginsberg, L.; Wood, N.W.; Schapira, A.H.; Warner, T.T. A clinical, genetic and biochemical study of SPG7 mutations in hereditary spastic paraplegia. Brain 2004, 127, 973–980. [Google Scholar] [CrossRef]
- Mahoney, C.J.; Dharmadasa, T.; Huynh, W.; Halpern, J.-P.; Vucic, S.; Mowat, D.; Kiernan, M.C. A novel phenotype of hereditary spastic paraplegia type 7 associated with a compound heterozygous mutation in paraplegin. Muscle Nerve 2020, 62, E44–E45. [Google Scholar] [CrossRef] [PubMed]
- Rothblum-Oviatt, C.; Wright, J.; Lefton-Greif, M.A.; McGrath-Morrow, S.A.; Crawford, T.O.; Lederman, H.M. Ataxia telangiectasia: A review. Orphanet J. Rare Dis. 2016, 11, 159. [Google Scholar] [CrossRef] [PubMed]
- Theis, M.; Donath, H.; Woelke, S.; Bakhtiar, S.; Salzmann-Manrique, E.; Zielen, S.; Kieslich, M. Peripheral polyneuropathy in children and young adults with ataxia–telangiectasia. Eur. J. Neurol. 2023, 30, 3842–3853. [Google Scholar] [CrossRef]
- Pommerening, H.; van Dullemen, S.; Kieslich, M.; Schubert, R.; Zielen, S.; Voss, S. Body composition, muscle strength and hormonal status in patients with ataxia telangiectasia: A cohort study. Orphanet J. Rare Dis. 2015, 10, 155. [Google Scholar] [CrossRef]
- Verhagen, M.M.M.; van Alfen, N.; Pillen, S.; Weemaes, C.M.R.; Yntema, J.L.; Hiel, J.P.; Ter Laak, H.; van Deuren, M.; Broeks, A.; Willemsen, M.P. Neuromuscular abnormalities in ataxia telangiectasia: A clinical, electrophysiological and muscle ultrasound study. Neuropediatrics 2007, 38, 117–121. [Google Scholar] [CrossRef]
- Schroeder, S.A.; Swift, M.; Sandoval, C.; Langston, C. Interstitial lung disease in patients with ataxia-telangiectasia. Pediatr. Pulmonol. 2005, 39, 537–543. [Google Scholar] [CrossRef]
- Félix, E.; Gimenes, A.C.; Costa-Carvalho, B.T. Effects of inspiratory muscle training on lung volumes, respiratory muscle strength, and quality of life in patients with ataxia telangiectasia. Pediatr. Pulmonol. 2014, 49, 238–244. [Google Scholar] [CrossRef]
- Nakamura, K.; Yoshida, K.; Makishita, H.; Kitamura, E.; Hashimoto, S.; Ikeda, S. A novel nonsense mutation in a Japanese family with ataxia with oculomotor apraxia type 2 (AOA2). J. Hum. Genet. 2009, 54, 746–748. [Google Scholar] [CrossRef]
- Shah, A.; Byrd, P.J.; Malcolm, A.; Nestor, T.; Ffr-Rcsi, S.R.; King, M.D. Atypical presentation of ataxia-oculomotor apraxia type 1. Dev. Med. Child Neurol. 2006, 48, 529–532. [Google Scholar] [CrossRef]
- Anheim, M.; Fleury, M.-C.; Franques, J.; Moreira, M.-C.; Delaunoy, J.-P.; Stoppa-Lyonnet, D.; Koenig, M.; Tranchant, C. Clinical and Molecular Findings of Ataxia With Oculomotor Apraxia Type 2 in 4 Families. Arch. Neurol. 2008, 65, 958–962. [Google Scholar] [CrossRef][Green Version]
- Morava, É.; Dinopoulos, A.; Kroes, H.Y.; Rodenburg, R.J.T.; Van Bokhoven, H.; Van Den Heuvel, L.P.; Smeitink, J.A.M. Mitochondrial Dysfunction in a Patient with Joubert Syndrome. Neuropediatrics 2005, 36, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Arancio, O.; Bongiovanni, L.G.; Bonadonna, G.; Tomelleri, G.; De Grandis, D. Congenital muscular dystrophy and cerebellar vermis agenesis in two brothers. Ital. J. Neuro. Sci. 1988, 9, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.E.; Vargas, W.; Pearson, T.S. Ataxia with Vitamin E Deficiency May Present with Cervical Dystonia. Tremor Other Hyperkinetic Mov. 2016, 6, 374. [Google Scholar] [CrossRef]
- Kleopa, K.A.; Kyriacou, K.; Zamba-Papanicolaou, E.; Kyriakides, T. Reversible inflammatory and vacuolar myopathy with vitamin E deficiency in celiac disease. Muscle Nerve 2005, 31, 260–265. [Google Scholar] [CrossRef]
- Rosen, J.M.; Kuntz, N.; Melin-Aldana, H.; Bass, L.M. Spasmodic Muscle Cramps and Weakness as Presenting Symptoms in Wilson Disease. Pediatrics 2013, 132, e1039–e1042. [Google Scholar] [CrossRef]
- Jobling, R.K.; Assoum, M.; Gakh, O.; Blaser, S.; Raiman, J.A.; Mignot, C.; Roze, E.; Dürr, A.; Brice, A.; Lévi, N.; et al. PMPCA mutations cause abnormal mitochondrial protein processing in patients with non-progressive cerebellar ataxia. Brain 2015, 138, 1505–1517. [Google Scholar] [CrossRef]
- Chang, J.C.; Ryan, M.R.; Stark, M.C.; Liu, S.; Purushothaman, P.; Bolan, F.; Johnson, C.A.; Champe, M.; Meng, H.; Lawlor, M.W.; et al. AAV8 gene therapy reverses cardiac pathology and prevents early mortality in a mouse model of Friedreich’s ataxia. Mol. Ther. Methods Clin. Dev. 2024, 32, 101193. [Google Scholar] [CrossRef]
- Puccio, H.; Simon, D.; Cossée, M.; Criqui-Filipe, P.; Tiziano, F.; Melki, J.; Hindelang, C.; Matyas, R.; Rustin, P.; Koenig, M. Mouse models for Friedreich ataxia exhibit cardiomyopathy, sensory nerve defect and Fe-S enzyme deficiency followed by intramitochondrial iron deposits. Nat. Genet. 2001, 27, 181–186. [Google Scholar] [CrossRef]
- Harding, A.E. Friedreich’s Ataxia: A Clinical and Genetic Study of 90 Families with an Analysis of Early Diagnostic Criteria and Intrafamilial Clustering of Clinical Features. Brain 1981, 104, 589–620. [Google Scholar] [CrossRef]
- Rötig, A.; de Lonlay, P.; Chretien, D.; Foury, F.; Koenig, M.; Sidi, D.; Munnich, A.; Rustin, P. Aconitase and mitochondrial iron–sulphur protein deficiency in Friedreich ataxia. Nat. Genet. 1997, 17, 215–217. [Google Scholar] [CrossRef]
- Vásquez-Trincado, C.; Dunn, J.; Han, J.I.; Hymms, B.; Tamaroff, J.; Patel, M.; Nguyen, S.; Dedio, A.; Wade, K.; Enigwe, C.; et al. Frataxin deficiency lowers lean mass and triggers the integrated stress response in skeletal muscle. JCI Insight 2022, 7, e155201. [Google Scholar] [CrossRef] [PubMed]
- Chandran, V.; Gao, K.; Swarup, V.; Versano, R.; Dong, H.; Jordan, M.C.; Geschwind, D.H. Inducible and reversible phenotypes in a novel mouse model of Friedreich’s Ataxia. eLife 2017, 6, e30054. [Google Scholar] [CrossRef] [PubMed]
- Hui, C.K.; Dedkova, E.N.; Montgomery, C.; Cortopassi, G. Dimethyl fumarate dose-dependently increases mitochondrial gene expression and function in muscle and brain of Friedreich’s ataxia model mice. Hum. Mol. Genet. 2021, 29, 3954–3965. [Google Scholar] [CrossRef]
- Piguet, F.; de Montigny, C.; Vaucamps, N.; Reutenauer, L.; Eisenmann, A.; Puccio, H. Rapid and Complete Reversal of Sensory Ataxia by Gene Therapy in a Novel Model of Friedreich Ataxia. Mol. Ther. 2018, 26, 1940–1952. [Google Scholar] [CrossRef]
- Katsu-Jiménez, Y.; Loría, F.; Corona, J.C.; Díaz-Nido, J. Gene Transfer of Brain-derived Neurotrophic Factor (BDNF) Prevents Neurodegeneration Triggered by FXN Deficiency. Mol. Ther. 2016, 24, 877–889. [Google Scholar] [CrossRef]
- Lim, F.; Palomo, G.M.; Mauritz, C.; Giménez-Cassina, A.; Illana, B.; Wandosell, F.; Díaz-Nido, J. Functional recovery in a Friedreich’s ataxia mouse model by frataxin gene transfer using an HSV-1 amplicon vector. Mol. Ther. 2007, 15, 1072–1078. [Google Scholar] [CrossRef]
- Salami, C.O.; Jackson, K.; Jose, C.; Alyass, L.; Cisse, G.-I.; De, B.P.; Stiles, K.M.; Chiuchiolo, M.J.; Sondhi, D.; Crystal, R.G.; et al. Stress-Induced Mouse Model of the Cardiac Manifestations of Friedreich’s Ataxia Corrected by AAV-mediated Gene Therapy. Hum. Gene. Ther. 2020, 31, 819–827. [Google Scholar] [CrossRef]
- Gérard, C.; Xiao, X.; Filali, M.; Coulombe, Z.; Arsenault, M.; Couet, J.; Li, J.; Drolet, M.-C.; Chapdelaine, P.; Chikh, A.; et al. An AAV9 coding for frataxin clearly improved the symptoms and prolonged the life of Friedreich ataxia mouse models. Mol. Ther. Methods Clin. Dev. 2014, 1, 14044. [Google Scholar] [CrossRef]
- Facchinello, N.; Laquatra, C.; Locatello, L.; Beffagna, G.; Brañas Casas, R.; Fornetto, C.; Dinarello, A.; Martorano, L.; Vettori, A.; Risato, G.; et al. Efficient clofilium tosylate-mediated rescue of POLG-related disease phenotypes in zebrafish. Cell Death Dis. 2021, 12, 100. [Google Scholar] [CrossRef]
- Sun, J.; Pan, C.Q.; Chew, T.W.; Liang, F.; Burmeister, M.; Low, B.C. BNIP-H Recruits the Cholinergic Machinery to Neurite Terminals to Promote Acetylcholine Signaling and Neuritogenesis. Dev. Cell 2015, 34, 555–568. [Google Scholar] [CrossRef]
- Jacobi, H.; Bauer, P.; Giunti, P.; Labrum, R.; Sweeney, M.G.; Charles, P.; Dürr, A.; Marelli, C.; Globas, C.; Linnemann, C.; et al. The natural history of spinocerebellar ataxia type 1, 2, 3, and 6. Neurology 2011, 77, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Jacobi, H.; Montcel, S.T.; Bauer, P.; Giunti, P.; Cook, A.; Labrum, R.; Parkinson, M.H.; Durr, A.; Brice, A.; Charles, P.; et al. Long-term disease progression in spinocerebellar ataxia types 1, 2, 3, and 6: A longitudinal cohort study. Lancet Neurol. 2015, 14, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Nuovo, S.; Bacigalupo, I.; Ginevrino, M.; Battini, R.; Bertini, E.; Borgatti, R.; Casella, A.; Micalizzi, A.; Nardella, M.; Romaniello, R.; et al. Age and sex prevalence estimate of Joubert syndrome in Italy. Neurology 2020, 94, e797–e801. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Huang, X.; Ye, Y.; Yang, D.; Xie, L.; Fu, D.; Peng, L.; Zhou, D.; Liao, J. Role of gender and age in features of Wilson’s disease. Front. Neurol. 2023, 14, 1176946. [Google Scholar] [CrossRef]
- Litwin, T.; Gromadzka, G.; Członkowska, A. Gender differences in Wilson’s disease. J. Neurol. Sci. 2012, 312, 31–35. [Google Scholar] [CrossRef]
- Kieffer, D.A.; Medici, V. Wilson disease: At the crossroads between genetics and epigenetics—A review of the evidence. Liver Res. 2017, 1, 121–130. [Google Scholar] [CrossRef]
| Observation | |||||||
|---|---|---|---|---|---|---|---|
| Fiber Size Variability | Fiber Type Grouping | Fatty Replacement | Mitochondrial Changes | Vacuolar Changes | Nuclear Changes | No Alterations | |
| Ataxias with DNA repair defects | |||||||
| A–T | - | - | - | - | - | - | - |
| AOA1/2 | + [80] | - | - | - | - | - | + [81,82] |
| Degenerative ataxias | |||||||
| FRDA | - | + [55,56] | + [55,56] | + [55,56] | - | - | - |
| MSS | ++ [12,21,23,24,26,27,28,30,32,33,34,35,36,37,38,39,40] | + [33,34,35,36,37] | ++ [21,24,27,30,32,34,35,37,38,39] | +++ [25,27,30,40] | +++ [12,23,25,26,28,30,31,32,34,35,36,37,38,39] | +++ [12,19,23,26,27,28,30,32,33,36,37,39] | - |
| CoQ10 | - | - | - | - | - | - | + [61] |
| ARCA-1 / SCAR8 | + [59] | - | + [59] | - | - | + [59] | - |
| SCAR2 | + [88] | - | - | - | - | + [88] | - |
| MIRAS | - | + [63,65] | - | ++ [62,63,64,65] | - | - | - |
| SPG7 | + [68] | + [67,68] | - | ++ [67,68,69,70] | + [71] | - | + [72,73] |
| Congenital ataxias | |||||||
| Joubert | - | - | + [84] | + [83] | - | - | + [83] |
| Cayman | - | - | - | - | - | - | - |
| Metabolic ataxias | |||||||
| AVED | + [85,86] | - | - | - | - | + [86] | - |
| Wilson’s | - | - | - | - | - | - | + [87] |
| Refsum’s | - | - | - | - | - | - | - |
| Disease | Preclinical Model (Name-Age) | Observations | Reference |
|---|---|---|---|
| Degenerative ataxias | |||
| MSS | Woozy mouse - 26 to 100 weeks of age | − Muscle atrophy − Variation in fiber size − Internalized nuclei − Rimmed vacuoles − Autophagic structures − Membrane-bound vacuolar structures − Swollen mitochondria | [26] |
| MSS | Woozy mouse - 6 to 26 weeks of age | − Muscle atrophy − Autophagic vacuoles − Degenerating mitochondria − Degenerating nuclei | [43] |
| MSS | Woozy mouse - 16 weeks of age | − Enlargements of the sarcoplasmic reticulum − Perinuclear autophagic vacuoles containing myelin-like membranous material | [44] |
| MSS | Woozy mouse - 26 weeks of age | − Reduction in the size of NMJs | [25] |
| MSS | Woozy mouse - 5 to 71 weeks of age | − Atrophic fibers − Autophagic vacuoles | [45] |
| MSS | Sil1Gt mouse - 3 to 15 months of age | − Variable fiber size − Internalized nuclei − Fatty replacement − Degenerating nuclei surrounded by membranous-bound structure | [46] |
| MSS | Morphant zebrafish - Embryos | − Markedly reduced normal patterns of birefringence − Disturbed myofibers formation | [47] |
| FRDA | FXN-MCK mice - 2 to 10 weeks of age | − No histological, biochemical, or ultrastructural defects | [90] |
| FRDA | Inducible FXN depletion (TG) mouse - 19 to 27 weeks of age | − Reduced growth of muscle mass, with smaller myofiber CSA, along with reduced translation of all proteins globally and no increase in autophagy | [93] |
| FRDA | Conditioned FXN depleted - 5.5 to 21.5 weeks of age | − Coordination and muscle impairment | [96] |
| MIRAS | polg knockout ENU zebrafish - Embryo and adults | − Altered fibers − Decrease in mitochondrial mass − Aberrant mitochondrial cristae | [101] |
| Ataxias with impaired DNA repair mechanism | |||
| A–T | ND | ||
| AOA1/2 | ND | ||
| Congenital ataxias | |||
| Cayman ataxia | atcay knockdown zebrafish - adults | − Disrupted muscle development | [102] |
| Joubert syndrome | ND | ||
| Metabolic ataxias | |||
| AVED | ND | ||
| Wilson’s disease | ND | ||
| Refsum’s disease | ND | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellia, F.; Federici, L.; Gatta, V.; Calabrese, G.; Sallese, M. Skeletal Muscle Pathology in Autosomal Recessive Cerebellar Ataxias: Insights from Marinesco–Sjögren Syndrome. Int. J. Mol. Sci. 2025, 26, 6736. https://doi.org/10.3390/ijms26146736
Bellia F, Federici L, Gatta V, Calabrese G, Sallese M. Skeletal Muscle Pathology in Autosomal Recessive Cerebellar Ataxias: Insights from Marinesco–Sjögren Syndrome. International Journal of Molecular Sciences. 2025; 26(14):6736. https://doi.org/10.3390/ijms26146736
Chicago/Turabian StyleBellia, Fabio, Luca Federici, Valentina Gatta, Giuseppe Calabrese, and Michele Sallese. 2025. "Skeletal Muscle Pathology in Autosomal Recessive Cerebellar Ataxias: Insights from Marinesco–Sjögren Syndrome" International Journal of Molecular Sciences 26, no. 14: 6736. https://doi.org/10.3390/ijms26146736
APA StyleBellia, F., Federici, L., Gatta, V., Calabrese, G., & Sallese, M. (2025). Skeletal Muscle Pathology in Autosomal Recessive Cerebellar Ataxias: Insights from Marinesco–Sjögren Syndrome. International Journal of Molecular Sciences, 26(14), 6736. https://doi.org/10.3390/ijms26146736







