Effect of Maternal Dietary DHA and Prenatal Stress Mouse Model on Autistic-like Behaviors, Lipid Peroxidation Activity, and GABA Expression in Offspring Pups
Abstract
1. Introduction
2. Results
2.1. Offspring Behavioral Results
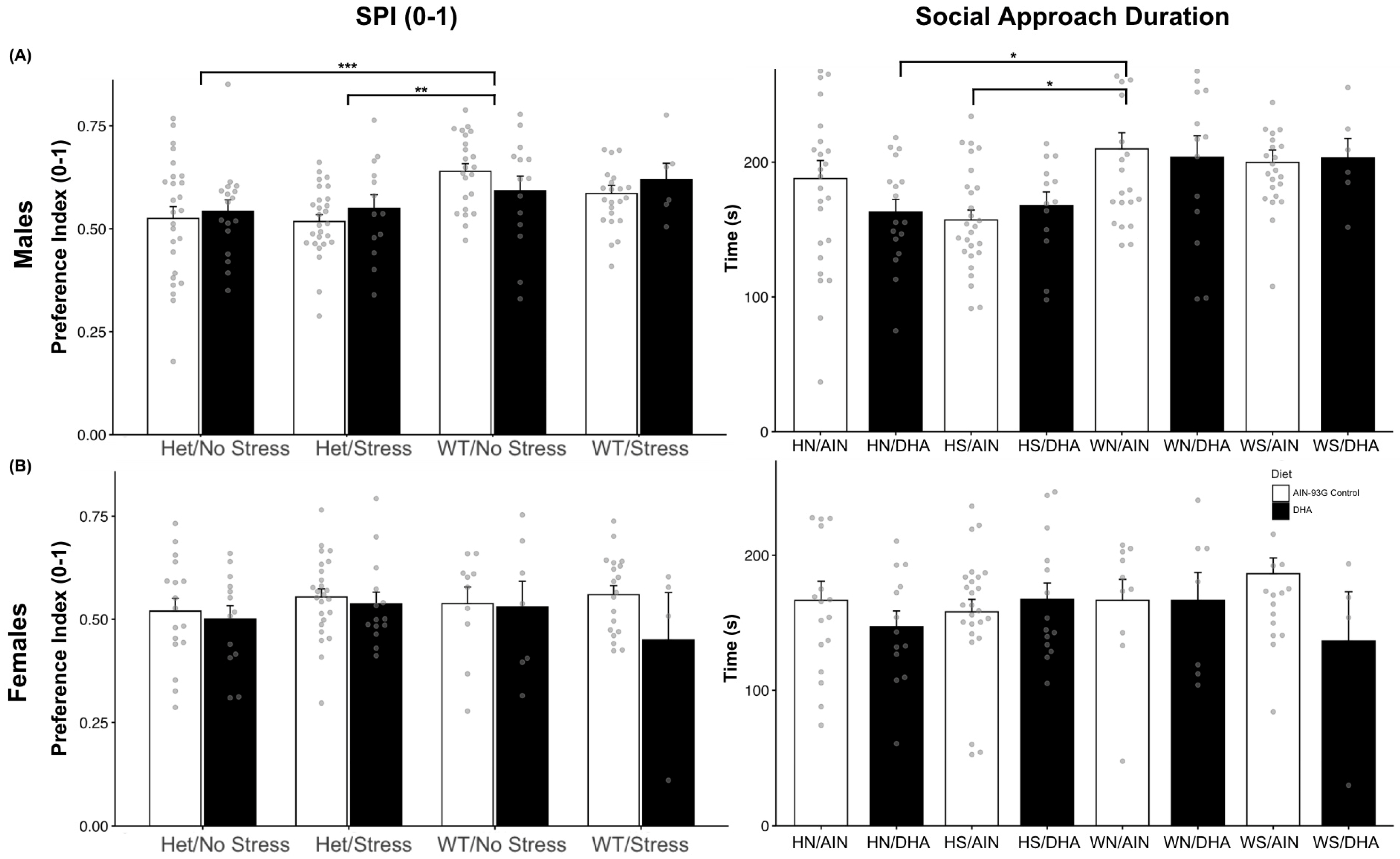
2.1.1. Social Interaction (Single Stranger Versus Novel Object)
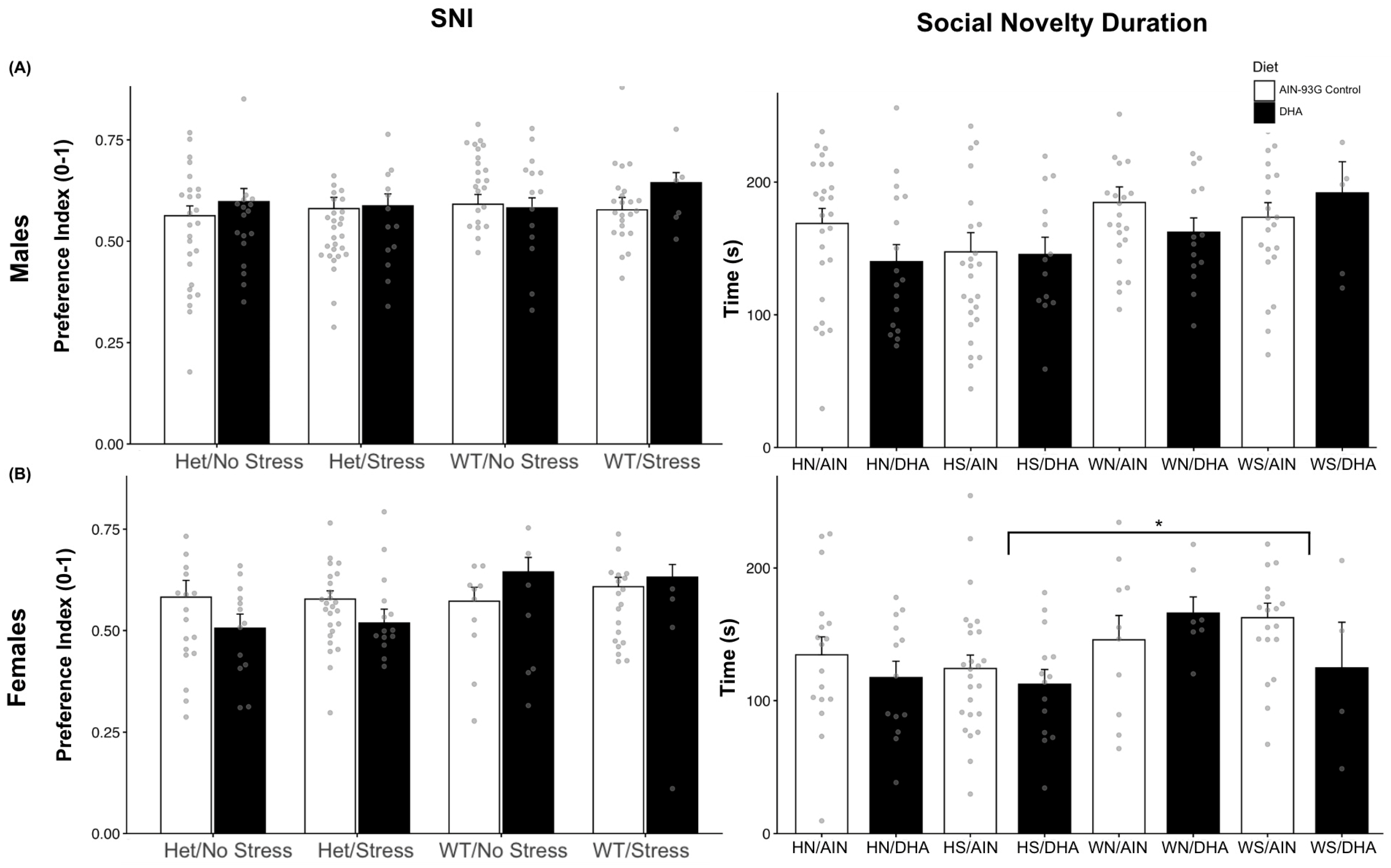
2.1.2. Social Novelty Interaction (Novel Stranger Versus Familiar Stranger)
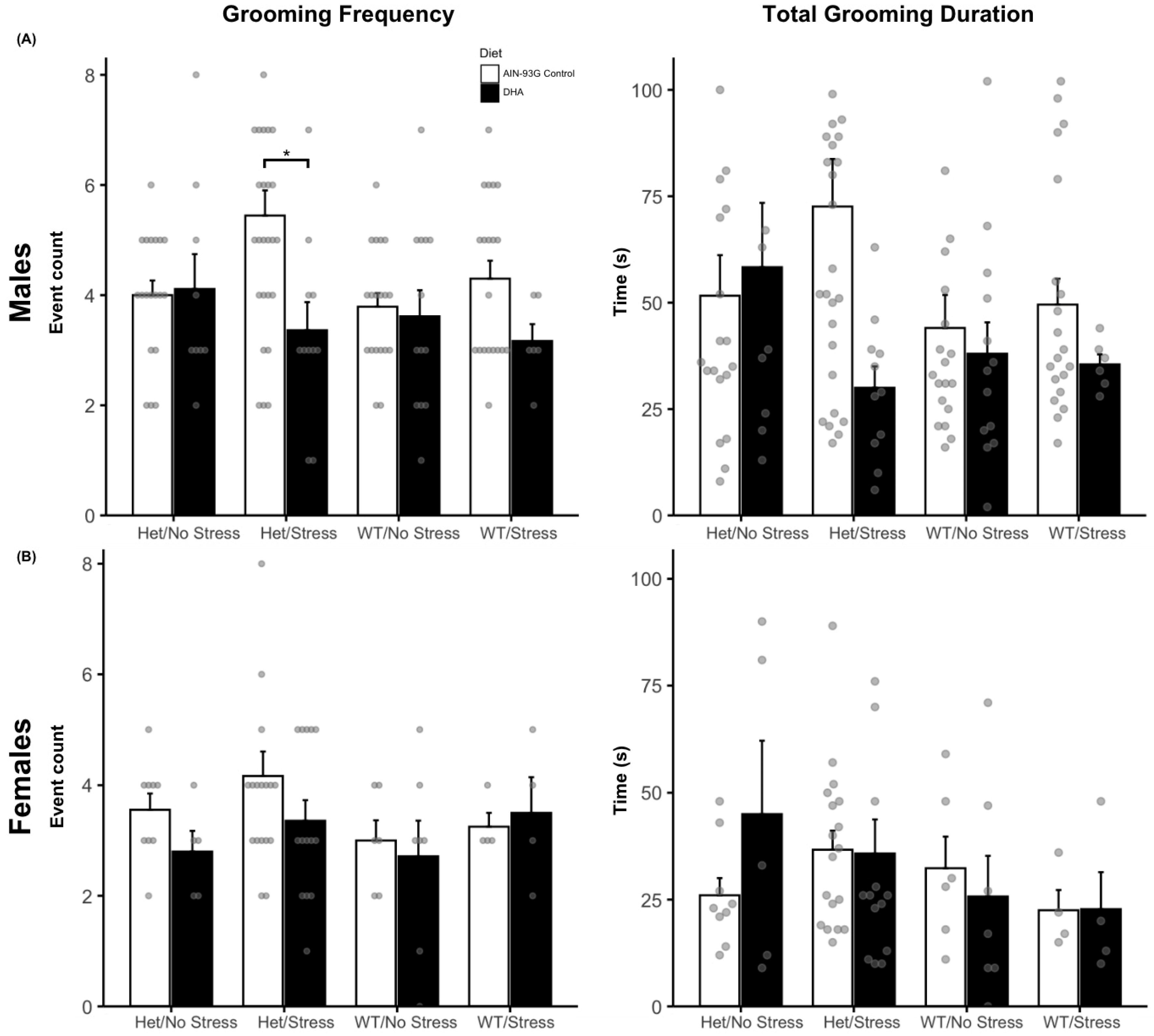
2.1.3. Grooming Behaviors
2.1.4. Marble Burying
2.1.5. Open Field
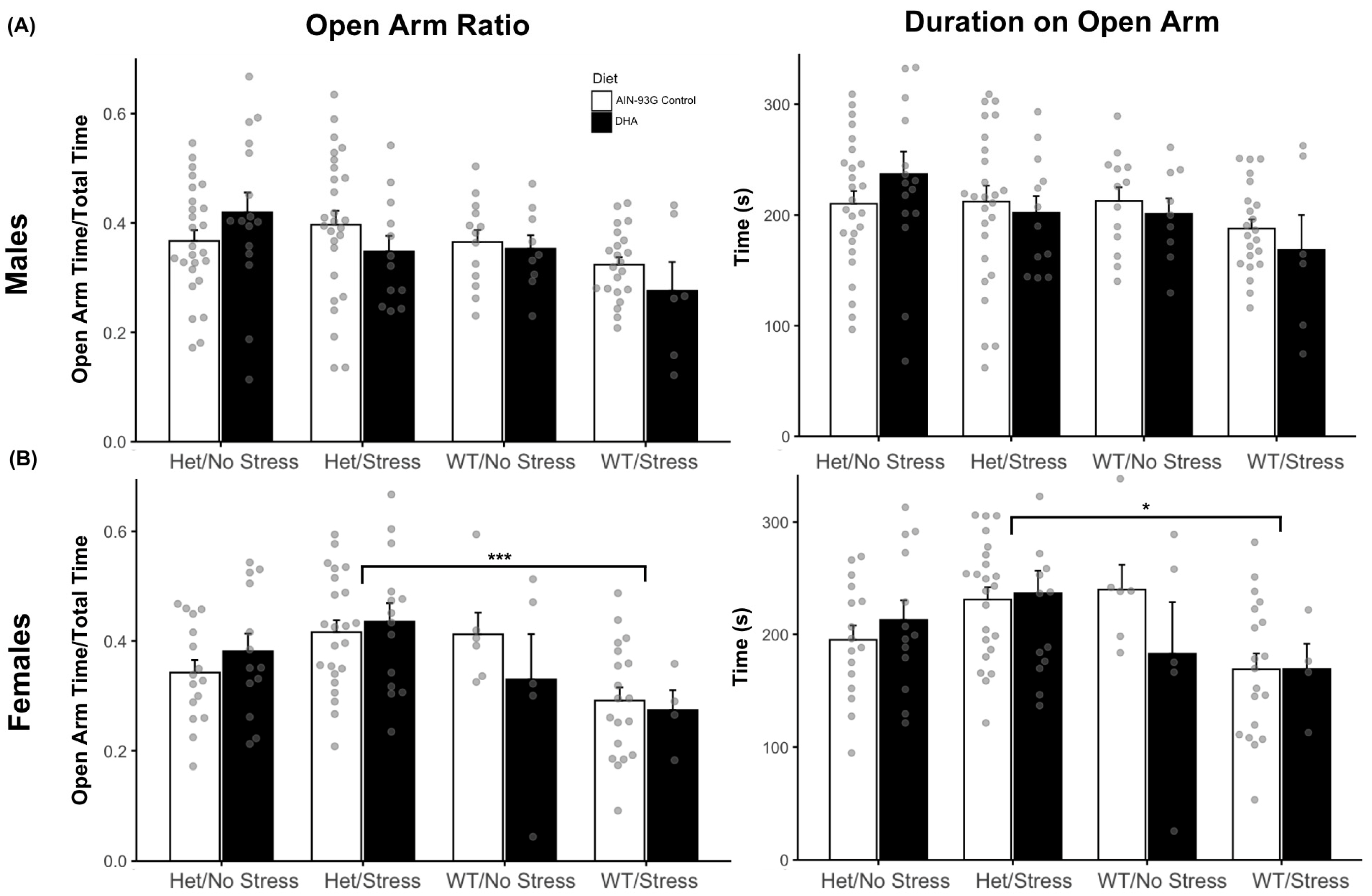
2.1.6. Elevated Plus Maze Results
2.2. HHE and 4-HNE Levels
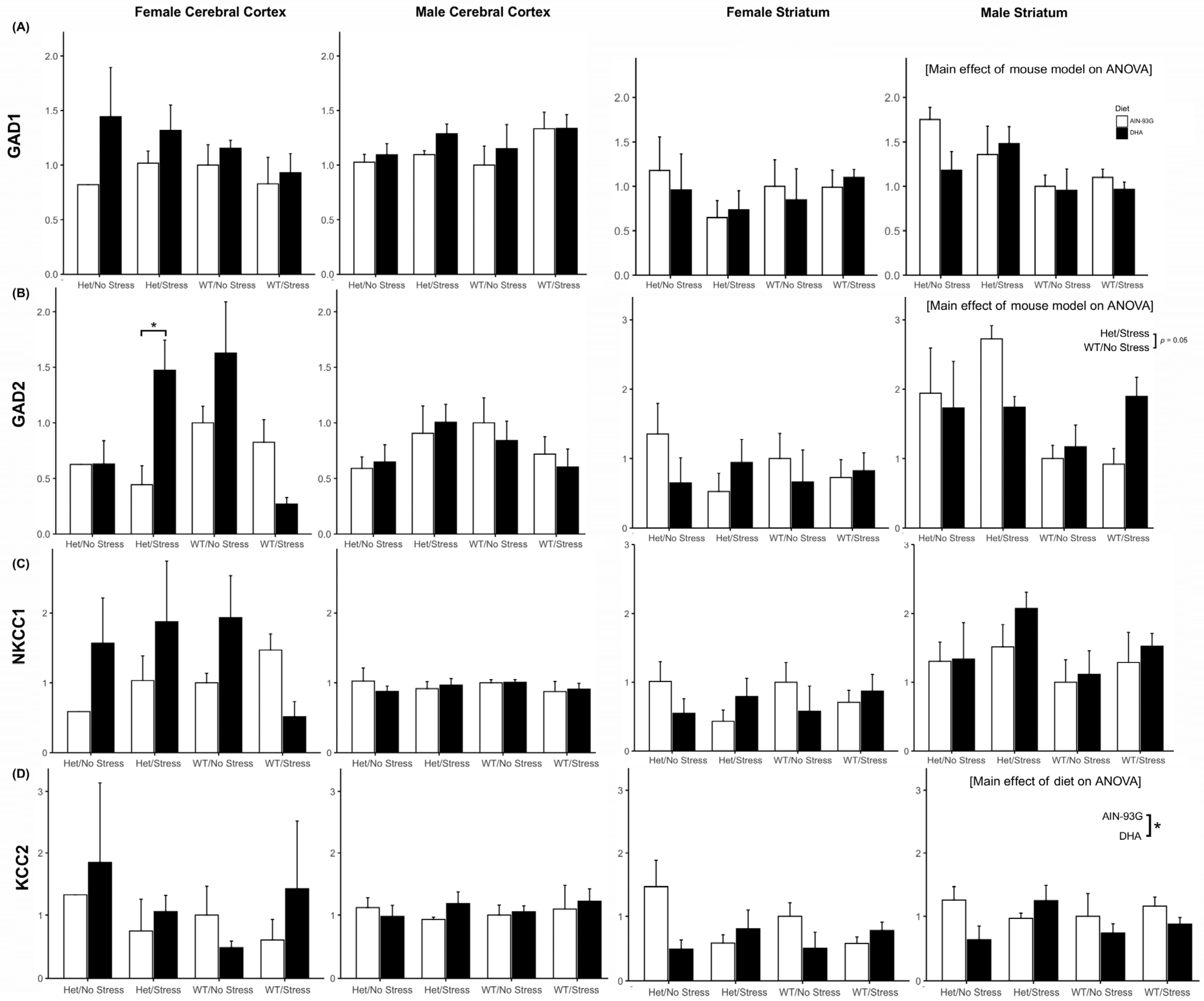
2.3. GABAergic System Gene Expression
2.3.1. Gad1 and Gad2 Expression
2.3.2. Nkcc1 and Kcc2 Expression
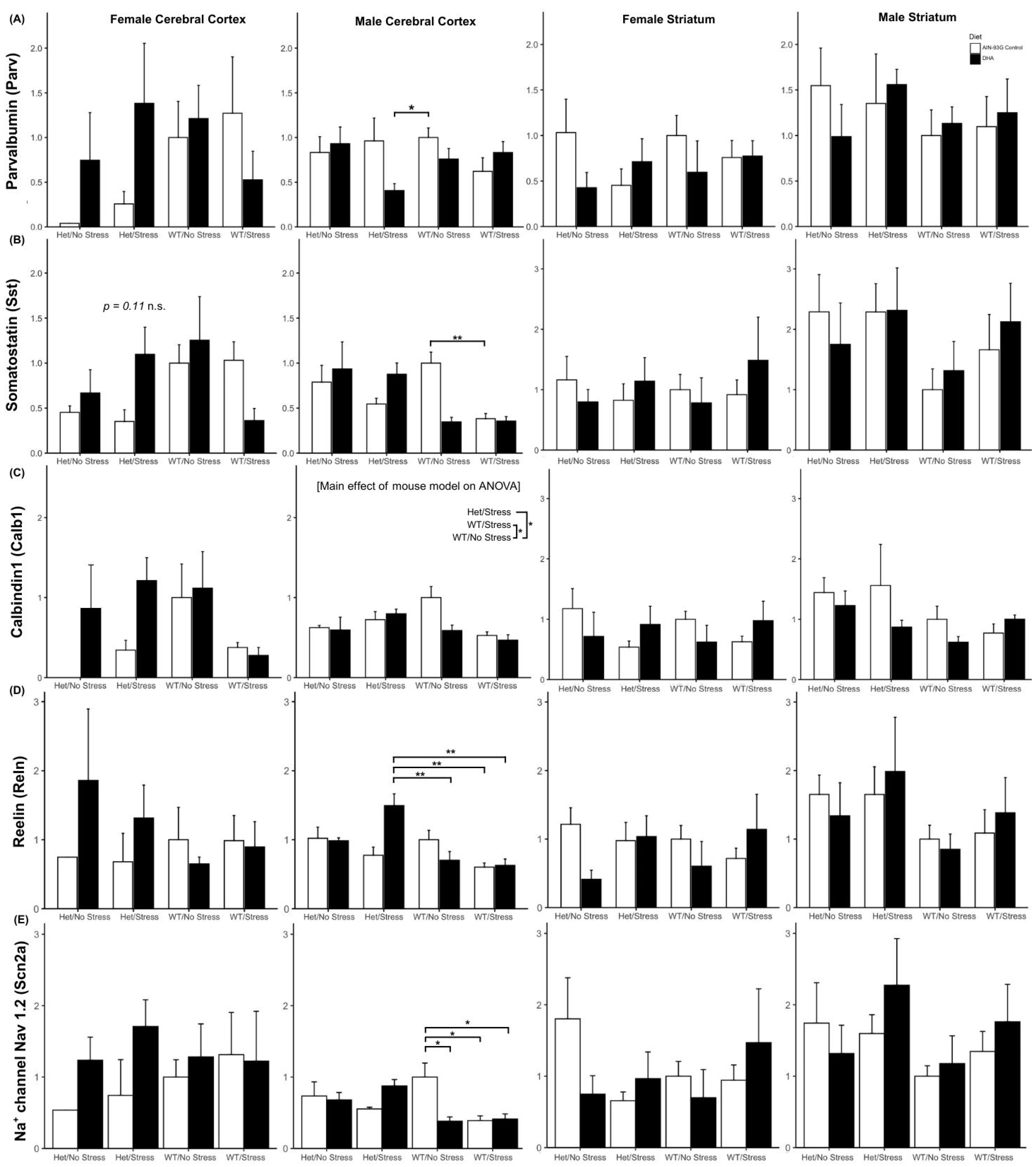
2.3.3. Interneuron Subtype Gene Expression
Other ASD-Relevant GABAergic Genes
3. Discussion
4. Materials and Methods
4.1. Animals, Diets, Stress, and Experimental Groups
4.1.1. Animals
4.1.2. Diet
4.1.3. Gene/Environment Interaction
4.1.4. Prenatal Chronic Variable Stress
4.2. Behavioral Assays
4.2.1. Social Approach
4.2.2. Open Field Test
4.2.3. Elevated Plus Maze
4.2.4. Repetitive Behaviors
4.3. Tissue Preparation
4.4. LC-MS/MS Analysis of 4-HHE and 4-HNE
4.5. Quantitative PCR
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jones, K.L.; Smith, R.M.; Edwards, K.S.; Givens, B.; Tilley, M.R.; Beversdorf, D.Q. Combined effect of maternal serotonin transporter genotype and prenatal stress in modulating offspring social interaction in mice. Int. J. Dev. Neurosci. 2010, 28, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Conduit, R.; Lockley, S.W.; Rajaratnam, S.M.; Cornish, K.M. The relationship between sleep and behavior in autism spectrum disorder (ASD): A review. J. Neurodev. Disord. 2014, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Prater, C.D.; Zylstra, R.G. Autism: A medical primer. Am. Fam. Physician 2002, 66, 1667–1674. [Google Scholar]
- DiCicco-Bloom, E.; Lord, C.; Zwaigenbaum, L.; Courchesne, E.; Dager, S.R.; Schmitz, C.; Schultz, R.T.; Crawley, J.; Young, L.J. The developmental neurobiology of autism spectrum disorder. J. Neurosci. 2006, 26, 6897–6906. [Google Scholar] [CrossRef]
- Hodges, H.; Fealko, C.; Soares, N. Autism spectrum disorder: Definition, epidemiology, causes, and clinical evaluation. Transl. Pediatr. 2020, 9 (Suppl. S1), S55–S65. [Google Scholar] [CrossRef]
- Shaw, K.A.; Williams, S.; Patrick, M.E.; Valencia-Prado, M.; Durkin, M.S.; Howerton, E.M.; Ladd-Acosta, C.M.; Pas, E.T.; Bakian, A.V.; Bartholomew, P.; et al. Prevalence and early identification of autism spectrum disorder among children aged 4 and 8 years—Autism and Developmental Disabilities Monitoring Network, 16 sites, United States, 2022. MMWR Surveill. Summ. 2025, 74, 1–22. [Google Scholar] [CrossRef]
- Zeidan, J.; Fombonne, E.; Scorah, J.; Ibrahim, A.; Durkin, M.S.; Saxena, S.; Yusuf, A.; Shih, A.; Elsabbagh, M. Global prevalence of autism: A systematic review update. Autism Res. Off. J. Int. Soc. Autism Res. 2022, 15, 778–790. [Google Scholar] [CrossRef]
- Leigh, J.P.; Du, J. Brief Report: Forecasting the Economic Burden of Autism in 2015 and 2025 in the United States. J. Autism Dev. Disord. 2015, 45, 4135–4139. [Google Scholar] [CrossRef]
- Manzari, N.; Matvienko-Sikar, K.; Baldoni, F.; O’Keeffe, G.W.; Khashan, A.S. Prenatal maternal stress and risk of neurodevelopmental disorders in the offspring: A systematic review and meta-analysis. Soc. Psychiatry Psychiatr. Epidemiol. 2019, 54, 1299–1309. [Google Scholar] [CrossRef]
- Kundakovic, M.; Jaric, I. The Epigenetic Link between Prenatal Adverse Environments and Neurodevelopmental Disorders. Genes 2017, 8, 104. [Google Scholar] [CrossRef]
- Say, G.N.; Karabekiroğlu, K.; Babadağı, Z.; Yüce, M. Maternal stress and perinatal features in autism and attention deficit/hyperactivity disorder. Pediatr. Int. 2016, 58, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Ronald, A.; Pennell, C.E.; Whitehouse, A.J.O. Prenatal Maternal Stress Associated with ADHD and Autistic Traits in early Childhood. Front. Psychol. 2011, 1, 223. [Google Scholar] [CrossRef] [PubMed]
- Charil, A.; Laplante, D.P.; Vaillancourt, C.; King, S. Prenatal stress and brain development. Brain Res. Rev. 2010, 65, 56–79. [Google Scholar] [CrossRef] [PubMed]
- Khashan, A.S.; Abel, K.M.; McNamee, R.; Pedersen, M.G.; Webb, R.T.; Baker, P.N.; Kenny, L.C.; Mortensen, P.B. Higher risk of offspring schizophrenia following antenatal maternal exposure to severe adverse life events. Arch. Gen. Psychiatry 2008, 65, 146–152. [Google Scholar] [CrossRef]
- Li, J.; Vestergaard, M.; Obel, C.; Christensen, J.; Precht, D.H.; Lu, M.; Olsen, J. A nationwide study on the risk of autism after prenatal stress exposure to maternal bereavement. Pediatrics 2009, 123, 1102–1107. [Google Scholar] [CrossRef]
- Breen, M.S.; Wingo, A.P.; Koen, N.; Donald, K.A.; Nicol, M.; Zar, H.J.; Ressler, K.J.; Buxbaum, J.D.; Stein, D.J. Gene expression in cord blood links genetic risk for neurodevelopmental disorders with maternal psychological distress and adverse childhood outcomes. Brain Behav. Immun. 2018, 73, 320–330. [Google Scholar] [CrossRef]
- Beversdorf, D.Q.; Manning, S.E.; Hillier, A.; Anderson, S.L.; Nordgren, R.E.; Walters, S.E.; Nagaraja, H.N.; Cooley, W.C.; Gaelic, S.E.; Bauman, M.L. Timing of prenatal stressors and autism. J. Autism Dev. Disord. 2005, 35, 471–478. [Google Scholar] [CrossRef]
- Kinney, D.K.; Munir, K.M.; Crowley, D.J.; Miller, A.M. Prenatal stress and risk for autism. Neurosci. Biobehav. Rev. 2008, 32, 1519–1532. [Google Scholar] [CrossRef]
- Varcin, K.J.; Alvares, G.A.; Uljarević, M.; Whitehouse, A.J.O. Prenatal maternal stress events and phenotypic outcomes in Autism Spectrum Disorder. Autism Res. 2017, 10, 1866–1877. [Google Scholar] [CrossRef]
- Mulder, E.J.H.; Robles De Medina, P.G.; Huizink, A.C.; Van Den Bergh, B.R.H.; Buitelaar, J.K.; Visser, G.H.A. Prenatal maternal stress: Effects on pregnancy and the (unborn) child. Early Hum. Dev. 2002, 70, 3–14. [Google Scholar] [CrossRef]
- Weinstock, M. Does prenatal stress impair coping and regulation of hypothalamic- pituitary-adrenal axis? Neurosci. Biobehav. Rev. 1997, 21, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kinney, D.K.; Miller, A.M.; Crowley, D.J.; Huang, E.; Gerber, E. Autism prevalence following prenatal exposure to hurricanes and tropical storms in Louisiana. J. Autism. Dev. Disord. 2008, 38, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Abdulamir, H.A.; Abdul-Rasheed, O.F.; Abdulghani, E.A. Serotonin and serotonin transporter levels in autistic children. Saudi Med. J. 2018, 39, 487–494. [Google Scholar] [CrossRef]
- Tordjman, S.; Gutknecht, L.; Carlier, M.; Spitz, E.; Antoine, C.; Slama, F.; Carsalade, V.; Cohen, D.J.; Ferrari, P.; Roubertoux, P.L.; et al. Role of the serotonin transporter gene in the behavioral expression of autism. Mol. Psychiatry 2001, 6, 434–439. [Google Scholar] [CrossRef]
- Huang, C.H.; Santangelo, S.L. Autism and serotonin transporter gene polymorphisms: A systematic review and meta-analysis. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2008, 147B, 903–913. [Google Scholar] [CrossRef]
- Brune, C.W.; Kim, S.J.; Salt, J.; Leventhal, B.L.; Lord, C.; Cook, E.H. 5-HTTLPR genotype-specific phenotype in children and adolescents with autism. Am. J. Psychiatry 2006, 163, 2148–2156. [Google Scholar] [CrossRef]
- Montañez, S.; Owens, W.A.; Gould, G.G.; Murphy, D.L.; Daws, L.C. Exaggerated effect of fluvoxamine in heterozygote serotonin transporter knockout mice. J. Neurochem. 2004, 86, 210–219. [Google Scholar] [CrossRef]
- Hecht, P.M.; Hudson, M.; Connors, S.L.; Tilley, M.R.; Liu, X.; Beversdorf, D.Q. Maternal serotonin transporter genotype affects risk for ASD with exposure to prenatal stress. Autism Res. 2016, 9, 1151–1160. [Google Scholar] [CrossRef]
- Sun, G.Y.; Simonyi, A.; Fritsche, K.L.; Chuang, D.Y.; Hannink, M.; Gu, Z.; Greenlief, C.M.; Yao, J.K.; Lee, J.C.; Beversdorf, D.Q. Docosahexaenoic acid (DHA): An essential nutrient and a nutraceutical for brain health and diseases. Prostaglandins Leukot. Essent. Fat. Acids 2018, 136, 3–13. [Google Scholar] [CrossRef]
- Belkouch, M.; Hachem, M.; Elgot, A.; Van ALo Picq, M.; Guichardant, M.; Lagarde, M.; Bernoud-Hubac, N. The pleiotropic effects of omega-3 docosahexaenoic acid on the hallmarks of Alzheimer’s disease. J. Nutr. Biochem. 2016, 38, 1–11. [Google Scholar] [CrossRef]
- Hashimoto, M.; Hossain, S.; Al Mamun, A.; Matsuzaki, K.; Arai, H. Docosahexaenoic acid: One molecule diverse functions. Crit. Rev. Biotechnol. 2017, 37, 579–597. [Google Scholar] [CrossRef] [PubMed]
- Bazan, N.G.; Molina, M.F.; Gordon, W.C. Docosahexaenoic Acid Signalolipidomics in Nutrition: Significance in Aging, Neuroinflammation, Macular Degeneration, Alzheimer’s, and Other Neurodegenerative Diseases. Annu. Rev. Nutr. 2011, 31, 321–351. [Google Scholar] [CrossRef] [PubMed]
- Avallone, R.; Vitale, G.; Bertolotti, M. Omega-3 Fatty Acids and Neurodegenerative Diseases: New Evidence in Clinical Trials. Int. J. Mol. Sci. 2019, 20, 4256. [Google Scholar] [CrossRef] [PubMed]
- Lauritzen, L.; Brambilla, P.; Mazzocchi, A.; Harsløf, L.; Ciappolino, V.; Agostoni, C. DHA Effects in Brain Development and Function. Nutrients 2016, 8, 6. [Google Scholar] [CrossRef]
- Kuratko, C.; Barrett, E.; Nelson, E.; Salem, N. The Relationship of Docosahexaenoic Acid (DHA) with Learning and Behavior in Healthy Children: A Review. Nutrients 2013, 5, 2777–2810. [Google Scholar] [CrossRef]
- Bradbury, J. Docosahexaenoic acid (DHA): An ancient nutrient for the modern human brain. Nutrients 2011, 3, 529–554. [Google Scholar] [CrossRef]
- DeMar, J.C.; Ma, K.; Bell, J.M.; Igarashi, M.; Greenstein, D.; Rapoport, S.I. One generation of n-3 polyunsaturated fatty acid deprivation increases depression and aggression test scores in rats. J. Lipid Res. 2006, 47, 172–180. [Google Scholar] [CrossRef]
- McCann, J.C.; Ames, B.N. Is docosahexaenoic acid, an n−3 long-chain polyunsaturated fatty acid, required for development of normal brain function? An overview of evidence from cognitive and behavioral tests in humans and animals. Am. J. Clin. Nutr. 2005, 82, 281–295. [Google Scholar] [CrossRef]
- McNamara, R.K. DHA Deficiency and Prefrontal Cortex Neuropathology in Recurrent Affective Disorders. J. Nutr. 2010, 140, 864–868. [Google Scholar] [CrossRef]
- Moriguchi, T.; Loewke, J.; Garrison, M.; Catalan, J.N.; Salem, N. Reversal of docosahexaenoic acid deficiency in the rat brain, retina, liver, and serum. J. Lipid Res. 2001, 42, 419–427. [Google Scholar] [CrossRef]
- Bhatia, H.S.; Agrawal, R.; Sharma, S.; Huo, Y.X.; Ying, Z.; Gomez-Pinilla, F. Omega-3 fatty acid deficiency during brain maturation reduces neuronal and behavioral plasticity in adulthood. PLoS ONE. 2011, 6, e28451. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Iwanaga, M.; Harada, E. Possible regulatory mechanism of DHA-induced anti-stress reaction in rats. Brain Res. 2003, 964, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Vinot, N.; Jouin, M.; Lhomme-Duchadeuil, A.; Guesnet, P.; Alessandri, J.-M.; Aujard, F.; Pifferi, F. Omega-3 Fatty Acids from Fish Oil Lower Anxiety, Improve Cognitive Functions and Reduce Spontaneous Locomotor Activity in a Non-Human Primate. Izquierdo I, ed. PLoS ONE. 2011, 6, e20491. [Google Scholar] [CrossRef]
- Ferraz, A.C.; Delattre, A.M.; Almendra, R.G.; Sonagli, M.; Borges, C.; Araujo, P.; Andersen, M.L.; Tufik, S.; Lima, M.M.S. Chronic ω-3 fatty acids supplementation promotes beneficial effects on anxiety, cognitive and depressive-like behaviors in rats subjected to a restraint stress protocol. Behav. Brain Res. 2011, 219, 116–122. [Google Scholar] [CrossRef]
- Tang, M.; Zhang, M.; Wang, L.; Li, H.; Cai, H.; Dang, R.; Jiang, P.; Liu, Y.; Xue, Y.; Wu, Y. Maternal dietary of n-3 polyunsaturated fatty acids affects the neurogenesis and neurochemical in female rat at weaning. Prostaglandins Leukot. Essent. Fat. Acids 2018, 128, 11–20. [Google Scholar] [CrossRef]
- Mazahery, H.; Stonehouse, W.; Delshad, M.; Kruger, M.; Conlon, C.; Beck, K.; von Hurst, P. Relationship between Long Chain n-3 Polyunsaturated Fatty Acids and Autism Spectrum Disorder: Systematic Review and Meta-Analysis of Case-Control and Randomised Controlled Trials. Nutrients 2017, 9, 155. [Google Scholar] [CrossRef]
- Matsui, F.; Hecht, P.; Yoshimoto, K.; Watanabe, Y.; Morimoto, M.; Fritsche, K.; Will, M.; Beversdorf, D. DHA Mitigates Autistic Behaviors Accompanied by Dopaminergic Change in a Gene/Prenatal Stress Mouse Model. Neuroscience 2018, 371, 407–419. [Google Scholar] [CrossRef]
- James, S.; Montgomery, P.; Williams, K. Omega-3 fatty acids supplementation for autism spectrum disorders (ASD). Cochrane Database Syst. Rev. 2011, 11, CD007992. [Google Scholar] [CrossRef]
- Mazahery, H.; Conlon, C.A.; Beck, K.L.; Mugridge, O.; Kruger, M.C.; Stonehouse, W.; Camargo, C.A.; Meyer, B.J.; Jones, B.; von Hurst, P.R. A randomised controlled trial of vitamin D and omega-3 long chain polyunsaturated fatty acids in the treatment of irritability and hyperactivity among children with autism spectrum disorder. J. Steroid Biochem. Mol. Biol. 2019, 187, 9–16. [Google Scholar] [CrossRef]
- Horvath, A.; Łukasik, J.; Szajewska, H. ω-3 Fatty Acid Supplementation Does Not Affect Autism Spectrum Disorder in Children: A Systematic Review and Meta-Analysis. J. Nutr. 2017, 147, 367–376. [Google Scholar] [CrossRef]
- De Crescenzo, F.; D’Alò, G.L.; Morgano, G.P.; Minozzi, S.; Mitrova, Z.; Saulle, R.; Cruciani, F.; Fulceri, F.; Davoli, M.; Scattoni, M.L.; et al. Impact of polyunsaturated fatty acids on patient-important outcomes in children and adolescents with autism spectrum disorder: A systematic review. Health Qual. Life Outcomes 2020, 18, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Politi, P.; Cena, H.; Comelli, M.; Marrone, G.; Allegri, C.; Emanuele, E.; Ucelli di Nemi, S. Behavioral Effects of Omega-3 Fatty Acid Supplementation in Young Adults with Severe Autism: An Open Label Study. Arch. Med. Res. 2008, 39, 682–685. [Google Scholar] [CrossRef] [PubMed]
- Mankad, D.; Dupuis, A.; Smile, S.; Roberts, W.; Brian, J.; Lui, T.; Genore, L.; Zaghloul, D.; Iaboni, A.; Marcon, P.M.A.; et al. A randomized, placebo controlled trial of omega-3 fatty acids in the treatment of young children with autism. Mol. Autism 2015, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Zou, X.; Jia, H.; Li, X.; Zhu, Z.; Liu, X.; Bucheli, P.; Ballevre, O.; Hou, Y.; Zhang, W.; et al. Maternal Docosahexaenoic Acid Feeding Protects Against Impairment of Learning and Memory and Oxidative Stress in Prenatally Stressed Rats: Possible Role of Neuronal Mitochondria Metabolism. Antioxid. Redox Signal. 2012, 16, 275–289. [Google Scholar] [CrossRef]
- Gao, J.; Wu, H.; Cao, Y.; Liang, S.; Sun, C.; Wang, P.; Wang, J.; Sun, H.; Wu, L. Maternal DHA supplementation protects rat offspring against impairment of learning and memory following prenatal exposure to valproic acid. J. Nutr. Biochem. 2016, 35, 87–95. [Google Scholar] [CrossRef]
- Yui, K.; Koshiba, M.; Nakamura, S.; Kobayashi, Y. Effects of Large Doses of Arachidonic Acid Added to Docosahexaenoic Acid on Social Impairment in Individuals with Autism Spectrum Disorders. J. Clin. Psychopharmacol. 2012, 32, 200–206. [Google Scholar] [CrossRef]
- Yang, B.; Fritsche, K.L.; Beversdorf, D.Q.; Gu, Z.; Lee, J.C.; Folk, W.R.; Greenlief, C.M.; Sun, G.Y. Yin-yang mechanisms regulating lipid peroxidation of docosahexaenoic acid and arachidonic acid in the central nervous system. Front. Neurol. 2019, 10, 642. [Google Scholar] [CrossRef]
- Yang, B.; Li, R.; Michael Greenlief, C.; Fritsche, K.L.; Gu, Z.; Cui, J.; Lee, J.C.; Beversdorf, D.Q.; Sun, G.Y. Unveiling anti-oxidative and anti-inflammatory effects of docosahexaenoic acid and its lipid peroxidation product on lipopolysaccharide-stimulated BV-2 microglial cells. J. Neuroinflamm. 2018, 15, 1–16. [Google Scholar] [CrossRef]
- Yang, B.; Li, R.; Woo, T.; Browning, J.D., Jr.; Song, H.; Gu, Z.; Cui, J.; Lee, J.C.; Fritsche, K.L.; Beversdorf, D.Q.; et al. Maternal Dietary Docosahexaenoic Acid Alters Lipid Peroxidation Products and (n-3)/(n-6) Fatty Acid Balance in Offspring Mice. Metabolites 2019, 9, 40. [Google Scholar] [CrossRef]
- Sun, G.Y.; Appenteng, M.K.; Li, R.; Woo, T.; Yang, B.; Qin, C.; Greenlief, C.M. Docosahexaenoic Acid (DHA) Supplementation Alters Phospholipid Species and Lipid Peroxidation Products in Adult Mouse Brain, Heart, and Plasma. Neuromol. Med. 2020, 1, 3. [Google Scholar] [CrossRef]
- Bonnin, A.; Goeden, N.; Chen, K.; Wilson, M.L.; King, J.; Shih, J.C.; Blakely, R.D.; Deneris, E.S.; Levitt, P. A transient placental source of serotonin for the fetal forebrain. Nature 2011, 472, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Bonnin, A.; Levitt, P. Fetal, maternal, and placental sources of serotonin and new implications for developmental programming of the brain. Neuroscience 2011, 197, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Muller, C.L.; Anacker, A.M.; Rogers, T.D.; Goeden, N.; Keller, E.H.; Forsberg, C.G.; Kerr, T.M.; La Wender, C.; Anderson, G.M.; Stanwood, G.D.; et al. Impact of maternal serotonin transporter genotype on placental serotonin, fetal forebrain serotonin, and neurodevelopment. Neuropsychopharmacology 2016, 42, 427–436. [Google Scholar] [CrossRef]
- Zhang, H.; Smith, G.N.; Liu, X.; Holden, J.J.A. Association of MAOA, 5-HTT, and NET promoter polymorphisms with gene expression and protein activity in human placentas. Physiol. Genom. 2010, 42, 85–92. [Google Scholar] [CrossRef]
- Sun, M.; Brivio, P.; Shan, L.; Docq, S.; Heltzel, L.C.M.W.; Smits, C.A.J.; Middelman, A.; Vrooman, R.; Spoelder, M.; Verheij, M.M.M.; et al. Offspring’s own serotonin transporter genotype, independently from the maternal one, increases anxiety- and depression-like behavior and alters neuroplasticity markers in rats. J. Affect. Disord. 2024, 350, 89–101. [Google Scholar] [CrossRef]
- Fatemi, S.H.; Halt, A.R.; Stary, J.M.; Kanodia, R.; Schulz, S.C.; Realmuto, G.R. Glutamic acid decarboxylase 65 and 67 kDa proteins are reduced in autistic parietal and cerebellar cortices. Biol. Psychiatry 2002, 52, 805–810. [Google Scholar] [CrossRef]
- Yip, J.; Soghomonian, J.-J.; Blatt, G.J. Decreased GAD67 mRNA levels in cerebellar Purkinje cells in autism: Pathophysiological implications. Acta Neuropathol. 2007, 113, 559–568. [Google Scholar] [CrossRef]
- Blatt, G.J.; Fatemi, S.H. Alterations in GABAergic Biomarkers in the Autism Brain: Research Findings and Clinical Implications. Anat. Rec. 2011, 294, 1646–1652. [Google Scholar] [CrossRef]
- Stevens, H.E.; Su, T.; Yanagawa, Y.; Vaccarino, F.M. Prenatal stress delays inhibitory neuron progenitor migration in the developing neocortex. Psychoneuroendocrinology 2013, 38, 509–521. [Google Scholar] [CrossRef]
- Maurer, S.V.; Hing, B.W.Q.; Lussier, S.; Radhakrishna, S.; Davis, J.L.B.; Abbott, P.W.; Michaelson, J.J.; Stevens, H.E. Prenatal stress alters mouse offspring dorsal striatal development and placental function in sex-specific ways. J. Psychiatr. Res. 2025, 182, 149–160. [Google Scholar] [CrossRef]
- Schroeder, R.E.; Sridharan, P.; Nguyen, L.; Loren, A.; Williams, N.S.; Kettimuthu, K.P.; Cintrón-Pérez, C.J.; Vásquez-Rosa, E.; Pieper, A.A.; Stevens, H.E. Maternal P7C3-A20 treatment protects offspring from neuropsychiatric sequelae of prenatal stress. Antiox. Redox Signal. 2021, 35, 511–530. [Google Scholar] [CrossRef] [PubMed]
- Uchida, T.; Furukawa, T.; Iwata, S.; Yanagawa, Y.; Fukuda, A. Selective loss of parvalbumin-positive GABAergic interneurons in the cerebral cortex of maternally stressed Gad1-heterozygous mouse offspring. Transl. Psychiatry 2014, 4, e371. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.L.; O’Connor, M.; Erlbacher, H.; Schlichte, S.L.; Stevens, H.E. The Impact of Maternal Antioxidants on Prenatal Stress Effects on Offspring Neurobiology and Behavior. Yale J. Biol. Med. 2022, 95, 87–104. [Google Scholar] [PubMed] [PubMed Central]
- Lussier, S.J.; Stevens, H.E. Delays in GABAergic interneuron development and behavioral inhibition after prenatal stress. Dev. Neurobiol. 2016, 76, 1078–1091. [Google Scholar] [CrossRef]
- Nadler, J.J.; Moy, S.S.; Dold, G.; DTrang Simmons, N.; Perez, A.; Young, N.B.; Barbaro, R.P.; Piven, J.; Magnuson, T.R.; Crawley, J.N. Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behav. 2004, 3, 303–314. [Google Scholar] [CrossRef]
- Shin, S.; Santi, A.; Huang, S. Conditional Pten knockout in parvalbumin- or somatostatin-positive neurons sufficiently leads to autism-related behavioral phenotypes. Mol. Brain 2021, 14, 24. [Google Scholar] [CrossRef]
- Kalueff, A.V.; Stewart, A.M.; Song, C.; Berridge, K.C.; Graybiel, A.M.; Fentress, J.C. Neurobiology of rodent self-grooming and its value for translational neuroscience. Nat. Rev. Neurosci. 2016, 17, 45–59. [Google Scholar] [CrossRef]
- Chang, Y.; Cole, T.B.; Costa, L.G. Behavioral phenotyping for autism spectrum disorders in mice. Curr. Protoc. Toxicol. 2017, 72, 11.22.1–11.22.21. [Google Scholar] [CrossRef]
- Woo, T.; King, C.; Ahmed, N.I.; Cordes, M.; Nistala, S.; Will, M.J.; Bloomer, C.; Kibiryeva, N.; Rivera, R.M.; Talebizadeh, Z.; et al. microRNA as a Maternal Marker for Prenatal Stress-Associated ASD, Evidence from a Murine Model. J. Pers. Med. 2023, 13, 1412. [Google Scholar] [CrossRef]
- Beversdorf, D.Q.; Stevens, H.E.; Jones, K.L. Prenatal stress, maternal immune dysregulation, and their association with autism spectrum disorders. Curr. Psychiatry Rep. 2018, 20, 76. [Google Scholar] [CrossRef]
- Mueller, B.; Bale, T. Impact of prenatal stress on long term body weight is dependent on timing and maternal sensitivity. Physiol. Behav. 2006, 88, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Moy, S.S.; Nadler, J.J.; Young, N.B.; Nonneman, R.J.; Grossman, A.W.; Murphy, D.L.; D’Ercole, A.J.; Crawley, J.N.; Magnuson, T.R.; Lauder, J.M. Social approach in genetically engineered mouse lines relevant to autism. Genes Brain Behav. 2009, 8, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Simon, P.; Dupuis, R.; Costentin, J. Thigmotaxis as an index of anxiety in mice. Influence of dopaminergic transmissions. Behav. Brain Res. 1994, 61, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Komada, M.; Takao, K.; Miyakawa, T. Elevated Plus Maze for Mice. J. Vis. Exp. JoVE 2008, 22, 1088. [Google Scholar] [CrossRef]
- Crawley, J.N. Mouse behavioral assays relevant to the symptoms of autism. Brain Pathol. 2007, 17, 448–459. [Google Scholar] [CrossRef]
- Silverman, J.L.; Yang, M.; Lord, C.; Crawley, J.N. Behavioural phenotyping assays for mouse models of autism. Nat. Rev. Neurosci. 2010, 11, 490–502. [Google Scholar] [CrossRef]

| Stressed DHA HT(HSD) | Stressed AIN HT (HAS) | Stressed DHA WT (WSD) | Stressed AIN WT (WSA) | |||||
|---|---|---|---|---|---|---|---|---|
| M | F | M | F | M | F | M | F | |
| Behavior | 14 (7) | 14 (8) | 18 (7) | 15 (6) | 6 (3) | 5(3) | 10 (5) | 11 (6) |
| Brain & Tissue | 4 (3) | 4 (3) | 4 (2) | 4 (2) | 4 (3) | 4 (2) | 4 (3) | 4 (3) |
| Non-stressed DHA HT (HND) | Non-stressed AIN HT (HNA) | Non-stressed DHA WT (WND) | Non-stressed AIN WT (WNA) | |||||
| M | F | M | F | M | F | M | F | |
| Behavior | 17 (7) | 12 (7) | 8 (5) | 8 (5) | 10 (4) | 7 (4) | 6 (4) | 6 (4) |
| Brain & Tissue | 4 (4) | 4 (4) | 4 (3) | 4 (3) | 4 (4) | 4 (3) | 4 (4) | 4 (4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woo, T.; Ahmed, N.I.; Appenteng, M.K.; King, C.; Li, R.; Fritsche, K.L.; Sun, G.Y.; Cui, J.; Will, M.J.; Maurer, S.V.; et al. Effect of Maternal Dietary DHA and Prenatal Stress Mouse Model on Autistic-like Behaviors, Lipid Peroxidation Activity, and GABA Expression in Offspring Pups. Int. J. Mol. Sci. 2025, 26, 6730. https://doi.org/10.3390/ijms26146730
Woo T, Ahmed NI, Appenteng MK, King C, Li R, Fritsche KL, Sun GY, Cui J, Will MJ, Maurer SV, et al. Effect of Maternal Dietary DHA and Prenatal Stress Mouse Model on Autistic-like Behaviors, Lipid Peroxidation Activity, and GABA Expression in Offspring Pups. International Journal of Molecular Sciences. 2025; 26(14):6730. https://doi.org/10.3390/ijms26146730
Chicago/Turabian StyleWoo, Taeseon, Nick I. Ahmed, Michael K. Appenteng, Candice King, Runting Li, Kevin L. Fritsche, Grace Y. Sun, Jiankun Cui, Matthew J. Will, Sara V. Maurer, and et al. 2025. "Effect of Maternal Dietary DHA and Prenatal Stress Mouse Model on Autistic-like Behaviors, Lipid Peroxidation Activity, and GABA Expression in Offspring Pups" International Journal of Molecular Sciences 26, no. 14: 6730. https://doi.org/10.3390/ijms26146730
APA StyleWoo, T., Ahmed, N. I., Appenteng, M. K., King, C., Li, R., Fritsche, K. L., Sun, G. Y., Cui, J., Will, M. J., Maurer, S. V., Stevens, H. E., Beversdorf, D. Q., & Greenlief, C. M. (2025). Effect of Maternal Dietary DHA and Prenatal Stress Mouse Model on Autistic-like Behaviors, Lipid Peroxidation Activity, and GABA Expression in Offspring Pups. International Journal of Molecular Sciences, 26(14), 6730. https://doi.org/10.3390/ijms26146730







