CNS Tumor with BCOR/BCORL1 Fusion: A Rare Tumor Entity
Abstract
1. Introduction
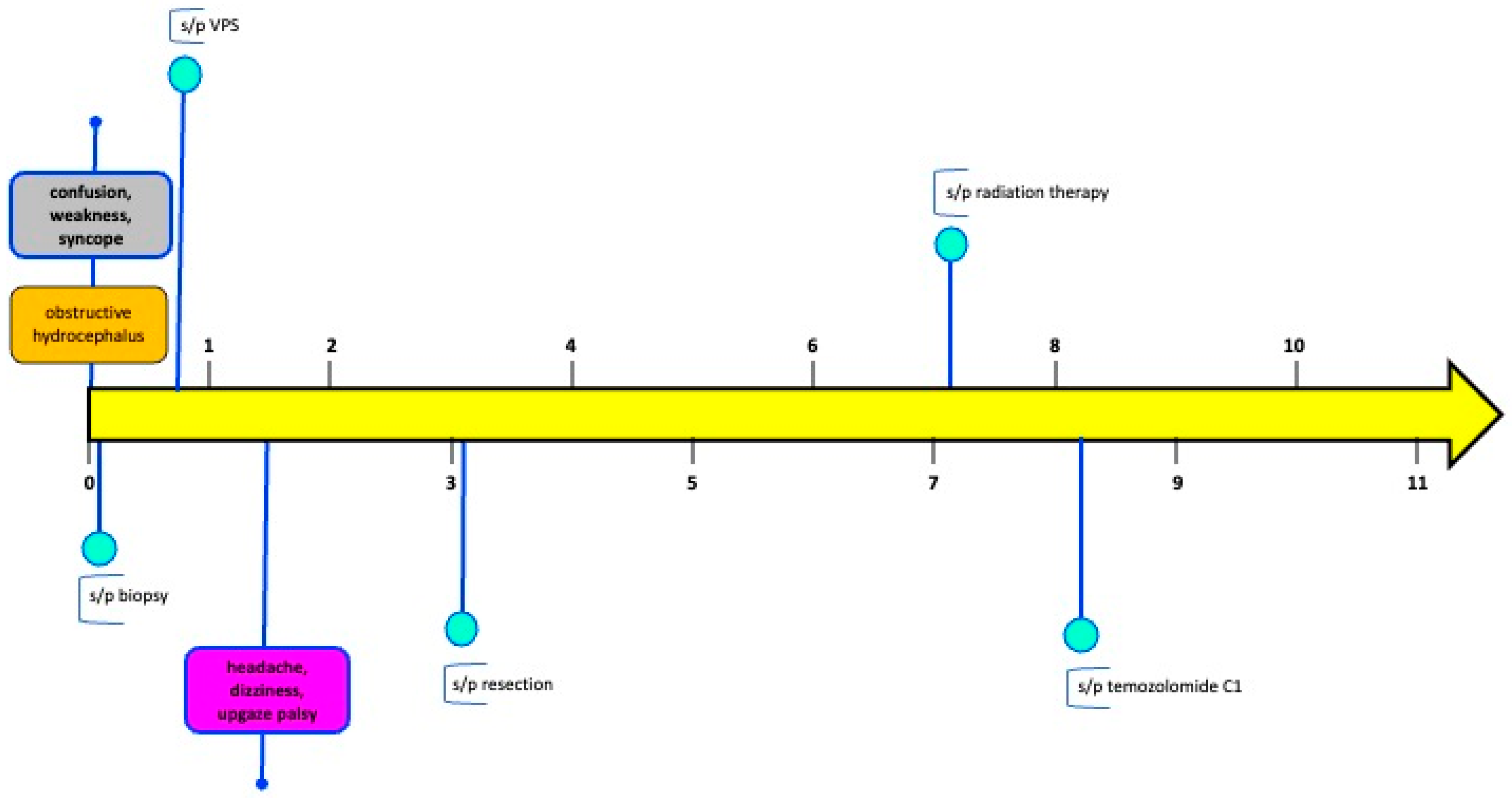
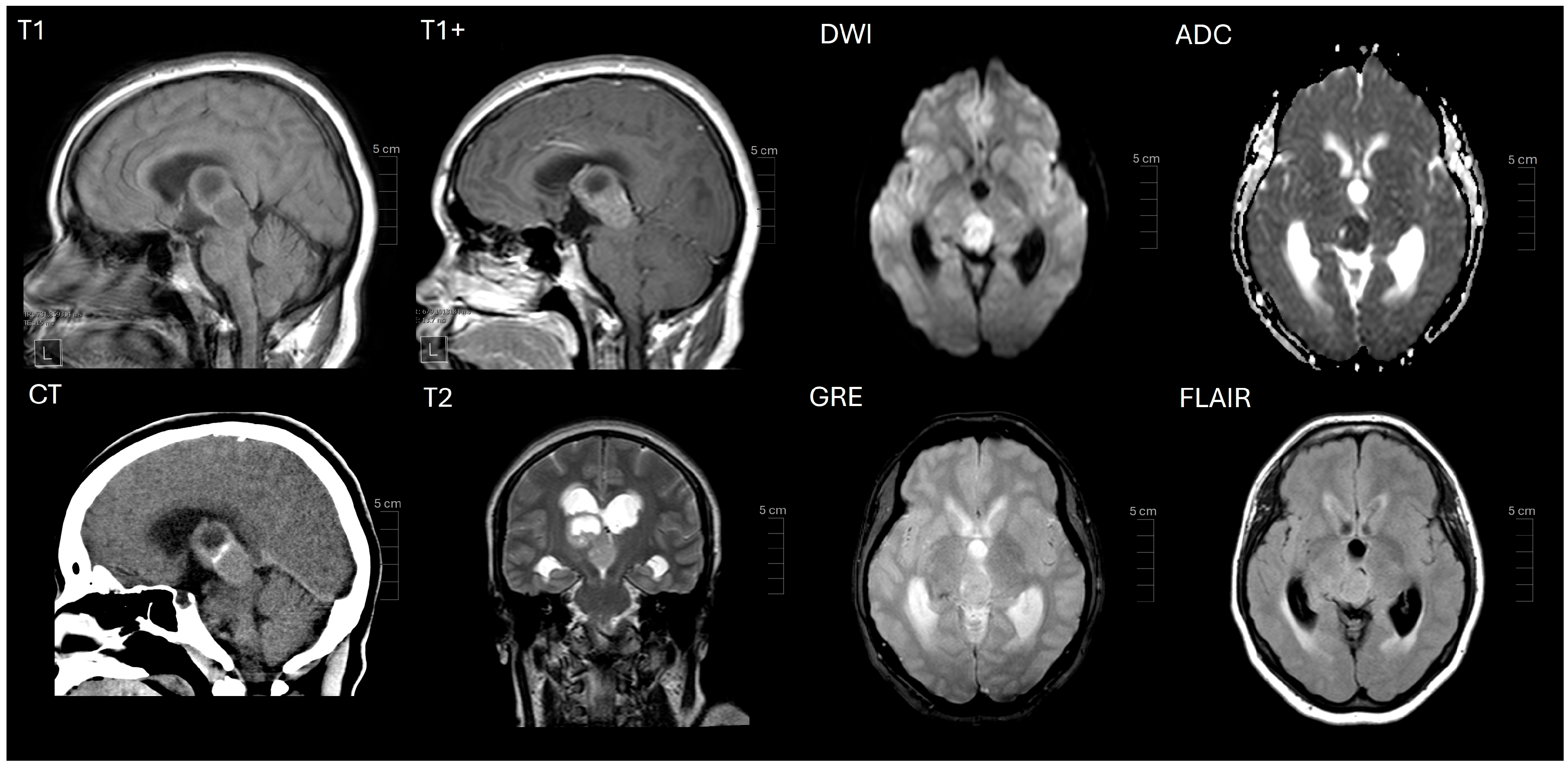
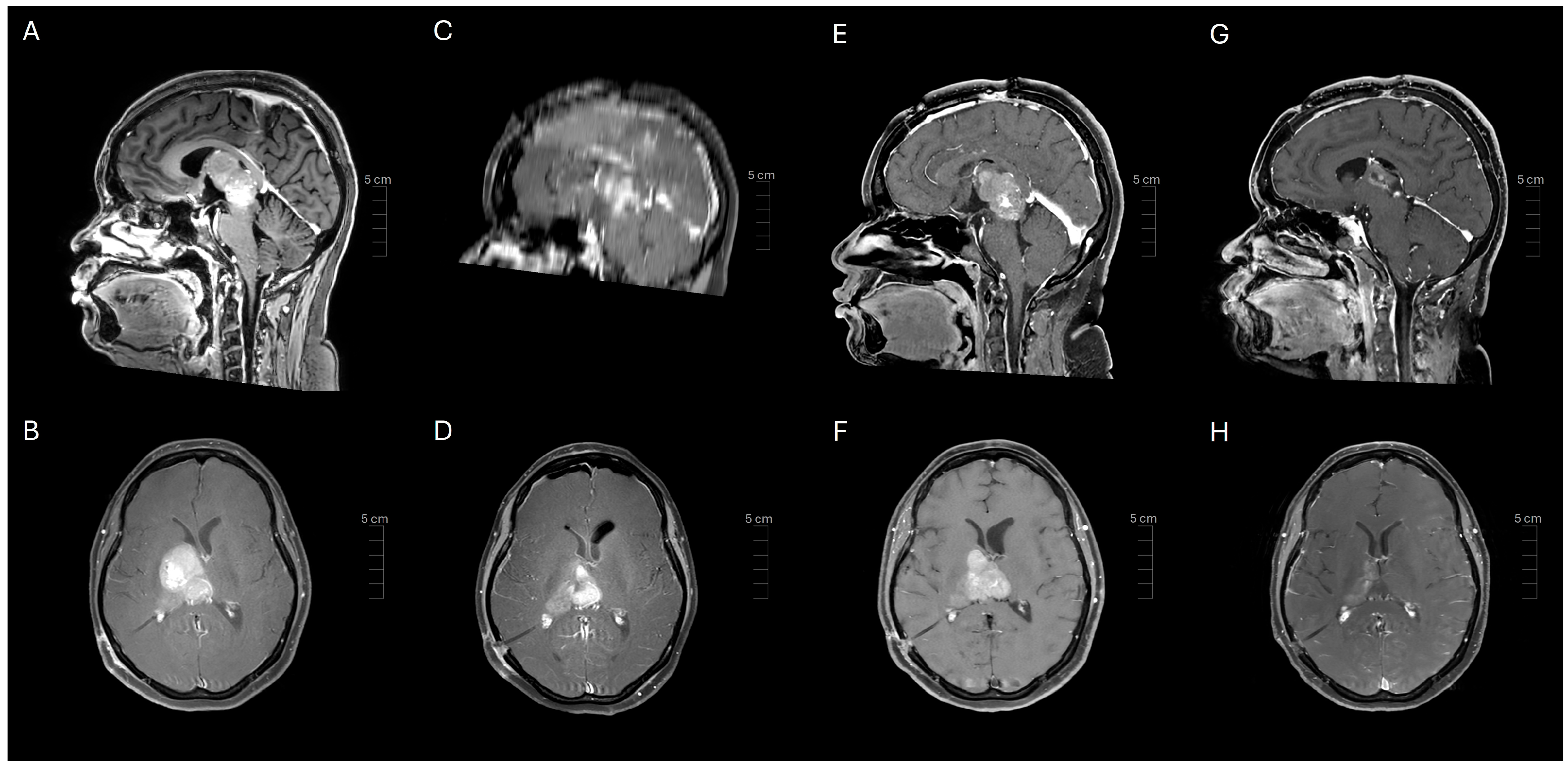
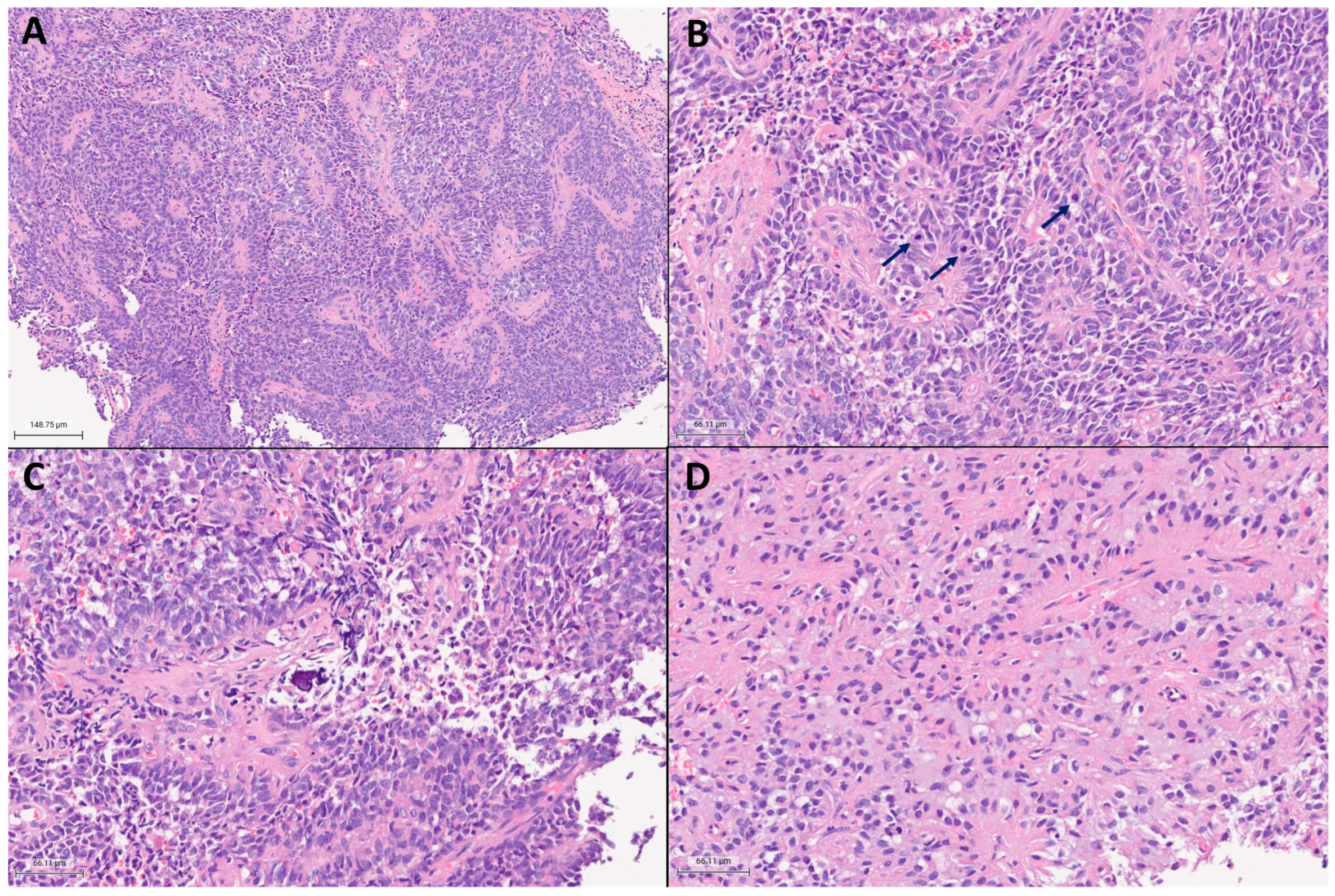
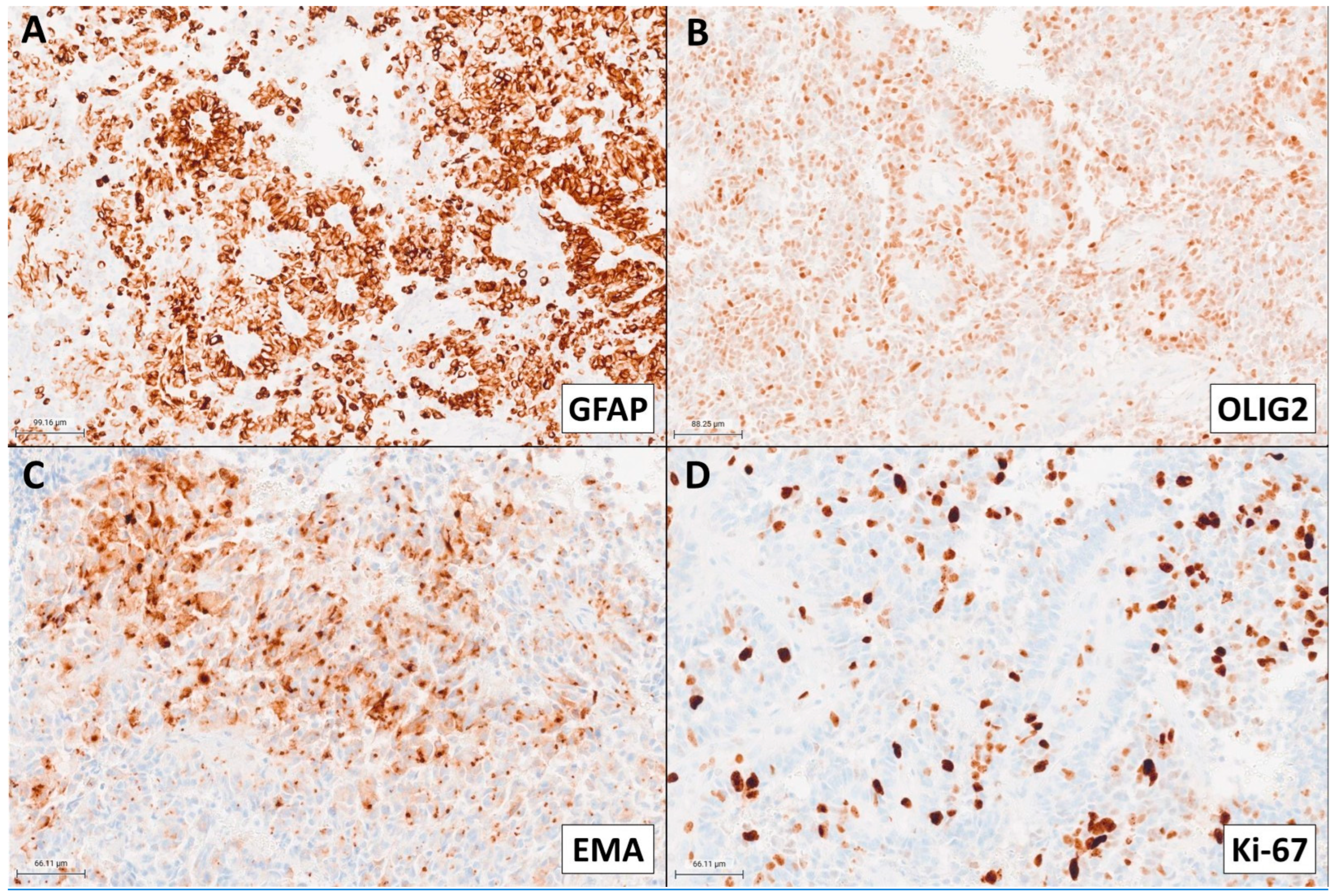
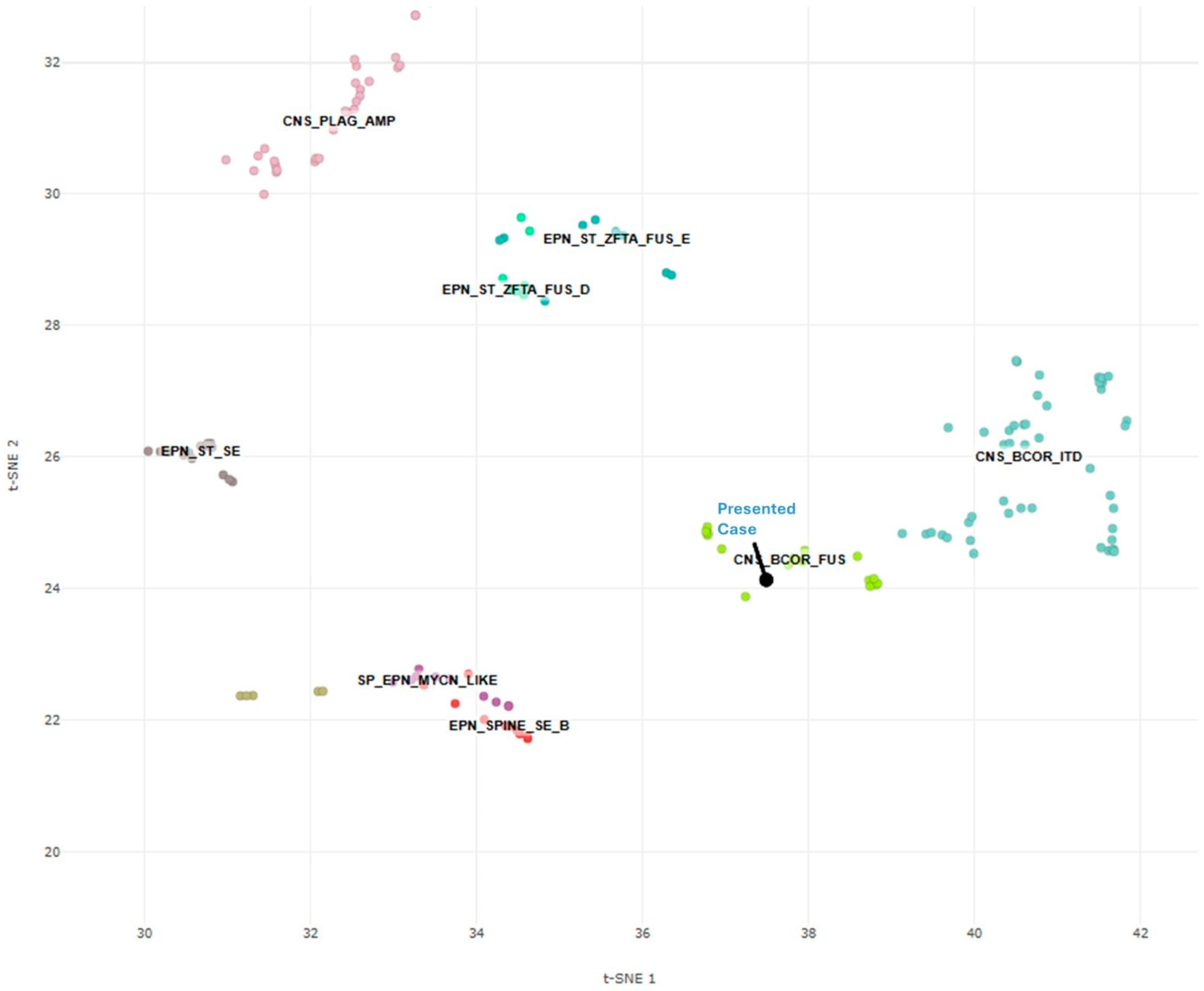
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CNS | central nervous system tumor |
| BCOR | BCL6 corepressor gene |
| CT | computed tomography |
| GTR | gross total resection |
| ITD | internal tandem duplication |
| MRI | magnetic resonance imaging |
| RT | radiation therapy |
| SRS | stereotactic radiosurgery |
| STR | subtotal resection |
| t-SNE | t-distributed stochastic neighbor embedding |
| TMZ | temozolomide |
| UMAP | uniform manifold approximation and projection |
| WHO | World Health Organization |
References
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Wesseling, P.; Aldape, K.; Brat, D.J.; Capper, D.; Cree, I.A.; Eberhart, C.; Figarella-Branger, D.; Fouladi, M.; Fuller, G.N.; et al. cIMPACT-NOW update 6: New entity and diagnostic principle recommendations of the cIMPACT-Utrecht meeting on future CNS tumor classification and grading. Brain Pathol. 2020, 30, 844–856. [Google Scholar] [CrossRef] [PubMed]
- Sturm, D.; Orr, B.A.; Toprak, U.H.; Hovestadt, V.; Jones, D.T.W.; Capper, D.; Sill, M.; Buchhalter, I.; Northcott, P.A.; Leis, I.; et al. New Brain Tumor Entities Emerge from Molecular Classification of CNS-PNETs. Cell 2016, 164, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Astolfi, A.; Fiore, M.; Melchionda, F.; Indio, V.; Bertuccio, S.N.; Pession, A. BCOR involvement in cancer. Epigenomics 2019, 11, 835–855. [Google Scholar] [CrossRef]
- Huynh, K.D.; Fischle, W.; Verdin, E.; Bardwell, V.J. BCoR, a novel corepressor involved in BCL-6 repression. Genes. Dev. 2000, 14, 1810–1823. [Google Scholar] [CrossRef]
- Gearhart, M.D.; Corcoran, C.M.; Wamstad, J.A.; Bardwell, V.J. Polycomb group and SCF ubiquitin ligases are found in a novel BCOR complex that is recruited to BCL6 targets. Mol. Cell Biol. 2006, 26, 6880–6889. [Google Scholar] [CrossRef]
- Junco, S.E.; Wang, R.; Gaipa, J.C.; Taylor, A.B.; Schirf, V.; Gearhart, M.D.; Bardwell, V.J.; Demeler, B.; Hart, P.J.; Kim, C.A. Structure of the polycomb group protein PCGF1 in complex with BCOR reveals basis for binding selectivity of PCGF homologs. Structure 2013, 21, 665–671. [Google Scholar] [CrossRef]
- Choi, W.I.; Jeon, B.N.; Yoon, J.H.; Koh, D.I.; Kim, M.H.; Yu, M.Y.; Lee, K.M.; Kim, Y.; Kim, K.; Hur, S.S.; et al. The proto-oncoprotein FBI-1 interacts with MBD3 to recruit the Mi-2/NuRD-HDAC complex and BCoR and to silence p21WAF/CDKN1A by DNA methylation. Nucleic Acids Res. 2013, 41, 6403–6420. [Google Scholar] [CrossRef]
- Pagan, J.K.; Arnold, J.; Hanchard, K.J.; Kumar, R.; Bruno, T.; Jones, M.J.; Richard, D.J.; Forrest, A.; Spurdle, A.; Verdin, E.; et al. A novel corepressor, BCoR-L1, represses transcription through an interaction with CtBP. J. Biol. Chem. 2007, 282, 15248–15257. [Google Scholar] [CrossRef]
- Schaefer, E.J.; Wang, H.C.; Karp, H.Q.; Meyer, C.A.; Cejas, P.; Gearhart, M.D.; Adelman, E.R.; Fares, I.; Apffel, A.; Lim, K.; et al. BCOR and BCORL1 Mutations Drive Epigenetic Reprogramming and Oncogenic Signaling by Unlinking PRC1.1 from Target Genes. Blood Cancer Discov. 2022, 3, 116–135. [Google Scholar] [CrossRef]
- Blackledge, N.P.; Rose, N.R.; Klose, R.J. Targeting Polycomb systems to regulate gene expression: Modifications to a complex story. Nat. Rev. Mol. Cell Biol. 2015, 16, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, V.; Tiacci, E.; Holmes, A.B.; Kohlmann, A.; Martelli, M.P.; Kern, W.; Spanhol-Rosseto, A.; Klein, H.U.; Dugas, M.; Schindela, S.; et al. Whole-exome sequencing identifies somatic mutations of BCOR in acute myeloid leukemia with normal karyotype. Blood 2011, 118, 6153–6163. [Google Scholar] [CrossRef] [PubMed]
- Damm, F.; Chesnais, V.; Nagata, Y.; Yoshida, K.; Scourzic, L.; Okuno, Y.; Itzykson, R.; Sanada, M.; Shiraishi, Y.; Gelsi-Boyer, V.; et al. BCOR and BCORL1 mutations in myelodysplastic syndromes and related disorders. Blood 2013, 122, 3169–3177. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Rajan, S.; Chung, H.J.; Raffeld, M.; Panneer Selvam, P.; Schweizer, L.; Perry, A.; Samuel, D.; Giannini, C.; Ragunathan, A.; et al. Molecular and clinicopathologic characteristics of gliomas with EP300::BCOR fusions. Acta Neuropathol. 2022, 144, 1175–1178. [Google Scholar] [CrossRef]
- Gojo, J.; Kjaersgaard, M.; Zezschwitz, B.V.; Capper, D.; Tietze, A.; Kool, M.; Haberler, C.; Pizer, B.; Hoff, K.V. Rare embryonal and sarcomatous central nervous system tumours: State-of-the art and future directions. Eur. J. Med. Genet. 2023, 66, 104660. [Google Scholar] [CrossRef]
- Yamazaki, A.; Arai, Y.; Fukuoka, K.; Nakano, Y.; Hama, N.; Nakata, S.; Makino, K.; Kuroda, J.I.; Shinojima, N.; Mukasa, A.; et al. Diffusely infiltrating glioma with CREBBP-BCORL1 fusion showing overexpression of not only BCORL1 but BCOR: A case report. Brain Tumor Pathol. 2022, 39, 171–178. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Waha, A.; Schittenhelm, J.; Gohla, G.; Schuhmann, M.U.; Pietsch, T. BCOR::CREBBP fusion in malignant neuroepithelial tumor of CNS expands the spectrum of methylation class CNS tumor with BCOR/BCOR(L1)-fusion. Acta Neuropathol. Commun. 2024, 12, 60. [Google Scholar] [CrossRef]
- Barresi, V.; Cardoni, A.; Miele, E.; Pedace, L.; Masotto, B.; Nardini, C.; Barresi, S.; Rossi, S. CNS tumor with CREBBP::BCORL1 Fusion and pathogenic mutations in BCOR and CREBBP: Expanding the spectrum of BCOR-altered tumors. Acta Neuropathol. Commun. 2024, 12, 8. [Google Scholar] [CrossRef]
- Tauziede-Espariat, A.; Uro-Coste, E.; Sievers, P.; Nicaise, Y.; Mariet, C.; Siegfried, A.; Pierron, G.; Guillemot, D.; Benzakoun, J.; Pallud, J.; et al. CNS tumor with EP300::BCOR fusion: Discussing its prevalence in adult population. Acta Neuropathol. Commun. 2023, 11, 26. [Google Scholar] [CrossRef]
- Tauziede-Espariat, A.; Pierron, G.; Siegfried, A.; Guillemot, D.; Uro-Coste, E.; Nicaise, Y.; Castel, D.; Catalaa, I.; Larrieu-Ciron, D.; Chaynes, P.; et al. The EP300:BCOR fusion extends the genetic alteration spectrum defining the new tumoral entity of “CNS tumors with BCOR internal tandem duplication”. Acta Neuropathol. Commun. 2020, 8, 178. [Google Scholar] [CrossRef]
- Torre, M.; Meredith, D.M.; Dubuc, A.; Solomon, D.A.; Perry, A.; Vasudevaraja, V.; Serrano, J.; Snuderl, M.; Ligon, K.L.; Alexandrescu, S. Recurrent EP300-BCOR Fusions in Pediatric Gliomas with Distinct Clinicopathologic Features. J. Neuropathol. Exp. Neurol. 2019, 78, 305–314. [Google Scholar] [CrossRef]
- Jaunmuktane, Z.; Capper, D.; Jones, D.T.W.; Schrimpf, D.; Sill, M.; Dutt, M.; Suraweera, N.; Pfister, S.M.; von Deimling, A.; Brandner, S. Methylation array profiling of adult brain tumours: Diagnostic outcomes in a large, single centre. Acta Neuropathol. Commun. 2019, 7, 24. [Google Scholar] [CrossRef]
- Phillips, J.J.; Gong, H.; Chen, K.; Joseph, N.M.; van Ziffle, J.; Bastian, B.C.; Grenert, J.P.; Kline, C.N.; Mueller, S.; Banerjee, A.; et al. The genetic landscape of anaplastic pleomorphic xanthoastrocytoma. Brain Pathol. 2019, 29, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Bremer, J.; Kottke, R.; Johann, P.D.; von Hoff, K.; Brazzola, P.; Grotzer, M.A.; Kool, M.; Rushing, E.; Gerber, N.U. A single supratentorial high-grade neuroepithelial tumor with two distinct BCOR mutations, exceptionally long complete remission and survival. Pediatr. Blood Cancer 2020, 67, e28384. [Google Scholar] [CrossRef]
- Mahlokozera, T.; Vellimana, A.K.; Li, T.; Mao, D.D.; Zohny, Z.S.; Kim, D.H.; Tran, D.D.; Marcus, D.S.; Fouke, S.J.; Campian, J.L.; et al. Biological and therapeutic implications of multisector sequencing in newly diagnosed glioblastoma. Neuro Oncol. 2018, 20, 472–483. [Google Scholar] [CrossRef]
- Binder, Z.A.; Thorne, A.H.; Bakas, S.; Wileyto, E.P.; Bilello, M.; Akbari, H.; Rathore, S.; Ha, S.M.; Zhang, L.; Ferguson, C.J.; et al. Epidermal Growth Factor Receptor Extracellular Domain Mutations in Glioblastoma Present Opportunities for Clinical Imaging and Therapeutic Development. Cancer Cell 2018, 34, 163–177.e167. [Google Scholar] [CrossRef]
- Lee, J.C.; Vivanco, I.; Beroukhim, R.; Huang, J.H.; Feng, W.L.; DeBiasi, R.M.; Yoshimoto, K.; King, J.C.; Nghiemphu, P.; Yuza, Y.; et al. Epidermal growth factor receptor activation in glioblastoma through novel missense mutations in the extracellular domain. PLoS Med. 2006, 3, e485. [Google Scholar] [CrossRef] [PubMed]
- Hayes, T.K.; Aquilanti, E.; Persky, N.S.; Yang, X.; Kim, E.E.; Brenan, L.; Goodale, A.B.; Alan, D.; Sharpe, T.; Shue, R.E.; et al. Comprehensive mutational scanning of EGFR reveals TKI sensitivities of extracellular domain mutants. Nat. Commun. 2024, 15, 2742. [Google Scholar] [CrossRef] [PubMed]
- Vivanco, I.; Robins, H.I.; Rohle, D.; Campos, C.; Grommes, C.; Nghiemphu, P.L.; Kubek, S.; Oldrini, B.; Chheda, M.G.; Yannuzzi, N.; et al. Differential sensitivity of glioma- versus lung cancer-specific EGFR mutations to EGFR kinase inhibitors. Cancer Discov. 2012, 2, 458–471. [Google Scholar] [CrossRef]
- Pisapia, D.J.; Ohara, K.; Bareja, R.; Wilkes, D.C.; Hissong, E.; Croyle, J.A.; Kim, J.H.; Saab, J.; MacDonald, T.Y.; Beg, S.; et al. Fusions involving BCOR and CREBBP are rare events in infiltrating glioma. Acta Neuropathol. Commun. 2020, 8, 80. [Google Scholar] [CrossRef]
- Xu, Y.; Hou, Y.; Gao, X.; Li, J.; Jiang, D.; Li, Z.; Hu, P.; Wang, Y.; Wen, Y.; Yao, X.; et al. Report two adult cases of high-grade neuroepithelial neoplasm harbouring EP300::BCOR fusions with comprehensive molecular detection. Brain Pathol. 2023, 33, e13177. [Google Scholar] [CrossRef] [PubMed]
- Fukuoka, K.; Kanemura, Y.; Shofuda, T.; Fukushima, S.; Yamashita, S.; Narushima, D.; Kato, M.; Honda-Kitahara, M.; Ichikawa, H.; Kohno, T.; et al. Significance of molecular classification of ependymomas: C11orf95-RELA fusion-negative supratentorial ependymomas are a heterogeneous group of tumors. Acta Neuropathol. Commun. 2018, 6, 134. [Google Scholar] [CrossRef] [PubMed]

| PMID | Age | Sex | Location | Fusion | Initial Diagnosis | Treatment | PFS (Months) | OS (Months) |
|---|---|---|---|---|---|---|---|---|
| Pisapia et al. [30] | 15 | M | right frontal, left temporal, left occipital | BCOR:CREBBP | diffuse astrocytic glioma, with molecular features of glioblastoma | RT with concomitant TMZ | 18 | 27 |
| Baressi et al. [18] | 45 | M | right frontal | CREBBP:BCORL1 | - | RT and TMZ | 1 | 1 |
| Yamazaki et al. [16] | 17 | F | right frontal | CREBBP:BCORL1 | - | resection | 18 | - |
| Tauziède-Espariat et al. [20] | 13 | M | right temporal | EP300:BCOR | - | GTR and chemotherapy | 16 | 16 |
| Tauziède-Espariat et al. [20] | 27 | M | left frontal | EP300:BCOR | - | GTR, chemotherapy and focal RT | 27 | 27 |
| Tauziède-Espariat et al. [19] | 64 | M | left frontal | EP300:BCOR | high-grade glioneuronal tumor | GTR | 12 | 12 |
| Tauziède-Espariat et al. [19] | 40 | M | within the right lateral ventricle | EP300:BCOR | high-grade glioneuronal tumor | STR, carboplatin and VP16 | 12 | 12 |
| Torre et al. [21] | 12 | M | right frontal | EP300:BCOR | oligodendroglioma | extraventricular drainage, STR | 4.5 | 6 |
| Torre et al. [21] | 10 | F | left basal ganglia and thalamus | EP300:BCOR | glioblastoma | STR | 1 | 7 |
| Torre et al. [21] | 18 | M | right medial occipital | EP300:BCOR | dysembryoplastic neuroepithelial tumor (DNET) versus glioneuronal tumor | STR | 15 | 42 |
| Fukuoka et al. [32] | 72 | M | occipital | EP300:BCORL1 | anaplastic ependymoma G3 | resection, RT (40 Gy), chemotherapy | 24 | 33 |
| Xu et al. [31] | 43 | F | right frontotemporal | EP300:BCOR | - | NTR, RT (5200 cGy), adjuvant chemotherapy | 8 | 8 |
| Xu et al. [31] | 54 | M | right frontal | EP300:BCOR; BCOR-L3MBTL2 | - | GTR, RT (6000 cGy) and adjuvant TMZ | 16 | 16 |
| Yamazaki et al. [16] | 18 | F | right frontal | - | - | resection | 33 | - |
| Yamazaki et al. [16] | 21 | F | right frontal | - | - | resection, RT (54 Gy), TMZ | 89 | - |
| Yamazaki et al. [16] | 25 | F | right frontal | - | - | resection, SRS, adjuvant chemotherapy with bevacizumab, surgery, later addition of ifosfamide, cisplatin, and etoposide | - | 115 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lou, J.; Yong, W.; Aldape, K.; Chu, E.; Hui, C.; Hsu, F.P.K.; Zheng, M.; Ribeiro, A.; Fote, G.; Na, D.; et al. CNS Tumor with BCOR/BCORL1 Fusion: A Rare Tumor Entity. Int. J. Mol. Sci. 2025, 26, 6729. https://doi.org/10.3390/ijms26146729
Lou J, Yong W, Aldape K, Chu E, Hui C, Hsu FPK, Zheng M, Ribeiro A, Fote G, Na D, et al. CNS Tumor with BCOR/BCORL1 Fusion: A Rare Tumor Entity. International Journal of Molecular Sciences. 2025; 26(14):6729. https://doi.org/10.3390/ijms26146729
Chicago/Turabian StyleLou, Jerry, William Yong, Kenneth Aldape, Eleanor Chu, Caressa Hui, Frank P. K. Hsu, Michelle Zheng, Anatevka Ribeiro, Gianna Fote, Daniel Na, and et al. 2025. "CNS Tumor with BCOR/BCORL1 Fusion: A Rare Tumor Entity" International Journal of Molecular Sciences 26, no. 14: 6729. https://doi.org/10.3390/ijms26146729
APA StyleLou, J., Yong, W., Aldape, K., Chu, E., Hui, C., Hsu, F. P. K., Zheng, M., Ribeiro, A., Fote, G., Na, D., & Yuen, C. A. (2025). CNS Tumor with BCOR/BCORL1 Fusion: A Rare Tumor Entity. International Journal of Molecular Sciences, 26(14), 6729. https://doi.org/10.3390/ijms26146729





