Molecular Mechanisms of Aminoglycoside-Induced Ototoxicity in Murine Auditory Cells: Implications for Otoprotective Drug Development
Abstract
1. Introduction
2. Results
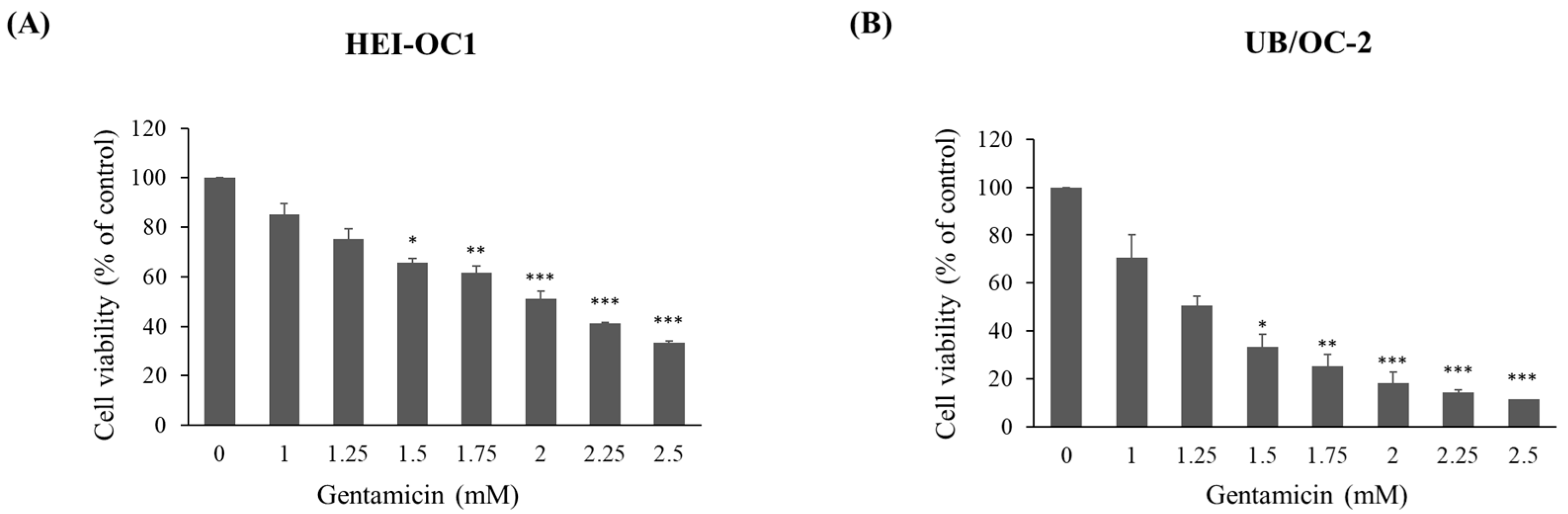
2.1. Cytotoxic Evaluation of Gentamicin
2.2. Transcriptome Analysis in HEI-OC1 Cells
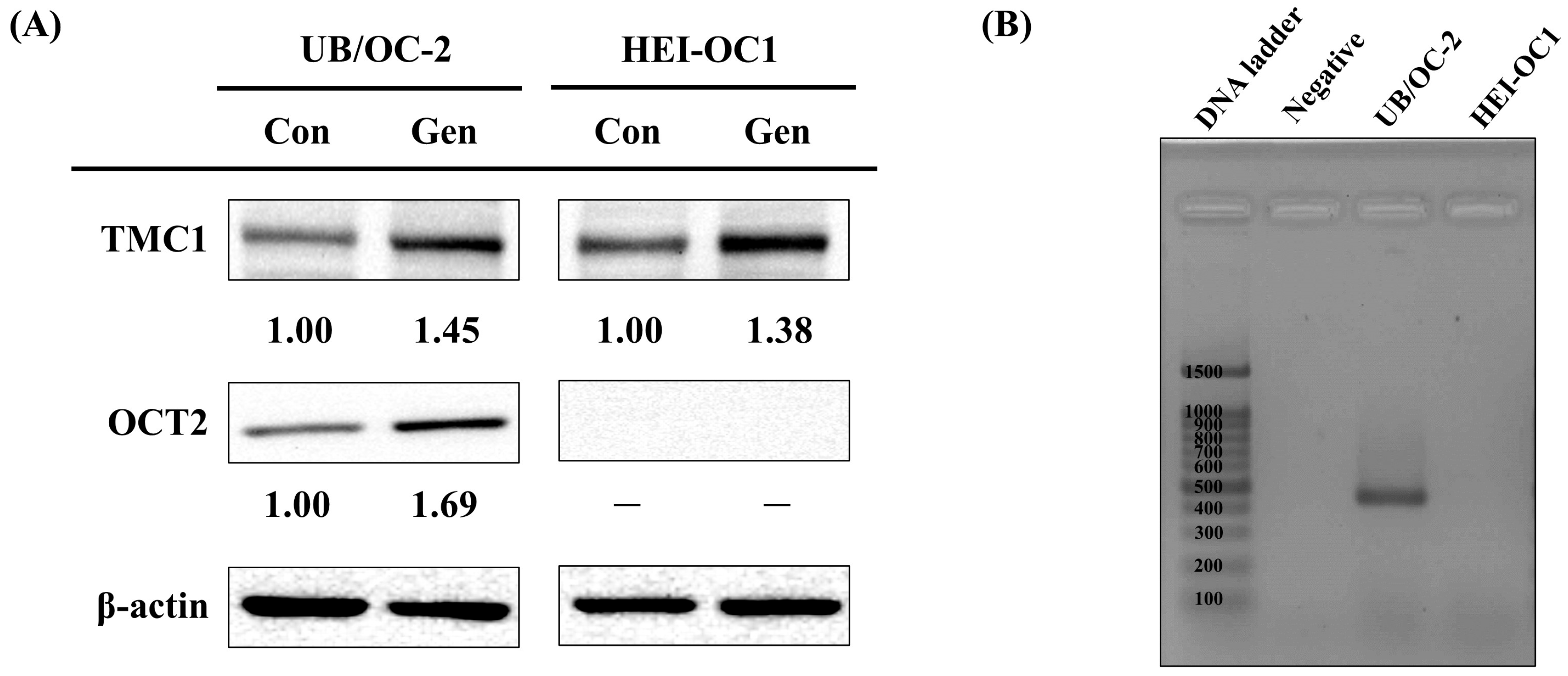
2.3. Gene Expression and Protein Analysis on TMC1 and OCT2
2.4. Protein Level Validation in UB/OC-2
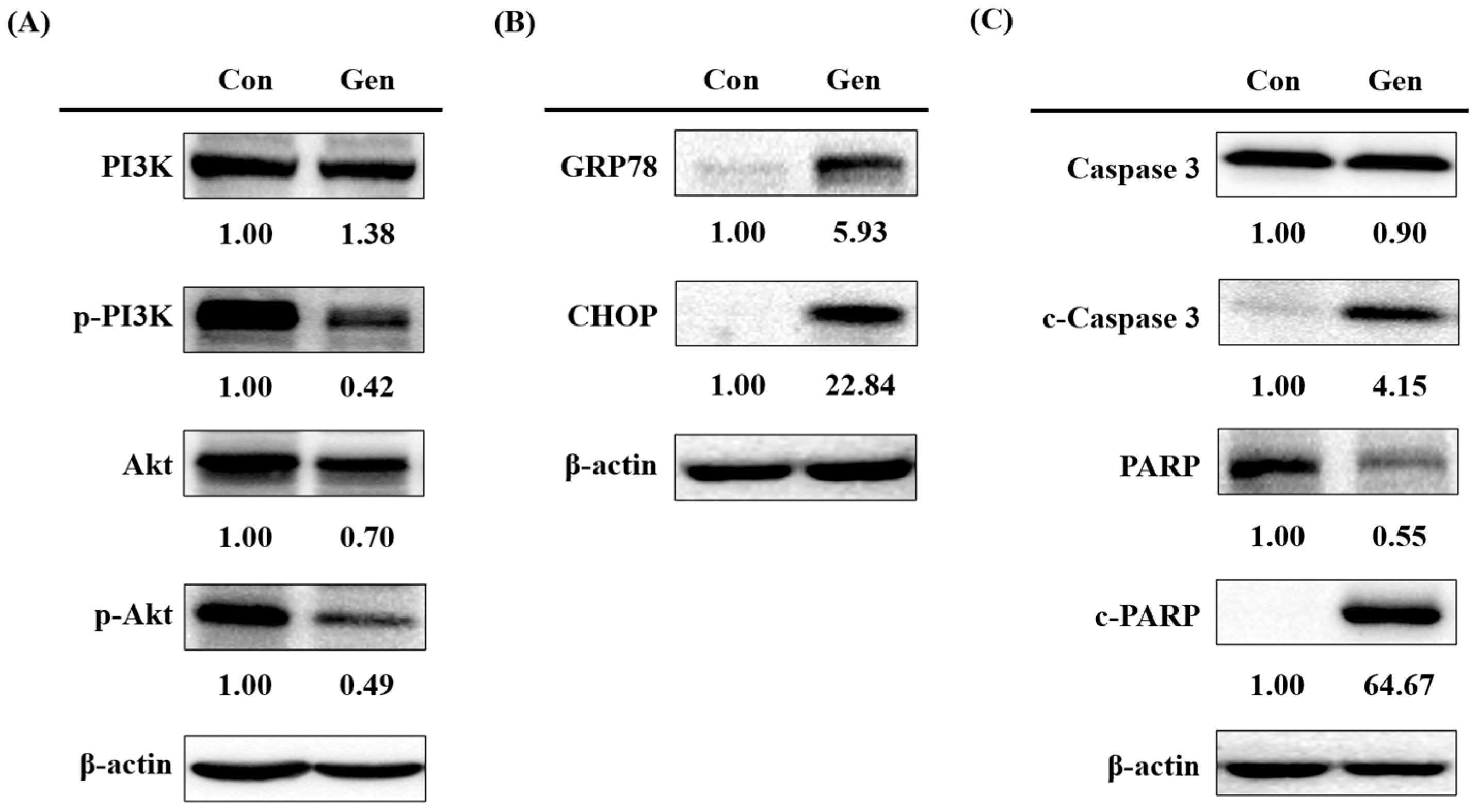
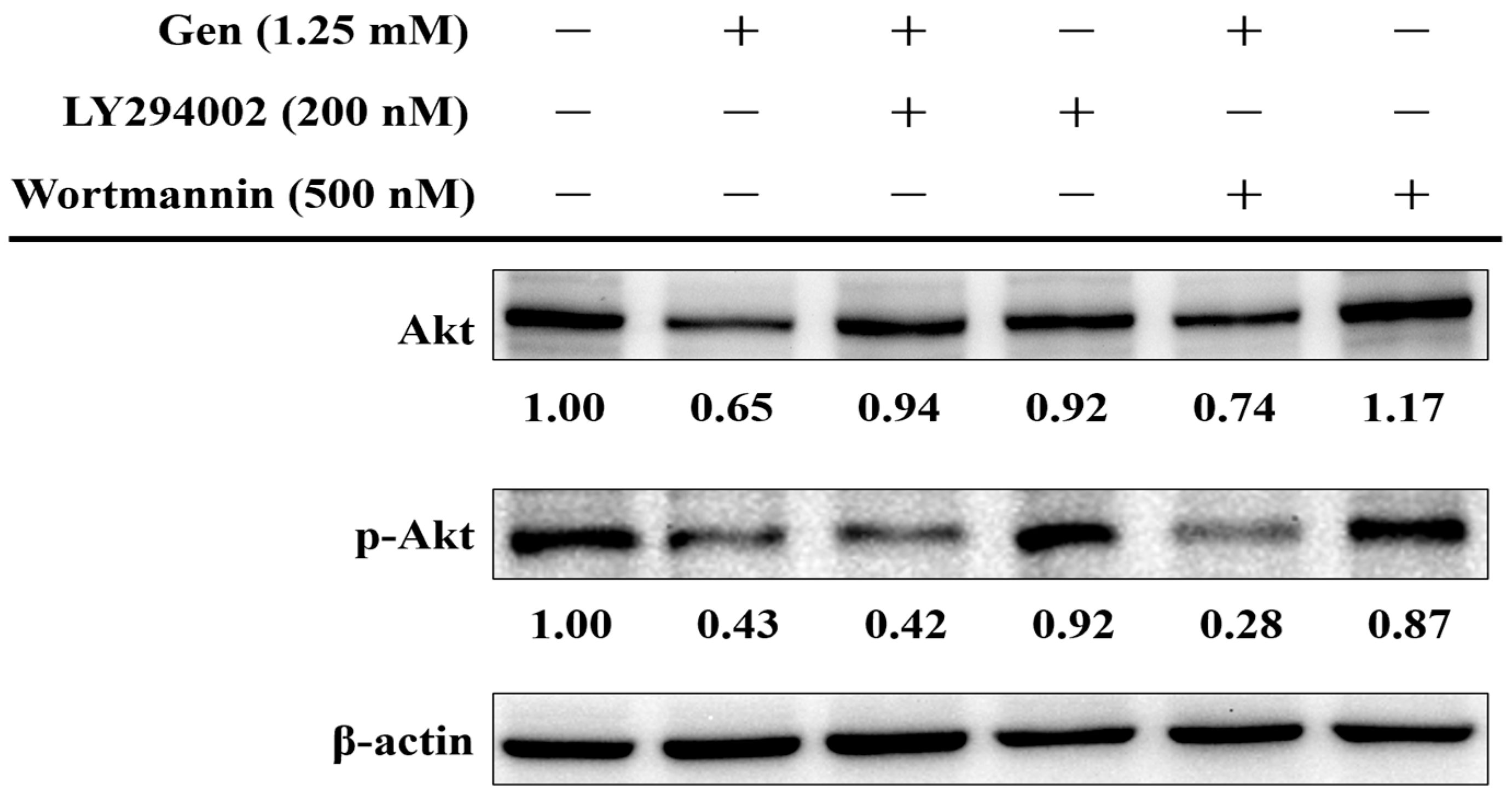
2.5. PI3K-Akt Pathway in Gentamicin-Induced Ototoxicity
2.6. GTTR Uptake and Protective Effects of STS/NAC
3. Discussion
3.1. UB/OC-2 vs. HEI-OC1 as In Vitro Models
3.2. Key Insights from Transcriptomic and Protein Expression Analyses
3.3. Further Implications of the PI3K-Akt Pathway in Gentamicin Ototoxicity
3.4. Implications of the GTTR Uptake Assay
3.5. Species-Specific Genetic Variation
3.6. Future Directions
3.7. Practical Implications
- Cell model selection:
- Assay window:
- Mechanistic insight:
- Otoprotectant evaluation:
4. Materials and Methods
- Methodological Overview
- Step 1—Cell-line validation.
- Step 2—Transcriptomic analysis.
- Step 3—Pathway validation and functional inhibition.
- Step 4—GTTR-based screening.
4.1. Cell Culture and Drug Treatment
4.2. Cell Viability Assay
4.3. RNA-Seq and Transcriptome Analysis
4.4. Western Blot Analysis
4.5. RNA Isolation and Reverse Transcription-PCR (RT-PCR)
4.6. Gentamicin-Conjugated Texas Red (GTTR) Uptake
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chadha, S.; Kamenov, K.; Cieza, A. The world report on hearing, 2021. Bull. World Health Organ. 2021, 99, 242. [Google Scholar] [CrossRef]
- Michels, T.C.; Duffy, M.T.; Rogers, D.J. Hearing loss in adults: Differential diagnosis and treatment. Am. Fam. Physician 2019, 100, 98–108. [Google Scholar] [PubMed]
- Cunningham, L.L.; Tucci, D.L. Hearing Loss in Adults. N. Engl. J. Med. 2017, 377, 2465–2473. [Google Scholar] [CrossRef] [PubMed]
- Hegde, V.; Shonuga, O.; Ellis, S.; Fragomen, A.; Kennedy, J.; Kudryashov, V.; Lane, J.M. A prospective comparison of 3 approved systems for autologous bone marrow concentration demonstrated nonequivalency in progenitor cell number and concentration. J. Orthop. Trauma 2014, 28, 591–598. [Google Scholar] [CrossRef]
- Lanvers-Kaminsky, C.; Ciarimboli, G. Pharmacogenetics of drug-induced ototoxicity caused by aminoglycosides and cisplatin. Pharmacogenomics 2017, 18, 1683–1695. [Google Scholar] [CrossRef] [PubMed]
- Hammill, T.L.; Campbell, K.C. Protection for medication-induced hearing loss: The state of the science. Int. J. Audiol. 2018, 57, S67–S75. [Google Scholar] [CrossRef]
- Xie, J.; Talaska, A.E.; Schacht, J. New developments in aminoglycoside therapy and ototoxicity. Hear. Res. 2011, 281, 28–37. [Google Scholar] [CrossRef]
- Krause, K.M.; Serio, A.W.; Kane, T.R.; Connolly, L.E. Aminoglycosides: An Overview. Cold Spring Harb. Perspect. Med. 2016, 6, a027029. [Google Scholar] [CrossRef]
- Lanvers-Kaminsky, C.; Zehnhoff-Dinnesen, A.A.; Parfitt, R.; Ciarimboli, G. Drug-induced ototoxicity: Mechanisms, Pharmacogenetics, and protective strategies. Clin. Pharmacol. Ther. 2017, 101, 491–500. [Google Scholar] [CrossRef]
- Rybak, L.P.; Whitworth, C.A. Ototoxicity: Therapeutic opportunities. Drug Discov. Today 2005, 10, 1313–1321. [Google Scholar] [CrossRef]
- Kalinec, G.M.; Webster, P.; Lim, D.J.; Kalinec, F. A cochlear cell line as an in vitro system for drug ototoxicity screening. Audiol. Neurootol. 2003, 8, 177–189. [Google Scholar] [CrossRef]
- Kalinec, G.; Thein, P.; Park, C.; Kalinec, F. HEI-OC1 cells as a model for investigating drug cytotoxicity. Hear. Res. 2016, 335, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Xiong, H.; Liu, Y.; Pang, J.; Lin, H.; Zhang, W.; Zheng, Y. Transcriptomic analysis highlights cochlear inflammation associated with age-related hearing loss in C57BL/6 mice using next generation sequencing. PeerJ 2020, 8, e9737. [Google Scholar] [CrossRef]
- Jagger, D.J.; Griesinger, C.B.; Rivolta, M.N.; Holley, M.C.; Ashmore, J.F. Calcium signalling mediated by the 9 acetylcholine receptor in a cochlear cell line from the immortomouse. J. Physiol. 2000, 527 Pt 1, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Fucile, S.; Sucapane, A.; Eusebi, F. Ca2+ permeability through rat cloned alpha9-containing nicotinic acetylcholine receptors. Cell Calcium 2006, 39, 349–355. [Google Scholar] [CrossRef]
- Kakuki, T.; Kohno, T.; Nishida, S.; Konno, T.; Kikuchi, S.; Ohwada, K.; Nakano, M.; Tezuka, M.; Takano, K.; Kojima, T. FOXO3/TGF-beta signal-dependent ciliogenesis and cell functions during differentiation of temperature-sensitive mouse cochlear precursor hair cells. Histochem. Cell Biol. 2022, 157, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Jagger, D.J.; Holley, M.C.; Ashmore, J.F. Ionic currents expressed in a cell line derived from the organ of Corti of the Immortomouse. Pflug. Arch. 1999, 438, 8–14. [Google Scholar] [CrossRef]
- Hsieh, C.Y.; Lin, J.N.; Kang, T.Y.; Wen, Y.H.; Yu, S.H.; Wu, C.C.; Wu, H.P. Otoprotective effects of fucoidan reduce cisplatin-induced ototoxicity in mouse cochlear UB/OC-2 cells. Int. J. Mol. Sci. 2023, 24, 3561. [Google Scholar] [CrossRef]
- Wen, Y.H.; Lin, J.N.; Wu, R.S.; Yu, S.H.; Hsu, C.J.; Tseng, G.F.; Wu, H.P. Otoprotective Effect of 2,3,4′,5-Tetrahydroxystilbene-2-O-beta-d-Glucoside on Gentamicin-Induced Apoptosis in Mouse Cochlear UB/OC-2 Cells. Molecules 2020, 25, 3070. [Google Scholar] [CrossRef]
- Gill, N.B.; Dowker-Key, P.D.; Hubbard, K.; Voy, B.H.; Whelan, J.; Hedrick, M.; Bettaieb, A. Ginsenoside Rc from Panax Ginseng Ameliorates Palmitate-Induced UB/OC-2 Cochlear Cell Injury. Int. J. Mol. Sci. 2023, 24, 7345. [Google Scholar] [CrossRef]
- Hsieh, C.Y.; Tsai, C.Y.; Chou, Y.F.; Hsu, C.J.; Wu, H.P.; Wu, C.C. Otoprotection against aminoglycoside- and cisplatin-induced ototoxicity focusing on the upstream drug uptake pathway. J. Chin. Med. Assoc. 2024, 87, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Steyger, P.S. Mechanisms of aminoglycoside- and cisplatin-induced ototoxicity. Am. J. Audiol. 2021, 30, 887–900. [Google Scholar] [CrossRef] [PubMed]
- Filipski, K.K.; Mathijssen, R.H.; Mikkelsen, T.S.; Schinkel, A.H.; Sparreboom, A. Contribution of organic cation transporter 2 (OCT2) to cisplatin-induced nephrotoxicity. Clin. Pharmacol. Ther. 2009, 86, 396–402. [Google Scholar] [CrossRef]
- Li, J.; Liu, C.; Kaefer, S.; Youssef, M.; Zhao, B. The mechanotransduction channel and organic cation transporter are critical for cisplatin ototoxicity in murine hair cells. Front. Mol. Neurosci. 2022, 15, 835448. [Google Scholar] [CrossRef]
- Ciarimboli, G.; Deuster, D.; Knief, A.; Sperling, M.; Holtkamp, M.; Edemir, B.; Pavenstadt, H.; Lanvers-Kaminsky, C.; am Zehnhoff-Dinnesen, A.; Schinkel, A.H.; et al. Organic cation transporter 2 mediates cisplatin-induced oto- and nephrotoxicity and is a target for protective interventions. Am. J. Pathol. 2010, 176, 1169–1180. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Shen, Y.; Hu, H.; Fan, C.; Zhang, A.; Ding, R.; Ye, B.; Xiang, M. Systematic Transcriptome Analysis of Noise-Induced Hearing Loss Pathogenesis Suggests Inflammatory Activities and Multiple Susceptible Molecules and Pathways. Front. Genet. 2020, 11, 968. [Google Scholar] [CrossRef]
- Chen, P.; Hao, J.J.; Li, M.W.; Bai, J.; Guo, Y.T.; Liu, Z.; Shi, P. Integrative Functional Transcriptomic Analyses Implicate Shared Molecular Circuits in Sensorineural Hearing Loss. Front. Cell. Neurosci. 2022, 16, 857344. [Google Scholar] [CrossRef]
- Brock, P.R.; Maibach, R.; Childs, M.; Rajput, K.; Roebuck, D.; Sullivan, M.J.; Laithier, V.; Ronghe, M.; Dall’Igna, P.; Hiyama, E.; et al. Sodium Thiosulfate for Protection from Cisplatin-Induced Hearing Loss. N. Engl. J. Med. 2018, 378, 2376–2385. [Google Scholar] [CrossRef]
- Pfister, M.; Zalaman, I.M.; Blumenstock, G.; Mauz, P.S.; Baumann, I. Impact of genotype and mutation type on health-related quality of life in patients with hereditary hemorrhagic telangiectasia. Acta Oto-Laryngol. 2009, 129, 862–866. [Google Scholar] [CrossRef]
- Rybak, L.P.; Whitworth, C.A.; Mukherjea, D.; Ramkumar, V. Mechanisms of cisplatin-induced ototoxicity and prevention. Hear. Res. 2007, 226, 157–167. [Google Scholar] [CrossRef]
- Shi, X.; Wang, J.; Lei, Y.; Cong, C.; Tan, D.; Zhou, X. Research progress on the PI3K/AKT signaling pathway in gynecological cancer. Mol. Med. Rep. 2019, 19, 4529–4535. [Google Scholar] [CrossRef]
- Spehr, M.; Munger, S.D. Olfactory receptors: G protein-coupled receptors and beyond. J. Neurochem. 2009, 109, 1570–1583. [Google Scholar] [CrossRef] [PubMed]
- Menini, A. Calcium signalling and regulation in olfactory neurons. Curr. Opin. Neurobiol. 1999, 9, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Fiori, M.C.; Krishnan, S.; Kjellgren, A.; Cuello, L.G.; Altenberg, G.A. Inhibition by Commercial Aminoglycosides of Human Connexin Hemichannels Expressed in Bacteria. Molecules 2017, 22, 2063. [Google Scholar] [CrossRef]
- Figueroa, V.A.; Retamal, M.A.; Cea, L.A.; Salas, J.D.; Vargas, A.A.; Verdugo, C.A.; Jara, O.; Martinez, A.D.; Saez, J.C. Extracellular gentamicin reduces the activity of connexin hemichannels and interferes with purinergic Ca(2+) signaling in HeLa cells. Front. Cell. Neurosci. 2014, 8, 265. [Google Scholar] [CrossRef]
- Chen, X.; Shi, C.; He, M.; Xiong, S.; Xia, X. Endoplasmic reticulum stress: Molecular mechanism and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 352. [Google Scholar] [CrossRef] [PubMed]
- Szegezdi, E.; Logue, S.E.; Gorman, A.M.; Samali, A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006, 7, 880–885. [Google Scholar] [CrossRef]
- Neuwelt, E.A.; Brummett, R.E.; Doolittle, N.D.; Muldoon, L.L.; Kroll, R.A.; Pagel, M.A.; Dojan, R.; Church, V.; Remsen, L.G.; Bubalo, J.S. First evidence of otoprotection against carboplatin-induced hearing loss with a two-compartment system in patients with central nervous system malignancy using sodium thiosulfate. J. Pharmacol. Exp. Ther. 1998, 286, 77–84. [Google Scholar] [CrossRef]
- Rivolta, M.N.; Grix, N.; Lawlor, P.; Ashmore, J.F.; Jagger, D.J.; Holley, M.C. Auditory hair cell precursors immortalized from the mammalian inner ear. Proc. Biol. Sci. 1998, 265, 1595–1603. [Google Scholar] [CrossRef]
- McCance, R.A. Proceedings of the Royal Society of London. Series B--Biological Sciences, Volume 119, 1935-1936: Experimental sodium chloride deficiency in man. Nutr. Rev. 1990, 48, 145–147. [Google Scholar] [CrossRef]
- Moriya, Y.; Itoh, M.; Okuda, S.; Yoshizawa, A.C.; Kanehisa, M. KAAS: An automatic genome annotation and pathway reconstruction server. Nucleic Acids Res. 2007, 35, W182–W185. [Google Scholar] [CrossRef] [PubMed]
- Alcock, B.P.; Raphenya, A.R.; Lau, T.T.Y.; Tsang, K.K.; Bouchard, M.; Edalatmand, A.; Huynh, W.; Nguyen, A.V.; Cheng, A.A.; Liu, S.; et al. CARD 2020: Antibiotic resistome surveillance with the comprehensive antibiotic resistance database. Nucleic Acids Res. 2020, 48, D517–D525. [Google Scholar] [CrossRef]
- McArthur, A.G.; Waglechner, N.; Nizam, F.; Yan, A.; Azad, M.A.; Baylay, A.J.; Bhullar, K.; Canova, M.J.; De Pascale, G.; Ejim, L.; et al. The comprehensive antibiotic resistance database. Antimicrob. Agents Chemother. 2013, 57, 3348–3357. [Google Scholar] [CrossRef]
- Luo, W.; Brouwer, C. Pathview: An R/Bioconductor package for pathway-based data integration and visualization. Bioinformatics 2013, 29, 1830–1831. [Google Scholar] [CrossRef]
- Boyle, E.I.; Weng, S.; Gollub, J.; Jin, H.; Botstein, D.; Cherry, J.M.; Sherlock, G. GO::TermFinder--open source software for accessing Gene Ontology information and finding significantly enriched Gene Ontology terms associated with a list of genes. Bioinformatics 2004, 20, 3710–3715. [Google Scholar] [CrossRef]
- Wiedenhoft, H.; Hayashi, L.; Coffin, A.B. PI3K and Inhibitor of Apoptosis Proteins Modulate Gentamicin- Induced Hair Cell Death in the Zebrafish Lateral Line. Front. Cell. Neurosci. 2017, 11, 326. [Google Scholar] [CrossRef] [PubMed]
- Hetz, C.; Saxena, S. ER stress and the unfolded protein response in neurodegeneration. Nat. Rev. Neurol. 2017, 13, 477–491. [Google Scholar] [CrossRef]
- Chung, W.H.; Pak, K.; Lin, B.; Webster, N.; Ryan, A.F. A PI3K pathway mediates hair cell survival and opposes gentamicin toxicity in neonatal rat organ of Corti. J. Assoc. Res. Otolaryngol. 2006, 7, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Han, E.; Park, S.; Hyun, K.; Lee, Y.; Baek, H.W.; Kim, H.J.; Rah, Y.C.; Choi, J. Protective Effects of (-)-Butaclamol Against Gentamicin-Induced Ototoxicity: In Vivo and In Vitro Approaches. Int. J. Mol. Sci. 2025, 26, 4201. [Google Scholar] [CrossRef]
- Derudas, M.; O’Reilly, M.; Kirkwood, N.K.; Kenyon, E.J.; Grimsey, S.; Kitcher, S.R.; Workman, S.; Bull, J.C.; Ward, S.E.; Kros, C.J.; et al. Charge and lipophilicity are required for effective block of the hair-cell mechano-electrical transducer channel by FM1-43 and its derivatives. Front. Cell Dev. Biol. 2023, 11, 1247324. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, D.; Zong, L.; Zhao, F.; Guan, L.; Zhang, P.; Shi, W.; Lan, L.; Wang, H.; Li, Q.; et al. A novel DFNA36 mutation in TMC1 orthologous to the Beethoven (Bth) mouse associated with autosomal dominant hearing loss in a Chinese family. PLoS ONE 2014, 9, e97064. [Google Scholar] [CrossRef] [PubMed]
- Hilgert, N.; Monahan, K.; Kurima, K.; Li, C.; Friedman, R.A.; Griffith, A.J.; Van Camp, G. Amino acid 572 in TMC1: Hot spot or critical functional residue for dominant mutations causing hearing impairment. J. Hum. Genet. 2009, 54, 188–190. [Google Scholar] [CrossRef]
- Corns, L.F.; Johnson, S.L.; Kros, C.J.; Marcotti, W. Tmc1 Point Mutation Affects Ca2+ Sensitivity and Block by Dihydrostreptomycin of the Mechanoelectrical Transducer Current of Mouse Outer Hair Cells. J. Neurosci. 2016, 36, 336–349. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Tsai, C.H.; Lu, Y.C.; Lin, H.C.; Hwang, J.H.; Lin, Y.H.; Yang, W.S.; Chen, P.J.; Liao, W.C.; Lee, Y.L.; et al. Contribution of adiponectin and its type 1 receptor to age-related hearing impairment. Neurobiol. Aging 2015, 36, 2085–2093. [Google Scholar] [CrossRef]
- Chan, Y.H.; Liu, T.C.; Liao, C.K.; Cheng, Y.F.; Tsai, C.H.; Lu, Y.C.; Hu, C.J.; Lin, H.J.; Lee, Y.L.; Wu, C.C.; et al. Consumption of betel quid contributes to sensorineural hearing impairment through arecoline-induced oxidative stress. Sci. Rep. 2019, 9, 14554. [Google Scholar] [CrossRef]
- Wen, Y.H.; Lin, H.Y.; Lin, J.N.; Tseng, G.F.; Hwang, C.F.; Lin, C.C.; Hsu, C.J.; Wu, H.P. 2,3,4′,5-Tetrahydroxystilbene-2-O-beta-D-glucoside ameliorates gentamicin-induced ototoxicity by modulating autophagy via Sesn2/AMPK/mTOR signaling. Int. J. Mol. Med. 2022, 49, 71. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef]
- Lagesen, K.; Hallin, P.; Rodland, E.A.; Staerfeldt, H.H.; Rognes, T.; Ussery, D.W. RNAmmer: Consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Res. 2007, 35, 3100–3108. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.P.; Lowe, T.M. tRNAscan-SE: Searching for tRNA Genes in Genomic Sequences. In Gene Prediction; Methods in Molecular Biology; Springer: New York, NY, USA, 2019; Volume 1962, pp. 1–14. [Google Scholar] [CrossRef]
- Delcher, A.L.; Harmon, D.; Kasif, S.; White, O.; Salzberg, S.L. Improved microbial gene identification with GLIMMER. Nucleic Acids Res. 1999, 27, 4636–4641. [Google Scholar] [CrossRef]
- Tatusov, R.L.; Fedorova, N.D.; Jackson, J.D.; Jacobs, A.R.; Kiryutin, B.; Koonin, E.V.; Krylov, D.M.; Mazumder, R.; Mekhedov, S.L.; Nikolskaya, A.N.; et al. The COG database: An updated version includes eukaryotes. BMC Bioinform. 2003, 4, 41. [Google Scholar] [CrossRef]
- Chen, T.W.; Gan, R.C.; Wu, T.H.; Huang, P.J.; Lee, C.Y.; Chen, Y.Y.; Chen, C.C.; Tang, P. FastAnnotator—An efficient transcript annotation web tool. BMC Genom. 2012, 13 (Suppl. S7), S9. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsieh, C.-Y.; Lin, J.-N.; Chou, Y.-F.; Hsu, C.-J.; Chen, P.-R.; Wen, Y.-H.; Wu, C.-C.; Sun, C.-H. Molecular Mechanisms of Aminoglycoside-Induced Ototoxicity in Murine Auditory Cells: Implications for Otoprotective Drug Development. Int. J. Mol. Sci. 2025, 26, 6720. https://doi.org/10.3390/ijms26146720
Hsieh C-Y, Lin J-N, Chou Y-F, Hsu C-J, Chen P-R, Wen Y-H, Wu C-C, Sun C-H. Molecular Mechanisms of Aminoglycoside-Induced Ototoxicity in Murine Auditory Cells: Implications for Otoprotective Drug Development. International Journal of Molecular Sciences. 2025; 26(14):6720. https://doi.org/10.3390/ijms26146720
Chicago/Turabian StyleHsieh, Cheng-Yu, Jia-Ni Lin, Yi-Fan Chou, Chuan-Jen Hsu, Peir-Rong Chen, Yu-Hsuan Wen, Chen-Chi Wu, and Chuan-Hung Sun. 2025. "Molecular Mechanisms of Aminoglycoside-Induced Ototoxicity in Murine Auditory Cells: Implications for Otoprotective Drug Development" International Journal of Molecular Sciences 26, no. 14: 6720. https://doi.org/10.3390/ijms26146720
APA StyleHsieh, C.-Y., Lin, J.-N., Chou, Y.-F., Hsu, C.-J., Chen, P.-R., Wen, Y.-H., Wu, C.-C., & Sun, C.-H. (2025). Molecular Mechanisms of Aminoglycoside-Induced Ototoxicity in Murine Auditory Cells: Implications for Otoprotective Drug Development. International Journal of Molecular Sciences, 26(14), 6720. https://doi.org/10.3390/ijms26146720






