Silencing of the Alkaline α-Galactosidase Gene CsAGA1 Impairs Root and Gall Development in Cucumber upon Meloidogyne incognita Infection
Abstract
1. Introduction
2. Results
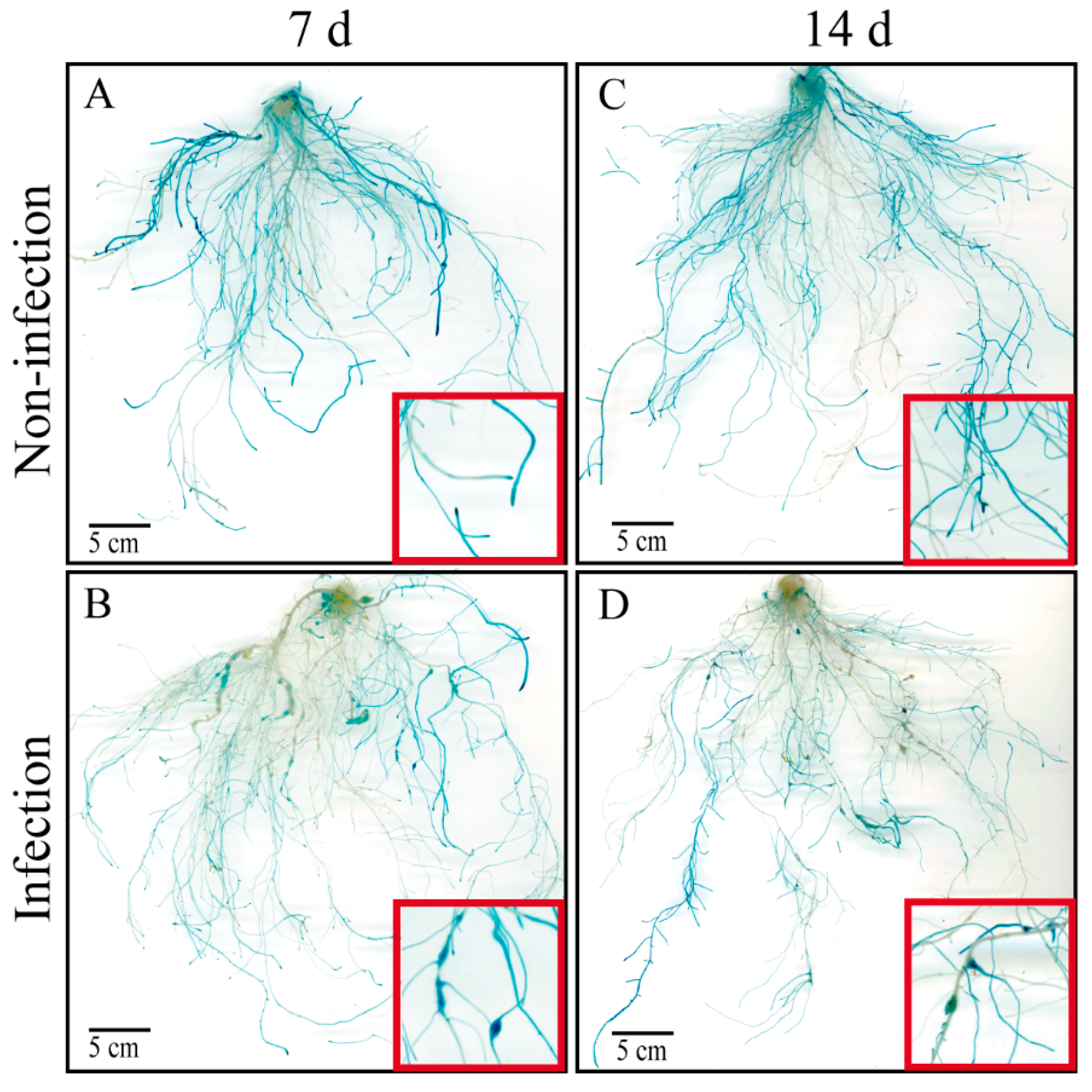
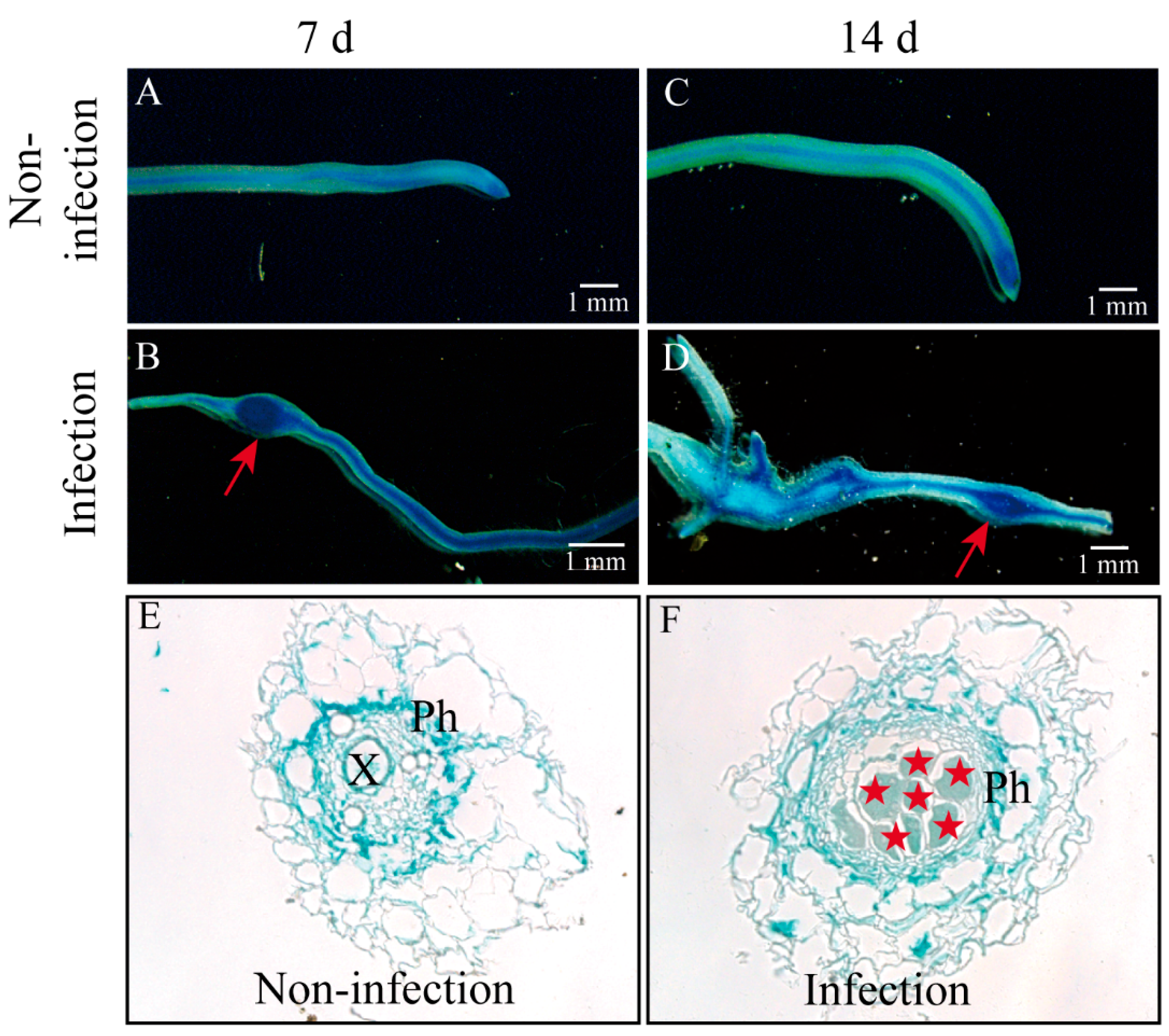
2.1. CsAGA1 Is Significantly Upregulated in GCs with M. incognita Infection
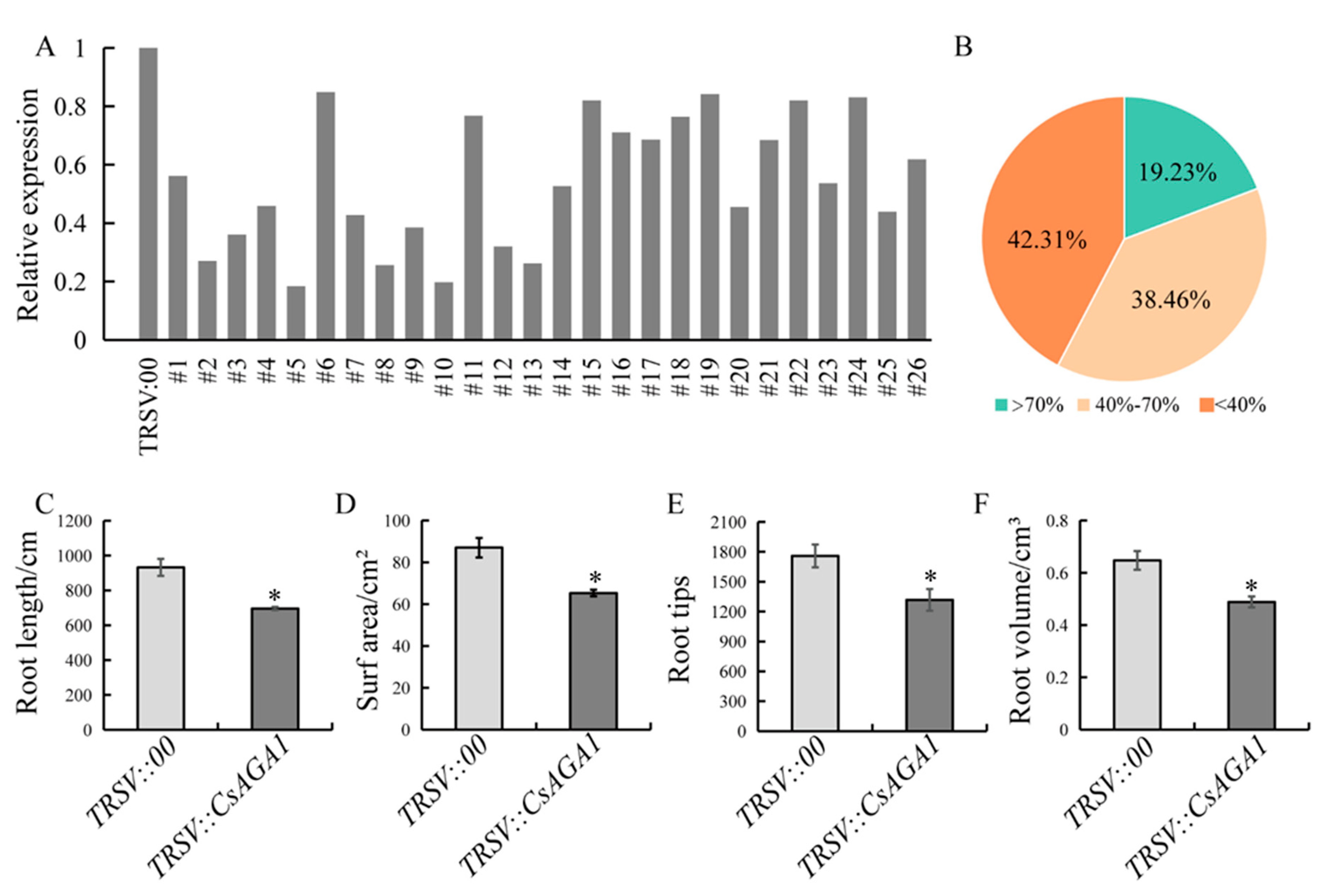
2.2. Down-Regulation of CsAGA1 Impairs Root Development in Cucumber
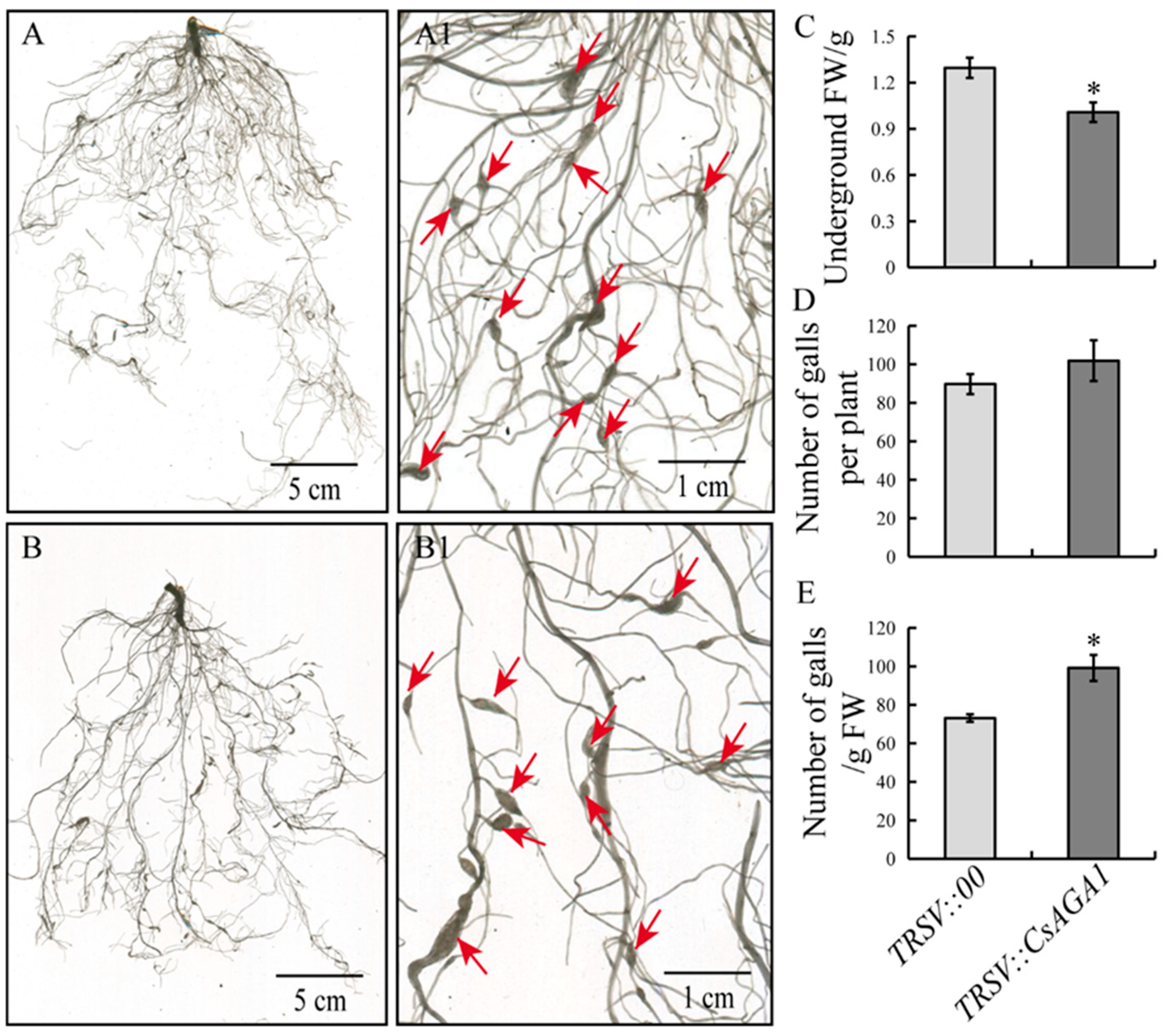
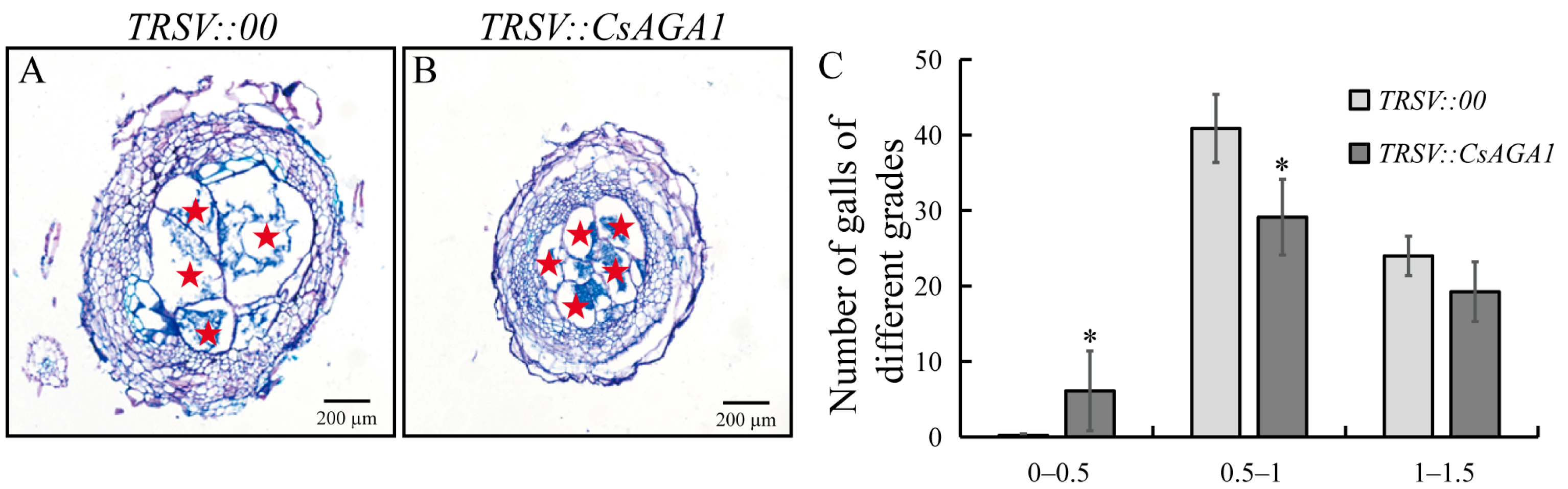
2.3. CsAGA1 Silencing Impairs GCs Development
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. RNA Expression Analysis
4.3. GUS Localization Analysis
4.4. VIGS Assay and Phenotypic Observation
4.5. Structure Observation of Paraffin
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bird, D.M.; Williamson, V.M.; Abad, P.; McCarter, J.; Danchin, E.G.; Castagnone-Sereno, P.; Opperman, C.H. The Genomes of Root-Knot Nematodes. Annu. Rev. Phytopathol. 2009, 47, 333–351. [Google Scholar] [CrossRef]
- Escobar, C.; Barcala, M.; Cabrera, J.; Fenoll, C. Chapter One—Overview of Root-Knot Nematodes and Giant Cells. Adv. Bot. Res. 2015, 73, 1–32. [Google Scholar]
- Moens, M.; Perry, R.N. Migratory Plant Endoparasitic Nematodes: A Group Rich in Contrasts and Divergence. Annu. Rev. Phytopathol. 2009, 47, 313–332. [Google Scholar] [CrossRef] [PubMed]
- Brinkman, E.P.; Duyts, H.; Karssen, G.; van der Stoel, C.D.; van der Putten, W.H. Plant-Feeding Nematodes in Coastal Sand Dunes: Occurrence, Host Specificity and Effects on Plant Growth. Plant Soil 2015, 397, 17–30. [Google Scholar] [CrossRef][Green Version]
- Caillaud, M.-C.; Dubreuil, G.; Quentin, M.; Perfus-Barbeoch, L.; Lecomte, P.; de Almeida Engler, J.; Abad, P.; Rosso, M.-N.; Favery, B. Root-Knot Nematodes Manipulate Plant Cell Functions During a Compatible Interaction. J. Plant Physiol. 2008, 165, 104–113. [Google Scholar] [CrossRef]
- Hofmann, J.; Grundler, F. How Do Nematodes Get Their Sweets? Solute Supply to Sedentary Plant-Parasitic Nematodes. Nematology 2007, 9, 451–458. [Google Scholar] [CrossRef]
- Marella, H.H.; Nielsen, E.; Schachtman, D.P.; Taylor, C.G. The Amino Acid Permeases Aap3 and Aap6 Are Involved in Root-Knot Nematode Parasitism of Arabidopsis. Mol. Plant Microbe Interact. 2013, 26, 44–54. [Google Scholar] [CrossRef]
- Kanwar, P.; Jha, G. Alterations in Plant Sugar Metabolism: Signatory of Pathogen Attack. Planta 2019, 249, 305–318. [Google Scholar] [CrossRef]
- Zou, J.; Kyndt, T.; Yu, J.; Zhou, J. Plant-Nematode Battle: Engagement of Complex Signaling Network. Trends Parasitol. 2024, 40, 846–857. [Google Scholar] [CrossRef]
- Cabello, S.; Lorenz, C.; Crespo, S.; Cabrera, J.; Ludwig, R.; Escobar, C.; Hofmann, J. Altered Sucrose Synthase and Invertase Expression Affects the Local and Systemic Sugar Metabolism of Nematode-Infected Arabidopsis thaliana Plants. J. Exp. Bot. 2014, 65, 201–212. [Google Scholar] [CrossRef]
- Bolouri Moghaddam, M.R.; van den Ende, W. Sugars and Plant Innate Immunity. J. Exp. Bot. 2012, 63, 3989–3998. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, S.; Zhang, X.; Gao, L.; Ruan, Y.L.; Tian, Y.; Ma, S. From Raffinose Family Oligosaccharides to Sucrose and Hexoses: Gene Expression Profiles Underlying Host-to-Nematode Carbon Delivery in Cucumis sativus Roots. Front. Plant Sci. 2022, 13, 823382. [Google Scholar] [CrossRef] [PubMed]
- Katrolia, P.; Rajashekhara, E.; Yan, Q.; Jiang, Z. Biotechnological Potential of Microbial A-Galactosidases. Crit. Rev. Biotechnol. 2014, 34, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Nie, J.; Li, X.; Scanlon, M.J.; Zhang, C.; Zheng, Y.; Ma, S.; Shan, N.; Fei, Z.; Turgeon, R.; et al. Transcriptomic and Functional Analysis of Cucumber (Cucumis sativus L.) Fruit Phloem During Early Development. Plant J. 2018, 96, 982–996. [Google Scholar] [CrossRef]
- Gu, H.; Lu, M.; Zhang, Z.; Xu, J.; Cao, W.; Miao, M. Metabolic Process of Raffinose Family Oligosaccharides During Cold Stress and Recovery in Cucumber Leaves. J. Plant Physiol. 2018, 224–225, 112–120. [Google Scholar] [CrossRef]
- Chrost, B.; Kolukisaoglu, U.; Schulz, B.; Krupinska, K. An Alpha-Galactosidase with an Essential Function During Leaf Development. Planta 2007, 225, 311–320. [Google Scholar] [CrossRef]
- Hua, B.; Zhang, M.; Zhang, J.; Dai, H.; Zhang, Z.; Miao, M. CsAGA1 and CsAGA2 Mediate Rfo Hydrolysis in Partially Distinct Manner in Cucumber Fruits. Int. J. Mol. Sci. 2021, 22, 13285. [Google Scholar] [CrossRef]
- Liu, H.; Liu, X.; Zhao, Y.; Nie, J.; Yao, X.; Lv, L.; Yang, J.; Ma, N.; Guo, Y.; Li, Y.; et al. Alkaline A-Galactosidase 2 (CsAGA2) Plays a Pivotal Role in Mediating Source-Sink Communication in Cucumber. Plant Physiol. 2022, 189, 1501–1518. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, D.; Dirk, L.M.A.; Downie, A.B.; Zhao, T. ZmAGA1 Hydrolyzes Rfos Late During the Lag Phase of Seed Germination, Shifting Sugar Metabolism toward Seed Germination over Seed Aging Tolerance. J. Agric. Food Chem. 2021, 69, 11606–11615. [Google Scholar] [CrossRef]
- Hara, M.; Tokunaga, K.; Kuboi, T. Isolation of a Drought-Responsive Alkaline α-Galactosidase Gene from New Zealand Spinach. Plant Biotechnol. 2008, 25, 497–501. [Google Scholar] [CrossRef]
- Daldoul, S.; Amar, A.B.; Gargouri, M.; Limam, H.; Mliki, A.; Wetzel, T. A Grapevine-Inducible Gene Vv-A-Gal/Sip Confers Salt and Desiccation Tolerance in Escherichia Coli and Tobacco at Germinative Stage. Biochem. Genet. 2018, 56, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Gross, K.C.; Pharr, D.M. A Potential Pathway for Galactose Metabolism in Cucumis sativus L., a Stachyose Transporting Species. Plant Physiol. 1982, 69, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Taji, T.; Ohsumi, C.; Iuchi, S.; Seki, M.; Kasuga, M.; Kobayashi, M.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Important Roles of Drought- and Cold-Inducible Genes for Galactinol Synthase in Stress Tolerance in Arabidopsis thaliana. Plant J. 2002, 29, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Pennycooke, J.C.; Jones, M.L.; Stushnoff, C. Down-Regulating α-Galactosidase Enhances Freezing Tolerance in Transgenic Petunia. Plant Physiol. 2003, 133, 901–909. [Google Scholar] [CrossRef]
- Li, Y.; Liu, H.; Yao, X.; Wang, J.; Feng, S.; Sun, L.; Ma, S.; Xu, K.; Chen, L.Q.; Sui, X. Hexose Transporter CsSWEET7a in Cucumber Mediates Phloem Unloading in Companion Cells for Fruit Development. Plant Physiol. 2021, 186, 640–654. [Google Scholar] [CrossRef]
- Jones, J.T.; Haegeman, A.; Danchin, E.G.J.; Gaur, H.S.; Helder, J.; Jones, M.G.K.; Kikuchi, T.; Manzanilla-López, R.; Palomares-Rius, J.E.; Wesemael, W.M.L.; et al. Top 10 Plant-Parasitic Nematodes in Molecular Plant Pathology. Mol. Plant Pathol. 2013, 14, 946–961. [Google Scholar] [CrossRef]
- Gommers, F.J.; Dropkin, V.H. Quantitative Histochemistry of Nematode-Induced Transfer Cells. Phytopathology 1977, 67, 869–873. [Google Scholar] [CrossRef]
- Sun, L.; Lian, L.; Yang, R.; Li, T.; Yang, M.; Zhao, W.; Huang, H.; Wang, S. Sugar Delivery at the Tomato Root and Root Galls after Meloidogyne Incognita Infestation. BMC Plant Biol. 2024, 24, 451. [Google Scholar] [CrossRef]
- Liu, H.; Wang, F.; Liu, B.; Kong, F.; Fang, C. Significance of Raffinose Family Oligosaccharides (RFOs) Metabolism in Plants. Adv. Biotechnol. 2024, 2, 13. [Google Scholar] [CrossRef]
- Ren, Y.; Li, M.; Guo, S.; Sun, H.; Zhao, J.; Zhang, J.; Liu, G.; He, H.; Tian, S.; Yu, Y.; et al. Evolutionary Gain of Oligosaccharide Hydrolysis and Sugar Transport Enhanced Carbohydrate Partitioning in Sweet Watermelon Fruits. Plant Cell 2021, 33, 1554–1573. [Google Scholar] [CrossRef]
- Zhang, J.; Gu, H.; Dai, H.; Zhang, Z.; Miao, M. Alternative Polyadenylation of the Stacyose Synthase Gene Mediates Source-Sink Regulation in Cucumber. J. Plant Physiol. 2020, 245, 153111. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.S.; Singh, M.; Aggrawal, P.; Laxmi, A. Glucose and Auxin Signaling Interaction in Controlling Arabidopsis thaliana Seedlings Root Growth and Development. PLoS ONE 2009, 4, e4502. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Hao, Y.; Cui, H. The Wuschel Related Homeobox Protein Wox7 Regulates the Sugar Response of Lateral Root Development in Arabidopsis thaliana. Mol. Plant 2016, 9, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Hu, T.; Li, X.; Song, C.P.; Zhu, J.K.; Chen, L.; Zhao, Y. Phosphorylation of Sweet Sucrose Transporters Regulates Plant Root: Shoot Ratio under Drought. Nat. Plants 2022, 8, 68–77. [Google Scholar] [CrossRef]
- Zhao, D.; You, Y.; Fan, H.; Zhu, X.; Wang, Y.; Duan, Y.; Xuan, Y.; Chen, L. The Role of Sugar Transporter Genes During Early Infection by Root-Knot Nematodes. Int. J. Mol. Sci. 2018, 19, 302. [Google Scholar] [CrossRef]
- Zhang, X.; Li, S.; Li, X.; Song, M.; Ma, S.; Tian, Y.; Gao, L. Peat-Based Hairy Root Transformation Using Rhizobium rhizogenes as a Rapid and Efficient Tool for Easily Exploring Potential Genes Related to Root-Knot Nematode Parasitism and Host Response. Plant Methods 2023, 19, 22. [Google Scholar] [CrossRef]
- Fang, L.; Wei, X.-Y.; Liu, L.-Z.; Zhou, L.-X.; Tian, Y.-P.; Geng, C.; Li, X.-D. A Tobacco Ringspot Virus-Based Vector System for Gene and Microrna Function Studies in Cucurbits. Plant Physiol. 2021, 186, 853–864. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, T.; Wang, X.; Wang, X.; Gao, L.; Tian, Y.; Ma, S. Silencing of the Alkaline α-Galactosidase Gene CsAGA1 Impairs Root and Gall Development in Cucumber upon Meloidogyne incognita Infection. Int. J. Mol. Sci. 2025, 26, 6686. https://doi.org/10.3390/ijms26146686
Ji T, Wang X, Wang X, Gao L, Tian Y, Ma S. Silencing of the Alkaline α-Galactosidase Gene CsAGA1 Impairs Root and Gall Development in Cucumber upon Meloidogyne incognita Infection. International Journal of Molecular Sciences. 2025; 26(14):6686. https://doi.org/10.3390/ijms26146686
Chicago/Turabian StyleJi, Tingting, Xingyi Wang, Xueyun Wang, Lihong Gao, Yongqiang Tian, and Si Ma. 2025. "Silencing of the Alkaline α-Galactosidase Gene CsAGA1 Impairs Root and Gall Development in Cucumber upon Meloidogyne incognita Infection" International Journal of Molecular Sciences 26, no. 14: 6686. https://doi.org/10.3390/ijms26146686
APA StyleJi, T., Wang, X., Wang, X., Gao, L., Tian, Y., & Ma, S. (2025). Silencing of the Alkaline α-Galactosidase Gene CsAGA1 Impairs Root and Gall Development in Cucumber upon Meloidogyne incognita Infection. International Journal of Molecular Sciences, 26(14), 6686. https://doi.org/10.3390/ijms26146686





