Abstract
A challenge in orthodontic treatment is the long time taken to move teeth, which extends the long treatment period. Accordingly, various treatment protocols and orthodontic materials have been developed to shorten the orthodontic treatment period. However, controlling biological reactions is considered necessary to further shorten this treatment period. Orthodontic force results in compression of the periodontal ligament in the direction of tooth movement, resulting in various reactions in the periodontal ligament that induce osteoclast development, alveolar bone absorption, and teeth movement. The aforementioned reactions include immune reactions. Cytokines are substances responsible for intercellular communication and are involved in various physiological actions, including immune and inflammatory reactions. They cause various cellular responses, including cell proliferation, differentiation, cell death, and functional expression. Various cytokines are involved in biological reactions during orthodontic tooth movement (OTM). It is important to understand the role of cytokines during OTM in order to elucidate their biological response. This review discusses the role of cytokines during OTM.
1. Introduction
Orthodontic tooth movement (OTM), which is achieved by applying orthodontic mechanical force to teeth, involves reorganization of the alveolar bone and periodontal ligament (PDL). During this process, immune reactions occur in the PDL and alveolar bone, playing an essential role in initiating and regulating bone remodeling.
OTM is crucially involved in the treatment of malocclusion [1,2]. Mechanical loading induces tooth displacement, which creates a PDL compression area in the direction of force action and a tension area on the other side [3]. This causes localized aseptic inflammation, which induces bone resorption and formation at the compression and tension areas, respectively (Figure 1) [4]. The PDL is the primary structure that senses and transmits orthodontic forces from the teeth to the alveolar bone [5]. In addition to bone resorption and formation, the extracellular matrix of the PDL is continuously remodeled during OTM, which is primarily regulated by the cellular components of the PDL [6,7]. Mechanical stimulation induces biological responses in numerous cell types within the PDL and alveolar bone. It is important to elucidate the highly complex cellular mechanisms underlying OTM, especially the involved immune reactions [8].
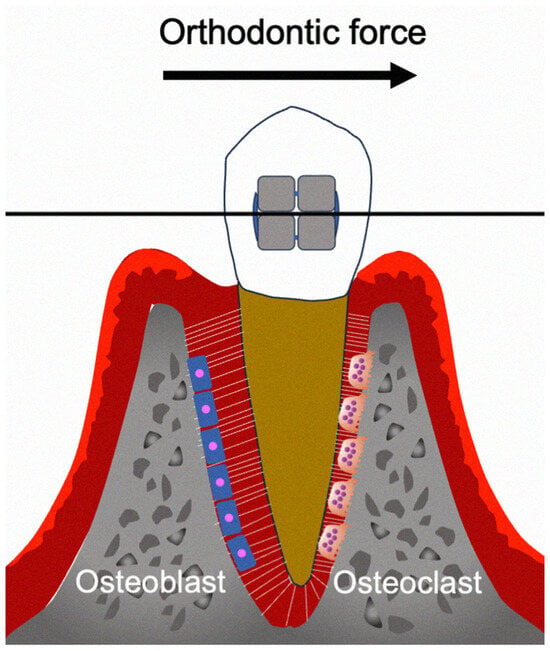
Figure 1.
Schema of the biological effects during OTM.
Osteoclasts are cells found on the bone surface during resorption and are therefore crucially involved in OTM. Macrophage colony-stimulating factor (M-CSF) and receptor activator of NF-κB ligand (RANKL) promote the differentiation of osteoclast precursor cells into bone-resorbing osteoclasts [9]. Additionally, tumor necrosis factor-α (TNF-α) induces osteoclast formation [10].
Cytokines monitor and control inflammatory and immune reactions through complicated networks; moreover, they serve as biomarkers for numerous disease types [11]. Additionally, cytokines control the maturation, growth, responsiveness, cytotoxic immunity, allergic immunity, and eosinophilia of specific cell populations [12]. Cytokines are central mediators of the biological cascade during OTM. When orthodontic force compresses the PDL on one side and stretches it on the other, various cytokines are secreted in response to mechanical stress [3]. Many cytokines are involved in the recruitment and activation of immune cells at the site of force application, thereby initiating and regulating bone remodeling during OTM [13,14]. They regulate osteoclast differentiation and bone resorption, with some promoting osteoclastogenesis and others acting as inhibitors [15]. Following the active phase of bone resorption, cytokines also participate in tissue repair and regeneration during OTM. Some cytokines, such as insulin-like growth factor (IGF) and bone morphogenetic proteins (BMPs), are involved in promoting osteoblast differentiation, angiogenesis, and bone formation, thereby facilitating the remodeling of periodontal tissues [16,17]. Importantly, the timing, balance, and local concentration of cytokine release are critical to ensure efficient and controlled OTM while avoiding adverse tissue destruction. Cytokine expression is dynamic and varies during different stages of OTM, reflecting the tightly regulated interplay between inflammatory activation and resolution phases.
Cytokine production in the gingival area, including gingival crevicular fluid (GCF), during OTM has been extensively studied to elucidate the cell metabolism at the local site as well as the periodontal tissue health and bone remodeling. Accordingly, cytokines may play a regulatory role in osteoclast formation. This review describes and discusses the role of OTM-related cytokines, with a focus on their effects on osteoclasts.
2. Cytokines Involved in Orthodontic Tooth Movement
In this review, we will describe the characteristics of the following cytokines; Interleukin (IL)-1, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-11, IL-12, IL-13, IL-16, IL-17, IL-18, IL-20, IL-23, IL-27, IL-33, IL-34, IFN-γ, TNF-α, M-CSF, TGFs, RANKL, and OPG, as well as their relationship to the immune system, bone metabolic diseases and their effects on osteoclast formation and OTM.
2.1. IL-1β
IL-1β is an important inflammatory cytokine produced by macrophages, T cells, monocytes, and dendritic cells [18]. It promotes osteoclast formation and bone resorption in rheumatoid arthritis, osteoporosis, and periodontal disease [6,19,20,21]. However, it cannot solely induce osteoclast differentiation from osteoclast precursor cells. Nonetheless, IL-1RI induction through c-Fos or IL-1RI receptor overexpression in bone marrow stromal cells can induce true osteoclast formation through a RANKL/RANK-independent mechanism [22]. IL-1 is not considered a mere substitute for RANKL, with reports suggesting that the in vivo effect of IL-1 on osteoclasts depends on its effect on osteoblasts [23].
As aforementioned, the PDL is crucially involved in the bone remodeling process by promoting bone resorption and formation in the compression and tension areas, respectively, in response to mechanical force during OTM. Notably, these processes are regulated by IL-1 [24]. The application of orthodontic forces to teeth has been shown to elevate IL-1β levels in GCF [25,26]. IL-1β is an important mediator in various acute-phase inflammatory and immune responses [27]; further, it is strongly involved in the periodontal tissue reorganizing process during the early stage of OTM [28,29]. Specifically, IL-1β prompts osteoclast differentiation, enhances their fusion and survival, and activates their function. Application of traction forces to the maxillary canine increases IL-1β levels in GCF on the distal tooth surface, peaking at 24–72 h after tooth movement initiation [30]. Additionally, the OTM rate is positively correlated with IL-1β levels in GCF [31]. The effect of IL-1β is regulated by its receptor antagonist, interleukin-1 receptor antagonist (IL-1RA); further, orthodontic mechanical force has been associated with an increase in both IL-1β secretion and the IL-1β/IL-1RA ratio [32]. IL-1, which is among the most potent cytokines in the early stage of OTM, induces cell proliferation and differentiation; further, it is secreted by various cell types such as fibroblasts, macrophages, cementoblasts, osteoblasts, and osteoclasts in response to external stimulation [28,33]. IL-1β may serve as a biomarker for the extent of OTM, which is dependent on the efficiency of alveolar bone remodeling [34]. At 24 h after orthodontic treatment initiation, there is an increase in both pocket fluid volume and IL-1 levels. Here, IL-1 levels in pocket fluid differed between adolescents and young adults, suggesting age-dependent differences in the tissue response. As a result, teeth have quicker mobility in teenagers than in young adults [35]. A study on the effects of low-level laser therapy on cytokine levels during OTM found that photobiomodulation was significantly related to elevated IL-1b levels in GCF [36]. Further, adolescents with obesity have shown higher orofacial pain severity at 24 h after OTM, as well as higher IL-1β levels before and during OTM, compared with adolescents without obesity [26]. Compared with the control group, rats who underwent ligature appliance as experimental OTM showed a greater increase in gene expression of IL-1β. Further, the ligation device group had greater linear bone resorption, bone mineral density, and trabecular number; lower values of fractional bone mass; and increased values of trabecular spacing compared with the other groups [37]. Autophagy inhibition with 3-methyladenine was shown to accelerate OTM and reduce bone mineral density, as well as increase the entropy of the PDL structure; contrastingly, autophagy enhancement with rapamycin yielded opposite results in OTM. In addition, there was a post-OTM increase in IL-1 levels, which was highest in the group treated with 3-methyladenine (autophagy inhibition) [38]. Taken together, IL-1 appears to directly enhance osteoclast formation and indirectly induce osteoclastogenic cytokines in OTM.
2.2. IL-2
IL-2 is a T helper 1 (Th1) cell-derived pro-inflammatory cytokine that is involved in macrophage stimulation, B cell activation, natural killer (NK) cell and T cell proliferation, and stimulation of osteoclast activity. Further, IL-2 is involved in the stimulation of osteoclast activity in bone resorption, which suggests an active pathological involvement in periodontal disease [39]. Contrastingly, low-dose IL-2 has been shown to attenuate osteoclast formation in collagen-induced arthritis via a c-Jun N-terminal kinase (JNK)-dependent pathway [40]. Notably, IL-2 polymorphism has been associated with the severity of chronic periodontitis [41]. A human study showed that during OTM, IL-2 levels were increased in GCF [42].
2.3. IL-4
IL-4 is primarily expressed in T helper 2 (Th2) cells, eosinophils, mast cells, basophils, and other immune cells; further, it plays a crucial role in regulating immune responses [43]. In addition, it plays a crucial role in allergic inflammation and parasitic infections, as well as stimulates B cell proliferation and the activation of eosinophils, basophils, and mast cells. IL-4 can antagonize Th1-induced inflammatory immune responses suppress the synthesis of numerous inflammatory cytokines [44,45]. Further, IL-4 prevents lipoprotein-induced periodontitis by inhibiting osteoclast formation [46]. IL-4 is a strong inhibitor of the RANKL-induced osteoclastogenic process [47]. Furthermore, IL-4 has been shown to inhibit TNF-α-induced osteoclast formation in vitro [48], as well as through RANKL expression by TNF-α-activated stromal cells and TNF-α-activated osteoclast precursors in vivo [49].
A mouse model of tooth movement showed that IL-4 inhibited OTM and osteoclast formation [50]. In a rat model of OTM, injection of exogenous IL-4 into the local site following tooth movement reduced relapse by inhibiting osteoclast formation [51]. In vitro cultivated primary osteoblasts from mouse calvariae that were subjected to micro-pulse vibration for 20 min showed an increase in IL-4 levels [52]. Accordingly, analyzing plasma proteins may facilitate the prediction and monitoring of the dynamics of bone and tissue remodeling, including during orthodontic treatment. A study applied two different orthodontic forces (low force and normal force) for bilateral buccal expansion around the maxillary second and third molars of rats. Notably, they observed no significant intergroup differences in the IL-4 levels [53].
2.4. IL-5
IL-5 is a type 2 cytokine produced by several immune cells, including Th2 cells, eosinophils, mast cells, and basophils [54]. Under normal conditions, IL-5 transgenic mice showed an increase in the number of eosinophils and the bone mass; further, IL-5 protects against inflammation- and hormone-induced bone loss [55]. Accordingly, IL-5 suppresses osteoclast formation.
GCF samples were taken 10 weeks after initial appliance placement at 4 h, 7 days, and 42 days after distal force was applied to the maxillary canines. IL-5 levels were measured in GCF using multiplex assays. However, the level of IL-5 was undetectable in the samples [56].
2.5. IL-6
IL-6 is a pro-inflammatory and pleiotropic cytokine that regulates the immune system [57]. It is crucially involved in inflammation, diabetes, atherosclerosis, hematopoiesis, autoimmunity, cancer, trauma, and rheumatoid arthritis [58,59,60,61,62]. It is released by several cell types, including B cells, T cells, monocytes, macrophages, endothelial cells, adipocytes, mesangial cells, fibroblasts, keratinocytes, and several tumor cells [63]. IL-6 indirectly affects osteoclasts; further, it promotes osteoclast formation and bone resorption by enhancing RANKL expression in IL-6-activated osteoblasts [64,65]. IL-6-neutralizing antibodies attenuate TNF-α- and IL-1β-induced osteoclast formation [66]. Accordingly, IL-6 promotes osteoclast bone resorption and plays a crucial role in the etiology of bone loss in acute and chronic inflammation [67], periodontitis [21], osteoporosis, and rheumatoid arthritis [64].
In a previous study, the maxillary canine that underwent distal movement was used as the experimental tooth, while the opposite canine was used as the control. Here, the IL-6 levels in GCF were higher in the experimental tooth than in the control tooth [68]. Adolescents have shown higher IL-6 levels in crevicular fluid than young adults [35]. Additionally, periodontitis induced elevated gingival IL-6 and CXCL2 levels in a rat model of tooth movement. Further, OTM promoted bacterial-induced periodontal tissue destruction and IL-6 gene expression in the gingiva. Similarly, increased gingival IL-6 and CXCL2 levels were observed in human periodontitis [69]. Compared with normal-weight individuals, individuals with obesity showed higher IL-6 levels in GCF during distal movement of canines [70]. Additionally, increased IL-6 levels have been reported in the GCF of individuals undergoing orthodontic treatment with clear aligners [71]. IL-6 signaling was activated in the PDL following orthodontic intervention in a rat orthodontic model. This signaling promoted OTM by inducing osteoclast bone resorption; further, IL-6 increased the number of osteoclasts by suppressing apoptosis as well as enhancing responsiveness to M-CSF and RANKL. Moreover, IL-6 signaling induces neuroinflammation in the trigeminal ganglion, which results in orthodontic pain. Taken together, IL-6 signaling regulates both tooth movement and pain during OTM [72]. Local static magnetic field stimulation has significantly increased the tooth movement distance and induced osteoclast formation in the compressed side of a rat model of OTM. IL-6 levels were increased in force-loaded PDL stem cells exposed to static magnetic field stimulation, as well as in the compressed side of a rat model of OTM. The OTM distance enhanced by static magnetic field stimulation was significantly reduced by administration of tocilizumab, which is an IL-6 inhibitor. Taken together, static magnetic field stimulation appears to induce IL-6 secretion by force-loaded PDL stem cells, which promotes OTM and osteoclast formation [73]. Exogenous application of direct current to piezoelectric biopolymers induces biochemical changes in the intra- and extracellular regions, significantly affecting the bone metabolism rate. Further, micro-current stimulation increased the rate of tooth movement and IL-6 levels in rats [74]. There were dynamic fluctuations in IL-6 and miR-146a expression patterns during orthodontic relapse in a rat model. Specifically, IL-6 protein levels peaked on day 7 following removal of the orthodontic appliance, which was accompanied by a decrease in miR-146a expression. In vitro studies have shown that miR-146a inhibition resulted in elevated IL-6 expression, indicating its regulatory function [75].
2.6. IL-7
IL-7 is expressed by stromal cells; further, it is crucially involved in the homeostatic survival and development of naive T cells, memory T cells, immature thymocytes, pro-B cells, and innate lymphoid cells. It contains four antiparallel α-helices that bind to type I cytokine receptors. Given this property, pharmacological treatment with IL-7 induces proliferation of pro-B cells, naive T cells, and memory T cells [76]. IL-7 induces bone loss through the induction of RANKL and TNF-a from T cells in vivo [77,78]. In rheumatoid arthritis, M1 macrophages show increased IL-7R expression, which increases their responsiveness to IL-7-induced osteoclast formation [79]. Notably, IL-7 enhances the generation of osteoclast precursors in vitro. Ovariectomized mice showed increased IL-7, but not RANKL, mRNA expression by osteoblasts and stromal cells [80]. Notably, patients wearing aligners did not show significant changes in IL-7 levels in GCF samples obtained from the lower incisors [81].
2.7. IL-8
IL-8, which is also known as CXCL8, is a CXC chemokine crucially involved in regulating inflammatory responses [82]. Further, it is a neutrophil chemotactic factor. IL-8 is synthesized by various cells, including macrophages, endothelial cells, airway smooth muscle cells, and epithelial cells [83]. IL-8 induces its effects by binding to its receptor (CXCR1 or CXCR2) [84]. IL-8 is expressed in numerous tumors and cancer cell lines; further, it promotes angiogenesis, tumor metastasis, and tumor growth in various human cancers [85]. Additionally, IL-8 levels are increased in the synovial fluid and serum of patients with rheumatoid arthritis [86]. IL-8 triggers RANKL expression in bone marrow stromal cells [87].
The study compared IL-8 levels in GCF during OTM using self-ligating brackets and conventional brackets. A study reported higher IL-8 levels in GCF in patients with conventional brackets than in those with self-ligating brackets [88]. In a rat OTM model, orthodontic compressive force enhanced IL-8 mRNA and protein expression from PDL cells in a force-dependent manner [89]. Another study on the effects of mechanical stress on inflammatory remodeling of periodontal tissues and IL-8 expression found that both inflammatory stimuli and orthodontic force induced IL-8 expression [90]. Orthodontic treatment with clear aligners significantly increased IL-8 levels in GCF [71]. Photobiomodulation increased tooth movement by modulating IL-8 levels, which were higher compared with those in the non-irradiated area. [36,91]. Orthodontic forces induced changes in IL-8 levels in human periodontal tissue. Following mechanical stimulation, there were differences in IL-8 levels at both tension and pressure sites, which may trigger the bone remodeling process. Early orthodontic forces significantly increased IL-8 levels after 1 h, which peaked on Day 6. Finally, local host responses to orthodontic forces have been shown to increase the accumulation of IL-8 and neutrophils, which may trigger the bone remodeling process [92].
2.8. IL-10
IL-10 is an anti-inflammatory cytokine crucially involved in suppressing Th1 cell responses by suppressing the expression and function of various inflammatory cytokines, including interferon gamma (IFN-γ), TNF, IL-1, and IL-6, in monocytes and T cells. IL-10 promotes immune events, including the cytotoxic activity of CD8+ T cells and NK cells, thymocyte proliferation, and immunoglobulin production by B cells [93,94,95]. Therefore, IL-10 plays both stimulatory and inhibitory roles in immune responses; further, IL-10 inhibits osteoclast differentiation in vitro and in vivo [96,97,98,99]. Additionally, IL-10 inhibits osteolysis in periodontitis and bone loss diseases [97,100,101].
A study reported a non-significant increase in IL-10 levels in GCF on day 7 after orthodontic treatment activation, which subsequently dissipated [102]. Moreover, IL-10 cytokine levels in GCF did not differ between patients treated with fixed orthodontics and those treated with Invisalign [103]. Compared with controls, mice treated with IL-1Ra showed decreased OTM and TRAP-positive osteoclasts, as well as increased IL-10 levels in the periodontal tissues [104]. An in vitro experiment showed that compressive force causes M2 polarization of macrophages, which increased IL-10 gene expression, via H3 histone acetylation [105].
2.9. IL-11
IL-11 is a multifunctional cytokine expressed in various tissues and cells, including epithelial cells, osteoclasts, osteoblasts, fibroblasts, hematopoietic cells, mesenchymal stem cells, central nervous system neurons, synovial cells, adipocytes, gastrointestinal tract, and chondrocytes. IL-11 belongs to the IL-6 cytokine family and mainly exists as a quadruplex structure. IL-11 induces various immune activities in both innate and adaptive immunity. IL-11 can directly regulate macrophage activity by inhibiting IL-1β, IL-12, and TNF-α, which are pro-inflammatory cytokines, in vitro. Taken together, IL-11 acts as an anti-inflammatory cytokine by regulating macrophage functions [106,107]. IL-11 is produced by bone marrow stromal cells. Further, IL-11 induces osteoclast formation and indirectly acts on osteoclast precursors. Moreover, IL-11 has been shown to induce bone destruction in a mouse calvarial bone organ culture model [108,109]. IL-11 is involved in several osteolytic bone diseases, such as rheumatoid arthritis and osteoporosis. Both diseases involve significantly increased serum IL-11 levels, which are positively correlated with the levels of bone resorption markers [110,111].
Patients undergoing orthodontic treatment with compressive force showed significantly increased IL-11 levels in the compressed PDL [112]. Another study found that vibration stimulation during OTM increased movement and IL-11 levels compared with the control group [113].
2.10. IL-12
IL-12 is a pro-inflammatory cytokine that is primarily produced by dendritic cells and phagocytes. IL-12 induces the conversion of naive CD4+ T cells to Th1 cells and induces IFN-γ production in Th1 cells [114]. IL-12 increases MHC I and MHC II expression on tumor cells, which promotes their recognition and lysis [115]. IL-12 exerts remarkable antitumor effects dependent on NK cells, NK T cells, and CD8+ T cells [116]. It has two subunits, p35 and p40, which form the p70 heterodimer via three disulfide bonds [117]. IL-12 was found to reduce RANK-induced osteoclast formation and RANKL interaction via a T cell-independent process [118]. Moreover, TNF-α-induced osteoclast formation was inhibited by apoptosis induction through the interaction of IL-12-induced FasL in non-adherent cells with TNF-α-induced Fas in adherent cells in bone marrow cell cultures in vitro [119]. IL-12 can inhibit osteoclast formation in spleen cell cultures in vitro via a T cell-dependent process [120].
In a mouse study on the effect of IL-12 on OTM, IL-12 was locally administered near the first molar every second day. IL-12-treated mice showed reduced OTM and increased apoptotic cells in the pressure area, which suggests that IL-12 attenuates OTM. This phenomenon can be attributed to IL-12-induced apoptosis of osteoclasts [121].
2.11. IL-13
IL-13 is expressed as a preform by granulocytes, including mast cells, basophils, and eosinophils; further, it is involved in immunoglobulin regulation, inflammation, antiparasitic responses, fibrosis, and allergic responses, with IgE being crucially involved [122]. IL-13 acts as a messenger in immune processes, especially in the induction of allergic responses [123]. IL-13 impairs maintenance of barrier function and wound healing [124]. Further, IL-13 exerts protective effects against bone destruction by suppressing bone resorption in mouse calvaria in vivo and inhibiting osteoclast formation by bone marrow macrophages and spleen cells in vitro [125]. IL-13 injection increases osteoprotegerin (OPG) expression and reduces bone destruction in the joints of mice with collagen-induced arthritis [126]. Primary osteoblasts obtained from mouse calvariae that were cultivated in vitro and subjected to micro-pulse vibration showed increased IL-13 levels [52].
In patients with fixed orthodontic appliances, there was a slight increase in IL-13 levels in GCF at 7 days after treatment initiation, which subsequently dissipated [102].
2.12. IL-16
IL-16 is a pro-inflammatory cytokine primarily known for its chemotactic properties, meaning it attracts specific immune cells, including CD4+ lymphocytes, monocytes, and eosinophils, to sites of inflammation or infection [127]. IL-16 directly induces differentiation of monocytes into osteoclasts through JNK/MAPK signaling-dependent activation of NFATc1 [128]. A previous study showed no significant difference between IL-16 levels in GCF between patients who underwent invisible and fixed orthodontic treatment [129].
2.13. IL-17
The IL-17 family, which comprises IL-17A (IL-17), IL-17B, IL-17C, IL-17D, IL-17E, and IL-17F, are crucially involved in regulating inflammation and the immune system [130]. IL-17A (commonly known as IL-17) is the most widely studied cytokine due to its pro-inflammatory actions. IL-17 is primarily produced by T helper 17 (Th17) cells, as well as other immune cells such as gamma-delta T cells, NK cells, NK T cells, macrophages, B cells, innate lymphocytes, mast cells, and neutrophils [131]. IL-17 activates multiple downstream signaling cascades, including ERK1/2, JNK, and p38, as well as NF-κB, STAT3, and Nrf2/keap1 [130]. IL-17 induces the expression of several pro-inflammatory cytokines and chemokines, including IL-1β, IL-6, IL-8, IL-23, TNF-α, CCL4, CCL20, CXCL1, and CXCL12 [132]. Th17 cells exert a direct or indirect osteoclastogenic effect via IL-17-induced activation of osteoclast-related molecules in various target cells. IL-17 treatment of CD14+ cells enhances the expression of osteoclast-related genes, including c-fms, TRAP, and RANK, which leads to an increase in the number of osteoclasts [133]. Furthermore, IL-17 directly promotes osteoclast formation and CD11b+ cell activation by increasing TNF-α and RANKL expression from IL-17-treated monocytes, even in the absence of osteoblasts or exogenous sRANKL [134].
In Wistar rats subjected to orthodontic force, there was a significant increase in IL-17-positive odontoclasts in the jiggling force during OTM [135]. Further, there was IL-17-induced activation of osteoclastogenesis and odontoclastogenesis in the rat OTM model. Taken together, the activation of Th17 cells may exacerbate root resorption through cytokine production upon exertion of excessive orthodontic forces to the PDL [136]. During orthodontic treatment, the levels of 1-25-dihydroxycholecalciferol and salivary cytokine IL-17A showed a negative correlation at various stages of OTM. This suggests that low vitamin D levels may extend the treatment duration and that vitamin D supplementation may be clinically useful in such patients [137]. IL-17 levels in human GCF were significantly up-regulated during tooth movement. Furthermore, IL-17 was positively correlated with MMP-1, MMP-2, MMP-3, MMP-8, MMP-9, and MMP-13 expression [138]. During OTM, there were significantly increased IL-17A and IL-17F levels in the GCF, which were positively correlated with RANKL expression. This suggests that Th17 cytokines are crucially involved in regulating OTM [139].
2.14. IL-18
IL-18, which is a stimulator of IFN-γ production by T cells and NK cells, is a pro-inflammatory cytokine crucially involved in regulating immune responses [140]. IL-18 is primarily expressed by activated macrophages and dendritic cells [141]. IL-18 plays an important role in several biological processes, including cell growth, innate and adaptive immunity, and inflammation regulation [142]. IL-18 induces its biological effects by binding to the IL-18 receptor, which is expressed on various cells, including B cells, T cells, NK cells, and macrophages [143]. Upon binding, it stimulates downstream signaling pathways, including the NF-κB and MAPKs pathways, which induce the synthesis of various cytokines and chemokines [144]. There is decreased RANKL-induced osteoclast formation in the presence of IL-18 in vitro [120]. Moreover, IL-18 inhibited TNF-α-induced osteoclast formation in bone marrow cell cultures through the interaction between TNF-α-induced Fas in adherent cells and IL-18-induced FasL in non-adherent cells [145]. IL-12 and IL-18 have a synergistic inhibitory effect on TNF-α-mediated osteoclast formation [146], which involves upregulation of FasL on non-adherent cells in bone marrow cell cultures [145]. In vivo, this effect was shown to involve a T cell-independent mechanism [147].
There was an increased IL-18 gene expression in the rat experimental tooth movement model [34]. Vibration stimulation has been shown to increase the levels of osteoclast biomarkers such as RANKL and RANKL/OPG, as well as inflammatory markers such as IL-18; moreover, it significantly improved tooth mobility and GCF volume [113]. Rats with ligature-induced periodontal disease with OTM have shown significantly higher IL-18 gene and protein expression than those without OTM. Orthodontic force loading regulates the inflammatory reactions in periodontal disease by up-regulating the production of various pro-inflammatory mediators, including IL-18 and its receptors, which increases bone resorption [148].
2.15. IL-20
IL-20 is a pleiotropic pro-inflammatory cytokine and an IL-10 family member that is produced in endothelial cells, epithelial cells, and monocytes [149]. IL-20 differentially regulates preosteoclast proliferation and apoptosis. Bone mesenchymal stem cells cultured in conditioned medium with IL-20 showed significantly enhanced osteoclast formation and bone resorption. This further demonstrates that IL-20 differentially regulates bone mesenchymal stem cells in osteoclastogenesis and exerts its effect by binding RANKL and RANK, as well as through the NF-κB, MAPK, and AKT signaling pathways [150]. Additionally, IL-20 is associated with periodontitis, with IL-20 contributing to periodontitis through osteoclast formation and collagen degradation [151].
In a rat model of OTM, intraperitoneal injection of IL-20 significantly increased the OTM rate and markedly activated the mechanical stress-sensing protein YAP [152]. Further, IL-20 expression was correlated with osteoclast activity in alveolar bone remodeling associated with OTM. Furthermore, local administration of IL-20 increased osteoclast activity and the OTM distance in rats, with these phenomena being reversed by anti-IL-20 antibody [153].
2.16. IL-23
IL-23 promotes the proliferation of Th17 cells, which express IL-17A, IL-17F, IL-22, IL-26, IFNγ, and TNF-α. Further, IL-23 signaling is significantly involved in the progression of chronic human diseases [154]. Specifically, IL-23 is involved in the pathogenesis of inflammatory arthritis, such as psoriatic arthritis, ankylosing spondylitis, and rheumatoid arthritis, with IL-23 levels in synovial fluid and serum being positively correlated with the severity and disease activity of rheumatoid arthritis. Furthermore, single-nucleotide polymorphisms in genes encoding the IL-23 and IL-23 receptor have been implicated in spondyloarthritis, such as psoriatic arthritis and ankylosing spondylitis [155,156]. IL-23 increases osteoclast formation in mice with collagen-induced arthritis [157].
Patients subjected to orthodontic force showed significantly higher IL-23 levels in the GCF at both the tension and pressure areas than controls. IL-23 levels are positively correlated with RANKL expression [139].
2.17. IL-27
IL-27 is a heterodimer that belongs to the IL-12 cytokine family and is composed of two subunits, IL-27p28 and Epstein–Barr virus-induced gene 3. IL-27 and its receptor are expressed on immune cells, including B cells, T cells, monocytes, macrophages, dendritic cells, and neutrophils, as well as non-immune cells, such as cardiac Sca-1 positive cells and renal tubular epithelial cells. The IL-27 receptor complex comprises glycoprotein 130 and IL-27ra. IL-27 exhibits both anti-inflammatory and pro-inflammatory effects. Regarding anti-inflammatory effects, IL-27 suppresses the proliferation and apoptosis of B cells, macrophages, monocytes, and dendritic cells. Contrastingly, IL-27 has been shown to exert pro-inflammatory effects through dendritic cells and various effector Th cells. Furthermore, given the relationship of IL-27 with inflammatory autoimmune diseases, IL-27 may not only inhibit autoimmunity development but also promote the pathogenesis of autoimmune diseases [158,159]. IL-27 has been shown to inhibit lipopolysaccharide-induced osteolysis in vivo. Notably, IL-27 inhibited RANKL-induced osteoclast formation by inhibiting NF-κB p65 and IκB phosphorylation [160]. Administration of exogenous IL-27 reduced soft tissue abscesses and peri-implant bone resorption during infection; however, this effect was observed in wild-type mice but not in IL-27Rα-/- mice. These results suggest that IL-27 is not essential for immunity but rather mediates redundant immune and bone cell functions during infection [161]. IL-27 significantly inhibits cell surface expression of RANKL and secretion of soluble RANKL by naive CD4+ T cells stimulated by T cell receptor engagement [162].
Compared with controls, orthodontic patients showed significantly higher IL-27 levels in GCF at both tension and compression sides in the early treatment stages; however, there was a negative correlation between IL-27 and RANKL expression during the later stages [139].
2.18. IL-33
There has been increasing evidence regarding the function of the IL-1 superfamily cytokine and damage-associated molecular patterns of IL-33. ST2, which is the receptor for IL-33, is expressed on the surface of various cells. IL-33 is a powerful driver of the type 2 immune response that promotes parasite clearance; moreover, it is involved in inflammatory diseases such as allergies and asthma. At steady state, full-length IL-33 is constitutively expressed in various cell types in human and mouse tissues and is localized to the nucleus. IL-33 has been shown to be produced by endothelial cells of the human vasculature; adventitial stromal cells of mice; epithelial cells of barrier tissues; fibroblastic reticular cells of lymphoid organs; and neurons, glial cells, and astrocytes of the nervous system [163]. IL-33 activation promotes the production of pro-inflammatory cytokines and induces osteoclast formation in periodontitis [164]. IL-33 inhibits RANKL-induced osteoclast formation by regulating Blimp-1 and IRF-8 expression in vitro [165]. In addition, IL-33 inhibited IκB phosphorylation and NF-κB nuclear translocation during TNF-α-induced osteoclast formation and bone resorption [166].
In a mouse model of OTM, IL-33 injection inhibited osteoclast differentiation induced by RANKL from bone marrow stromal cells in vitro. Further, it reduced the number of TRAP-positive cells in the PDL during OTM [167]. High orthodontic stress induces IL-33 expression in periodontal tissues, with IL-33 exerting a negative effect on cementum formation in mice by suppressing cementoblast differentiation, mineralization, and the expression of cementogenesis-related proteins [168].
2.19. IL-34
IL-34 is a pro-inflammatory cytokine that binds to four receptors (CSF-1R, syndecan-1, PTP-ζ, and TREM2). It was first identified as a ligand for the M-CSF receptor. Although there is no sequence homology between IL-34 and CSF-1, they have similar active regions. IL-34 is crucially involved in the onset and progression of various inflammatory diseases by binding to the CSF-1 receptor at the gap between the immunoglobulin-like structural domains D2 and D3 [169,170]. IL-34 inhibited RANKL-induced osteoclast formation in vitro. Moreover, local injections of mouse recombinant IL-34 significantly increased the number of osteoclasts, enhanced alveolar bone loss, and elevated cathepsin K activity in a mouse model of ligature-induced periodontitis. Notably, anti-IL-34 neutralizing monoclonal antibodies reduced the number of osteoclasts and attenuated alveolar bone loss in periodontitis lesions [171]. Serum IL-34 levels are associated with increased disease severity in rheumatoid arthritis. IL-34 is an endogenous factor that repopulates hypermetabolic M34 macrophages and promotes cross-regulation with effector T cells to promote inflammatory bone resorption in rheumatoid arthritis [172]. Wistar rats subjected to orthodontic force showed a significant increase in IL-34-positive odontoclasts during OTM [135].
2.20. IFN-γ
IFN-γ inhibits viral replication in response to stimulation with phytohemagglutinin. It was first identified as a soluble factor secreted by human leukocytes. IFN-γ has antiviral and immunomodulatory properties. IFN-γ directly inhibits several steps of the viral life cycle, including host cell entry, viral gene replication, and viral gene transcription, across several cell types. Regarding immune regulation, IFN-γ promotes Th1 responses in viral infections. Additionally, IFN-γ activates macrophages by stimulating nitric oxide release, enhancing production of reactive oxygen species, and increasing phagocytic activity. Furthermore, IFN-γ promotes antigen presentation and increases the expression of MHC I and II, which are antigen-presenting molecules, on antigen-presenting cells. IFN-γ, which is mainly produced by NK cells and T cells, induces immune cell proliferation and activation, cytokine expression, maturation, and effector function; additionally, it induces tumor growth arrest and apoptosis [173,174,175,176]. IFN-γ is crucially involved in bone metabolism. Specifically, IFN-γ promotes osteoblast differentiation and inhibits bone marrow adipocyte formation. The role of IFN-γ is dependent on the stage of osteoclast formation. In addition, IFN-γ is crucially involved in the pathogenesis of bone diseases, including postmenopausal osteoporosis, rheumatoid arthritis, and acquired immune deficiency syndrome. IFN-γ can enhance local inflammation in gingival tissues. IFN-γ promotes alveolar bone loss and osteoclast differentiation. The truncated tryptophanyl-tRNA synthetase-dependent action of IFN-γ promotes multinucleation of myeloid lineage, which is a crucial step in osteoclastogenesis. IFN-γ directly inhibits TNF-α-induced osteoclast formation in vitro and in vivo and induces apoptosis via the interaction of TNF-α-induced Fas in adherent cells with IFN-γ-induced Fas ligand in non-adherent cells in bone marrow cell culture [177,178,179,180].
We previously showed increased local IFN-γ mRNA expression in PDL during OTM in mice. IFN-γ-administrated mice showed lower tooth movement and osteoclast numbers on the pressure side than phosphate-buffered saline (PBS)-treated control mice. IFN-γ, which is increased in PDL during OTM, may inhibit osteoclast formation and tooth movement induced by mechanical loading [181]. Patients with grade C periodontitis showed decreased IFN-γ levels in GCF and plasma during orthodontic treatment, which may reduce the proportion of IFN-γ-positive Th1 cells [182]. IFN-γ-treated rats whose mandibular first molars were proximally moved using Ni-Ti sealed coil springs showed an increase in Tr.N and BV/TV, as well as a decrease in Tr.Sep, indicating an anti-bone resorption effect of IFN-γ [183]. Tooth movement was significantly reduced in T cell-deficient immunodeficient mice. Wild-type (WT) mice showed an increased number of TRAP-positive cells around the alveolar bone after OTM; however, this was not observed in immunodeficient mice. Taken together, T cells are required for OTM in a manner dependent on Th1-related cytokines such as IFN-γ [184]. Rats administered substance P showed a significant increase in tooth movement, as well as activated osteoclast and osteoblast activity. Finally, IFN-γ levels in peripheral blood showed an increase and decrease in periodontal tissues at the early and later stages, respectively. This demonstrates that systemic administration of substance P accelerates OTM [185].
2.21. TNF-α
TNF-α is an important regulator of inflammatory responses. This cytokine exerts pleiotropic effects on various cell types; further, it has been implicated in the pathogenesis of several inflammatory and autoimmune diseases [186,187]. Structurally, TNF-α is a homotrimeric protein and is primarily produced by activated NK cells, T cells, and macrophages [188,189]. Inflammation through TNF-α-mediated cell death may be beneficial in infection by providing cell-extrinsic signals that help elicit an appropriate immune response [187,190]. Functionally, it triggers the induction of various inflammatory molecules, including other cytokines and chemokines [191]. TNF-α exists in two forms, soluble and transmembrane. Transmembrane TNF-α is initially synthesized, followed by processing by TNF-α-converting enzyme, which is a membrane-bound disintegrin metalloprotease, to release soluble TNF-α [192]. TNF-α mediates osteoclast formation in vitro and in vivo [193]. TNF-α-induced osteoclast recruitment is crucially involved in the pathogenic process of inflammatory diseases [191,194]. TNF-α has been implicated in postmenopausal osteoporosis [195], periodontal diseases [196], and rheumatoid arthritis [197]. There are two cell surface receptors for TNF-α, including TNF receptor type 1 (TNFR1) and TNF receptor type 2 (TNFR2). Binding to these receptors induces biological responses, with each receptor mediating distinct intracellular signals [198]. In vitro analysis of osteoclast formation from bone marrow cells obtained from TNFR1- or TNFR2-deficient mice revealed that TNFR1 and TNFR2 induced and inhibited osteoclast differentiation, respectively [198,199].
OTM increases TNF-α levels in the human gingival sulcus [102,200,201,202,203,204,205,206]. TNF-α is expressed in the periodontal tissues of rats under pathological conditions caused by excessive orthodontic forces [207]. Upon application of tooth movement appliances, TNFR2-deficient mice showed less amount of tooth movement than wild-type mice [208]. These results suggest that TNFR2 is crucially involved in OTM. TNFR1-deficient mice had fewer osteoclasts than wild-type mice [209]. Mice without both TNFR1 and TNFR2 (TNFRsKO) showed a significant reduction in tooth movement, which further confirms the role of TNFRs [210]. A previous study performed OTM with or without injection of docosahexaenoic acid (DHA) in the local site of the gingiva around the mouse tooth; subsequently, the effects of DHA on osteoclast formation at the pressure side of the alveolar bones were examined. GPR120 is a DHA receptor and a lipid sensor. In this study, DHA suppressed OTM in WT mice but not in GPR120-deficient mice. This indicates that DHA attenuates TNF-α-induced osteoclast formation and bone resorption via GPR120. This suggests that TNF-α is crucially involved in OTM. Taken together, DHA may inhibit TNF-α-induced osteoclast recruitment and bone resorption during OTM [211]. After mesial movement of first molars, WT mice showed a significantly higher number of RANK-positive cells on the compression side than TNFRsKO mice. TNF-α directly induces RANKL expression in osteocytes and promotes osteoclast formation [212,213]. The results suggested that TNF-α induces RANK expression in vitro and at baseline in vivo, as well as RANK expression on the compression side during OTM [214]. WT mice showed a significantly higher number of RANKL-positive osteocytes in the alveolar bone after OTM than TNFRsKO mice, which suggests that TNF-α induces RANKL expression in osteocytes during OTM [215]. Compared with 8-week-old mice, 78-week-old mice showed reduced TNF-α-induced bone resorption, osteoclast formation, and calvarial expression of TRAP and cathepsin K. Furthermore, 78-week-old mice showed reduced osteoclast formation and reduced OTM, with the latter being attributed to reduced TNF-α-induced osteoclast formation [216]. Compared with WT mice, TNFRsKO mice showed fewer VEGF-positive cells in the mesial periodontal membrane of the distal buccal root during OTM, as well as reduced VEGF mRNA expression. Taken together, these results suggest that TNF-α plays a crucial role in VEGF expression during OTM [217]. Osteocyte death in TNFRsKO mice was reduced during OTM. Necroptosis markers in osteocytes on the compression side in WT mice were detected, whereas such osteocytes were almost undetectable in TNFRsKO mice. Moreover, the conditioned medium from osteocytes undergoing necroptosis significantly enhanced osteoclast formation. Taken together, TNF-α-induced osteocyte necroptosis promotes osteoclast formation and alveolar bone resorption on the compression side during OTM, and is accompanied by the release of inflammatory factors such as damage-associated molecular patterns (DAMPs) [218].
2.22. M-CSF
M-CSF is a protein constitutively produced by various cells, including macrophages, osteoblasts, lymphocytes, monocytes, endothelial cells, fibroblasts, and osteocytes. It was first identified as a growth factor of hematopoietic cells that induces the differentiation of bone marrow progenitor cells into macrophages [219]. M-CSF is essential for the proliferation and differentiation of osteoclast precursor cells; moreover, it induces the proliferation, differentiation, and survival of macrophages, monocytes, and bone marrow precursor cells [220]. In osteoporotic (op/op) mice, M-CSF deficiency caused by thymidine insertion into the Csf-1 gene reduces osteoclast and macrophage function [221]. Therefore, M-CSF is considered an essential factor for osteoclast formation and activation [222]. Furthermore, administration of the M-CSF receptor c-fms antibody completely inhibited osteoclast formation as well as TNF-α-induced bone destruction and inflammatory arthritis [223]. Furthermore, neutralizing anti-c-fms antibody suppressed osteoclast formation and bone loss compared with that in PBS-treated ovariectomized mice [224].
Local injection of anti-c-fms antibody during mechanical loading significantly inhibited OTM and significantly reduced the number of osteoclasts [210]. Furthermore, M-CSF injection promoted OTM and osteoclast formation [225]. Notably, administration of anti-c-fms antibodies inhibited osteoclast formation in mice as well as reduced orthodontic relapse after OTM [226].
2.23. TGFs
There are two main types of TGFs: TGF-α and TGF-β. The TGF-β superfamily is a large and highly diverse group of structurally related proteins that act as cytokines and growth factors. The BMP subfamily is within the broader TGF-β superfamily. TGF-β regulates both innate and adaptive immune responses. Further, it has several biological functions in cell biology, cancer biology, and the immune response [227,228]. TGF-β is recognized as a key cytokine with a dual role in both immunity and tolerance. TGF-β was originally considered an immunomodulatory cytokine since it inhibits inflammatory cytotoxic cells and Th cells, as well as promotes immunosuppressive Treg cells. However, it was subsequently found to induce Th17 cells, which have both pathogenic and immunoregulatory functions [229]. Another study showed that low TGF-β levels promote osteoclast differentiation, with both M-CSF levels and the RANKL/OPG ratio being higher. Contrastingly, high TGF-β levels suppress the RANKL/OPG ratio since TGF-β inhibits RANKL expression and enhances OPG expression in osteoblasts [230]. Combined with dose-dependent inhibition of M-CSF expression by TGF-β, osteoclast formation is inhibited [231]. B cells have been shown to secrete TGF-β, which inhibits osteoclast formation and shortens the lifespan of mature osteoclasts [232]. Osteoclastogenesis inhibition by TGF-β is primarily achieved by reducing RANKL secretion by osteoblasts; contrastingly, TGF-β significantly increased osteoclastogenesis in hematopoietic cell cultures stimulated with recombinant RANKL or TNF-α [233]. It has been reported that TGF-α increased osteoclast formation by stimulating osteoclast precursors in human marrow culture [234]. The dual effect of BMPs on bone resorption and mineralization highlights the essential role of BMP signaling in bone homeostasis. BMPs are well known to induce osteoblast formation and bone formation. Furthermore, BMPs also induced osteoclast formation and bone resorption [235].
A human study on tooth extraction following OTM showed increased TGF-β expression in both the tension and compression areas [13]. Further, increased TGF-β levels in GCF have been reported during OTM in humans [236]. Notably, low-level laser therapy in OTM did not increase TGF-β1 levels [36]. Bioinformatics analysis of gingival crevicular fluid during OTM demonstrated increased TGF-β levels [237]. In a rat model of OTM, orthodontic force and assisted vibration were applied to the maxillary right first molar, followed by injection of the TGF-β receptor inhibitor SB431542 into the palatal and buccal submucosa. SB431542 inhibited the vibration-induced promotion of tooth movement and an increase in the number of osteoclasts. Further, assisted vibration increased the number of TGF-β-positive bone cells in the compressed alveolar bone during OTM. Taken together, assisted vibration promoted OTM by promoting the production of TGF-β in bone cells and subsequent osteoclast formation [238]. Rats injected with platelet-rich plasma showed less tooth movement compared to the control group on day 3. However, all groups showed maximum tooth movement on day 14, with no significant between-group differences. Further, there were no significant between-group differences in the number of osteoclasts and osteoblasts, as well as in TGF-β, ALP, and TRAP expression [239]. It has been reported that the expression of BMPs increased on the tension side during OTM, stimulating the differentiation of mesenchymal stem cells to osteoblasts [240]. BMP-3 expression is gradually increased on the tension side until day 14 in rodent models of OTM, the mid-stage in OTM [241]. One study examined the effect of BMP2 injection on the pressure and tension side of orthodontic tooth in rats and found that local injection of BMP-2 inhibited OTM. BMP-2 enhanced osteoclast formation, although bone resorption was not dominant during OTM [242].
2.24. RANKL and OPG
RANKL is also termed the osteoclast differentiation factor, TNF-related activation-inducing cytokine, TNF ligand superfamily member 11, and OPG ligand [243]. RANKL is highly produced in activated T lymphocytes, osteoblasts, and osteocytes [244]. RANK has extracellular and intracellular domains, with each containing four cysteine-rich pseudo-repeats at the amino terminus and three TRAF-binding domains at the carboxy terminus [245]. RANK is primarily expressed by osteoclast precursors, mature osteoclasts, and immune cells such as dendritic cells, microglia, and macrophages [246]. Signaling by RANKL-RANK binding activates osteoclast differentiation and function, inhibits osteoclast apoptosis, and induces bone resorption, while the RANKL decoy receptor OPG inhibits RANKL-RANK binding [222]. Osteocytes, which act as mechanosensors, are an important source of RANKL [247]. Notably, OPG expression by osteocytes is significantly reduced in osteocyte-specific B-catenin knockout mice. Additionally, osteocyte OPG-knockout mice exhibit an osteopenic phenotype, indicating that OPG expression in osteocytes is crucially involved in regulating bone mass [248].
RANKL-injected rats showed enhanced OTM, which was consistent with a significant increase in TRAP activity [249]. Similarly, local injection of RANKL in mice increases osteoclast activity and promotes tooth movement [250]. In humans, RANKL and OPG levels in GCF were higher and lower, respectively, in OTM teeth than in control teeth [251]. Similar findings were reported in a rat OTM model, in which two different orthodontic forces were applied to the bilateral buccal expansions around the maxillary second and third molars. The low-force group showed increased sRANKL expression and sRANKL/OPG ratio compared with the normal-force and control groups [53]. Compared with WT mice, OPG-deficient mice exhibited greater tooth movement distances, enhanced alveolar bone resorption, greater osteoclast numbers, and higher serum TRAP levels [252]. Osteocyte-derived RANKL promotes osteoclast formation during OTM [215]. Osteocyte-specific RANKL-deficient mice showed reduced OTM due to inhibition of osteoclast formation in periodontal tissue [253].
3. Summary and Limitations
During OTM, various cytokines play regulatory roles—some promote the process, while others inhibit it. Cytokines such as IL-1, IL-2, IL-6, IL-8, IL-10, IL-11, IL-13, IL-17, IL-18, IL-20, IL-23, IL-27, IL-33, IL-34, IFN-γ, TNF-α, M-CSF, TGF-β, RANKL, and OPG have been identified during OTM through analyses of GCF, immunostaining, and RNA expression. Among these, IL-1, IL-6, IL-20, TNF-α, M-CSF, TGF-β, and RANKL enhance OTM, while IL-4, IL-12, IL-33, IFN-γ, and OPG suppress it. These findings are based on studies involving cytokine administration, cytokine-neutralizing antibody administration, and cytokine-deficient mice. Some of the cytokines discussed in this review promote osteoclastogenesis, including IL-1, IL-6, IL-7, IL-8, IL-11, IL-16, IL-17, IL-20, IL-23, TNF-α, M-CSF, TGF-β, and RANKL. Others inhibit osteoclastogenesis, such as IL-4, IL-5, IL-10, IL-12, IL-13, IL-18, IL-27, IL-33, IL-34, IFN-γ, TGF-β, and OPG. These observations suggest that cytokines that enhance osteoclast formation may promote OTM, while those that inhibit osteoclastogenesis may suppress it (Figure 2).
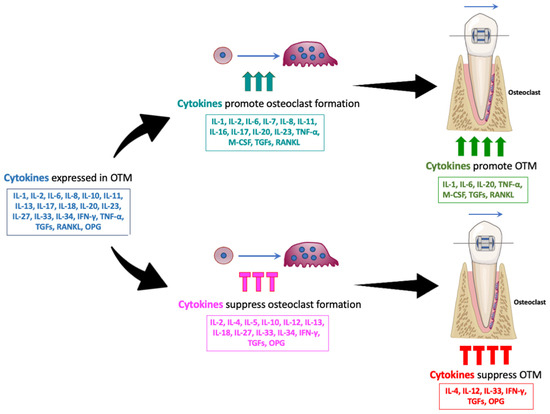
Figure 2.
Various cytokines influence OTM, with some promoting and others suppressing the process. Multiple cytokines, including IL-1, IL-2, IL-6, IL-8, IL-10, IL-11, IL-13, IL-17, IL-18, IL-20, IL-23, IL-27, IL-33, IL-34, IFN-γ, TNF-α, M-CSF, TGFs, RANKL, and OPG, have been identified during OTM through analyses of GCF, immunostaining, and RNA expression. Cytokines such as IL-1, IL-6, IL-7, IL-8, IL-11, IL-16, IL-17, IL-20, IL-23, TNF-α, M-CSF, TGFs, and RANKL promote osteoclastogenesis. Others, including IL-4, IL-5, IL-10, IL-12, IL-13, IL-18, IL-27, IL-33, IL-34, IFN-γ, TGFs, and OPG, inhibit osteoclastogenesis. Among these, IL-1, IL-6, IL-20, TNF-α, M-CSF, TGF-β, and RANKL were reported to promote OTM, while IL-4, IL-12, IL-33, IFN-γ, and OPG were reported to suppress it. These findings suggest that cytokines that enhance osteoclast formation may promote OTM, while those that inhibit osteoclastogenesis may suppress it.
First, there is a limitation in research on the expression of cytokines depending on the strength of the orthodontic force. There are differences in the strength, application method, and duration of the orthodontic force in experimental animals such as mice, rats, rabbits, and dogs. These variables have not been standardized across studies. Therefore, it is unclear how these cytokines interact with each other during different stages of OTM. It is necessary to standardize the animal species, the method of applying the orthodontic force, and the duration, and to examine the expression and effects of cytokines by varying the strength of the orthodontic force. Furthermore, understanding how these cytokines interact with each other during different stages of OTM may provide insight into identifying which of the cytokines to serve as biomarkers in GCF to monitor the progress of orthodontic treatment.
The main limitation of this review is the lack of studies directly investigating the effects of individual cytokines on OTM in humans. Most of the studies that investigated how cytokines affect OTM in this review were animal studies, which allow for experimental manipulations such as administering cytokines, administering antibodies that suppress cytokines, or the use of cytokine-deficient mice. Therefore, studies that directly investigate the effects of cytokines on OTM in humans are also needed. In addition, even in human studies of cytokine expression during orthodontic treatment, there is still variability due to differences in methods used, such as the method of orthodontic force application, location, duration of force application, and patient age. To address this issue, human studies with more standardized and controlled methodologies are needed.
Recent research indicates that epigenetic mechanisms significantly regulate cytokine expression and cellular responses within the PDL during OTM. The differentiation and function of periodontal tissue cells, such as periodontal ligament cells, osteoblasts, osteoclasts, as well as cytokine expression, may be regulated by epigenetic mechanisms, which include histone modification and non-coding RNA [105,254,255]. In particular, the importance of epigenetic regulation in stem cell differentiation and bone homeostasis has been recognized, and these mechanisms are thought to be involved in cytokine expression during OTM [256]. Research into epigenetic regulation of cytokines during OTM will be of great significance in promoting a deeper understanding of the mechanism of tooth movement and improving the efficiency, predictability, and safety of orthodontic treatment. Therefore, epigenetic regulation represents an important direction for future research (Table 1).

Table 1.
Effects of cytokines on osteoclast formation and orthodontic tooth movement.
4. Conclusions
This review discusses the various cytokines involved in OTM, either by suppressing or promoting tooth movement. The influence of cytokines on OTM involves the formation of osteoclasts; i.e., cytokines that inhibit and promote osteoclast formation probably inhibit and promote OTM, respectively. These cytokines are expressed by various cells during OTM; moreover, they act on bone-related cells such as osteoblasts, osteoclasts, and osteocytes. Further studies, including single-cell sequencing analysis, are warranted to further elucidate the role of cytokines in OTM.
Author Contributions
Conceptualization, H.K. (Hideki Kitaura); writing—original draft preparation, H.K. (Hideki Kitaura); writing—review and editing, H.K. (Hideki Kitaura), F.O., A.M., J.M., A.L., Z.F., K.N., K.M. and H.K. (Hiroyasu Kanetaka); funding acquisition, H.K. (Hideki Kitaura). All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported in part by JSPS KAKENHI grants from the Japan Society for the Promotion of Science (No. 22K10236 and 25K02830 to H.K. (Hideki Kitaura)).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available from the corresponding authors upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Li, Y.; Guo, W.; Li, X.; Zhang, J.; Sun, M.; Tang, Z.; Ran, W.; Yang, K.; Huang, G.; Li, L. Expert consensus on the clinical application of recombinant adenovirus human p53 for head and neck cancers. Int. J. Oral Sci. 2021, 13, 38. [Google Scholar] [CrossRef]
- Maltha, J.C.; Kuijpers-Jagtman, A.M. Mechanobiology of orthodontic tooth movement: An update. J. World Fed. Orthod. 2023, 12, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, V.; Davidovitch, Z. Cellular, molecular, and tissue-level reactions to orthodontic force. Am. J. Orthod. Dentofac. Orthop. 2006, 129, 469.e1–469.e32. [Google Scholar] [CrossRef]
- Chaushu, S.; Klein, Y.; Mandelboim, O.; Barenholz, Y.; Fleissig, O. Immune Changes Induced by Orthodontic Forces: A Critical Review. J. Dent. Res. 2022, 101, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, P.M.; Dalstra, M.; Melsen, B. The finite element method: A tool to study orthodontic tooth movement. J. Dent. Res. 2005, 84, 428–433. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Fukasawa, S. Is Inflammation a Friend or Foe for Orthodontic Treatment?: Inflammation in Orthodontically Induced Inflammatory Root Resorption and Accelerating Tooth Movement. Int. J. Mol. Sci. 2021, 22, 2388. [Google Scholar] [CrossRef]
- Yu, Q.Y.; Huang, Y.P.; Li, W.R. Extracellular Matrix Remodelling of the Periodontium under Orthodontic Force. Chin. J. Dent. Res. 2024, 27, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Min, Q.; Li, X.; Liu, L.; Lv, Y.; Xu, W.; Liu, X.; Wang, H. Immune System Acts on Orthodontic Tooth Movement: Cellular and Molecular Mechanisms. BioMed Res. Int. 2022, 2022, 9668610. [Google Scholar] [CrossRef]
- Teitelbaum, S.L. Bone resorption by osteoclasts. Science 2000, 289, 1504–1508. [Google Scholar] [CrossRef]
- Kobayashi, K.; Takahashi, N.; Jimi, E.; Udagawa, N.; Takami, M.; Kotake, S.; Nakagawa, N.; Kinosaki, M.; Yamaguchi, K.; Shima, N.; et al. Tumor necrosis factor alpha stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL-RANK interaction. J. Exp. Med. 2000, 191, 275–286. [Google Scholar] [CrossRef]
- Liu, C.; Chu, D.; Kalantar-Zadeh, K.; George, J.; Young, H.A.; Liu, G. Cytokines: From Clinical Significance to Quantification. Adv. Sci. 2021, 8, e2004433. [Google Scholar] [CrossRef] [PubMed]
- Borish, L.C.; Steinke, J.W. 2. Cytokines and chemokines. J. Allergy Clin. Immunol. 2003, 111 (Suppl. S2), S460–S475. [Google Scholar] [CrossRef] [PubMed]
- Garlet, T.P.; Coelho, U.; Silva, J.S.; Garlet, G.P. Cytokine expression pattern in compression and tension sides of the periodontal ligament during orthodontic tooth movement in humans. Eur. J. Oral Sci. 2007, 115, 355–362. [Google Scholar] [CrossRef]
- Yamaguchi, M. RANK/RANKL/OPG during orthodontic tooth movement. Orthod. Craniofacial Res. 2009, 12, 113–119. [Google Scholar] [CrossRef]
- Rauner, M.; Sipos, W.; Pietschmann, P. Osteoimmunology. Int. Arch. Allergy Immunol. 2007, 143, 31–48. [Google Scholar] [CrossRef]
- Danz, J.C.; Degen, M. Selective modulation of the bone remodeling regulatory system through orthodontic tooth movement—A review. Front. Oral Health 2025, 6, 1472711. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.J.; Macari, S.; Coimbra, C.C.; Pereira, T.; Barrioni, B.R.; Gomez, R.S.; Silva, T.A.; Paiva, S.M. Aerobic and resistance training improve alveolar bone quality and interferes with bone-remodeling during orthodontic tooth movement in mice. Bone 2020, 138, 115496. [Google Scholar] [CrossRef]
- Dawalibi, A.; Alosaimi, A.A.; Mohammad, K.S. Balancing the Scales: The Dual Role of Interleukins in Bone Metastatic Microenvironments. Int. J. Mol. Sci. 2024, 25, 8163. [Google Scholar] [CrossRef]
- Han, P.; Liu, X.; He, J.; Han, L.; Li, J. Overview of mechanisms and novel therapies on rheumatoid arthritis from a cellular perspective. Front. Immunol. 2024, 15, 1461756. [Google Scholar] [CrossRef]
- Patel, J.P.; Konanur Srinivasa, N.K.; Gande, A.; Anusha, M.; Dar, H.; Baji, D.B. The Role of Biologics in Rheumatoid Arthritis: A Narrative Review. Cureus 2023, 15, e33293. [Google Scholar] [CrossRef]
- Ramadan, D.E.; Hariyani, N.; Indrawati, R.; Ridwan, R.D.; Diyatri, I. Cytokines and Chemokines in Periodontitis. Eur. J. Dent. 2020, 14, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Jin, H.M.; Kim, K.; Song, I.; Youn, B.U.; Matsuo, K.; Kim, N. The mechanism of osteoclast differentiation induced by IL-1. J. Immunol. 2009, 183, 1862–1870. [Google Scholar] [CrossRef]
- Fox, S.W.; Fuller, K.; Chambers, T.J. Activation of osteoclasts by interleukin-1: Divergent responsiveness in osteoclasts formed in vivo and in vitro. J. Cell Physiol. 2000, 184, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Dipalma, G.; Inchingolo, A.D.; Fiore, A.; Balestriere, L.; Nardelli, P.; Casamassima, L.; Di Venere, D.; Palermo, A.; Inchingolo, F.; Inchingolo, A.M. The Differential Impact of Clear Aligners and Fixed Orthodontic Appliances on Periodontal Health: A Systematic Review. Children 2025, 12, 138. [Google Scholar] [CrossRef]
- Motyl, S.; Manfredini, D.; Oruba, Z.; Bugajska, J.; Sztefko, K.; Stos, W.; Osiewicz, M.; Loster, B.W.; Lobbezoo, F. Evaluation of interleukin-1 beta and the ratio of interleukin-1 beta to interleukin-1 receptor antagonist in gingival crevicular fluid during orthodontic canine retraction. Dent. Med. Probl. 2021, 58, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Soares Bonato, R.C.; Abel Mapengo, M.A.; de Azevedo-Silva, L.J.; Janson, G.; de Carvalho Sales-Peres, S.H. Tooth movement, orofacial pain, and leptin, interleukin-1beta, and tumor necrosis factor-alpha levels in obese adolescents. Angle Orthod. 2022, 92, 95–100. [Google Scholar] [CrossRef]
- Dinarello, C.A. Interleukin-1 and its biologically related cytokines. Adv. Immunol. 1989, 44, 153–205. [Google Scholar] [CrossRef]
- d’Apuzzo, F.; Cappabianca, S.; Ciavarella, D.; Monsurro, A.; Silvestrini-Biavati, A.; Perillo, L. Biomarkers of periodontal tissue remodeling during orthodontic tooth movement in mice and men: Overview and clinical relevance. Sci. World J. 2013, 2013, 105873. [Google Scholar] [CrossRef]
- Davidovitch, Z.; Nicolay, O.F.; Ngan, P.W.; Shanfeld, J.L. Neurotransmitters, cytokines, and the control of alveolar bone remodeling in orthodontics. Dent. Clin. N. Am. 1988, 32, 411–435. [Google Scholar] [CrossRef]
- Lee, K.J.; Park, Y.C.; Yu, H.S.; Choi, S.H.; Yoo, Y.J. Effects of continuous and interrupted orthodontic force on interleukin-1beta and prostaglandin E2 production in gingival crevicular fluid. Am. J. Orthod. Dentofac. Orthop. 2004, 125, 168–177. [Google Scholar] [CrossRef]
- Iwasaki, L.R.; Haack, J.E.; Nickel, J.C.; Reinhardt, R.A.; Petro, T.M. Human interleukin-1 beta and interleukin-1 receptor antagonist secretion and velocity of tooth movement. Arch. Oral Biol. 2001, 46, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Varella, A.M.; Revankar, A.V.; Patil, A.K. Low-level laser therapy increases interleukin-1beta in gingival crevicular fluid and enhances the rate of orthodontic tooth movement. Am. J. Orthod. Dentofac. Orthop. 2018, 154, 535–544.e5. [Google Scholar] [CrossRef]
- Bletsa, A.; Berggreen, E.; Brudvik, P. Interleukin-1alpha and tumor necrosis factor-alpha expression during the early phases of orthodontic tooth movement in rats. Eur. J. Oral Sci. 2006, 114, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, C.C.; Khoo, E.; Tran, J.; Chartres, I.; Liu, Y.; Thant, L.M.; Khabensky, I.; Gart, L.P.; Cisneros, G.; Alikhani, M. Cytokine expression and accelerated tooth movement. J. Dent. Res. 2010, 89, 1135–1141. [Google Scholar] [CrossRef]
- Kumar, B.D.; Singh, N.; Verma, S.K.; Singh, S.; Thakur, S. A Study to Evaluate Il1 And Il6 Gingival Crevicular Fluid Levels in Adolescents and Young Adults During the Early Phase of Orthodontic Tooth Movement. J. Pharm. Bioallied Sci. 2022, 14, S494–S497. [Google Scholar] [CrossRef]
- Reis, C.L.B.; de Souza Furtado, T.C.; Mendes, W.D.; Matsumoto, M.A.N.; Alves, S.Y.F.; Stuani, M.B.S.; Borsatto, M.C.; Corona, S.A.M. Photobiomodulation impacts the levels of inflammatory mediators during orthodontic tooth movement? A systematic review with meta-analysis. Lasers Med. Sci. 2022, 37, 771–787. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, A.V.B.; Marcantonio, C.C.; de Molon, R.S.; Leguizamon, N.D.P.; Silva, R.C.L.; Deschner, J.; Cerri, P.S.; Cirelli, J.A. Experimental models of orthodontic tooth movement and their effects on periodontal tissues remodelling. Arch. Oral Biol. 2021, 130, 105216. [Google Scholar] [CrossRef]
- Chen, L.; Hua, Y. Autophagy of periodontal ligament inhibits inflammation and reduces the decline of bone density during orthodontic tooth movement of mice. Arch. Oral Biol. 2021, 121, 104960. [Google Scholar] [CrossRef]
- de Mello-Neto, J.M.; Elangovan, G.; Ervolino, E.; Johnson, N.W.; Gustafsson, A.; da Silva Figueredo, C.M. Higher expression of Th1/Th2-related cytokines in the intestine of Wistar rats with ligature-induced periodontitis. J. Periodontal Res. 2023, 58, 588–595. [Google Scholar] [CrossRef]
- Sun, H.; Zhao, Y.; Wang, K.; Zhu, L.; Dong, J.; Zhao, J.; Wang, Y.; Li, H.; Sun, X.; Lu, Y. Low dose IL-2 suppress osteoclastogenesis in collagen-induced arthritis via JNK dependent pathway. Immun. Inflamm. Dis. 2020, 8, 727–735. [Google Scholar] [CrossRef]
- Wang, X.; Feng, C. The Association between IL2 Genotypes and Risk and Severity of Chronic Periodontitis in a Chinese Han Population: A Case-control Study. Immunol. Invest. 2022, 51, 924–930. [Google Scholar] [CrossRef] [PubMed]
- Basaran, G.; Ozer, T.; Kaya, F.A.; Hamamci, O. Interleukins 2, 6, and 8 levels in human gingival sulcus during orthodontic treatment. Am. J. Orthod. Dentofac. Orthop. 2006, 130, 7.e1–7.e6. [Google Scholar] [CrossRef]
- Pan, K.; Li, Q.; Guo, Z.; Li, Z. Healing action of Interleukin-4 (IL-4) in acute and chronic inflammatory conditions: Mechanisms and therapeutic strategies. Pharmacol. Ther. 2025, 265, 108760. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.A.; Hural, J. Functions of IL-4 and Control of Its Expression. Crit. Rev. Immunol. 2017, 37, 181–212. [Google Scholar] [CrossRef] [PubMed]
- Iwaszko, M.; Bialy, S.; Bogunia-Kubik, K. Significance of Interleukin (IL)-4 and IL-13 in Inflammatory Arthritis. Cells 2021, 10, 3000. [Google Scholar] [CrossRef]
- Lima Teixeira, J.F.; Henning, P.; Cintra Magalhaes, F.A.; Coletto-Nunes, G.; Floriano-Marcelino, T.; Westerlund, A.; Moverare-Skrtic, S.; Oliveira, G.; Lerner, U.H.; Souza, P.P.C. Osteoprotective effect by interleukin-4 (IL-4) on lipoprotein-induced periodontitis. Cytokine 2023, 172, 156399. [Google Scholar] [CrossRef]
- Zhou, P.; Zheng, T.; Zhao, B. Cytokine-mediated immunomodulation of osteoclastogenesis. Bone 2022, 164, 116540. [Google Scholar] [CrossRef]
- Kitaura, H.; Nagata, N.; Fujimura, Y.; Hotokezaka, H.; Tatamiya, M.; Nakao, N.; Yoshida, N.; Nakayama, K. Interleukin-4 directly inhibits tumor necrosis factor-alpha-mediated osteoclast formation in mouse bone marrow macrophages. Immunol. Lett. 2003, 88, 193–198. [Google Scholar] [CrossRef]
- Fujii, T.; Kitaura, H.; Kimura, K.; Hakami, Z.W.; Takano-Yamamoto, T. IL-4 inhibits TNF-alpha-mediated osteoclast formation by inhibition of RANKL expression in TNF-alpha-activated stromal cells and direct inhibition of TNF-alpha-activated osteoclast precursors via a T-cell-independent mechanism in vivo. Bone 2012, 51, 771–780. [Google Scholar] [CrossRef]
- Hakami, Z.; Kitaura, H.; Kimura, K.; Ishida, M.; Sugisawa, H.; Ida, H.; Jafari, S.; Takano-Yamamoto, T. Effect of interleukin-4 on orthodontic tooth movement and associated root resorption. Eur. J. Orthod. 2015, 37, 87–94. [Google Scholar] [CrossRef]
- Wu, M.; Liu, J. Inhibitory effect of exogenous IL-4 on orthodontic relapse in rats. Oral Dis. 2022, 28, 469–479. [Google Scholar] [CrossRef]
- Garcia-Lopez, S.; Villanueva, R.E.; Masso-Rojas, F.; Paez-Arenas, A.; Meikle, M.C. Micro-vibrations at 30 Hz on bone cells cultivated in vitro produce soluble factors for osteoclast inhibition and osteoblast activity. Arch. Oral Biol. 2020, 110, 104594. [Google Scholar] [CrossRef]
- Danz, J.C.; Kantarci, A.; Bornstein, M.M.; Katsaros, C.; Stavropoulos, A. Impact of Orthodontic Forces on Plasma Levels of Markers of Bone Turnover and Inflammation in a Rat Model of Buccal Expansion. Front. Physiol. 2021, 12, 637606. [Google Scholar] [CrossRef] [PubMed]
- Omata, Y.; Frech, M.; Saito, T.; Schett, G.; Zaiss, M.M.; Tanaka, S. Inflammatory Arthritis and Bone Metabolism Regulated by Type 2 Innate and Adaptive Immunity. Int. J. Mol. Sci. 2022, 23, 1104. [Google Scholar] [CrossRef] [PubMed]
- Andreev, D.; Kachler, K.; Liu, M.; Chen, Z.; Krishnacoumar, B.; Ringer, M.; Frey, S.; Kronke, G.; Voehringer, D.; Schett, G.; et al. Eosinophils preserve bone homeostasis by inhibiting excessive osteoclast formation and activity via eosinophil peroxidase. Nat. Commun. 2024, 15, 1067. [Google Scholar] [CrossRef] [PubMed]
- Grant, M.; Wilson, J.; Rock, P.; Chapple, I. Induction of cytokines, MMP9, TIMPs, RANKL and OPG during orthodontic tooth movement. Eur. J. Orthod. 2013, 35, 644–651. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Pandolfi, F.; Franza, L.; Carusi, V.; Altamura, S.; Andriollo, G.; Nucera, E. Interleukin-6 in Rheumatoid Arthritis. Int. J. Mol. Sci. 2020, 21, 5238. [Google Scholar] [CrossRef]
- Hirano, T. IL-6 in inflammation, autoimmunity and cancer. Int. Immunol. 2021, 33, 127–148. [Google Scholar] [CrossRef]
- Tyrrell, D.J.; Goldstein, D.R. Ageing and atherosclerosis: Vascular intrinsic and extrinsic factors and potential role of IL-6. Nat. Rev. Cardiol. 2021, 18, 58–68. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, F.; Hou, L. IL-6 and diabetic kidney disease. Front. Immunol. 2024, 15, 1465625. [Google Scholar] [CrossRef] [PubMed]
- Grebenciucova, E.; VanHaerents, S. Interleukin 6: At the interface of human health and disease. Front. Immunol. 2023, 14, 1255533. [Google Scholar] [CrossRef]
- Kang, S.; Narazaki, M.; Metwally, H.; Kishimoto, T. Historical overview of the interleukin-6 family cytokine. J. Exp. Med. 2020, 217, e20190347. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; He, C. TNF-alpha and IL-6: The Link between Immune and Bone System. Curr. Drug Targets 2020, 21, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Udagawa, N.; Takahashi, N.; Katagiri, T.; Tamura, T.; Wada, S.; Findlay, D.M.; Martin, T.J.; Hirota, H.; Taga, T.; Kishimoto, T.; et al. Interleukin (IL)-6 induction of osteoclast differentiation depends on IL-6 receptors expressed on osteoblastic cells but not on osteoclast progenitors. J. Exp. Med. 1995, 182, 1461–1468. [Google Scholar] [CrossRef] [PubMed]
- Devlin, R.D.; Reddy, S.V.; Savino, R.; Ciliberto, G.; Roodman, G.D. IL-6 mediates the effects of IL-1 or TNF, but not PTHrP or 1,25(OH)2D3, on osteoclast-like cell formation in normal human bone marrow cultures. J. Bone Min. Res. 1998, 13, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.Y.; Wu, H.K.; Chen, Y.H.; Hsu, Y.P.; Cheng, M.T.; Yu, C.H.; Chen, S.K. Interleukin-6 transiently promotes proliferation of osteoclast precursors and stimulates the production of inflammatory mediators. Mol. Biol. Rep. 2022, 49, 3927–3937. [Google Scholar] [CrossRef]
- Jayaprakash, P.K.; Basavanna, J.M.; Grewal, H.; Modi, P.; Sapawat, P.; Bohara, P.D. Elevated levels of Interleukin (IL)-1beta, IL-6, tumor necrosis factor-alpha, epidermal growth factor, and beta2-microglobulin levels in gingival crevicular fluid during human Orthodontic tooth movement (OTM). J. Fam. Med. Prim. Care 2019, 8, 1602–1606. [Google Scholar] [CrossRef]
- Rath-Deschner, B.; Nogueira, A.V.B.; Beisel-Memmert, S.; Nokhbehsaim, M.; Eick, S.; Cirelli, J.A.; Deschner, J.; Jager, A.; Damanaki, A. Interaction of periodontitis and orthodontic tooth movement—An in vitro and in vivo study. Clin. Oral Investig. 2022, 26, 171–181. [Google Scholar] [CrossRef]
- Uzun, M.; Cesur, M.G.; Erdogan, O. Evaluation of the effects of obesity on orthodontic tooth movement. Korean J. Orthod. 2025, 55, 3–14. [Google Scholar] [CrossRef]
- Altindal, D.; Tunca, Y.; Tunca, M. Evaluation of IL-8 and IL-6 levels in gingival crevicular fluid of individuals undergoing clear aligner therapy. Angle Orthod. 2025, 95, 212–218. [Google Scholar] [CrossRef]
- Toyama, N.; Ono, T.; Ono, T.; Nakashima, T. The interleukin-6 signal regulates orthodontic tooth movement and pain. Biochem. Biophys. Res. Commun. 2023, 684, 149068. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Li, Z.; Liu, L.; Zhao, J.; Ge, W.; Zhang, K.; Zhou, Z.; Liu, Y. Static magnetic field-induced IL-6 secretion in periodontal ligament stem cells accelerates orthodontic tooth movement. Sci. Rep. 2024, 14, 9851. [Google Scholar] [CrossRef]
- Dwivedi, P.; Agrawal, A.; Gupta, S.C.; Chou, T. The Effect of Varied Time Interval and Micro-Current (Direct) on the Level of Biomarker (IL-6) and Rate of Tooth Movement: An Animal Study. Indian. J. Dent. Res. 2023, 34, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.Y.; Li, S.Q.; Jia, X.; Wu, M.Y.; Song, B.; Li, J.Y.; Guo, Y.; Gao, R. Expression patterns of interleukin-6 and microRNA-146A during orthodontic relapse in a rat model. J. Physiol. Pharmacol. 2024, 75, 447–456. [Google Scholar]
- Winer, H.; Rodrigues, G.O.L.; Hixon, J.A.; Aiello, F.B.; Hsu, T.C.; Wachter, B.T.; Li, W.; Durum, S.K. IL-7: Comprehensive review. Cytokine 2022, 160, 156049. [Google Scholar] [CrossRef]
- Toraldo, G.; Roggia, C.; Qian, W.P.; Pacifici, R.; Weitzmann, M.N. IL-7 induces bone loss in vivo by induction of receptor activator of nuclear factor kappa B ligand and tumor necrosis factor alpha from T cells. Proc. Natl. Acad. Sci. USA 2003, 100, 125–130. [Google Scholar] [CrossRef]
- Xu, H.; Cai, L.; Li, Z.; Zhang, L.; Wang, G.; Xie, R.; Jiang, Y.; Yuan, Y.; Nie, H. Dual effect of IL-7/IL-7R signalling on the osteoimmunological system: A potential therapeutic target for rheumatoid arthritis. Immunology 2021, 164, 161–172. [Google Scholar] [CrossRef]
- Kim, S.J.; Chang, H.J.; Volin, M.V.; Umar, S.; Van Raemdonck, K.; Chevalier, A.; Palasiewicz, K.; Christman, J.W.; Volkov, S.; Arami, S.; et al. Macrophages are the primary effector cells in IL-7-induced arthritis. Cell Mol. Immunol. 2020, 17, 728–740. [Google Scholar] [CrossRef]
- Sato, T.; Watanabe, K.; Masuhara, M.; Hada, N.; Hakeda, Y. Production of IL-7 is increased in ovariectomized mice, but not RANKL mRNA expression by osteoblasts/stromal cells in bone, and IL-7 enhances generation of osteoclast precursors in vitro. J. Bone Min. Metab. 2007, 25, 19–27. [Google Scholar] [CrossRef]
- Chami, V.O.; Nunes, L.; Capelli Junior, J. Expression of cytokines in gingival crevicular fluid associated with tooth movement induced by aligners: A pilot study. Dent. Press. J. Orthod. 2018, 23, 41–46. [Google Scholar] [CrossRef]
- Matsushima, K.; Yang, D.; Oppenheim, J.J. Interleukin-8: An evolving chemokine. Cytokine 2022, 153, 155828. [Google Scholar] [CrossRef] [PubMed]
- Park, S.E.; Park, K.; Kim, E.; Kim, C.Y.; Hwang, S.M.; Lee, J.M.; Suh, J.Y.; Lee, Y.; Kim, M.O.; Kim, Y.G. CXCL5/CXCL8 induces neutrophilic inflammation in peri-implantitis. J. Periodontal Res. 2024, 59, 698–711. [Google Scholar] [CrossRef]
- Cambier, S.; Gouwy, M.; Proost, P. The chemokines CXCL8 and CXCL12: Molecular and functional properties, role in disease and efforts towards pharmacological intervention. Cell Mol. Immunol. 2023, 20, 217–251. [Google Scholar] [CrossRef] [PubMed]
- Fousek, K.; Horn, L.A.; Palena, C. Interleukin-8: A chemokine at the intersection of cancer plasticity, angiogenesis, and immune suppression. Pharmacol. Ther. 2021, 219, 107692. [Google Scholar] [CrossRef]
- Malhotra, H.; Garg, V.; Singh, G. Biomarker Approach Towards Rheumatoid Arthritis Treatment. Curr. Rheumatol. Rev. 2021, 17, 162–175. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, Z.; Lan, T.; Liang, P.; Tao, Q. Upregulation of interleukin-8 and activin A induces osteoclastogenesis in ameloblastoma. Int. J. Mol. Med. 2019, 43, 2329–2340. [Google Scholar] [CrossRef]
- Nassar, E.A.; Almasoud, N.N.; Al-Qurashi, M.S.; Alsulaiman, A.A.; Hassan, K.S. An Evaluation of Microbial Flora, Alkaline Phosphatase and IL-8 Levels in GCF of Orthodontic Patients with Self-Ligating and Conventional Brackets. Clin. Cosmet. Investig. Dent. 2021, 13, 343–352. [Google Scholar] [CrossRef]
- Asano, M.; Yamaguchi, M.; Nakajima, R.; Fujita, S.; Utsunomiya, T.; Yamamoto, H.; Kasai, K. IL-8 and MCP-1 induced by excessive orthodontic force mediates odontoclastogenesis in periodontal tissues. Oral Dis. 2011, 17, 489–498. [Google Scholar] [CrossRef]
- Yang, J.H.; Li, Z.C.; Kong, W.D.; Zhang, W.; Jia, Y.P.; Zhang, Y.L.; Liu, L.B.; Han, X.P. Effect of orthodontic force on inflammatory periodontal tissue remodeling and expression of IL-6 and IL-8 in rats. Asian Pac. J. Trop. Med. 2013, 6, 757–761. [Google Scholar] [CrossRef]
- Fernandes, M.R.U.; Suzuki, S.S.; Suzuki, H.; Martinez, E.F.; Garcez, A.S. Photobiomodulation increases intrusion tooth movement and modulates IL-6, IL-8 and IL-1beta expression during orthodontically bone remodeling. J. Biophotonics 2019, 12, e201800311. [Google Scholar] [CrossRef] [PubMed]
- Tuncer, B.B.; Ozmeric, N.; Tuncer, C.; Teoman, I.; Cakilci, B.; Yucel, A.; Alpar, R.; Balos, K. Levels of interleukin-8 during tooth movement. Angle Orthod. 2005, 75, 631–636. [Google Scholar]
- Saraiva, M.; Vieira, P.; O’Garra, A. Biology and therapeutic potential of interleukin-10. J. Exp. Med. 2020, 217, e20190418. [Google Scholar] [CrossRef]
- Nagata, K.; Nishiyama, C. IL-10 in Mast Cell-Mediated Immune Responses: Anti-Inflammatory and Proinflammatory Roles. Int. J. Mol. Sci. 2021, 22, 4972. [Google Scholar] [CrossRef] [PubMed]
- Rasquinha, M.T.; Sur, M.; Lasrado, N.; Reddy, J. IL-10 as a Th2 Cytokine: Differences Between Mice and Humans. J. Immunol. 2021, 207, 2205–2215. [Google Scholar] [CrossRef]
- Gao, X.; Ge, J.; Zhou, W.; Xu, L.; Geng, D. IL-10 inhibits osteoclast differentiation and osteolysis through MEG3/IRF8 pathway. Cell. Signal. 2022, 95, 110353. [Google Scholar] [CrossRef]
- Rios-Arce, N.D.; Dagenais, A.; Feenstra, D.; Coughlin, B.; Kang, H.J.; Mohr, S.; McCabe, L.R.; Parameswaran, N. Loss of interleukin-10 exacerbates early Type-1 diabetes-induced bone loss. J. Cell Physiol. 2020, 235, 2350–2365. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wan, Z.; Yang, L.; Song, S.; Fu, Z.; Tang, K.; Chen, L.; Song, Y. Exosomes derived from reparative M2-like macrophages prevent bone loss in murine periodontitis models via IL-10 mRNA. J. Nanobiotechnology 2022, 20, 110. [Google Scholar] [CrossRef]
- Zhu, Z.; Chen, G.; Yu, S.; Huang, X.; Lu, X.; Feng, G.; Yi, M.; Wang, J.; Liu, Y.; Chen, L. Circadian clock disruption stimulates bone loss via regulatory T cell-Mediated regulation of IL-10 expression. Int. Immunopharmacol. 2024, 139, 112589. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, B.; Yan, F.; Guo, J.; Zhu, X.; Ma, S.; Yang, W. Interleukin-10 inhibits bone resorption: A potential therapeutic strategy in periodontitis and other bone loss diseases. Biomed. Res. Int. 2014, 2014, 284836. [Google Scholar] [CrossRef]
- Tzaneti, A.; Athanasopoulou, E.; Fessatou, S.; Fotis, L. Chronic Nonbacterial Osteomyelitis in Inflammatory Bowel Disease. Life 2023, 13, 2347. [Google Scholar] [CrossRef] [PubMed]
- Nunes, L.; Quintanilha, L.; Perinetti, G.; Capelli, J.J. Effect of orthodontic force on expression levels of ten cytokines in gingival crevicular fluid. Arch. Oral Biol. 2017, 76, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Alnazeh, A.A.; Kamran, M.A.; Aseeri, Y.; Alrwuili, M.R.; Aljabab, M.A.; Baig, E.A.; Hameed, M.S. Levels of Inflammatory and Bone Metabolic Markers in the Gingival Crevicular Fluid of Individuals Undergoing Fixed Orthodontic Treatment in Comparison to Those Utilizing Invisalign. Medicina 2023, 59, 2107. [Google Scholar] [CrossRef]
- Salla, J.T.; Taddei, S.R.; Queiroz-Junior, C.M.; Andrade Junior, I.; Teixeira, M.M.; Silva, T.A. The effect of IL-1 receptor antagonist on orthodontic tooth movement in mice. Arch. Oral Biol. 2012, 57, 519–524. [Google Scholar] [CrossRef]
- Wang, Y.; Groeger, S.; Yong, J.; Ruf, S. Orthodontic Compression Enhances Macrophage M2 Polarization via Histone H3 Hyperacetylation. Int. J. Mol. Sci. 2023, 24, 3117. [Google Scholar] [CrossRef] [PubMed]
- Fung, K.Y.; Louis, C.; Metcalfe, R.D.; Kosasih, C.C.; Wicks, I.P.; Griffin, M.D.W.; Putoczki, T.L. Emerging roles for IL-11 in inflammatory diseases. Cytokine 2022, 149, 155750. [Google Scholar] [CrossRef]
- Cook, S.A. Understanding interleukin 11 as a disease gene and therapeutic target. Biochem. J. 2023, 480, 1987–2008. [Google Scholar] [CrossRef]
- Han, Y.; Gao, H.; Gan, X.; Liu, J.; Bao, C.; He, C. Roles of IL-11 in the regulation of bone metabolism. Front. Endocrinol. 2023, 14, 1290130. [Google Scholar] [CrossRef]
- Dong, B.; Zhu, J.; Chen, X.; Jiang, H.; Deng, Y.; Xu, L.; Wang, Y.; Li, S. The Emerging Role of Interleukin-(IL)-11/IL-11R in Bone Metabolism and Homeostasis: From Cytokine to Osteokine. Aging Dis. 2023, 14, 2113–2126. [Google Scholar] [CrossRef]
- Zou, R.; Huang, X.; Xu, P. The study of gp130/the inflammatory factors regulating osteoclast differentiation in rheumatoid arthritis. Biochem. Biophys. Rep. 2021, 26, 100934. [Google Scholar] [CrossRef]
- Tuerlings, M.; van Hoolwerff, M.; Houtman, E.; Suchiman, E.; Lakenberg, N.; Mei, H.; van der Linden, E.; Nelissen, R.; Ramos, Y.; Coutinho de Almeida, R.; et al. RNA Sequencing Reveals Interacting Key Determinants of Osteoarthritis Acting in Subchondral Bone and Articular Cartilage: Identification of IL11 and CHADL as Attractive Treatment Targets. Arthritis Rheumatol. 2021, 73, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zheng, W.; Liu, J.S.; Wang, J.; Yang, P.; Li, M.L.; Zhao, Z.H. Expression of osteoclastogenesis inducers in a tissue model of periodontal ligament under compression. J. Dent. Res. 2011, 90, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Xu, C.; Li, Y.; Hao, C.; Zheng, J.; Jin, X.; Yu, J.; Zhu, Y.; Guan, Z.; Yin, Q. The gingival crevicular fluid biomarkers with micropulse vibration device: A pilot study. Heliyon 2024, 10, e31982. [Google Scholar] [CrossRef]
- Ullrich, K.A.; Schulze, L.L.; Paap, E.M.; Muller, T.M.; Neurath, M.F.; Zundler, S. Immunology of IL-12: An update on functional activities and implications for disease. EXCLI J. 2020, 19, 1563–1589. [Google Scholar] [CrossRef]
- Nguyen, K.G.; Vrabel, M.R.; Mantooth, S.M.; Hopkins, J.J.; Wagner, E.S.; Gabaldon, T.A.; Zaharoff, D.A. Localized Interleukin-12 for Cancer Immunotherapy. Front. Immunol. 2020, 11, 575597. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, E.; Carson, W.E., 3rd. Analysis of potential biomarkers of response to IL-12 therapy. J. Leukoc. Biol. 2022, 112, 557–567. [Google Scholar] [CrossRef]
- Floss, D.M.; Moll, J.M.; Scheller, J. IL-12 and IL-23-Close Relatives with Structural Homologies but Distinct Immunological Functions. Cells 2020, 9, 2184. [Google Scholar] [CrossRef]
- Nagata, N.; Kitaura, H.; Yoshida, N.; Nakayama, K. Inhibition of RANKL-induced osteoclast formation in mouse bone marrow cells by IL-12: Involvement of IFN-gamma possibly induced from non-T cell population. Bone 2003, 33, 721–732. [Google Scholar] [CrossRef]
- Kitaura, H.; Nagata, N.; Fujimura, Y.; Hotokezaka, H.; Yoshida, N.; Nakayama, K. Effect of IL-12 on TNF-alpha-mediated osteoclast formation in bone marrow cells: Apoptosis mediated by Fas/Fas ligand interaction. J. Immunol. 2002, 169, 4732–4738. [Google Scholar] [CrossRef]
- Horwood, N.J.; Udagawa, N.; Elliott, J.; Grail, D.; Okamura, H.; Kurimoto, M.; Dunn, A.R.; Martin, T.; Gillespie, M.T. Interleukin 18 inhibits osteoclast formation via T cell production of granulocyte macrophage colony-stimulating factor. J. Clin. Invest. 1998, 101, 595–603. [Google Scholar] [CrossRef]
- Yoshimatsu, M.; Kitaura, H.; Fujimura, Y.; Kohara, H.; Morita, Y.; Eguchi, T.; Yoshida, N. Inhibitory effects of IL-12 on experimental tooth movement and root resorption in mice. Arch. Oral Biol. 2012, 57, 36–43. [Google Scholar] [CrossRef]
- Bernstein, Z.J.; Shenoy, A.; Chen, A.; Heller, N.M.; Spangler, J.B. Engineering the IL-4/IL-13 axis for targeted immune modulation. Immunol. Rev. 2023, 320, 29–57. [Google Scholar] [CrossRef] [PubMed]
- Tubau, C.; Puig, L. Therapeutic targeting of the IL-13 pathway in skin inflammation. Expert. Rev. Clin. Immunol. 2021, 17, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Roeb, E. Interleukin-13 (IL-13)-A Pleiotropic Cytokine Involved in Wound Healing and Fibrosis. Int. J. Mol. Sci. 2023, 24, 12884. [Google Scholar] [CrossRef]
- Palmqvist, P.; Lundberg, P.; Persson, E.; Johansson, A.; Lundgren, I.; Lie, A.; Conaway, H.H.; Lerner, U.H. Inhibition of hormone and cytokine-stimulated osteoclastogenesis and bone resorption by interleukin-4 and interleukin-13 is associated with increased osteoprotegerin and decreased RANKL and RANK in a STAT6-dependent pathway. J. Biol. Chem. 2006, 281, 2414–2429. [Google Scholar] [CrossRef]
- Chen, Z.; Cheng, Z.; Tang, Z.; Li, M.; Tang, X.; Zhang, H.; Sun, L. Interleukin-13 reduces bone erosion in rheumatoid arthritis by up-regulating osteoprotegerin expression in fibroblast-like synoviocytes: An in vitro and in vivo study. Clin. Exp. Rheumatol. 2023, 41, 2151–2161. [Google Scholar] [CrossRef] [PubMed]
- Cruikshank, W.W.; Kornfeld, H.; Center, D.M. Interleukin-16. J. Leukoc. Biol. 2000, 67, 757–766. [Google Scholar] [CrossRef]
- Chang, Y.; Hsiao, Y.M.; Hu, C.C.; Chang, C.H.; Li, C.Y.; Ueng, S.W.N.; Chen, M.F. Synovial Fluid Interleukin-16 Contributes to Osteoclast Activation and Bone Loss through the JNK/NFATc1 Signaling Cascade in Patients with Periprosthetic Joint Infection. Int. J. Mol. Sci. 2020, 21, 2904. [Google Scholar] [CrossRef]
- Luo, H.; Fang, S.; Liu, Q.; Dang, W.; Wang, Y. Comparison of interleukin expression in gingival crevicular fluid between patients with invisible orthodontics treat-ment and fixed orthodontics treatment. Hua Xi Kou Qiang Yi Xue Za Zhi 2022, 40, 293–296. [Google Scholar] [CrossRef]
- Huangfu, L.; Li, R.; Huang, Y.; Wang, S. The IL-17 family in diseases: From bench to bedside. Signal Transduct. Target. Ther. 2023, 8, 402. [Google Scholar] [CrossRef]
- Mills, K.H.G. IL-17 and IL-17-producing cells in protection versus pathology. Nat. Rev. Immunol. 2023, 23, 38–54. [Google Scholar] [CrossRef] [PubMed]
- McGeachy, M.J.; Cua, D.J.; Gaffen, S.L. The IL-17 Family of Cytokines in Health and Disease. Immunity 2019, 50, 892–906. [Google Scholar] [CrossRef]
- Adamopoulos, I.E.; Chao, C.C.; Geissler, R.; Laface, D.; Blumenschein, W.; Iwakura, Y.; McClanahan, T.; Bowman, E.P. Interleukin-17A upregulates receptor activator of NF-kappaB on osteoclast precursors. Arthritis Res. Ther. 2010, 12, R29. [Google Scholar] [CrossRef]
- Yago, T.; Nanke, Y.; Ichikawa, N.; Kobashigawa, T.; Mogi, M.; Kamatani, N.; Kotake, S. IL-17 induces osteoclastogenesis from human monocytes alone in the absence of osteoblasts, which is potently inhibited by anti-TNF-alpha antibody: A novel mechanism of osteoclastogenesis by IL-17. J. Cell Biochem. 2009, 108, 947–955. [Google Scholar] [CrossRef]
- Ueda, M.; Hikida, T.; Shimizu, M.; Kikuta, J.; Takagi, K.; Tsukada, M.; Yamaguchi, M. Involvement of interleukins-17 and -34 in exacerbated orthodontic root resorption by jiggling force during rat experimental tooth movement. J. World Fed. Orthod. 2020, 9, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, N.; Yamaguchi, M.; Nakajima, R.; Utsunomiya, T.; Yamamoto, H.; Kasai, K. T-helper 17 cells mediate the osteo/odontoclastogenesis induced by excessive orthodontic forces. Oral Dis. 2012, 18, 375–388. [Google Scholar] [CrossRef]
- Sagar, S.; Ramani, P.; Moses, S.; Gheena, S.; Selvaraj, J. Correlation of salivary cytokine IL-17A and 1,25 dihydroxycholecalciferol in patients undergoing orthodontic treatment. Odontology 2024, 112, 966–975. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Yang, L.; Zheng, W.; Zhang, B. Matrix metalloproteinases and Th17 cytokines in the gingival crevicular fluid during orthodontic tooth movement. Eur. J. Paediatr. Dent. 2021, 22, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Yang, L.; Zheng, W.; Zhang, B. Th17 Cytokines and its Correlation with Receptor Activator of Nuclear Factor kappa B Ligand During Orthodontic Tooth Movement. Iran. J. Immunol. 2020, 17, 137–143. [Google Scholar] [CrossRef]
- Landy, E.; Carol, H.; Ring, A.; Canna, S. Biological and clinical roles of IL-18 in inflammatory diseases. Nat. Rev. Rheumatol. 2024, 20, 33–47. [Google Scholar] [CrossRef]
- Vecchie, A.; Bonaventura, A.; Toldo, S.; Dagna, L.; Dinarello, C.A.; Abbate, A. IL-18 and infections: Is there a role for targeted therapies? J. Cell Physiol. 2021, 236, 1638–1657. [Google Scholar] [CrossRef] [PubMed]
- Ihim, S.A.; Abubakar, S.D.; Zian, Z.; Sasaki, T.; Saffarioun, M.; Maleknia, S.; Azizi, G. Interleukin-18 cytokine in immunity, inflammation, and autoimmunity: Biological role in induction, regulation, and treatment. Front. Immunol. 2022, 13, 919973. [Google Scholar] [CrossRef]
- Novick, D. IL-18 and IL-18BP: A Unique Dyad in Health and Disease. Int. J. Mol. Sci. 2024, 25, 13505. [Google Scholar] [CrossRef]
- Rex, D.A.B.; Agarwal, N.; Prasad, T.S.K.; Kandasamy, R.K.; Subbannayya, Y.; Pinto, S.M. A comprehensive pathway map of IL-18-mediated signalling. J. Cell Commun. Signal 2020, 14, 257–266. [Google Scholar] [CrossRef]
- Kitaura, H.; Tatamiya, M.; Nagata, N.; Fujimura, Y.; Eguchi, T.; Yoshida, N.; Nakayama, K. IL-18 induces apoptosis of adherent bone marrow cells in TNF-alpha mediated osteoclast formation in synergy with IL-12. Immunol. Lett. 2006, 107, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Horwood, N.J.; Elliott, J.; Martin, T.J.; Gillespie, M.T. IL-12 alone and in synergy with IL-18 inhibits osteoclast formation in vitro. J. Immunol. 2001, 166, 4915–4921. [Google Scholar] [CrossRef] [PubMed]
- Morita, Y.; Kitaura, H.; Yoshimatsu, M.; Fujimura, Y.; Kohara, H.; Eguchi, T.; Yoshida, N. IL-18 inhibits TNF-alpha-induced osteoclastogenesis possibly via a T cell-independent mechanism in synergy with IL-12 in vivo. Calcif. Tissue Int. 2010, 86, 242–248. [Google Scholar] [CrossRef]
- Nogueira, A.V.; de Molon, R.S.; Nokhbehsaim, M.; Deschner, J.; Cirelli, J.A. Contribution of biomechanical forces to inflammation-induced bone resorption. J. Clin. Periodontol. 2017, 44, 31–41. [Google Scholar] [CrossRef]
- Blumberg, H.; Conklin, D.; Xu, W.F.; Grossmann, A.; Brender, T.; Carollo, S.; Eagan, M.; Foster, D.; Haldeman, B.A.; Hammond, A.; et al. Interleukin 20: Discovery, receptor identification, and role in epidermal function. Cell 2001, 104, 9–19. [Google Scholar] [CrossRef]
- Meng, B.; Wu, D.; Cheng, Y.; Huang, P.; Liu, Y.; Gan, L.; Liu, C.; Cao, Y. Interleukin-20 differentially regulates bone mesenchymal stem cell activities in RANKL-induced osteoclastogenesis through the OPG/RANKL/RANK axis and the NF-kappaB, MAPK and AKT signalling pathways. Scand. J. Immunol. 2020, 91, e12874. [Google Scholar] [CrossRef]
- Yemenoglu, H.; Senkal, R.; Kose, O.; Yilmaz, A.; Mataraci Karakas, S.; Akyildiz, K. The effect of interleukin-20 on periodontal tissue destruction in individuals with periodontitis. J. Periodontal Res. 2024, 59, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ai, Y.; Sun, X.; Meng, B.; Chen, X.; Wu, D.; Gan, L.; Yang, B.; Fu, C.; Wu, Y.; et al. Interleukin-20 Acts as a Promotor of Osteoclastogenesis and Orthodontic Tooth Movement. Stem Cells Int. 2021, 2021, 5539962. [Google Scholar] [CrossRef]
- Meng, B.; Yang, B.; Qu, Y.; Liu, Y.; Wu, D.; Fu, C.; He, Y.; Chen, X.; Liu, C.; Kou, X.; et al. Dual Role of Interleukin-20 in Different Stages of Osteoclast Differentiation and Its Osteoimmune Regulation during Alveolar Bone Remodeling. Int. J. Mol. Sci. 2023, 24, 3810. [Google Scholar] [CrossRef]
- Sewell, G.W.; Kaser, A. Interleukin-23 in the Pathogenesis of Inflammatory Bowel Disease and Implications for Therapeutic Intervention. J. Crohns Colitis 2022, 16, ii3–ii19. [Google Scholar] [CrossRef] [PubMed]
- Furuya, H.; Nguyen, C.T.; Gu, R.; Hsieh, S.L.; Maverakis, E.; Adamopoulos, I.E. Interleukin-23 Regulates Inflammatory Osteoclastogenesis via Activation of CLEC5A(+) Osteoclast Precursors. Arthritis Rheumatol. 2023, 75, 1477–1489. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Pang, D.D.; Dai, S.M. Expression Profile of Osteoclasts Following the Stimulation With Interleukin-23 in Mice. Arch. Rheumatol. 2020, 35, 533–544. [Google Scholar] [CrossRef]
- Chen, S.Y.; Tsai, T.C.; Li, Y.T.; Ding, Y.C.; Wang, C.T.; Hsieh, J.L.; Wu, C.L.; Wu, P.T.; Shiau, A.L. Interleukin-23 Mediates Osteoclastogenesis in Collagen-Induced Arthritis by Modulating MicroRNA-223. Int. J. Mol. Sci. 2022, 23, 9718. [Google Scholar] [CrossRef]
- Xu, W.D.; Wang, D.C.; Zhao, M.; Huang, A.F. An updated advancement of bifunctional IL-27 in inflammatory autoimmune diseases. Front. Immunol. 2024, 15, 1366377. [Google Scholar] [CrossRef]
- Yoshida, H.; Hunter, C.A. The immunobiology of interleukin-27. Annu. Rev. Immunol. 2015, 33, 417–443. [Google Scholar] [CrossRef]
- Li, X.; Luo, W.; Hu, J.; Chen, Y.; Yu, T.; Yang, J.; Dong, S.; Tian, X.; Sun, L. Interleukin-27 prevents LPS-induced inflammatory osteolysis by inhibiting osteoclast formation and function. Am. J. Transl. Res. 2019, 11, 1154–1169. [Google Scholar]
- Morita, Y.; Saito, M.; Rangel-Moreno, J.; Franchini, A.M.; Owen, J.R.; Martinez, J.C.; Daiss, J.L.; de Mesy Bentley, K.L.; Kates, S.L.; Schwarz, E.M.; et al. Systemic IL-27 administration prevents abscess formation and osteolysis via local neutrophil recruitment and activation. Bone Res. 2022, 10, 56. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, S.; Okumura, M.; Chiba, Y.; Fukawa, T.; Nakamura, C.; Nimura, N.; Mizuguchi, J.; Wada, S.; Yoshimoto, T. IL-27 suppresses RANKL expression in CD4+ T cells in part through STAT3. Immunol. Lett. 2011, 138, 47–53. [Google Scholar] [CrossRef]
- Dwyer, G.K.; D’Cruz, L.M.; Turnquist, H.R. Emerging Functions of IL-33 in Homeostasis and Immunity. Annu. Rev. Immunol. 2022, 40, 15–43. [Google Scholar] [CrossRef]
- Alarcon-Sanchez, M.A.; Romero-Castro, N.S.; Reyes-Fernandez, S.; Sanchez-Tecolapa, E.U.; Heboyan, A. Expression of IL-33 in subjects with periodontitis: A systematic review and meta-analysis. Eur. J. Med. Res. 2024, 29, 440. [Google Scholar] [CrossRef] [PubMed]
- Kiyomiya, H.; Ariyoshi, W.; Okinaga, T.; Kaneuji, T.; Mitsugi, S.; Sakurai, T.; Habu, M.; Yoshioka, I.; Tominaga, K.; Nishihara, T. IL-33 inhibits RANKL-induced osteoclast formation through the regulation of Blimp-1 and IRF-8 expression. Biochem. Biophys. Res. Commun. 2015, 460, 320–326. [Google Scholar] [CrossRef]
- Ohori, F.; Kitaura, H.; Ogawa, S.; Shen, W.R.; Qi, J.; Noguchi, T.; Marahleh, A.; Nara, Y.; Pramusita, A.; Mizoguchi, I. IL-33 Inhibits TNF-alpha-Induced Osteoclastogenesis and Bone Resorption. Int. J. Mol. Sci. 2020, 21, 1130. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Feng, J.; Li, B.; Bai, D.; Xu, H. Inhibition of osteoclastogenesis by interleukin-33 administration in the periodontal ligament under mechanical loading. J. Periodontal Res. 2022, 57, 1003–1013. [Google Scholar] [CrossRef]
- Dong, X.; Feng, J.; Wen, J.; Bai, D.; Xu, H. Effect of interleukin-33 on cementoblast-mediated cementum repair during orthodontic tooth movement. Arch. Oral Biol. 2020, 112, 104663. [Google Scholar] [CrossRef]
- Si, Y.; Zhang, J.; Bao, S.; Wise, S.G.; Wang, Y.; Zhang, Y.; Tang, Y. IL-32 and IL-34 in hepatocellular carcinoma. Front. Med. 2022, 9, 1051113. [Google Scholar] [CrossRef]
- Lelios, I.; Cansever, D.; Utz, S.G.; Mildenberger, W.; Stifter, S.A.; Greter, M. Emerging roles of IL-34 in health and disease. J. Exp. Med. 2020, 217, e20190290. [Google Scholar] [CrossRef]
- Duarte, C.; Yamada, C.; Ngala, B.; Garcia, C.; Akkaoui, J.; Birsa, M.; Ho, A.; Nusbaum, A.; AlQallaf, H.; John, V.; et al. Effects of IL-34 and anti-IL-34 neutralizing mAb on alveolar bone loss in a ligature-induced model of periodontitis. Mol. Oral Microbiol. 2024, 39, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Van Raemdonck, K.; Umar, S.; Palasiewicz, K.; Volin, M.V.; Elshabrawy, H.A.; Romay, B.; Tetali, C.; Ahmed, A.; Amin, M.A.; Zomorrodi, R.K.; et al. Interleukin-34 Reprograms Glycolytic and Osteoclastic Rheumatoid Arthritis Macrophages via Syndecan 1 and Macrophage Colony-Stimulating Factor Receptor. Arthritis Rheumatol. 2021, 73, 2003–2014. [Google Scholar] [CrossRef]
- Ng, C.T.; Fong, L.Y.; Abdullah, M.N.H. Interferon-gamma (IFN-gamma): Reviewing its mechanisms and signaling pathways on the regulation of endothelial barrier function. Cytokine 2023, 166, 156208. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Wu, M.; Liu, Z. Dysregulation in IFN-gamma signaling and response: The barricade to tumor immunotherapy. Front. Immunol. 2023, 14, 1190333. [Google Scholar] [CrossRef]
- Casanova, J.L.; MacMicking, J.D.; Nathan, C.F. Interferon-gamma and infectious diseases: Lessons and prospects. Science 2024, 384, eadl2016. [Google Scholar] [CrossRef] [PubMed]
- Mezouar, S.; Mege, J.L. Changing the paradigm of IFN-gamma at the interface between innate and adaptive immunity: Macrophage-derived IFN-gamma. J. Leukoc. Biol. 2020, 108, 419–426. [Google Scholar] [CrossRef]
- Tang, M.; Tian, L.; Luo, G.; Yu, X. Interferon-Gamma-Mediated Osteoimmunology. Front. Immunol. 2018, 9, 1508. [Google Scholar] [CrossRef]
- Tan, J.; Dai, A.; Pan, L.; Zhang, L.; Wang, Z.; Ke, T.; Sun, W.; Wu, Y.; Ding, P.H.; Chen, L. Inflamm-Aging-Related Cytokines of IL-17 and IFN-gamma Accelerate Osteoclastogenesis and Periodontal Destruction. J. Immunol. Res. 2021, 2021, 9919024. [Google Scholar] [CrossRef]
- Biros, E.; Malabu, U.H.; Vangaveti, V.N.; Birosova, E.; Moran, C.S. The IFN-gamma/miniTrpRS signaling axis: An insight into the pathophysiology of osteoporosis and therapeutic potential. Cytokine Growth Factor Rev. 2022, 64, 7–11. [Google Scholar] [CrossRef]
- Kohara, H.; Kitaura, H.; Fujimura, Y.; Yoshimatsu, M.; Morita, Y.; Eguchi, T.; Masuyama, R.; Yoshida, N. IFN-gamma directly inhibits TNF-alpha-induced osteoclastogenesis in vitro and in vivo and induces apoptosis mediated by Fas/Fas ligand interactions. Immunol. Lett. 2011, 137, 53–61. [Google Scholar] [CrossRef]
- Kohara, H.; Kitaura, H.; Yoshimatsu, M.; Fujimura, Y.; Morita, Y.; Eguchi, T.; Yoshida, N. Inhibitory effect of interferon-gamma on experimental tooth movement in mice. J. Interferon Cytokine Res. 2012, 32, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Xin, T.Y.; Zhu, Z.L.; Chen, L.J.; Han, B.; Yang, R.L.; Shi, J. Profile of Inflammatory Cytokines in Gingival Crevicular Fluid and Plasma in Patients With Grade C Periodontitis During Orthodontic Treatment: A Longitudinal Case Series Report. Orthod. Craniofacial Res. 2025, 28, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Mermut, S.; Bengi, A.O.; Akin, E.; Kurkcu, M.; Karacay, S. Effects of interferon-gamma on bone remodeling during experimental tooth movement. Angle Orthod. 2007, 77, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Liu, F.; Kou, X.; Liu, D.; Yang, R.; Wang, X.; Song, Y.; He, D.; Gan, Y.; Zhou, Y. T Cells Are Required for Orthodontic Tooth Movement. J. Dent. Res. 2015, 94, 1463–1470. [Google Scholar] [CrossRef]
- An, S.; Zhang, Y.; Chen, Q.; Xiong, B.; Hao, J.; Zheng, Y.; Zhou, X.; Wang, J. Effect of systemic delivery of Substance P on experimental tooth movement in rats. Am. J. Orthod. Dentofac. Orthop. 2019, 155, 642–649. [Google Scholar] [CrossRef]
- Jang, D.I.; Lee, A.H.; Shin, H.Y.; Song, H.R.; Park, J.H.; Kang, T.B.; Lee, S.R.; Yang, S.H. The Role of Tumor Necrosis Factor Alpha (TNF-alpha) in Autoimmune Disease and Current TNF-alpha Inhibitors in Therapeutics. Int. J. Mol. Sci. 2021, 22, 2719. [Google Scholar] [CrossRef]
- van Loo, G.; Bertrand, M.J.M. Death by TNF: A road to inflammation. Nat. Rev. Immunol. 2023, 23, 289–303. [Google Scholar] [CrossRef]
- Berkhout, L.C.; I’Ami, M.J.; Kruithof, S.; Vogelzang, E.H.; Hooijberg, F.; Hart, M.H.L.; Bentlage, A.E.H.; Thomas, D.; Vermeire, S.; Vidarsson, G.; et al. Formation and clearance of TNF-TNF inhibitor complexes during TNF inhibitor treatment. Br. J. Pharmacol. 2024, 181, 1165–1181. [Google Scholar] [CrossRef]
- Idriss, H.T.; Naismith, J.H. TNF alpha and the TNF receptor superfamily: Structure-function relationship(s). Microsc. Res. Tech. 2000, 50, 184–195. [Google Scholar] [CrossRef]
- Huyghe, J.; Priem, D.; Bertrand, M.J.M. Cell death checkpoints in the TNF pathway. Trends Immunol. 2023, 44, 628–643. [Google Scholar] [CrossRef]
- Veerasubramanian, P.K.; Wynn, T.A.; Quan, J.; Karlsson, F.J. Targeting TNF/TNFR superfamilies in immune-mediated inflammatory diseases. J. Exp. Med. 2024, 221, e20240806. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, T.; Mitoma, H.; Harashima, S.; Tsukamoto, H.; Shimoda, T. Transmembrane TNF-alpha: Structure, function and interaction with anti-TNF agents. Rheumatology 2010, 49, 1215–1228. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Getting, S.J.; Locke, I.C. Regulation of TNF-Induced Osteoclast Differentiation. Cells 2021, 11, 132. [Google Scholar] [CrossRef] [PubMed]
- Guberna, L.; Nyssen, O.P.; Chaparro, M.; Gisbert, J.P. Frequency and Effectiveness of Empirical Anti-TNF Dose Intensification in Inflammatory Bowel Disease: Systematic Review with Meta-Analysis. J. Clin. Med. 2021, 10, 2132. [Google Scholar] [CrossRef]
- Yao, Q.; He, L.; Bao, C.; Yan, X.; Ao, J. The role of TNF-alpha in osteoporosis, bone repair and inflammatory bone diseases: A review. Tissue Cell 2024, 89, 102422. [Google Scholar] [CrossRef]
- Neurath, N.; Kesting, M. Cytokines in gingivitis and periodontitis: From pathogenesis to therapeutic targets. Front. Immunol. 2024, 15, 1435054. [Google Scholar] [CrossRef]
- Siegmund, D.; Wajant, H. TNF and TNF receptors as therapeutic targets for rheumatic diseases and beyond. Nat. Rev. Rheumatol. 2023, 19, 576–591. [Google Scholar] [CrossRef]
- Kitaura, H.; Marahleh, A.; Ohori, F.; Noguchi, T.; Nara, Y.; Pramusita, A.; Kinjo, R.; Ma, J.; Kanou, K.; Mizoguchi, I. Role of the Interaction of Tumor Necrosis Factor-alpha and Tumor Necrosis Factor Receptors 1 and 2 in Bone-Related Cells. Int. J. Mol. Sci. 2022, 23, 1481. [Google Scholar] [CrossRef]
- Abu-Amer, Y.; Erdmann, J.; Alexopoulou, L.; Kollias, G.; Ross, F.P.; Teitelbaum, S.L. Tumor necrosis factor receptors types 1 and 2 differentially regulate osteoclastogenesis. J. Biol. Chem. 2000, 275, 27307–27310. [Google Scholar] [CrossRef]
- Lowney, J.J.; Norton, L.A.; Shafer, D.M.; Rossomando, E.F. Orthodontic forces increase tumor necrosis factor alpha in the human gingival sulcus. Am. J. Orthod. Dentofac. Orthop. 1995, 108, 519–524. [Google Scholar] [CrossRef]
- Uematsu, S.; Mogi, M.; Deguchi, T. Interleukin (IL)-1 beta, IL-6, tumor necrosis factor-alpha, epidermal growth factor, and beta 2-microglobulin levels are elevated in gingival crevicular fluid during human orthodontic tooth movement. J. Dent. Res. 1996, 75, 562–567. [Google Scholar] [CrossRef]
- Li, H.; Chen, Y.; Sun, W. Effects of Kangfuxinye on NF-kappaB and Inflammatory Cytokines in Gingival Crevicular Fluid of Patients with Orthodontic Gingivitis Caused by Orthodontic Treatment. Cell Mol. Biol. 2023, 69, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, P.; Kharbanda, O.P.; Monga, N.; Miglani, R.; Kapila, S. Effect of orthodontic forces on cytokine and receptor levels in gingival crevicular fluid: A systematic review. Prog. Orthod. 2014, 15, 65. [Google Scholar] [CrossRef]
- Debnath, P.; Bangi, S.L.; Hussain, M.F.; Rafiq, S.; Tousifulla, S.; Abdu, M.; Gupta, S. The Evaluation of Gingival Crevicular Fluid Biomarkers as Predictors of Gingival Enlargement in Patients Undergoing Fixed Orthodontic Treatment: A Prospective Study. Cureus 2024, 16, e74281. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Mi, D.; Wu, H.; Fu, Y.; Liu, L.; Chen, X.; Dong, Y.; Zhang, W. Changes in Matrix Metalloproteinase-8, Interleukin-6 and Tumor Necrosis Factor-A in Gingival Crevicular Fluid during Rapid Maxillary Expansion in Adolescent Patients. Iran. J. Public. Health 2021, 50, 1944–1952. [Google Scholar] [CrossRef]
- Basaran, G.; Ozer, T.; Kaya, F.A.; Kaplan, A.; Hamamci, O. Interleukine-1beta and tumor necrosis factor-alpha levels in the human gingival sulcus during orthodontic treatment. Angle Orthod. 2006, 76, 830–836. [Google Scholar] [PubMed]
- Ogasawara, T.; Yoshimine, Y.; Kiyoshima, T.; Kobayashi, I.; Matsuo, K.; Akamine, A.; Sakai, H. In situ expression of RANKL, RANK, osteoprotegerin and cytokines in osteoclasts of rat periodontal tissue. J. Periodontal Res. 2004, 39, 42–49. [Google Scholar] [CrossRef]
- Yoshimatsu, M.; Shibata, Y.; Kitaura, H.; Chang, X.; Moriishi, T.; Hashimoto, F.; Yoshida, N.; Yamaguchi, A. Experimental model of tooth movement by orthodontic force in mice and its application to tumor necrosis factor receptor-deficient mice. J. Bone Min. Metab. 2006, 24, 20–27. [Google Scholar] [CrossRef]
- Andrade, I., Jr.; Silva, T.A.; Silva, G.A.; Teixeira, A.L.; Teixeira, M.M. The role of tumor necrosis factor receptor type 1 in orthodontic tooth movement. J. Dent. Res. 2007, 86, 1089–1094. [Google Scholar] [CrossRef]
- Kitaura, H.; Yoshimatsu, M.; Fujimura, Y.; Eguchi, T.; Kohara, H.; Yamaguchi, A.; Yoshida, N. An anti-c-Fms antibody inhibits orthodontic tooth movement. J. Dent. Res. 2008, 87, 396–400. [Google Scholar] [CrossRef]
- Ma, J.; Kitaura, H.; Ogawa, S.; Ohori, F.; Noguchi, T.; Marahleh, A.; Nara, Y.; Pramusita, A.; Kinjo, R.; Kanou, K.; et al. Docosahexaenoic acid inhibits TNF-alpha-induced osteoclast formation and orthodontic tooth movement through GPR120. Front. Immunol. 2022, 13, 929690. [Google Scholar] [CrossRef]
- Marahleh, A.; Kitaura, H.; Ohori, F.; Kishikawa, A.; Ogawa, S.; Shen, W.R.; Qi, J.; Noguchi, T.; Nara, Y.; Mizoguchi, I. TNF-alpha Directly Enhances Osteocyte RANKL Expression and Promotes Osteoclast Formation. Front. Immunol. 2019, 10, 2925. [Google Scholar] [CrossRef] [PubMed]
- Marahleh, A.; Kitaura, H.; Ohori, F.; Noguchi, T.; Mizoguchi, I. The osteocyte and its osteoclastogenic potential. Front. Endocrinol. 2023, 14, 1121727. [Google Scholar] [CrossRef]
- Noguchi, T.; Kitaura, H.; Ogawa, S.; Qi, J.; Shen, W.R.; Ohori, F.; Marahleh, A.; Nara, Y.; Pramusita, A.; Mizoguchi, I. TNF-alpha stimulates the expression of RANK during orthodontic tooth movement. Arch. Oral Biol. 2020, 117, 104796. [Google Scholar] [CrossRef] [PubMed]
- Marahleh, A.; Kitaura, H.; Ohori, F.; Noguchi, T.; Nara, Y.; Pramusita, A.; Kinjo, R.; Ma, J.; Kanou, K.; Mizoguchi, I. Effect of TNF-alpha on osteocyte RANKL expression during orthodontic tooth movement. J. Dent. Sci. 2021, 16, 1191–1197. [Google Scholar] [CrossRef]
- Kanou, K.; Kitaura, H.; Noguchi, T.; Ohori, F.; Marahleh, A.; Kinjo, R.; Ma, J.; Ren, J.; Ogasawara, K.; Mizoguchi, I. Effect of age on orthodontic tooth movement in mice. J. Dent. Sci. 2024, 19, 828–836. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, T.; Kitaura, H.; Marahleh, A.; Ohori, F.; Nara, Y.; Pramusita, A.; Kinjo, R.; Ma, J.; Kanou, K.; Mizoguchi, I. Tumor necrosis factor-alpha enhances the expression of vascular endothelial growth factor in a mouse orthodontic tooth movement model. J. Dent. Sci. 2022, 17, 415–420. [Google Scholar] [CrossRef]
- Ohori, F.; Kitaura, H.; Marahleh, A.; Ma, J.; Miura, M.; Ren, J.; Narita, K.; Fan, Z.; Lin, A.; Mizoguchi, I. Osteocyte necroptosis drives osteoclastogenesis and alveolar bone resorption during orthodontic tooth movement. Sci. Rep. 2025, 15, 19413. [Google Scholar] [CrossRef]
- Sehgal, A.; Irvine, K.M.; Hume, D.A. Functions of macrophage colony-stimulating factor (CSF1) in development, homeostasis, and tissue repair. Semin. Immunol. 2021, 54, 101509. [Google Scholar] [CrossRef]
- Mun, S.H.; Park, P.S.U.; Park-Min, K.H. The M-CSF receptor in osteoclasts and beyond. Exp. Mol. Med. 2020, 52, 1239–1254. [Google Scholar] [CrossRef]
- Begg, S.K.; Radley, J.M.; Pollard, J.W.; Chisholm, O.T.; Stanley, E.R.; Bertoncello, I. Delayed hematopoietic development in osteopetrotic (op/op) mice. J. Exp. Med. 1993, 177, 237–242. [Google Scholar] [CrossRef]
- Udagawa, N.; Koide, M.; Nakamura, M.; Nakamichi, Y.; Yamashita, T.; Uehara, S.; Kobayashi, Y.; Furuya, Y.; Yasuda, H.; Fukuda, C.; et al. Osteoclast differentiation by RANKL and OPG signaling pathways. J. Bone Min. Metab. 2021, 39, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Kitaura, H.; Zhou, P.; Kim, H.J.; Novack, D.V.; Ross, F.P.; Teitelbaum, S.L. M-CSF mediates TNF-induced inflammatory osteolysis. J. Clin. Investig. 2005, 115, 3418–3427. [Google Scholar] [CrossRef]
- Nara, Y.; Kitaura, H.; Ogawa, S.; Shen, W.R.; Qi, J.; Ohori, F.; Noguchi, T.; Marahleh, A.; Pramusita, A.; Kinjo, R.; et al. Anti-c-fms Antibody Prevents Osteoclast Formation and Bone Resorption in Co-Culture of Osteoblasts and Osteoclast Precursors In Vitro and in Ovariectomized Mice. Int. J. Mol. Sci. 2020, 21, 6120. [Google Scholar] [CrossRef]
- Brooks, P.J.; Heckler, A.F.; Wei, K.; Gong, S.G. M-CSF accelerates orthodontic tooth movement by targeting preosteoclasts in mice. Angle Orthod. 2011, 81, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Kitaura, H.; Shen, W.R.; Kishikawa, A.; Ogawa, S.; Ohori, F.; Noguchi, T.; Marahleh, A.; Nara, Y.; Mizoguchi, I. Establishment of an orthodontic retention mouse model and the effect of anti-c-Fms antibody on orthodontic relapse. PLoS ONE 2019, 14, e0214260. [Google Scholar] [CrossRef] [PubMed]
- Massague, J.; Sheppard, D. TGF-beta signaling in health and disease. Cell 2023, 186, 4007–4037. [Google Scholar] [CrossRef]
- Larson, C.; Oronsky, B.; Carter, C.A.; Oronsky, A.; Knox, S.J.; Sher, D.; Reid, T.R. TGF-beta: A master immune regulator. Expert. Opin. Ther. Targets 2020, 24, 427–438. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, X.; Wan, Y.Y. Intricacies of TGF-beta signaling in Treg and Th17 cell biology. Cell Mol. Immunol. 2023, 20, 1002–1022. [Google Scholar] [CrossRef]
- Takai, H.; Kanematsu, M.; Yano, K.; Tsuda, E.; Higashio, K.; Ikeda, K.; Watanabe, K.; Yamada, Y. Transforming growth factor-beta stimulates the production of osteoprotegerin/osteoclastogenesis inhibitory factor by bone marrow stromal cells. J. Biol. Chem. 1998, 273, 27091–27096. [Google Scholar] [CrossRef]
- Karst, M.; Gorny, G.; Galvin, R.J.; Oursler, M.J. Roles of stromal cell RANKL, OPG, and M-CSF expression in biphasic TGF-beta regulation of osteoclast differentiation. J. Cell Physiol. 2004, 200, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Weitzmann, M.N.; Cenci, S.; Haug, J.; Brown, C.; DiPersio, J.; Pacifici, R. B lymphocytes inhibit human osteoclastogenesis by secretion of TGFbeta. J. Cell Biochem. 2000, 78, 318–324. [Google Scholar] [CrossRef]
- Quinn, J.M.; Itoh, K.; Udagawa, N.; Hausler, K.; Yasuda, H.; Shima, N.; Mizuno, A.; Higashio, K.; Takahashi, N.; Suda, T.; et al. Transforming growth factor beta affects osteoclast differentiation via direct and indirect actions. J. Bone Min. Res. 2001, 16, 1787–1794. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Macdonald, B.R.; Hon, J.; Winkler, M.E.; Derynck, R.; Mundy, G.R.; Roodman, G.D. Recombinant Human Transforming Growth-Factor-Alpha-Stimulates the Formation of Osteoclast-Like Cells in Long-Term Human Marrow Cultures. J. Clin. Investig. 1986, 78, 894–898. [Google Scholar] [CrossRef]
- Heubel, B.; Nohe, A. The Role of BMP Signaling in Osteoclast Regulation. J. Dev. Biol. 2021, 9, 24. [Google Scholar] [CrossRef]
- Uematsu, S.; Mogi, M.; Deguchi, T. Increase of transforming growth factor-beta 1 in gingival crevicular fluid during human orthodontic tooth movement. Arch. Oral Biol. 1996, 41, 1091–1095. [Google Scholar] [CrossRef]
- Chen, Y.; Mei, L.; Qian, Y.; Zhou, X.; Zhao, Z.; Zheng, W.; Li, Y. Integrated bioinformatic analysis of protein landscape in gingival crevicular fluid unveils sequential bioprocess in orthodontic tooth movement. Prog. Orthod. 2024, 25, 37. [Google Scholar] [CrossRef]
- Sasaki, K.; Takeshita, N.; Fukunaga, T.; Seiryu, M.; Sakamoto, M.; Oyanagi, T.; Maeda, T.; Takano-Yamamoto, T. Vibration accelerates orthodontic tooth movement by inducing osteoclastogenesis via transforming growth factor-beta signalling in osteocytes. Eur. J. Orthod. 2022, 44, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Akbulut, S.; Yagci, A.; Yay, A.H.; Yalcin, B. Experimental investigation of effects of platelet-rich plasma on early phases of orthodontic tooth movement. Am. J. Orthod. Dentofac. Orthop. 2019, 155, 71–79. [Google Scholar] [CrossRef]
- Jeon, H.H.; Teixeira, H.; Tsai, A.N. Mechanistic Insight into Orthodontic Tooth Movement Based on Animal Studies: A Critical Review. J. Clin. Med. 2021, 10, 1733. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, M.; Tian, X.; Wang, M.; Zhang, F. Experimental animal study on BMP-3 expression in periodontal tissues in the process of orthodontic tooth movement. Exp. Ther. Med. 2019, 17, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.L.; Fan, J.D.; Wang, A.A.; Jin, X.; Zhang, Z.B.; Hu, X.T.; Liu, L.; Zhao, Y.T.; Li, Y.F. Effect of local application of bone morphogenetic protein-2 on experimental tooth movement and biological remodeling in rats. Front. Physiol. 2023, 14, 1111857. [Google Scholar] [CrossRef]
- Yasuda, H.; Shima, N.; Nakagawa, N.; Yamaguchi, K.; Kinosaki, M.; Mochizuki, S.; Tomoyasu, A.; Yano, K.; Goto, M.; Murakami, A.; et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc. Natl. Acad. Sci. USA 1998, 95, 3597–3602. [Google Scholar] [CrossRef]
- Kim, J.M.; Lin, C.; Stavre, Z.; Greenblatt, M.B.; Shim, J.H. Osteoblast-Osteoclast Communication and Bone Homeostasis. Cells 2020, 9, 2073. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, T.; Hayashi, M.; Takayanagi, H. New insights into osteoclastogenic signaling mechanisms. Trends Endocrinol. Metab. 2012, 23, 582–590. [Google Scholar] [CrossRef]
- Ono, T.; Hayashi, M.; Sasaki, F.; Nakashima, T. RANKL biology: Bone metabolism, the immune system, and beyond. Inflamm. Regen. 2020, 40, 2. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, T.; Hayashi, M.; Fukunaga, T.; Kurata, K.; Oh-Hora, M.; Feng, J.Q.; Bonewald, L.F.; Kodama, T.; Wutz, A.; Wagner, E.F.; et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat. Med. 2011, 17, 1231–1234. [Google Scholar] [CrossRef]
- Kramer, I.; Halleux, C.; Keller, H.; Pegurri, M.; Gooi, J.H.; Weber, P.B.; Feng, J.Q.; Bonewald, L.F.; Kneissel, M. Osteocyte Wnt/beta-catenin signaling is required for normal bone homeostasis. Mol. Cell Biol. 2010, 30, 3071–3085. [Google Scholar] [CrossRef]
- Chang, J.H.; Chen, P.J.; Arul, M.R.; Dutra, E.H.; Nanda, R.; Kumbar, S.G.; Yadav, S. Injectable RANKL sustained release formulations to accelerate orthodontic tooth movement. Eur. J. Orthod. 2020, 42, 317–325. [Google Scholar] [CrossRef]
- Li, C.; Chung, C.J.; Hwang, C.J.; Lee, K.J. Local injection of RANKL facilitates tooth movement and alveolar bone remodelling. Oral Dis. 2019, 25, 550–560. [Google Scholar] [CrossRef]
- Nishijima, Y.; Yamaguchi, M.; Kojima, T.; Aihara, N.; Nakajima, R.; Kasai, K. Levels of RANKL and OPG in gingival crevicular fluid during orthodontic tooth movement and effect of compression force on releases from periodontal ligament cells in vitro. Orthod. Craniofacial Res. 2006, 9, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Miyazawa, K.; Tabuchi, M.; Yabumoto, T.; Kadota, M.; Yoshizako, M.; Yamane, C.; Kawatani, M.; Osada, H.; Maeda, H.; et al. Effect of Reveromycin A on experimental tooth movement in OPG-/- mice. J. Dent. Res. 2012, 91, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Shoji-Matsunaga, A.; Ono, T.; Hayashi, M.; Takayanagi, H.; Moriyama, K.; Nakashima, T. Osteocyte regulation of orthodontic force-mediated tooth movement via RANKL expression. Sci. Rep. 2017, 7, 8753. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Mi, S.C.; Li, X.Y.; Liu, Y.T.; Han, N.N.; Xu, J.J.; Liu, Y.; Li, S.; Guo, L.J. MicroRNA-155 targets SOCS1 to inhibit osteoclast differentiation during orthodontic tooth movement. BMC Oral Health 2023, 23, 63–70. [Google Scholar] [CrossRef]
- Cultrera, G.; Lo Giudice, A.; Santonocito, S.; Ronsivalle, V.; Conforte, C.; Reitano, G.; Leonardi, R.; Isola, G. MicroRNA Modulation during Orthodontic Tooth Movement: A Promising Strategy for Novel Diagnostic and Personalized Therapeutic Interventions. Int. J. Mol. Sci. 2022, 23, 15501. [Google Scholar] [CrossRef]
- Han, Y.N.; Yang, Q.L.; Huang, Y.P.; Li, X.B.; Zhu, Y.Y.; Jia, L.F.; Zheng, Y.F.; Li, W.R. Mechanical force inhibited hPDLSCs proliferation with the downregulation of via DNA methylation. Oral Dis. 2021, 27, 1268–1282. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).