Association of First-Trimester Maternal Biomarkers with Preeclampsia and Related Maternal and Fetal Severe Adverse Events
Abstract
1. Introduction
2. Results
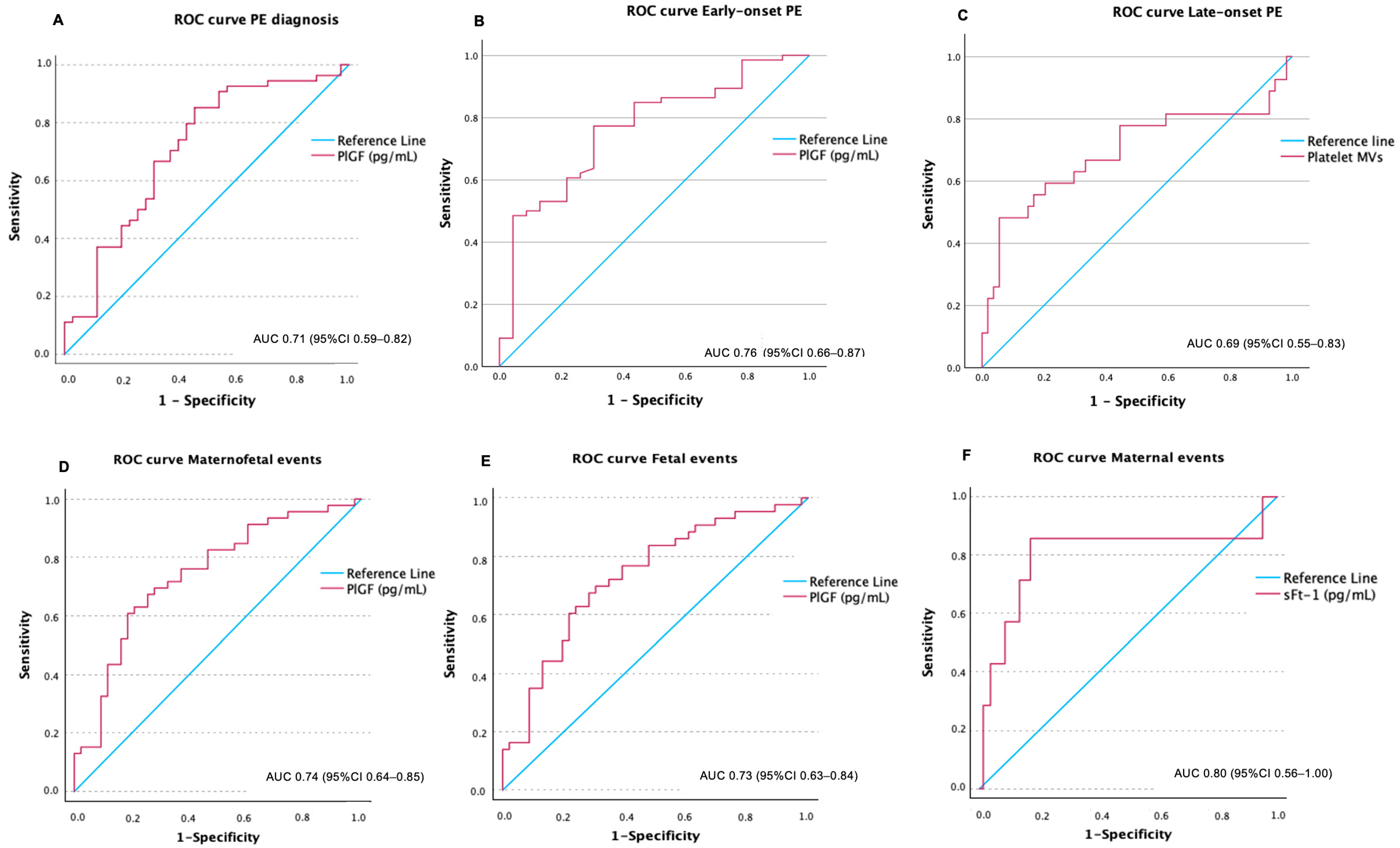
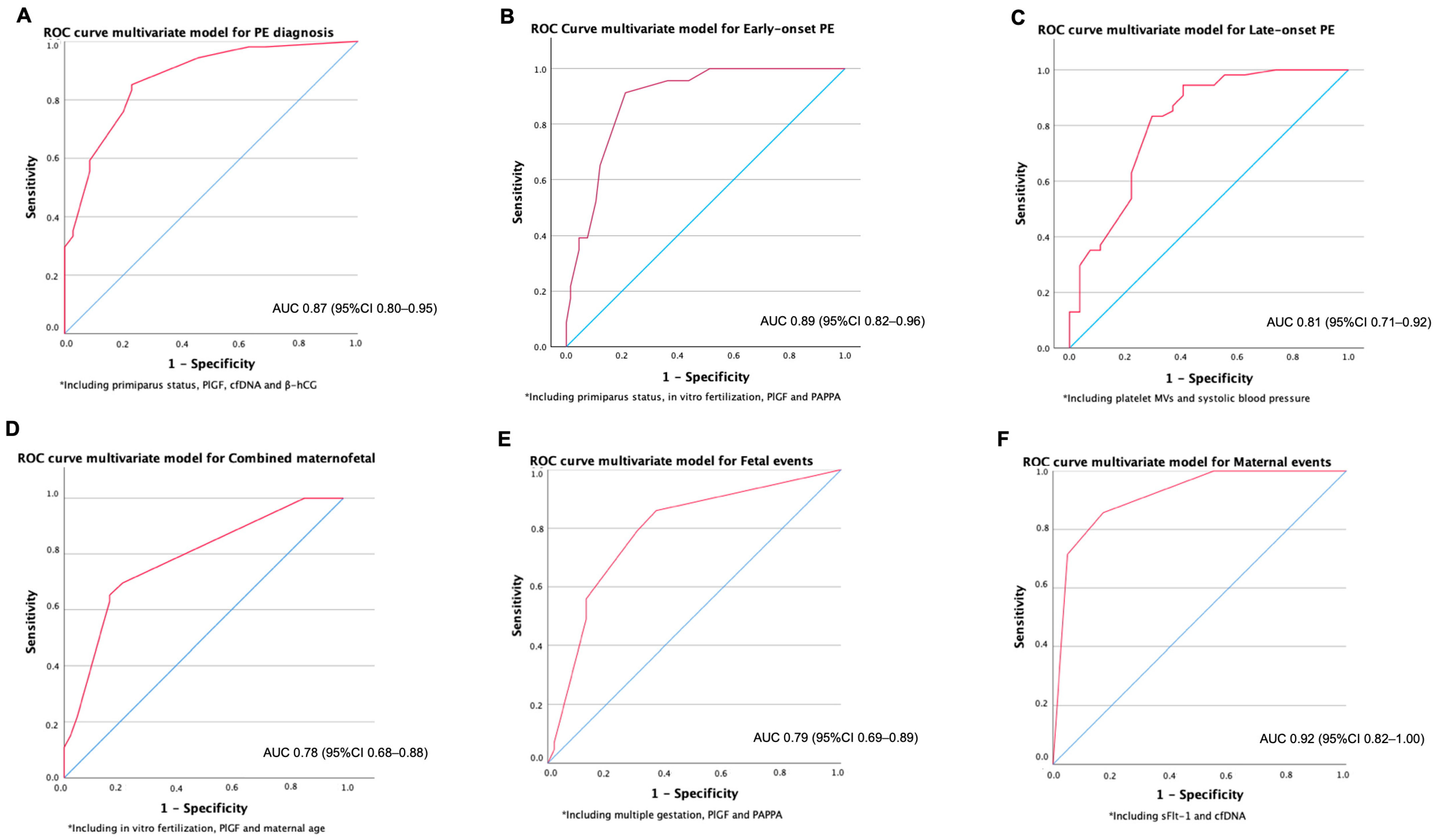
2.1. Association of Biomarkers at First Trimester with a Later Diagnosis of PE
2.2. Association of Biomarkers at First Trimester with Early-Onset PE
2.3. Association of Biomarkers at First Trimester with Late-Onset PE
2.4. Maternal and Fetal-Neonatal Severe Adverse Events
3. Discussion
Strengths and Limitations
4. Material and Methods
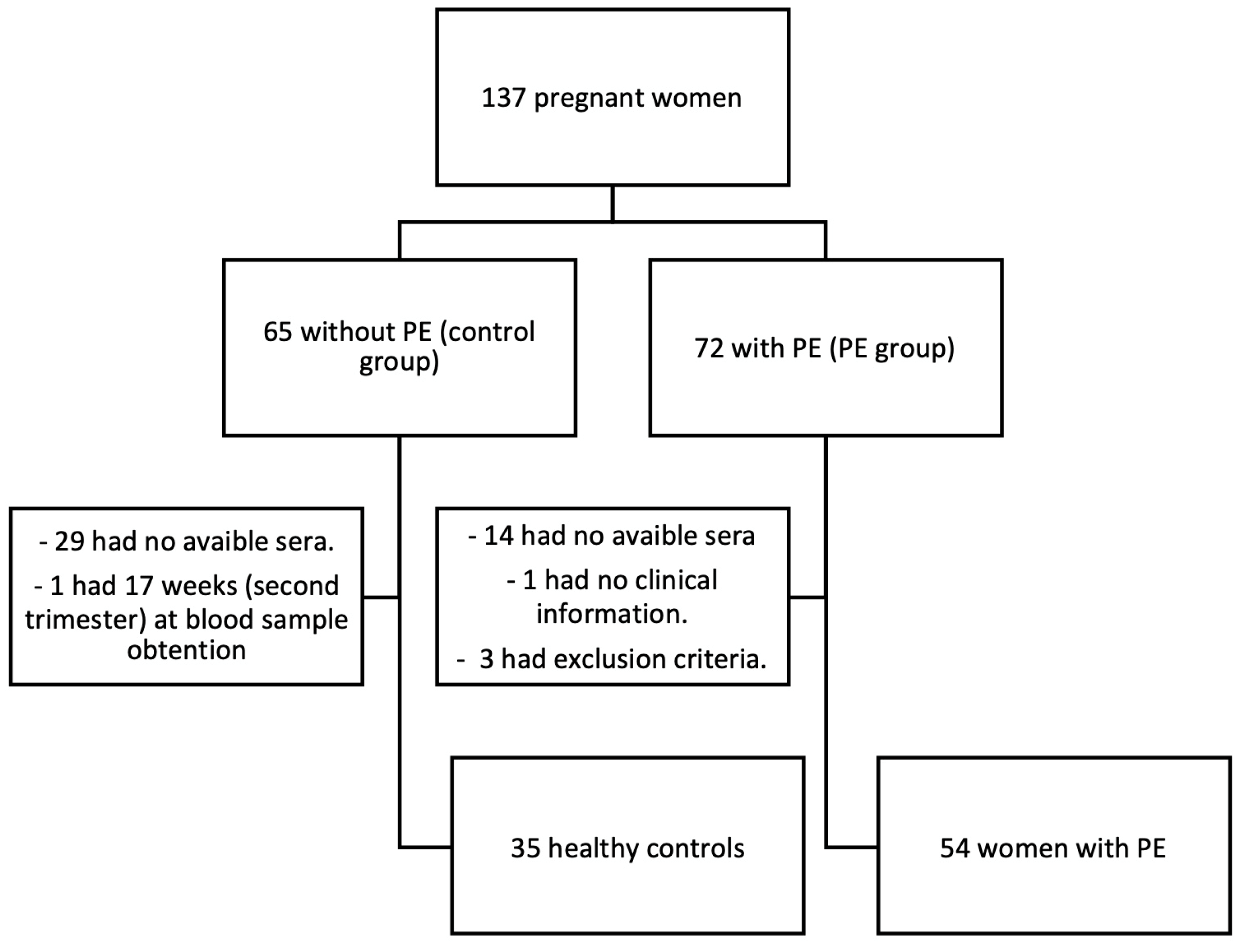
4.1. Study Design and Population
4.2. Sample Size
4.3. Events Definitions
4.4. Data Sources and Quality Control
4.5. Sample Collection, Storage and Biomarker Analyses
4.6. Statistical Analysis
4.7. Ethics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dimitriadis, E.; Rolnik, D.L.; Zhou, W.; Estrada-Gutierrez, G.; Koga, K.; Francisco, R.P.V.; Whitehead, C.; Hyett, J.; da Silva Costa, F.; Nicolaides, K.; et al. Pre-Eclampsia. Nat. Rev. Dis. Primer. 2023, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Mol, B.W.J.; Roberts, C.T.; Thangaratinam, S.; Magee, L.A.; de Groot, C.J.M.; Hofmeyr, G.J. Pre-Eclampsia. Lancet Lond. Engl. 2016, 387, 999–1011. [Google Scholar] [CrossRef] [PubMed]
- Say, L.; Chou, D.; Gemmill, A.; Tunçalp, Ö.; Moller, A.-B.; Daniels, J.; Gülmezoglu, A.M.; Temmerman, M.; Alkema, L. Global Causes of Maternal Death: A WHO Systematic Analysis. Lancet Glob. Health 2014, 2, e323–e333. [Google Scholar] [CrossRef] [PubMed]
- Poon, L.C.; Shennan, A.; Hyett, J.A.; Kapur, A.; Hadar, E.; Divakar, H.; McAuliffe, F.; da Silva Costa, F.; von Dadelszen, P.; McIntyre, H.D.; et al. The International Federation of Gynecology and Obstetrics (FIGO) Initiative on Preeclampsia (PE): A Pragmatic Guide for First Trimester Screening and Prevention. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 2019, 145 (Suppl. S1), S1–S33. [Google Scholar] [CrossRef]
- Magee, L.A.; Brown, M.A.; Hall, D.R.; Gupte, S.; Hennessy, A.; Karumanchi, S.A.; Kenny, L.C.; McCarthy, F.; Myers, J.; Poon, L.C.; et al. The 2021 International Society for the Study of Hypertension in Pregnancy Classification, Diagnosis & Management Recommendations for International Practice. Pregnancy Hypertens. 2022, 27, 148–169. [Google Scholar] [CrossRef]
- Pittara, T.; Vyrides, A.; Lamnisos, D.; Giannakou, K. Pre-Eclampsia and Long-Term Health Outcomes for Mother and Infant: An Umbrella Review. BJOG Int. J. Obstet. Gynaecol. 2021, 128, 1421–1430. [Google Scholar] [CrossRef]
- Myatt, L. The Prediction of Preeclampsia: The Way Forward. Am. J. Obstet. Gynecol. 2022, 226 (Suppl. S2), S1102–S1107.e8. [Google Scholar] [CrossRef]
- MacDonald, T.M.; Walker, S.P.; Hannan, N.J.; Tong, S.; Kaitu’u-Lino, T.J. Clinical Tools and Biomarkers to Predict Preeclampsia. EBioMedicine 2022, 75, 103780. [Google Scholar] [CrossRef]
- Roberts, J.M.; Rich-Edwards, J.W.; McElrath, T.F.; Garmire, L.; Myatt, L. Subtypes of Preeclampsia: Recognition and Determining Clinical Usefulness. Hypertension 2021, 77, 1430–1441. [Google Scholar] [CrossRef]
- Lisonkova, S.; Joseph, K.S. Incidence of Preeclampsia: Risk Factors and Outcomes Associated with Early- versus Late-Onset Disease. Am. J. Obstet. Gynecol. 2013, 209, e1–e544. [Google Scholar] [CrossRef]
- Melchiorre, K.; Giorgione, V.; Thilaganathan, B. The Placenta and Preeclampsia: Villain or Victim? Am. J. Obstet. Gynecol. 2022, 226 (Suppl. S2), S954–S962. [Google Scholar] [CrossRef] [PubMed]
- Chaemsaithong, P.; Sahota, D.S.; Poon, L.C. First Trimester Preeclampsia Screening and Prediction. Am. J. Obstet. Gynecol. 2022, 226 (Suppl. S2), S1071–S1097.e2. [Google Scholar] [CrossRef] [PubMed]
- Paul, T.D.; Hastie, R.; Tong, S.; Keenan, E.; Hiscock, R.; Brownfoot, F.C. Prediction of Adverse Maternal Outcomes in Preeclampsia at Term. Pregnancy Hypertens. 2019, 18, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Cristodoro, M.; Messa, M.; Tossetta, G.; Marzioni, D.; Dell’Avanzo, M.; Inversetti, A.; Di Simone, N. First Trimester Placental Biomarkers for Pregnancy Outcomes. Int. J. Mol. Sci. 2024, 25, 6136. [Google Scholar] [CrossRef]
- Miranda, M.L.; Macher, H.C.; Muñoz-Hernández, R.; Vallejo-Vaz, A.; Moreno-Luna, R.; Villar, J.; Guerrero, J.M.; Stiefel, P. Role of Circulating Cell-Free DNA Levels in Patients with Severe Preeclampsia and HELLP Syndrome. Am. J. Hypertens. 2013, 26, 1377–1380. [Google Scholar] [CrossRef]
- Contro, E.; Bernabini, D.; Farina, A. Cell-Free Fetal DNA for the Prediction of Pre-Eclampsia at the First and Second Trimesters: A Systematic Review and Meta-Analysis. Mol. Diagn. Ther. 2017, 21, 125–135. [Google Scholar] [CrossRef]
- Ling, L.; Huang, H.; Zhu, L.; Mao, T.; Shen, Q.; Zhang, H. Evaluation of Plasma Endothelial Microparticles in Pre-Eclampsia. J. Int. Med. Res. 2014, 42, 42–51. [Google Scholar] [CrossRef]
- McElrath, T.F.; Cantonwine, D.E.; Gray, K.J.; Mirzakhani, H.; Doss, R.C.; Khaja, N.; Khalid, M.; Page, G.; Brohman, B.; Zhang, Z.; et al. Late First Trimester Circulating Microparticle Proteins Predict the Risk of Preeclampsia < 35 Weeks and Suggest Phenotypic Differences among Affected Cases. Sci. Rep. 2020, 10, 17353. [Google Scholar] [CrossRef]
- Zwertbroek, E.F.; Broekhuijsen, K.; Langenveld, J.; van Baaren, G.-J.; van den Berg, P.P.; Bremer, H.A.; Ganzevoort, W.; van Loon, A.J.; Mol, B.W.J.; van Pampus, M.G.; et al. Prediction of Progression to Severe Disease in Women with Late Preterm Hypertensive Disorders of Pregnancy. Acta Obstet. Gynecol. Scand. 2017, 96, 96–105. [Google Scholar] [CrossRef]
- Ukah, U.V.; Payne, B.; Hutcheon, J.A.; Ansermino, J.M.; Ganzevoort, W.; Thangaratinam, S.; Magee, L.A.; von Dadelszen, P. Assessment of the fullPIERS Risk Prediction Model in Women with Early-Onset Preeclampsia. Hypertension 2018, 71, 659–665. [Google Scholar] [CrossRef]
- Bian, X.; Biswas, A.; Huang, X.; Lee, K.J.; Li, T.K.-T.; Masuyama, H.; Ohkuchi, A.; Park, J.S.; Saito, S.; Tan, K.H.; et al. Short-Term Prediction of Adverse Outcomes Using the sFlt-1 (Soluble Fms-Like Tyrosine Kinase 1)/PlGF (Placental Growth Factor) Ratio in Asian Women with Suspected Preeclampsia. Hypertension 2019, 74, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.; Palmer, K.; Rolnik, D.L.; Wallace, E.M.; Mol, B.W.; Da Silva Costa, F. Role of Placental, Fetal and Maternal Cardiovascular Markers in Predicting Adverse Outcome in Women with Suspected or Confirmed Pre-Eclampsia. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2022, 59, 596–605. [Google Scholar] [CrossRef]
- Sun, Y.; Meng, C.; Yu, L. Correlation of Serum IGF-1 and sFlt-1 Levels with Adverse Pregnancy Outcomes in Patients with Severe Preeclampsia. Altern. Ther. Health Med. 2023, 29, 364–369. [Google Scholar] [PubMed]
- Townsend, R.; Khalil, A.; Premakumar, Y.; Allotey, J.; Snell, K.I.E.; Chan, C.; Chappell, L.C.; Hooper, R.; Green, M.; Mol, B.W.; et al. Prediction of Pre-Eclampsia: Review of Reviews. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2019, 54, 16–27. [Google Scholar] [CrossRef]
- Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin Summary, Number 222. Obstet. Gynecol. 2020, 135, 1492–1495. [CrossRef] [PubMed]
- Overview|Hypertension in Pregnancy: Diagnosis and Management|Guidance|NICE. Available online: https://www.nice.org.uk/guidance/ng133 (accessed on 12 October 2023).
- Tan, M.Y.; Wright, D.; Syngelaki, A.; Akolekar, R.; Cicero, S.; Janga, D.; Singh, M.; Greco, E.; Wright, A.; Maclagan, K.; et al. Comparison of Diagnostic Accuracy of Early Screening for Pre-Eclampsia by NICE Guidelines and a Method Combining Maternal Factors and Biomarkers: Results of SPREE. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2018, 51, 743–750. [Google Scholar] [CrossRef]
- Wright, D.; Wright, A.; Nicolaides, K.H. The Competing Risk Approach for Prediction of Preeclampsia. Am. J. Obstet. Gynecol. 2020, 223, 12–23.e7. [Google Scholar] [CrossRef]
- Huang, X.; Jia, L.; Jia, Y.; Xu, X.; Wang, R.; Wei, M.; Li, H.; Peng, H.; Wei, Y.; He, Q.; et al. sFlt-1-Enriched Exosomes Induced Endothelial Cell Dysfunction and a Preeclampsia-like Phenotype in Mice. Cytokine 2023, 166, 156190. [Google Scholar] [CrossRef]
- Zhai, Y.; Liu, Y.; Qi, Y.; Long, X.; Gao, J.; Yao, X.; Chen, Y.; Wang, X.; Lu, S.; Zhao, Z. The Soluble VEGF Receptor sFlt-1 Contributes to Endothelial Dysfunction in IgA Nephropathy. PLoS ONE 2020, 15, e0234492. [Google Scholar] [CrossRef]
- Mauricio, R.; Singh, K.; Sanghavi, M.; Ayers, C.R.; Rohatgi, A.; Vongpatanasin, W.; de Lemos, J.A.; Khera, A. Soluble Fms-like Tyrosine Kinase-1 (sFlt-1) Is Associated with Subclinical and Clinical Atherosclerotic Cardiovascular Disease: The Dallas Heart Study. Atherosclerosis 2022, 346, 46–52. [Google Scholar] [CrossRef]
- Hammadah, M.; Georgiopoulou, V.V.; Kalogeropoulos, A.P.; Weber, M.; Wang, X.; Samara, M.A.; Wu, Y.; Butler, J.; Tang, W.H.W. Elevated Soluble Fms-Like Tyrosine Kinase-1 and Placental-Like Growth Factor Levels Are Associated with Development and Mortality Risk in Heart Failure. Circ. Heart Fail. 2016, 9, e002115. [Google Scholar] [CrossRef]

| Women Stratified Based on Whether They Developed or Did Not Develop Preeclampsia | Women Who Later Developed Preeclampsia, Stratified by Time of Diagnosis | |||||
|---|---|---|---|---|---|---|
| Women Who Did Not Develop Preeclampsia | Women Who Developed Preeclampsia | p-Value | Early-Onset Preeclampsia | Late-Onset Preeclampsia | p-Value | |
| n = 35 | n = 54 | -- | n = 23 | n = 31 | -- | |
| Age, years | 34.45 (32.0–39.4) | 34.6 (30.0–37.8) | 0.383 | 34.87 (31.7–36.9) | 34.54 (29.7–38.2) | 0.923 |
| Gestational week at blood sample obtention, weeks | 10.57 (10.2–11.2) | 10.22 (9.9–10.7) | 0.07 | 10.2 (9.9–10.7) | 10.3 (9.9–10.9) | 1.0 |
| Multiple gestation | 1 (2.9%) | 6 (11.1%) | 0.238 | 3 (13.0%) | 3 (9.7%) | 0.697 |
| Pregnancy from IVF | 2 (5.7%) | 11 (20.4%) | 0.056 | 8 (34.8%) | 3 (9.7%) | 0.024 |
| Primiparous mothers | 5 (14.3%) | 26 (48.1%) | 0.001 | 15 (65.2%) | 11 (35.5%) | 0.031 |
| Number of prior gestations | 2.0 (2.0–3.0) | 2.0 (1.0–2.0) | 0.022 | 1.0 (0.0–1.0) | 1.0 (0.0–2.0) | 0.031 |
| Prior miscarriage/abortion | 11 (31.4%) | 20 (37.0%) | 0.587 | 4 (17.4%) | 16 (51.6%) | 0.010 |
| Number of prior miscarriages/abortions | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) | 0.465 | 0.0 (0.0–0.0) | 1.0 (0.0–1.0) | 0.026 |
| Preeclampsia in prior gestation(s) | 1 (2.9%) | 2 (3.7%) | 1.000 | 0 (0.0%) | 2(6.5%) | 0.214 |
| Fetal sex, male | 16 (45.7%) | 28 (51.9%) | 0.572 | 12 (52.2%) | 16 (51.6%) | 0.967 |
| If 2 fetuses, fetal sex of the 2nd fetus, male | 1 (16.7%) | 5 (83.3%) | 1.000 | 2 (40%) | 3 (60%) | 0.273 |
| If 3 fetuses, fetal sex of the 3rd fetus, male | 1 (100%) | -- | -- | -- | -- | -- |
| Body mass index, kg/m2 | 24.3 (21.4–27.8) | 24.8 (22.3–31.4) | 0.090 | 25.2 (22.7–30.8) | 24.6 (22.3–35.8) | 0.871 |
| Systolic blood pressure, mmHg | 110.0 (100.0–117.0) | 111.5 (108.3–125.0) | 0.006 | 115.0 (109.0–120.0) | 111.0 (106.0–130.0) | 0.705 |
| Diastolic blood pressure, mmHg | 62.0 (60.0–70.0) | 70.0 (60.0–79.3) | 0.004 | 70.0 [60.0–75.0] | 70.0 [60.0–80.0] | 0.691 |
| Treatment with aspirin | 2 (5.7%) | 10 (18.5%) | 0.084 | 5 (21.7%) | 5 (16.1%) | 0.6 |
| Treatment with heparin | 2 (5.7%) | 2(3.7%) | 0.644 | 1 (4.3%) | 1 (3.2%) | 0.829 |
| Hb, g/dL | 12.81 ± 1.0 | 12.88 ± 1.0 | 0.453 | 12.94 ± 1.1 | 12.85 ± 1.0 | 0.774 |
| Platelets, 103/mL | 255 (201–295) | 279 (247–302) | 0.097 | 261 (219–302) | 284 (255–304) | 0.351 |
| Women Who Did Not Develop Preeclampsia | Women Who Developed Preeclampsia | p-Value for Control vs. PE | Early-Onset PE | Late-Onset PE | p-Value for Early vs. Late-Onset PE | p-Value for Control vs. Early-Onset PE | p-Value for Control vs. Late-Onset PE | |
|---|---|---|---|---|---|---|---|---|
| n = 35 | n = 54 | n = 23 | n = 31 | -- | ||||
| sFlt-1, pg/mL | 1366 [1221–1857] | 1261 [1003.6–1776.5] | 0.320 | 1090 [909–1802] | 1366 [1144–1768] | 0.349 | 0.190 | 0.644 |
| PlGF, pg/mL | 37.12 [23.97–50.4] | 25.94 [17.3–34.6] | <0.001 | 19.26 [14.86–28.92] | 29.13 [21.48–36.21] | 0.005 | <0.001 | 0.052 |
| sFlt-1/PlGF | 42.32 [32.7–50.8] | 50.09 [38.8–84.4] | 0.015 | 61.67 [40.79–110.5] | 48.39 [36.8–58.94] | 0.049 | 0.005 | 0.156 |
| β -hCG, ng/mL | 61 [38.5–77.7] | 36 [25–63.8] | 0.02 | 35.9 [27.6–64.8] | 37 [23.9–63.4] | 0.937 | 0.007 | 0.010 |
| PAPP-A, mUI/mL | 1.77 [0.78–2.38] | 1.01 [0.61–2.52] | 0.248 | 0.7 [0.45–1.53] | 1.65 [0.73–3.35] | 0.037 | 0.013 | 0.807 |
| cfDNA, ng/mL | 849 [512–1580] | 1210 [761–2060] | 0.020 | 1170 [715–1910] | 1580 [777–2240] | 0.588 | 0.117 | 0.022 |
| Total MVs *, (U/µL) | 4775 [2781–7216] | 5023 [2475–13,841] | 0.540 | 3268 [1808–6067] | 7445 [3103–18,584] | 0.030 | 0.259 | 0.065 |
| Endothelial MVs *, (AU/µL) | 28.16 [17.9–44.12] | 45.29 [8.2–327.1] | 0.373 | 26.9 [8.12–245.6] | 93.2 [12.5–339] | 0.254 | 0.667 | 0.089 |
| Platelet MVs *, (U/µL) | 669.3 [411.6–1282.8] | 829.1 [331.7–4686.8] | 0.259 | 495.9 [300–833.9] | 1512.8 [668.5–7697] | 0.023 | 0.291 | 0.010 |
| Women Stratified Based on Whether They Developed or Did Not Develop Preeclampsia | Women Who Later Developed Preeclampsia, Stratified by Moment of Diagnosis | |||||
|---|---|---|---|---|---|---|
| Women Who Did Not Develop Preeclampsia | Women Who Developed Preeclampsia | p-Value | Early-Onset PE | Late-Onset PE | p-Value | |
| n = 35 | n = 54 | -- | n = 23 | n = 31 | -- | |
| Composite maternal–fetal outcome | 4 (11.4%) | 42 (77.8%) | <0.001 | 21 (91.3%) | 21 (67.7%) | 0.039 |
| Maternal outcomes | -- | -- | -- | -- | -- | -- |
| Composite maternal outcome | 0 | 7 (13%) | 0.039 | 2 (8.7%) | 5 (16.1%) | 0.685 |
| Individual events: | -- | -- | -- | -- | -- | -- |
| 0 | 12 (22.2%) | 0.001 | 7 (30.4%) | 5 (16.1%) | 0.211 |
| 0 | 0 | -- | 0 | 0 | -- |
| 0 | 0 | -- | 0 | 0 | -- |
| 0 | 5 (9.3%) | 0.152 | 2 (8.7%) | 3 (9.7%) | 1.000 |
| 0 | 3 (5.6%) | 0.276 | 1 (4.3%) | 2 (6.5%) | 1.000 |
| 0 | 1 (1.9%) | 1.000 | 0 | 1(3.2%) | 1.000 |
| 11 (31.4%) | 44 (81.5%) | <0.001 | 21 (91.3%) | 23 (74.2%) | 0.161 |
| 0 | 1 (1.9%) | 1.000 | 0 | 1(3.2%) | 1.000 |
| 0 | 0 | -- | 0 | 0 | -- |
| Other maternal events: | -- | -- | -- | -- | -- | -- |
| 1(2.9%) | 3 (5.6%) | 1.000 | 1 (4.3%) | 2 (6.5%) | 1.000 |
| Fetal outcomes | -- | -- | -- | -- | -- | -- |
| Composite fetal outcome | 4(11.4%) | 39 (72.2%) | <0.001 | 21 (91.3%) | 18 (58.1%) | 0.007 |
| Individual events: | -- | -- | -- | -- | -- | -- |
| 0 | 24 (44.4%) | <0.001 | 14 (60.9%) | 10 (32.3%) | 0.036 |
| 2 (5.7%) | 18 (33.3%) | 0.002 | 14 (60.9%) | 4 (12.9%) | <0.001 |
| 0 | 0 | -- | 0 | 0 | -- |
| 0 | 1 (1.9%) | 1.000 | 0 | 1 (3.2%) | 1.000 |
Other fetal events: | -- | -- | -- | -- | -- | -- |
| 4.5 (3–6) | 10 (4–19) | 0.379 | 4 (0–17) | 0 | <0.001 |
| 3 (2–3) | 5 (3–20) | <0.001 | 21 (13–28) | 4 (3–4) | <0.001 |
| Women Without Severe Adverse Events | Women with Severe Adverse Events | p-Value | |
|---|---|---|---|
| n = 82 | n = 7 | -- | |
| sFlt-1, pg/mL | 1278.0 [1049.0–1752.0] | 2126.0 [1922.0–2325.0] | 0.009 |
| PlGF, pg/mL | 28.9 [20.4–40.9] | 29.6 [19.1–38.2] | 0.819 |
| sFlt-1/PlGF | 46.0 [35.0–56.2] | 54.1 [40.9–99.0] | 0.148 |
| cfDNA, ng/mL | 1010.0 [648.0–1725.0] | 1965.0 [1680.0–3080.0] | 0.041 |
| Total MVs, (U/µL) | 4862.3 [2517.1–9345.0] | 5410.2 [2795.5–13,841.2] | 0.745 |
| Endothelial MVs, (U/µL) | 34.1 [14.3–78.9] | 80.4 [14.2–4597.2] | 0.471 |
| Platelet MVs, (U/µL) | 812.3 [403.9–1575.2] | 1073.5 [436.8–7697.6] | 0.576 |
| β-hCG, ng/mL | 40.8 [29.3–65.8] | 81.4 [37.3–94.7] | 0.048 |
| PAPP-A, mUI/mL | 1.2 [0.6–2.3] | 3.2 [1.0–6.0] | 0.046 |
| Total microvesicles AV+; endothelial microvesicles AV+ CD31+ CD41−; platelet microvesicles AV+ CD31+ CD41+. | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camacho-Carrasco, A.; Montenegro-Martínez, J.; Miranda-Guisado, M.L.; Muñoz-Hernández, R.; Salsoso, R.; Fatela-Cantillo, D.; García-Díaz, L.; Stiefel García-Junco, P.; Mate, A.; Vázquez, C.M.; et al. Association of First-Trimester Maternal Biomarkers with Preeclampsia and Related Maternal and Fetal Severe Adverse Events. Int. J. Mol. Sci. 2025, 26, 6684. https://doi.org/10.3390/ijms26146684
Camacho-Carrasco A, Montenegro-Martínez J, Miranda-Guisado ML, Muñoz-Hernández R, Salsoso R, Fatela-Cantillo D, García-Díaz L, Stiefel García-Junco P, Mate A, Vázquez CM, et al. Association of First-Trimester Maternal Biomarkers with Preeclampsia and Related Maternal and Fetal Severe Adverse Events. International Journal of Molecular Sciences. 2025; 26(14):6684. https://doi.org/10.3390/ijms26146684
Chicago/Turabian StyleCamacho-Carrasco, Ana, Jorge Montenegro-Martínez, María Luisa Miranda-Guisado, Rocío Muñoz-Hernández, Rocío Salsoso, Daniel Fatela-Cantillo, Lutgardo García-Díaz, Pablo Stiefel García-Junco, Alfonso Mate, Carmen M. Vázquez, and et al. 2025. "Association of First-Trimester Maternal Biomarkers with Preeclampsia and Related Maternal and Fetal Severe Adverse Events" International Journal of Molecular Sciences 26, no. 14: 6684. https://doi.org/10.3390/ijms26146684
APA StyleCamacho-Carrasco, A., Montenegro-Martínez, J., Miranda-Guisado, M. L., Muñoz-Hernández, R., Salsoso, R., Fatela-Cantillo, D., García-Díaz, L., Stiefel García-Junco, P., Mate, A., Vázquez, C. M., Alfaro-Lara, V., Vallejo-Vaz, A. J., & Beltrán-Romero, L. M. (2025). Association of First-Trimester Maternal Biomarkers with Preeclampsia and Related Maternal and Fetal Severe Adverse Events. International Journal of Molecular Sciences, 26(14), 6684. https://doi.org/10.3390/ijms26146684









