Retinoic Acid Induced 1 and Smith–Magenis Syndrome: From Genetics to Biology and Possible Therapeutic Strategies
Abstract
1. Introduction
2. Haploinsufficiency Disorders
2.1. Smith–Magenis and Potocki–Lupski Syndromes
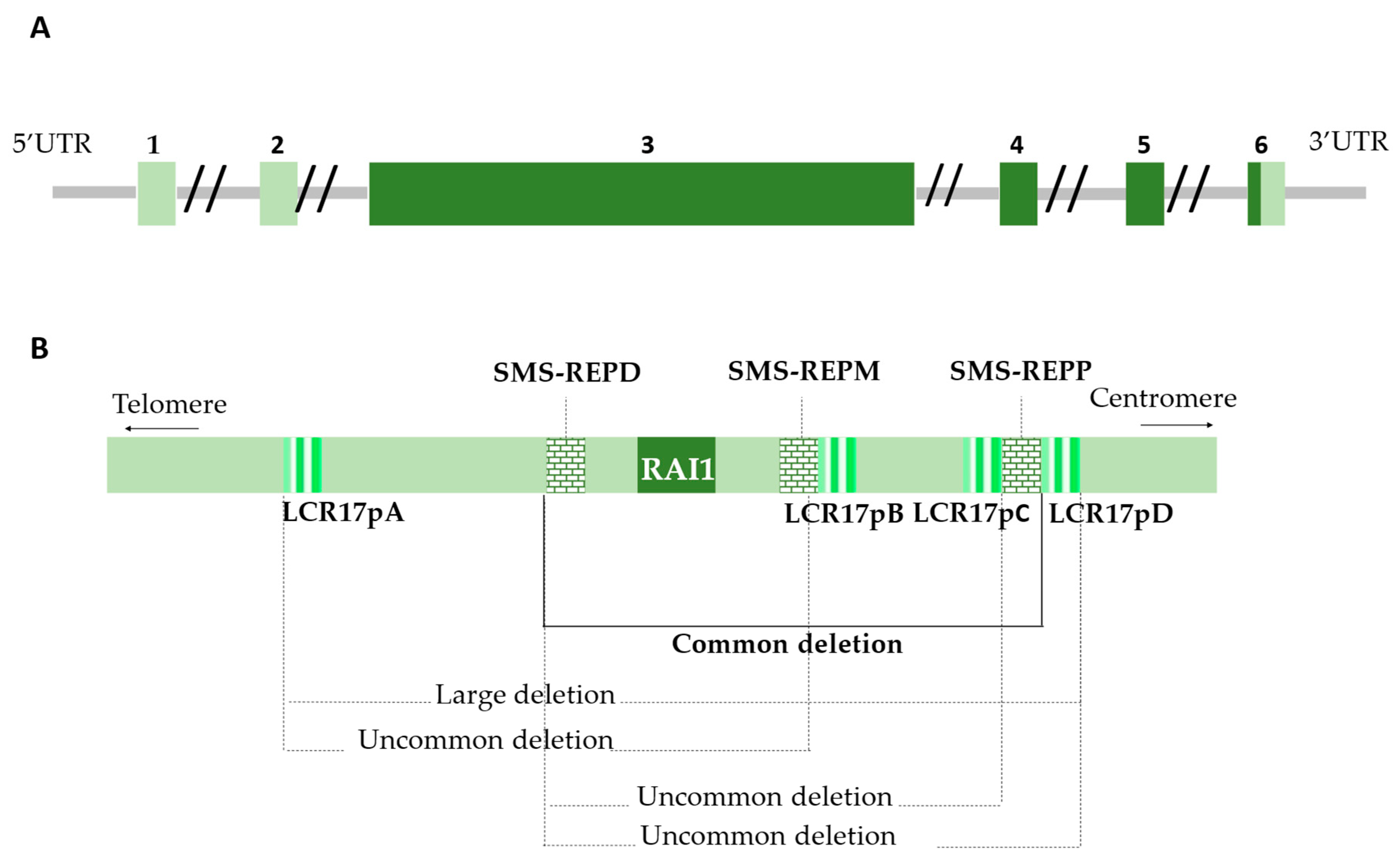
2.1.1. The 17p11.2 Chromosomal Region
2.1.2. Divergent Outcomes in 17p11.2 Imbalances
2.1.3. SMS and PTLS Epidemiology and Clinical Features
3. Retinoic Acid Induced 1 (RAI1)
3.1. Genetics
3.2. Biological Functions
3.2.1. Retinoic Acid Induced 1 Protein Structure
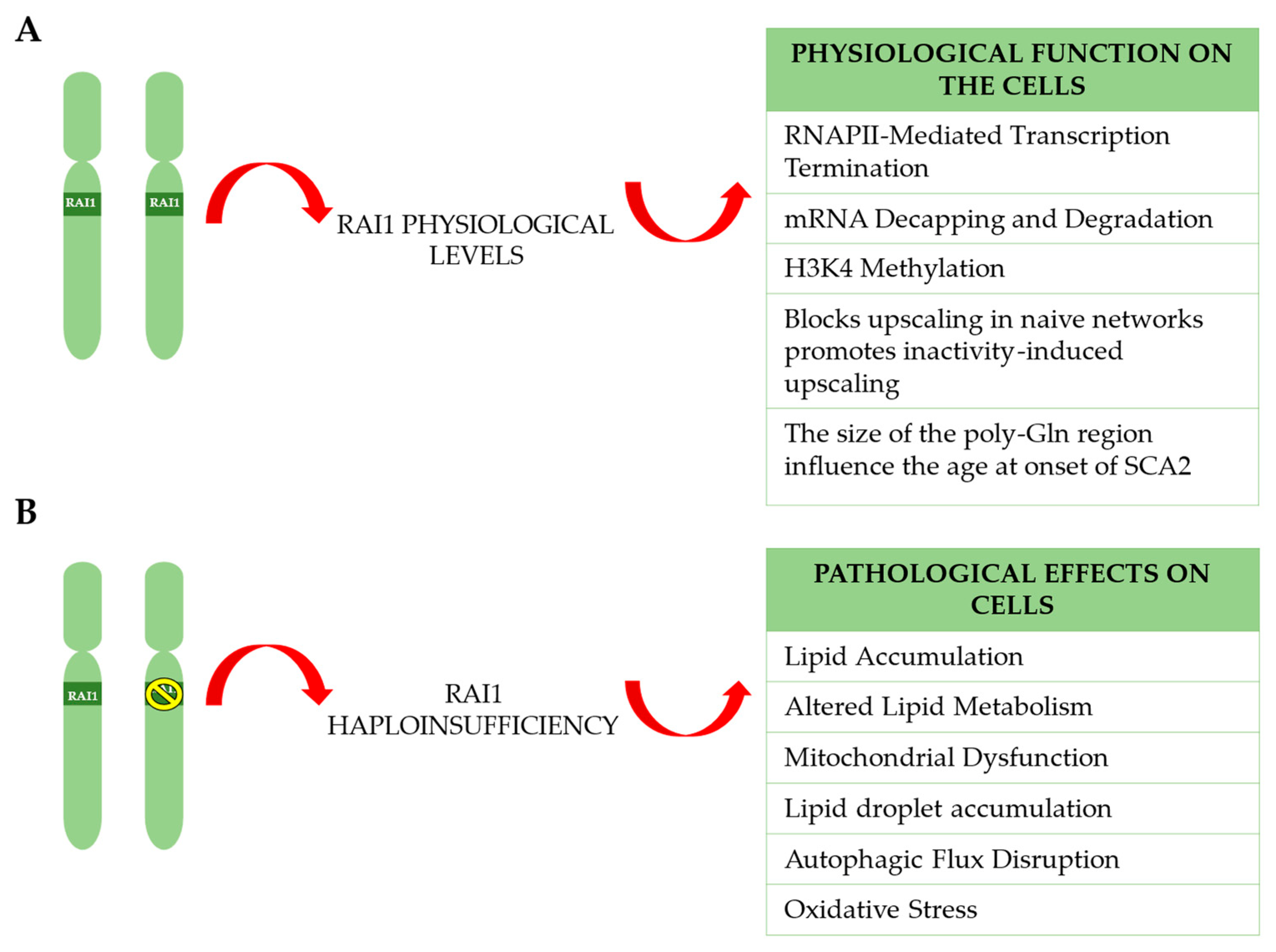
3.2.2. RAI1’s Role in RNAPII-Mediated Transcription Termination in Eukaryotic Cells
3.2.3. RAI1 in mRNA Decapping and Degradation
3.2.4. RAI1 Key Regulator of H3K4 Methylation Ensures Normal Brain Development
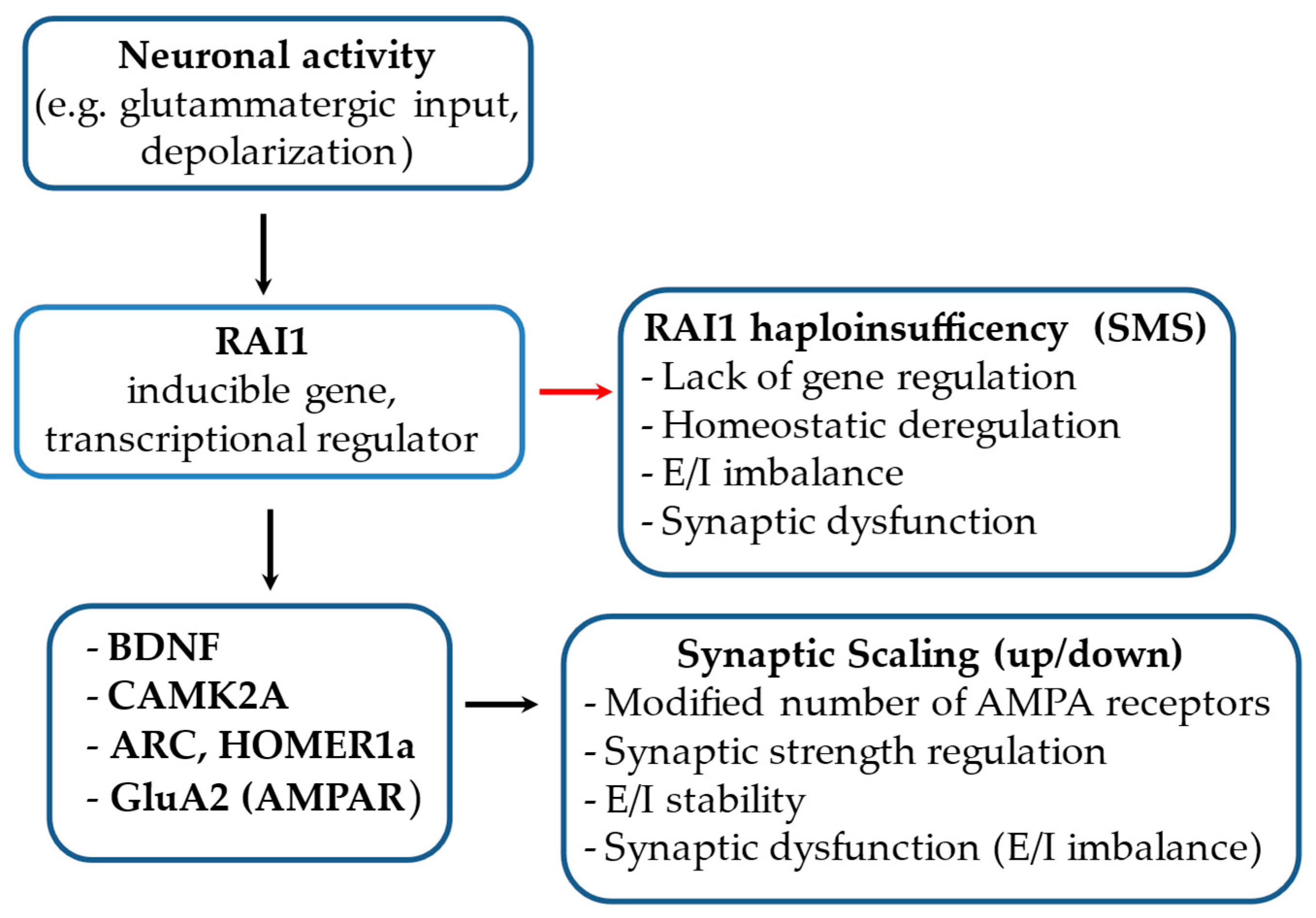
3.2.5. RAI1 Blocks Upscaling in Naive Networks and Promotes Inactivity-Induced Upscaling
3.2.6. Role of RAI1 in Modulating Age at Onset in SCA2 and Convergence with ATXN2-Associated Pathways
4. Pathophysiological Mechanisms in RAI1 Haploinsufficiency
4.1. Lipid Accumulation and Altered Lipid Metabolism
4.2. Autophagic Flux Disruption
4.3. Mitochondrial Dysfunction and Oxidative Stress
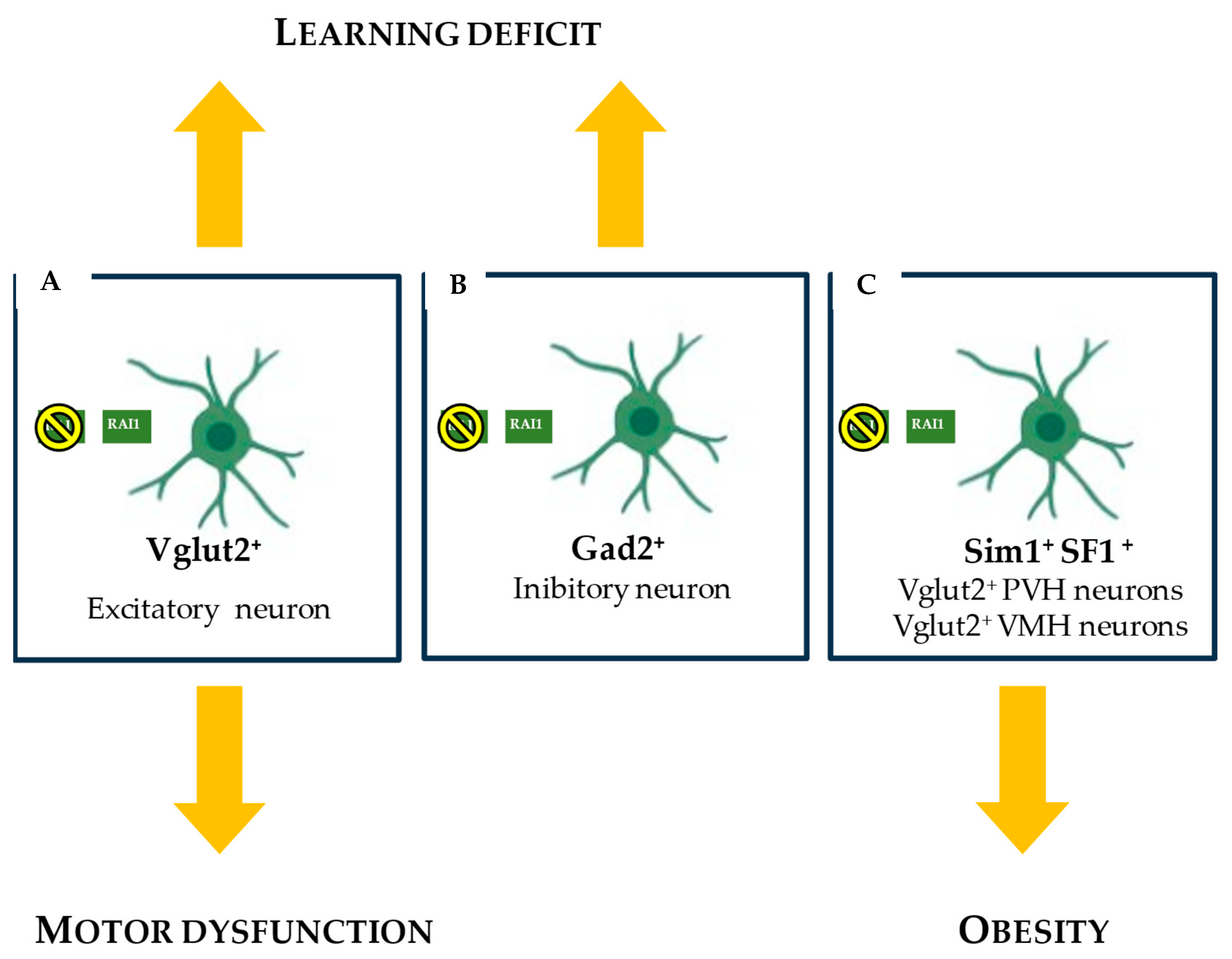
5. RAI1 and Smith–Magenis Syndrome Neurobiology
RAI1 and BDNF in SMS Cells: Disruption of Appetite Regulation
6. Gene Therapies and Alternative Treatment Approaches for SMS
6.1. CRISPRa Enhancing Expression from the Endogenous RAI1 Functional Allele in a Tissue-Specific Manner
6.2. RNA Therapeutic Oligonucleotides: SINEUPs
6.3. Synthetic Drugs
6.3.1. N-Acetylcysteine Modifies the SMS Cell Phenotype
6.3.2. Avenue Strategies Treating Obesity Targeting BDNF
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALDH3A2 | Aldehyde dehydrogenase family 3 member A2 |
| ASAH1 | N-Acylsphingosine Amidohydrolase 1 |
| ASDs | Autism spectrum disorders |
| ATXN2 | Spinocerebellar Ataxia Type 2 |
| ATXN3 | Spinocerebellar Ataxia Type 3 |
| AAO | Age at onset |
| BDNF | Brain-derived neurotrophic factor |
| BMAL1 | Basic helix–loop–helix ARNT-like protein 1 |
| CACNA1A | Calcium voltage-gated channel subunit Alpha1 A |
| CHD8 | Chromodomain Helicase DNA-Binding 8 gene |
| CLN3 | CLN3 lysosomal/endosomal transmembrane protein |
| CLOCK | Clock circadian regulator |
| CNS | Central nervous system |
| CoREST | Co-repressor for element-1-silencing transcription factor |
| CRISPRa | CRISPR-mediated activation |
| CTNS | Cystinosin |
| CTSH | Cathepsin H |
| dCas9 | Nuclease-deficient Cas9 |
| DFNB3 | Deafness, autosomal recessive 3 |
| DHCR7 | 7-dehydrocholesterol reductase |
| EEG | Electroencephalogram |
| EHMT1/2 | Histone-lysine N-methyltransferase |
| Ephd | Extended plant homeodomain |
| FLCN | Folliculin |
| GDNF | Glial cell line-derived neurotrophic factor |
| GO | Gene ontology |
| GSH | Glutathione |
| HDAC4 | Histone deacetylase 4 |
| HEXA | Hexosaminidase Subunit Alpha |
| HEXB | Hexosaminidase Subunit Beta |
| hiNPCs | Human induced neuronal progenitor cells |
| HGMD | Human Gene Mutation Database |
| H3K4 | Histone H3 lysine 4 |
| H3K4me | Histone H3 lysine 4 methylation |
| HMG20A | High mobility group 20A, Homo sapiens |
| HSPG2 | Heparan Sulfate Proteoglycan 2 |
| iBRAF | High mobility group protein 20A, mouse |
| IgA | Immunoglobulin A |
| IGF2 | Insulin-like growth factor 2 |
| IGF2R | Insulin-like growth factor 2 receptor |
| KD | Knockdown |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| KMT2A | Histone-lysine N-methyltransferase 2A |
| KOW5 | K homology (KH) domain-containing protein with an Oligomeric structure and a weak homology to the RNA-binding proteins |
| LC3-II | Microtubule-associated protein light chain 3-II |
| LC–MS/MS | Liquid chromatography–tandem mass spectrometry |
| LDL | Low-density lipoprotein |
| LGMN | Legumain |
| LLGL1 | Lethal(2) giant larvae protein homolog 1 |
| LSD1 | Lysine-specific histone demethylase 1 |
| MIO15A | Unconventional myosin-XV |
| MLL1 | Mixed-lineage leukemia 1 |
| mTORC1 | Mammalian target of rapamycin complex 1 |
| MYO15A | Unconventional myosin-XV |
| NAC | N-acetylcysteine |
| NAHR | Non-allelic homologous recombination |
| NDDs | Neurodevelopmental disorders |
| NOS1 | Nitric oxide synthase 1 |
| PHF14 | PHD finger protein 14 |
| PER | Period |
| PVH | Paraventricular nucleus of hypothalamus |
| rAAV | Recombinant adeno-associated viral vector |
| Rat1 | 5′-3′ exoribonuclease 2 |
| RNAPII | RNA polymerase II |
| Rtt103 | Regulator of Ty1 transposition protein 103 |
| SCA2 | Spinocerebellar Ataxia Type 2 |
| SHMT1 | Serine hydroxymethyltransferase, cytosolic |
| SLC16A1 | Solute carrier family 16 member 1 |
| SLC17A5 | Solute carrier family 17 member 5 |
| SORT1 | Sortilin 1 |
| Spt4/5 | Transcription elongation factor SPT4/5 |
| Spt6 | Transcription elongation factor SPT6 |
| SQLE | Squalene Epoxidase |
| SREBF1 | Sterol regulatory element-binding protein 1 |
| TCF20 | Transcription factor 20 |
| TET3 | Methylcytosine dioxygenase |
| TIM | Timeless |
| TNFRSF13B | Tumor necrosis factor receptor superfamily member 13B |
| TRKB | Tropomyosin receptor kinase B signaling |
| TTR | Transthyretin |
| TTX | Tetrodotoxin |
| UTRs | Untranslated regions |
| VMH | Ventromedial nucleus of hypothalamus |
| Xrn2 | 5′-3′ exoribonuclease |
References
- Wilkie, A.O.M. The Molecular Basis of Genetic Dominance. J. Med. Genet. 1994, 31, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Javed, S.; Selliah, T.; Lee, Y.J.; Huang, W.H. Dosage-sensitive genes in autism spectrum disorders: From neurobiology to therapy. Neurosci. Biobehav. Rev. 2020, 118, 538–567. [Google Scholar] [CrossRef]
- Falco, M.; Amabile, S.; Acquaviva, F. Rai1 Gene Mutations: Mechanisms of Smith–Magenis Syndrome. Appl. Clin. Genet. 2017, 10, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, T.; Murayama, Y.; Ehara, H.; Goto, M.; Aoki, M.; Sekine, S.I. Structural Basis of Eukaryotic Transcription Termination by the Rat1 Exonuclease Complex. Nat. Commun. 2024, 15, 7854. [Google Scholar] [CrossRef] [PubMed]
- Klama, S.; Hirsch, A.G.; Schneider, U.M.; Zander, G.; Seel, A.; Krebber, H. A Guard Protein Mediated Quality Control Mechanism Monitors 5’-Capping of Pre-MRNAs. Nucleic Acids Res. 2022, 50, 11301–11314. [Google Scholar] [CrossRef]
- Garay, P.M.; Wallner, M.A.; Iwase, S. Yin-Yang Actions of Histone Methylation Regulatory Complexes in the Brain. Epigenomics 2016, 8, 1689–1708. [Google Scholar] [CrossRef]
- Garay, P.M.; Chen, A.; Tsukahara, T.; Rodríguez Díaz, J.C.; Kohen, R.; Althaus, J.C.; Wallner, M.A.; Giger, R.J.; Jones, K.S.; Sutton, M.A.; et al. RAI1 Regulates Activity-Dependent Nascent Transcription and Synaptic Scaling. Cell Rep. 2020, 32, 108002. [Google Scholar] [CrossRef]
- Williams, S.R.; Zies, D.; Mullegama, S.V.; Grotewiel, M.S.; Elsea, S.H. Smith-Magenis Syndrome Results in Disruption of CLOCK Gene Transcription and Reveals an Integral Role for RAI1 in the Maintenance of Circadian Rhythmicity. Am. J. Hum. Genet. 2012, 90, 941–949. [Google Scholar] [CrossRef]
- Turco, E.M.; Giovenale, A.M.G.; Sireno, L.; Mazzoni, M.; Cammareri, A.; Marchioretti, C.; Goracci, L.; Di Veroli, A.; Marchesan, E.; D’Andrea, D.; et al. Retinoic Acid-Induced 1 Gene Haploinsufficiency Alters Lipid Metabolism and Causes Autophagy Defects in Smith-Magenis Syndrome. Cell Death Dis. 2022, 13, 981. [Google Scholar] [CrossRef]
- Huang, W.H.; Guenthner, C.J.; Xu, J.; Nguyen, T.; Schwarz, L.A.; Wilkinson, A.W.; Gozani, O.; Chang, H.Y.; Shamloo, M.; Luo, L. Molecular and Neural Functions of Rai1, the Causal Gene for Smith-Magenis Syndrome. Neuron 2016, 92, 392–406. [Google Scholar] [CrossRef]
- Elsea, S.H.; Williams, S.R. Smith-Magenis Syndrome: Haploinsufficiency of RAI1 Results in Altered Gene Regulation in Neurological and Metabolic Pathways. Expert Rev. Mol. Med. 2011, 13, e14. [Google Scholar] [CrossRef] [PubMed]
- Javed, S.; Chang, Y.-T.; Cho, Y.; Lee, Y.-J.; Chang, H.-C.; Haque, M.; Lin, Y.C.; Huang, W.-H. Smith-Magenis Syndrome Protein RAI1 Regulates Body Weight Homeostasis through Hypothalamic BDNF-Producing Neurons and Neurotrophin Downstream Signalling. eLife 2023, 12, 90333. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.C.; Lee, Y.J.; Javed, S.; Haque, M.; Chang, Y.T.; Lin, Y.C.; Oram, C.; Huang, W.H. RAAV-CRISPRa Therapy Corrects Rai1 Haploinsufficiency and Rescues Selective Disease Features in Smith-Magenis Syndrome Mice. J. Biol. Chem. 2023, 299, 102728. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.C.M.; Mcgavran, L.; Robinson, J.; Waldstein, G.; Macfarlane, J.; Zonona, J.; Reiss, J.; Lahr, M.; Allen, L.; Magenis, E. Interstitial Deletion of (17)(P11.2p11.2) in Nine Patients. Am. J. Med. Genet. 1986, 24, 393–414. [Google Scholar] [CrossRef]
- Espinoza, S.; Bon, C.; Valentini, P.; Pierattini, B.; Matey, A.T.; Damiani, D.; Pulcrano, S.; Sanges, R.; Persichetti, F.; Takahashi, H.; et al. SINEUPs: A Novel Toolbox for RNA Therapeutics. Essays Biochem. 2021, 65, 775–789. [Google Scholar]
- Johnson, A.F.; Nguyen, H.T.; Veitia, R.A. Causes and Effects of Haploinsufficiency. Biol. Rev. 2019, 94, 1774–1785. [Google Scholar] [CrossRef]
- Deutschbauer, A.M.; Jaramillo, D.F.; Proctor, M.; Kumm, J.; Hillenmeyer, M.E.; Davis, R.W.; Nislow, C.; Giaever, G. Mechanisms of Haploinsufficiency Revealed by Genome-Wide Profiling in Yeast. Genetics 2005, 169, 1915–1925. [Google Scholar] [CrossRef]
- Veitia, R.A. Exploring the Etiology of Haploinsufficiency. Bioessays 2002, 24, 175–184. [Google Scholar] [CrossRef]
- Fuller, Z.L.; Berg, J.J.; Mostafavi, H.; Sella, G.; Przeworski, M. Measuring Intolerance to Mutation in Human Genetics. Nat. Genet. 2019, 51, 772–776. [Google Scholar] [CrossRef]
- Gropman, A.L.; Adams, D.R. Atypical Patterns of Inheritance. Semin. Pediatr. Neurol. 2007, 14, 34–45. [Google Scholar] [CrossRef]
- Dang, V.T.; Kassahn, K.S.; Marcos, A.E.; Ragan, M.A. Identification of Human Haploinsufficient Genes and Their Genomic Proximity to Segmental Duplications. Eur. J. Hum. Genet. 2008, 16, 1350–1357. [Google Scholar] [CrossRef] [PubMed]
- Lupski, J.R. Genomic Disorders: Structural Features of the Genome Can Lead to DNA Rearrangements and Human Disease Traits. Trends Genet. 1998, 14, 417–422. [Google Scholar] [CrossRef]
- Correa, C.L.; Brems, H.; Lázaro, C.; Marynen, P.; Legius, E. Unequal Meiotic Crossover: A Frequent Cause of NF1 Microdeletions. Am. J. Hum. Genet. 2000, 66, 1969–1974. [Google Scholar] [CrossRef]
- Urbán, Z.; Helms, C.; Fekete, G.; Csiszár, K.; Bonnet, D.; Munnich, A.; Donis-Keller, H.; Boyd, C.D. 7q11.23 deletions in Williams syndrome arise as a consequence of unequal meiotic crossover. Am. J. Hum. Genet. 1996, 59, 958–962. [Google Scholar][Green Version]
- Ji, Y.; Eichler, E.E.; Schwartz, S.; Nicholls, R.D. Structure of Chromosomal Duplicons and their Role in Mediating Human Genomic Disorders. Genome Res. 2000, 10, 597–610. [Google Scholar] [CrossRef]
- Stankiewicz, P.; Shaw, C.J.; Dapper, J.D.; Wakui, K.; Shaffer, L.G.; Withers, M.; Elizondo, L.; Park, S.-S.; Lupski, J.R. Genome Architecture Catalyzes Nonrecurrent Chromosomal Rearrangements. Am. J. Hum. Genet. 2003, 72, 1101–1116. [Google Scholar] [CrossRef] [PubMed]
- Baumer, A.; Dutly, F.; Balmer, D.; Riegel, M.; Tükel, T.; Krajewska-Walasek, M.; Schinzel, A.A. High Level of Unequal Meiotic Crossovers at the Origin of the 22q11.2 and 7q11.23 Deletions. Hum. Mol. Genet. 1998, 7, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Devriendt, K.; Vermeesch, J.R. Chromosomal phenotypes and submicroscopic abnormalities. Hum. Genom. 2004, 1, 126. [Google Scholar] [CrossRef]
- Juyal, R.C.; Figuera, L.E.; Hauge, X.; Elsea, S.H.; Lupski, J.R.; Greenberg, F.; Baldini, A.; Patel, P.I. Molecular analyses of 17p11.2 deletions in 62 Smith-Magenis syndrome patients. Am. J. Hum. Genet. 1996, 58, 998–1007. [Google Scholar]
- Chen, K.-S.; Gunaratne, P.H.; Hoheisel, J.D.; Young, I.G.; Miklos, G.L.G.; Greenberg, F.; Shaffer, L.G.; Campbell, H.D.; Lupski, J.R. The human homologue of the Drosophila melanogaster flightless-I gene (flil) maps within the Smith-Magenis microdeletion critical region in 17p11.2. Am. J. Hum. Genet. 1995, 56, 175–182. [Google Scholar]
- Otaño-Joos, M.; Mechtersheimer, G.; Ohl, S.; Wilgenbus, K.K.; Scheurlen, W.; Lehnert, T.; Willeke, F.; Otto, H.F.; Lichter, P.; Joos, S. Detection of Chromosomal Imbalances in Leiomyosarcoma by Comparative Genomic Hybridization and Interphase Cytogenetics. Cytogenet. Cell Genet. 2000, 90, 86–92. [Google Scholar] [CrossRef]
- Fioretos, T.; Ströbeck, B.; Sandberg, T.; Johansson, B.; Billströ, R.; Borg, Å.; Nilsson, P.-G.; Van Den Berghe, H.; Hagemeijer, A.; Mitelman, F.; et al. Isochromosome 17q in Blast Crisis of Chronic Myeloid Leukemia and in Other Hematologic Malignancies Is the Result of Clustered Breakpoints in 17p11 and Is not Associated With Coding TP53 Mutations. Blood J. Am. Soc. Hematol. 1999, 94, 225–232. [Google Scholar] [CrossRef]
- Tarkkanen, M.; Karhu, R.; Kallioniemi, A.; Elomaa, I.; Kivioja, A.H.; Nevalainen, J.; Rohling, T.; Rkki Karaharju, F.; Hyytinen, E.; Knuutila, S.; et al. Gains and Losses of DNA Sequences in Osteosarcomas by Comparative Genomic Hybridization. Cancer Res. 1995, 55, 1334–1338. [Google Scholar] [PubMed]
- Scheurlen, W.G.; Schwabe, G.C.; Seranski, P.; Joos, S.; Harbott, J.; Metzke, S.; Döhner, H.; Poustka, A.; Wilgenbus, K.; Haas, O.A. Mapping of the Breakpoints on the Short Arm of Chromosome 17 in Neoplasms with an i(17q). Genes Chromosomes Cancer 1999, 25, 230–240. [Google Scholar] [CrossRef]
- Zody, M.C.; Garber, M.; Adams, D.J.; Sharpe, T.; Harrow, J.; Lupski, J.R.; Nicholson, C.; Searle, S.M.; Wilming, L.; Young, S.K.; et al. DNA Sequence of Human Chromosome 17 and Analysis of Rearrangement in the Human Lineage. Nature 2006, 440, 1045–1049. [Google Scholar] [CrossRef] [PubMed]
- Park, S.S.; Stankiewicz, P.; Bi, W.; Shaw, C.; Lehoczky, J.; Dewar, K.; Birren, B.; Lupski, J.R. Structure and Evolution of the Smith-Magenis Syndrome Repeat Gene Clusters, SMS-REPs. Genome Res. 2002, 12, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Shaw, C.J.; Lupski, J.R. Non-Recurrent 17p11.2 Deletions Are Generated by Homologous and Non-Homologous Mechanisms. Hum. Genet. 2005, 116, 1–7. [Google Scholar] [CrossRef]
- Slager, R.E.; Newton, T.L.; Vlangos, C.N.; Finucane, B.; Elsea, S.H. Mutations in RAI1 Associated with Smith-Magenis Syndrome. Nat. Genet. 2003, 33, 466–468. [Google Scholar] [CrossRef]
- Potocki, L.; Bi, W.; Treadwell-Deering, D.; Carvalho, C.M.B.; Eifert, A.; Friedman, E.M.; Glaze, D.; Krull, K.; Lee, J.A.; Lewis, R.A.; et al. Characterization of Potocki-Lupski syndrome (dup(17)(p11.2p11.2)) and delineation of a dosage-sensitive critical interval that can convey an autism phenotype. Am. J. Hum. Genet. 2007, 80, 633–649. [Google Scholar] [CrossRef]
- Haybaeck, J.; Postruznik, M.; Miller, C.L.; Dulay, J.R.; Llenos, I.C.; Weis, S. Increased Expression of Retinoic Acid-Induced Gene 1 in the Dorsolateral Prefrontal Cortex in Schizophrenia, Bipolar Disorder, and Major Depression. Neuropsychiatr. Dis. Treat. 2015, 11, 279–289. [Google Scholar] [CrossRef]
- Smith, A.C.M.; Groptnan, A.L.; Bailey-Wilson, J.E.; Goker-Alpan, O.; Elsea, S.H.; Blancato, J.; Lupski, J.R.; Potocki, L. Hypercholesterolemia in Children with Smith-Magenis Syndrome: Del (17)(P11.2p11.2). Genet. Med. 2002, 4, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Oliver, C.; Berg, K.; Moss, J.; Arron, K.; Burbidge, C. Delineation of Behavioral Phenotypes in Genetic Syndromes: Characteristics of Autism Spectrum Disorder, Affect and Hyperactivity. J. Autism Dev. Disord. 2011, 41, 1019–1032. [Google Scholar] [CrossRef]
- Gropman, A.; Duncan, W.C.; Smith, A.C. Neurologic and developmental features of the Smith-Magenis syndrome (del 17p11.2). Pediatr Neurol. 2006, 34, 337–350. [Google Scholar] [CrossRef]
- Burns, B.; Schmidt, K.; Williams, S.R.; Kim, S.; Girirajan, S.; Elsea, S.H.; Greenberg, F.; Guzzetta, V.; De Oca-Luna, R.M.; Magenis, R.E.; et al. Rai1 Haploinsufficiency Causes Reduced Bdnf Expression Resulting in Hyperphagia, Obesity and Altered Fat Distribution in Mice and Humans with No Evidence of Metabolic Syndrome. Hum. Mol. Genet. 2010, 19, 4026–4042. [Google Scholar] [CrossRef]
- Turner, D.J.; Miretti, M.; Rajan, D.; Fiegler, H.; Carter, N.P.; Blayney, M.L.; Beck, S.; Hurles, M.E. Germline Rates of de Novo Meiotic Deletions and Duplications Causing Several Genomic Disorders. Nat. Genet. 2008, 40, 90–95. [Google Scholar] [CrossRef]
- Carpenter, N.J. Molecular Cytogenetics. Semin. Pediatr. Neurol. 2001, 8, 135–146. [Google Scholar] [CrossRef]
- Mosca-Boidron, A.L.; Bouquillon, S.; Faivre, L.; Callier, P.; Andrieux, J.; Marle, N.; Bonnet, C.; Vincent-Delorme, C.; Berri, M.; Plessis, G.; et al. What Can We Learn from Old Microdeletion Syndromes Using Array-CGH Screening? Clin. Genet. 2012, 82, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Slavotinek, A.M. Novel Microdeletion Syndromes Detected by Chromosome Microarrays. Hum. Genet. 2008, 124, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Laje, G.; Morse, R.; Richter, W.; Ball, J.; Pao, M.; Smith, A.C.M. Autism Spectrum Features in Smith-Magenis Syndrome. Am. J. Med. Genet. C Semin. Med. Genet. 2010, 154, 456–462. [Google Scholar] [CrossRef]
- Greenberg, F.; Guzzetta, V.; De Oca-Luna, R.M.; Magenis, R.E.; Smith, A.C.M.; Richter, S.F.; Kondo, I.; Dobyns, W.B.; Patel, P.I.; Lupski, J.R. Molecular Analysis of the Smith-Magenis Syndrome: A Possible Contiguous-Gene Syndrome Associated with Del(17)(P11.2). Am. J. Hum. Genet. 1991, 49, 1207–1218. [Google Scholar]
- Watson, C.T.; Marques-Bonet, T.; Sharp, A.J.; Mefford, H.C. The Genetics of Microdeletion and Microduplication Syndromes: An Update. Annu. Rev. Genom. Hum. Genet. 2014, 15, 215–244. [Google Scholar] [CrossRef] [PubMed]
- Sharp, A.J.; Hansen, S.; Selzer, R.R.; Cheng, Z.; Regan, R.; Hurst, J.A.; Stewart, H.; Price, S.M.; Blair, E.; Hennekam, R.C.; et al. Discovery of Previously Unidentified Genomic Disorders from the Duplication Architecture of the Human Genome. Nat. Genet. 2006, 38, 1038–1042. [Google Scholar] [CrossRef]
- Elsea, S.H.; Girirajan, S. Smith-Magenis Syndrome. Eur. J. Hum. Genet. 2008, 16, 412–421. [Google Scholar] [CrossRef] [PubMed]
- De Leersnyder, H. Smith-Magenis Syndrome. Handb. Clin. Neurol. 2013, 111, 295–296. [Google Scholar] [CrossRef] [PubMed]
- Ricard, G.; Molina, J.; Chrast, J.; Gu, W.; Gheldof, N.; Pradervand, S.; Schütz, F.; Young, J.I.; Lupski, J.R.; Reymond, A.; et al. Phenotypic Consequences of Copy Number Variation: Insights from Smith-Magenis and Potocki-Lupski Syndrome Mouse Models. PLoS Biol. 2010, 8, e1000543. [Google Scholar] [CrossRef]
- Walz, K.; Caratini-Rivera, S.; Bi, W.; Fonseca, P.; Mansouri, D.L.; Lynch, J.; Vogel, H.; Noebels, J.L.; Bradley, A.; Lupski, J.R. Modeling Del(17)(P11.2p11.2) and Dup(17)(P11.2p11.2) Contiguous Gene Syndromes by Chromosome Engineering in Mice: Phenotypic Consequences of Gene Dosage Imbalance. Mol. Cell. Biol. 2003, 23, 3646–3655. [Google Scholar] [CrossRef] [PubMed]
- Lacaria, M.; Gu, W.; Lupski, J.R. Circadian Abnormalities in Mouse Models of Smith-Magenis Syndrome: Evidence for Involvement of RAI1. Am. J. Med. Genet. A 2013, 161, 1561–1568. [Google Scholar] [CrossRef]
- Bi, W.; Yan, J.; Shi, X.; Yuva-Paylor, L.A.; Antalffy, B.A.; Goldman, A.; Yoo, J.W.; Noebels, J.L.; Armstrong, D.L.; Paylor, R.; et al. Rai1 Deficiency in Mice Causes Learning Impairment and Motor Dysfunction, Whereas Rai1 Heterozygous Mice Display Minimal Behavioral Phenotypes. Hum. Mol. Genet. 2007, 16, 1802–1813. [Google Scholar] [CrossRef]
- Edelman, E.A.; Girirajan, S.; Finucane, B.; Patel, P.I.; Lupski, J.R.; Smith, A.C.M.; Elsea, S.H. Gender, Genotype, and Phenotype Differences in Smith-Magenis Syndrome: A Meta-Analysis of 105 Cases. Clin. Genet. 2007, 71, 540–550. [Google Scholar] [CrossRef]
- Walz, K.; Spencer, C.; Kaasik, K.; Lee, C.C.; Lupski, J.R.; Paylor, R. Behavioral Characterization of Mouse Models for Smith–Magenis Syndrome and Dup(17)(P11.2p11.2). Hum. Mol. Genet. 2004, 13, 367–378. [Google Scholar] [CrossRef]
- Girirajan, S.; Elsas, L.J., 2nd; Devriendt, K.; Elsea, S.H. RAI1 Variations in Smith-Magenis Syndrome Patients without 17p11.2 Deletions. J. Med. Genet. 2005, 42, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Girirajan, S.; Vlangos, C.N.; Szomju, B.B.; Edelman, E.; Trevors, C.D.; Dupuis, L.; Nezarati, M.; Bunyan, D.J.; Elsea, S.H. Genotype-Phenotype Correlation in Smith-Magenis Syndrome: Evidence That Multiple Genes in 17p11.2 Contribute to the Clinical Spectrum. Genet. Med. 2006, 8, 417–427. [Google Scholar] [CrossRef]
- Vlangos, C.N.; Wilson, M.; Blancato, J.; Smith, A.C.M.; Elsea, S.H. Diagnostic FISH Probes for Del(17)(P11.2p11.2) Associated with Smith-Magenis Syndrome Should Contain the RAI1 Gene. Am. J. Med. Genet. A 2005, 132A, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, K.; McCool, C.; Lupski, J.R.; Glaze, D.; Potocki, L. Objective Measures of Sleep Disturbances in Children with Potocki–Lupski Syndrome. Am. J. Med. Genet. A 2019, 179, 1982–1986. [Google Scholar] [CrossRef] [PubMed]
- Yusupov, R.; Roberts, A.E.; Lacro, R.V.; Sandstrom, M.; Ligon, A.H. Potocki-Lupski Syndrome: An Inherited Dup(17)(P11.2p11.2) with Hypoplastic Left Heart. Am. J. Med. Genet. A 2011, 155, 367–371. [Google Scholar] [CrossRef]
- Sanchez-Valle, A.; Pierpont, M.E.; Potocki, L. The Severe End of the Spectrum: Hypoplastic Left Heart in Potocki-Lupski Syndrome. Am. J. Med. Genet. A 2011, 155, 363–366. [Google Scholar] [CrossRef]
- Kolbasin, L.N.; Dubrovskaya, T.A.; Salnikova, G.B.; Solovieva, E.N.; Donnikov, M.Y.; Illarionov, R.A.; Glotov, A.S.; Kovalenko, L.V.; Belotserkovtseva, L.D. Family Case of Potocki-Lupski Syndrome. Mol. Cytogenet. 2024, 17, 6. [Google Scholar] [CrossRef]
- Imai, Y.; Matsui, T.; Tohyama, M.; Wanaka, A.; Takagi, T. Cloning of a Retinoic Acid-Induced Gene, GT1, in the Embryonal Carcinoma Cell Line P19: Neuron-Specific Expression in the Mouse Brain. Brain Res. Mol. Brain Res. 1995, 31, 1–9. [Google Scholar] [CrossRef]
- Toulouse, A.; Rochefort, D.; Roussel, J.; Joober, R.; Rouleau, G.A. Molecular Cloning and Characterization of Human RAI1, a Gene Associated with Schizophrenia. Genomics 2003, 82, 162–171. [Google Scholar] [CrossRef]
- Nagase, T.; Nakayama, M.; Nakajima, D.; Kikuno, R.; Ohara, O. Prediction of the Coding Sequences of Uni-dentified Human Genes. XX. The Complete Sequences of 100 New CDNA Clones from Brain Which Code for Large Proteins in Vitro. DNA Res. 2001, 8, 85–95. [Google Scholar] [CrossRef]
- Carmona-Mora, P.; Walz, K. Retinoic Acid Induced 1, RAI1: A Dosage Sensitive Gene Related to Neurobehavioral Alterations Including Autistic Behavior. Curr. Genom. 2010, 11, 607–617. [Google Scholar] [CrossRef]
- Carmona-Mora, P.; Canales, C.P.; Cao, L.; Perez, I.C.; Srivastava, A.K.; Young, J.I.; Walz, K. RAI1 Transcription Factor Activity Is Impaired in Mutants Associated with Smith-Magenis Syndrome. PLoS ONE 2012, 7, e45155. [Google Scholar] [CrossRef]
- Zori, R.T.; Lupski, J.R.; Heju, Z.; Greenberg, F.; Killian, J.M.; Gray, B.A.; Driscoll, D.J.; Patel, P.I.; Zackowski, J.L. Clinical, Cytogenetic, and Molecular Evidence for an Infant with Smith- Magenis Syndrome Born from a Mother Having a Mosaic 17p11.2p12 Deletion. Am. J. Med. Genet. 1993, 47, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.P.; Bidichandani, S.I.; Figuera, L.E.; Juyal, R.C.; Saxon, P.J.; Baldini, A.; Patel, P.I. Molecular Analysis of Deletion (17)(P11.2p11.2) in a Family Segregating a 17p Paracentric Inversion: Implications for Carriers of Paracentric Inversions. Am. J. Hum. Genet. 1997, 60, 1184–1193. [Google Scholar] [PubMed]
- Rinaldi, B.; Villa, R.; Sironi, A.; Garavelli, L.; Finelli, P.; Bedeschi, M.F. Smith-Magenis Syndrome—Clinical Review, Biological Background and Related Disorders. Genes 2022, 13, 335. [Google Scholar] [CrossRef]
- Greenberg, F.; Lewis, R.A.; Potocki, L.; Glaze, D.; Parke, J.; Killian, J.; Murphy, M.A.; Williamson, D.; Brown, F.; Dutton, R.; et al. Multi-Disciplinary Clinical Study of Smith-Magenis Syndrome (Deletion 17p11.2). Am. J. Med. Genet. 1996, 62, 247–254. [Google Scholar] [CrossRef]
- Zhang, J.; van Oostrom, D.; Li, J.X.; Savelkoul, H.F.J. Innate Mechanisms in Selective IgA Deficiency. Front. Immunol. 2021, 12, 649112. [Google Scholar] [CrossRef]
- Menko, F.H.; van Steensel, M.A.; Giraud, S.; Friis-Hansen, L.; Richard, S.; Ungari, S.; Nordenskjöld, M.; Hansen, T.v.O.; Solly, J.; Maher, E.R. Birt-Hogg-Dubé Syndrome: Diagnosis and Management. Lancet Oncol. 2009, 10, 1199–1206. [Google Scholar] [CrossRef]
- Dardour, L.; Verleyen, P.; Lesage, K.; Holvoet, M.; Devriendt, K. Bilateral Renal Tumors in an Adult Man with Smith-Magenis Syndrome: The Role of the FLCN Gene. Eur. J. Med. Genet. 2016, 59, 499–501. [Google Scholar] [CrossRef]
- Vocke, C.D.; Fleming, L.R.; Piskorski, A.M.; Amin, A.; Phornphutkul, C.; de la Monte, S.; Vilboux, T.; Duncan, F.; Pellegrino, J.; Braddock, B.; et al. A Diagnosis of Birt–Hogg–Dubé Syndrome in Individuals with Smith–Magenis Syndrome: Recommendation for Cancer Screening. Am. J. Med. Genet. A 2023, 191, 490–497. [Google Scholar] [CrossRef]
- Bindu, P.S. Sjogren-Larsson Syndrome: Mechanisms and Management. Appl. Clin. Genet. 2020, 13, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhang, L.; Chen, S.; Li, Y. A Case of Smith–Magenis Syndrome with Skin Manifestations Caused by a Novel Locus Mutation in the RAI1 Gene. J. Int. Med. Res. 2023, 51. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Jacobson, A. Eukaryotic MRNA Decapping Factors: Molecular Mechanisms and Activity. FEBS J. 2023, 290, 5057–5085. [Google Scholar] [CrossRef]
- Zhai, L.T.; Xiang, S. MRNA Quality Control at the 5′ End. J. Zhejiang Univ. Sci. B 2014, 15, 438–443. [Google Scholar] [CrossRef]
- Shi, H.; Wei, J.; He, C. Where, When, and How: Context-Dependent Functions of RNA Methylation Writers, Readers, and Erasers. Mol. Cell 2019, 74, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Costa-Mattioli, M.; Sossin, W.S.; Klann, E.; Sonenberg, N. Translational Control of Long-Lasting Synaptic Plasticity and Memory. Neuron 2009, 61, 10–26. [Google Scholar] [CrossRef]
- Larizza, L.; Finelli, P. Developmental Disorders with Intellectual Disability Driven by Chromatin Dysregulation: Clinical Overlaps and Molecular Mechanisms. Clin. Genet. 2019, 95, 231–240. [Google Scholar] [CrossRef]
- Harris, J.R.; Gao, C.W.; Britton, J.F.; Applegate, C.D.; Bjornsson, H.T.; Fahrner, J.A. Five Years of Experience in the Epigenetics and Chromatin Clinic: What Have We Learned and Where Do We Go from Here? Hum. Genet. 2024, 143, 607–624. [Google Scholar] [CrossRef]
- Pulst, S.M.; Santos, N.; Wang, D.; Yang, H.; Huynh, D.; Velazquez, L.; Figueroa, K.P. Spinocerebellar Ataxia Type 2: PolyQ Repeat Variation in the CACNAIA Calcium Channel Modifies Age of Onset. Brain 2005, 128, 2297–2303. [Google Scholar] [CrossRef]
- Hayes, S.; Turecki, G.; Brisebois, K.; Lopes-Cendes, I.; Gaspar, C.; Riess, O.; Ranum, L.P.W.; Pulst, S.-M.; Rouleau, G.A. CAG Repeat Length in RAI1 Is Associated with Age at Onset Variability in Spinocerebellar Ataxia Type 2 (SCA2). Hum. Mol. Genet. 2000, 9, 1753–1758. [Google Scholar] [CrossRef]
- Malik, I.; Kelley, C.P.; Wang, E.T.; Todd, P.K. Molecular Mechanisms Underlying Nucleotide Repeat Expansion Disorders. Nat. Rev. Mol. Cell Biol. 2021, 22, 589–607. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Z.; Hou, X.; Chen, Z.; Shen, L.; Xia, K.; Tang, B.; Jiang, H.; Wang, J. Effect of CAG Repeats on the Age at Onset of Patients with Spinocerebellar Ataxia Type 2 in China. J. Cent. South Univ. (Med. Sci.) 2021, 46, 793–799. [Google Scholar] [CrossRef]
- Lastres-Becker, I.; Brodesser, S.; Lütjohann, D.; Azizov, M.; Buchmann, J.; Hintermann, E.; Sandhoff, K.; Schürmann, A.; Nowock, J.; Auburger, G. Insulin Receptor and Lipid Metabolism Pathology in Ataxin-2 Knock-out Mice. Hum. Mol. Genet. 2008, 17, 1465–1481. [Google Scholar] [CrossRef]
- Li, L.; Wang, M.; Huang, L.; Zheng, X.; Wang, L.; Miao, H. Ataxin-2: A Powerful RNA-Binding Protein. Discov. Oncol. 2024, 15, 298. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, H.; Hosoda, N.; Tsuiji, H.; Hoshino, S.I. Direct Evidence That Ataxin-2 Is a Translational Activator Mediating Cytoplasmic Polyadenylation. J. Biol. Chem. 2020, 295, 15810–18525. [Google Scholar] [CrossRef]
- Scherz-Shouval, R.; Elazar, Z. ROS, Mitochondria and the Regulation of Autophagy. Trends Cell Biol. 2007, 17, 422–427. [Google Scholar] [CrossRef]
- Chu, C.T. Mechanisms of Selective Autophagy and Mitophagy: Implications for Neurodegenerative Diseases. Neurobiol. Dis. 2019, 122, 23–34. [Google Scholar] [CrossRef]
- Liu, W.J.; Ye, L.; Huang, W.F.; Guo, L.J.; Xu, Z.G.; Wu, H.L.; Yang, C.; Liu, H.F. P62 Links the Autophagy Pathway and the Ubiqutin-Proteasome System upon Ubiquitinated Protein Degradation. Cell. Mol. Biol. Lett. 2016, 21, 29. [Google Scholar] [CrossRef]
- Quiles, J.M.; Gustafsson, Å.B. Mitochondrial Quality Control and Cellular Proteostasis: Two Sides of the Same Coin. Front. Physiol. 2020, 11, 515. [Google Scholar] [CrossRef]
- Chhunchha, B.; Kubo, E.; Singh, D.P. Clock Protein Bmal1 and Nrf2 Cooperatively Control Aging or Oxidative Response and Redox Homeostasis by Regulating Rhythmic Expression of Prdx6. Cells 2020, 9, 1861. [Google Scholar] [CrossRef]
- Wilking, M.; Ndiaye, M.; Mukhtar, H.; Ahmad, N. Circadian Rhythm Connections to Oxidative Stress: Implications for Human Health. Antioxid. Redox Signal. 2013, 19, 192–208. [Google Scholar] [CrossRef] [PubMed]
- Villalpando-Rodriguez, G.E.; Gibson, S.B. Reactive Oxygen Species (ROS) Regulates Different Types of Cell Death by Acting as a Rheostat. Oxid. Med. Cell. Longev. 2021, 2021, 9912436. [Google Scholar] [CrossRef]
- Bi, W.; Ohyama, T.; Nakamura, H.; Yan, J.; Visvanathan, J.; Justice, M.J.; Lupski, J.R. Inactivation of Rai1 in Mice Recapitulates Phenotypes Observed in Chromosome Engineered Mouse Models for Smith-Magenis Syndrome. Hum. Mol. Genet. 2005, 14, 983–995. [Google Scholar] [CrossRef] [PubMed]
- Tahir, R.; Kennedy, A.; Elsea, S.H.; Dickinson, A.J. Retinoic Acid Induced-1 (Rai1) Regulates Craniofacial and Brain Development in Xenopus. Mech. Dev. 2014, 133, 91–104. [Google Scholar] [CrossRef]
- Machaalani, R.; Chen, H. Brain Derived Neurotrophic Factor (BDNF), Its Tyrosine Kinase Receptor B (TrkB) and Nicotine. Neurotoxicology 2018, 65, 186–195. [Google Scholar] [CrossRef]
- Rosentha, A.; Lin, J.C. Modulation of Neurotrophin Signaling by Monoclonal Antibodies. Handb. Exp. Pharmacol. 2014, 220, 497–512. [Google Scholar] [CrossRef]
- Kase, Y.; Kase, Y.; Shimazaki, T.; Okano, H. Current Understanding of Adult Neurogenesis in the Mammalian Brain: How Does Adult Neurogenesis Decrease with Age? Inflamm. Regen. 2020, 40, 10. [Google Scholar] [CrossRef] [PubMed]
- De Leersnyder, H. Inverted Rhythm of Melatonin Secretion in Smith-Magenis Syndrome: From Symptoms to Treatment. Trends Endocrinol. Metab. 2006, 17, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Bendixen, L.; Jensen, T.I.; Bak, R.O. CRISPR-Cas-Mediated Transcriptional Modulation: The Therapeutic Promises of CRISPRa and CRISPRi. Mol. Ther. 2023, 31, 1920–1937. [Google Scholar] [CrossRef]
- Gilbert, L.A.; Larson, M.H.; Morsut, L.; Liu, Z.; Brar, G.A.; Torres, S.E.; Stern-Ginossar, N.; Brandman, O.; Whitehead, E.H.; Doudna, J.A.; et al. XCRISPR-Mediated Modular RNA-Guided Regulation of Transcription in Eukaryotes. Cell 2013, 154, 442. [Google Scholar] [CrossRef]
- Matharu, N.; Rattanasopha, S.; Tamura, S.; Maliskova, L.; Wang, Y.; Bernard, A.; Hardin, A.; Eckalbar, W.L.; Vaisse, C.; Ahituv, N. CRISPR-Mediated Activation of a Promoter or Enhancer Rescues Obesity Caused by Haploinsufficiency. Science (1979) 2019, 363, eaau0629. [Google Scholar] [CrossRef] [PubMed]
- Carrieri, C.; Cimatti, L.; Biagioli, M.; Beugnet, A.; Zucchelli, S.; Fedele, S.; Pesce, E.; Ferrer, I.; Collavin, L.; Santoro, C.; et al. Long Non-Coding Antisense RNA Controls Uchl1 Translation through an Embedded SINEB2 Repeat. Nature 2012, 491, 454–457. [Google Scholar] [CrossRef] [PubMed]
- Bon, C.; Luffarelli, R.; Russo, R.; Fortuni, S.; Pierattini, B.; Santulli, C.; Fimiani, C.; Persichetti, F.; Cotella, D.; Mallamaci, A.; et al. SINEUP Non-Coding RNAs Rescue Defective Frataxin Expression and Activity in a Cellular Model of Friedreich’s Ataxia. Nucleic Acids Res. 2019, 47, 10728–10743. [Google Scholar] [CrossRef] [PubMed]
- Indrieri, A.; Grimaldi, C.; Zucchelli, S.; Tammaro, R.; Gustincich, S.; Franco, B. Synthetic Long Non-Coding RNAs [SINEUPs] Rescue Defective Gene Expression in Vivo. Sci. Rep. 2016, 6, 27315. [Google Scholar] [CrossRef]
- Espinoza, S.; Scarpato, M.; Damiani, D.; Managò, F.; Mereu, M.; Contestabile, A.; Peruzzo, O.; Carninci, P.; Santoro, C.; Papaleo, F.; et al. SINEUP Non-Coding RNA Targeting GDNF Rescues Motor Deficits and Neurodegeneration in a Mouse Model of Parkinson’s Disease. Mol. Ther. 2020, 28, 642–652. [Google Scholar] [CrossRef]
- Di Leva, F.; Arnoldi, M.; Santarelli, S.; Massonot, M.; Lemée, M.V.; Bon, C.; Pellegrini, M.; Castellini, M.E.; Zarantonello, G.; Messina, A.; et al. SINEUP RNA Rescues Molecular Phenotypes Associated with CHD8 Suppression in Autism Spectrum Disorder Model Systems. Mol. Ther. 2025, 33, 1180–1196. [Google Scholar] [CrossRef]
- Raffaele, M.; Barbagallo, I.; Licari, M.; Carota, G.; Sferrazzo, G.; Spampinato, M.; Sorrenti, V.; Vanella, L. N-Acetylcysteine (NAC) Ameliorates Lipid-Related Metabolic Dysfunction in Bone Marrow Stromal Cells-Derived Adipocytes. Evid.-Based Complement. Altern. Med. 2018, 2018, 5310961. [Google Scholar] [CrossRef]
- Javed, S.; Lee, Y.J.; Xu, J.; Huang, W.H. Temporal Dissection of Rai1 Function Reveals Brain-Derived Neurotrophic Factor as a Potential Therapeutic Target for Smith-Magenis Syndrome. Hum. Mol. Genet. 2022, 31, 275–288. [Google Scholar] [CrossRef]
- Morrill, S.A.; Amon, A. Why Haploinsufficiency Persists. Proc. Natl. Acad. Sci. USA 2019, 116, 11866–11871. [Google Scholar] [CrossRef]
- Veitia, R.A.; Birchler, J.A. Dominance and Gene Dosage Balance in Health and Disease: Why Levels Matter! J. Pathol. 2010, 220, 174–185. [Google Scholar] [CrossRef]
- Babcock, M.; Yatsenko, S.; Hopkins, J.; Brenton, M.; Cao, Q.; De Jong, P.; Stankiewicz, P.; Lupski, J.R.; Sikela, J.M.; Morrow, B.E. Hominoid Lineage Specific Amplification of Low-Copy Repeats on 22q11.2 (LCR22s) Associated with Velo-Cardio-Facial/Digeorge Syndrome. Hum. Mol. Genet. 2007, 16, 2560–2571. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.S. The Conundrum of a Jumping Translocation (JT) in CVS from Twins and Review of JTs. Am. J. Med. Genet. A 2010, 152, 2924–2936. [Google Scholar] [CrossRef] [PubMed]
- Müller, A.R.; Zinkstok, J.R.; Rommelse, N.N.J.; van de Ven, P.M.; Roes, K.C.B.; Wijburg, F.A.; de Rooij-Askes, E.; Linders, C.; Boot, E.; van Eeghen, A.M. Methylphenidate for Attention-Deficit/Hyperactivity Disorder in Patients with Smith–Magenis Syndrome: Protocol for a Series of N-of-1 Trials. Orphanet J. Rare Dis. 2021, 16, 380. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Covarelli, J.; Vinciarelli, E.; Mirarchi, A.; Prontera, P.; Arcuri, C. Retinoic Acid Induced 1 and Smith–Magenis Syndrome: From Genetics to Biology and Possible Therapeutic Strategies. Int. J. Mol. Sci. 2025, 26, 6667. https://doi.org/10.3390/ijms26146667
Covarelli J, Vinciarelli E, Mirarchi A, Prontera P, Arcuri C. Retinoic Acid Induced 1 and Smith–Magenis Syndrome: From Genetics to Biology and Possible Therapeutic Strategies. International Journal of Molecular Sciences. 2025; 26(14):6667. https://doi.org/10.3390/ijms26146667
Chicago/Turabian StyleCovarelli, Jasmine, Elisa Vinciarelli, Alessandra Mirarchi, Paolo Prontera, and Cataldo Arcuri. 2025. "Retinoic Acid Induced 1 and Smith–Magenis Syndrome: From Genetics to Biology and Possible Therapeutic Strategies" International Journal of Molecular Sciences 26, no. 14: 6667. https://doi.org/10.3390/ijms26146667
APA StyleCovarelli, J., Vinciarelli, E., Mirarchi, A., Prontera, P., & Arcuri, C. (2025). Retinoic Acid Induced 1 and Smith–Magenis Syndrome: From Genetics to Biology and Possible Therapeutic Strategies. International Journal of Molecular Sciences, 26(14), 6667. https://doi.org/10.3390/ijms26146667





