Simulated Microgravity Attenuates Stretch Sensitivity of Mechanically Gated Channels in Rat Ventricular Myocytes
Abstract
1. Introduction
2. Results
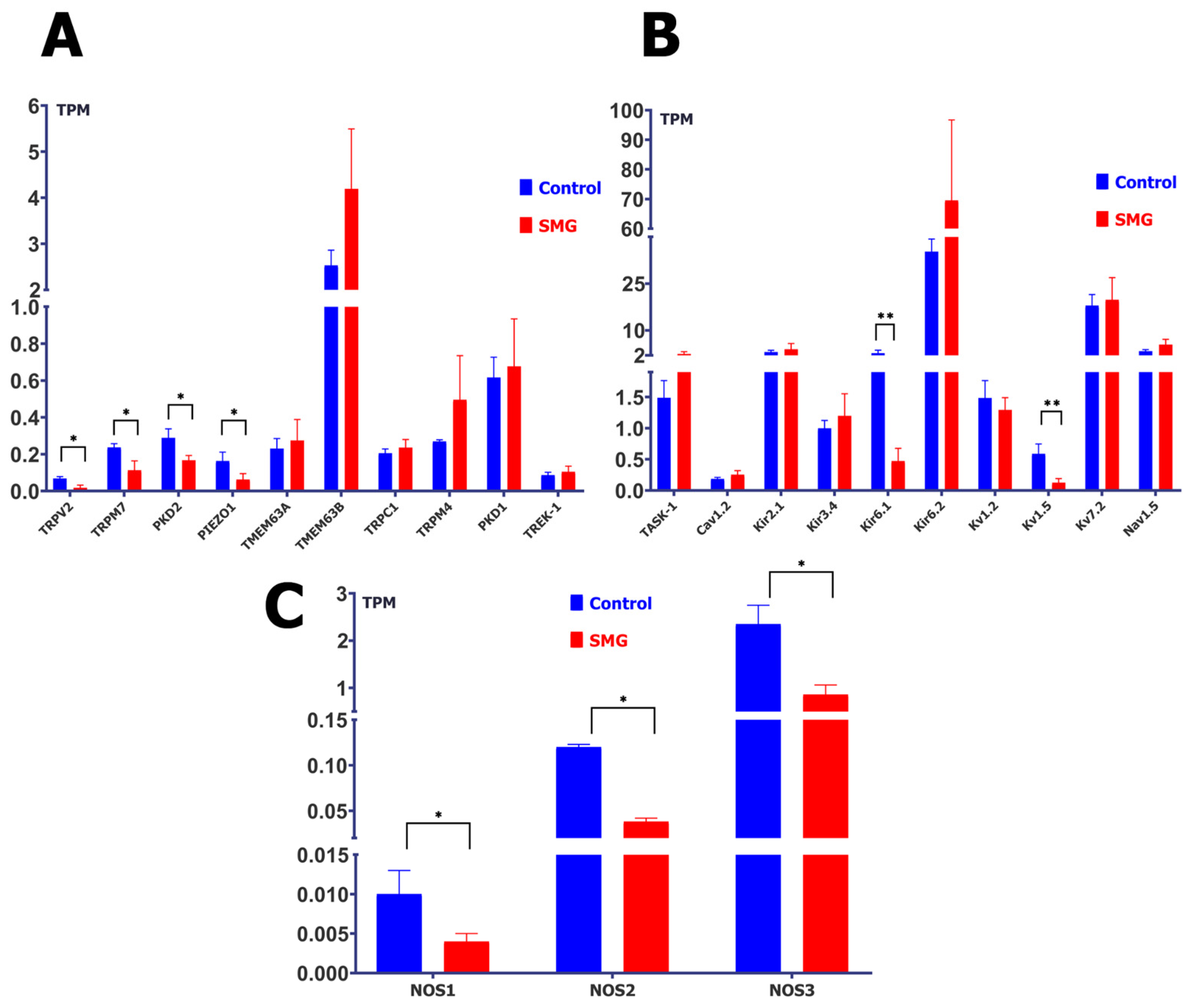
2.1. Simulated Microgravity Changes the Abundance of Protein Transcripts of MGCs, MSCs, and Nitric Oxide Synthase (NOS)
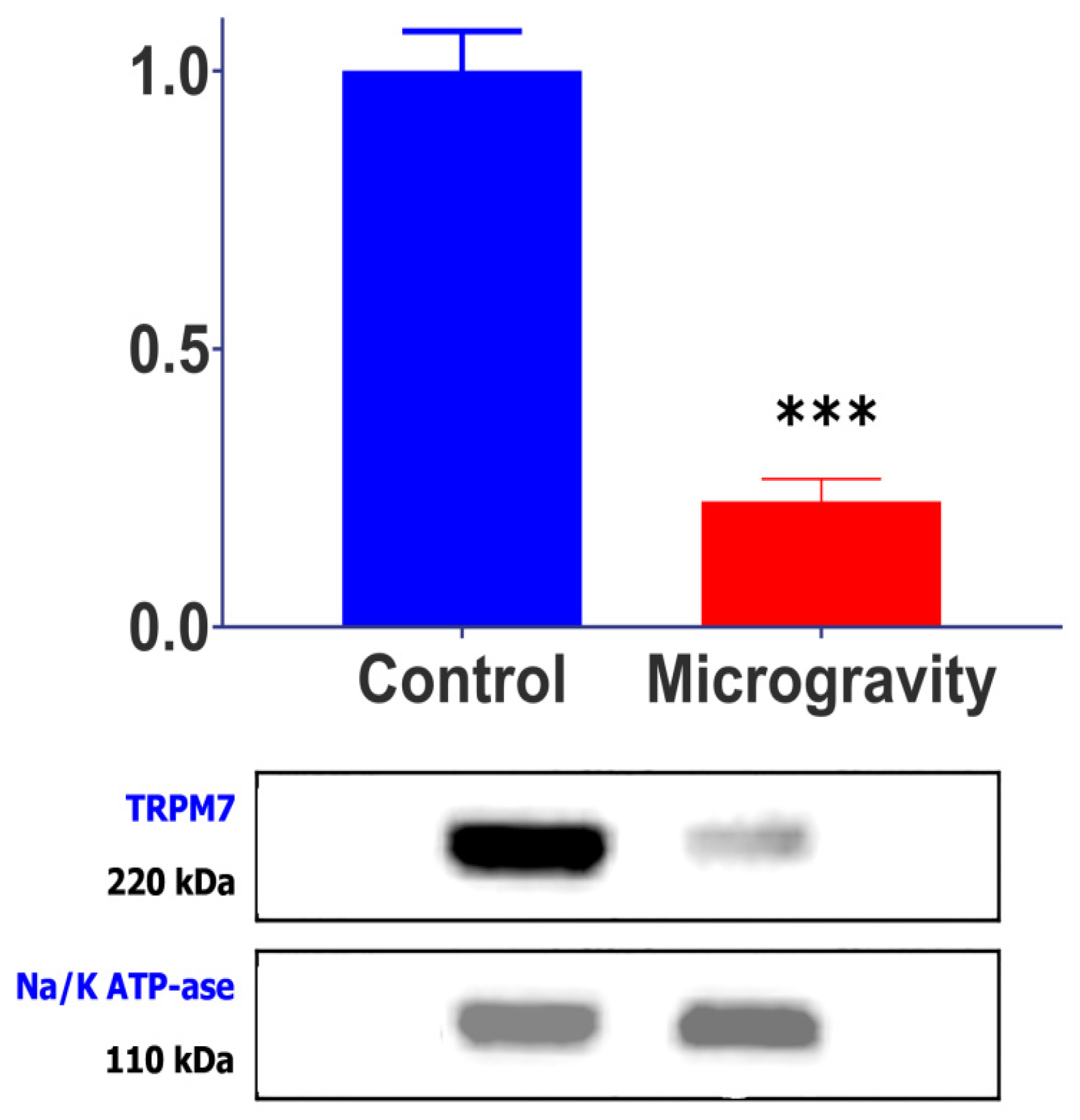
2.2. SMG Causes a Decrease in TRPM7 Channel Protein
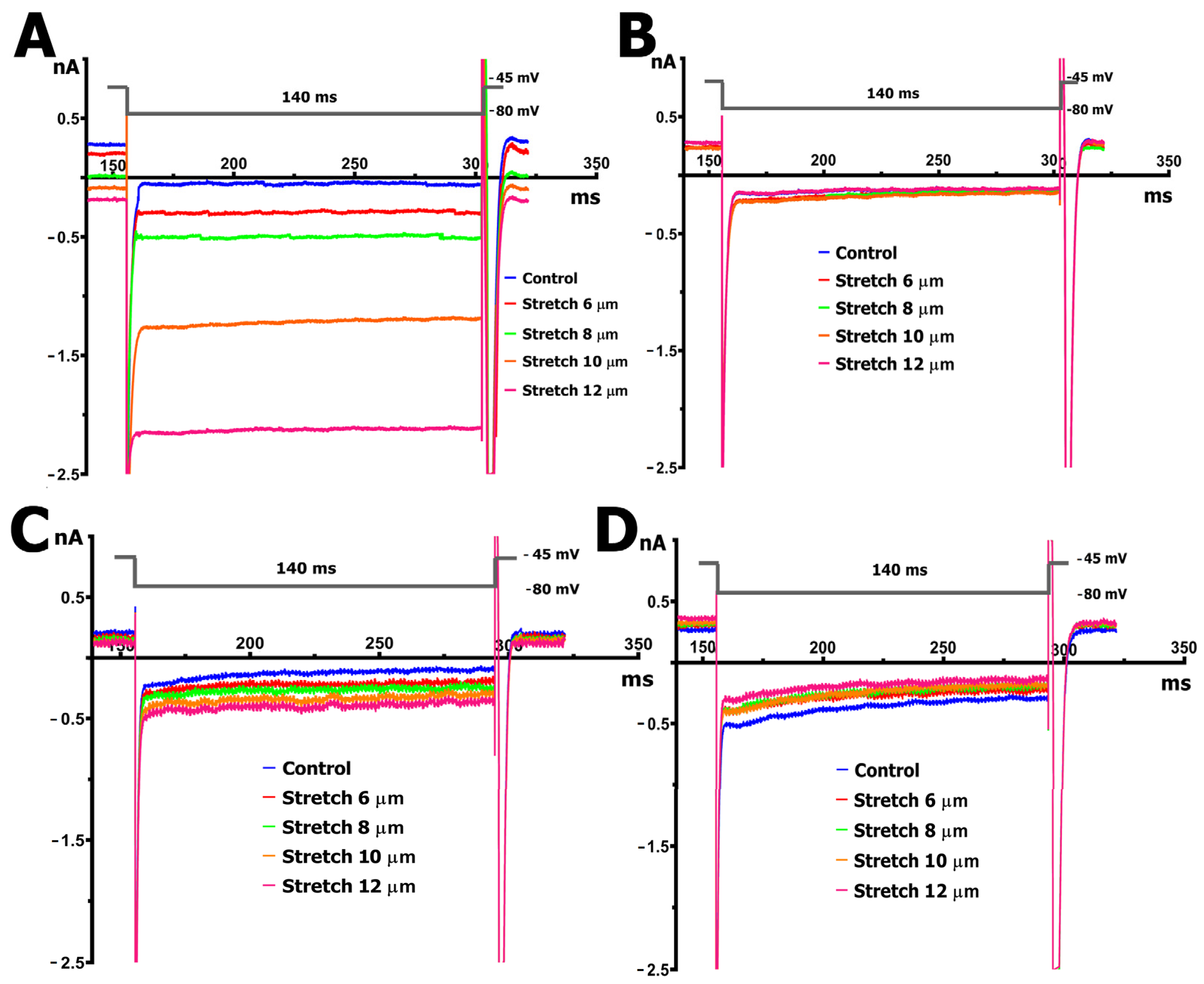
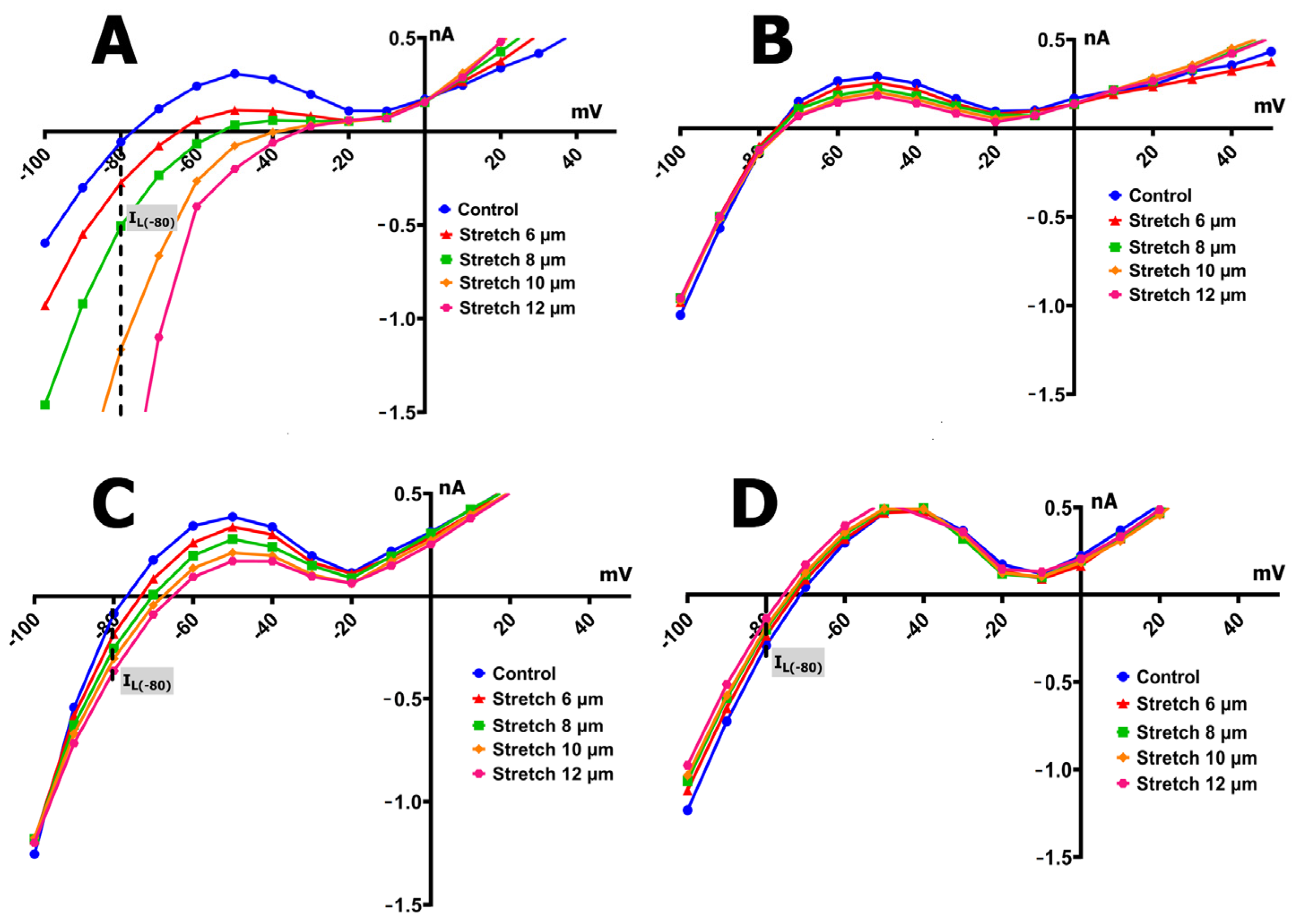
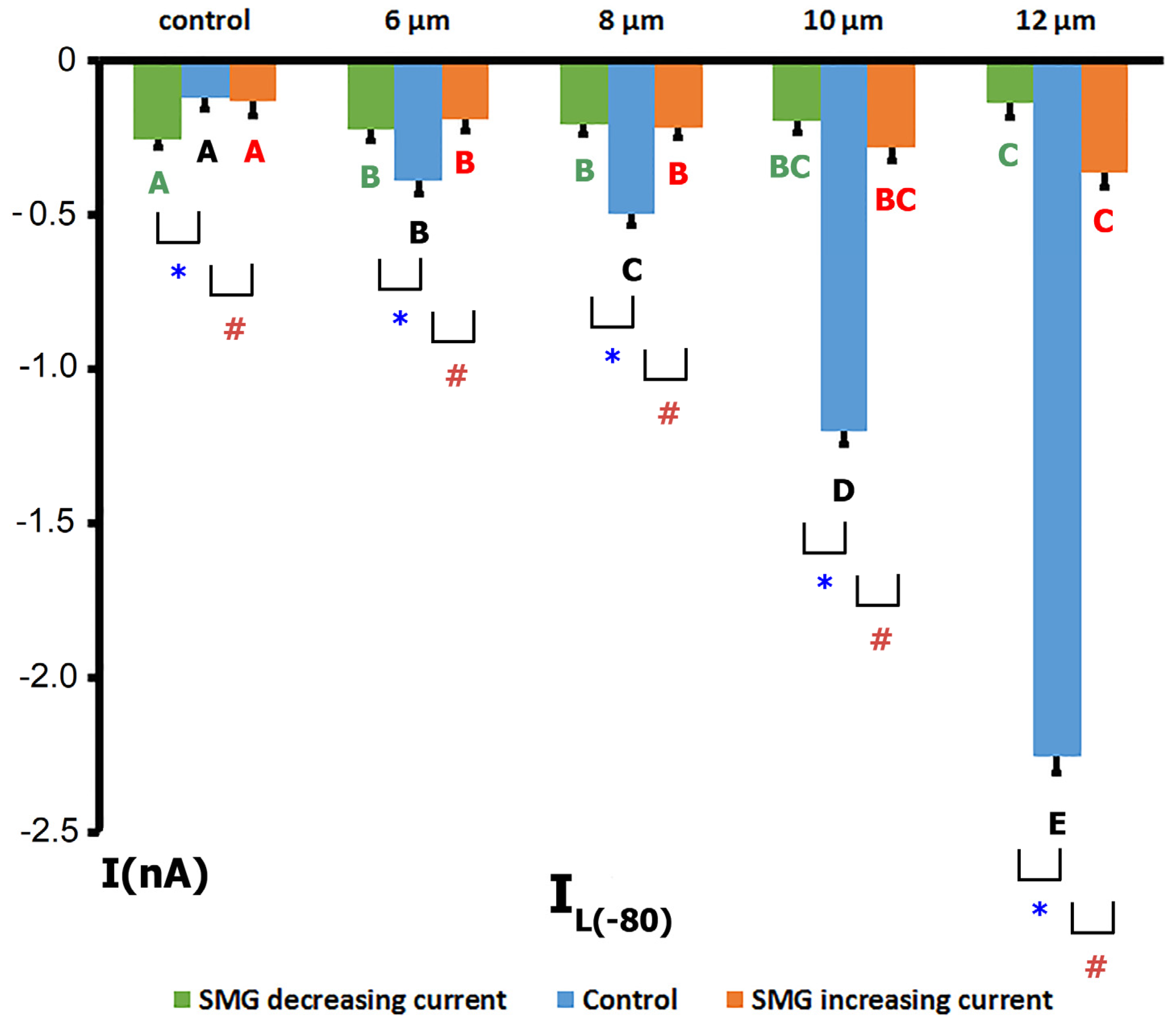
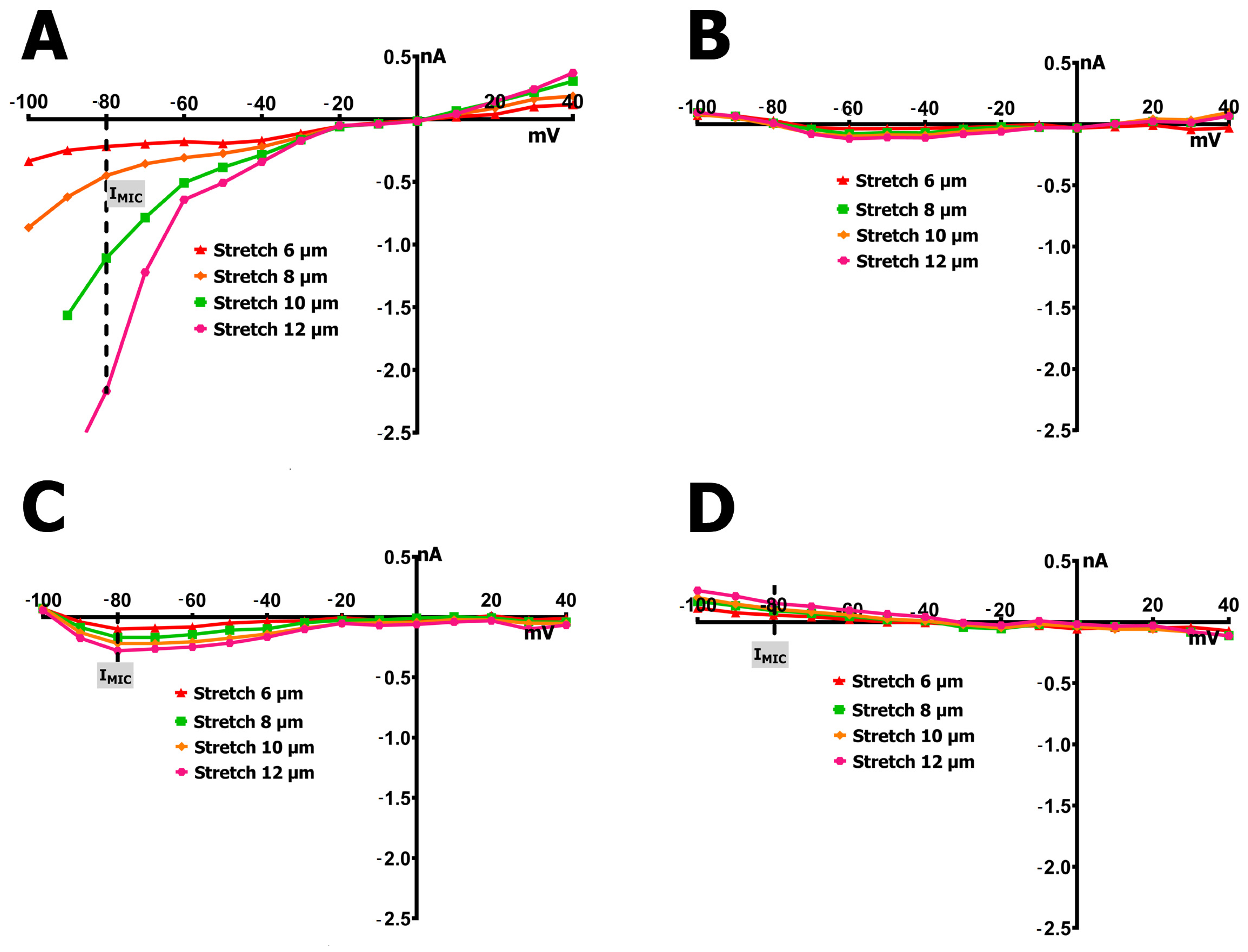
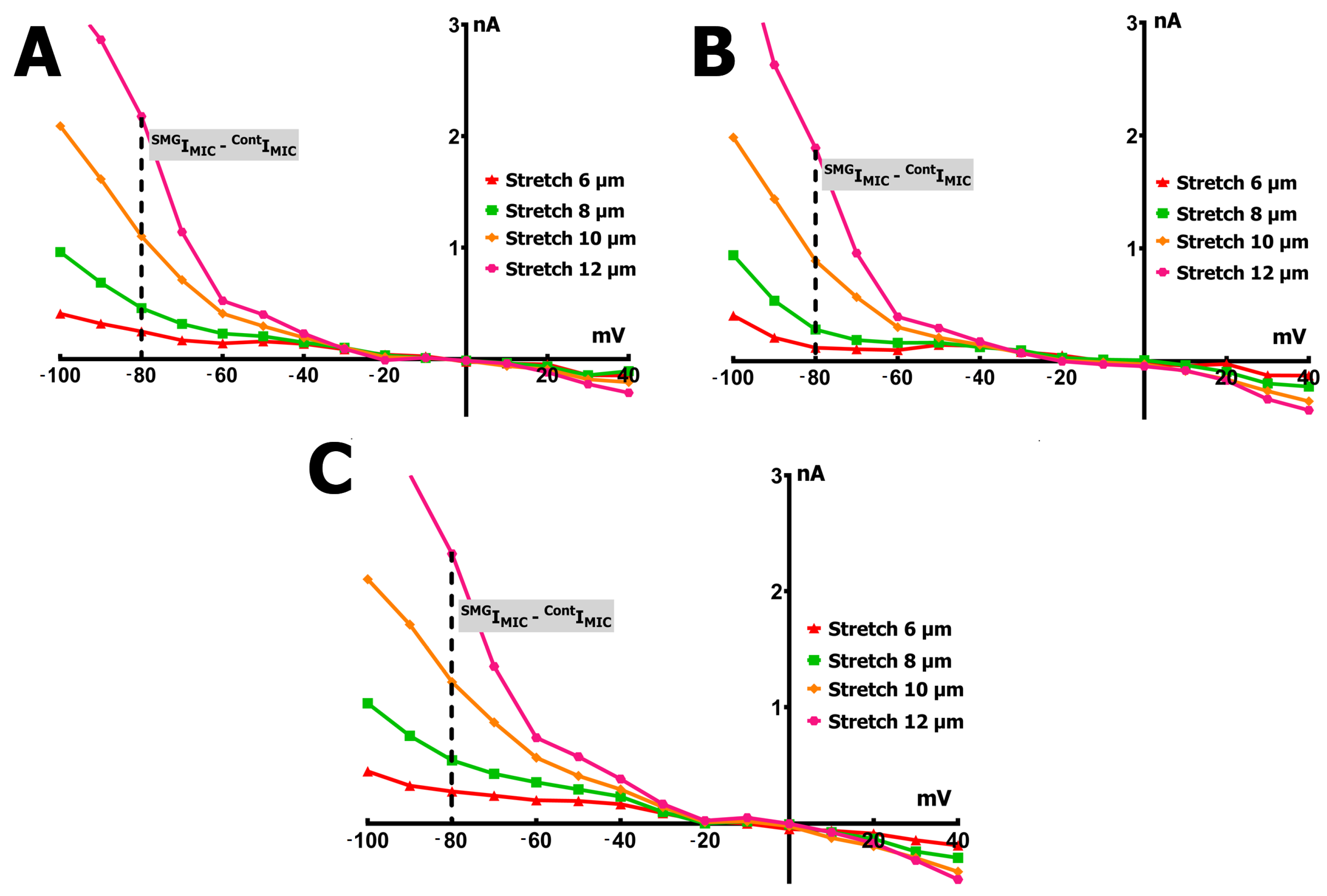
2.3. SMG Alters IMGC Sensitivity to Stretch
- An 8 µm stretch depolarized V0 to −55 ± 3 mV and increased IL to −0.498 ± 0.036 nA, corresponding to a differential current of −0.411 ± 0.050 nA (n = 9; S8IMGC).
- A 10 µm stretch shifted V0 to −41 ± 3 mV and SIL to −1.205 ± 0.042 nA, with a resulting S10IMGC of −1.12 ± 0.063 nA (n = 8).
- A 12 µm stretch caused the most pronounced depolarization, with V0 reaching −31 ± 3 mV and SIL increasing to −2.256 ± 0.053 nA. The corresponding differential current was −2.23 ± 0.055 nA (n = 6; S12IMGC).
3. Discussion
3.1. Simulated Microgravity Affects the Gene Expression of Cardiomyocyte MGCs and MSCs Proteins
3.2. SMG Changes the Electrical Response to Stretch
4. Materials and Methods
4.1. Animals
4.2. Simulation of Microgravity
4.3. Solutions
4.4. Isolated Cardiomyocyte Preparation
4.5. RNA Isolation, Sequencing and Analysis
4.6. Western Blot
4.7. Mechanical Stretch of the Ventricular Myocytes
4.8. Whole-Cell Patch-Clamp
4.9. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AC | Adenylate Cyclase |
| ANOVA | Analysis of Variance |
| AP | Action Potential |
| APD | Action Potential Duration |
| ATP | Adenosine Triphosphate |
| BAPTA | 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid |
| Ca2+ | Calcium |
| EGTA | Ethylene Glycol Tetraacetic Acid |
| ER | Endoplasmic Reticulum |
| ESC | Embryonic Stem Cell |
| FASTQ | Text-based format for storing nucleotide sequences and quality scores |
| FASTQC | Quality Control tool for High-Throughput Sequence Data |
| HEK | Human Embryonic Kidney |
| HEPES | 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid |
| HISAT | Hierarchical Indexing for Spliced Alignment of Transcripts |
| IK | Delayed Rectifier Potassium Current |
| IL | Late Membrane Current |
| IMGC | Mechanically Gated Current |
| ISAC | Stretch-Activated Current |
| KATP | ATP-sensitive Potassium Channel |
| KB | Kraftbrühe |
| KOH | Potassium Hydroxide |
| KV | Voltage-Gated Potassium Channel |
| MEF | Mechanoelectrical Feedback |
| MG | Microgravity |
| MGC | Mechanically Gated Channel |
| NFAT | Nuclear Factor of Activated T-cells |
| NMDA | N-Methyl-D-Aspartate |
| NO | Nitric Oxide |
| NOS | Nitric Oxide Synthase |
| PDE | Phosphodiesterase |
| PSS | Physiological Salt Solution |
| RNA | Ribonucleic Acid |
| SD | Standard Deviation |
| SEM | Standard Error of the Mean |
| SMG | Simulated Microgravity |
| TASK | TWIK-related Acid-sensitive K+ channel |
| TBST | Tris-Buffered Saline with Tween 20 |
| TPM | Transcripts Per Kilobase Million |
| TREK | TWIK-Related K+ Channel |
| TRIS | Tris(hydroxymethyl)aminomethane |
| TRP | Transient Receptor Potential |
| TTX | Tetrodotoxin |
References
- Ravens, U. Mechano-electric feedback and arrhythmias. Prog. Biophys. Mol. Biol. 2003, 82, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Craelius, W.; Chen, V.; el-Sherif, N. Stretch activated ion channels in ventricular myocytes. Biosci. Rep. 1988, 8, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Kamkin, A.; Kiseleva, I.; Wagner, K.D.; Leiterer, K.P.; Theres, H.; Scholz, H.; Günther, J.; Lab, M.J. Mechano-electric feedback in right atrium after left ventricular infarction in rats. J. Mol. Cell. Cardiol. 2000, 32, 465–477. [Google Scholar] [CrossRef]
- Kiseleva, I.; Kamkin, A.; Wagner, K.D.; Theres, H.; Ladhoff, A.; Scholz, H.; Günther, J.; Lab, M.J. Mechanoelectric feedback after left ventricular infarction in rats. Cardiovasc. Res. 2000, 45, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Kamkin, A.; Kiseleva, I.; Isenberg, G. Stretch-activated currents in ventricular myocytes: Amplitude and arrhythmogenic effects increase with hypertrophy. Cardiovasc. Res. 2000, 48, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Kamkin, A.; Kiseleva, I.; Isenberg, G. Ion selectivity of stretch-activated cation currents in mouse ventricular myocytes. Pflugers Arch. 2003, 446, 220–231. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Youm, J.B.; Sung, H.K.; Lee, S.H.; Ryu, S.Y.; Ho, W.K.; Earm, Y.E. Stretch-activated and background non-selective cation channels in rat atrial myocytes. J. Physiol. 2000, 523, 607–619. [Google Scholar] [CrossRef]
- Sachs, F. Mechanical transduction in biological systems. Crit. Rev. Biomed. Eng. 1988, 16, 141–169. [Google Scholar]
- Ohmori, H. Gating properties of the mechano-electrical transducer channel in the dissociated vestibular hair cell of the chick. J. Physiol. 1987, 387, 589–609. [Google Scholar] [CrossRef]
- Sachs, F.; Morris, C.E. Mechanosensitive ion channels in nonspecialized cells. Rev. Physiol. Biochem. Pharmacol. 1998, 132, 1–77. [Google Scholar]
- Belin, S.; Maki, B.A.; Catlin, J.; Rein, B.A.; Popescu, G.K. Membrane Stretch Gates NMDA Receptors. J. Neurosci. 2022, 42, 5672–5680. [Google Scholar] [CrossRef] [PubMed]
- Kamkin, A.G.; Kamkina, O.V.; Kazansky, V.E.; Mitrokhin, V.M.; Bilichenko, A.; Nasedkina, E.A.; Shileiko, S.A.; Rodina, A.S.; Zolotareva, A.D.; Zolotarev, V.I.; et al. Identification of RNA reads encoding different channels in isolated rat ventricular myocytes and the effect of cell stretching on L-type Ca2+ current. Biol. Direct 2023, 18, 70. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, B.; Tabarean, I.V.; Juranka, P.; Morris, C.E. Mechanosensitivity of N-type calcium channel currents. Biophys. J. 2002, 83, 2560–2574. [Google Scholar] [CrossRef]
- Morris, C.E.; Prikryl, E.A.; Joós, B. Mechanosensitive gating of Kv channels. PLoS ONE 2015, 10, e0118335. [Google Scholar] [CrossRef]
- Ingber, D. How cells (might) sense microgravity. FASEB J. 1999, 13 (Suppl. S1), S3–S15. [Google Scholar] [CrossRef] [PubMed]
- Baran, R.; Marchal, S.; Garcia Campos, S.; Rehnberg, E.; Tabury, K.; Baselet, B.; Wehland, M.; Grimm, D.; Baatout, S. The cardiovascular system in space: Focus on in vivo and in vitro studies. Biomedicines 2021, 10, 59. [Google Scholar] [CrossRef]
- Arbeille, P.; Fomina, G.; Pottier, J.M.; Achaibou, F.; Kotovskaya, A. Influence of the thigh cuffs countermeasure on the cardiovascular adaptation to 0.g (14 and 21 day Mir spaceflights). J. Gravit. Physiol. 1995, 2, P9–P10. [Google Scholar]
- Arbeille, P.; Sigaudo, D.; Pavy-Le Traon, A.; Herault, S.; Porcher, M.; Gharib, C. Femoral to cerebral arterial blood flow redistribution and femoral vein distension during orthostatic tests after 4 days in the head-down tilt position or confinement. Eur. J. Appl. Physiol. 1998, 78, 208–218. [Google Scholar] [CrossRef]
- Mulvagh, S.L.; Charles, J.B.; Riddle, J.M.; Rehbein, T.L.; Bungo, M.W. Echocardiographic evaluation of the cardiovascular effects of short-duration spaceflight. J. Clin. Pharmacol. 1991, 31, 921–924. [Google Scholar] [CrossRef]
- Herault, S.; Fomina, G.; Alferova, I.; Kotovskaya, A.; Poliakov, V.; Arbeille, P. Cardiac, arterial and venous adaptation to weightlessness during 6-month MIR spaceflights with and without thigh cuffs (bracelets). Eur. J. Appl. Physiol. 2000, 81, 384–390. [Google Scholar] [CrossRef]
- Buckey, J.C., Jr.; Gaffney, F.A.; Lane, L.D.; Levine, B.D.; Watenpaugh, D.E.; Wright, S.J.; Yancy, C.W., Jr.; Meyer, D.M.; Blomqvist, C.G. Central venous pressure in space. J. Appl. Physiol. 1996, 81, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Prisk, G.K.; Guy, H.J.; Elliott, A.R.; Deutschman, R.A.; West, J.B. Pulmonary diffusing capacity, capillary blood volume, and cardiac output during sustained microgravity. J. Appl. Physiol. 1993, 75, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Norsk, P.; Asmar, A.; Damgaard, M.; Christensen, N.J. Fluid shifts, vasodilatation and ambulatory blood pressure reduction during long duration spaceflight. J. Physiol. 2015, 593, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.S.; South, D.A.; Wood, M.L.; Bungo, M.W.; Meck, J.V. Comparison of echocardiographic changes after short- and long-duration spaceflight. Aviat. Space Environ. Med. 2002, 73, 532–536. [Google Scholar]
- Perhonen, M.A.; Franco, F.; Lane, L.D.; Buckey, J.C.; Blomqvist, C.G.; Zerwekh, J.E.; Peshock, R.M.; Weatherall, P.T.; Levine, B.D. Cardiac atrophy after bed rest and spaceflight. J. Appl. Physiol. 2001, 91, 645–653. [Google Scholar] [CrossRef]
- Anzai, T.; Frey, M.A.; Nogami, A. Cardiac arrhythmias during long-duration spaceflights. J. Arrhythm. 2014, 30, 139–149. [Google Scholar] [CrossRef]
- Goldstein, M.A.; Edwards, R.J.; Schroeter, J.P. Cardiac morphology after conditions of microgravity during COSMOS 2044. J. Appl. Physiol. 1992, 73, S94–S100. [Google Scholar] [CrossRef]
- Gazenko, O.G.; Genin, A.M.; Il’in, E.A.; Serova, L.V.; Tigranyan, R.A.; Oganov, V.S. Physiological mechanisms of adaptation to weightlessness. Data from experiments with animals in earth-orbit biosatellites. Biol. Bull. Acad. Sci. 1980, 7, 1–12. [Google Scholar]
- Liu, C.; Zhong, G.; Zhou, Y.; Yang, Y.; Tan, Y.; Li, Y.; Gao, X.; Sun, W.; Li, J.; Jin, X.; et al. Alteration of calcium signalling in cardiomyocyte induced by simulated microgravity and hypergravity. Cell Prolif. 2020, 53, e12783. [Google Scholar] [CrossRef]
- Xiong, J.; Li, Y.; Nie, J. Effects of simulated microgravity on nitric oxide level in cardiac myocytes and its mechanism. Sci. China Ser. C-Life Sci. 2003, 46, 302–309. [Google Scholar] [CrossRef]
- Goldermann, M.; Hanke, W. Ion channel are sensitive to gravity changes. Microgravity Sci. Technol. 2001, 13, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Richard, S.; Henggeler, D.; Ille, F.; Vadrucci Beck, S.; Moeckli, M.; Forster, I.C.; Franco-Obregón, A.; Egli, M. A semi-automated electrophysiology system for recording from Xenopus oocytes under microgravity conditions. Microgravity Sci. Technol. 2012, 24, 237–244. [Google Scholar] [CrossRef]
- Schaffhauser, D.F.; Andrini, O.; Ghezzi, C.; Forster, I.C.; Franco-Obregón, A.; Egli, M.; Dittrich, P.S. Microfluidic platform for electrophysiological studies on Xenopus laevis oocytes under varying gravity levels. Lab Chip 2011, 11, 3471–3478. [Google Scholar] [CrossRef] [PubMed]
- Wuest, S.L.; Gantenbein, B.; Ille, F.; Egli, M. Electrophysiological experiments in microgravity: Lessons learned and future challenges. npj Microgravity 2018, 4, 7. [Google Scholar] [CrossRef]
- Xue, J.H.; Zhang, L.F.; Ma, J.; Xie, M.J. Differential regulation of L-type Ca2+ channels in cerebral and mesenteric arteries after simulated microgravity in rats and its intervention by standing. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H691–H701. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, L.; Bai, Y.G.; Song, J.B.; Cheng, J.H.; Ma, H.Z.; Ma, J.; Xie, M.J. miR-137 and its target T-type CaV3.1 channel modulate dedifferentiation and proliferation of cerebrovascular smooth muscle cells in simulated microgravity rats by regulating calcineurin/NFAT pathway. Cell Prolif. 2020, 53, e12774. [Google Scholar] [CrossRef]
- Franco-Obregón, A.; Cambria, E.; Greutert, H.; Wernas, T.; Hitzl, W.; Egli, M.; Sekiguchi, M.; Boos, N.; Hausmann, O.; Ferguson, S.J.; et al. TRPC6 in simulated microgravity of intervertebral disc cells. Eur. Spine J. 2018, 27, 2621–2630. [Google Scholar] [CrossRef]
- Hu, Y.; Tian, H.; Chen, W.; Liu, Y.; Cao, Y.; Pei, H.; Ming, C.; Shan, C.; Chen, X.; Dai, Z.; et al. The critical role of the piezo1/β-catenin/ATF4 Axis on the stemness of Gli1+ BMSCs during simulated microgravity-induced bone loss. Adv. Sci. 2023, 10, 2303375. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; Wang, R.; Zhao, X.; Pan, Y.; Zhang, Q.; Li, S.; Fan, J.; Wang, Y.; Sun, X. PIEZO1 Promotes the Migration of Endothelial Cells via Enhancing CXCR4 Expression under Simulated Microgravity. Int. J. Mol. Sci. 2024, 25, 7254. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Fu, Z.; Xing, C.; Wang, Z.; Yang, H.; Li, J.; Liu, M.; Dong, L.; Zhang, X.; et al. Long-term simulated microgravity fosters carotid aging-like changes via Piezo1. Cardiovasc. Res. 2024, 120, 548–559. [Google Scholar] [CrossRef]
- Mirzoev, T.M.; Shenkman, B.S. Mechanosensory Structures in the Mechanotransduction System of Muscle Fibers. J. Evol. Biochem. Physiol. 2023, 59, 1341–1359. [Google Scholar] [CrossRef]
- Patel, A.J.; Honoré, E.; Lesage, F.; Fink, M.; Romey, G.; Lazdunski, M. Inhalational anesthetics activate two-pore-domain background K+ channels. Nat. Neurosci. 1998, 2, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, T.; Obukhov, A.G.; Schaefer, M.; Harteneck, C.; Gudermann, T.; Schultz, G. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature 1999, 397, 259–263. [Google Scholar] [CrossRef]
- Spassova, M.A.; Hewavitharana, T.; Xu, W.; Soboloff, J.; Gill, D.L. A common mechanism underlies stretch activation and receptor activation of TRPC6 channels. Proc. Natl. Acad. Sci. USA 2006, 103, 16586–16591. [Google Scholar] [CrossRef] [PubMed]
- Watenpaugh, D.E. Analogs of microgravity: Head-down tilt and water immersion. J. Appl. Physiol. 2016, 120, 904–914. [Google Scholar] [CrossRef] [PubMed]
- Zhong, G.; Li, Y.; Li, H.; Sun, W.; Cao, D.; Li, J.; Zhao, D.; Song, J.; Jin, X.; Song, H.; et al. Simulated microgravity and recovery-induced remodeling of the left and right ventricle. Front. Physiol. 2016, 7, 274. [Google Scholar] [CrossRef]
- Nadler, M.J.; Hermosura, M.C.; Inabe, K.; Perraud, A.L.; Zhu, Q.; Stokes, A.J.; Kurosaki, T.; Kinet, J.P.; Penner, R.; Scharenberg, A.M.; et al. LTRPC7 is a Mg·ATP-regulated divalent cation channel required for cell viability. Nature 2001, 411, 590–595. [Google Scholar] [CrossRef]
- Li, M.; Jiang, J.; Yue, L. Functional characterization of homo- and heteromeric channel kinases TRPM6 and TRPM7. J. Gen. Physiol. 2006, 127, 525–537. [Google Scholar] [CrossRef]
- Monteilh-Zoller, M.K.; Hermosura, M.C.; Nadler, M.J.; Scharenberg, A.M.; Penner, R.; Fleig, A. TRPM7 provides an ion channel mechanism for cellular entry of trace metal ions. J. Gen. Physiol. 2003, 121, 49–60. [Google Scholar] [CrossRef]
- Li, M.; Du, J.; Jiang, J.; Ratzan, W.; Su, L.T.; Runnels, L.W.; Yue, L. Molecular determinants of Mg2+ and Ca2+ permeability and pH sensitivity in TRPM6 and TRPM7. J. Biol. Chem. 2007, 282, 25817–25830. [Google Scholar] [CrossRef]
- Numata, T.; Shimizu, T.; Okada, Y. TRPM7 is a stretch- and swelling-activated cation channel involved in volume regulation in human epithelial cells. Am. J. Physiol. Cell Physiol. 2007, 292, C460–C467. [Google Scholar] [CrossRef]
- Ortega, A.; Roselló-Lletí, E.; Tarazon, E.; Gil-Cayuela, C.; Lago, F.; González-Juanatey, J.R.; Martinez-Dolz, L.; Portoles, M.; Rivera, M. TRPM7 is down-regulated in both left atria and left ventricle of ischaemic cardiomyopathy patients and highly related to changes in ventricular function. ESC Heart Fail. 2016, 3, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Hu, S.; Huang, L.; Zhou, J.; Xiang, H.; Yang, H.; Cheng, H.; Tang, Y. Protective effect of inhibiting TRPM7 expression on hypoxia post-treatment H9C2 cardiomyocytes. Clin. Hemorheol. Microcirc. 2021, 77, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.Y.; Gong, H.; Kesteven, S.; Guo, Y.; Wu, J.; Li, J.V.; Cheng, D.; Zhou, Z.; Iismaa, S.E.; Kaidonis, X.; et al. Piezo1 is the cardiac mechanosensor that initiates the cardiomyocyte hypertrophic response to pressure overload in adult mice. Nat. Cardiovasc. Res. 2022, 1, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Nilius, B.; Flockerzi, V. Mammalian Transient Receptor Potential (TRP) Cation Channels; Springer: Berlin, Germany, 2014; pp. 681–699. [Google Scholar]
- Zhao, X.; Yan, X.; Liu, Y.; Zhang, P.; Ni, X. Co-expression of mouse TMEM63A, TMEM63B and TMEM63C confers hyperosmolarity activated ion currents in HEK293 cells. Cell Biochem. Funct. 2016, 34, 238–241. [Google Scholar] [CrossRef]
- Hof, T.; Chaigne, S.; Récalde, A.; Salle, L.; Brette, F.; Guinamard, R. Transient receptor potential channels in cardiac health and disease. Nat. Rev. Cardiol. 2019, 16, 344–360. [Google Scholar] [CrossRef]
- Gueffier, M.; Zintz, J.; Lambert, K.; Finan, A.; Aimond, F.; Chakouri, N.; Hédon, C.; Granier, M.; Launay, P.; Thireau, J.; et al. The TRPM4 channel is functionally important for the beneficial cardiac remodeling induced by endurance training. J. Muscle Res. Cell Motil. 2017, 38, 3–16. [Google Scholar] [CrossRef]
- Limberg, S.H.; Netter, M.F.; Rolfes, C.; Rinné, S.; Schlichthörl, G.; Zuzarte, M.; Vassiliou, T.; Moosdorf, R.; Wulf, H.; Daut, J.; et al. TASK-1 channels may modulate action potential duration of human atrial cardiomyocytes. Cell. Physiol. Biochem. 2011, 28, 613–624. [Google Scholar] [CrossRef]
- Jones, S.A.; Morton, M.J.; Hunter, M.; Boyett, M.R. Expression of TASK-1, a pH-sensitive twin-pore domain K+ channel, in rat myocytes. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H181–H185. [Google Scholar] [CrossRef]
- Duan, W.; Hicks, J.; Makara, M.A.; Ilkayeva, O.; Abraham, D.M. TASK-1 and TASK-3 channels modulate pressure overload-induced cardiac remodeling and dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H566–H580. [Google Scholar] [CrossRef]
- Vishwakarma, V.K.; Upadhyay, P.K.; Chaurasiya, H.S.; Srivasatav, R.K.; Ansari, T.M.; Srivastava, V. Mechanistic pathways of ATP-sensitive potassium channels referring to cardio-protective effects and cellular functions. Drug Res. 2019, 69, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Jepps, T.A.; Barrese, V.; Miceli, F. Kv7 channels: Structure, physiology, and pharmacology. Front. Physiol. 2021, 12, 679317. [Google Scholar] [CrossRef]
- Cui, M.; Cantwell, L.; Zorn, A.; Logothetis, D.E. Kir Channel Molecular Physiology, Pharmacology, and Therapeutic Implications. In Pharmacology of Potassium Channels; Handbook of Experimental Pharmacology; Gamper, N., Wang, K., Eds.; Springer: Cham, Switzerland, 2021; Volume 267. [Google Scholar]
- Bodnár, M.; Schlichthörl, G.; Daut, J. The potassium current carried by TREK-1 channels in rat cardiac ventricular muscle. Pflugers Arch. 2015, 467, 1069–1079. [Google Scholar] [CrossRef]
- Wettwer, E.; Terlau, H. Pharmacology of voltage-gated potassium channel Kv1.5—Impact on cardiac excitability. Curr. Opin. Pharmacol. 2014, 15, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Ravens, U.; Wettwer, E. Ultra-rapid delayed rectifier channels: Molecular basis and therapeutic implications. Cardiovasc. Res. 2011, 89, 776–785. [Google Scholar] [CrossRef] [PubMed]
- Benitah, J.P.; Alvarez, J.L.; Gómez, A.M. L-type Ca2+ current in ventricular cardiomyocytes. J. Mol. Cell. Cardiol. 2010, 48, 26–36. [Google Scholar] [CrossRef]
- Abriel, H. Roles and regulation of the cardiac sodium channel Nav1.5: Recent insights from experimental studies. Cardiovasc. Res. 2007, 76, 381–389. [Google Scholar] [CrossRef]
- Mitrokhin, V.M.; Kamkina, O.V.; Kamkin, A.G.; Rodina, A.S.; Zolotareva, A.D.; Zolotarev, V.I.; Kazansky, V.E.; Gorbacheva, L.R.; Bilichenko, A.S.; Shileiko, S.A.; et al. Simulated microgravity and hypergravity affect the expression level of soluble guanylate cyclase, adenylate cyclase, and phosphodiesterase genes in rat ventricular cardiomyocytes. Bull. Exp. Biol. Med. 2024, 176, 359–362. [Google Scholar] [CrossRef]
- Mitrokhin, V.; Bilichenko, A.; Kazanski, V.; Schobik, R.; Shileiko, S.; Revkova, V.; Kalsin, V.; Kamkina, O.; Kamkin, A.; Mladenov, M. Transcriptomic Profile of the Mechanosensitive Ion Channelome in Human Cardiac Fibroblasts. Exp. Biol. Med. 2023, 248, 2341–2350. [Google Scholar] [CrossRef]
- Ashley, E.A.; Sears, C.E.; Bryant, S.M.; Watkins, C.H.; Casadei, B. Cardiac nitric oxide synthase 1 regulates basal and β-adrenergic contractility in murine ventricular myocytes. Circulation 2002, 105, 3011–3016. [Google Scholar] [CrossRef]
- Barouch, L.A.; Harrison, R.W.; Skaf, M.W.; Rosas, G.O.; Cappola, T.P.; Kobeissi, Z.A.; Hobai, I.A.; Lemmon, C.A.; Burnett, A.L.; O’Rourke, B.; et al. Nitric oxide regulates the heart by spatial confinement of nitric oxide synthase isoforms. Nature 2002, 416, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Kamkin, A.G.; Kamkina, O.V.; Shim, A.L.; Bilichenko, A.; Mitrokhin, V.M.; Kazansky, V.E.; Filatova, T.S.; Abramochkin, D.V.; Mladenov, M.I. The role of activation of two different sGC binding sites by NO-dependent and NO-independent mechanisms in the regulation of SACs in rat ventricular cardiomyocytes. Physiol. Rep. 2022, 10, e15246. [Google Scholar] [CrossRef] [PubMed]
- Tidball, J.G.; Lavergne, E.; Lau, K.S.; Spencer, M.J.; Stull, J.T.; Wehling, M. Mechanical loading regulates NOS expression and activity in developing and adult skeletal muscle. Am. J. Physiol. Cell Physiol. 1998, 275, C260–C266. [Google Scholar] [CrossRef]
- Kazansky, V.E.; Kamkin, A.G.; Makarenko, E.Y.; Lysenko, N.; Sutiagin, P.V.; Kiseleva, I.S. Role of nitric oxide in the regulation of mechanosensitive ionic channels in cardiomyocytes: Contribution of NO-synthases. Bull. Exp. Biol. Med. 2010, 150, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Boycott, H.E.; Nguyen, M.N.; Vrellaku, B.; Gehmlich, K.; Robinson, P. Nitric oxide and mechano-electrical transduction in cardiomyocytes. Front. Physiol. 2020, 11, 606740. [Google Scholar] [CrossRef]
- Gödecke, A.; Heinicke, T.; Kamkin, A.; Kiseleva, I.; Strasser, R.H.; Decking, U.K.; Stumpe, T.; Isenberg, G.; Schrader, J. Inotropic response to β-adrenergic receptor stimulation and anti-adrenergic effect of ACh in endothelial NO synthase-deficient mouse hearts. J. Physiol. 2001, 532, 195–204. [Google Scholar] [CrossRef]
- Andrews, S. FASTQC. A Quality Control Tool for High Throughput Sequence Data. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 15 May 2025).
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R.; 1000 Genome Project Data Processing Subgroup. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq—A Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Seddon, M.; Shah, A.M.; Casadei, B. Cardiomyocytes as effectors of nitric oxide signalling. Cardiovasc. Res. 2007, 75, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Dyachenko, V.; Rueckschloss, U.; Isenberg, G. Modulation of cardiac mechanosensitive ion channels involves superoxide, nitric oxide and peroxynitrite. Cell Calcium 2009, 45, 55–64. [Google Scholar] [CrossRef]
- Li, X.T.; Dyachenko, V.; Zuzarte, M.; Putzke, C.; Preisig-Müller, R.; Isenberg, G.; Daut, J. The stretch-activated potassium channel TREK-1 in rat cardiac ventricular muscle. Cardiovasc. Res. 2006, 69, 86–97. [Google Scholar]
- Kamkin, A.; Kiseleva, I. Mechano-Electric Feedback in the Heart: Evidence from Intracellular Microelectrode Recordings on Multicellular Preparations and Single Cells from Healthy and Diseased Tissue. In Mechanosensitivity in Cells and Tissues; Kamkin, A., Kiseleva, I., Eds.; Academia: Moscow, Russia, 2005; pp. 165–202. [Google Scholar]
- Lozinsky, I.; Kamkin, A. Mechanosensitive Alterations of Action Potentials and Membrane Currents in Healthy and Diseased Cardiomyocytes: Cardiac Tissue and Isolated Cell. In Mechanosensitivity of the Heart. Mechanosensitivity in Cells and Tissues; Kamkin, A., Kiseleva, I., Eds.; Springer: Dordrecht, The Netherlands, 2010; Volume 3, pp. 185–238. [Google Scholar]
- Gillis, K.D. Techniques for membrane capacitance measurement. In Single-Channel Recording, 2nd ed.; Sakmann, B., Neher, E., Eds.; Plenum Press: New York, NY, USA, 1995; pp. 155–198. [Google Scholar]
| Antibodies | Dilution | Origin | Country of Origin |
|---|---|---|---|
| TRPM 7 | 1:1000 | Rabbit | ABclonal, San Diego, CA, USA |
| α-subunit of Na/K-ATPase | 1:100,000 | Rabbit | Abcam Limited, Cambridge, UK |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bilichenko, A.S.; Zolotareva, A.D.; Kamkina, O.V.; Zolotarev, V.I.; Rodina, A.S.; Kazansky, V.E.; Mitrokhin, V.M.; Mladenov, M.I.; Kamkin, A.G. Simulated Microgravity Attenuates Stretch Sensitivity of Mechanically Gated Channels in Rat Ventricular Myocytes. Int. J. Mol. Sci. 2025, 26, 6653. https://doi.org/10.3390/ijms26146653
Bilichenko AS, Zolotareva AD, Kamkina OV, Zolotarev VI, Rodina AS, Kazansky VE, Mitrokhin VM, Mladenov MI, Kamkin AG. Simulated Microgravity Attenuates Stretch Sensitivity of Mechanically Gated Channels in Rat Ventricular Myocytes. International Journal of Molecular Sciences. 2025; 26(14):6653. https://doi.org/10.3390/ijms26146653
Chicago/Turabian StyleBilichenko, Andrey S., Alexandra D. Zolotareva, Olga V. Kamkina, Valentin I. Zolotarev, Anastasia S. Rodina, Viktor E. Kazansky, Vadim M. Mitrokhin, Mitko I. Mladenov, and Andre G. Kamkin. 2025. "Simulated Microgravity Attenuates Stretch Sensitivity of Mechanically Gated Channels in Rat Ventricular Myocytes" International Journal of Molecular Sciences 26, no. 14: 6653. https://doi.org/10.3390/ijms26146653
APA StyleBilichenko, A. S., Zolotareva, A. D., Kamkina, O. V., Zolotarev, V. I., Rodina, A. S., Kazansky, V. E., Mitrokhin, V. M., Mladenov, M. I., & Kamkin, A. G. (2025). Simulated Microgravity Attenuates Stretch Sensitivity of Mechanically Gated Channels in Rat Ventricular Myocytes. International Journal of Molecular Sciences, 26(14), 6653. https://doi.org/10.3390/ijms26146653






