Mechanotransduction-Driven Modulation of L-Type Calcium Channels: Roles of Nitric Oxide, S-Nitrosylation, and cGMP in Rat Ventricular Cardiomyocytes
Abstract
1. Introduction
2. Results
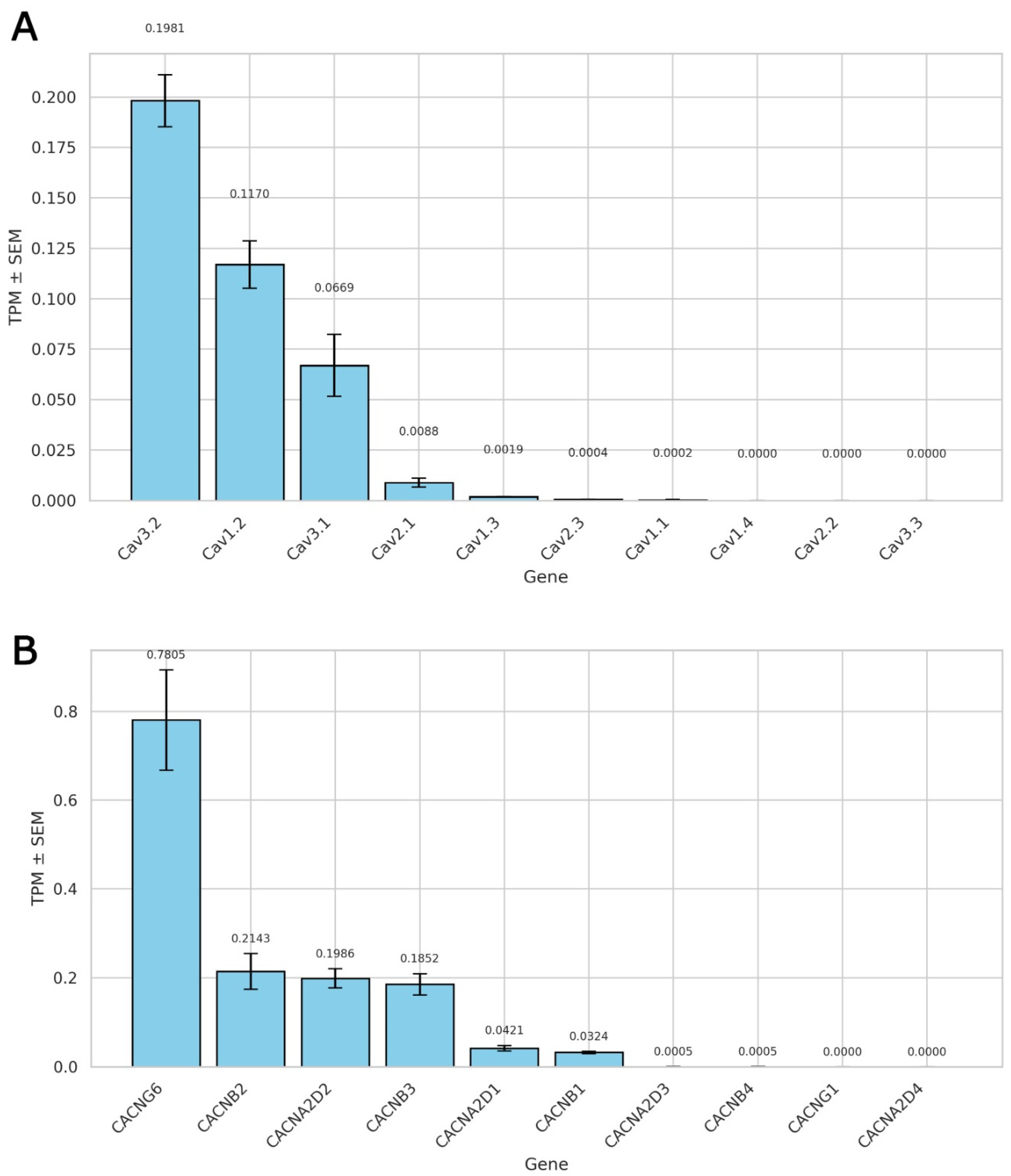
2.1. Expression Profile of Voltage-Gated Calcium Channels and Their Auxiliary Subunits
2.1.1. L-Type Calcium Channel Predominance in Rat Ventricular Cardiomyocytes
2.1.2. Auxiliary Subunit Expression Profile of L-Type Calcium Channels
2.1.3. Implications for T-Type Channel Contribution to Recorded Currents
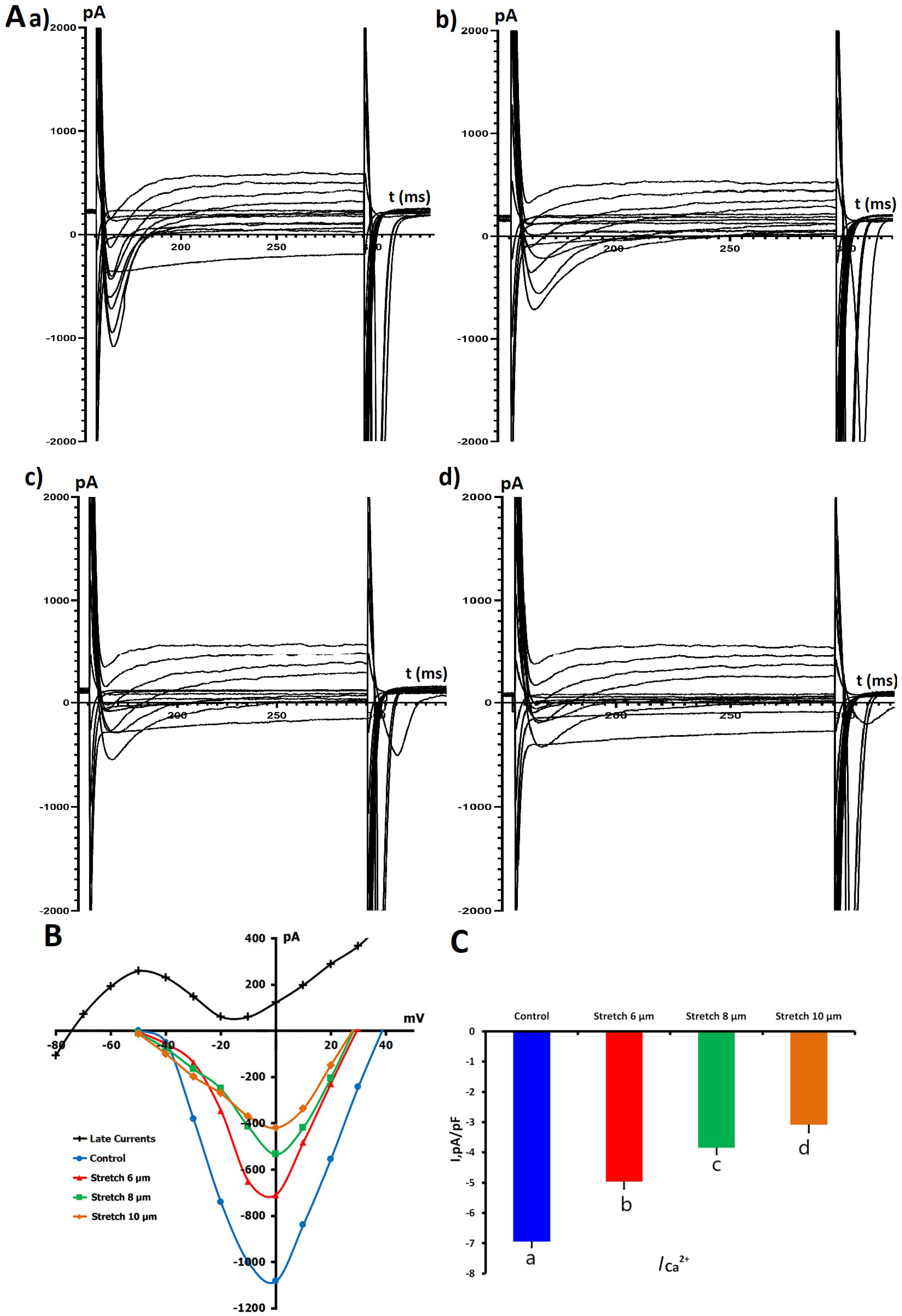
2.1.4. Impact of Mechanical Stretch on L-Type Ca2+ Currents
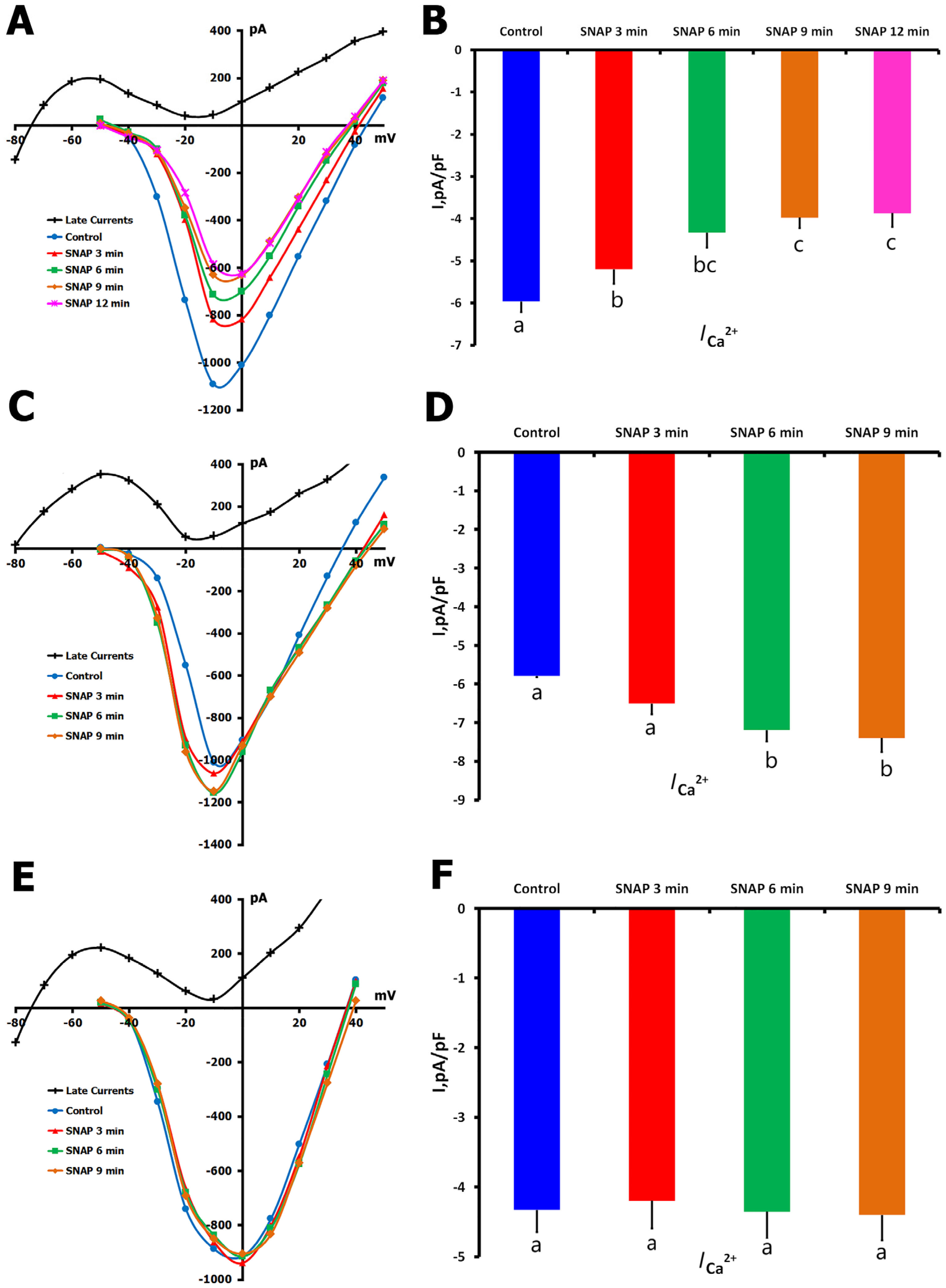
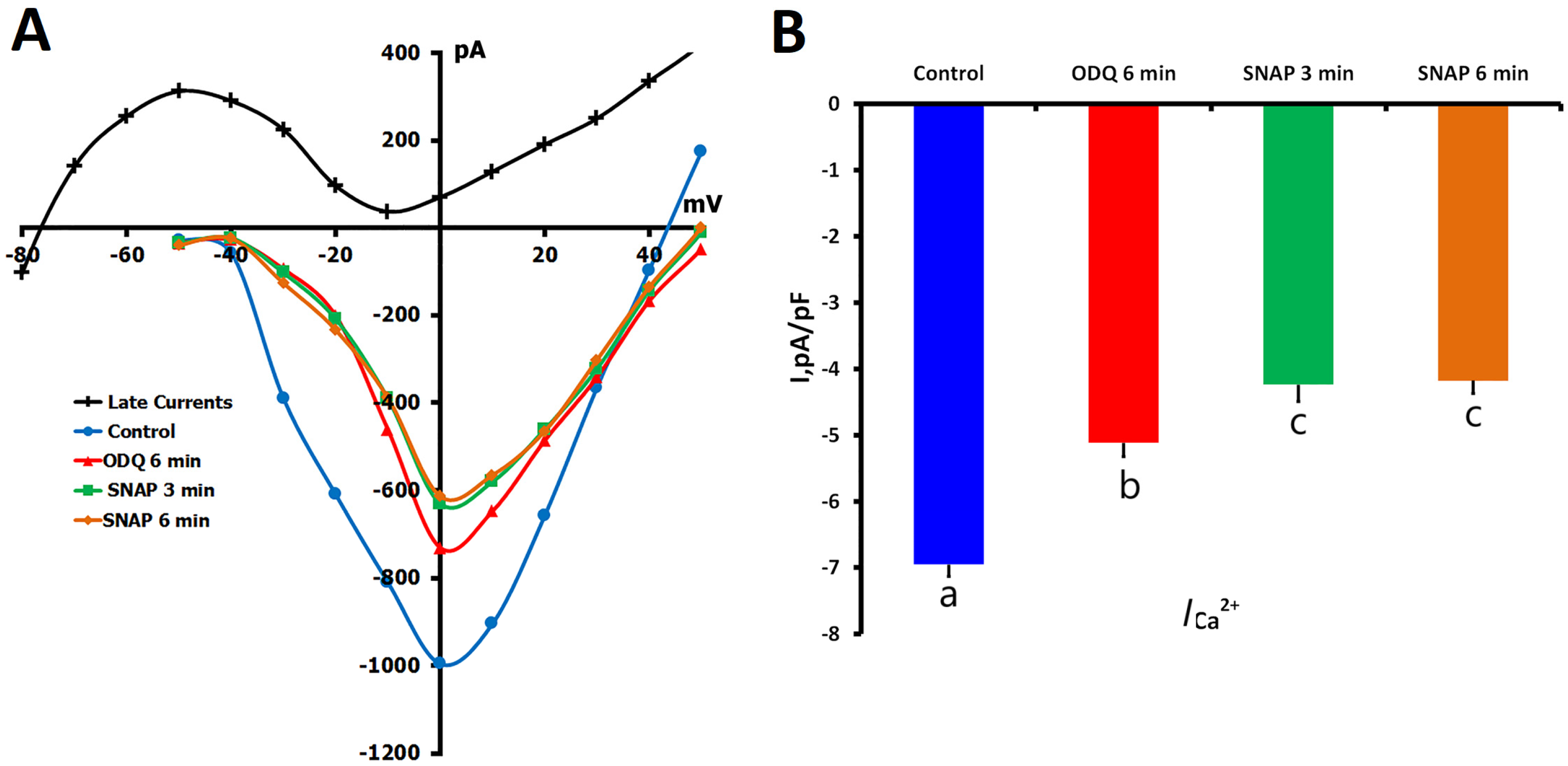
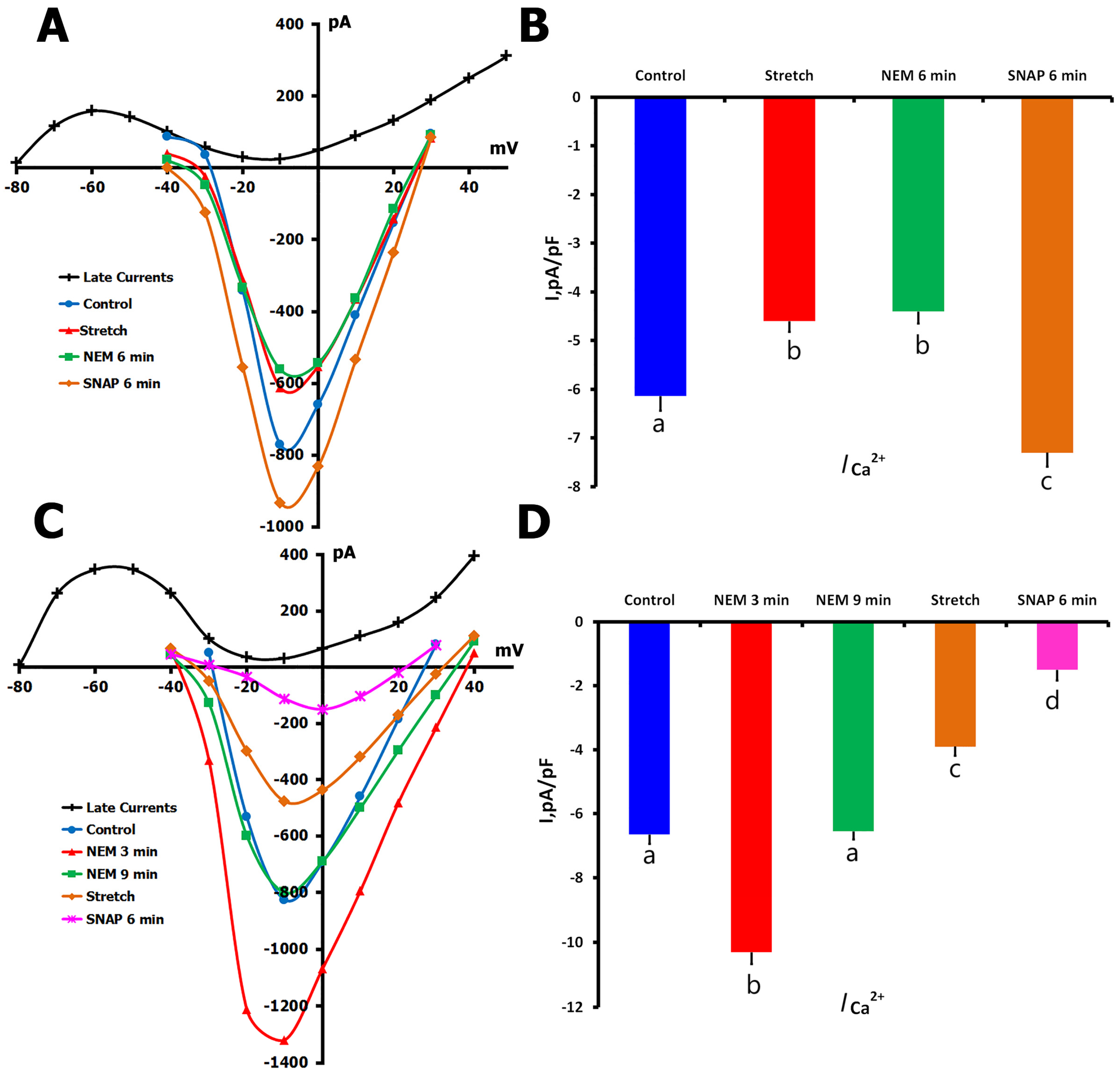
2.1.5. Effects of NO Donor SNAP on ICa,L
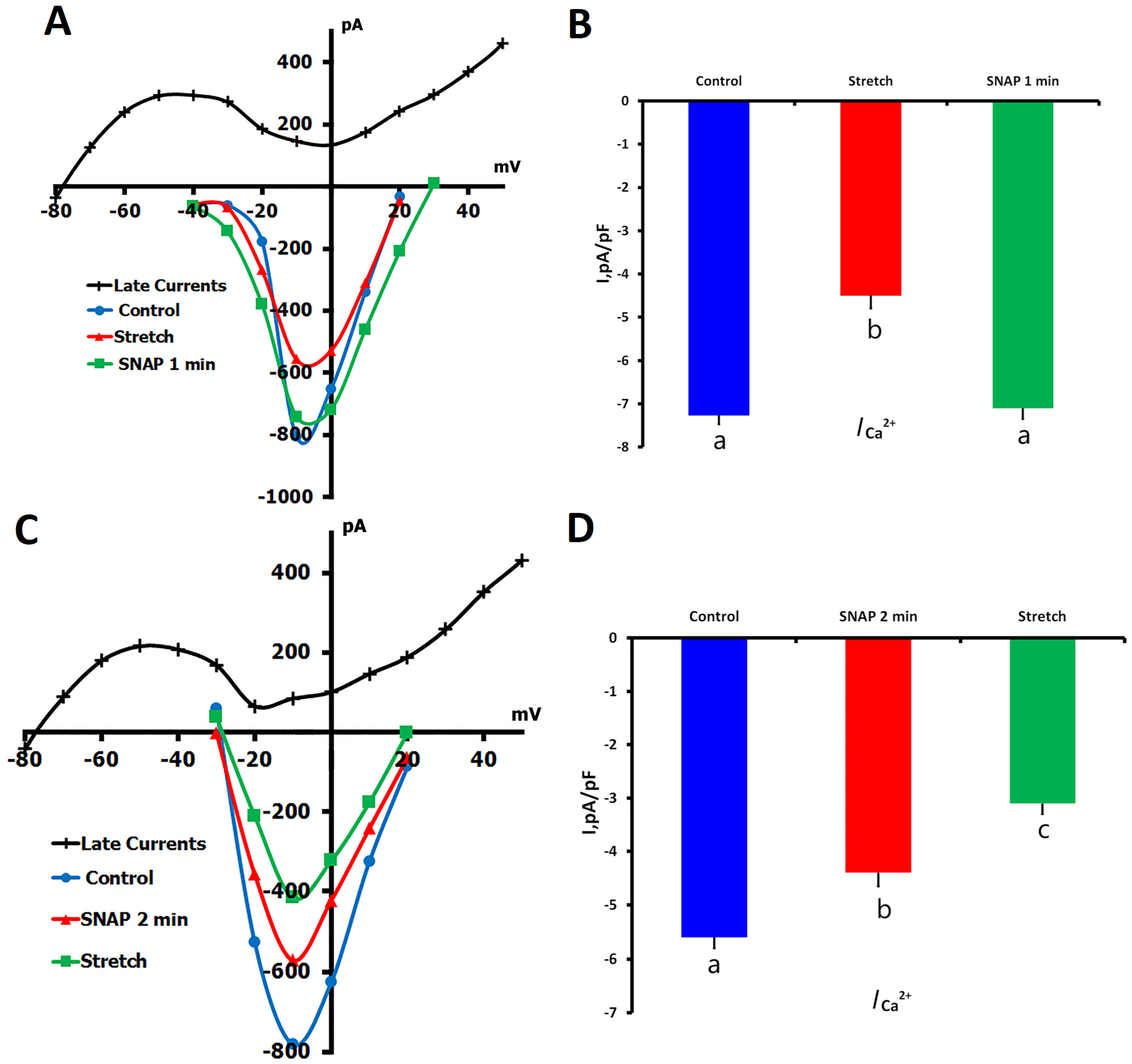
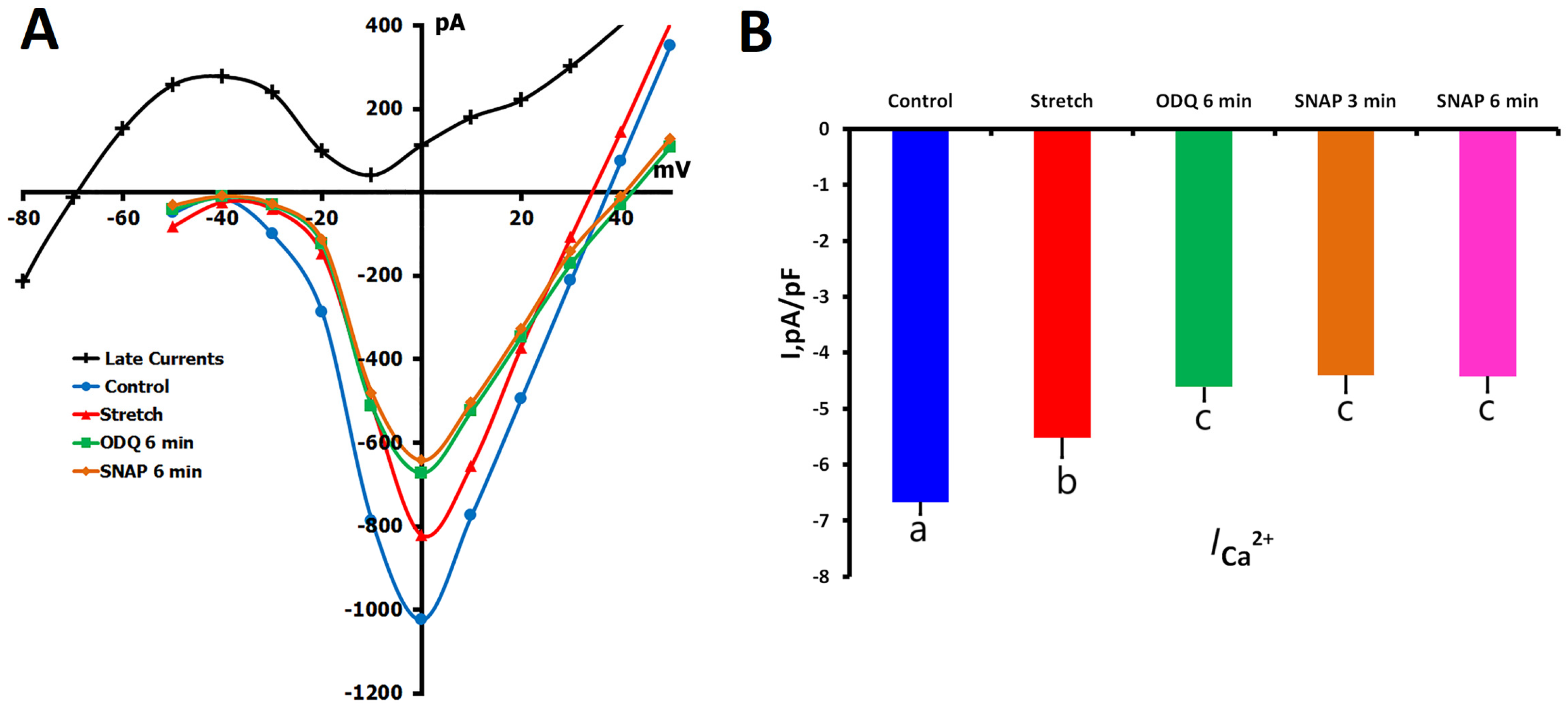
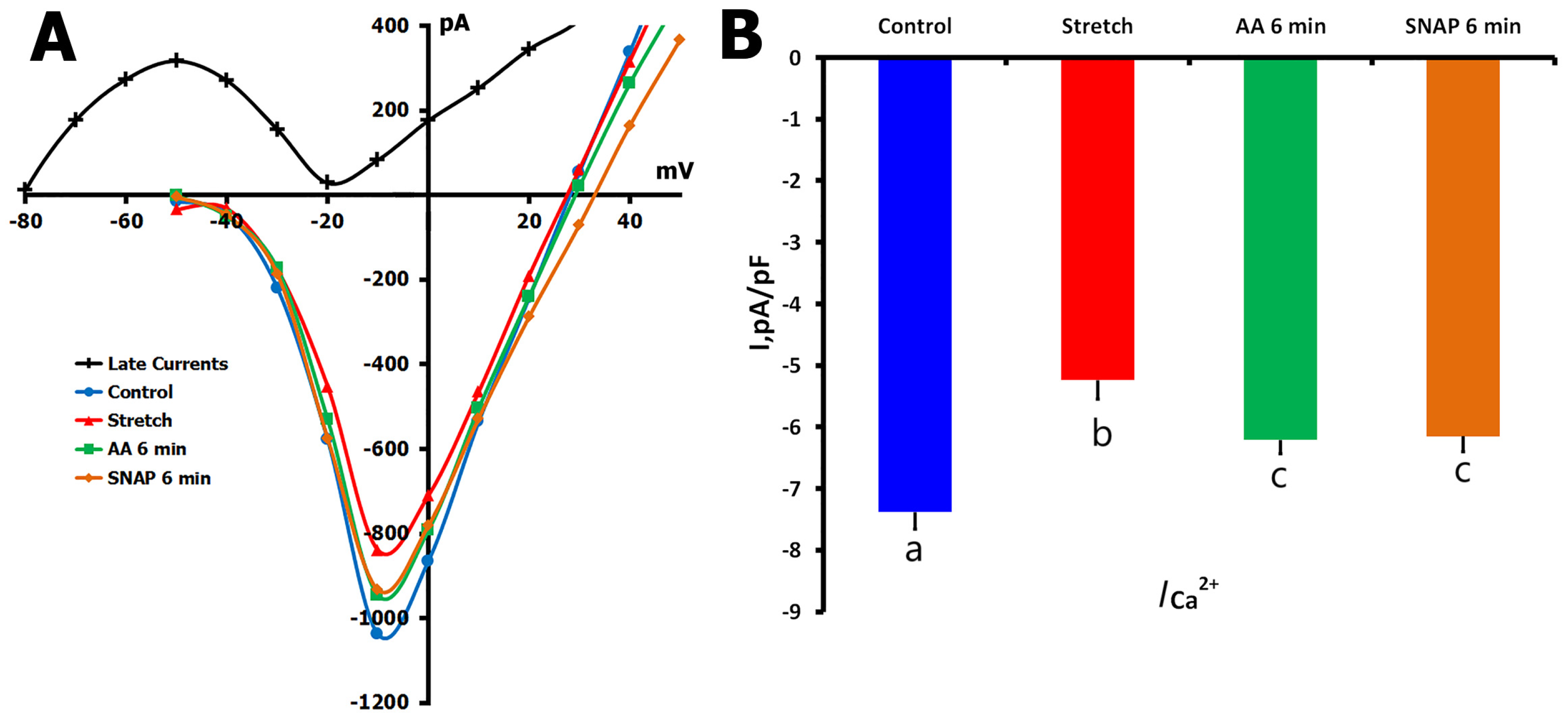
2.1.6. Effects of SNAP on ICa,L During Mechanical Stretch
- (1)
- SNAP application during sustained mechanical stretch;
- (2)
- Mechanical stretch was applied to cells pretreated with SNAP.
2.2. Effects of sGC Inhibition by ODQ on ICa,L Regulation
2.2.1. ODQ Inhibition of ICa,L in Unstretched Cells
2.2.2. ODQ Effects on ICa,L During Mechanical Stretch
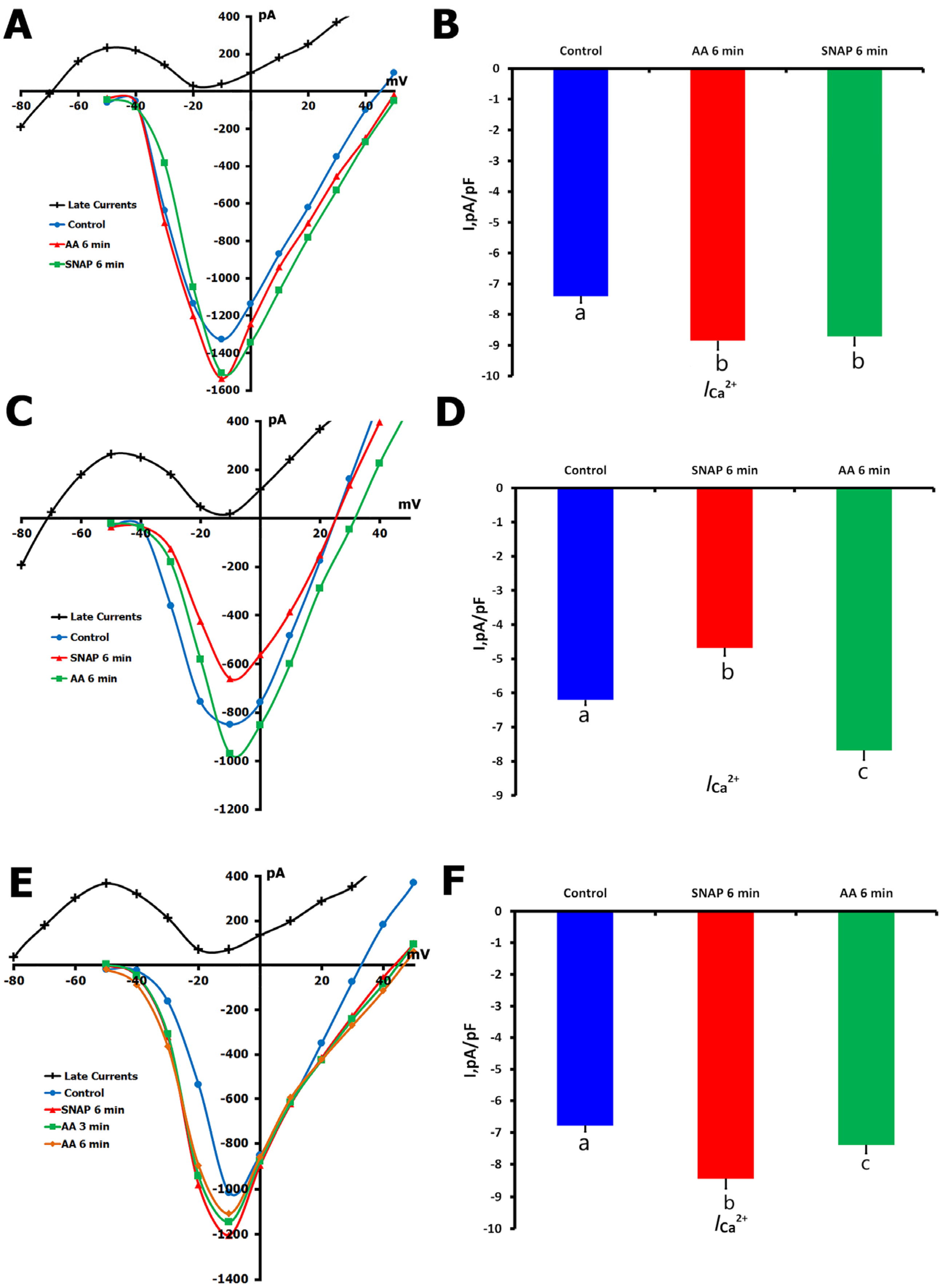
2.3. Effects of Ascorbic Acid (AA) on ICa,L Regulation
2.3.1. AA Modulation of ICa,L in Unstretched Cells
2.3.2. AA Effects on Stretch-Reduced ICa,L
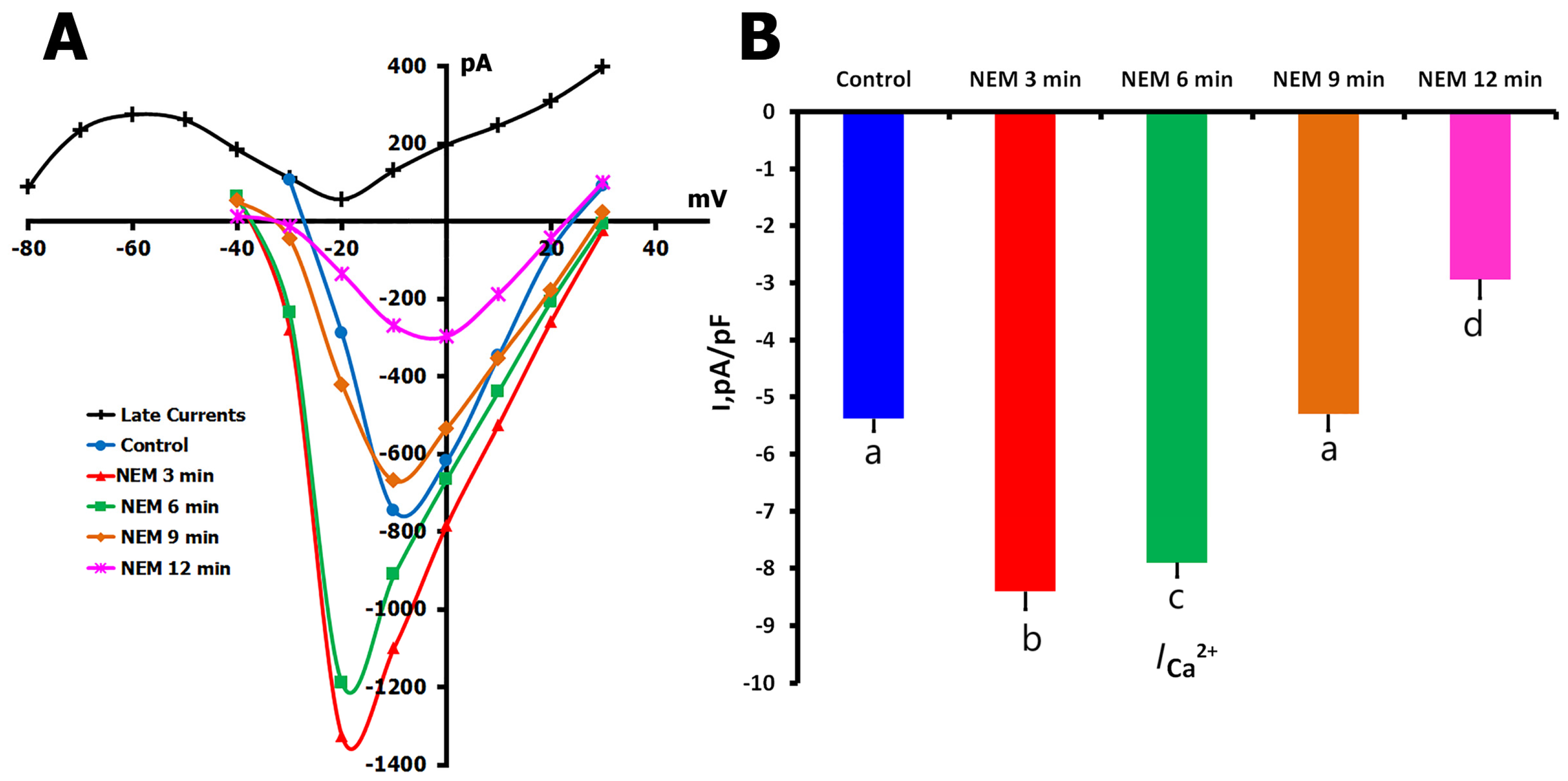
2.4. Effects of NEM on ICa,L Regulation
2.4.1. Biphasic NEM Effects on ICa,L in Unstretched Cells
2.4.2. NEM Effects on Stretch-Modified ICa,L
3. Discussion
3.1. Expression Pattern and Mechanosensitivity
3.2. NO Signaling Complexity and Mechanisms of Regulation
3.3. Integration with Cellular Ca2+ Handling
3.4. Regulation Through Multiple Pathways
3.5. Limitations and Future Directions
4. Materials and Methods
4.1. Animals and Cardiomyocyte Isolation
4.2. Patch-Clamp Recordings
4.3. Mechanical Stretch Protocol
4.4. Pharmacological Interventions
4.5. RNA Sequencing
4.6. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AA | Ascorbic Acid |
| ATP | Adenosine Triphosphate |
| BAPTA | 1,2-Bis(o-aminophenoxy)ethane-N,N,N′,N′-Tetraacetic Acid |
| BSA | Bovine Serum Albumin |
| CMs | Cardiomyocytes |
| cGMP | Cyclic Guanosine Monophosphate |
| EGTA | Ethylene Glycol Tetraacetic Acid |
| GEO | Gene Expression Omnibus |
| hiPSC-CMs | Human-Induced Pluripotent Stem Cell–Derived Cardiomyocytes |
| ICa,L | L-type Calcium Current |
| iPSCs | Induced Pluripotent Stem Cells |
| IV curve | Current–Voltage Curve |
| KB medium | Kraftbrühe Medium |
| MGCs | Mechanically Gated Channels |
| mRNA | Messenger Ribonucleic Acid |
| MSCs | Mechano-Sensitive Channels |
| NEB | New England Biolabs |
| NEM | N-Ethylmaleimide |
| NO | Nitric Oxide |
| NOS | Nitric Oxide Synthase |
| NOS1/2/3 | Nitric Oxide Synthase Isoforms (neuronal, inducible, endothelial) |
| ODQ | 1H-[1,2,4]Oxadiazolo[4,3-a]quinoxalin-1-one |
| PSS | Physiological Salt Solution |
| RNA | Ribonucleic Acid |
| ROS | Reactive Oxygen Species |
| RT-qPCR | Real-Time Quantitative Polymerase Chain Reaction (if mentioned, inferred from context) |
| sGC | Soluble Guanylyl Cyclase |
| SEM | Standard Error of the Mean |
| SIN-1 | 3-Morpholinosydnonimine |
| SNAP | S-nitroso-N-acetylpenicillamine |
| TPM | Transcripts Per Million |
| TRIzol | Commercial Reagent for RNA Isolation |
References
- Bers, D.M. Cardiac excitation–contraction coupling. Nature 2002, 415, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Lyford, G.L.; Strege, P.R.; Shepard, A.; Ou, Y.; Ermilov, L.; Miller, S.M.; Gibbons, S.J.; Farrugia, G. α(1C) (Ca(v)1.2) L-type calcium channel mediates mechanosensitive calcium regulation. Am. J. Physiol.-Cell Physiol. 2002, 283, C1001–C1008. [Google Scholar] [CrossRef]
- Vincent, P.F.; Bouleau, Y.; Petit, C.; Dulon, D. A synaptic F-actin network controls otoferlin-dependent exocytosis in auditory inner hair cells. eLife 2015, 4, e10988. [Google Scholar] [CrossRef]
- Ziolo, M.T. The fork in the nitric oxide road: Cyclic GMP or nitrosylation? Nitric Oxide 2008, 18, 153–156. [Google Scholar] [CrossRef]
- Boycott, H.E.; Nguyen, M.N.; Vrellaku, B.; Gehmlich, K.; Robinson, P. Nitric oxide and mechano-electrical transduction in cardiomyocytes. Front. Physiol. 2020, 11, 1629. [Google Scholar] [CrossRef]
- Petroff, M.G.; Kim, S.H.; Pepe, S.; Dessy, C.; Marbán, E.; Balligand, J.L.; Sollott, S.J. Endogenous nitric oxide mechanisms mediate the stretch dependence of Ca2⁺ release in cardiomyocytes. Nat. Cell Biol. 2001, 3, 867–873. [Google Scholar] [CrossRef]
- Gonzalez, D.R.; Treuer, A.V.; Sun, Q.A.; Stamler, J.S.; Hare, J.M. S-nitrosylation of cardiac ion channels. J. Cardiovasc. Pharmacol. 2009, 54, 188–195. [Google Scholar] [CrossRef]
- Chen, L.; Chetkovich, D.M.; Petralia, R.S.; Swanson, G.T.; Wenthold, R.J.; Bredt, D.S. Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature 2000, 408, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Greger, I.H.; Khatri, L.; Kong, X.; Ziff, E.B. AMPA receptor tetramerization is mediated by Q/R editing. Neuron 2003, 40, 763–774. [Google Scholar] [CrossRef]
- Klugbauer, N.; Marais, E.; Hofmann, F. Calcium channel α2δ subunits: Differential expression, function, and drug binding. J. Bioenerg. Biomembr. 2003, 35, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Sandoz, G.; López-Gonzalez, I.; Grunwald, D.; Bichet, D.; Altafaj, X.; Weiss, N.; Mourre, C. Cavβ-subunit displacement is a key step in L-type calcium channel activation. J. Physiol. 2004, 555, 685–700. [Google Scholar]
- Calabrese, B.; Tabarean, I.V.; Juranka, P.; Morris, C.E. Mechanosensitivity of N-type calcium channel currents. Biophys. J. 2002, 83, 2560–2574. [Google Scholar] [CrossRef] [PubMed]
- Beyder, A.; Rae, J.L.; Bernard, C.E.; Strege, P.R.; Sachs, F.; Farrugia, G. Mechanosensitivity of Nav1.5, a voltage-sensitive sodium channel. J. Physiol. 2010, 588, 4969–4985. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Jan, L.Y.; Jan, Y.N. Mechanosensitive ion channels: Structural features relevant to mechanotransduction mechanisms. Annu. Rev. Neurosci. 2020, 43, 207–229. [Google Scholar] [CrossRef]
- Youm, J.B.; Han, J.; Kim, N.; Zhang, Y.H.; Kim, E.; Leem, C.H. Role of stretch-activated channels in the heart: Action potential and Ca2⁺ transients. In Mechanosensitivity in Cells and Tissues; Kamkin, A., Kiseleva, I., Eds.; Academia: Moscow, Russia, 2005; pp. 103–125. [Google Scholar]
- Youm, J.B.; Han, J.; Kim, N.; Zhang, Y.H.; Kim, E.; Joo, H.; Leem, C.H. Role of stretch-activated channels on the stretch-induced changes of rat atrial myocytes. Prog. Biophys. Mol. Biol. 2006, 90, 186–206. [Google Scholar] [CrossRef]
- Sasaki, N.; Mitsuiye, T.; Noma, A. Effects of mechanical stretch on membrane currents of single ventricular myocytes of guinea-pig heart. Jpn. J. Physiol. 1992, 42, 957–970. [Google Scholar] [CrossRef]
- Kamkin, A.; Kiseleva, I.; Isenberg, G. Stretch-activated currents in ventricular myocytes: Amplitude and arrhythmogenic effects increase with hypertrophy. Cardiovasc. Res. 2000, 48, 409–420. [Google Scholar] [CrossRef]
- Kamkin, A.; Kiseleva, I.; Isenberg, G. Ion selectivity of stretch-activated cation currents in mouse ventricular myocytes. Pflügers Arch.-Eur. J. Physiol. 2003, 446, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Vandecasteele, G.; Eschenhagen, T.; Fischmeister, R. Role of the NO-cGMP pathway in the muscarinic regulation of the L-type Ca2⁺ current in human atrial myocytes. J. Physiol. 1998, 506, 653–663. [Google Scholar] [CrossRef]
- Vulcu, S.D.; Wegener, J.W.; Nawrath, H. Differences in the nitric oxide-soluble guanylyl cyclase signaling pathway in the myocardium of neonatal and adult rats. Eur. J. Pharmacol. 2000, 406, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Méry, P.; Pavoine, C.; Belhassen, L.; Pecker, F.; Fischmeister, R. Nitric oxide regulates cardiac Ca2⁺ current. J. Biol. Chem. 1993, 268, 26286–26295. [Google Scholar] [CrossRef]
- Barouch, L.A.; Harrison, R.W.; Skaf, M.W.; Rosas, G.O.; Cappola, T.P.; Kobeissi, Z.A.; Hobai, I.A.; Lemmon, C.A.; Burnett, A.L.; O’Rourke, B.; et al. Nitric oxide regulates the heart by spatial confinement of nitric oxide synthase isoforms. Nature 2002, 416, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef]
- Cittadini, A.; Monti, M.G.; Iaccarino, G.; Castiello, M.C.; Baldi, A.; Bossone, E.; Longobardi, S.; Marra, A.M.; Petrillo, V.; Saldamarco, L.; et al. SOCS1 gene transfer accelerates the transition to heart failure through the inhibition of the gp130/JAK/STAT pathway. Cardiovasc. Res. 2012, 96, 381–390. [Google Scholar] [CrossRef]
- Burkard, N.; Rokita, A.G.; Kaufmann, S.G.; Hallhuber, M.; Wu, R.; Hu, K.; Hofmann, U.; Stilli, D.; Dirschinger, R.; Ruth, P.; et al. Conditional neuronal nitric oxide synthase overexpression impairs myocardial contractility. Circ. Res. 2007, 100, e32–e34. [Google Scholar] [CrossRef]
- Evangelista, A.M.; Kohr, M.J.; Murphy, E. S-nitrosylation: Specificity, occupancy, and interaction with other post-translational modifications. Antioxid. Redox Signal. 2013, 19, 1209–1219. [Google Scholar] [CrossRef]
- Murphy, E.; Kohr, M.; Sun, J.; Nguyen, T.; Steenbergen, C. S-nitrosylation: A radical way to protect the heart. J. Mol. Cell. Cardiol. 2012, 52, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Kazanski, V.; Kamkin, A.; Makarenko, E.; Lysenko, N.; Sutiagin, P.V.; Kiseleva, I. Role of nitric oxide in the regulation of mechanosensitive ionic channels in cardiomyocytes: Contribution of NO-synthases. Bull. Exp. Biol. Med. 2010, 150, 263–267. [Google Scholar] [CrossRef]
- Kazanski, V.; Kamkin, A.; Makarenko, E.; Lysenko, N.; Lapina, N.; Kiseleva, I. The role of nitric oxide in the regulation of mechanically gated channels in the heart. In Mechanosensitivity and Mechanotransduction; Kamkin, A., Kiseleva, I., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 109–140. [Google Scholar]
- Gödecke, A.; Heinicke, T.; Kamkin, A.; Kiseleva, I.; Strasser, R.H.; Decking, U.K.; Schrader, J. Inotropic response to β-adrenergic receptor stimulation and anti-adrenergic effect of ACh in endothelial NO synthase-deficient mouse hearts. J. Physiol. 2001, 532, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Qi, H.; Ma, Y.; Liu, S.; Zhao, M.; Guo, Z.; Li, Y.; Zhang, Y.; Ren, T. A flexible and highly sensitive organic electrochemical transistor-based biosensor for continuous and wireless nitric oxide detection. Proc. Natl. Acad. Sci. USA 2022, 119, e2208060119. [Google Scholar] [CrossRef]
- Gomes, F.O.; Maia, L.B.; Loureiro, J.A.; Pereira, M.C.; Delerue-Matos, C.; Moura, I.; Moura, J.J.G. Biosensor for direct bioelectrocatalysis detection of nitric oxide using nitric oxide reductase incorporated in carboxylated single-walled carbon nanotubes/lipidic bilayer nanocomposite. Bioelectrochemistry 2019, 127, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Liu, J.M.; Du, L.; Tang, A.; Shang, Y.; Wang, S.Q.; Li, X.; Li, Y. Nitric oxide signaling in stretch-induced apoptosis of neonatal rat cardiomyocytes. FASEB J. 2006, 20, 1883–1885. [Google Scholar] [CrossRef][Green Version]
- Han, X.; Kobzik, L.; Balligand, J.L.; Kelly, R.A.; Smith, T.W. Nitric oxide synthase (NOS3)-mediated cholinergic modulation of Ca2⁺ current in adult rabbit atrioventricular nodal cells. Circ. Res. 1996, 78, 998–1008. [Google Scholar] [CrossRef]
- Massion, P.B.; Pelat, M.; Belge, C.; Balligand, J.L. Modulation of cardiac contraction, relaxation and rate by the endothelial nitric oxide synthase (eNOS): Lessons from genetically modified mice. J. Physiol. 2003, 546, 63–75. [Google Scholar] [CrossRef]
- Belus, A.; White, E. Streptomycin and intracellular calcium modulate the response of single guinea-pig ventricular myocytes to axial stretch. J. Physiol. 2003, 546, 501–509. [Google Scholar] [CrossRef]
- Sigurdson, W.; Ruknudin, A.; Sachs, F. Calcium imaging of mechanically induced fluxes in tissue-cultured chick heart: Role of stretch-activated ion channels. Am. J. Physiol. 1992, 262, H1110–H1115. [Google Scholar] [CrossRef]
- Isenberg, G.; Kazanski, V.; Kondratev, D.; Wendt-Gallitelli, M.F.; Kiseleva, I.; Kamkin, A. Differential effects of stretch and compression on membrane currents and [Na⁺]c in ventricular myocytes. Prog. Biophys. Mol. Biol. 2003, 82, 43–56. [Google Scholar] [CrossRef]
- Wendt-Gallitelli, M.F.; Voigt, T.; Isenberg, G. Microheterogeneity of subsarcolemmal sodium gradients. Electron probe microanalysis in guinea-pig ventricular myocytes. J. Physiol. 1993, 472, 33–44. [Google Scholar] [CrossRef]
- Tang, Q.Y.; Qi, Z.; Naruse, K.; Sokabe, M. Characterization of a functionally expressed stretch-activated BKCa channel cloned from chick ventricular myocytes. J. Membr. Biol. 2003, 196, 185–200. [Google Scholar] [CrossRef] [PubMed]
- Hongo, K.; Pascarel, C.; Cazorla, O.; Gannier, F.; Le Guennec, J.Y.; White, E. Gadolinium blocks the delayed rectifier potassium current in isolated guinea-pig ventricular myocytes. Exp. Physiol. 1997, 82, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Wahler, G.M.; Dollinger, S.J. Nitric oxide donor SIN-1 inhibits mammalian cardiac calcium current through cGMP-dependent protein kinase. Am. J. Physiol. 1995, 268, C45–C54. [Google Scholar] [CrossRef] [PubMed]
- Campbell, D.L.; Stamler, J.S.; Strauss, H.C. Redox modulation of L-type calcium channels in ferret ventricular myocytes. J. Gen. Physiol. 1996, 108, 277–293. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Hui, W.; Huang, M.; Guo, Y.; Yu, M.; Yang, X.; Liu, Y.; Chen, X. Dynamic Changes in Ion Channels during Myocardial Infarction and Therapeutic Challenges. Int. J. Mol. Sci. 2024, 25, 6467. [Google Scholar] [CrossRef]
- Bechard, E.; Bride, J.; Le Guennec, J.-Y.; Brette, F.; Demion, M. TREK-1 in the Heart: Potential Physiological and Pathophysiological Roles. Front. Physiol. 2022, 13, 1095102. [Google Scholar] [CrossRef]
- Del Canto, I.; Santamaría, L.; Genovés, P.; Such-Miquel, L.; Arias-Mutis, O.; Zarzoso, M.; Soler, C.; Parra, G.; Tormos, Á.; Alberola, A.; et al. Effects of the Inhibition of Late Sodium Current by GS967 on Stretch-Induced Changes in Cardiac Electrophysiology. Cardiovasc. Drugs Ther. 2018, 32, 413–425. [Google Scholar] [CrossRef]
- Ji, S.; John, S.A.; Lu, Y.; Weiss, J.N. Mechanosensitivity of the Cardiac Muscarinic Potassium Channel: A Novel Property Conferred by Kir3.4 Subunit. J. Biol. Chem. 1998, 273, 1324–1328. [Google Scholar] [CrossRef]
- Isenberg, G.; Klöckner, U. Calcium currents of isolated bovine ventricular myocytes are fast and of large amplitude. Pflügers Arch.-Eur. J. Physiol. 1982, 395, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Kamkin, A.G.; Kamkina, O.V.; Kazansky, V.E.; Mitrokhin, V.M.; Bilichenko, A.; Nasedkina, E.A.; Shileiko, S.A.; Rodina, A.S.; Zolotareva, A.D.; Zolotarev, V.I.; et al. Identification of RNA reads encoding different channels in isolated rat ventricular myocytes and the effect of cell stretching on L-type Ca2⁺ current. Biol. Direct 2023, 18, 70. [Google Scholar] [CrossRef]
- Andrews, S. FASTQC: A Quality Control Tool for High Throughput Sequence Data [Internet]. 2010. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 1 November 2024).
- Bolger, M.A.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamkina, O.V.; Rodina, A.S.; Kamkin, A.; Bilichenko, A.S.; Kazansky, V.E.; Zolotareva, A.D.; Zolotarev, V.I.; Shileiko, S.A.; Mitrokhin, V.M.; Mladenov, M.I. Mechanotransduction-Driven Modulation of L-Type Calcium Channels: Roles of Nitric Oxide, S-Nitrosylation, and cGMP in Rat Ventricular Cardiomyocytes. Int. J. Mol. Sci. 2025, 26, 7539. https://doi.org/10.3390/ijms26157539
Kamkina OV, Rodina AS, Kamkin A, Bilichenko AS, Kazansky VE, Zolotareva AD, Zolotarev VI, Shileiko SA, Mitrokhin VM, Mladenov MI. Mechanotransduction-Driven Modulation of L-Type Calcium Channels: Roles of Nitric Oxide, S-Nitrosylation, and cGMP in Rat Ventricular Cardiomyocytes. International Journal of Molecular Sciences. 2025; 26(15):7539. https://doi.org/10.3390/ijms26157539
Chicago/Turabian StyleKamkina, Olga V., Anastasia S. Rodina, Andre Kamkin, Andrei S. Bilichenko, Viktor E. Kazansky, Alexandra D. Zolotareva, Valentin I. Zolotarev, Stanislav A. Shileiko, Vadim M. Mitrokhin, and Mitko I. Mladenov. 2025. "Mechanotransduction-Driven Modulation of L-Type Calcium Channels: Roles of Nitric Oxide, S-Nitrosylation, and cGMP in Rat Ventricular Cardiomyocytes" International Journal of Molecular Sciences 26, no. 15: 7539. https://doi.org/10.3390/ijms26157539
APA StyleKamkina, O. V., Rodina, A. S., Kamkin, A., Bilichenko, A. S., Kazansky, V. E., Zolotareva, A. D., Zolotarev, V. I., Shileiko, S. A., Mitrokhin, V. M., & Mladenov, M. I. (2025). Mechanotransduction-Driven Modulation of L-Type Calcium Channels: Roles of Nitric Oxide, S-Nitrosylation, and cGMP in Rat Ventricular Cardiomyocytes. International Journal of Molecular Sciences, 26(15), 7539. https://doi.org/10.3390/ijms26157539





