Thiamine Compounds Alleviate Oxidative Stress, Over-Expression of Pro-Inflammatory Markers and Behavioral Abnormalities in a Mouse Predation Model of PTSD
Abstract
1. Introduction
2. Results
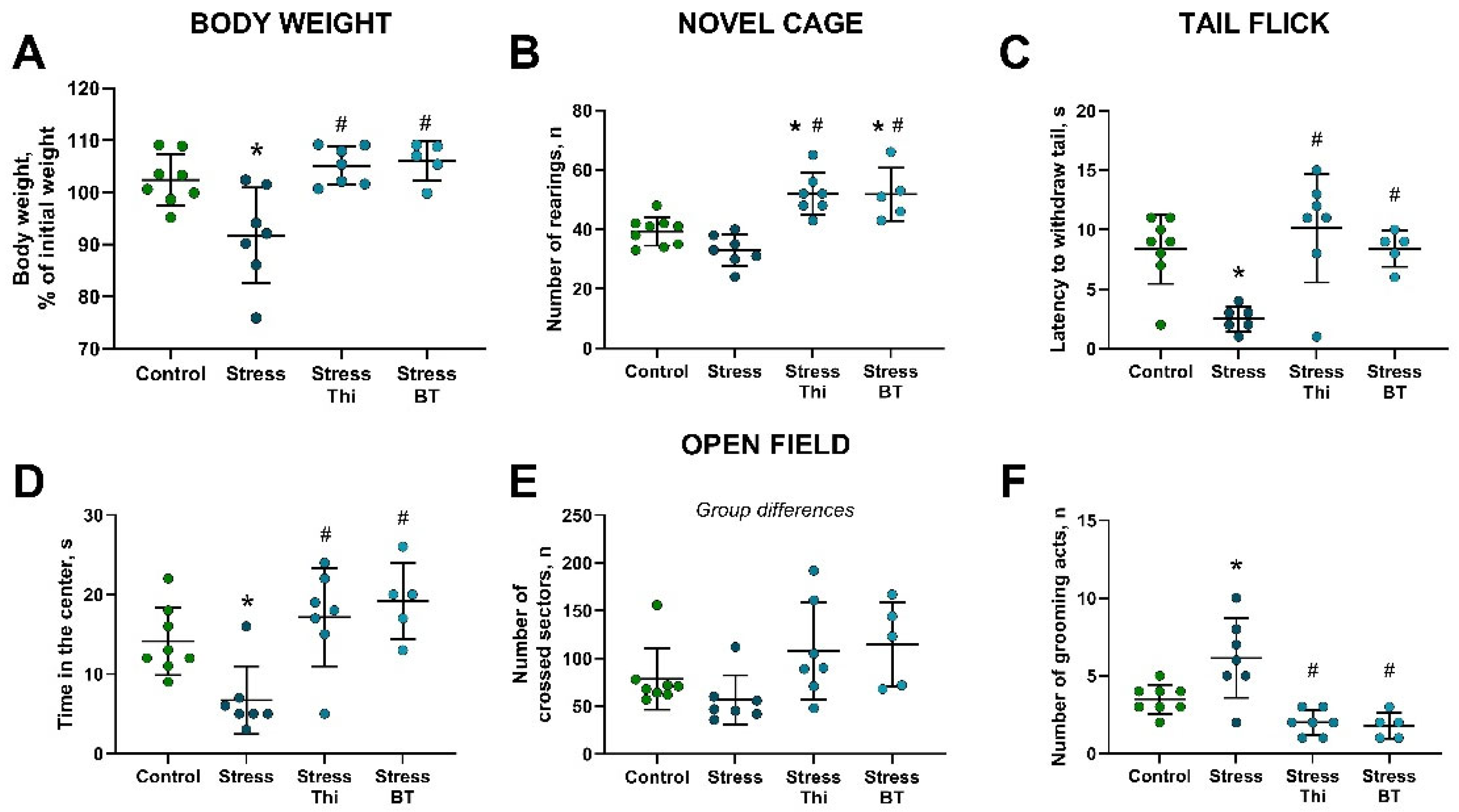
2.1. Chronic Dosing with Thiamine or Benfotiamine Ameliorated Parameters on Anxiety-like Behavior, Locomotor Activity, and Pain Sensitivity Altered by Rat Exposure
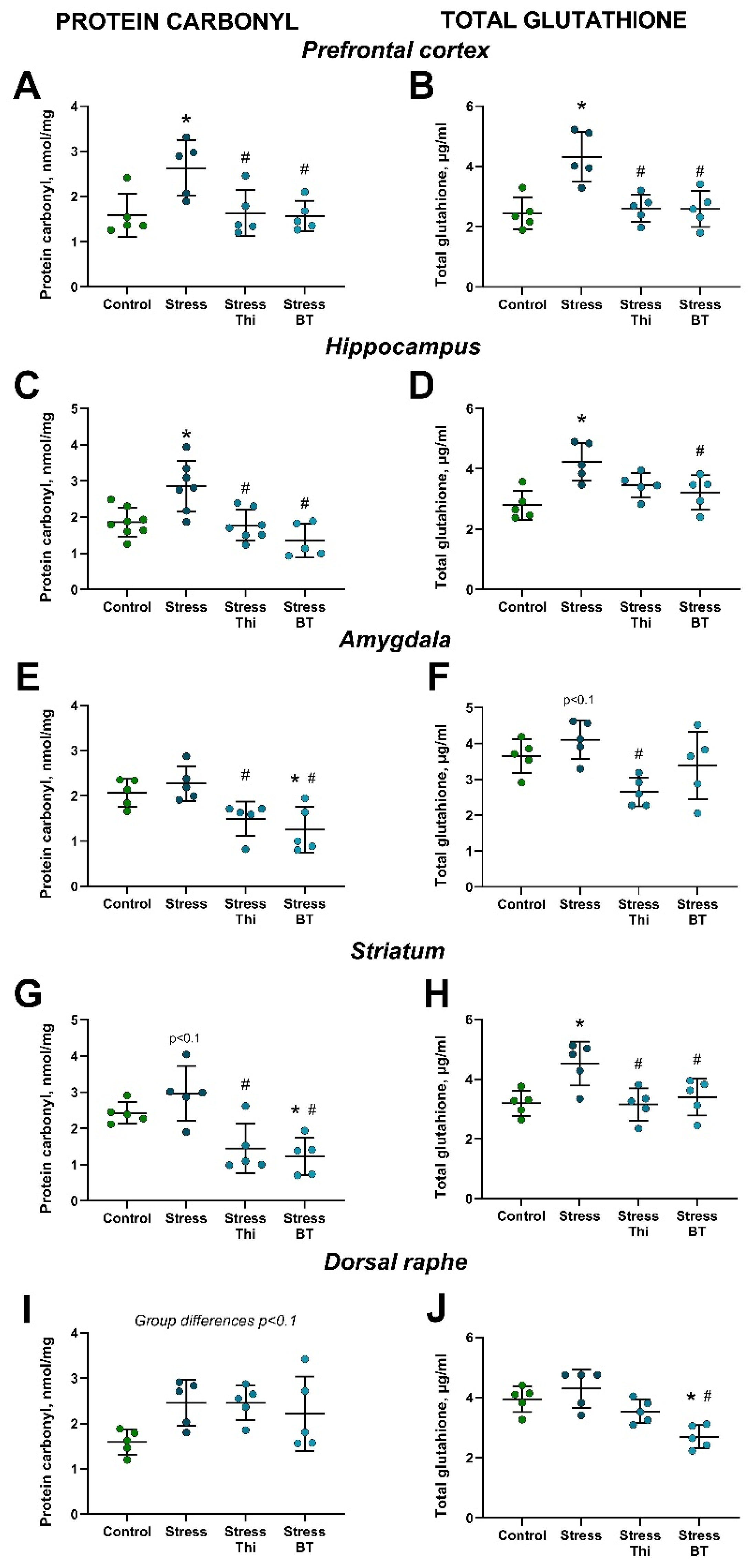
2.2. Increased Brain Concentration of Oxidative Stress Markers in Mice Subjected to Rat Exposure Stress and Normalizing Effects of Thiamine Compounds
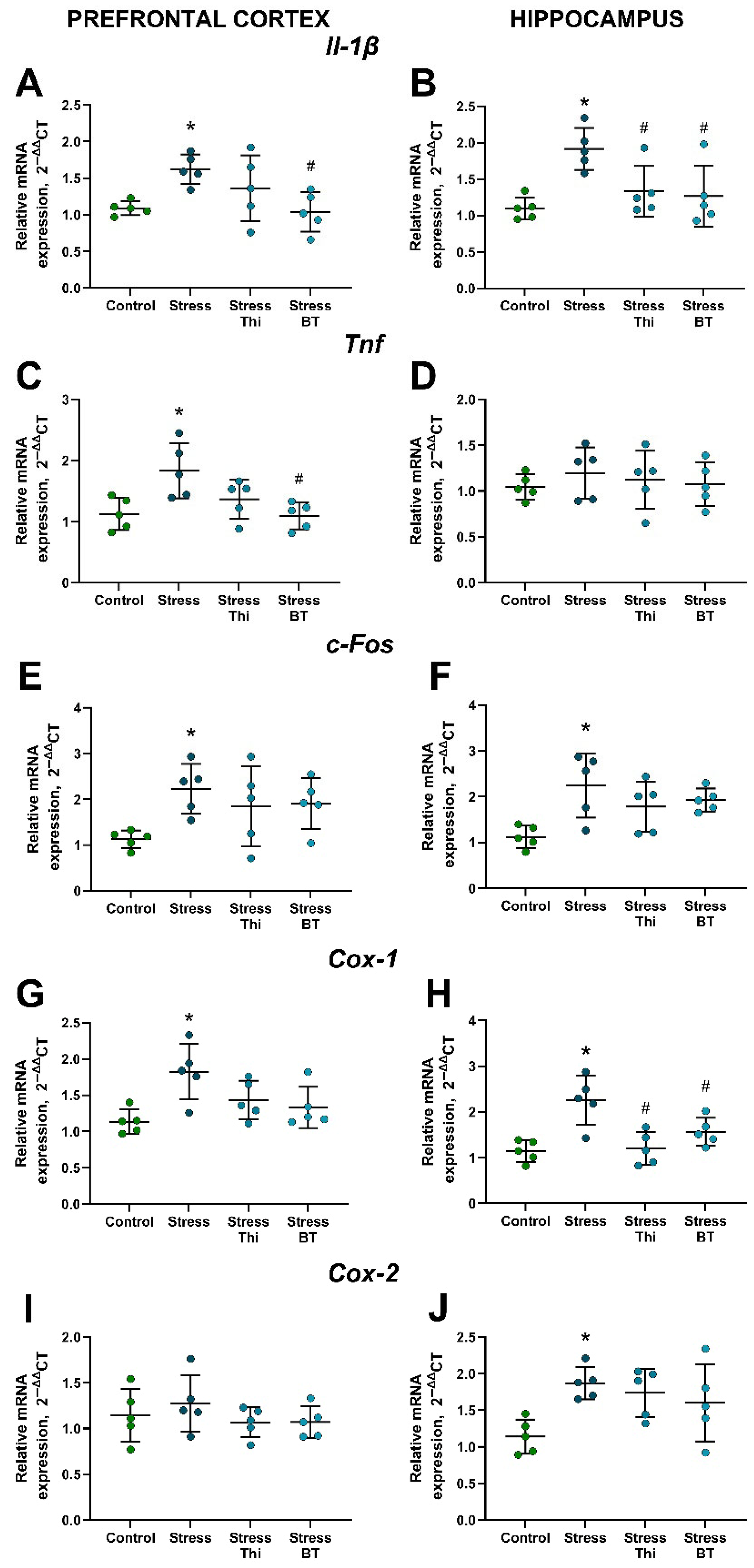
2.3. Upregulated Brain Gene Expression of Inflammatory Markers, Cyclooxygenases, and c-Fos in Mice Subjected to Rat Exposure Stress Is Counteracted by the Treatment with Thiamine Compounds
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
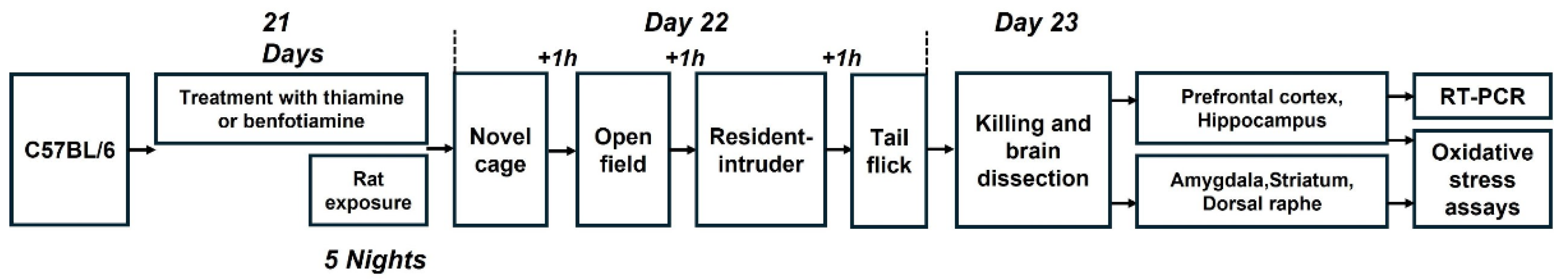
4.2. Study Flow
4.3. Rat Exposure Stress
4.4. Novel Cage
4.5. Open Field Test
4.6. Resident–Intruder Test
4.7. Tail-Flick Test
4.8. Killing of Mice and Sample Collection
4.9. Quantitative Real-Time PCR
4.10. Protein Carbonyl Assay
4.11. Total Glutathione Assay
4.12. Drug Administration
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hyland, P.; Shevlin, M.; Fyvie, C.; Karatzias, T. Posttraumatic Stress Disorder and Complex Posttraumatic Stress Disorder in DSM-5 and ICD-11: Clinical and Behavioral Correlates. J. Trauma. Stress 2018, 31, 174–180. [Google Scholar] [CrossRef]
- Mann, S.K.; Marwaha, R.; Torrico, T.J. Posttraumatic Stress Disorder. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Koenen, K.C.; Ratanatharathorn, A.; Ng, L.; McLaughlin, K.A.; Bromet, E.J.; Stein, D.J.; Karam, E.G.; Meron Ruscio, A.; Benjet, C.; Scott, K.; et al. Posttraumatic Stress Disorder in the World Mental Health Surveys. Psychol. Med. 2017, 47, 2260–2274. [Google Scholar] [CrossRef] [PubMed]
- Jericho, B.; Luo, A.; Berle, D. Trauma-focused Psychotherapies for Post-traumatic Stress Disorder: A Systematic Review and Network Meta-analysis. Acta Psychiatr. Scand. 2022, 145, 132–155. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C.; Roberts, N.P.; Gibson, S.; Bisson, J.I. Dropout from Psychological Therapies for Post-Traumatic Stress Disorder (PTSD) in Adults: Systematic Review and Meta-Analysis. Eur. J. Psychotraumatol. 2020, 11, 1709709. [Google Scholar] [CrossRef] [PubMed]
- Hoskins, M.D.; Bridges, J.; Sinnerton, R.; Nakamura, A.; Underwood, J.F.G.; Slater, A.; Lee, M.R.D.; Clarke, L.; Lewis, C.; Roberts, N.P.; et al. Pharmacological Therapy for Post-Traumatic Stress Disorder: A Systematic Review and Meta-Analysis of Monotherapy, Augmentation and Head-to-Head Approaches. Eur. J. Psychotraumatol. 2021, 12, 1802920. [Google Scholar] [CrossRef]
- Williams, T.; Phillips, N.J.; Stein, D.J.; Ipser, J.C. Pharmacotherapy for Post Traumatic Stress Disorder (PTSD). Cochrane Database Syst. Rev. 2022, 3, CD002795. [Google Scholar] [CrossRef]
- Lund, C.; Tomlinson, M.; De Silva, M.; Fekadu, A.; Shidhaye, R.; Jordans, M.; Petersen, I.; Bhana, A.; Kigozi, F.; Prince, M.; et al. PRIME: A Programme to Reduce the Treatment Gap for Mental Disorders in Five Low- and Middle-Income Countries. PLoS Med. 2012, 9, e1001359. [Google Scholar] [CrossRef]
- Verbitsky, A.; Dopfel, D.; Zhang, N. Rodent Models of Post-Traumatic Stress Disorder: Behavioral Assessment. Transl. Psychiatry 2020, 10, 132. [Google Scholar] [CrossRef]
- Buynitsky, T.; Mostofsky, D.I. Restraint Stress in Biobehavioral Research: Recent Developments. Neurosci. Biobehav. Rev. 2009, 33, 1089–1098. [Google Scholar] [CrossRef]
- Lisieski, M.J.; Eagle, A.L.; Conti, A.C.; Liberzon, I.; Perrine, S.A. Single-Prolonged Stress: A Review of Two Decades of Progress in a Rodent Model of Post-Traumatic Stress Disorder. Front. Psychiatry 2018, 9, 196. [Google Scholar] [CrossRef]
- Golden, S.A.; Covington, H.E.; Berton, O.; Russo, S.J. A Standardized Protocol for Repeated Social Defeat Stress in Mice. Nat. Protoc. 2011, 6, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, L.K.; Nakashima, B.R.; Hong, H.; Watanabe, K. The Smell of Danger: A Behavioral and Neural Analysis of Predator Odor-Induced Fear. Neurosci. Biobehav. Rev. 2005, 29, 1157–1167. [Google Scholar] [CrossRef] [PubMed]
- Zoladz, P.R.; Park, C.R.; Fleshner, M.; Diamond, D.M. Psychosocial Predator-Based Animal Model of PTSD Produces Physiological and Behavioral Sequelae and a Traumatic Memory Four Months Following Stress Onset. Physiol. Behav. 2015, 147, 183–192. [Google Scholar] [CrossRef]
- Vignisse, J.; Sambon, M.; Gorlova, A.; Pavlov, D.; Caron, N.; Malgrange, B.; Shevtsova, E.; Svistunov, A.; Anthony, D.C.; Markova, N.; et al. Thiamine and Benfotiamine Prevent Stress-Induced Suppression of Hippocampal Neurogenesis in Mice Exposed to Predation without Affecting Brain Thiamine Diphosphate Levels. Mol. Cell. Neurosci. 2017, 82, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Gorlova, A.; Ortega, G.; Waider, J.; Bazhenova, N.; Veniaminova, E.; Proshin, A.; Kalueff, A.V.; Anthony, D.C.; Lesch, K.-P.; Strekalova, T. Stress-Induced Aggression in Heterozygous TPH2 Mutant Mice Is Associated with Alterations in Serotonin Turnover and Expression of 5-HT6 and AMPA Subunit 2A Receptors. J. Affect. Disord. 2020, 272, 440–451. [Google Scholar] [CrossRef]
- Strekalova, T.; Svirin, E.; Waider, J.; Gorlova, A.; Cespuglio, R.; Kalueff, A.; Pomytkin, I.; Schmitt-Boehrer, A.G.; Lesch, K.-P.; Anthony, D.C. Altered Behaviour, Dopamine and Norepinephrine Regulation in Stressed Mice Heterozygous in TPH2 Gene. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 108, 110155. [Google Scholar] [CrossRef]
- Svirin, E.; Veniaminova, E.; Costa-Nunes, J.P.; Gorlova, A.; Umriukhin, A.; Kalueff, A.V.; Proshin, A.; Anthony, D.C.; Nedorubov, A.; Tse, A.C.K.; et al. Predation Stress Causes Excessive Aggression in Female Mice with Partial Genetic Inactivation of Tryptophan Hydroxylase-2: Evidence for Altered Myelination-Related Processes. Cells 2022, 11, 1036. [Google Scholar] [CrossRef]
- Strekalova, T.; Moskvin, O.; Jain, A.Y.; Gorbunov, N.; Gorlova, A.; Sadovnik, D.; Umriukhin, A.; Cespuglio, R.; Yu, W.S.; Tse, A.C.K.; et al. Molecular Signature of Excessive Female Aggression: Study of Stressed Mice with Genetic Inactivation of Neuronal Serotonin Synthesis. J. Neural Transm. 2023, 130, 1113–1132. [Google Scholar] [CrossRef]
- Weidner, M.T.; Lardenoije, R.; Eijssen, L.; Mogavero, F.; De Groodt, L.P.M.T.; Popp, S.; Palme, R.; Förstner, K.U.; Strekalova, T.; Steinbusch, H.W.M.; et al. Identification of Cholecystokinin by Genome-Wide Profiling as Potential Mediator of Serotonin-Dependent Behavioral Effects of Maternal Separation in the Amygdala. Front. Neurosci. 2019, 13, 460. [Google Scholar] [CrossRef]
- Dmytriv, T.R.; Tsiumpala, S.A.; Semchyshyn, H.M.; Storey, K.B.; Lushchak, V.I. Mitochondrial Dysfunction as a Possible Trigger of Neuroinflammation at Post-Traumatic Stress Disorder (PTSD). Front. Physiol. 2023, 14, 1222826. [Google Scholar] [CrossRef]
- Peng, Z.; Wang, H.; Zhang, R.; Chen, Y.; Xue, F.; Nie, H.; Chen, Y.; Wu, D.; Wang, Y.; Wang, H.; et al. Gastrodin Ameliorates Anxiety-like Behaviors and Inhibits IL-1beta Level and P38 MAPK Phosphorylation of Hippocampus in the Rat Model of Posttraumatic Stress Disorder. Physiol. Res. 2013, 62, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Sur, B.; Yeom, M.; Shim, I.; Lee, H.; Hahm, D.-H. Effects of Systemic Administration of Ibuprofen on Stress Response in a Rat Model of Post-Traumatic Stress Disorder. Korean J. Physiol. Pharmacol. 2016, 20, 357–366. [Google Scholar] [CrossRef]
- Alzoubi, K.H.; Al Subeh, Z.Y.; Khabour, O.F. Molecular Targets for the Interactive Effect of Etazolate during Post-Traumatic Stress Disorder: Role of Oxidative Stress, BDNF and Histones. Behav. Brain Res. 2019, 369, 111930. [Google Scholar] [CrossRef]
- Peruzzolo, T.L.; Pinto, J.V.; Roza, T.H.; Shintani, A.O.; Anzolin, A.P.; Gnielka, V.; Kohmann, A.M.; Marin, A.S.; Lorenzon, V.R.; Brunoni, A.R.; et al. Inflammatory and Oxidative Stress Markers in Post-Traumatic Stress Disorder: A Systematic Review and Meta-Analysis. Mol. Psychiatry 2022, 27, 3150–3163. [Google Scholar] [CrossRef] [PubMed]
- Gill, J.; Vythilingam, M.; Page, G.G. Low Cortisol, High DHEA, and High Levels of Stimulated TNF-Alpha, and IL-6 in Women with PTSD. J. Trauma. Stress 2008, 21, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Vidović, A.; Gotovac, K.; Vilibić, M.; Sabioncello, A.; Jovanović, T.; Rabatić, S.; Folnegović-Šmalć, V.; Dekaris, D. Repeated Assessments of Endocrine- and Immune-Related Changes in Posttraumatic Stress Disorder. Neuroimmunomodulation 2011, 18, 199–211. [Google Scholar] [CrossRef]
- Lindqvist, D.; Wolkowitz, O.M.; Mellon, S.; Yehuda, R.; Flory, J.D.; Henn-Haase, C.; Bierer, L.M.; Abu-Amara, D.; Coy, M.; Neylan, T.C.; et al. Proinflammatory Milieu in Combat-Related PTSD Is Independent of Depression and Early Life Stress. Brain Behav. Immun. 2014, 42, 81–88. [Google Scholar] [CrossRef]
- Miller, K.; Driscoll, D.; Smith, L.M.; Ramaswamy, S. The Role of Inflammation in Late-Life Post-Traumatic Stress Disorder. Mil. Med. 2017, 182, e1815–e1818. [Google Scholar] [CrossRef]
- de Oliveira, J.F.; Wiener, C.D.; Jansen, K.; Portela, L.V.; Lara, D.R.; Souza, L.D.d.M.; da Silva, R.A.; Moreira, F.P.; Oses, J.P. Serum Levels of Interleukins IL-6 and IL-10 in Individuals with Posttraumatic Stress Disorder in a Population-Based Sample. Psychiatry Res. 2018, 260, 111–115. [Google Scholar] [CrossRef]
- Tezcan, E.; Atmaca, M.; Kuloglu, M.; Ustundag, B. Free Radicals in Patients with Post-Traumatic Stress Disorder. Eur. Arch. Psychiatry Clin. Neurosci. 2003, 253, 89–91. [Google Scholar] [CrossRef]
- Atli, A.; Bulut, M.; Bez, Y.; Kaplan, İ.; Özdemir, P.G.; Uysal, C.; Selçuk, H.; Sir, A. Altered Lipid Peroxidation Markers Are Related to Post-Traumatic Stress Disorder (PTSD) and Not Trauma Itself in Earthquake Survivors. Eur. Arch. Psychiatry Clin. Neurosci. 2016, 266, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Ogłodek, E.A. Changes in the Concentrations of Inflammatory and Oxidative Status Biomediators (MIP-1 α, PMN Elastase, MDA, and IL-12) in Depressed Patients with and without Posttraumatic Stress Disorder. Pharmacol. Rep. 2018, 70, 110–118. [Google Scholar] [CrossRef]
- Seo, J.-H.; Park, H.-S.; Park, S.-S.; Kim, C.-J.; Kim, D.-H.; Kim, T.-W. Physical Exercise Ameliorates Psychiatric Disorders and Cognitive Dysfunctions by Hippocampal Mitochondrial Function and Neuroplasticity in Post-Traumatic Stress Disorder. Exp. Neurol. 2019, 322, 113043. [Google Scholar] [CrossRef]
- Reed, E.C.; Case, A.J. Defining the Nuanced Nature of Redox Biology in Post-Traumatic Stress Disorder. Front. Physiol. 2023, 14, 1130861. [Google Scholar] [CrossRef]
- Miller, M.W.; Lin, A.P.; Wolf, E.J.; Miller, D.R. Oxidative Stress, Inflammation, and Neuroprogression in Chronic PTSD. Harv. Rev. Psychiatry 2018, 26, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Schnurr, P.P.; Hamblen, J.L.; Wolf, J.; Coller, R.; Collie, C.; Fuller, M.A.; Holtzheimer, P.E.; Kelly, U.; Lang, A.J.; McGraw, K.; et al. The Management of Posttraumatic Stress Disorder and Acute Stress Disorder: Synopsis of the 2023 U.S. Department of Veterans Affairs and U.S. Department of Defense Clinical Practice Guideline. Ann. Intern. Med. 2024, 177, 363–374. [Google Scholar] [CrossRef]
- Rajkumar, R.P. Potassium Channels in Animal Models of Post-Traumatic Stress Disorder: Mechanistic and Therapeutic Implications. Front. Cell Neurosci. 2024, 18, 1441514. [Google Scholar] [CrossRef]
- Richter-Levin, G.; Stork, O.; Schmidt, M.V. Animal Models of PTSD: A Challenge to Be Met. Mol. Psychiatry 2019, 24, 1135–1156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, Y.; Du, Y.; Sun, H.; Zhang, W.; Wang, A.; Li, Q.; Li, C.; Wang, Y.; Du, Z.; et al. Effect of Ketamine on Mood Dysfunction and Spatial Cognition Deficits in PTSD Mouse Models via HCN1–BDNF Signaling. J. Affect. Disord. 2021, 286, 248–258. [Google Scholar] [CrossRef]
- Sambon, M.; Gorlova, A.; Demelenne, A.; Alhama-Riba, J.; Coumans, B.; Lakaye, B.; Wins, P.; Fillet, M.; Anthony, D.C.; Strekalova, T.; et al. Dibenzoylthiamine Has Powerful Antioxidant and Anti-Inflammatory Properties in Cultured Cells and in Mouse Models of Stress and Neurodegeneration. Biomedicines 2020, 8, 361. [Google Scholar] [CrossRef]
- Bozic, I.; Lavrnja, I. Thiamine and Benfotiamine: Focus on Their Therapeutic Potential. Heliyon 2023, 9, e21839. [Google Scholar] [CrossRef]
- Markova, N.; Bazhenova, N.; Anthony, D.C.; Vignisse, J.; Svistunov, A.; Lesch, K.-P.; Bettendorff, L.; Strekalova, T. Thiamine and Benfotiamine Improve Cognition and Ameliorate GSK-3β-Associated Stress-Induced Behaviours in Mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 75, 148–156. [Google Scholar] [CrossRef]
- Gorlova, A.; Pavlov, D.; Anthony, D.C.; Ponomarev, E.D.; Sambon, M.; Proshin, A.; Shafarevich, I.; Babaevskaya, D.; Lesch, K.-P.; Bettendorff, L.; et al. Thiamine and Benfotiamine Counteract Ultrasound-Induced Aggression, Normalize AMPA Receptor Expression and Plasticity Markers, and Reduce Oxidative Stress in Mice. Neuropharmacology 2019, 156, 107543. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, D.; Gorlova, A.; Bettendorff, L.; Kalueff, A.A.; Umriukhin, A.; Proshin, A.; Lysko, A.; Landgraf, R.; Anthony, D.C.; Strekalova, T. Enhanced Conditioning of Adverse Memories in the Mouse Modified Swim Test Is Associated with Neuroinflammatory Changes—Effects That Are Susceptible to Antidepressants. Neurobiol. Learn. Mem. 2020, 172, 107227. [Google Scholar] [CrossRef] [PubMed]
- Sambon, M.; Wins, P.; Bettendorff, L. Neuroprotective Effects of Thiamine and Precursors with Higher Bioavailability: Focus on Benfotiamine and Dibenzoylthiamine. Int. J. Mol. Sci. 2021, 22, 5418. [Google Scholar] [CrossRef]
- de Munter, J.; Pavlov, D.; Gorlova, A.; Sicker, M.; Proshin, A.; Kalueff, A.V.; Svistunov, A.; Kiselev, D.; Nedorubov, A.; Morozov, S.; et al. Increased Oxidative Stress in the Prefrontal Cortex as a Shared Feature of Depressive- and PTSD-Like Syndromes: Effects of a Standardized Herbal Antioxidant. Front. Nutr. 2021, 8, 661455. [Google Scholar] [CrossRef]
- Probert, F.; Gorlova, A.; Deikin, A.; Bettendorff, L.; Veniaminova, E.; Nedorubov, A.; Chaprov, K.D.; Ivanova, T.A.; Anthony, D.C.; Strekalova, T. In FUS [1−359]-tg Mice O,S-Dibenzoyl Thiamine Reduces Muscle Atrophy, Decreases Glycogen Synthase Kinase 3 Beta, and Normalizes the Metabolome. Biomed. Pharmacother. 2022, 156, 113986. [Google Scholar] [CrossRef] [PubMed]
- Belambri, S.A.; Rolas, L.; Raad, H.; Hurtado-Nedelec, M.; Dang, P.M.-C.; El-Benna, J. NADPH Oxidase Activation in Neutrophils: Role of the Phosphorylation of Its Subunits. Eur. J. Clin. Invest. 2018, 48 (Suppl. S2), e12951. [Google Scholar] [CrossRef]
- Pan, X.; Kaminga, A.C.; Wen, S.W.; Liu, A. Catecholamines in Post-Traumatic Stress Disorder: A Systematic Review and Meta-Analysis. Front. Mol. Neurosci. 2018, 11, 450. [Google Scholar] [CrossRef]
- Gibson, G.E.; Luchsinger, J.A.; Cirio, R.; Chen, H.; Franchino-Elder, J.; Hirsch, J.A.; Bettendorff, L.; Chen, Z.; Flowers, S.A.; Gerber, L.M.; et al. Benfotiamine and Cognitive Decline in Alzheimer’s Disease: Results of a Randomized Placebo-Controlled Phase IIa Clinical Trial. J. Alzheimer’s Dis. 2020, 78, 989–1010. [Google Scholar] [CrossRef]
- Bönhof, G.J.; Sipola, G.; Strom, A.; Herder, C.; Strassburger, K.; Knebel, B.; Reule, C.; Wollmann, J.-C.; Icks, A.; Al-Hasani, H.; et al. BOND Study: A Randomised Double-Blind, Placebo-Controlled Trial over 12 Months to Assess the Effects of Benfotiamine on Morphometric, Neurophysiological and Clinical Measures in Patients with Type 2 Diabetes with Symptomatic Polyneuropathy. BMJ 2022, 12, e057142. [Google Scholar] [CrossRef] [PubMed]
- Serhiyenko, V.A.; Chemerys, O.M.; Pankiv, V.I.; Serhiyenko, A.A. Post-Traumatic Stress Disorder, Metabolic Syndrome, Diabetic Distress, and Vitamin B1/Benfotiamine. Int. Neurol. J. 2025, 21, 96–107. [Google Scholar] [CrossRef]
- Tesarz, J.; Baumeister, D.; Andersen, T.E.; Vaegter, H.B. Pain Perception and Processing in Individuals with Posttraumatic Stress Disorder: A Systematic Review with Meta-Analysis. PR9 2020, 5, e849. [Google Scholar] [CrossRef]
- Akagawa, M. Protein Carbonylation: Molecular Mechanisms, Biological Implications, and Analytical Approaches. Free Radic. Res. 2021, 55, 307–320. [Google Scholar] [CrossRef]
- Ghanizadeh, A.; Akhondzadeh, S.; Hormozi, M.; Makarem, A.; Abotorabi-Zarchi, M.; Firoozabadi, A. Glutathione-Related Factors and Oxidative Stress in Autism, A Review. CMC 2012, 19, 4000–4005. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-H.; Langenbach, R.; Bosetti, F. Genetic Deletion or Pharmacological Inhibition of Cyclooxygenase-1 Attenuate Lipopolysaccharide-Induced Inflammatory Response and Brain Injury. FASEB J. 2008, 22, 1491–1501. [Google Scholar] [CrossRef]
- Choi, S.-H.; Bosetti, F. Cyclooxygenase-1 Null Mice Show Reduced Neuroinflammation in Response to β-Amyloid. Aging 2009, 1, 234–244. [Google Scholar] [CrossRef]
- Roberts, B.L.; Karatsoreos, I.N. Brain-Body Responses to Chronic Stress: A Brief Review. Fac. Rev. 2021, 10, 83. [Google Scholar] [CrossRef]
- McEwen, B.S. Physiology and Neurobiology of Stress and Adaptation: Central Role of the Brain. Physiol. Rev. 2007, 87, 873–904. [Google Scholar] [CrossRef]
- Koolhaas, J.M.; Bartolomucci, A.; Buwalda, B.; De Boer, S.F.; Flügge, G.; Korte, S.M.; Meerlo, P.; Murison, R.; Olivier, B.; Palanza, P.; et al. Stress Revisited: A Critical Evaluation of the Stress Concept. Neurosci. Biobehav. Rev. 2011, 35, 1291–1301. [Google Scholar] [CrossRef]
- Zoladz, P.R.; Diamond, D.M. Predator-Based Psychosocial Stress Animal Model of PTSD: Preclinical Assessment of Traumatic Stress at Cognitive, Hormonal, Pharmacological, Cardiovascular and Epigenetic Levels of Analysis. Exp. Neurol. 2016, 284, 211–219. [Google Scholar] [CrossRef]
- Miles, S.R.; Hale, W.J.; Mintz, J.; Wachen, J.S.; Litz, B.T.; Dondanville, K.A.; Yarvis, J.S.; Hembree, E.A.; Young-McCaughan, S.; Peterson, A.L.; et al. Hyperarousal Symptoms Linger after Successful PTSD Treatment in Active Duty Military. Psychol. Trauma Theory Res. Pract. Policy 2023, 15, 1398–1405. [Google Scholar] [CrossRef]
- Chiu, H.T.S.; Low, D.C.W.; Chan, A.H.T.; Meiser-Stedman, R. Relationship between Anxiety Sensitivity and Post-Traumatic Stress Symptoms in Trauma-Exposed Adults: A Meta-Analysis. J. Anxiety Disord. 2024, 103, 102857. [Google Scholar] [CrossRef]
- Teleanu, D.M.; Niculescu, A.-G.; Lungu, I.I.; Radu, C.I.; Vladâcenco, O.; Roza, E.; Costăchescu, B.; Grumezescu, A.M.; Teleanu, R.I. An Overview of Oxidative Stress, Neuroinflammation, and Neurodegenerative Diseases. IJMS 2022, 23, 5938. [Google Scholar] [CrossRef]
- Eraly, S.A.; Nievergelt, C.M.; Maihofer, A.X.; Barkauskas, D.A.; Biswas, N.; Agorastos, A.; O’Connor, D.T.; Baker, D.G. Assessment of Plasma C-Reactive Protein as a Biomarker of Posttraumatic Stress Disorder Risk. JAMA Psychiatry 2014, 71, 423. [Google Scholar] [CrossRef]
- Passos, I.C.; Vasconcelos-Moreno, M.P.; Costa, L.G.; Kunz, M.; Brietzke, E.; Quevedo, J.; Salum, G.; Magalhães, P.V.; Kapczinski, F.; Kauer-Sant’Anna, M. Inflammatory Markers in Post-Traumatic Stress Disorder: A Systematic Review, Meta-Analysis, and Meta-Regression. Lancet Psychiatry 2015, 2, 1002–1012. [Google Scholar] [CrossRef]
- Gamble-George, J.C.; Baldi, R.; Halladay, L.; Kocharian, A.; Hartley, N.; Silva, C.G.; Roberts, H.; Haymer, A.; Marnett, L.J.; Holmes, A.; et al. Cyclooxygenase-2 Inhibition Reduces Stress-Induced Affective Pathology. eLife 2016, 5, e14137. [Google Scholar] [CrossRef]
- Strekalova, T.; Liu, Y.; Kiselev, D.; Khairuddin, S.; Chiu, J.L.Y.; Lam, J.; Chan, Y.-S.; Pavlov, D.; Proshin, A.; Lesch, K.-P.; et al. Chronic Mild Stress Paradigm as a Rat Model of Depression: Facts, Artifacts, and Future Perspectives. Psychopharmacology 2022, 239, 663–693. [Google Scholar] [CrossRef]
- Wang, M.; Duan, F.; Wu, J.; Min, Q.; Huang, Q.; Luo, M.; He, Z. Effect of Cyclooxygenase-2 Inhibition on the Development of Post-traumatic Stress Disorder in Rats. Mol. Med. Rep. 2018, 17, 4925–4932. [Google Scholar] [CrossRef]
- Couch, Y.; Anthony, D.C.; Dolgov, O.; Revischin, A.; Festoff, B.; Santos, A.I.; Steinbusch, H.W.; Strekalova, T. Microglial activation, increased TNF and SERT expression in the prefrontal cortex define stress-altered behaviour in mice susceptible to anhedonia. Brain Behav. Immun. 2013, 29, 136–146. [Google Scholar] [CrossRef]
- Santos, P.L.; Brito, R.G.; Matos, J.P.S.C.F.; Quintans, J.S.S.; Quintans-Júnior, L.J. Fos Protein as a Marker of Neuronal Activity: A Useful Tool in the Study of the Mechanism of Action of Natural Products with Analgesic Activity. Mol. Neurobiol. 2018, 55, 4560–4579. [Google Scholar] [CrossRef]
- Numa, C.; Nagai, H.; Taniguchi, M.; Nagai, M.; Shinohara, R.; Furuyashiki, T. Social Defeat Stress-Specific Increase in c-Fos Expression in the Extended Amygdala in Mice: Involvement of Dopamine D1 Receptor in the Medial Prefrontal Cortex. Sci. Rep. 2019, 9, 16670. [Google Scholar] [CrossRef]
- Azevedo, H.; Ferreira, M.; Mascarello, A.; Osten, P.; Guimarães, C.R.W. Brain-Wide Mapping of c-Fos Expression in the Single Prolonged Stress Model and the Effects of Pretreatment with ACH-000029 or Prazosin. Neurobiol. Stress 2020, 13, 100226. [Google Scholar] [CrossRef]
- Knox, D.; Stanfield, B.R.; Staib, J.M.; David, N.P.; Keller, S.M.; DePietro, T. Neural Circuits via Which Single Prolonged Stress Exposure Leads to Fear Extinction Retention Deficits. Learn. Mem. 2016, 23, 689–698. [Google Scholar] [CrossRef]
- Valle, R.D.; Mohammadmirzaei, N.; Knox, D. Single Prolonged Stress Alters Neural Activation in the Periacqueductal Gray and Midline Thalamic Nuclei during Emotional Learning and Memory. Learn. Mem. 2019, 26, 403–411. [Google Scholar] [CrossRef]
- Jones, M.E.; Lebonville, C.L.; Barrus, D.; Lysle, D.T. The Role of Brain Interleukin-1 in Stress-Enhanced Fear Learning. Neuropsychopharmacology 2015, 40, 1289–1296. [Google Scholar] [CrossRef]
- Levkovitz, Y.; Fenchel, D.; Kaplan, Z.; Zohar, J.; Cohen, H. Early Post-Stressor Intervention with Minocycline, a Second-Generation Tetracycline, Attenuates Post-Traumatic Stress Response in an Animal Model of PTSD. Eur. Neuropsychopharmacol. 2015, 25, 124–132. [Google Scholar] [CrossRef]
- Jope, R.S.; Yuskaitis, C.J.; Beurel, E. Glycogen Synthase Kinase-3 (GSK3): Inflammation, Diseases, and Therapeutics. Neurochem. Res. 2007, 32, 577–595. [Google Scholar] [CrossRef]
- Dib, P.; Zhang, Y.; Ihnat, M.A.; Gallucci, R.M.; Standifer, K.M. TNF-Alpha as an Initiator of Allodynia and Anxiety-Like Behaviors in a Preclinical Model of PTSD and Comorbid Pain. Front. Psychiatry 2021, 12, 721999. [Google Scholar] [CrossRef]
- Bitsch, R.; Wolf, M.; Möller, J.; Heuzeroth, L.; Grüneklee, D. Bioavailability assessment of the lipophilic benfotiamine as compared to a water-soluble thiamin derivative. Ann. Nutr. Metab. 1991, 35, 292–296. [Google Scholar] [CrossRef]
- Tapias, V.; Jainuddin, S.; Ahuja, M.; Stack, C.; Elipenahli, C.; Vignisse, J.; Gerges, M.; Starkova, N.; Xu, H.; Starkov, A.A.; et al. Benfotiamine Treatment Activates the Nrf2/ARE Pathway and Is Neuroprotective in a Transgenic Mouse Model of Tauopathy. Hum. Mol. Genet. 2018, 27, 2874–2892. [Google Scholar] [CrossRef]
- Marchetti, V.; Menghini, R.; Rizza, S.; Vivanti, A.; Feccia, T.; Lauro, D.; Fukamizu, A.; Lauro, R.; Federici, M. Benfotiamine Counteracts Glucose Toxicity Effects on Endothelial Progenitor Cell Differentiation via Akt/FoxO Signaling. Diabetes 2006, 55, 2231–2237. [Google Scholar] [CrossRef]
- Raut, A.; Ratka, A. Oxidative Damage and Sensitivity to Nociceptive Stimulus and Opioids in Aging Rats. Neurobiol. Aging 2009, 30, 910–919. [Google Scholar] [CrossRef]
- Ji, R.-R.; Nackley, A.; Huh, Y.; Terrando, N.; Maixner, W. Neuroinflammation and Central Sensitization in Chronic and Widespread Pain. Anesthesiology 2018, 129, 343–366. [Google Scholar] [CrossRef]
- Vaegter, H.B.; Andersen, T.E.; Harvold, M.; Andersen, P.G.; Graven-Nielsen, T. Increased Pain Sensitivity in Accident-Related Chronic Pain Patients with Comorbid Posttraumatic Stress. Clin. J. Pain. 2018, 34, 313–321. [Google Scholar] [CrossRef]
- Lanius, R.A.; Terpou, B.A.; McKinnon, M.C. The Sense of Self in the Aftermath of Trauma: Lessons from the Default Mode Network in Posttraumatic Stress Disorder. Eur. J. Psychotraumatol. 2020, 11, 1807703. [Google Scholar] [CrossRef]
- Vieira, J.S.; De Souza, G.R.; Kalil-Cutti, B.; Giusti-Paiva, A.; Vilela, F.C. Post-Traumatic Stress Disorder Increases Pain Sensitivity by Reducing Descending Noradrenergic and Serotoninergic Modulation. Behav. Brain Res. 2021, 411, 113367. [Google Scholar] [CrossRef]
- Siever, L.J. Neurobiology of Aggression and Violence. AJP 2008, 165, 429–442. [Google Scholar] [CrossRef]
- Clinton, S.M.; Watson, S.J.; Akil, H. High Novelty-Seeking Rats Are Resilient to Negative Physiological Effects of the Early Life Stress. Stress 2014, 17, 97–107. [Google Scholar] [CrossRef]
- Dief, A.E.; Samy, D.M.; Dowedar, F.I. Impact of Exercise and Vitamin B1 Intake on Hippocampal Brain-Derived Neurotrophic Factor and Spatial Memory Performance in a Rat Model of Stress. J. Nutr. Sci. Vitaminol. 2015, 61, 1–7. [Google Scholar] [CrossRef]
- Saiki, M.; Matsui, T.; Soya, M.; Kashibe, T.; Shima, T.; Shimizu, T.; Naruto, T.; Kitayoshi, T.; Akimoto, K.; Ninomiya, S.; et al. Thiamine Tetrahydrofurfuryl Disulfide Promotes Voluntary Activity through Dopaminergic Activation in the Medial Prefrontal Cortex. Sci. Rep. 2018, 8, 10469. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xu, H.; Wen, W.; Wu, L.; Xu, M.; Luo, J. Thiamine Deficiency Causes Long-Lasting Neurobehavioral Deficits in Mice. Brain Sci. 2020, 10, 565. [Google Scholar] [CrossRef] [PubMed]
- Depeint, F.; Bruce, W.R.; Shangari, N.; Mehta, R.; O’Brien, P.J. Mitochondrial function and toxicity: Role of the B vitamin family on mitochondrial energy metabolism. Chem. Biol. Interact. 2006, 163, 94–112. [Google Scholar] [CrossRef]
- Gibson, G.E.; Blass, J.P. Thiamine-dependent processes and treatment strategies in neurodegeneration. Antioxid. Redox Signal. 2007, 10, 1605–1619. [Google Scholar] [CrossRef]
- Mrowicka, M.; Mrowicki, J.; Dragan, G.; Majsterek, I. The importance of thiamine (vitamin B1) in humans. Biosci. Rep. 2023, 43, BSR20230374. [Google Scholar] [CrossRef]
- Cornish, S.; Mehl-Madrona, L. The Role of Vitamins and Minerals in Psychiatry. Integr. Med. Insights 2008, 3, 33–42. [Google Scholar] [CrossRef]
- Moti, M.; Amini, L.; Haghani, H.; Nateghi, M.R. The Effects of Thiamine Supplementation on General Health and Infertility Treatment Outcomes in Women with Polycystic Ovary Syndrome: A Triple-Blinded Randomized Placebo-Controlled Clinical Trial. Int. J. Fertil. Steril. 2024, 18, 128–134. [Google Scholar] [CrossRef]
- Wang, R.; Zeng, Y.; Xu, J.; He, M. Thiamine Use Is Associated with Better Outcomes for Traumatic Brain Injury Patients. Front. Nutr. 2024, 11, 1362817. [Google Scholar] [CrossRef]
- Jia, W.; Wang, H.; Li, C.; Shi, J.; Yong, F.; Jia, H. Association between Dietary Vitamin B1 Intake and Cognitive Function among Older Adults: A Cross-Sectional Study. J. Transl. Med. 2024, 22, 165. [Google Scholar] [CrossRef]
- Strekalova, T.; Steinbusch, H.W.M. Measuring Behavior in Mice with Chronic Stress Depression Paradigm. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2010, 34, 348–361. [Google Scholar] [CrossRef]
- Strekalova, T.; Radford-Smith, D.; Dunstan, I.K.; Gorlova, A.; Svirin, E.; Sheveleva, E.; Burova, A.; Morozov, S.; Lyundup, A.; Berger, G.; et al. Omega-3 Alleviates Behavioral and Molecular Changes in a Mouse Model of Stress-Induced Juvenile Depression. Neurobiol. Stress. 2024, 31, 100646. [Google Scholar] [CrossRef]
- Strekalova, T.; Spanagel, R.; Dolgov, O.; Bartsch, D. Stress-Induced Hyperlocomotion as a Confounding Factor in Anxiety and Depression Models in Mice. Behav. Pharmacol. 2005, 16, 171–180. [Google Scholar] [CrossRef]
- Malatynska, E.; Steinbusch, H.W.M.; Redkozubova, O.; Bolkunov, A.; Kubatiev, A.; Yeritsyan, N.B.; Vignisse, J.; Bachurin, S.; Strekalova, T. Anhedonic-like Traits and Lack of Affective Deficits in 18-Month-Old C57BL/6 Mice: Implications for Modeling Elderly Depression. Exp. Gerontol. 2012, 47, 552–564. [Google Scholar] [CrossRef]
- Schroeter, C.A.; Gorlova, A.; Sicker, M.; Umriukhin, A.; Burova, A.; Shulgin, B.; Morozov, S.; Costa-Nunes, J.P.; Strekalova, T. Unveiling the Mechanisms of a Remission in Major Depressive Disorder (MDD)-like Syndrome: The Role of Hippocampal Palmitoyltransferase Expression and Stress Susceptibility. Biomolecules 2025, 15, 67. [Google Scholar] [CrossRef]
- Anthony, D.C.; Probert, F.; Gorlova, A.; Hebert, J.; Radford-Smith, D.; Nefedova, Z.; Umriukhin, A.; Nedorubov, A.; Cespuglio, R.; Shulgin, B.; et al. Impact of Serotonin Transporter Absence on Brain Insulin Receptor Expression, Plasma Metabolome Changes, and ADHD-like Behavior in Mice fed a Western Diet. Biomolecules 2024, 14, 884. [Google Scholar] [CrossRef]
- Morozova, A.; Zubkov, E.; Strekalova, T.; Kekelidze, Z.; Storozeva, Z.; Schroeter, C.A.; Bazhenova, N.; Lesch, K.P.; Cline, B.H.; Chekhonin, V. Ultrasound of alternating frequencies and variable emotional impact evokes depressive syndrome in mice and rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 68, 52–63. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strekalova, T.; Gorlova, A.; Costa-Nunes, J.; Litavrin, A.; de Munter, J.P.M.; Lyundup, A.; Umriukhin, A.; Proshin, A.; Kalueff, A.V.; Grünblatt, E.; et al. Thiamine Compounds Alleviate Oxidative Stress, Over-Expression of Pro-Inflammatory Markers and Behavioral Abnormalities in a Mouse Predation Model of PTSD. Int. J. Mol. Sci. 2025, 26, 6627. https://doi.org/10.3390/ijms26146627
Strekalova T, Gorlova A, Costa-Nunes J, Litavrin A, de Munter JPM, Lyundup A, Umriukhin A, Proshin A, Kalueff AV, Grünblatt E, et al. Thiamine Compounds Alleviate Oxidative Stress, Over-Expression of Pro-Inflammatory Markers and Behavioral Abnormalities in a Mouse Predation Model of PTSD. International Journal of Molecular Sciences. 2025; 26(14):6627. https://doi.org/10.3390/ijms26146627
Chicago/Turabian StyleStrekalova, Tatyana, Anna Gorlova, Joao Costa-Nunes, Aleksandr Litavrin, Johannes P. M. de Munter, Alexei Lyundup, Aleksei Umriukhin, Andrey Proshin, Allan V. Kalueff, Edna Grünblatt, and et al. 2025. "Thiamine Compounds Alleviate Oxidative Stress, Over-Expression of Pro-Inflammatory Markers and Behavioral Abnormalities in a Mouse Predation Model of PTSD" International Journal of Molecular Sciences 26, no. 14: 6627. https://doi.org/10.3390/ijms26146627
APA StyleStrekalova, T., Gorlova, A., Costa-Nunes, J., Litavrin, A., de Munter, J. P. M., Lyundup, A., Umriukhin, A., Proshin, A., Kalueff, A. V., Grünblatt, E., & Walitza, S. (2025). Thiamine Compounds Alleviate Oxidative Stress, Over-Expression of Pro-Inflammatory Markers and Behavioral Abnormalities in a Mouse Predation Model of PTSD. International Journal of Molecular Sciences, 26(14), 6627. https://doi.org/10.3390/ijms26146627




