Hyaluronan-Containing Injectable Magnesium–Calcium Phosphate Cements Demonstrated Improved Performance, Cytocompatibility, and Ability to Support Osteogenic Differentiation In Vitro
Abstract
1. Introduction
2. Results
2.1. Characterisation of Cement Powders
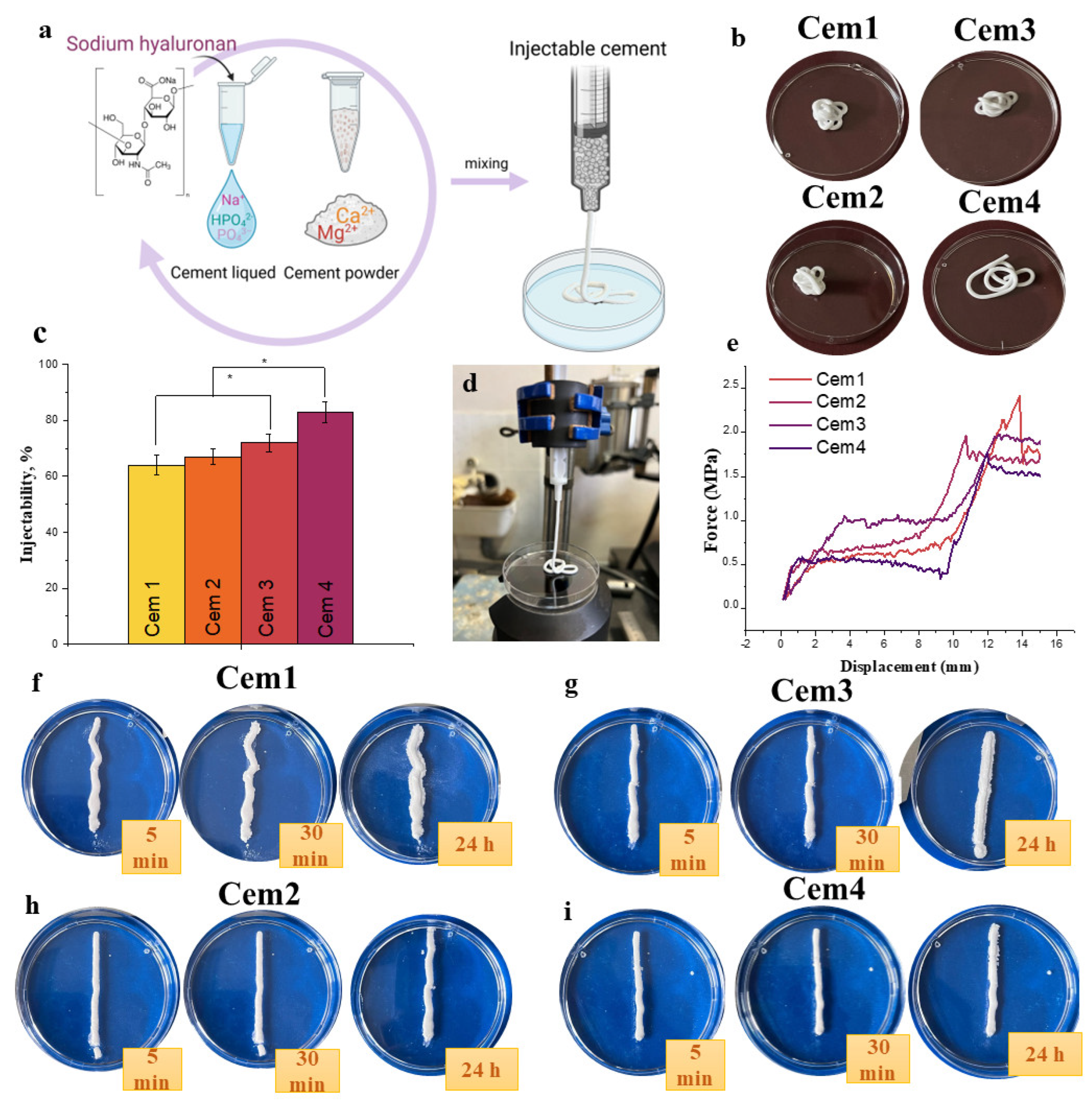
2.2. Characterisation of Cement Liquids
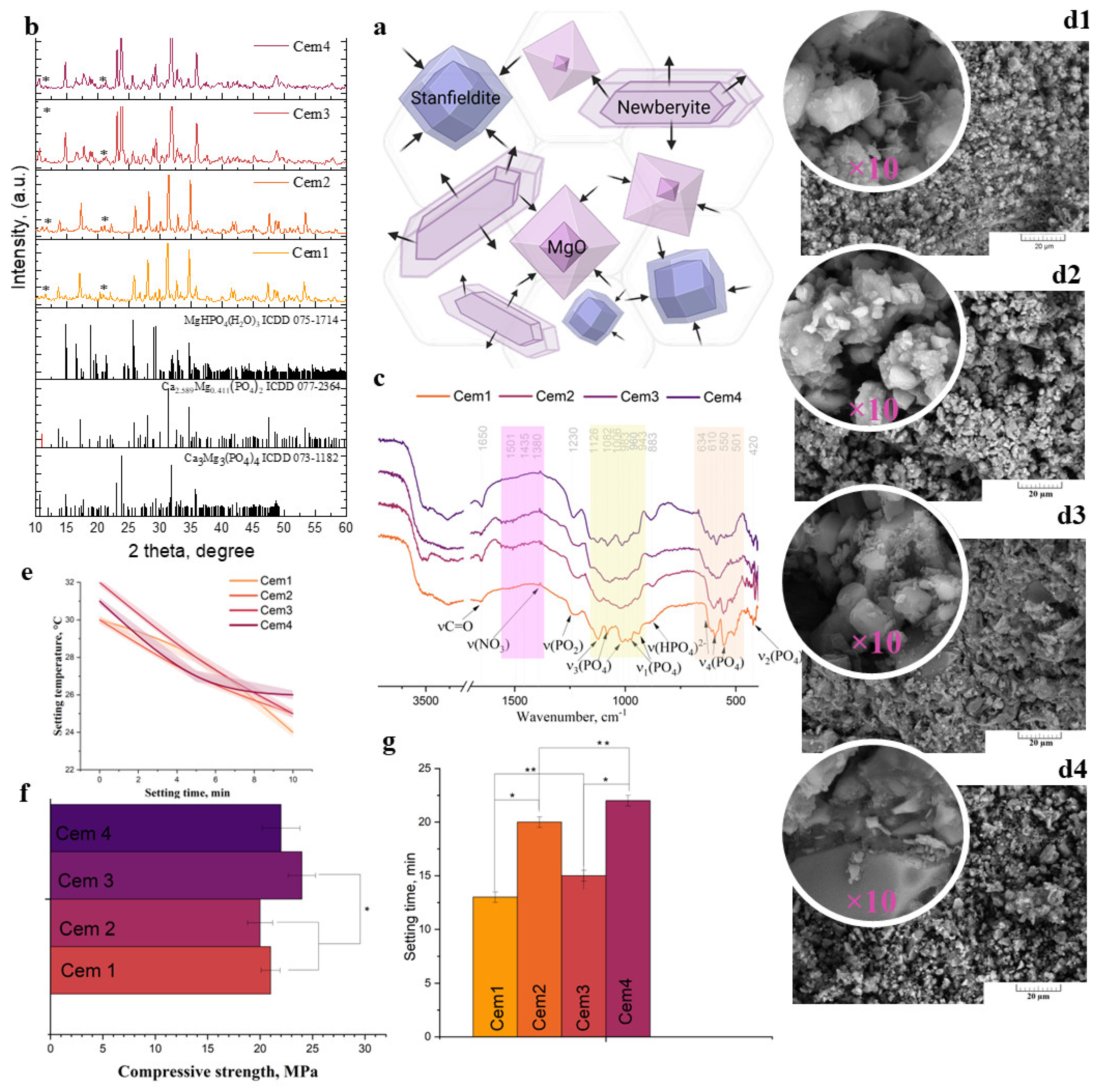
2.3. Characterisation of Cement Materials
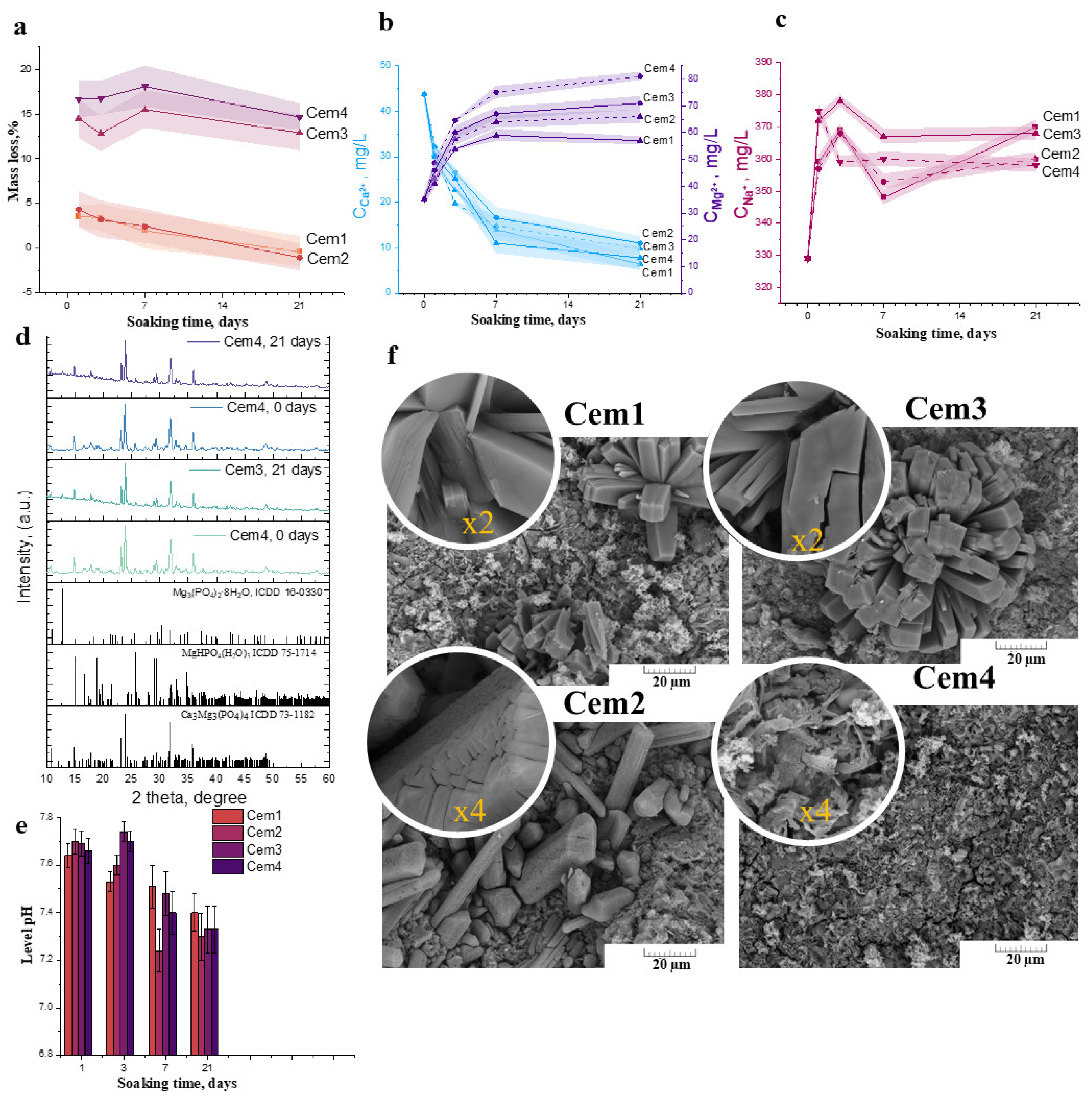
2.4. In Vitro Dissolution Assays
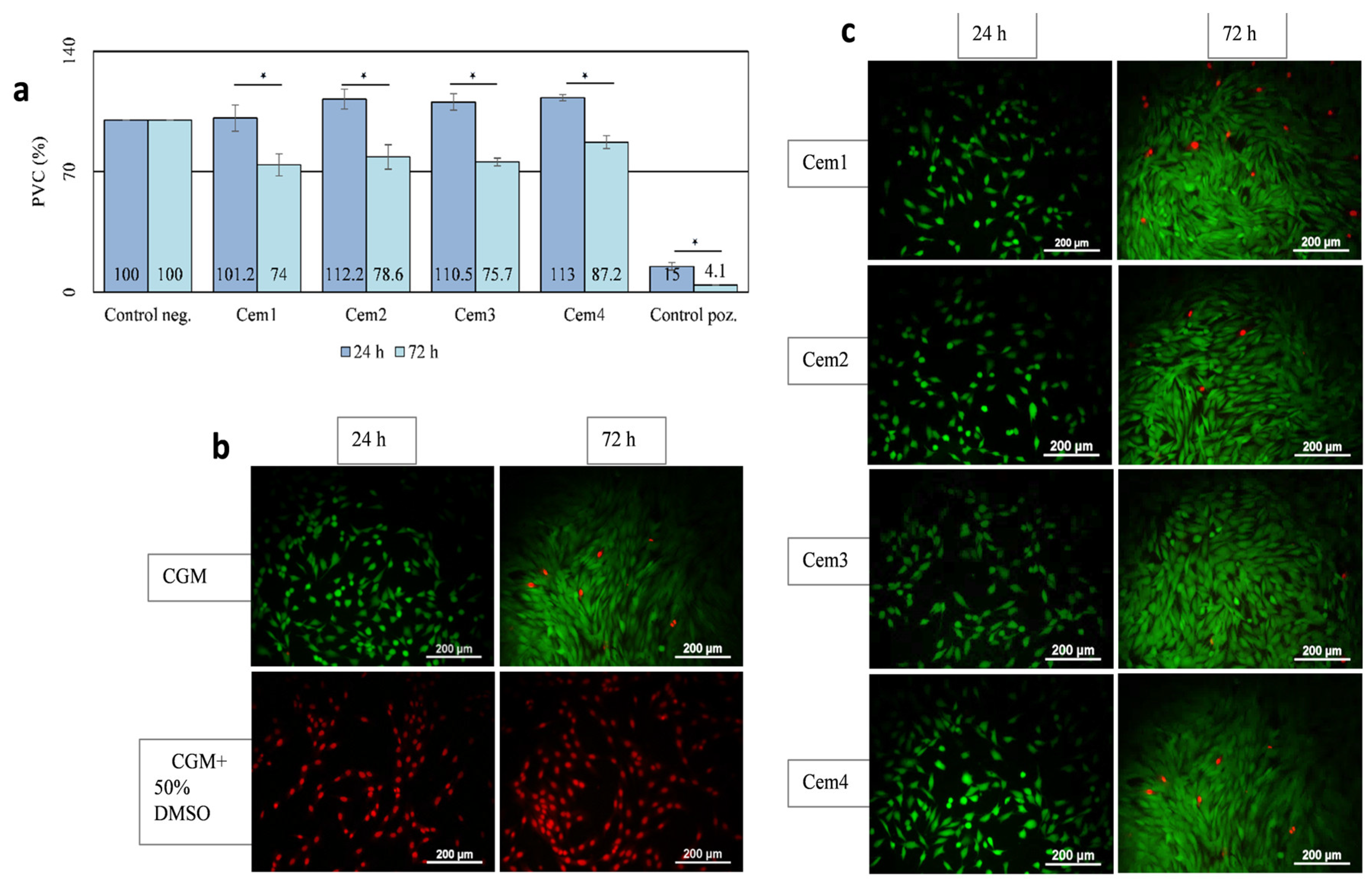
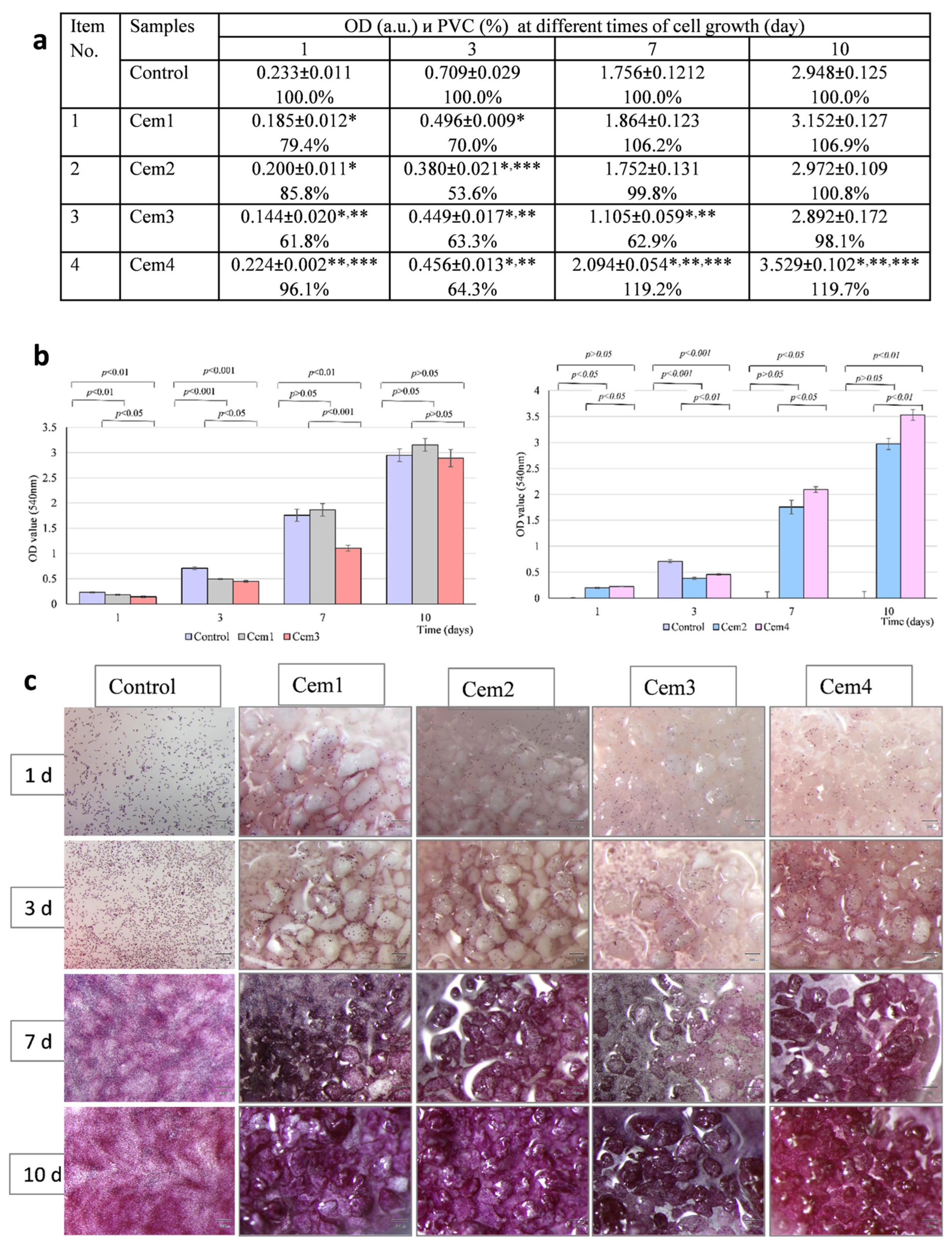
2.5. In Vitro Cytotoxicity of MCPC Samples
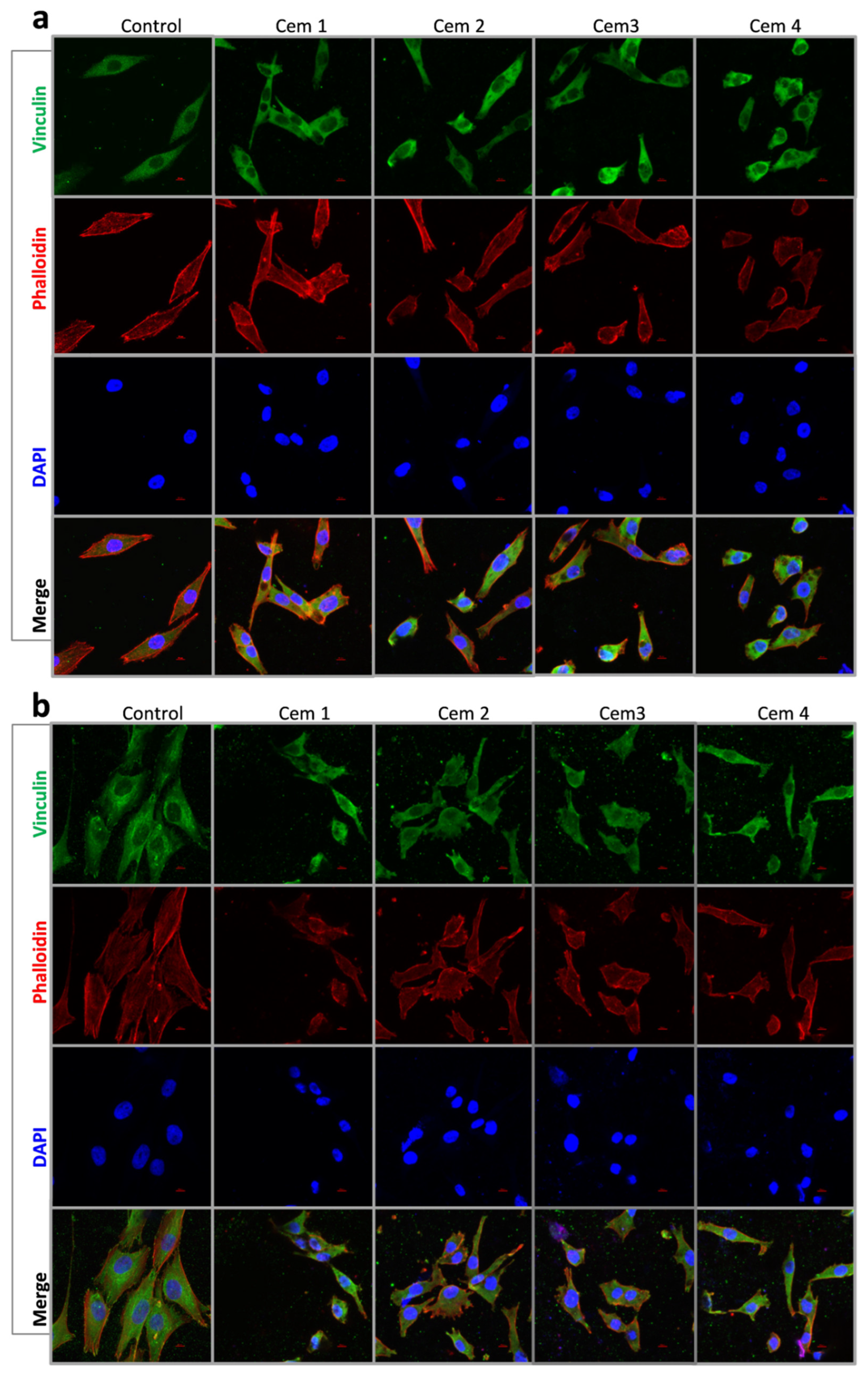
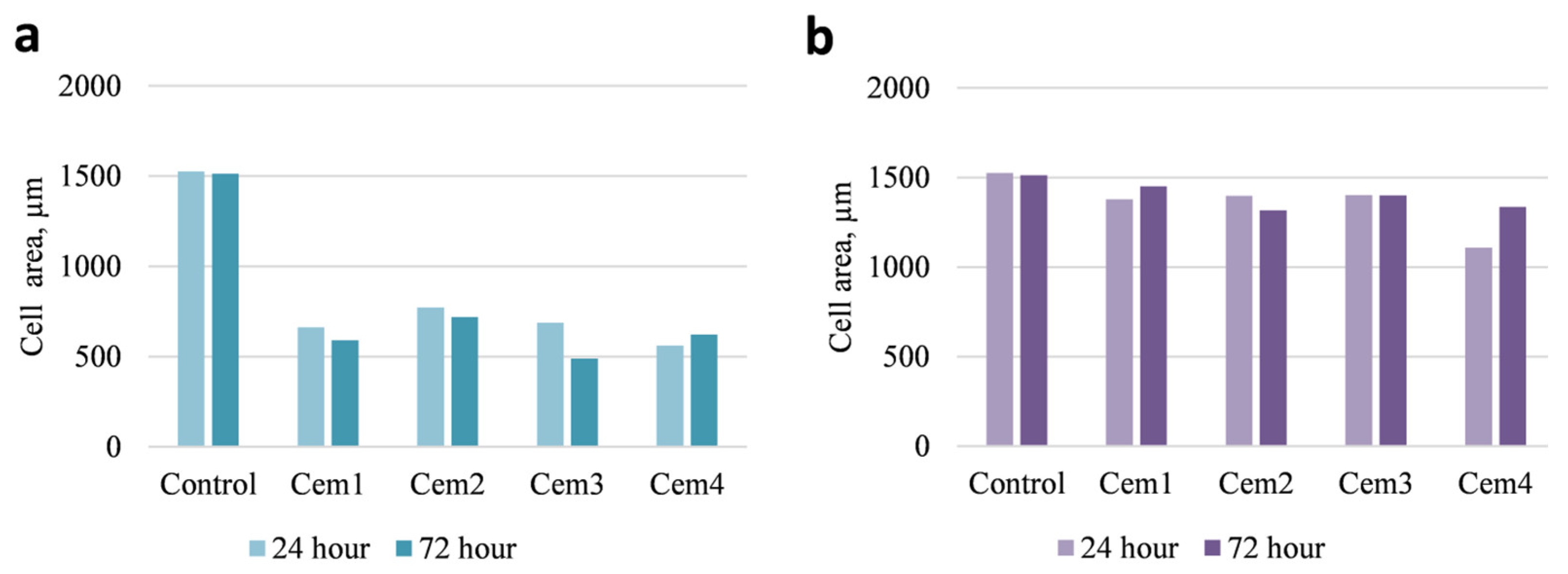
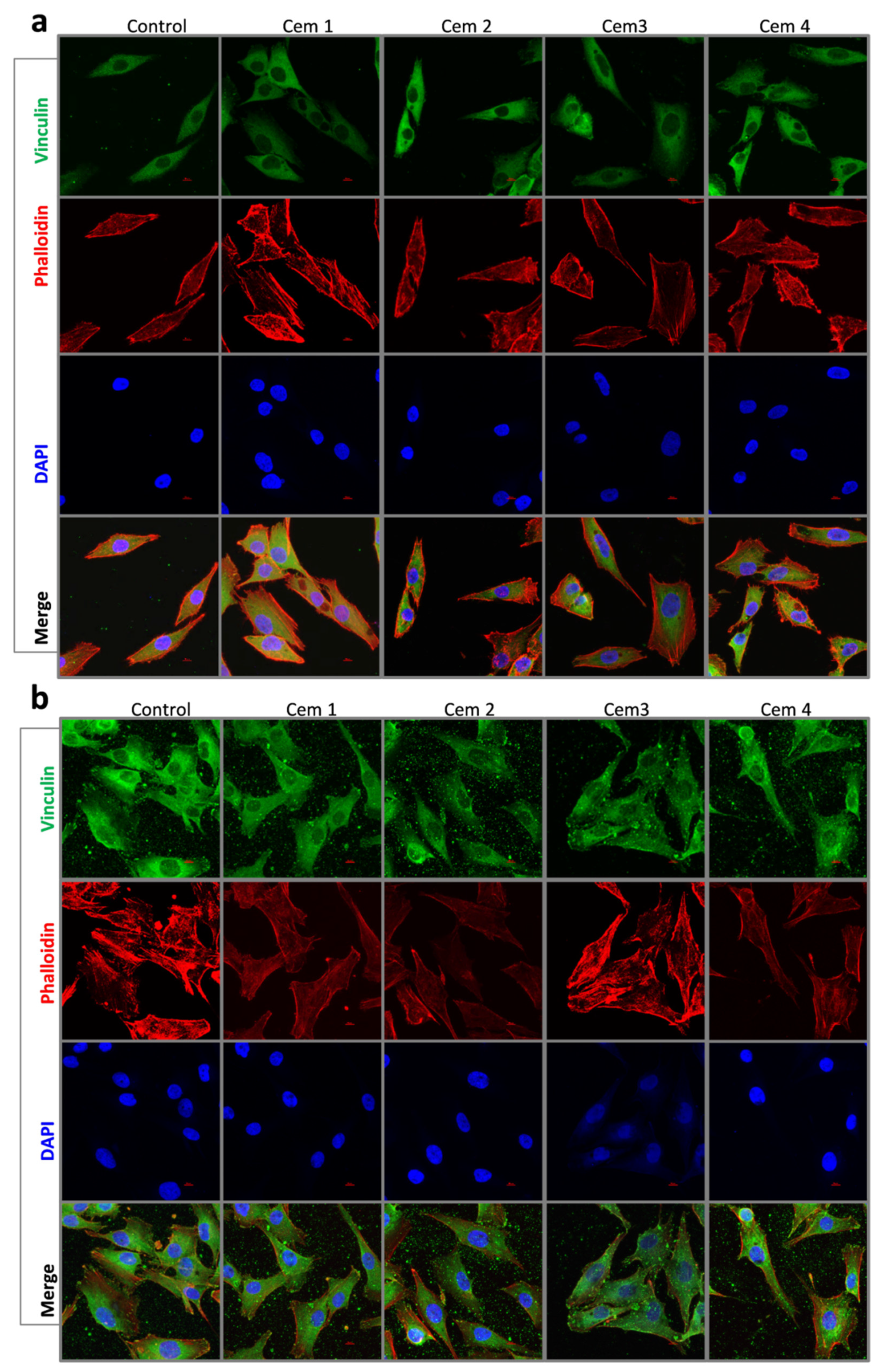
2.6. Effect of MCPC Samples on In Vitro Cytocompatibility
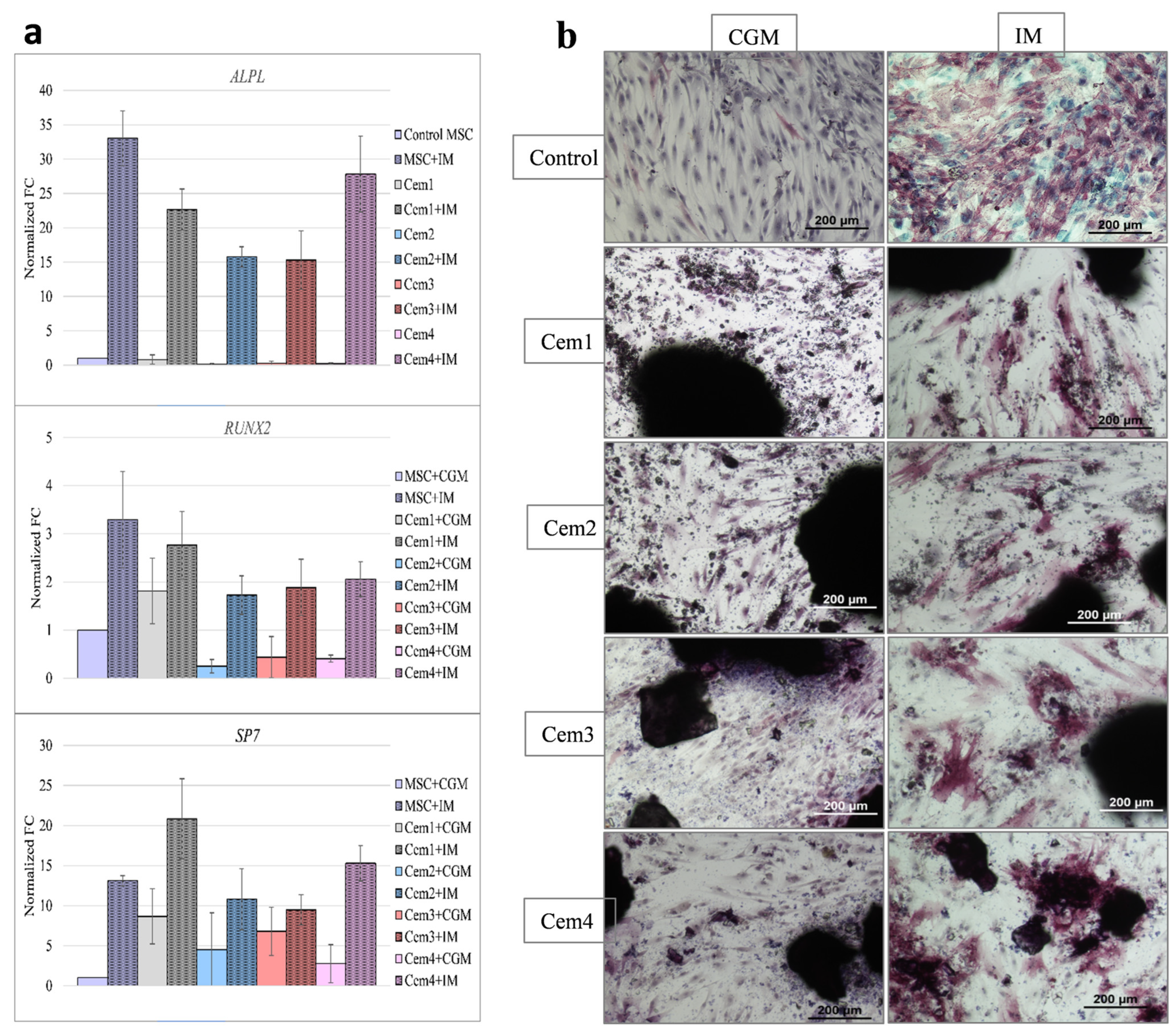
2.7. Effect of MCPC on the Osteogenic Differentiation of BM hMSCs
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Preparation of Cement Powders, Cement Liquid, and Samples
4.3. Characterisation of the Materials
4.4. Mechanical Testing
4.5. Injectability and Washout Resistance
4.6. Dissolution Assays
4.7. In Vitro Assay
4.7.1. Cell Cultures
4.7.2. Cell Viability Determination Methods
MTT Test
Live/Dead Assay
4.7.3. Assessment of Cytotoxicity of MCPC Samples via the Indirect Contact Method
4.7.4. Investigation of Adhesion Properties of the Surface of MCPC Samples
4.7.5. Cytocompatibility Study of MCPC Samples via Direct Contact Method
4.7.6. Investigation of the Ability of MCPC Samples to Support Osteogenic Differentiation of BM hMSCs
Gene Expression Analysis
4.7.7. Statistical Processing of Results
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gelli, R.; Ridi, F. An Overview of Magnesium-Phosphate-Based Cements as Bone Repair Materials. J. Funct. Biomater. 2023, 14, 424. [Google Scholar] [CrossRef] [PubMed]
- Zupan, J.; Strazar, K.; Kocijan, R.; Nau, T.; Grillari, J.; Marolt Presen, D. Age-Related Alterations and Senescence of Mesenchymal Stromal Cells: Implications for Regenerative Treatments of Bones and Joints. Mech. Ageing Dev. 2021, 198, 111539. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Chabok, R.; Guan, X.; Chawla, A.; Li, Y.; Khademhosseini, A.; Jang, H.L. Synergistic Interplay between the Two Major Bone Minerals, Hydroxyapatite and Whitlockite Nanoparticles, for Osteogenic Differentiation of Mesenchymal Stem Cells. Acta Biomater. 2018, 69, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Savvidou, O.; Papakonstantinou, O.; Lakiotaki, E.; Melissaridou, D.; Korkolopoulou, P.; Papagelopoulos, P.J. Post-Traumatic Myositis Ossificans: A Benign Lesion That Simulates Malignant Bone and Soft Tissue Tumours. EFORT Open Rev. 2021, 6, 572–583. [Google Scholar] [CrossRef]
- Ladegaard, T.H.; Sørensen, M.S.; Petersen, M.M. Solitary versus Multiple Bone Metastases in the Appendicular Skeleton: Should the Surgical Treatment Be Different? Bone Jt. J. 2023, 105-B, 1206–1215. [Google Scholar] [CrossRef]
- Wang, W.; Yeung, K.W.K. Bone Grafts and Biomaterials Substitutes for Bone Defect Repair: A Review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef]
- Anastasieva, E.A.; Sadovoy, M.A.; Voropaeva, V.V.; Kirilova, I.A. Reconstruction of Bone Defects after Tumor Resection by Auto- and Allografts (Review of Literature). Traumatol. Orthop. Russ. 2017, 23, 148–155. [Google Scholar] [CrossRef][Green Version]
- Kareem, R.O.; Bulut, N.; Kaygili, O. Hydroxyapatite Biomaterials: A Comprehensive Review of Their Properties, Structures, Medical Applications, and Fabrication Methods. J. Chem. Rev. 2024, 6, 1–26. [Google Scholar]
- Ogueri, K.S.; Jafari, T.; Escobar Ivirico, J.L.; Laurencin, C.T. Polymeric Biomaterials for Scaffold-Based Bone Regenerative Engineering. Regen. Eng. Transl. Med. 2019, 5, 128–154. [Google Scholar] [CrossRef]
- Jonathan, E.M.; Ohifuemen, A.O.; Jacob, J.N.; Isaac, A.Y.; Ifijen, I.H. Polymeric Biodegradable Biomaterials for Tissue Bioengineering and Bone Rejuvenation. In TMS Annual Meeting & Exhibition; Minerals, Metals and Materials Series; Springer Nature: Cham, Switzerland, 2023; pp. 267–277. [Google Scholar] [CrossRef]
- Goldberg, M.A.; Smirnov, V.V.; Krokhicheva, P.A.; Barinov, S.M.; Komlev, V.S. The Creation and Application Outlook of Calcium Phosphate and Magnesium Phosphate Bone Cements with Antimicrobial Properties (Review). Inorg. Mater. Appl. Res. 2021, 12, 195–203. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium Orthophosphate (CaPO4) Containing Composites for Biomedical Applications: Formulations, Properties, and Applications. J. Compos. Sci. 2024, 8, 218. [Google Scholar] [CrossRef]
- Bakhtiari, H.; Nouri, A.; Khakbiz, M.; Tolouei-Rad, M. Fatigue Behaviour of Load-Bearing Polymeric Bone Scaffolds: A Review. Acta Biomater. 2023, 172, 16–37. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Yang, R.; Cooper, P.R.; Khurshid, Z.; Shavandi, A.; Ratnayake, J. Bone Grafts and Substitutes in Dentistry: A Review of Current Trends and Developments. Molecules 2021, 26, 3007. [Google Scholar] [CrossRef]
- Santoro, A.; Voto, A.; Fortino, L.; Guida, R.; Laudisio, C.; Cillo, M.; D’Ursi, A.M. Bone Defect Treatment in Regenerative Medicine: Exploring Natural and Synthetic Bone Substitutes. Int. J. Mol. Sci. 2025, 26, 3085. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, P.; Li, D.J.; Auston, D.A.; Mir, H.S.; Yoon, R.S.; Koval, K.J. Autograft, Allograft, and Bone Graft Substitutes: Clinical Evidence and Indications for Use in the Setting of Orthopaedic Trauma Surgery. J. Orthop. Trauma. 2019, 33, 203–213. [Google Scholar] [CrossRef]
- Sohn, H.S.; Oh, J.K. Review of Bone Graft and Bone Substitutes with an Emphasis on Fracture Surgeries. Biomater. Res. 2019, 23, s40824-019-0157-y. [Google Scholar] [CrossRef]
- Brochu, B.M.; Sturm, S.R.; Kawase De Queiroz Goncalves, J.A.; Mirsky, N.A.; Sandino, A.I.; Panthaki, K.Z.; Panthaki, K.Z.; Nayak, V.V.; Daunert, S.; Witek, L.; et al. Advances in Bioceramics for Bone Regeneration: A Narrative Review. Biomimetics 2024, 9, 690. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, G.; Long, Z.; Li, M.; Ji, C.; Wang, F.; Li, H.; Lu, J.; Wang, Z.; Li, J. Novel 3D-Printed Prosthetic Composite for Reconstruction of Massive Bone Defects in Lower Extremities after Malignant Tumor Resection. J. Bone Oncol. 2019, 16, 100220. [Google Scholar] [CrossRef]
- Goldberg, M.; Gafurov, M.; Makshakova, O.; Smirnov, V.; Komlev, V.; Barinov, S.; Kudryavtsev, E.; Sergeeva, N.; Achmedova, S.; Mamin, G.; et al. Influence of Al on the Structure and in Vitro Behavior of Hydroxyapatite Nanopowders. J. Phys. Chem. B 2019, 123, 9143–9154. [Google Scholar] [CrossRef]
- Peranidze, K.; Safronova, T.V.; Kildeeva, N.R. Fibrous Polymer-Based Composites Obtained by Electrospinning for Bone Tissue Engineering. Polymers 2021, 14, 96. [Google Scholar] [CrossRef]
- Povernov, P.A.; Shibryaeva, L.S.; Lusova, L.R.; Popov, A.A. Modern Polymer Composite Materials for Bone Surgery: Problems and Prospects. Fine Chem. Technol. 2023, 17, 514–536. [Google Scholar] [CrossRef]
- Madar Saheb, M.A.; Kanagaraj, M.; Kannan, S. Exploring the Biomedical Potential of PLA/Dysprosium Phosphate Composites via Extrusion-Based 3D Printing: Design, Morphological, Mechanical, and Multimodal Imaging and Finite Element Modeling. ACS Appl. Bio. Mater. 2023, 6, 5414–5425. [Google Scholar] [CrossRef] [PubMed]
- Fadeeva, I.V.; Deyneko, D.V.; Knotko, A.V.; Olkhov, A.A.; Slukin, P.V.; Davydova, G.A.; Trubitsyna, T.A.; Preobrazhenskiy, I.I.; Gosteva, A.N.; Antoniac, I.V.; et al. Antibacterial Composite Material Based on Polyhydroxybutyrate and Zn-Doped Brushite Cement. Polymers 2023, 15, 2106. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S. Self-Setting Calcium Orthophosphate Formulations. J. Funct. Biomater. 2013, 4, 209–311. [Google Scholar] [CrossRef]
- Fadeeva, I.V.; Goldberg, M.A.; Preobrazhensky, I.I.; Mamin, G.V.; Davidova, G.A.; Agafonova, N.V.; Fosca, M.; Russo, F.; Barinov, S.M.; Cavalu, S.; et al. Improved Cytocompatibility and Antibacterial Properties of Zinc-Substituted Brushite Bone Cement Based on β-Tricalcium Phosphate. J. Mater. Sci. Mater. Med. 2021, 32, 99. [Google Scholar] [CrossRef] [PubMed]
- Brückner, T.; Meininger, M.; Groll, J.; Kübler, A.C.; Gbureck, U. Magnesium Phosphate Cement as Mineral Bone Adhesive. Materials 2019, 12, 3819. [Google Scholar] [CrossRef]
- Schröter, L.; Kaiser, F.; Stein, S.; Gbureck, U.; Ignatius, A. Biological and Mechanical Performance and Degradation Characteristics of Calcium Phosphate Cements in Large Animals and Humans. Acta Biomater. 2020, 117, 1–20. [Google Scholar] [CrossRef]
- Vezenkova, A.; Locs, J. Sudoku of Porous, Injectable Calcium Phosphate Cements—Path to Osteoinductivity. Bioact. Mater. 2022, 17, 109–124. [Google Scholar] [CrossRef]
- Meng, D.; Dong, L.; Yuan, Y.; Jiang, Q. In Vitro and in Vivo Analysis of the Biocompatibility of Two Novel and Injectable Calcium Phosphate Cements. Regen. Biomater. 2019, 6, 13–19. [Google Scholar] [CrossRef]
- Low, K.L.; Tan, S.H.; Zein, S.H.S.; Roether, J.A.; Mouriño, V.; Boccaccini, A.R. Calcium Phosphate-Based Composites as Injectable Bone Substitute Materials. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 94, 273–286. [Google Scholar] [CrossRef]
- Demir-Oğuz, Ö.; Boccaccini, A.R.; Loca, D. Injectable Bone Cements: What Benefits the Combination of Calcium Phosphates and Bioactive Glasses Could Bring? Bioact. Mater. 2023, 19, 217–236. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M.A.; Smirnov, V.V.; Antonova, O.S.; Tut’kova, Y.B.; Obolkina, T.O.; Khairutdinova, D.R.; Krokhicheva, P.A.; Barinov, S.M.; Komlev, V.S. Ceramic Materials in the Tricalcium Phosphate–Trimagnesium Phosphate System. Inorg. Mater. 2020, 56, 314–320. [Google Scholar] [CrossRef]
- Wu, J.; Cheng, X.; Wu, J.; Chen, J.; Pei, X. The Development of Magnesium-Based Biomaterials in Bone Tissue Engineering: A Review. J. Biomed. Mater. Res. B Appl. Biomater. 2024, 112, e35326. [Google Scholar] [CrossRef]
- Ostrowski, N.; Roy, A.; Kumta, P.N. Magnesium Phosphate Cement Systems for Hard Tissue Applications: A Review. ACS Biomater. Sci. Eng. 2016, 2, 1067–1083. [Google Scholar] [CrossRef] [PubMed]
- Klammert, U.; Ignatius, A.; Wolfram, U.; Reuther, T.; Gbureck, U. In Vivo Degradation of Low Temperature Calcium and Magnesium Phosphate Ceramics in a Heterotopic Model. Acta Biomater. 2011, 7, 3469–3475. [Google Scholar] [CrossRef] [PubMed]
- Krokhicheva, P.A.; Goldberg, M.A.; Fomin, A.S.; Khayrutdinova, D.R.; Antonova, O.S.; Baikin, A.S.; Konovalov, A.A.; Leonov, A.V.; Mikheev, I.V.; Merzlyak, E.M.; et al. Enhanced Bone Repair by Silver-Doped Magnesium Calcium Phosphate Bone Cements. Ceram. Int. 2023, 49, 19249–19264. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, X.; Lin, D.; Shi, H.; Yuan, Y.; Tang, W.; Zhou, H.; Guo, H.; Qian, J.; Liu, C. Magnesium Modification of a Calcium Phosphate Cement Alters Bone Marrow Stromal Cell Behavior via an Integrin-Mediated Mechanism. Biomaterials 2015, 53, 251–264. [Google Scholar] [CrossRef]
- Haque, M.A.; Chen, B. In Vitro and in Vivo Research Advancements on the Magnesium Phosphate Cement Biomaterials: A Review. Materialia 2020, 13, 100852. [Google Scholar] [CrossRef]
- O’Neill, R.; McCarthy, H.O.; Montufar, E.B.; Ginebra, M.P.; Wilson, D.I.; Lennon, A.; Dunne, N. Critical Review: Injectability of Calcium Phosphate Pastes and Cements. Acta Biomater. 2017, 50, 1–19. [Google Scholar] [CrossRef]
- Liao, H.; Walboomers, X.F.; Habraken, W.J.E.M.; Zhang, Z.; Li, Y.; Grijpma, D.W.; Mikos, A.G.; Wolke, J.G.C.; Jansen, J.A. Injectable Calcium Phosphate Cement with PLGA, Gelatin and PTMC Microspheres in a Rabbit Femoral Defect. Acta Biomater. 2011, 7, 1752–1759. [Google Scholar] [CrossRef]
- Cai, P.; Lu, S.; Yu, J.; Xiao, L.; Wang, J.; Liang, H.; Huang, L.; Han, G.; Bian, M.; Zhang, S.; et al. Injectable Nanofiber-Reinforced Bone Cement with Controlled Biodegradability for Minimally-Invasive Bone Regeneration. Bioact. Mater. 2023, 21, 267–283. [Google Scholar] [CrossRef] [PubMed]
- Jacquart, S.; Girod-Fullana, S.; Brouillet, F.; Pigasse, C.; Siadous, R.; Fatnassi, M.; Grimoud, J.; Rey, C.; Roques, C.; Combes, C. Injectable Bone Cement Containing Carboxymethyl Cellulose Microparticles as a Silver Delivery System Able to Reduce Implant-Associated Infection Risk. Acta Biomater. 2022, 145, 342–357. [Google Scholar] [CrossRef]
- Chiang, T.Y.; Ho, C.C.; Chen, D.C.H.; Lai, M.H.; Ding, S.J. Physicochemical Properties and Biocompatibility of Chitosan Oligosaccharide/Gelatin/Calcium Phosphate Hybrid Cements. Mater. Chem. Phys. 2010, 120, 282–288. [Google Scholar] [CrossRef]
- Tang, Y.; Chen, L.; Wu, Z.; Zhao, K.; Tan, Q. Fabrication of Injectable and Expandable PMMA/PAASf Bone Cements. Compos. Sci. Technol. 2017, 146, 203–209. [Google Scholar] [CrossRef]
- Yu, L.; Xia, K.; Gong, C.; Chen, J.; Li, W.; Zhao, Y.; Guo, W.; Dai, H. An Injectable Bioactive Magnesium Phosphate Cement Incorporating Carboxymethyl Chitosan for Bone Regeneration. Int. J. Biol. Macromol. 2020, 160, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Safwat, E.; Hassan, M.L.; Saniour, S.; Zaki, D.Y.; Eldeftar, M.; Saba, D.; Zazou, M. Injectable TEMPO-Oxidized Nanofibrillated Cellulose/Biphasic Calcium Phosphate Hydrogel for Bone Regeneration. J. Biomater. Appl. 2018, 32, 1371–1381. [Google Scholar] [CrossRef]
- Oğuz Öznur, D.; Ege, D. Rheological and Mechanical Properties of Thermoresponsive Methylcellulose/Calcium Phosphate-Based Injectable Bone Substitutes. Materials 2018, 11, 604. [Google Scholar] [CrossRef]
- Abatangelo, G.; Vindigni, V.; Avruscio, G.; Pandis, L.; Brun, P. Hyaluronic Acid: Redefining Its Role. Cells 2020, 9, 1743. [Google Scholar] [CrossRef] [PubMed]
- The Effect of Hyaluronic Acid on Brushite Cement Cohesion|Elsevier Enhanced Reader. Available online: https://reader.elsevier.com/reader/sd/pii/S1742706109001494?token=2F73BCB3D7DAED8AF912678DE19DEDA54A512A59F9BBF7D57ED096BD58597CA18CDEC2F54387C6DFA6D6DD837F95B4DA&originRegion=eu-west-1&originCreation=20211107234429 (accessed on 8 November 2021).
- Zhang, Y.; Jia, S.; Tian, F.; Gao, X.; Wang, Z.; Zhu, L.; Hao, D. Effects of Calcium Phosphate Cement Combined with Hyaluronic Acid/Curcumin on the Proliferation and Osteogenesis of Osteoblasts. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2021, 35, 104–110. [Google Scholar] [CrossRef]
- Cui, X.; Huang, C.; Chen, Z.; Zhang, M.; Liu, C.; Su, K.; Wang, J.; Li, L.; Wang, R.; Li, B.; et al. Hyaluronic Acid Facilitates Bone Repair Effects of Calcium Phosphate Cement by Accelerating Osteogenic Expression. Bioact. Mater. 2021, 6, 3801–3811. [Google Scholar] [CrossRef]
- Balazs, E.A.; Laurent, T.C.; Jeanloz, R.W. Nomenclature of Hyaluronic Acid. Biochem. J. 1986, 235, 903. [Google Scholar] [CrossRef] [PubMed]
- Nganvongpanit, K.; Boonsri, B.; Sripratak, T.; Markmee, P. Effects of One-Time and Two-Time Intra-Articular Injection of Hyaluronic Acid Sodium Salt after Joint Surgery in Dogs. J. Vet. Sci. 2013, 14, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Felson, D.T.; Anderson, J.J. Hyaluronate Sodium Injections for Osteoarthritis: Hope, Hype, and Hard Truths. Arch. Intern. Med. 2002, 162, 245–247. [Google Scholar] [CrossRef]
- BjØrnland, T.; GjÆrum, A.A.; MØystad, A. Osteoarthritis of the Temporomandibular Joint: An Evaluation of the Effects and Complications of Corticosteroid Injection Compared with Injection with Sodium Hyaluronate. J. Oral. Rehabil. 2007, 34, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Blaine, T.; Moskowitz, R.; Udell, J.; Skyhar, M.; Levin, R.; Friedlander, J.; Daley, M.; Altman, R. Treatment of Persistent Shoulder Pain with Sodium Hyaluronate: A Randomized, Controlled Trial. A Multicenter Study. J. Bone Jt. Surg.-Ser. A 2008, 90, 970–979. [Google Scholar] [CrossRef]
- Kai, D.; Li, D.; Zhu, X.; Zhang, L.; Fan, H.; Zhang, X. Addition of Sodium Hyaluronate and the Effect on Performance of the Injectable Calcium Phosphate Cement. J. Mater. Sci. Mater. Med. 2009, 20, 1595–1602. [Google Scholar] [CrossRef]
- Krokhicheva, P.A.; Goldberg, M.A.; Khairutdinova, D.R.; Fomin, A.S.; Sentsova, A.M.; Antonova, O.S.; Kondratiev, A.V.; Leonov, A.V.; Baikin, A.S.; Konovalov, A.A.; et al. Magnesium–Calcium Phosphate Cements with Sodium Hyaluronate. Inorg. Mater. Appl. Res. 2023, 14, 384–391. [Google Scholar] [CrossRef]
- Hammerli, J.; Hermann, J.; Tollan, P.; Naab, F. Measuring in Situ CO2 and H2O in Apatite via ATR-FTIR. Contrib. Mineral. Petrol. 2021, 176, 105. [Google Scholar] [CrossRef]
- Suchanek, W.L.; Shuk, P.; Byrappa, K.; Riman, R.E.; TenHuisen, K.S.; Janas, V.F. Mechanochemical-Hydrothermal Synthesis of Carbonated Apatite Powders at Room Temperature. Biomaterials 2002, 23, 699–710. [Google Scholar] [CrossRef]
- Cacciotti, I.; Bianco, A. High Thermally Stable Mg-Substituted Tricalcium Phosphate via Precipitation. Ceram. Int. 2011, 37, 127–137. [Google Scholar] [CrossRef]
- El Makhloufy, S.; Oubouaza, R.; Ouasri, A.; Belaaouad, S. X-Ray Diffraction and Infrared Spectroscopy Data Review Analyses of the Calcium Phosphates. Biointerface Res. Appl. Chem. 2022, 12, 732–755. [Google Scholar]
- Goldberg, M.A.; Krohicheva, P.A.; Fomin, A.S.; Khairutdinova, D.R.; Antonova, O.S.; Baikin, A.S.; Smirnov, V.V.; Fomina, A.A.; Leonov, A.V.; Mikheev, I.V.; et al. In Situ Magnesium Calcium Phosphate Cements Formation: From One Pot Powders Precursors Synthesis to in Vitro Investigations. Bioact. Mater. 2020, 5, 644–658. [Google Scholar] [CrossRef] [PubMed]
- Mirkiani, S.; Mesgar, A.S.; Mohammadi, Z.; Matinfar, M. Synergetic Reinforcement of Brushite Cements by Monetite/Apatite Whisker-like Fibers and Carboxymethylcellulose. Materialia 2022, 21, 101329. [Google Scholar] [CrossRef]
- Haque, M.A.; Chen, B. Research Progresses on Magnesium Phosphate Cement: A Review. Constr. Build. Mater. 2019, 211, 885–898. [Google Scholar] [CrossRef]
- Wekwejt, M.; Jesiołkiewicz, R.; Mielewczyk-Gryń, A.; Kozień, D.; Ronowska, A.; Kozłowska, J.; Gbureck, U. Injectable Bone Cement Based on Magnesium Potassium Phosphate and Cross-Linked Alginate Hydrogel Designed for Minimally Invasive Orthopedic Procedures. Sci. Rep. 2024, 14, 20279. [Google Scholar] [CrossRef]
- Wu, F.; Wei, J.; Guo, H.; Chen, F.; Hong, H.; Liu, C. Self-Setting Bioactive Calcium-Magnesium Phosphate Cement with High Strength and Degradability for Bone Regeneration. Acta Biomater. 2008, 4, 1873–1884. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cheng, Z.; Chen, D.; Li, G.; Chen, J.; Wang, K.; Xu, L.; Huang, J. An Injectable Porous Bioactive Magnesium Phosphate Bone Cement Foamed with Calcium Carbonate and Citric Acid for Periodontal Bone Regeneration. J. Mech. Behav. Biomed. Mater. 2023, 142, 105805. [Google Scholar] [CrossRef] [PubMed]
- Klammert, U.; Reuther, T.; Blank, M.; Reske, I.; Barralet, J.E.; Grover, L.M.; Kübler, A.C.; Gbureck, U. Phase Composition, Mechanical Performance and in Vitro Biocompatibility of Hydraulic Setting Calcium Magnesium Phosphate Cement. Acta Biomater. 2010, 6, 1529–1535. [Google Scholar] [CrossRef]
- Wu, J.; Liu, F.; Wang, Z.; Liu, Y.; Zhao, X.; Fang, C.; Leung, F.; Yeung, K.W.K.; Wong, T.M. The Development of a Magnesium-Releasing and Long-Term Mechanically Stable Calcium Phosphate Bone Cement Possessing Osteogenic and Immunomodulation Effects for Promoting Bone Fracture Regeneration. Front. Bioeng. Biotechnol. 2022, 9, 803723. [Google Scholar] [CrossRef]
- Wu, F.; Su, J.; Wei, J.; Guo, H.; Liu, C. Injectable Bioactive Calcium-Magnesium Phosphate Cement for Bone Regeneration. Biomed. Mater. 2008, 3, 044105. [Google Scholar] [CrossRef]
- Krokhicheva, P.A.; Goldberg, M.A.; Fomin, A.S.; Khayrutdinova, D.R.; Antonova, O.S.; Sadovnikova, M.A.; Mikheev, I.V.; Leonov, A.V.; Merzlyak, E.M.; Kovalishina, D.A.; et al. Gadolinium-Doped Injectable Magnesium-Calcium Phosphate Bone Cements for Noninvasive Visualization. J. Magnes. Alloys 2024, 12, 3698–3716. [Google Scholar] [CrossRef]
- Zhai, P.; Peng, X.; Li, B.; Liu, Y.; Sun, H.; Li, X. The Application of Hyaluronic Acid in Bone Regeneration. Int. J. Biol. Macromol. 2020, 151, 1224–1239. [Google Scholar] [CrossRef]
- Patrascu, J.M.; Krüger, J.P.; Böss, H.G.; Ketzmar, A.K.; Freymann, U.; Sittinger, M.; Notter, M.; Endres, M.; Kaps, C. Polyglycolic Acid-Hyaluronan Scaffolds Loaded with Bone Marrow-Derived Mesenchymal Stem Cells Show Chondrogenic Differentiation in Vitro and Cartilage Repair in the Rabbit Model. J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101, 1310–1320. [Google Scholar] [CrossRef]
- Araújo, J.C.; Sader, M.S.; Moreira, E.L.; Moraes, V.C.A.; LeGeros, R.Z.; Soares, G.A. Maximum Substitution of Magnesium for Calcium Sites in Mg-β-TCP Structure Determined by X-Ray Powder Diffraction with the Rietveld Refinement. Mater. Chem. Phys. 2009, 118, 337–340. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, W.; Schnitzler, V.; Tancret, F.; Bouler, J.M. Calcium Phosphate Cements for Bone Substitution: Chemistry, Handling and Mechanical Properties. Acta Biomater. 2014, 10, 1035–1049. [Google Scholar] [CrossRef]
- Afonina, A.; Kizalaite, A.; Zarkov, A.; Drabavicius, A.; Goto, T.; Sekino, T.; Kareiva, A.; Grigoraviciute-Puroniene, I. Synthesis of Whitlockite Nanopowders with Different Magnesium Content. Ceram. Int. 2022, 48, 32125–32130. [Google Scholar] [CrossRef]
- Bohner, M.; Baroud, G. Injectability of Calcium Phosphate Pastes. Biomaterials 2005, 26, 1553–1563. [Google Scholar] [CrossRef]
- Bernards; Chapman; Mirza, S. Lethality of Embolized Norian Bone Cement Varies with the Time between Mixing and Embolization. In Proceedings of the 50th Annual Meeting of the Orthopaedic Research Society, San Francisco, CA, USA, 7–10 March 2004. [Google Scholar]
- Hou, P.; Sun, Y.; Yang, W.; Wu, H.; Sun, L.; Xiu, X.; Xiu, C.; Zhang, X.; Zhang, W. Magnesium Promotes Osteogenesis via Increasing OPN Expression and Activating CaM/CaMKIV/CREB1 Pathway. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 1594–1603. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, A.; Romero-Gavilán, F.; García-Arnáez, I.; Martinez-Ramos, C.; Ozturan, S.; Izquierdo, R.; Azkargorta, M.; Elortza, F.; Gurruchaga, M.; Suay, J.; et al. Characterization of Magnesium Doped Sol-Gel Biomaterial for Bone Tissue Regeneration: The Effect of Mg Ion in Protein Adsorption. Mater. Sci. Eng.C 2021, 125, 112114. [Google Scholar] [CrossRef]
- Wang, X.; Sun, H.; Song, M.; Yan, G.; Wang, Q. The Biodegradability and in Vitro Cytological Study on the Composite of PLGA Combined With Magnesium Metal. Front. Bioeng. Biotechnol. 2022, 10, 859280. [Google Scholar] [CrossRef]
- Xu, H.; Tian, F.; Liu, Y.; Liu, R.; Li, H.; Gao, X.; Ju, C.; Lu, B.; Wu, W.; Wang, Z.; et al. Magnesium Malate-Modified Calcium Phosphate Bone Cement Promotes the Repair of Vertebral Bone Defects in Minipigs via Regulating CGRP. J. Nanobiotechnol. 2024, 22, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Fang, X.; Yan, J.; Pan, C.; Ge, W.; Wang, J.; Shen, S.G.; Lin, K.; Zhang, L. Magnesium-Containing Bioceramics Stimulate Exosomal MiR-196a-5p Secretion to Promote Senescent Osteogenesis through Targeting Hoxa7/MAPK Signaling Axis. Bioact Mater 2024, 33, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Coelho, C.C.; Padrão, T.; Costa, L.; Pinto, M.T.; Costa, P.C.; Domingues, V.F.; Quadros, P.A.; Monteiro, F.J.; Sousa, S.R. The Antibacterial and Angiogenic Effect of Magnesium Oxide in a Hydroxyapatite Bone Substitute. Sci Rep 2020, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- ISO 9917-1:2025; Dentistry—Water-based cements—Part 1: Acid-base cements. ISO: Geneva, Switzerland, 2025.
- ISO 10993-5:2009; Biological evaluation of medical devices. Part 12 and Part 5. ISO: Geneva, Switzerland, 2009.
- Amable, P.R.; Teixeira, M.V.T.; Carias, R.B.V.; Granjeiro, J.M.; Borojevic, R. Identification of Appropriate Reference Genes for Human Mesenchymal Cells during Expansion and Differentiation. PLoS ONE 2013, 8, e73792. [Google Scholar] [CrossRef]
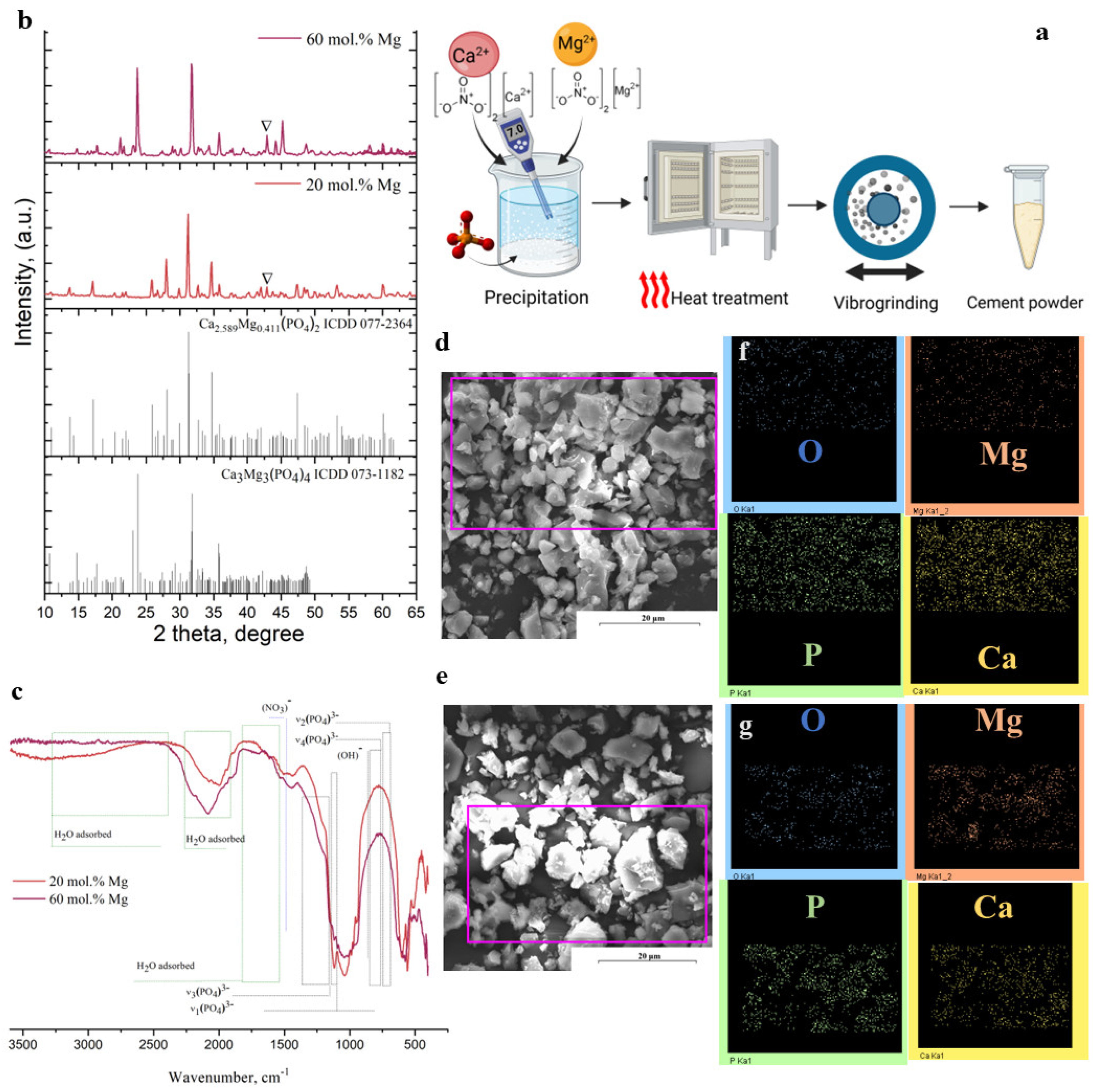
| Cement Powder | Phase Composition | Particle Size Distribution (μm) | Content, wt.% (ICP) | (Ca + Mg)/P | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mg-Wt | St | MgO | D10 | D50 | D90 | Ca | Mg | P | ||
| 20 mol.% Mg | 84 | 0 | 16 | 0.8 | 5.8 | 18.6 | 18.8 | 15.8 | 20.4 | 1.67 |
| 60 mol.%Mg | 0 | 90 | 10 | 1.2 | 11.7 | 57.6 | 18.3 | 15.8 | 20.5 | 1.66 |
| Cement Liquid | Concentration of Polymer NaHA, % | Viscosity, mPa·Sec | Surface Tension, mN/m | |
|---|---|---|---|---|
| 50 rmp | 200 rpm | |||
| A | - | 2.4 ± 0.5 | 6.00 ± 0.5 | 79.5 |
| A+1%NaHA | 1.00 | 12 ± 0.5 | 24.5 ± 0.5 | 83.0 |
| № | Gene Symbol | Encoded Protein | F and R Primer Sequences 5′-3′ |
|---|---|---|---|
| 1 | RUNX2 | Runt-associated transcription factor 2 is one of the key regulators of osteoblastic cells | F: tca-acg-atc-tga-gat-ttg-tgg-g R: ggg-gag-gat-ttg-tga-aga-cgg |
| 2 | SP7 | Osterix is a transcription factor involved in the differentiation of mesenchymal progenitors into osteoblasts and osteocytes | F: ccc-acc-tac-cca-tct-gac-tt R: gct-gcc-cac-tat-ttc-cca-ct |
| 3 | ALPL | Alkaline phosphatase is a membrane-bound glycosylated enzyme involved in matrix mineralisation | F: acc-acc-acg-aga-gtg-aac-ca R: cgt-tgt-ctg-agt-acc-agt-ccc |
| 4 | GAPDH | Glyceraldehyde-3-phosphate dehydrogenase, housekeeping gene | F: gaa-ggt-gaa-ggt-cgg-agt-c R: gaa-gat-ggt-gat-ggg-att-tc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sergeeva, N.S.; Krokhicheva, P.A.; Sviridova, I.K.; Goldberg, M.A.; Khayrutdinova, D.R.; Akhmedova, S.A.; Kirsanova, V.A.; Antonova, O.S.; Fomin, A.S.; Mikheev, I.V.; et al. Hyaluronan-Containing Injectable Magnesium–Calcium Phosphate Cements Demonstrated Improved Performance, Cytocompatibility, and Ability to Support Osteogenic Differentiation In Vitro. Int. J. Mol. Sci. 2025, 26, 6624. https://doi.org/10.3390/ijms26146624
Sergeeva NS, Krokhicheva PA, Sviridova IK, Goldberg MA, Khayrutdinova DR, Akhmedova SA, Kirsanova VA, Antonova OS, Fomin AS, Mikheev IV, et al. Hyaluronan-Containing Injectable Magnesium–Calcium Phosphate Cements Demonstrated Improved Performance, Cytocompatibility, and Ability to Support Osteogenic Differentiation In Vitro. International Journal of Molecular Sciences. 2025; 26(14):6624. https://doi.org/10.3390/ijms26146624
Chicago/Turabian StyleSergeeva, Natalia S., Polina A. Krokhicheva, Irina K. Sviridova, Margarita A. Goldberg, Dinara R. Khayrutdinova, Suraya A. Akhmedova, Valentina A. Kirsanova, Olga S. Antonova, Alexander S. Fomin, Ivan V. Mikheev, and et al. 2025. "Hyaluronan-Containing Injectable Magnesium–Calcium Phosphate Cements Demonstrated Improved Performance, Cytocompatibility, and Ability to Support Osteogenic Differentiation In Vitro" International Journal of Molecular Sciences 26, no. 14: 6624. https://doi.org/10.3390/ijms26146624
APA StyleSergeeva, N. S., Krokhicheva, P. A., Sviridova, I. K., Goldberg, M. A., Khayrutdinova, D. R., Akhmedova, S. A., Kirsanova, V. A., Antonova, O. S., Fomin, A. S., Mikheev, I. V., Leonov, A. V., Karalkin, P. A., Rodionov, S. A., Barinov, S. M., Komlev, V. S., & Kaprin, A. D. (2025). Hyaluronan-Containing Injectable Magnesium–Calcium Phosphate Cements Demonstrated Improved Performance, Cytocompatibility, and Ability to Support Osteogenic Differentiation In Vitro. International Journal of Molecular Sciences, 26(14), 6624. https://doi.org/10.3390/ijms26146624








