Dengue and Flavivirus Co-Infections: Challenges in Diagnosis, Treatment, and Disease Management
Abstract
1. Introduction
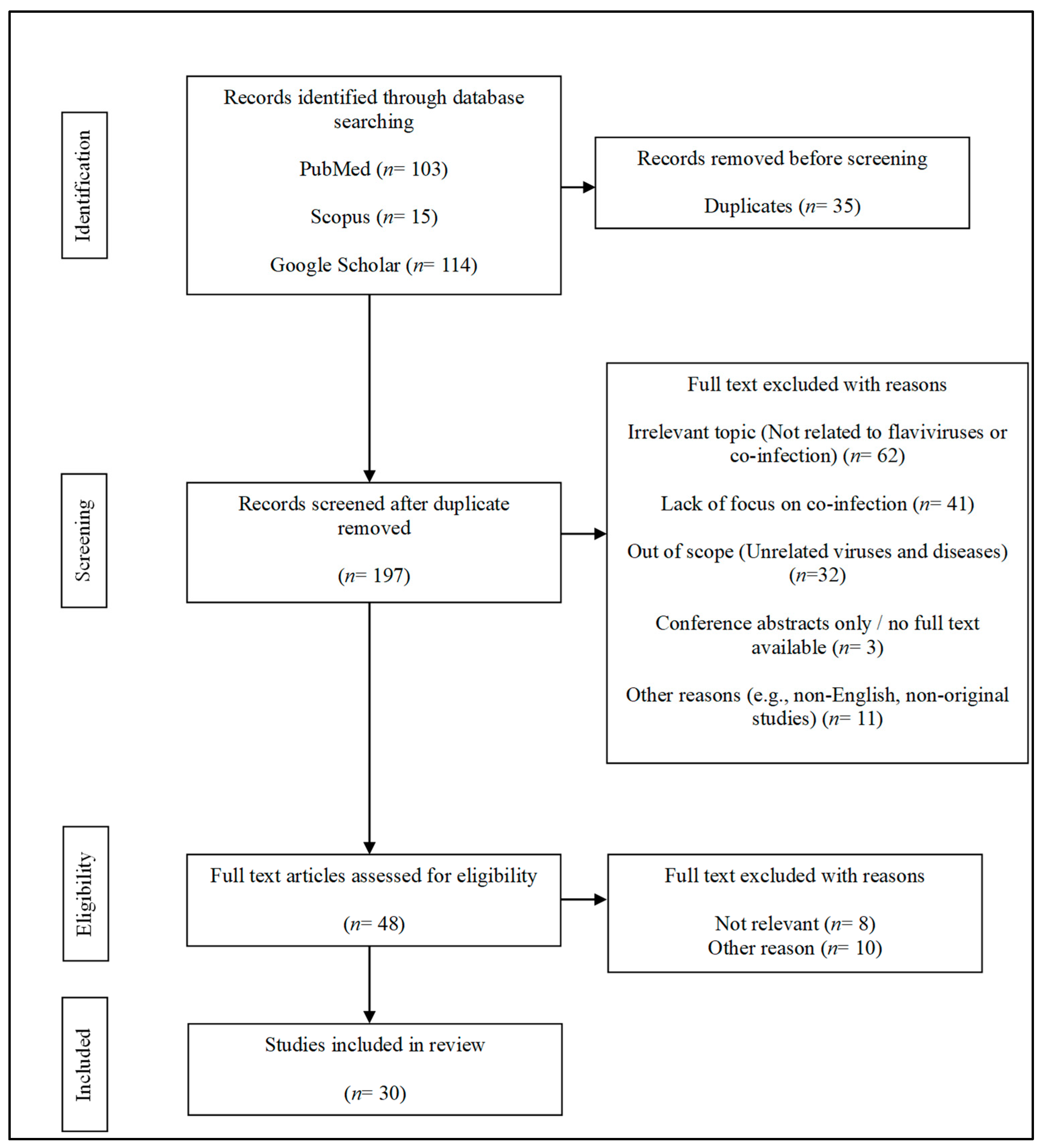
2. Methods
3. Co-Infection: Occurrence and Clinical Relevance
4. Severity and Implications of Co-Infections of DENV Serotypes and with Other Flaviviruses
4.1. Co-Infections of DENV Serotypes
4.2. Co-Infections of DENV with Other Flaviviruses
5. Implications of Better Understanding Flaviviral Co-Infections
5.1. Better Disease Diagnosis
5.2. Improved Disease Management and Treatment
5.3. Development of Vaccines for Flavivirus Diseases
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organisation. WHO Launches Global Strategic Plan to Fight Rising Dengue and Other Aedes-Borne Arboviral Diseases. Available online: https://www.who.int/news/item/03-10-2024-who-launches-global-strategic-plan-to-fight-rising-dengue-and-other-aedes-borne-arboviral-diseases?utm_source=chatgpt.com (accessed on 18 February 2025).
- Kuhn, R.J.; Zhang, W.; Rossmann, M.G.; Pletnev, S.V.; Corver, J.; Lenches, E.; Jones, C.T.; Mukhopadhyay, S.; Chipman, P.R.; Strauss, E.G.; et al. Structure of Dengue Virus: Implications for Flavivirus Organization, Maturation, and Fusion. Cell 2002, 108, 717–725. [Google Scholar] [CrossRef]
- Sirisena, P.D.N.N.; Mahilkar, S.; Sharma, C.; Jain, J.; Sunil, S. Concurrent Dengue Infections: Epidemiology & Clinical Implications. Indian J. Med. Res. 2021, 154, 669. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, H. Dynamics Analysis of a Zika–Dengue Co-Infection Model with Dengue Vaccine and Antibody-Dependent Enhancement. Phys. A Stat. Mech. Appl. 2019, 522, 248–273. [Google Scholar] [CrossRef]
- Suppiah, J.; Ching, S.M.; Amin-Nordin, S.; Mat-Nor, L.A.; Ahmad-Najimudin, N.A.; Low, G.K.K.; Abdul-Wahid, M.Z.; Thayan, R.; Chee, H.Y. Clinical Manifestations of Dengue in Relation to Dengue Serotype and Genotype in Malaysia: A Retrospective Observational Study. PLoS Negl. Trop. Dis. 2018, 12, e0006817. [Google Scholar] [CrossRef] [PubMed]
- Colombo, T.E.; Vedovello, D.; Mondini, A.; Reis, A.F.N.; Cury, A.A.F.; de Oliveira, F.H.; Cruz, L.E.A.A.; Bronzoni, R.V.d.M.; Nogueira, M.L. Co-Infection of Dengue Virus by Serotypes 1 and 4 in Patient from Medium Sized City from Brazil. Rev. Inst. Med. Trop. Sao Paulo 2013, 55, 275–281. [Google Scholar] [CrossRef]
- Dong, L.; Xing, L. Editorial: The Biological Mechanism and Health Effect of Co-Infection with Multiple Pathogens. Front. Cell Infect. Microbiol. 2024, 14, 1370067. [Google Scholar] [CrossRef] [PubMed]
- ten Bosch, Q.A.; Singh, B.K.; Hassan, M.R.A.; Chadee, D.D.; Michael, E. The Role of Serotype Interactions and Seasonality in Dengue Model Selection and Control: Insights from a Pattern Matching Approach. PLoS Negl. Trop. Dis. 2016, 10, e0004680. [Google Scholar] [CrossRef]
- Khan, E.; Prakoso, D.; Imtiaz, K.; Malik, F.; Farooqi, J.Q.; Long, M.T.; Barr, K.L. The Clinical Features of Co-Circulating Dengue Viruses and the Absence of Dengue Hemorrhagic Fever in Pakistan. Front. Public Health 2020, 8, 541004. [Google Scholar] [CrossRef]
- Senaratne, U.T.N.; Senaratne, U.T.N.; Murugananthan, K.; Murugananthan, K.; Sirisena, P.D.N.N.; Carr, J.M.; Noordeen, F. Dengue Virus Co-Infections with Multiple Serotypes Do Not Result in a Different Clinical Outcome Compared to Mono-Infections. Epidemiol. Infect. 2020, 148, e119. [Google Scholar] [CrossRef]
- Loroño-Pino, M.A.; Cropp, C.B.; Farfán, J.A.; Vorndam, A.V.; Rodríguez-Angulo, E.M.; Rosado-Paredes, E.P.; Flores-Flores, L.F.; Beaty, B.J.; Gubler, D.J. Common Occurrence of concurrent Infections by Multiple Dengue Virus Serotypes. Am. J. Trop. Med. Hyg. 1999, 61, 725–730. [Google Scholar] [CrossRef]
- Lewis, J.; Gallichotte, E.N.; Randall, J.; Glass, A.; Foy, B.D.; Ebel, G.D.; Kading, R.C. Intrinsic Factors Driving Mosquito Vector Competence and Viral Evolution: A Review. Front. Cell Infect. Microbiol. 2023, 13, 1330600. [Google Scholar] [CrossRef] [PubMed]
- Rückert, C.; Weger-Lucarelli, J.; Garcia-Luna, S.M.; Young, M.C.; Byas, A.D.; Murrieta, R.A.; Fauver, J.R.; Ebel, G.D. Impact of Simultaneous Exposure to Arboviruses on Infection and Transmission by Aedes Aegypti Mosquitoes. Nat. Commun. 2017, 8, 15412. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Barbosa, H.; Lyke, K.E.; Leguia, M.; Weiskopf, D.; Khanam, A.; Gutiérrez-Barbosa, H.; Lyke, K.E.; Chua, J. V Immune-Mediated Pathogenesis in Dengue Virus Infection. Viruses 2022, 14, 2575. [Google Scholar] [CrossRef]
- Martins, V.D.C.A.; De Bastos, M.S.; Ramasawmy, R.; De Figueiredo, R.P.; Gimaque, J.B.L.; Braga, W.S.M.; Nogueira, M.L.; Nozawa, S.; Naveca, F.G.; Figueiredo, L.T.M.; et al. Clinical and Virological Descriptive Study in the 2011 Outbreak of Dengue in the Amazonas, Brazil. PLoS ONE 2014, 9, e100535. [Google Scholar] [CrossRef]
- Bhatt, P.; Jayaram, A.; Varma, M.; Mukhopadhyay, C. Kinetics of Dengue Viremia and Its Association with Disease Severity: An Ambispective Study. VirusDisease 2024, 35, 250–259. [Google Scholar] [CrossRef]
- Phanthanawiboon, S.; Ekalaksananan, T.; Chuerduangphui, J.; Suwannatrai, A.T.; Aromseree, S.; Alexander, N.; Overgaard, H.J.; Thongchai, P.; Burassakarn, A.; Pientong, C. Prevalence and Characteristics of Dengue Virus Co-Infection in Patients and Mosquitoes Collected from Patients’ Houses. PLoS ONE 2025, 20, e0314553. [Google Scholar] [CrossRef]
- Sujitha, S.; Murugesan, R. Double Trouble: A Review of Dengue Virus Co-Infections and Their Impact on Disease Severity. Int. J. Entomol. Res. 2024, 9, 117–121. [Google Scholar]
- Dhanoa, A.; Hassan, S.S.; Ngim, C.F.; Lau, C.F.; Chan, T.S.; Adnan, N.A.A.; Eng, W.W.H.; Gan, H.M.; Rajasekaram, G. Impact of Dengue Virus (DENV) Co-Infection on Clinical Manifestations, Disease Severity and Laboratory Parameters. BMC Infect. Dis. 2016, 16, 406. [Google Scholar] [CrossRef]
- Dupont-Rouzeyrol, M.; O’Connor, O.; Calvez, E.; Daures, M.; John, M.; Grangeon, J.P.; Gourinat, A.C. Co-Infection with Zika and Dengue Viruses in 2 Patients, New Caledonia, 2014. Emerg. Infect. Dis. 2015, 21, 381. [Google Scholar] [CrossRef]
- Villamil-Gómez, W.E.; González-Camargo, O.; Rodriguez-Ayubi, J.; Zapata-Serpa, D.; Rodriguez-Morales, A.J. Dengue, Chikungunya and Zika Co-Infection in a Patient from Colombia. J. Infect. Public Health 2016, 9, 684–686. [Google Scholar] [CrossRef]
- Teng, Y.; Bi, D.; Xie, G.; Jin, Y.; Huang, Y.; Lin, B.; An, X.; Tong, Y.; Feng, D. Model-Informed Risk Assessment for Zika Virus Outbreaks in the Asia-Pacific Regions. J. Infect. 2017, 74, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.F.M.; Prudente, C.O.M.; de Queiróz, K.B.P. Motor Development of Children Exposed to The Zika Virus: Systematic Reviews. Rev. Bras. Saúde Matern. Infant. 2023, 22, 739–751. [Google Scholar] [CrossRef]
- Priyamvada, L.; Quicke, K.M.; Hudson, W.H.; Onlamoon, N.; Sewatanon, J.; Edupuganti, S.; Pattanapanyasat, K.; Chokephaibulkit, K.; Mulligan, M.J.; Wilson, P.C.; et al. Human Antibody Responses after Dengue Virus Infection Are Highly Cross-Reactive to Zika Virus. Proc. Natl. Acad. Sci. USA 2016, 113, 7852–7857. [Google Scholar] [CrossRef] [PubMed]
- Paul, L.M.; Carlin, E.R.; Jenkins, M.M.; Tan, A.L.; Barcellona, C.M.; Nicholson, C.O.; Michael, S.F.; Isern, S. Dengue Virus Antibodies Enhance Zika Virus Infection. Clin. Transl. Immunol. 2016, 5, e117. [Google Scholar] [CrossRef] [PubMed]
- Dejnirattisai, W.; Supasa, P.; Wongwiwat, W.; Rouvinski, A.; Barba-Spaeth, G.; Duangchinda, T.; Sakuntabhai, A.; Cao-Lormeau, V.M.; Malasit, P.; Rey, F.A.; et al. Dengue Virus Sero-Cross-Reactivity Drives Antibody-Dependent Enhancement of Infection with Zika Virus. Nat. Immunol. 2016, 17, 1102–1108. [Google Scholar] [CrossRef]
- Vannice, K.S.; Durbin, A.; Hombach, J. Status of Vaccine Research and Development of Vaccines for Dengue. Vaccine 2016, 34, 2934–2938. [Google Scholar] [CrossRef]
- Katzelnick, L.C.; Gresh, L.; Halloran, M.E.; Mercado, J.C.; Kuan, G.; Gordon, A.; Balmaseda, A.; Harris, E. Antibody-Dependent Enhancement of Severe Dengue Disease in Humans. Science 2017, 358, 929–932. [Google Scholar] [CrossRef]
- Halstead, S.B. Dengvaxia Sensitizes Seronegatives to Vaccine Enhanced Disease Regardless of Age. Vaccine 2017, 35, 6355–6358. [Google Scholar] [CrossRef]
- Pessoa, R.; Patriota, J.V.; De Lourdes De Souza, M.; Felix, A.C.; Mamede, N.; Sanabani, S.S. Investigation into an Outbreak of Dengue-like Illness in Pernambuco, Brazil, Revealed a Cocirculation of Zika, Chikungunya, and Dengue Virus Type 1. Medicine 2016, 95, e3201. [Google Scholar] [CrossRef]
- Chia, P.Y.; Yew, H.S.; Ho, H.; Chow, A.; Sadarangani, S.P.; Chan, M.; Kam, Y.W.; Chong, C.Y.; Thoon, K.C.; Yung, C.F.; et al. Clinical Features of Patients with Zika and Dengue Virus Co-Infection in Singapore. J. Infect. 2017, 74, 611–615. [Google Scholar] [CrossRef][Green Version]
- Heinen, L.B.d.S.; Zuchi, N.; Serra, O.P.; Cardoso, B.F.; Gondim, B.H.F.; dos Santos, M.A.M.; Souto, F.J.D.; de Paula, D.A.J.; Dutra, V.; Dezengrini-Slhessarenko, R. Saint Louis Encephalitis Virus in Mato Grosso, Central-Western Brazil. Rev. Inst. Med. Trop. Sao Paulo 2015, 57, 215. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.K.; Kumar, S.; Maurya, V.K.; Bhatt, M.L.B. The Global Distribution and Burden of Dengue and Japanese Encephalitis Co-Infection in Acute Encephalitis Syndrome. Curr. Top. Negl. Trop. Dis. 2019, 496, 504–507. [Google Scholar]
- Kumar, N.; Sharma, S.; Barua, S.; Tripathi, B.N.; Rouse, B.T. Virological and Immunological Outcomes of Coinfections. Clin. Microbiol. Rev. 2018, 31, e00111-17. [Google Scholar] [CrossRef] [PubMed]
- Ioos, S.; Mallet, H.P.; Leparc Goffart, I.; Gauthier, V.; Cardoso, T.; Herida, M. Current Zika Virus Epidemiology and Recent Epidemics. Med. Mal. Infect. 2014, 44, 302–307. [Google Scholar] [CrossRef]
- WHO. Handbook for Clinical Management of Dengue WHO and Special Programme for Research and Training in Tropical Diseases (TDR) Report; WHO: Geneva, Switzerland, 2012; p. 111. [Google Scholar]
- Made Susila Utama, I.; Lukman, N.; Sukmawati, D.D.; Alisjahbana, B.; Alam, A.; Murniati, D.; Made Gede Dwi Lingga Utama, I.; Puspitasari, D.; Kosasih, H.; Laksono, I.; et al. Dengue Viral Infection in Indonesia: Epidemiology, Diagnostic Challenges, and Mutations from an Observational Cohort Study. PLoS Negl Trop Dis 2019, 13, e0007785. [Google Scholar] [CrossRef]
- Lin, D.C.D.; Weng, S.C.; Tsao, P.N.; Chu, J.J.H.; Shiao, S.H. Co-Infection of Dengue and Zika Viruses Mutually Enhances Viral Replication in the Mosquito Aedes Aegypti. Parasit. Vectors 2023, 16, 160. [Google Scholar] [CrossRef]
- Agarwal, A.; Parida, M.; Dash, P.K. Impact of Transmission Cycles and Vector Competence on Global Expansion and Emergence of Arboviruses. Rev. Med. Virol. 2017, 27, e1941. [Google Scholar] [CrossRef]
- Wu, P.; Yu, X.; Wang, P.; Cheng, G. Arbovirus Lifecycle in Mosquito: Acquisition, Propagation and Transmission. Expert. Rev. Mol. Med. 2019, 21, e1. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, J.; Wei, Y.H.; Song, Z.; Hu, K.; Chen, Y.; Zhou, G.; Zhong, D.; Zheng, X. Vector Competence for DENV-2 Among Aedes Albopictus (Diptera: Culicidae) Populations in China. Front. Cell Infect. Microbiol. 2021, 11, 649975. [Google Scholar] [CrossRef]
- Bellone, R.; Failloux, A.B. The Role of Temperature in Shaping Mosquito-Borne Viruses Transmission. Front. Microbiol. 2020, 11, 584846. [Google Scholar] [CrossRef]
- Tahir, F.; Bansal, D.; Rehman, A.U.; Ajjur, S.B.; Skariah, S.; Belhaouari, S.B.; Al-Romaihi, H.; Al-Thani, M.H.J.; Farag, E.; Sultan, A.A.; et al. Assessing the Impact of Climate Conditions on the Distribution of Mosquito Species in Qatar. Front. Public Health 2023, 10, 970694. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.J.; Pascual, M.; Wimberly, M.C.; Johnson, L.R.; Murdock, C.C. Humidity—The Overlooked Variable in the Thermal Biology of Mosquito-Borne Disease. Ecol. Lett. 2023, 26, 1029. [Google Scholar] [CrossRef]
- Tuiskunen Bäck, A.; Lundkvist, Å. Dengue Viruses—An Overview. Infect. Ecol. Epidemiol. 2013, 3, 19839. [Google Scholar] [CrossRef]
- Soo, K.M.; Khalid, B.; Ching, S.M.; Chee, H.Y. Meta-Analysis of Dengue Severity during Infection by Different Dengue Virus Serotypes in Primary and Secondary Infections. PLoS ONE 2016, 11, e0154760. [Google Scholar] [CrossRef]
- Tazeen, A.; Afreen, N.; Abdullah, M.; Deeba, F.; Haider, S.H.; Kazim, S.N.; Ali, S.; Naqvi, I.H.; Broor, S.; Ahmed, A.; et al. Occurrence of Co-Infection with Dengue Viruses during 2014 in New Delhi, India. Epidemiol. Infect. 2017, 145, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, B.; Sunder, A.; Pathak, S. Clinicolaboratory Profile of Expanded Dengue Syndrome—Our Experience in a Teaching Hospital. J. Fam. Med. Prim. Care 2019, 8, 1022. [Google Scholar] [CrossRef] [PubMed]
- Turuk, J.; Palo, S.; Rath, S.; Subhadra, S.; Sabat, J.; Sahoo, P.; Panda, S.; Pati, S. Viral Characteristics and Clinical Presentation in Dengue Co-Infection- Findings from a Facility Based Observational Study in Odisha, India. J. Fam. Med. Prim. Care 2021, 10, 2958. [Google Scholar] [CrossRef]
- Briseño-García, B.; Gómez-Dantés, H.; Argott-Ramírez, E.; Montesano, R.; Vázquez-Martínez, A.L.; Ibáñez-Bernal, S.; Madrigal-Ayala, G.; Ruíz-Matus, C.; Flisser, A.; Tapia-Conyer, R. Potential Risk for Dengue Hemorrhagic Fever: The Isolation of Serotype Dengue-3 in Mexico. Emerg. Infect. Dis. 1996, 2, 133. [Google Scholar] [CrossRef]
- Laille, M.; Deubel, V.; Sainte-Marie, F.F. Demonstration of Concurrent Dengue 1 and Dengue 3 Infection in Six Patients by the Polymerase Chain Reaction. J. Med. Virol. 1991, 34, 51–54. [Google Scholar] [CrossRef]
- Mishra, B.; Turuk, J.; Sahu, S.J.; Khajuria, A.; Kumar, S.; Dey, A.; Praharaj, A.K. Co-Circulation of All Four Dengue Virus Serotypes: First Report from Odisha. Indian. J. Med. Microbiol. 2017, 35, 293–295. [Google Scholar] [CrossRef]
- Thai, K.T.D.; Nishiura, H.; Hoang, P.L.; Tran, N.T.T.; Phan, G.T.; Le, H.Q.; Tran, B.Q.; Van Nguyen, N.; de Vries, P.J. Age-Specificity of Clinical Dengue during Primary and Secondary Infections. PLoS Negl. Trop. Dis. 2011, 5, e1180. [Google Scholar] [CrossRef] [PubMed]
- Anoop, M.; Issac, A.; Mathew, T.; Philip, S.; Kareem, N.A.; Unnikrishnan, R.; Sreekumar, E. Genetic Characterization of Dengue Virus Serotypes Causing Concurrent Infection in an Outbreak in Ernakulam, Kerala, South India. Indian. J. Exp. Biol. 2010, 48, 849–857. [Google Scholar]
- Fahri, S.; Yohan, B.; Trimarsanto, H.; Sayono, S.; Hadisaputro, S.; Dharmana, E.; Syafruddin, D.; Sasmono, R.T. Molecular Surveillance of Dengue in Semarang, Indonesia Revealed the Circulation of an Old Genotype of Dengue Virus Serotype-1. PLoS Negl. Trop. Dis. 2013, 7, e2354. [Google Scholar] [CrossRef] [PubMed]
- Lardo, S.; Utami, Y.; Yohan, B.; Tarigan, S.M.M.U.; Santoso, W.D.; Nainggolan, L.; Sasmono, R.T. Concurrent Infections of Dengue Viruses Serotype 2 and 3 in Patient with Severe Dengue from Jakarta, Indonesia. Asian Pac. J. Trop. Med. 2016, 9, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Chahar, H.S.; Bharaj, P.; Dar, L.; Guleria, R.; Kabra, S.K.; Broor, S. Co-Infections with Chikungunya Virus and Dengue Virus in Delhi, India. Emerg. Infect. Dis. 2009, 15, 1077. [Google Scholar] [CrossRef] [PubMed]
- Pinto Junior, V.L.; Luz, K.; Parreira, R.; Ferrinho, P. Zika Virus: A Review to Clinicians. Acta Med. Port. 2015, 28, 760–765. [Google Scholar] [CrossRef]
- Stamm, L.V. Zika Virus in the Americas: An Obscure Arbovirus Comes Calling. JAMA Dermatol. 2016, 152, 621–622. [Google Scholar] [CrossRef]
- Suwanmanee, S.; Luplertlop, N. Dengue and Zika Viruses: Lessons Learned from the Similarities between These Aedes Mosquito-Vectored Arboviruses. J. Microbiol. 2017, 55, 81–89. [Google Scholar] [CrossRef]
- Mercado-Reyes, M.; Acosta-Reyes, J.; Navarro-Lechuga, E.; Corchuelo, S.; Rico, A.; Parra, E.; Tolosa, N.; Pardo, L.; González, M.; Martìn-Rodriguez-Hernández, J.; et al. Dengue, Chikungunya and Zika Virus Coinfection: Results of the National Surveillance during the Zika Epidemic in Colombia. Epidemiol. Infect. 2019, 147, e77. [Google Scholar] [CrossRef]
- Bang, Y.X.; Sher, G.L.; Richard, T.H.T.; Farhad, F.V. A Case Series of Atypical Presentation of Zika Virus Infection in Singapore. BMC Infect. Dis. 2016, 16, 681. [Google Scholar] [CrossRef]
- Borawake, K.; Prayag, P.; Wagh, A.; Dole, S. Dengue Encephalitis. Indian J. Crit. Care Med. 2011, 15, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Kathuria, R.; Choudhary, P.; Kashyap, A.; Ansari, E.; Kamal, M. Dengue Virus and Japanese Encephalitis Virus Co-Infection: A Case Report. IP Int. J. Med. Paediatr. Oncol. 2020, 6, 169–171. [Google Scholar] [CrossRef]
- Sivamani, K.; Dhir, V.; Singh, S.; Sharma, A. Diagnostic Dilemma—Dengue or Japanese Encephalitis? Neurol. India 2017, 65, 105–107. [Google Scholar] [CrossRef] [PubMed]
- Laureti, M.; Narayanan, D.; Rodriguez-Andres, J.; Fazakerley, J.K.; Kedzierski, L. Flavivirus Receptors: Diversity, Identity, and Cell Entry. Front. Immunol. 2018, 9, 396205. [Google Scholar] [CrossRef]
- Salas-Benito, J.S.; De Nova-Ocampo, M. Viral Interference and Persistence in Mosquito-Borne Flaviviruses. J. Immunol. Res. 2015, 2015, 73404. [Google Scholar] [CrossRef]
- Singh, K.P.; Mishra, G.; Jain, P.; Pandey, N.; Nagar, R.; Gupta, S.; Prakash, S.; Prakash, O.; Khan, D.N.; Shrivastav, S.; et al. Co-Positivity of Anti-Dengue Virus and Anti-Japanese Encephalitis Virus IgM in Endemic Area: Co-Infection or Cross Reactivity? Asian Pac. J. Trop. Med. 2014, 7, 124–129. [Google Scholar] [CrossRef]
- Mondini, A.; Bronzoni, R.V.d.M.; Cardeal, I.L.S.; dos Santos, T.M.I.L.; Lázaro, E.; Nunes, S.H.P.; Silva, G.C.D.; Madrid, M.C.F.S.; Rahal, P.; Figueiredo, L.T.; et al. Simultaneous Infection by DENV-3 and SLEV in Brazil. J. Clin. Virol. 2007, 40, 84–86. [Google Scholar] [CrossRef]
- Bifani, A.M.; Siriphanitchakorn, T.; Choy, M.M. Intra-Host Diversity of Dengue Virus in Mosquito Vectors. Front. Cell Infect. Microbiol. 2022, 12, 888804. [Google Scholar] [CrossRef]
- Yong, Y.K.; Wong, W.F.; Vignesh, R.; Chattopadhyay, I.; Velu, V.; Tan, H.Y.; Zhang, Y.; Larsson, M.; Shankar, E.M. Dengue Infection—Recent Advances in Disease Pathogenesis in the Era of COVID-19. Front. Immunol. 2022, 13, 889196. [Google Scholar] [CrossRef]
- Herencia, J.S.S. Lessons Learned and Recent Advances in Dengue Research. In Dengue Fever in a One Health Perspective; Intech Open: London, UK, 2020. [Google Scholar] [CrossRef]
- WHO; TDR. Dengue Guidelines, for Diagnosis, Treatment, Prevention and Control; World Health Organization: Geneva, Switzerland, 2009; Volume 41, p. 29. [Google Scholar]
- Beltrán-Silva, S.L.; Chacón-Hernández, S.S.; Moreno-Palacios, E.; Pereyra-Molina, J.Á. Clinical and Differential Diagnosis: Dengue, Chikungunya and Zika. Rev. Méd Hosp. General. México 2018, 81, 146–153. [Google Scholar] [CrossRef]
- World Health Organization. Comprehensive Guideline for Prevention and Control of Dengue and Dengue Haemorrhagic Fever; Revised and Expanded Edition; World Health Organization: Geneva, Switzerland, 2011; ISBN 978-92-9022-387-0. [Google Scholar]
- Prommool, T.; Sethanant, P.; Phaenthaisong, N.; Tangthawornchaikul, N.; Songjaeng, A.; Avirutnan, P.; Mairiang, D.; Luangaram, P.; Srisawat, C.; Kasinrerk, W.; et al. High Performance Dengue Virus Antigen-Based Serotyping-NS1-ELISA (plus): A Simple Alternative Approach to Identify Dengue Virus Serotypes in Acute Dengue Specimens. PLoS Negl. Trop. Dis. 2021, 15, e0009065. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.G.; Jaenisch, T.; Gaczkowski, R.; Hang, V.T.T.; Sekaran, S.D.; Kroeger, A.; Vazquez, S.; Ruiz, D.; Martinez, E.; Mercado, J.C.; et al. Multi-Country Evaluation of the Sensitivity and Specificity of Two Commercially-Available NS1 ELISA Assays for Dengue Diagnosis. PLoS Negl. Trop. Dis. 2010, 4, e811. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.K.; Wong, W.Y.; Yang, H.T.; Huber, R.G.; Bond, P.J.; Ng, L.C.; Maurer-Stroh, S.; Hapuarachchi, H.C. Flavivirus Cross-Reactivity to Dengue Nonstructural Protein 1 Antigen Detection Assays. Diagnostics 2019, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Coronel-Ruiz, C.; Velandia-Romero, M.L.; Calvo, E.; Camacho-Ortega, S.; Parra-Alvarez, S.; Beltrán-Zuñiga, E.; Calderón-Pelaez, M.-A.; Cortés-Muñoz, F.; Rojas-Hernandez, J.P.; Velasco-Alvarez, S.; et al. Improving Dengue Case Confirmation by Combining Rapid Diagnostic Test, Clinical, and Laboratory Variables. medRxiv 2021, 2021.05.21.21257609. [Google Scholar] [CrossRef]
- Luvira, V.; Thawornkuno, C.; Lawpoolsri, S.; Thippornchai, N.; Duangdee, C.; Ngamprasertchai, T.; Leaungwutiwong, P. Diagnostic Performance of Dengue NS1 and Antibodies by Serum Concentration Technique. Trop. Med. Infect. Dis. 2023, 8, 117. [Google Scholar] [CrossRef]
- Lai, S.C.; Huang, Y.Y.; Wey, J.J.; Tsai, M.H.; Chen, Y.L.; Shu, P.Y.; Chang, S.F.; Hung, Y.J.; Hou, J.N.; Lin, C.C. Development of Novel Dengue NS1 Multiplex Lateral Flow Immunoassay to Differentiate Serotypes in Serum of Acute Phase Patients and Infected Mosquitoes. Front. Immunol. 2022, 13, 852452. [Google Scholar] [CrossRef]
- World Health Organisation Dengue and Severe Dengue. Available online: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue?utm_source=chatgpt.com (accessed on 19 February 2025).
- Yung, C.F.; Lee, K.S.; Thein, T.L.; Tan, L.K.; Gan, V.C.; Wong, J.G.X.; Lye, D.C.; Ng, L.C.; Leo, Y.S. Dengue Serotype-Specific Differences in Clinical Manifestation, Laboratory Parameters and Risk of Severe Disease in Adults, Singapore. Am. J. Trop. Med. Hyg. 2015, 92, 999. [Google Scholar] [CrossRef]
- San Diego, M.J.; Sayo, A.; EDRADA, E. 645. Risk of Disease Severity and Disease Outcomes with Serotype-Specific Dengue Virus among Hospitalized Dengue Patients in a Tertiary Infectious Diseases Hospital: A Five-Year Retrospective Study. Open Forum Infect. Dis. 2023, 10, ofad500.709. [Google Scholar] [CrossRef]
- Rocha, B.A.M.; Guilarde, A.O.; Argolo, A.F.L.T.; Tassara, M.P.; da Silveira, L.A.; Junqueira, I.C.; Turchi, M.D.; Féres, V.C.R.; Martelli, C.M.T. Dengue-Specific Serotype Related to Clinical Severity during the 2012/2013 Epidemic in Centre of Brazil. Infect. Dis. Poverty 2017, 6, 73–83. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, K.; Sidhu, S.K.; Devi, P.; Kaur, M.; Soneja, S.; Singh, N. Coinfection of Chikungunya and Dengue Viruses: A Serological Study from North Western Region of Punjab, India. J. Lab. Physicians 2018, 10, 443. [Google Scholar] [CrossRef]
- Cao-Lormeau, V.M.; Blake, A.; Mons, S.; Lastère, S.; Roche, C.; Vanhomwegen, J.; Dub, T.; Baudouin, L.; Teissier, A.; Larre, P.; et al. Guillain-Barré Syndrome Outbreak Associated with Zika Virus Infection in French Polynesia: A Case-Control Study. Lancet 2016, 387, 1531–1539. [Google Scholar] [CrossRef]
- Timiryasova, T.M.; Bonaparte, M.I.; Luo, P.; Zedar, R.; Hu, B.T.; Hildreth, S.W. Optimization and Validation of a Plaque Reduction Neutralization Test for the Detection of Neutralizing Antibodies to Four Serotypes of Dengue Virus Used in Support of Dengue Vaccine Development. Am. J. Trop. Med. Hyg. 2013, 88, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Loyola, S.; Huaman, A.; Popuche, D.; Castillo, E.; Ampuero, J.S.; Silva, M.; Guevara, C.; Watts, D.M. Evaluation of Two Serological Assays for Diagnosing Zika Virus Infection. Diagnostics 2021, 11, 1696. [Google Scholar] [CrossRef] [PubMed]
- Panning, M. Zika Virus Serology: More Diagnostic Targets, More Reliable Answers? EBioMedicine 2017, 16, 12–13. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shan, C.; Xie, X.; Ren, P.; Loeffelholz, M.J.; Yang, Y.; Furuya, A.; Dupuis, A.P.; Kramer, L.D.; Wong, S.J.; Shi, P.Y. A Rapid Zika Diagnostic Assay to Measure Neutralizing Antibodies in Patients. EBioMedicine 2017, 17, 157–162. [Google Scholar] [CrossRef]
- Favresse, J.; Gillot, C.; Oliveira, M.; Cadrobbi, J.; Elsen, M.; Eucher, C.; Laffineur, K.; Rosseels, C.; Van Eeckhoudt, S.; Nicolas, J.B.; et al. Head-to-Head Comparison of Rapid and Automated Antigen Detection Tests for the Diagnosis of SARS-CoV-2 Infection. J. Clin. Med. 2021, 10, 265. [Google Scholar] [CrossRef]
- Bruzzone, B.; De Pace, V.; Caligiuri, P.; Ricucci, V.; Guarona, G.; Pennati, B.M.; Boccotti, S.; Orsi, A.; Domnich, A.; Da Rin, G.; et al. Comparative Diagnostic Performance of Rapid Antigen Detection Tests for COVID-19 in a Hospital Setting. Int. J. Infect. Dis. 2021, 107, 215. [Google Scholar] [CrossRef]
- Chao, D.Y.; Davis, B.S.; Chang, G.J.J. Development of Multiplex Real-Time Reverse Transcriptase PCR Assays for Detecting Eight Medically Important Flaviviruses in Mosquitoes. J. Clin. Microbiol. 2007, 45, 584–589. [Google Scholar] [CrossRef]
- Pabbaraju, K.; Wong, S.; Gill, K.; Fonseca, K.; Tipples, G.A.; Tellier, R. Simultaneous Detection of Zika, Chikungunya and Dengue Viruses by a Multiplex Real-Time RT-PCR Assay. J. Clin. Virol. 2016, 83, 66–71. [Google Scholar] [CrossRef]
- Xu, Z.; Peng, Y.; Yang, M.; Li, X.; Wang, J.; Zou, R.; Liang, J.; Fang, S.; Liu, Y.; Yang, Y. Simultaneous Detection of Zika, Chikungunya, Dengue, Yellow Fever, West Nile, and Japanese Encephalitis Viruses by a Two-Tube Multiplex Real-Time RT-PCR Assay. J. Med. Virol. 2022, 94, 2528–2536. [Google Scholar] [CrossRef]
- Wee, S.; Alli-Shaik, A.; Kek, R.; Swa, H.L.F.; Tien, W.P.; Lim, V.W.; Leo, Y.S.; Ng, L.C.; Hapuarachchi, H.C.; Gunaratne, J. Multiplex Targeted Mass Spectrometry Assay for One-Shot Flavivirus Diagnosis. Proc. Natl. Acad. Sci. USA 2019, 116, 6754–6759. [Google Scholar] [CrossRef] [PubMed]
- Shariq, M.; Khan, M.F.; Raj, R.; Ahsan, N.; Singh, R.; Kumar, P. CRISPR-based Diagnostic Approaches: Implications for Rapid Management of Future Pandemics (Review). Mol. Med. Rep. 2023, 27, 118. [Google Scholar] [CrossRef]
- Letizia, A.G.; Pratt, C.B.; Wiley, M.R.; Fox, A.T.; Mosore, M.; Agbodzi, B.; Yeboah, C.; Kumordjie, S.; Di Paola, N.; Assana, K.C.; et al. Retrospective Genomic Characterization of a 2017 Dengue Virus Outbreak, Burkina Faso. Emerg. Infect. Dis. 2022, 28, 1198–1210. [Google Scholar] [CrossRef] [PubMed]
- Reteng, P.; Nguyen Thuy, L.; Tran Thi Minh, T.; Mares-Guia, M.A.M.d.M.; Torres, M.C.; de Filippis, A.M.B.; Orba, Y.; Kobayashi, S.; Hayashida, K.; Sawa, H.; et al. A Targeted Approach with Nanopore Sequencing for the Universal Detection and Identification of Flaviviruses. Sci. Rep. 2021, 11, 19031. [Google Scholar] [CrossRef] [PubMed]
- Frazer, J.L.; Norton, R. Dengue: A Review of Laboratory Diagnostics in the Vaccine Age. J. Med. Microbiol. 2024, 73, 1833. [Google Scholar] [CrossRef]
- Kelly-Cirino, C.D.; Nkengasong, J.; Kettler, H.; Tongio, I.; Gay-Andrieu, F.; Escadafal, C.; Piot, P.; Peeling, R.W.; Gadde, R.; Boehme, C. Importance of Diagnostics in Epidemic and Pandemic Preparedness. BMJ Glob. Health 2019, 4, 1179. [Google Scholar] [CrossRef]
- Badolato-Corrêa, J.; Sánchez-Arcila, J.C.; Alves de Souza, T.M.; Santos Barbosa, L.; Conrado Guerra Nunes, P.; da Rocha Queiroz Lima, M.; Gandini, M.; Bispo de Filippis, A.M.; Venâncio da Cunha, R.; Leal de Azeredo, E.; et al. Human T Cell Responses to Dengue and Zika Virus Infection Compared to Dengue/Zika Coinfection. Immun. Inflamm. Dis. 2018, 6, 194–206. [Google Scholar] [CrossRef]
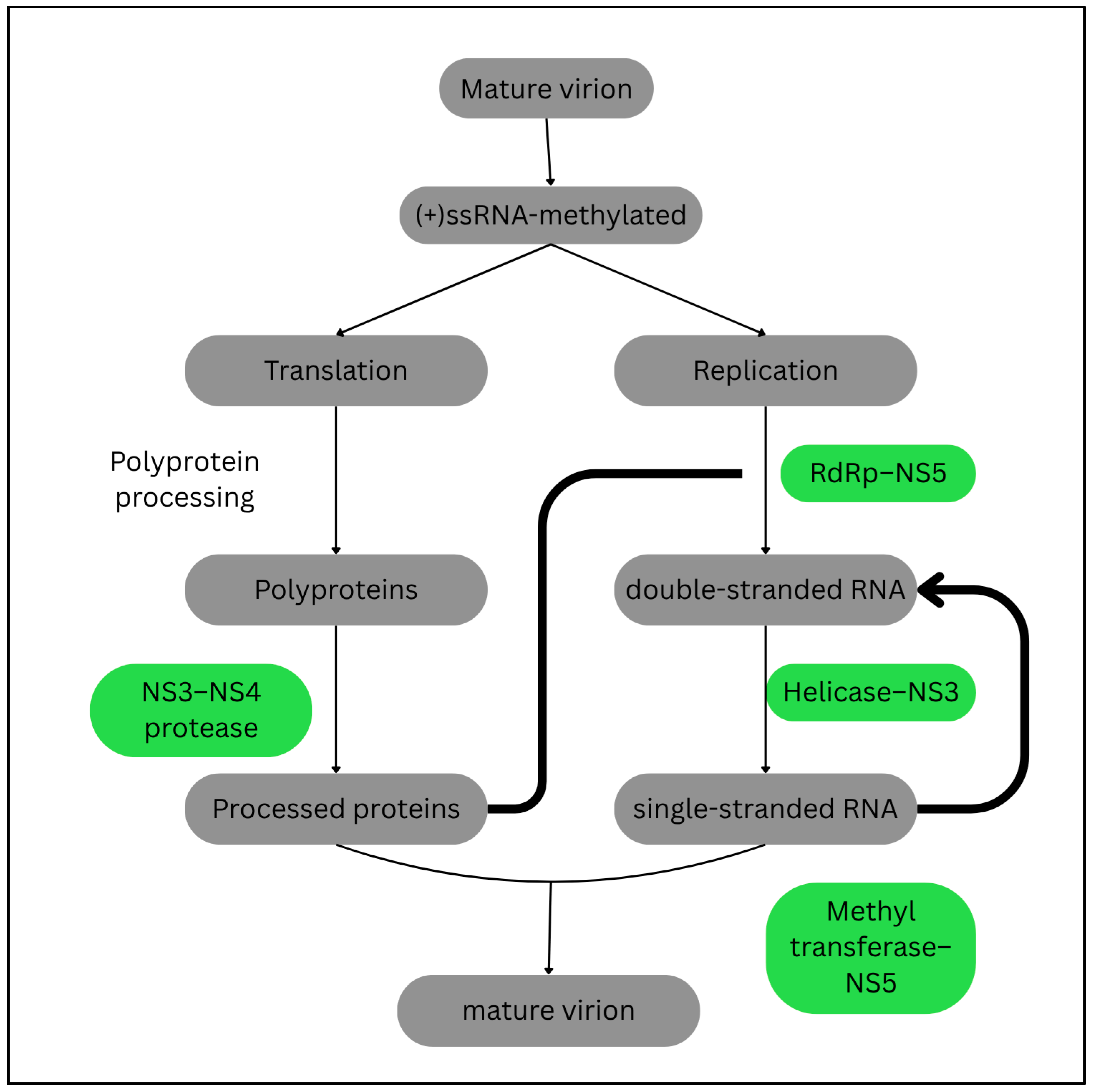
- Hollidge, B.S.; Weiss, S.R.; Soldan, S.S. The Role of Interferon Antagonist, Non-Structural Proteins in the Pathogenesis and Emergence of Arboviruses. Viruses 2011, 3, 629. [Google Scholar] [CrossRef]
- Jayachandran, B.; Chanda, K.; Balamurali, M.M. Overview of Pathogenesis, Diagnostics, and Therapeutics of Infectious Diseases: Dengue and Zika. ACS Omega 2021, 6, 22487. [Google Scholar] [CrossRef]
- Blackard, J.T.; Cohen, D.E.; Mayer, K.H. Human Immunodeficiency Virus Superinfection and Recombination: Current State of Knowledge and Potential Clinical Consequences. Clin. Infect. Dis. 2002, 34, 1108–1114. [Google Scholar] [CrossRef]
- Chuang, F.K.; Liao, C.L.; Hu, M.K.; Chiu, Y.L.; Lee, A.R.; Huang, S.M.; Chiu, Y.L.; Tsai, P.L.; Su, B.C.; Chang, T.H.; et al. Antiviral Activity of Compound L3 against Dengue and Zika Viruses In Vitro and In Vivo. Int. J. Mol. Sci. 2020, 21, 4050. [Google Scholar] [CrossRef] [PubMed]
- Bonyah, E.; Khan, M.A.; Okosun, K.O.; Gómez-Aguilar, J.F. On the Co-Infection of Dengue Fever and Zika Virus. Optim. Control Appl. Methods 2019, 40, 394–421. [Google Scholar] [CrossRef]
- Chew, M.F.; Poh, K.S.; Poh, C.L. Peptides as Therapeutic Agents for Dengue Virus. Int. J. Med. Sci. 2017, 14, 1342–1359. [Google Scholar] [CrossRef] [PubMed]
- Dando, T.M.; Perry, C.M. Enfuvirtide. Drugs 2012, 63, 2755–2766. [Google Scholar] [CrossRef]
- Songprakhon, P.; Thaingtamtanha, T.; Limjindaporn, T.; Puttikhunt, C.; Srisawat, C.; Luangaram, P.; Dechtawewat, T.; Uthaipibull, C.; Thongsima, S.; Yenchitsomanus, P.-T.; et al. Peptides Targeting Dengue Viral Nonstructural Protein 1 Inhibit Dengue Virus Production. Sci. Rep. 2020, 10, 12933. [Google Scholar] [CrossRef]
- Kaptein, S.J.F.; Goethals, O.; Kiemel, D.; Marchand, A.; Kesteleyn, B.; Bonfanti, J.F.; Bardiot, D.; Stoops, B.; Jonckers, T.H.M.; Dallmeier, K.; et al. A Pan-Serotype Dengue Virus Inhibitor Targeting the NS3–NS4B Interaction. Nature 2021, 598, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Ajmal, A.; Shahab, M.; Waqas, M.; Zheng, G.; Zulfat, M.; Bin Jardan, Y.A.; Wondmie, G.F.; Bourhia, M.; Ali, I. In Silico Design of Peptide Inhibitors for Dengue Virus to Treat Dengue Virus-Associated Infections. Sci. Rep. 2024, 14, 13130. [Google Scholar] [CrossRef]
- Hrobowski, Y.M.; Garry, R.F.; Michael, S.F. Peptide Inhibitors of Dengue Virus and West Nile Virus Infectivity. Virol. J. 2005, 2, 49. [Google Scholar] [CrossRef]
- Behrouz, S.; Kühl, N.; Klein, C.D. N-Sulfonyl Peptide-Hybrids as a New Class of Dengue Virus Protease Inhibitors. Eur. J. Med. Chem. 2023, 251, 115227. [Google Scholar] [CrossRef]
- Huang, Y.-J.; Cheng, T.-L.; Wang, Y.-T.; Chen, C.-S.; Leu, Y.-L.; Chang, C.-S.; Ho, C.-H.; Chao, S.-W.; Li, C.-T.; Chuang, C.-H. Exploring the Therapeutic Potential of DV-B-120 as an Inhibitor of Dengue Virus Infection. J. Virol. 2024, 98, e0125823. [Google Scholar] [CrossRef]
- Lin, C.S.; Lu, C.H.; Lin, T.H.; Kiu, Y.T.; Kan, J.Y.; Chang, Y.J.; Hung, P.Y.; Koval’skaya, A.V.; Tsypyshev, D.O.; Tsypysheva, I.P.; et al. Inhibition of Dengue Viruses by N-Methylcytisine Thio Derivatives through Targeting Viral Envelope Protein and NS2B-NS3 Protease. Bioorg Med. Chem. Lett. 2024, 99, 129623. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.T.N.; Hvasta, M.G.; Kudlacek, S.T.; Thiono, D.J.; Tripathy, A.; Nicely, N.I.; de Silva, A.M.; Kuhlman, B. A Conserved Set of Mutations for Stabilizing Soluble Envelope Protein Dimers from Dengue and Zika Viruses to Advance the Development of Subunit Vaccines. J. Biol. Chem. 2022, 298, 102079. [Google Scholar] [CrossRef]
- Martínez, M.A. RNA Interference and Viruses: Current Innovations and Future Trends; Caister Academic Press: Poole, UK, 2010. [Google Scholar]
- Wu, X.; Hong, H.; Yue, J.; Wu, Y.; Li, X.; Jiang, L.; Li, L.; Li, Q.; Gao, G.; Yang, X. Inhibitory Effect of Small Interfering RNA on Dengue Virus Replication in Mosquito Cells. Virol. J. 2010, 7, 270. [Google Scholar] [CrossRef]
- Ma, Y.; Chan, C.Y.; He, M.L. RNA Interference and Antiviral Therapy. World J. Gastroenterol. WJG 2007, 13, 5169. [Google Scholar] [CrossRef] [PubMed]
- Leggewie, M.; Schnettler, E. The Aedes Aegypti RNA Interference Response against Zika Virus in the Context of Co-Infection with Dengue and Chikungunya Viruses. PLoS Neglected Trop. Dis. 2023, 17, e0011456. [Google Scholar] [CrossRef] [PubMed]
- Contreras, D.; Arumugaswami, V. Zika Virus Infectious Cell Culture System and the In Vitro Prophylactic Effect of Interferons. J. Vis. Exp. 2016, 2016, 54767. [Google Scholar] [CrossRef]
- Munjal, A.; Khandia, R.; Dhama, K.; Sachan, S.; Karthik, K.; Tiwari, R.; Malik, Y.S.; Kumar, D.; Singh, R.K.; Iqbal, H.M.N.; et al. Advances in Developing Therapies to Combat Zika Virus: Current Knowledge and Future Perspectives. Front. Microbiol. 2017, 8, 1469. [Google Scholar] [CrossRef]
- Lai, Z.-Z.; Ho, Y.-J.; Lu, J.-W.; Jang, J.; Chu, H.; Mok, C.K. Harringtonine Inhibits Zika Virus Infection through Multiple Mechanisms. Molecules 2020, 25, 4082. [Google Scholar] [CrossRef]
- Felicetti, T.; Manfroni, G.; Cecchetti, V.; Cannalire, R. Broad-Spectrum Flavivirus Inhibitors: A Medicinal Chemistry Point of View. ChemMedChem 2020, 15, 2391–2419. [Google Scholar] [CrossRef]
- Vicenti, I.; Martina, M.G.; Boccuto, A.; De Angelis, M.; Giavarini, G.; Dragoni, F.; Marchi, S.; Trombetta, C.M.; Crespan, E.; Maga, G.; et al. System-Oriented Optimization of Multi-Target 2,6-Diaminopurine Derivatives: Easily Accessible Broad-Spectrum Antivirals Active against Flaviviruses, Influenza Virus and SARS-CoV-2. Eur. J. Med. Chem. 2021, 224, 113683. [Google Scholar] [CrossRef]
- Michiaki, M.; Tomohiro, I.; Satofumi, K.; Motoyasu, T.; Yasufumi, W. Therapeutic Agent for Flavivirus Infection. US20220193124A1, 24 April 2020. [Google Scholar]
- Valencia, H.J.; de Aguiar, M.C.A.M.; Costa, M.A.; Mendonça, D.C.; Reis, E.V.; Arias, N.E.C.; Drumond, B.P.; Bonjardim, C.A. Evaluation of Kinase Inhibitors as Potential Therapeutics for Flavivirus Infections. Arch. Virol. 2021, 166, 1433–1438. [Google Scholar] [CrossRef] [PubMed]
- Wilder-Smith, A. TAK-003 Dengue Vaccine as a New Tool to Mitigate Dengue in Countries with a High Disease Burden. Lancet Glob. Health 2024, 12, e179–e180. [Google Scholar] [CrossRef] [PubMed]
- Dengvaxia® Vaccine Overview and Updates|Sanofi. Available online: https://www.sanofi.com/en/our-company/sustainability/responsible-business-values/information-on-dengvaxia (accessed on 26 June 2025).
- Wilder-Smith, A. Controlled Human Infection Study Underpins Efficacy of the Tetravalent Live-Attenuated Dengue Vaccine TV005. J. Clin. Investig. 2024, 134, e177610. [Google Scholar] [CrossRef] [PubMed]
- Zika Vaccine VLA1601—Vax-Before-Travel. Available online: https://www.vax-before-travel.com/vaccines/zika-vaccine-vla1601 (accessed on 26 June 2025).
- Japanese Encephalitis Virus Vaccine SA14-14-2 Uses, Side Effects & Warnings. Available online: https://www.drugs.com/mtm/japanese-encephalitis-virus-vaccine-sa14-14-2.html (accessed on 26 June 2025).
- Oreshkova, N.; Myeni, S.K.; Mishra, N.; Albulescu, I.C.; Dalebout, T.J.; Snijder, E.J.; Bredenbeek, P.J.; Dallmeier, K.; Kikkert, M. A Yellow Fever 17D Virus Replicon-Based Vaccine Platform for Emerging Coronaviruses. Vaccines 2021, 9, 1492. [Google Scholar] [CrossRef]
- Wang, Y.; Ling, L.; Zhang, Z.; Marin-Lopez, A. Current Advances in Zika Vaccine Development. Vaccines 2022, 10, 1816. [Google Scholar] [CrossRef]
- Valneva Commences Trial of Second-Generation Zika Vaccine. Available online: https://www.clinicaltrialsarena.com/news/valneva-trial-zika-vaccine/ (accessed on 26 June 2025).
- University of Miami to Begin Phase 2 Zika Vaccine Trial. Available online: https://news.miami.edu/stories/2017/03/university-to-begin-phase-2-zika-vaccine-trial.html (accessed on 26 June 2025).
- Wollner, C.J.; Richner, J.M. MRNA Vaccines against Flaviviruses. Vaccines 2021, 9, 148. [Google Scholar] [CrossRef]
- Rahman, N.A.A.; Fuaad, A.A.H.A.; Azami, N.A.M.; Amin, M.C.I.M.; Azmi, F. Next-Generation Dengue Vaccines: Leveraging Peptide-Based Immunogens and Advanced Nanoparticles as Delivery Platforms. J. Pharm. Sci. 2024, 113, 2044–2054. [Google Scholar] [CrossRef]
- Geerling, E.; Steffen, T.L.; Brien, J.D.; Pinto, A.K. Current Flavivirus Research Important for Vaccine Development. Vaccines 2020, 8, 477. [Google Scholar] [CrossRef]
- Adekola, H.A.; Onajobi, I.B.; Egberongbe, H.O.; Samson, O.J.; Kareem, W.A.; Osipitan, G.O.; Adekola, R.A. Vaccine Candidates for Arboviruses with Pandemic Potential: A Mini Review. Microbiol Infect Dis AMJ Microbiol. Infect. Dis. 2023, 8, 9. [Google Scholar] [CrossRef]
- Doets, K.; Pijlman, G.P. Subgenomic Flavivirus RNA as Key Target for Live-Attenuated Vaccine Development. J. Virol. 2024, 98, e0010023. [Google Scholar] [CrossRef]
- Li, S.-H.; Dong, H.; Li, X.-F.; Xie, X.; Zhao, H.; Deng, Y.-Q.; Wang, X.-Y.; Ye, Q.; Zhu, S.-Y.; Wang, H.-J.; et al. Rational Design of a Flavivirus Vaccine by Abolishing Viral RNA 2′- O Methylation. J. Virol. 2013, 87, 5812–5819. [Google Scholar] [CrossRef] [PubMed]
- Dejnirattisai, W.; Jumnainsong, A.; Onsirisakul, N.; Fitton, P.; Vasanawathana, S.; Limpitikul, W.; Puttikhunt, C.; Edwards, C.; Duangchinda, T.; Supasa, S.; et al. Cross-Reacting Antibodies Enhance Dengue Virus Infection in Humans. Science 2010, 328, 745–748. [Google Scholar] [CrossRef] [PubMed]
- Pierson, T.C.; Diamond, M.S. The Continued Threat of Emerging Flaviviruses. Nat. Microbiol. 2020, 5, 796–812. [Google Scholar] [CrossRef] [PubMed]
| Disease | Dengue | Chikugunya | Zika |
|---|---|---|---|
| Fever | ++++ | +++ | +++ |
| Myalgia/Arthralgia | +++ | ++++ | ++ |
| Oedema in limbs | − | − | ++ |
| Maculopapular exanthema | ++ | ++ | +++ |
| Retro-orbital pain | ++ | + | ++ |
| Conjunctivitis | − | + | +++ |
| Lymphadenopathy | ++ | ++ | + |
| Hepatomegaly | − | +++ | − |
| Bleeding | + | − | − |
| Category | Specific Aspect | Summary | References | Evidence Strengths/Controversies |
|---|---|---|---|---|
| 1. Occurrence of Co-infection | DENV–DENV co-infection | Co-infections of multiple DENV serotypes have been increasingly reported, particularly during outbreaks in endemic regions. In some areas, the incidences were likely underreported due to limited surveillance and diagnostic sensitivity. | [10,11] | Supported by outbreak data; lacked consistent reporting and large-scale prevalence studies. |
| DENV–ZIKV co-infection | Frequently reported due to overlapping endemicity. Documented in New Caledonia (2014), Brazil (2015), and Colombia (2016). | [22,23,24] | Well documented in regional surveillance; supported by ecological overlap. | |
| Other flavivirus co-infections | Co-infections involving JEV, SLEV, and WNV were less commonly reported, hence their clinical impacts need to be further explored. | [34,35] | Limited data; often incidental reports. Requires more studies. | |
| 2. Transmission mechanism, vector, and ecological factors | Vector feeding behaviors | Aedes mosquitoes often take multiple blood meals in short intervals, increasing the risk of acquiring more than one virus. | [11,12,13,14] | Strong entomological support; field validation limited. |
| Vector competence | Aedes aegypti can maintain dual infections due to midgut permissiveness and immune factors. Influenced by virus–virus interactions and host behaviors. | [12,15] | Laboratory models support the mechanism, which is complex in field settings. | |
| Environmental overlap | Urban environments promote co-transmission due to poor sanitation, high population density, and the presence of shared vector. | [25] | Strong ecological and epidemiological correlation. | |
| 3. Clinical relevance and disease severity | Disease outcome | Co-infections may result in higher viremia, leukocyte infection, and cytokine storms, potentially leading to DHF or DSS. | [16,17,18] | Suggested in clinical observations, however, not consistently seen in all cohorts. |
| Discrepancies in disease severity | Some studies reported more severe symptoms in co-infections than mono-infections. Others found no significant difference from monoinfection. | [19,20,21] | Conflicting data; clinical heterogeneity and diagnostic variability contribute. | |
| Timing of infection | Sequential infection timing can lead to either viral interference or enhanced replication. | [36] | Experimental studies supported both outcomes, depending on the infection timing and infecting serotypes. | |
| 4. Immunological interactions and diagnosis | Antibody cross-reactivity | Due to ~60% nucleotide similarity between DENV and ZIKV. | [26,27,28] | Well established in serology analyses; significant implications in clinical management. |
| Antibody-dependent enhancement (ADE) | Cross-reactive, non-neutralizing antibodies may enhance viral entry, worsening disease severity. Impacts on vaccine development. | [29,30,31] | Strong theoretical and experimental evidence. | |
| Diagnostic limitations | Overlapping clinical symptoms lead to frequent misdiagnosis. The current WHO guidelines may miss out on co-infection cases. | [34,35,36,37] | Widely acknowledged limitations; highlights urgent need for differential diagnostic tools. |
| Diagnostic Test | Immunological Target |
|---|---|
| Polymerase chain reaction | RNA detection |
| Rapid tests | NS1, IgM, and IgG |
| Virus isolation | Virus |
| Immunofluorescence (IF) | Virus, IgM, and IgG |
| Plaque assay (PA) and fluorescent focus assay (FFA) | Virus titer |
| Enzyme-linked immunosorbent assay (ELISA) | NS1, IgG, IgM, and IgA |
| Neutralization test | Neutralizing antibodies (IgG) |
| Assay Type | Sensitivity Range | Specificity Range | Key Limitations |
|---|---|---|---|
| NS1 ELISA | 34–76% | 95–100% | Low for DENV-2 and secondary DENV infections. |
| NS1 RDT | 37.8–80% | 85–98% | Cross-reactivity at high viral loads. |
| NS1 + IgM | 90.3% | 96.2% | Optimal for acute-phase diagnosis. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sufi Aiman Sabrina, R.; Muhammad Azami, N.A.; Yap, W.B. Dengue and Flavivirus Co-Infections: Challenges in Diagnosis, Treatment, and Disease Management. Int. J. Mol. Sci. 2025, 26, 6609. https://doi.org/10.3390/ijms26146609
Sufi Aiman Sabrina R, Muhammad Azami NA, Yap WB. Dengue and Flavivirus Co-Infections: Challenges in Diagnosis, Treatment, and Disease Management. International Journal of Molecular Sciences. 2025; 26(14):6609. https://doi.org/10.3390/ijms26146609
Chicago/Turabian StyleSufi Aiman Sabrina, Rosmen, Nor Azila Muhammad Azami, and Wei Boon Yap. 2025. "Dengue and Flavivirus Co-Infections: Challenges in Diagnosis, Treatment, and Disease Management" International Journal of Molecular Sciences 26, no. 14: 6609. https://doi.org/10.3390/ijms26146609
APA StyleSufi Aiman Sabrina, R., Muhammad Azami, N. A., & Yap, W. B. (2025). Dengue and Flavivirus Co-Infections: Challenges in Diagnosis, Treatment, and Disease Management. International Journal of Molecular Sciences, 26(14), 6609. https://doi.org/10.3390/ijms26146609






