Radiation-Sensitive Nano-, Micro-, and Macro-Gels and Polymer Capsules for Use in Radiotherapy Dosimetry
Abstract
1. Introduction
2. Results and Discussion
2.1. Stability of Fricke Solution and Interactions of Ingredients
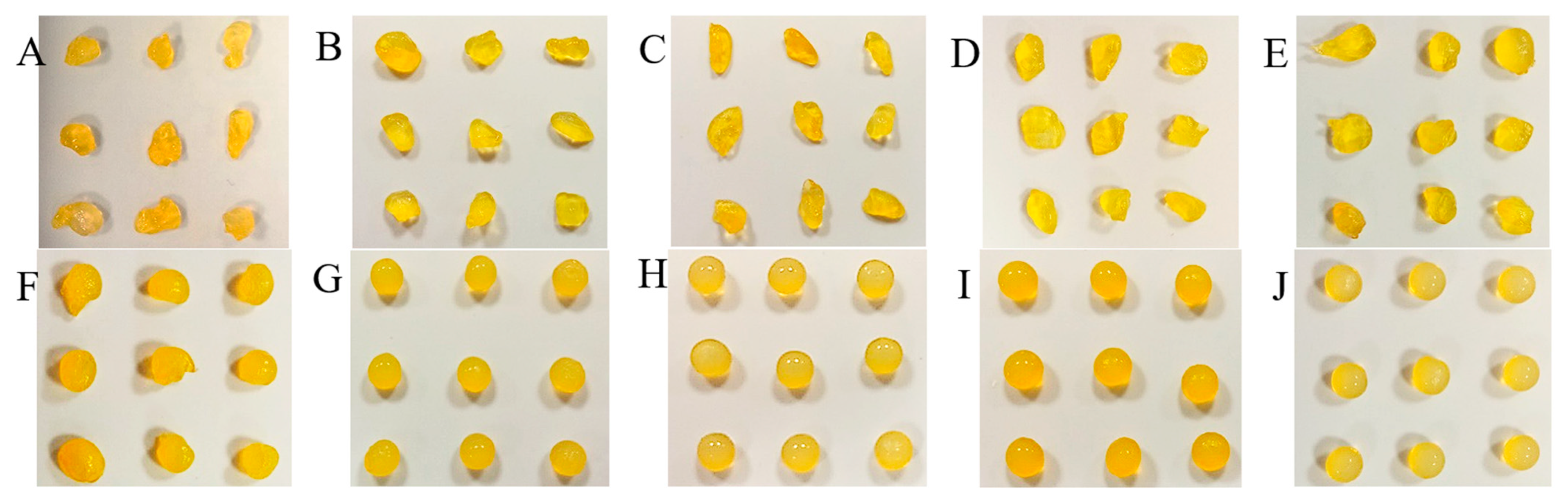
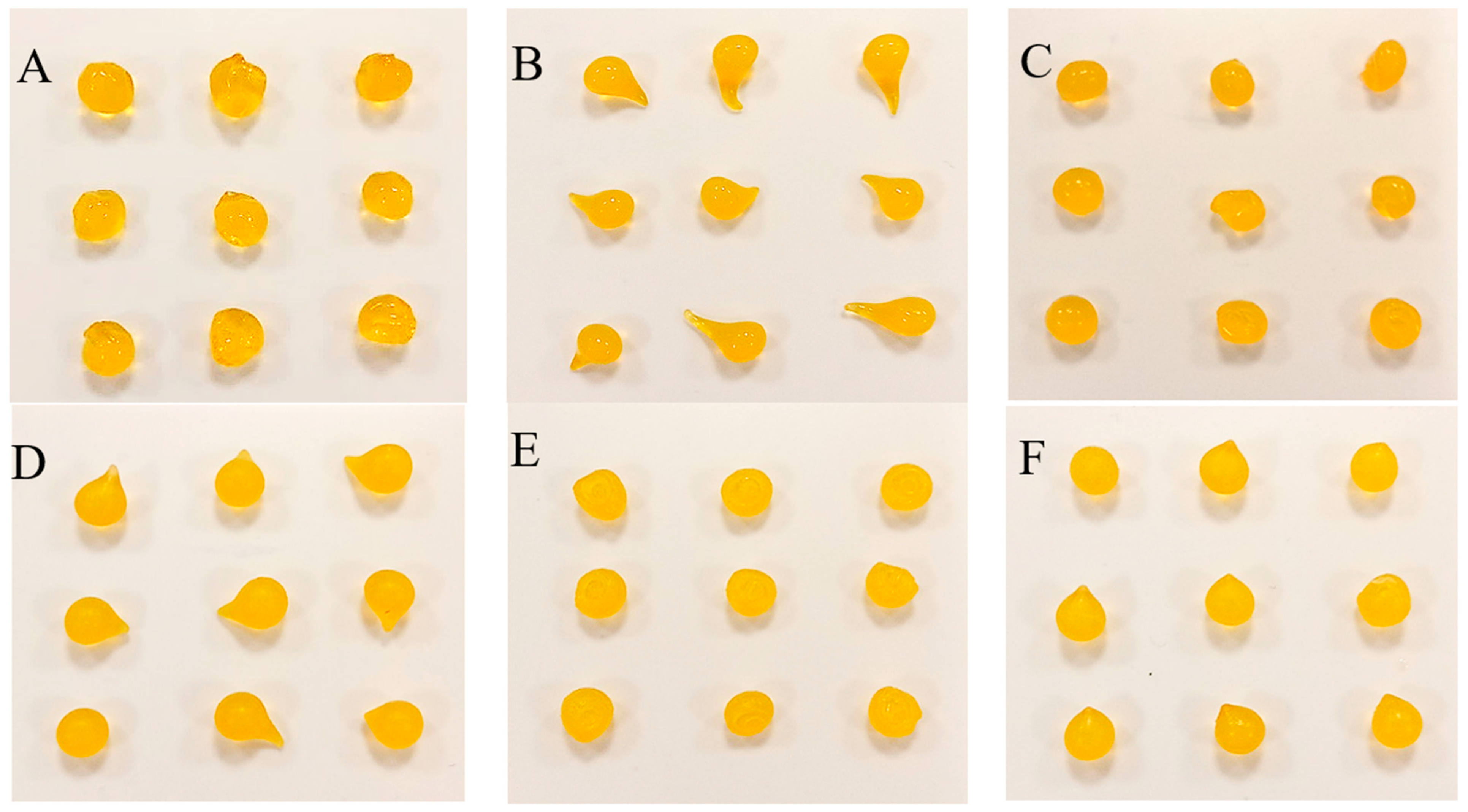
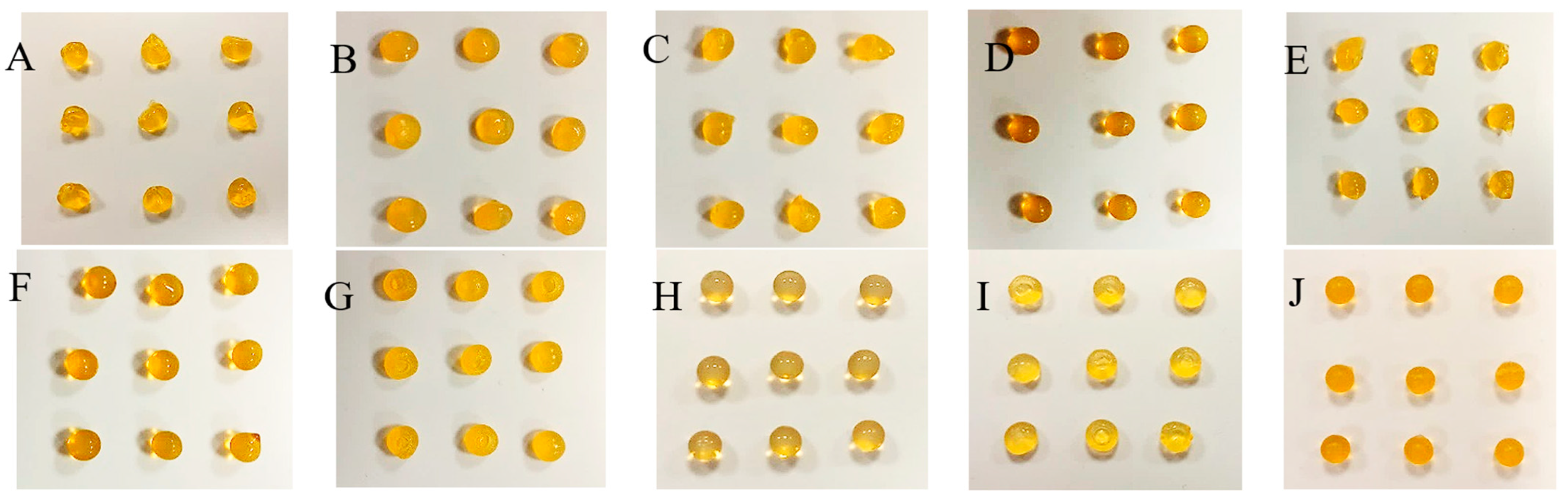
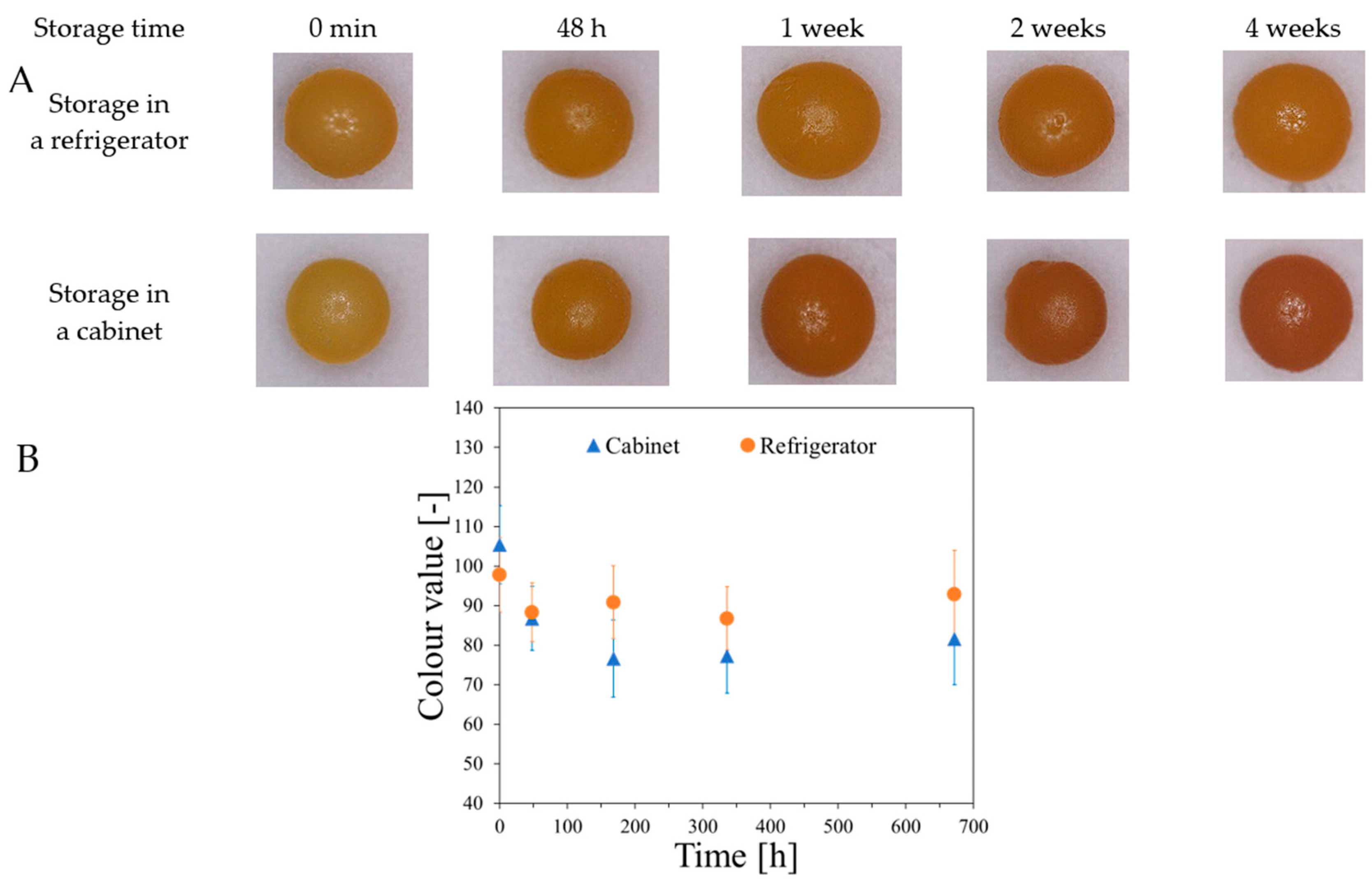
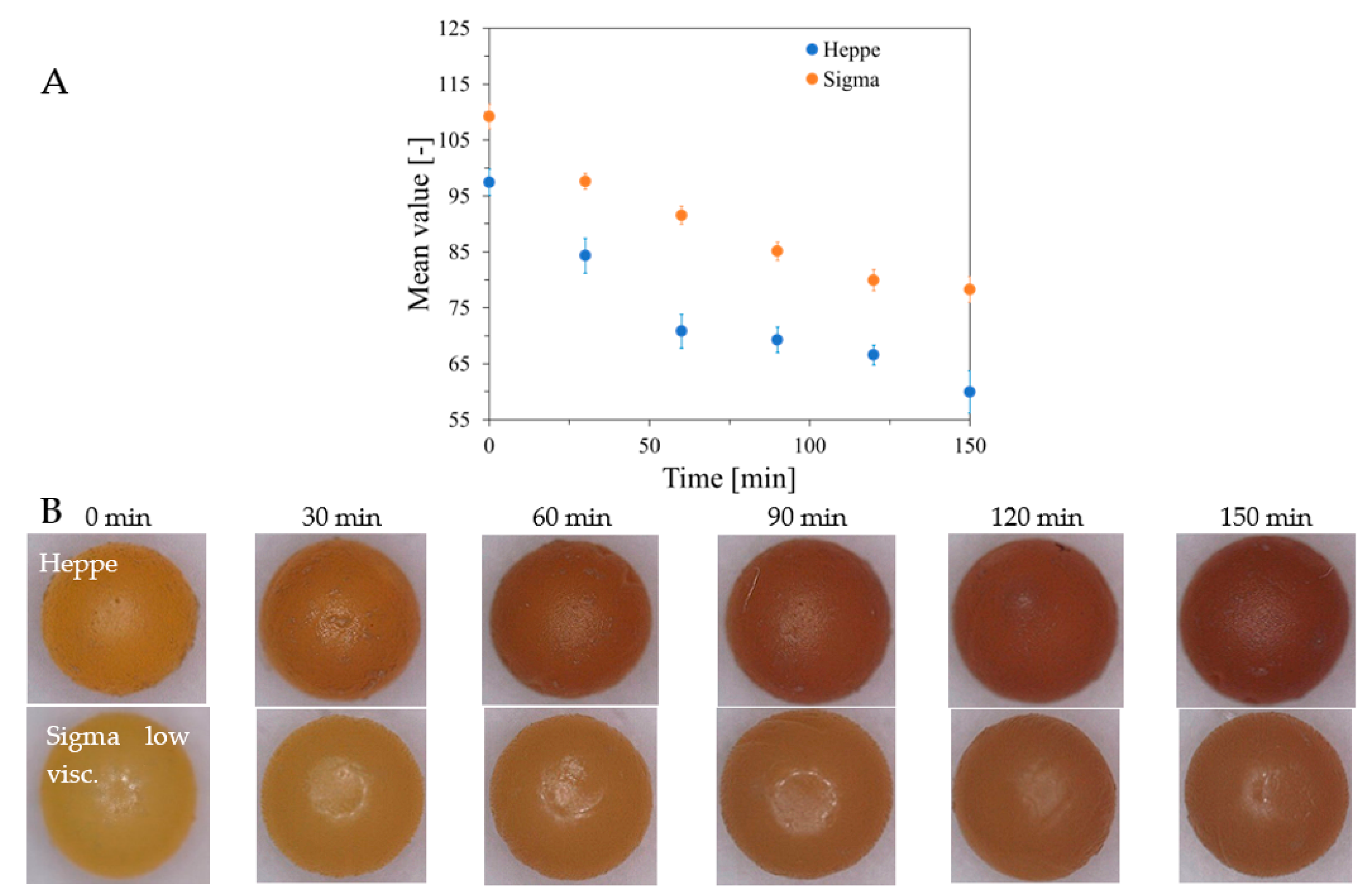
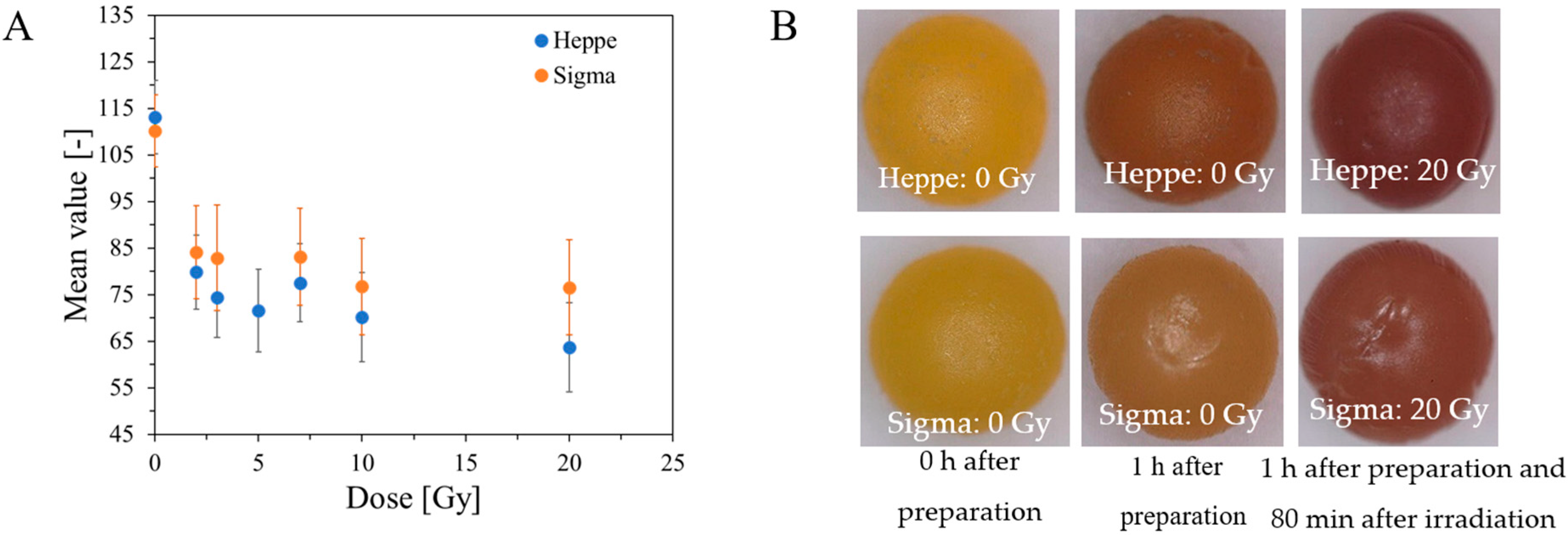
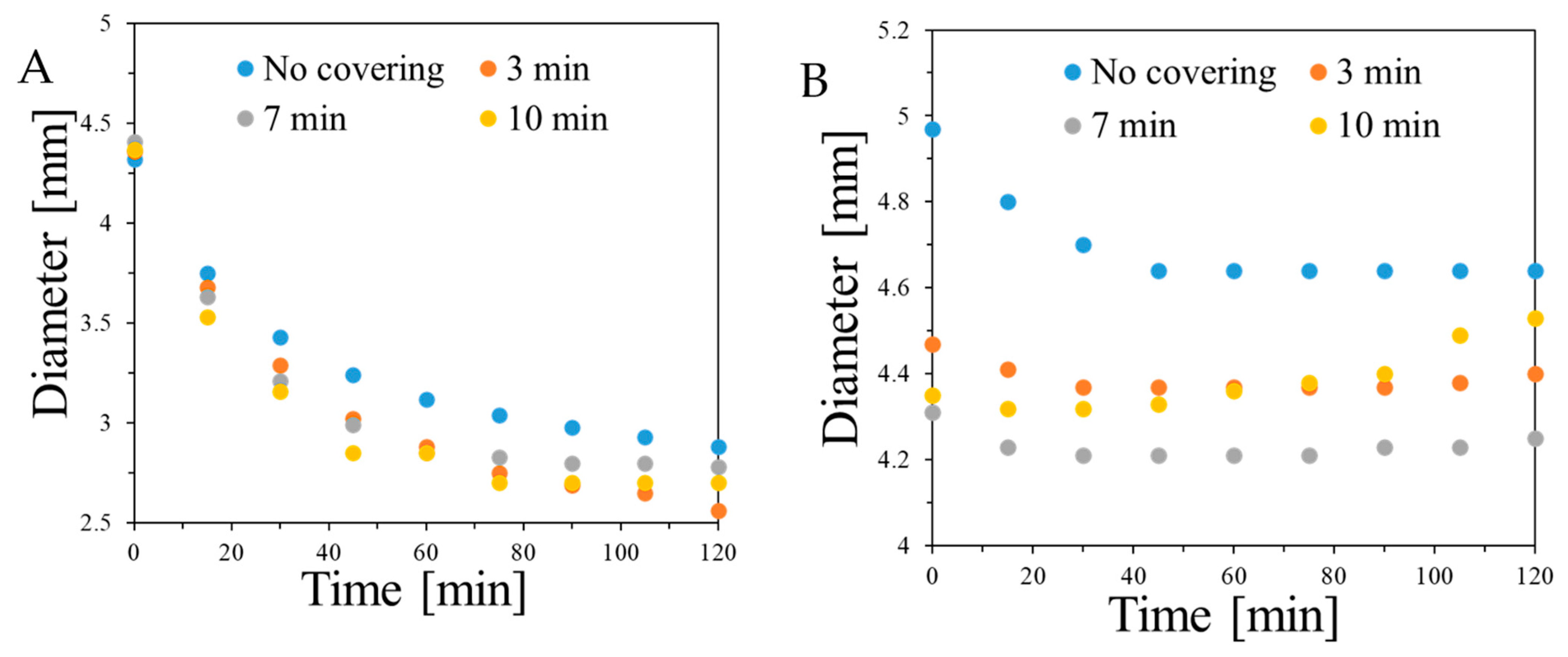
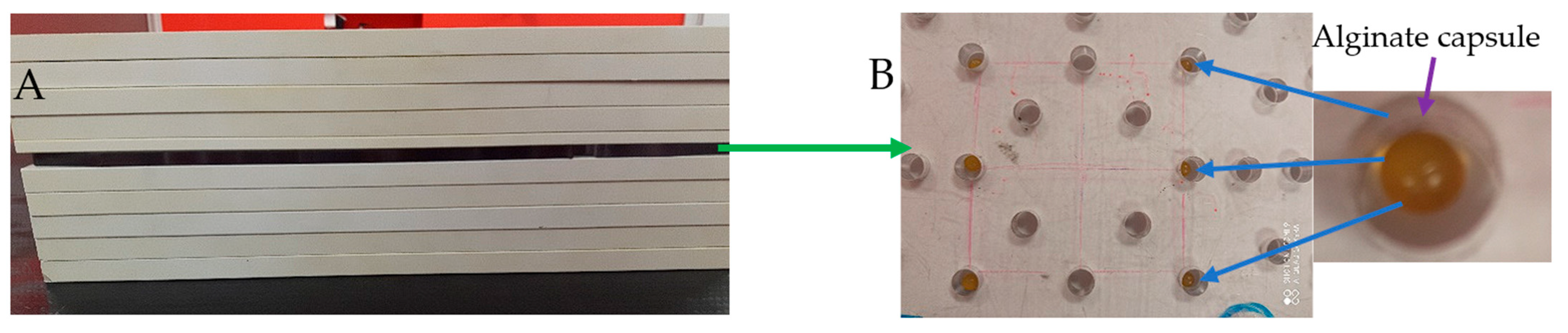
2.2. Features of Capsules
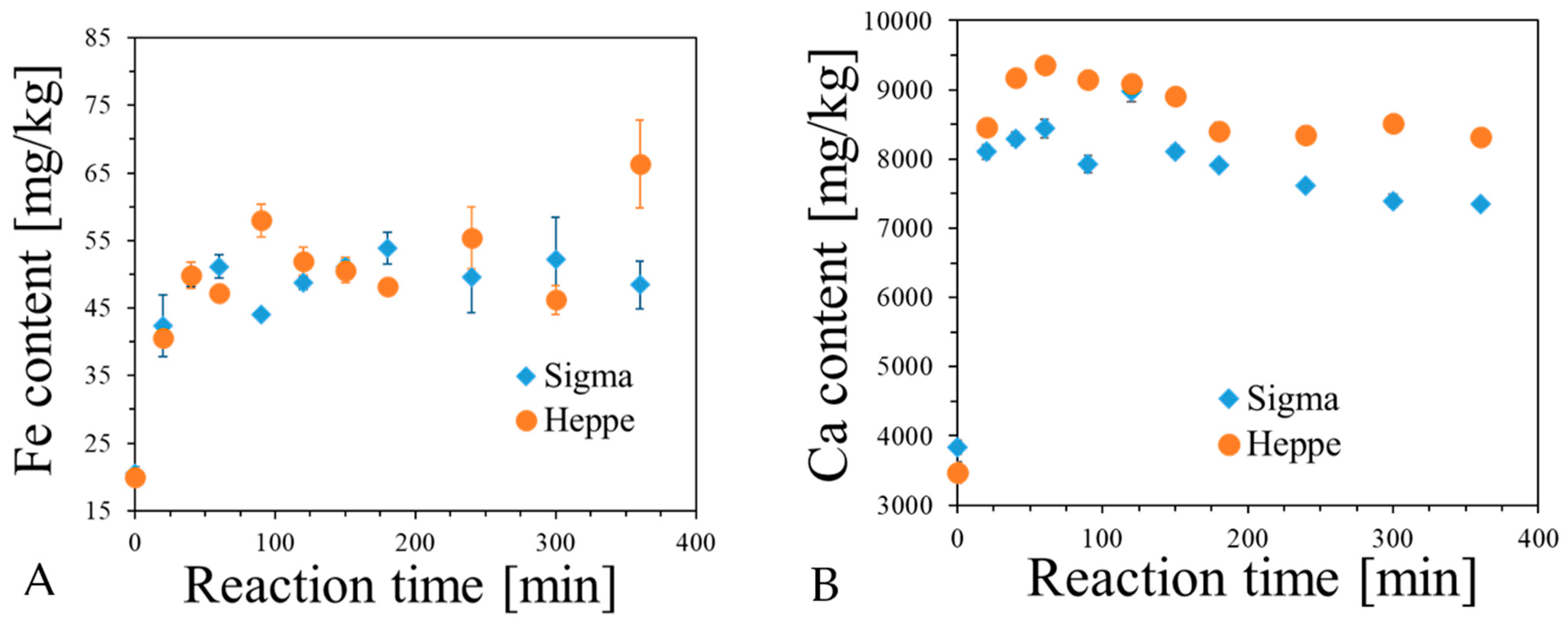
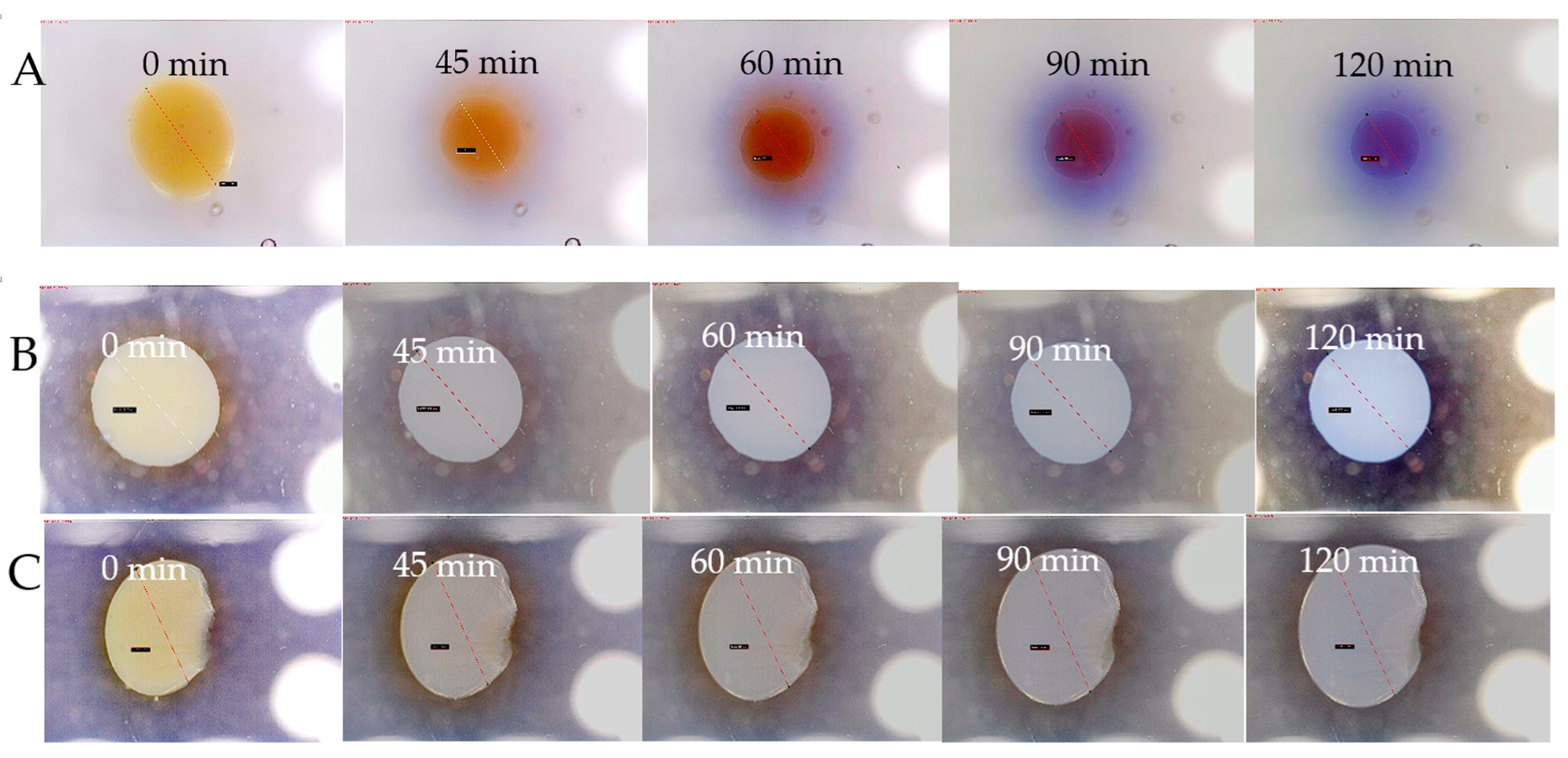
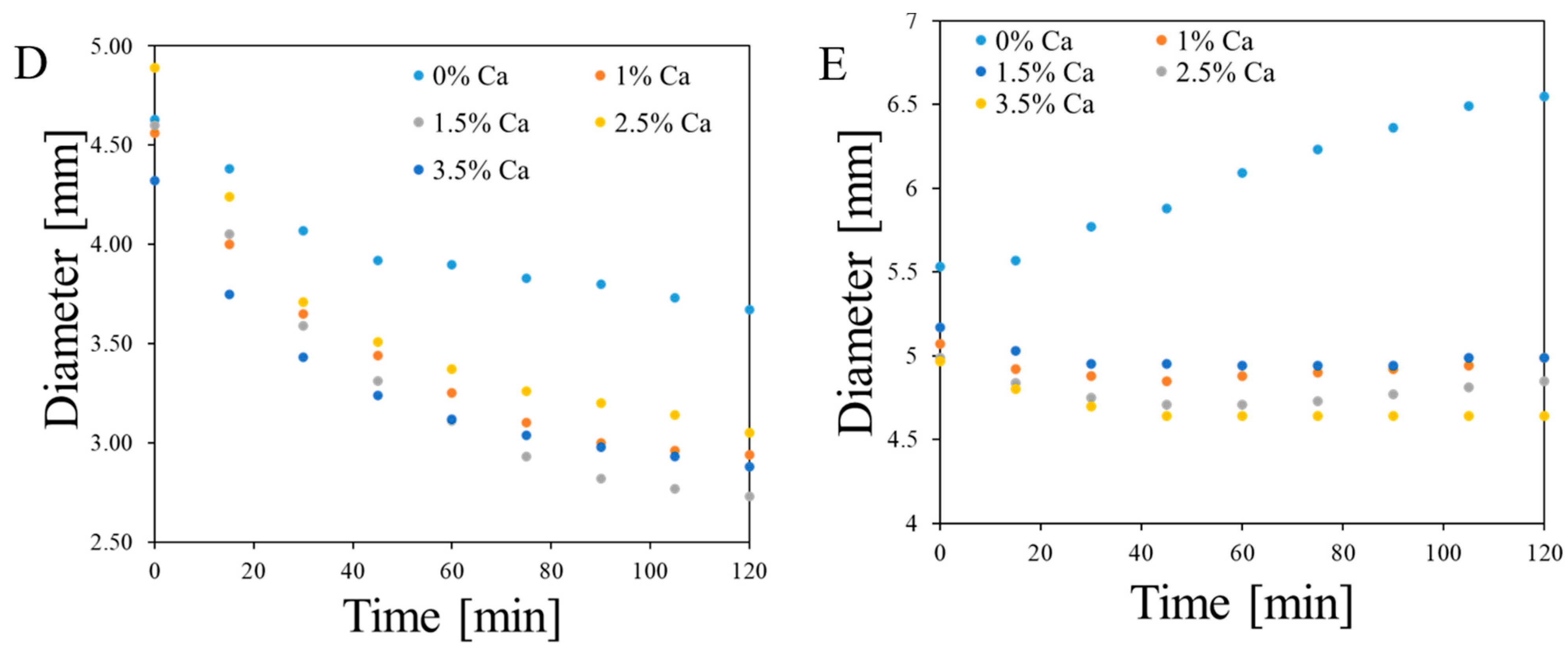
2.3. Analysis of Fe, Ca and XO in Alginate Capsules
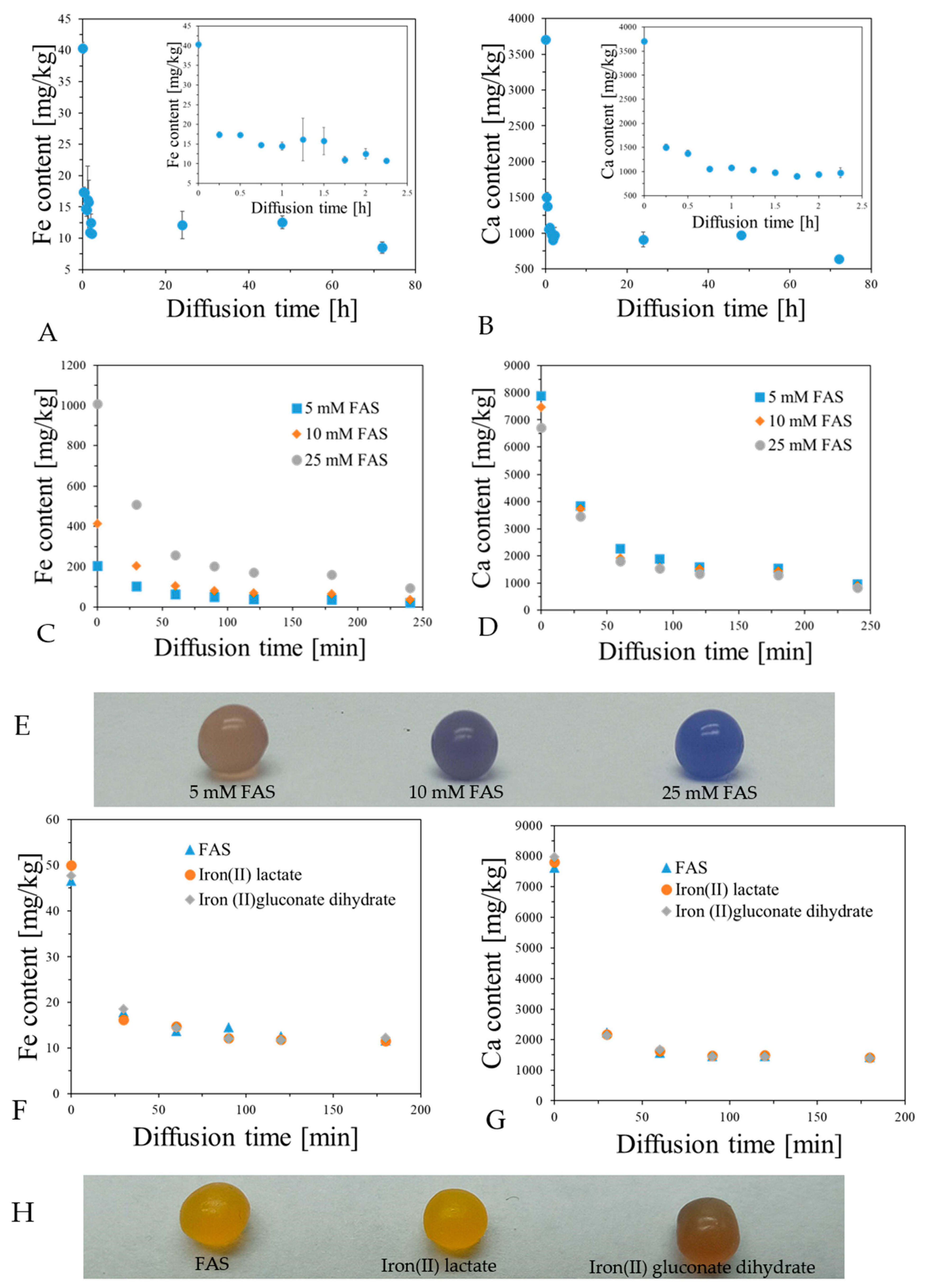
2.4. Macro-Gel and Characteristics of Nano- and Micro-Gels
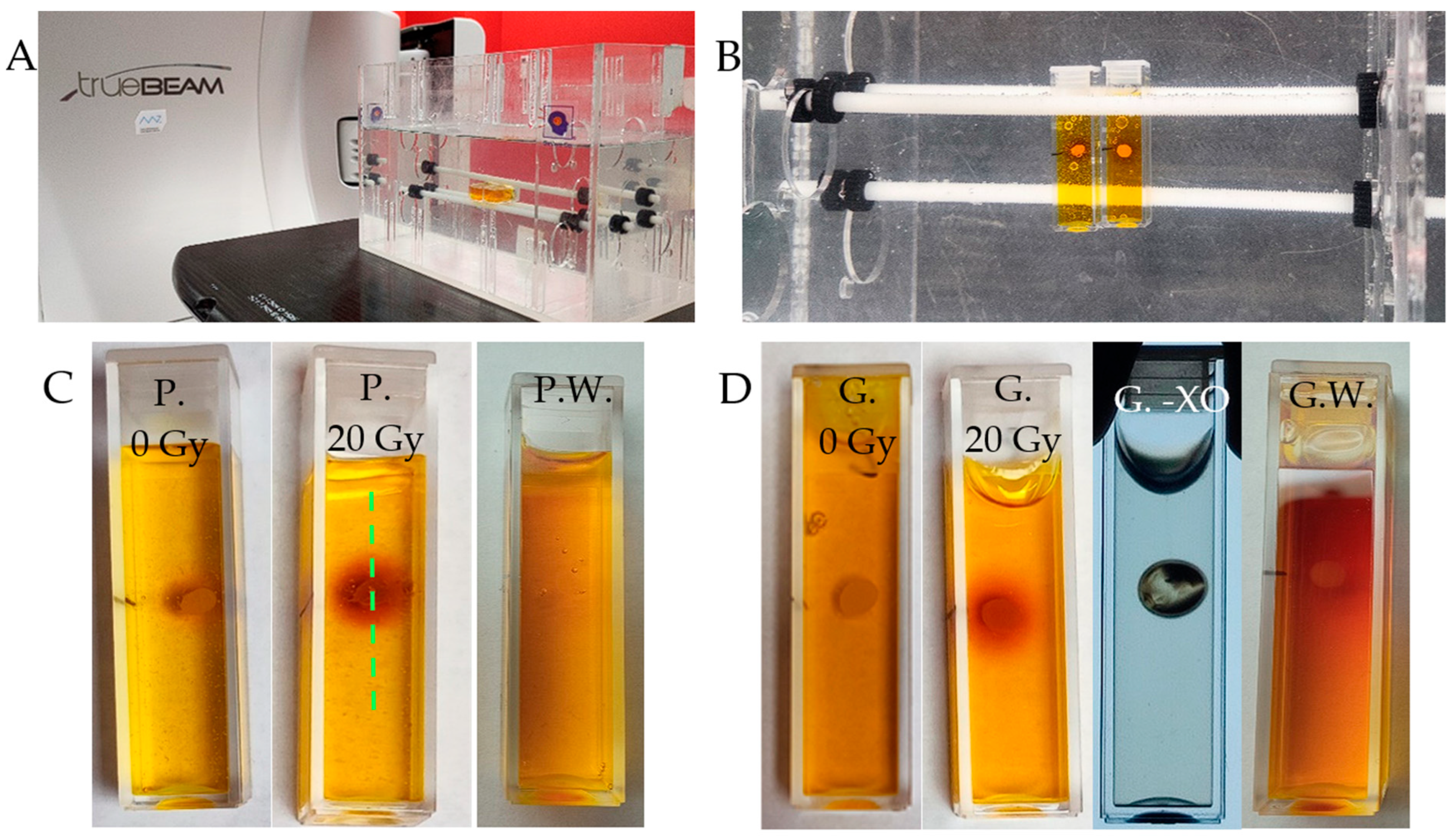
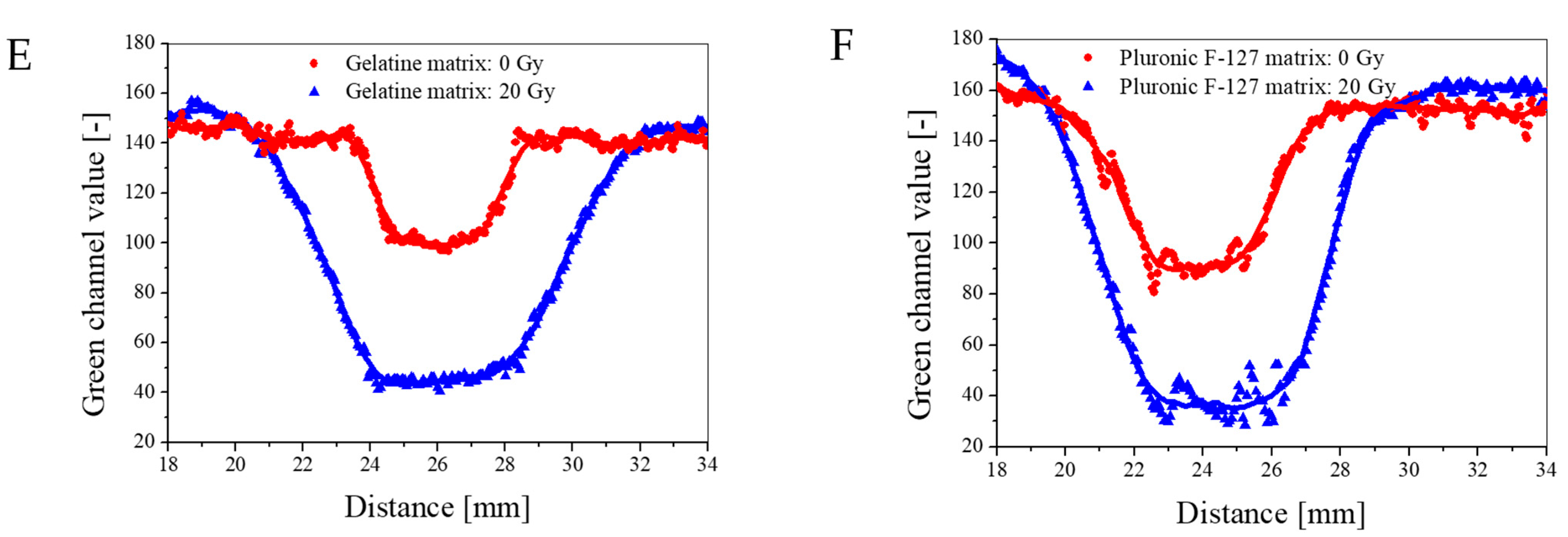
2.5. 1D Capsule Dosimeter
3. Materials and Methods
3.1. Type of Water
3.2. Preparation of Capsules
3.3. Preparation of Macro-Gel
3.4. Preparation of Nano- and Micro-Gels
3.5. Preparation of 1D Dosimeter
3.6. Preparation of 3D Dosimeter
3.7. Particle Charge Measurements
3.8. Diffusion Measurements
3.9. UV–Vis Measurements
3.10. Dynamic Light Scattering Measurements
3.11. Assessment of Metal Content in Capsules
3.12. Irradiation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Slotman, B.J.; Solberg, T.D.; Verellen, D. (Eds.) Extracranial Stereotactic Radiotherapy and Radiosurgery; Taylor & Francis: Abingdon-on-Thames, UK, 2005. [Google Scholar]
- Trifiletti, D.M.; Chao, S.T.; Sahgal, A.; Sheehan, J.P. (Eds.) Stereotactic Radiosurgery and Stereotactic Body Radiation Therapy. A Comprehensive Guide; Springer Nature: Basel, Switzerland, 2019. [Google Scholar]
- Swiss Society of Radiobiology and Medical Physics. Quality Control of Medical Electron Accelerators. Recommendations Number 11; Swiss Society of Radiobiology and Medical Physics: Geneva, Switzerland, 2015; ISBN 3-908-125-57-X. [Google Scholar]
- Fricke, H.; Morse, S. The chemical action of Roentgen rays on dilute ferrosulphate solutions as a measure of dose. Am. J. Roent. Radium Ther. Nucl. Med. 1927, 18, 430–432. [Google Scholar]
- Gore, J.C.; Kang, Y.S.; Schultz, R.J. Measurement of radiation dose distributions by nuclear magnetic resonance (NMR) imaging. Phys. Med. Biol. 1984, 29, 1189–1199. [Google Scholar] [CrossRef]
- Baldock, C.; De Deene, Y.; Doran, S.; Ibbott, G.; Jirasek, A.; Lepage, M.; McAuley, K.B.; Oldham, M.; Schreiner, L.J. Polymer gel dosimetry. Phys. Med. Biol. 2010, 55, R1–R63. [Google Scholar] [CrossRef] [PubMed]
- De Deene, Y. Radiation dosimetry by use of radiosensitive hydrogels and polymers: Mechanisms, state-of-the-art and perspective from 3D to 4D. Gels 2022, 8, 599. [Google Scholar] [CrossRef] [PubMed]
- Marrale, M.; Collura, G.; Gallo, S.; Nici, S.; Tranchina, L.; Abbate, B.F.; Marineo, S.; Caracappa, S.; d’Errico, F. Analysis of spatial diffusion of ferric ions in PVA-GTA gel dosimeters through magnetic resonance imaging. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2017, 396, 50–55. [Google Scholar] [CrossRef]
- Rabaeh, K.A.; Eyadeh, M.M.; Hailat, T.F.; Madas, B.G.; Aldweri, F.M.; Almomani, A.M.; Awad, S.I. Improvement on the performance of chemically cross-linked Fricke methylthymol-blue radiochromic gel dosimeter by addition of dimethyl sulfoxide. Radiat. Meas. 2021, 141, 106540. [Google Scholar] [CrossRef]
- Babic, S.; Battista, J.; Jordan, K. An apparent threshold dose response in ferrous xylenol-orange gel dosimeters when scanned with a yellow light source. Phys. Med. Biol. 2008, 53, 1637–1650. [Google Scholar] [CrossRef]
- Gallo, S.; Artuso, E.; Brambilla, M.G.; Gambarini, G.; Lenardi, C.; Monti, A.F.; Torresin, A.; Pignoli, E.; Veronese, I. Characterization of radiochromic poly(vinyl-alcohol)–glutaraldehyde Fricke gels for dosimetry in external x-ray radiation therapy. J. Phys. D Appl. Phys. 2019, 52, 225601. [Google Scholar] [CrossRef]
- Scotti, M.; Arosio, P.; Brambilla, E.; Gallo, S.; Lenardi, C.; Locarno, S.; Orsini, F.; Pignoli, E.; Pedicone, L.; Veronese, I. How Xylenol Orange and Ferrous Ammonium Sulphate Influence the Dosimetric Properties of PVA–GTA Fricke Gel Dosimeters: A Spectrophotometric Study. Gels 2022, 8, 204. [Google Scholar] [CrossRef]
- Piotrowski, M.; Maras, P.; Wach, R.; Kadlubowski, S.; Kozicki, M. Impact of salt on thermal stability and dose response of the Fricke-XO-Pluronic F-127 3D radiotherapy dosimeter. Materials 2022, 15, 5223. [Google Scholar] [CrossRef]
- Piotrowski, M.; Maras, P.; Kadłubowski, S.; Kozicki, M. Study of the optimal composition and storage conditions of the Fricke−XO−Pluronic F−127 radiochromic dosimeter. Materials 2022, 15, 984. [Google Scholar] [CrossRef] [PubMed]
- Dudek, M.; Piotrowski, M.; Maras, P.; Jaszczak, M.; Kozicki, M. Anisotropic diffusion of Fe ions in Fricke-XO-Pluronic F-127 and Fricke-XO-gelatine 3D radiotherapy dosimeters. Phys. Med. Biol. 2021, 66, 155005. [Google Scholar] [CrossRef] [PubMed]
- Maryanski, M.J.; Audet, C.; Gore, J.C. Effects of crosslinking and temperature on the dose response of a BANG polymer gel dosimeter. Phys. Med. Biol. 1997, 42, 303–311. [Google Scholar] [CrossRef]
- Vandecasteele, J.; De Deene, Y. On the validity of 3D polymer gel dosimetry, II: Physico-chemical effects. Phys. Med. Biol. 2013, 58, 43–61. [Google Scholar] [CrossRef]
- Fong, P.M.; Keil, D.C.; Does, M.D.; Gore, J.C. Polymer gels for magnetic resonance imaging of radiation dose distributions at normal room atmosphere. Phys. Med. Biol. 2001, 46, 3105–3113. [Google Scholar] [CrossRef] [PubMed]
- Johnston, H.; Hilts, M.; Carrick, J.; Jirasek, A. An x-ray CT polymer gel dosimetry prototype: II. Gel characterization and clinical application. Phys. Med. Biol. 2012, 57, 3155–3175. [Google Scholar] [CrossRef]
- Pappas, E.; Maris, T.; Angelopoulos, A.; Paparigopoulou, M.; Sakelliou, L.; Sandilos, P.; Voyiatzi, S.; Vlachos, L. A new polymer gel for magnetic resonance imaging (MRI) radiation dosimetry. Phys. Med. Biol. 1999, 44, 2677. [Google Scholar] [CrossRef]
- Kozicki, M.; Jaszczak, M.; Maras, P.; Dudek, M.; Cłapa, M. On the development of a VIPARnd radiotherapy 3D polymer gel dosimeter. Phys. Med. Biol. 2017, 62, 986–1008. [Google Scholar] [CrossRef]
- Kozicki, M.; Berg, A.; Maras, P.; Jaszczak, M.; Dudek, M. Clinical radiotherapy application of N-vinylpyrrolidone-containing 3D polymer gel dosimeters with remote external MR-reading. Phys. Medica 2020, 69, 134–146. [Google Scholar] [CrossRef]
- Kozicki, M.; Jaszczak, M.; Maras, P.; Dudek, M. A chemical evolution of NVP-containing VIPAR-family 3D polymer gel dosimeters–a brief overview. J. Phys. Conf. Ser. 2019, 1305, 012067. [Google Scholar] [CrossRef]
- Jaszczak, M.; Wach, R.; Maras, P.; Dudek, M.; Kozicki, M. Substituting gelatine with Pluronic F-127 matrix in 3D polymer gel dosimeters can improve nuclear magnetic resonance, thermal and optical properties. Phys. Med. Biol. 2018, 63, 175010. [Google Scholar] [CrossRef] [PubMed]
- Kozicki, M.; Maras, P.; Karwowski, A.C. Software for 3D radiotherapy dosimetry. Validation. Phys. Med. Biol. 2014, 59, 4111–4136. [Google Scholar] [CrossRef]
- Moftah, B.; Basfar, A.A.; Almousa, A.A.; Kafi, A.; Rabaeh, K.A. Novel 3D polymer gel dosimeters based on N-(3-Methoxypropyl)acrylamide (NMPAGAT) for quality assurance in radiation oncology. Radiat. Meas. 2020, 135, 106372. [Google Scholar] [CrossRef]
- Mather, M.L.; Whittaker, A.K.; Baldock, C. Ultrasound evaluation of polymer gel dosimeters. Phys. Med. Biol. 2002, 47, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- Atkins, T.J.; Humphrey, V.F.; Duck, F.A.; Tooley, M.A. Investigation of ultrasonic properties of MAGIC gels for pulse-echo gel dosimetry. J. Phys. Conf. Ser. 2010, 250, 012075. [Google Scholar] [CrossRef]
- Kwiatos, K.; Maras, P.; Kadlubowski, S.; Stempień, Z.; Dudek, M.; Kozicki, M. Tetrazolium salts-Pluronic F-127 gels for 3D radiotherapy dosimetry. Phys. Med. Biol. 2018, 63, 095012. [Google Scholar] [CrossRef]
- Hayashi, S.-I.; Ono, K.; Fujino, K.; Ikeda, S.; Tanaka, K. Novel radiochromic gel dosimeter based on a polyvinyl alcohol-Iodide complex. Radiat. Meas. 2020, 131, 106226. [Google Scholar] [CrossRef]
- Jaszczak, M.; Sasiadek, E.; Kadlubowski, S.; Dudek, M.; Kozicki, M. Preliminary study on a new 3D radiochromic KI-Pluronic F-127 gel dosimeter for radiotherapy. Radiat. Phys. Chem. 2021, 185, 109507. [Google Scholar] [CrossRef]
- Babic, S.; Battista, J.; Jordan, K. Radiochromic leuco dye micelle hydrogels: II. Low diffusion rate leuco crystal violet gel. Phys. Med. Biol. 2009, 54, 6791–6808. [Google Scholar] [CrossRef]
- Brown, S.; Venning, A.; De Deene, Y.; Vial, P.; Oliver, L.; Adamovics, J.; Baldock, C. Radiological properties of the PRESAGE and PAGAT polymer dosimeters. Appl. Radiat. Isot. 2008, 66, 1970–1974. [Google Scholar] [CrossRef]
- Tajaldeen, A.; Alghamdi, S. Investigation of dosimetric impact of organ motion in static and dynamic conditions for three stereotactic ablative body radiotherapy techniques: 3D conformal radiotherapy, intensity modulated radiation therapy, and volumetric modulated arc therapy by using PRESAGE 3D dosimeters. Exp. Oncol. 2019, 41, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Alqathami, M.; Adamovics, J.; Benning, R.; Qiao, G.; Geso, M.; Blencowe, A. Evaluation of ultra-sensitive leucomalachite dye derivatives for use in the PRESAGE® dosimeter. Radiat. Phys. Chem. 2013, 85, 204–209. [Google Scholar] [CrossRef]
- Kozicki, M.; Jaszczak, M.; Maras, P.; Naglik, R.; Dudek, M.; Kadlubowski, S.; Wach, R. Preliminary study on a 3D lung mimicking dosimeter based on Pluronic F-127 matrix. Radiat. Phys. Chem. 2021, 185, 109479. [Google Scholar] [CrossRef]
- De Deene, Y.; Vergote, K.; Claeys, C.; De Wagter, C. Three dimensional radiation dosimetry in lung-equivalent regions by use of a radiation sensitive gel foam: Proof of principle. Med. Phys. 2006, 33, 2586–2597. [Google Scholar] [CrossRef]
- Haraldsson, P.; Karlsson, A.; Wieslander, E.; Gustavsson, H.; Bäck, S.Å.J. Dose response evaluation of a low-density normoxic polymer gel dosimeter using MRI. Phys. Med. Biol. 2006, 51, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.V.; Valdetaro, L.B.; Poulsen, P.R.; Balling, P.; Petersen, J.B.B.; Muren, L.P. Dose-response of deformable radiochromic dosimeters for spot scanning proton therapy. Phys. Imaging Radiat. Oncol. 2020, 16, 134–137. [Google Scholar] [CrossRef]
- De Deene, Y.; Skyt, P.S.; Hil, R.; Booth, J.T. FlexyDos3D: A deformable anthropomorphic 3D radiation dosimeter: Radiation properties. Phys. Med. Biol. 2015, 60, 1543–1563. [Google Scholar] [CrossRef]
- Warman, J.M.; de Haas, M.P.; Luthjens, L.H.; Yao, T.; Navarro-Campos, J.; Yuksel, S.; Aarts, J.; Thiele, S.; Houter, J.; Zandt, W.I.H. FluoroTome 1: An apparatus for tomographic imaging of radio-fluorogenic (RFG) gels. Polymers 2019, 11, 1729. [Google Scholar] [CrossRef]
- Maeyama, T.; Hase, S. Nanoclay gel-based radio-fluorogenic gel dosimeters using various fluorescence probes. Radiat. Phys. Chem. 2018, 151, 42–46. [Google Scholar] [CrossRef]
- Sandwall, P.A.; Bastow, B.P.; Spitz, H.B.; Elson, H.R.; Lamba, M.; Connick, W.B.; Fenichel, H. Radio-fluorogenic gel dosimetry with coumarin. Bioengineering 2018, 5, 53. [Google Scholar] [CrossRef]
- Kozicki, M.; Bartosiak, M.; Maras, P.; Wach, R.; Kadlubowski, S. First combined, double-density LCV-Pluronic F-127 radiochromic dosimeter mimicking lungs and muscles. Adv. Mater. Technol. 2023, 8, 2201023. [Google Scholar] [CrossRef]
- Maras, P.; Jaszczak, M.; Kozicki, M. Basic features of VIC-T dosimeter with spiral CT readout. CT scanning conditions and data processing with a new polyGeVero-CT software package. Radiat. Phys. Chem. 2021, 189, 109730. [Google Scholar] [CrossRef]
- Schreiner, L.J. Review of Fricke gel dosimeters. J. Phys. Conf. Ser. 2004, 3, 9–21. [Google Scholar] [CrossRef]
- Penev, K.; Mequanint, K. Controlling sensitivity and stability of ferrous–xylenol orange–gelatine 3D gel dosimeters by doping with phenanthroline-type ligands and glyoxal. Phys. Med. Biol. 2013, 58, 1823–1838. [Google Scholar] [CrossRef]
- Kozicki, M.; Kwiatos, K.; Kadlubowski, S.; Dudek, M. TTC-Pluronic 3D radiochromic gel dosimetry of ionizing radiation. Phys. Med. Biol. 2017, 62, 5668–5690. [Google Scholar] [CrossRef]
- Maeyama, T.; Fukunishi, N.; Ishikawa, K.L.; Fukasaku, K.; Fukuda, S. Organic-gelatine-free nanocomposite Fricke gel dosimeter. J. Phys. Chem. B 2017, 121, 4238–4246. [Google Scholar] [CrossRef]
- Maeyama, T.; Fukunishi, N.; Ishikawa, K.L.; Furuta, T.; Fukasaku, K.; Takagi, S.; Noda, S.; Himeno, R.; Fukuda, S. A diffusion-free and linear energy transfer-independent nanocomposite Fricke gel dosimeter. Radiat. Phys. Chem. 2014, 96, 92–96. [Google Scholar] [CrossRef]
- Locarno, S.; Gallo, S.; Arosio, P.; Biordi, C.; Dallasega, D.; Gargano, M.; Ludwig, N.; Orsini, F.; Pignoli, E.; Veronese, I.; et al. Dosimetric Double Network Hydrogel Based on Poly(vinyl-alcohol)/Phenylalanine-Derivatives with Enhanced Mechanical Properties. ACS Appl. Polym. Mater. 2023, 5, 1902–1914. [Google Scholar] [CrossRef]
- Alexandridis, P.; Hatton, T.A. Poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) block copolymer surfactants in aqueous solutions and at interfaces: Thermodynamics, structure, dynamics, and modelling. Colloids Surf. A Physicochem. Eng. Asp. 1995, 96, 1–46. [Google Scholar] [CrossRef]
- Almeida, H.; Amaral, M.H.; Lobão, P.; Lobo, J.M.S. Pluronic F-127 and Pluronic lecithin organogel (PLO): Main features and their applications in topical and transdermal administration of drugs. J. Pharm. Pharm. Sci. 2012, 15, 592–605. [Google Scholar] [CrossRef]
- Diniz, I.M.; Chen, C.; Xu, X.; Ansari, S.; Zadeh, H.H.; Marques, M.M.; Shi, S.; Moshaverinia, A. Pluronic F-127 hydrogel as a promising scaffold for encapsulation of dental-derived mesenchymal stem cells. J. Mater. Sci. Mater. Med. 2015, 26, 153. [Google Scholar] [CrossRef]
- Bigelow, C.C.; Cragg, L.H. Critical concentration effects in polymer-polymer-solvent systems. Can. J. Chem. 1958, 36, 199–205. [Google Scholar] [CrossRef]
- Kozicki, M.; Kołodziejczyk, M.; Szynkowska, M.; Pawlaczyk, A.; Lesniewska, E.; Matusiak, A.; Adamus, A.; Karolczak, A. Hydrogels made from chitosan and silver nitrate. Carbohydr. Polym. 2016, 140, 74–87. [Google Scholar] [CrossRef]
- Kozicki, M.; Pawlaczyk, A.; Adamska, A.; Szynkowska-Jóźwik, M.; Sąsiadek-Andrzejczak, E. Golden and silver–golden chitosan hydrogels and fabrics modified with golden chitosan hydrogels. Int. J. Mol. Sci. 2022, 23, 5406. [Google Scholar] [CrossRef] [PubMed]
- Frent, O.D.; Vicas, L.G.; Duteanu, N.; Morgovan, C.M.; Jurca, T.; Pallag, A.; Muresan, M.E.; Filip, S.M.; Lucaciu, R.-L.; Marian, E. Sodium alginate—Natural microencapsulation material of polymeric microparticles. Int. J. Mol. Sci. 2022, 23, 121018. [Google Scholar] [CrossRef] [PubMed]
- Chuang, J.-J.; Huang, Y.-Y.; Lo, S.-H.; Hsu, T.-F.; Huang, W.-Y.; Huang, S.-L.; Lin, Y.-S. Effects of pH on the shape of alginate particles and its release behavior. Int. J. Polym. Sci. 2017, 2017, 3902704. [Google Scholar] [CrossRef]
- Ferradini, C.; Jay-Gerin, J. The effect of pH on water radiolysis: A still open question—A minireview. Res. Chem. Intermed. 2000, 26, 549–565. [Google Scholar] [CrossRef]
- Morgan, B.; Lahav, O. The effect of pH on the kinetics of spontaneous Fe(II) oxidation by O2 in aqueous solution—Basic principles and a simple heuristic description. Chemosphere 2007, 68, 2080–2084. [Google Scholar] [CrossRef]
- Stumm, W.; Lee, G.F. Oxygenation of ferrous iron. Ind. Eng. Chem. 1961, 53, 143–146. [Google Scholar] [CrossRef]
- Irianto, H.E.; Giyatmi, G.; Fransiska, D.; Nuraelah, A. Physical and chemical characteristics of alginate extracted from Sargassum sp. IOP Conf. Ser. Earth Environ. Sci. 2023, 1177, 012029. [Google Scholar] [CrossRef]
- Kong, L. Copper requirement and acquisition by marine microalgae. Microorganisms 2022, 10, 1853. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, K.; Zeng, Y.; Hu, X.; Zhang, X.; Chang, S.; Zhang, H. Low-diffusion Fricke gel dosimeters with core-shell structure based on spatial confinement. Materials 2021, 14, 3992. [Google Scholar] [CrossRef] [PubMed]
- Technical Reports Series No. 398, Absorbed Dose Determination in External Beam Radiotherapy. In An International Code of Practice for Dosimetry Based on Standards of Absorbed Dose to Water; International Atomic Energy Agency: Vienna, Austria, 2000.


| No. | Alginate Type | Alginate Concentration [%] | CaCl2 Concentration [%] | Comment |
|---|---|---|---|---|
| 1 | Low viscosity, Sigma | 2.7 | 2.5 | Acceptable |
| 2 | 3.5 | |||
| 3 | 3.0 | 2.5 | Very good | |
| 4 | 3.5 | |||
| 5 | 3.5 | 1.5 | Acceptable | |
| 6 | 2.5 | Very good | ||
| 7 | 3.5 | |||
| 8 | Medium viscosity, Sigma | 2.7 | 1.5 | Acceptable |
| 9 | Medium viscosity, Heppe | 2.7 | 1.5 | Very good |
| 10 | 2.5 | |||
| 11 | 3.0 | 1 | Acceptable | |
| 12 | 1.5 | Very good | ||
| 13 | 2.5 | |||
| 14 | 3.5 | |||
| 15 | 3.5 | 1.5 | ||
| 16 | 2.5 | |||
| 17 | 3.5 |
| Type of Water | Water Hardness [°dH] | pH [-] | Conductivity [µS/cm] |
|---|---|---|---|
| Tap water | 12.17 | 7.46 | 378.9 |
| Distilled | 0.35 | 6.95 | 11.15 |
| Re-distilled | - | 5.77 | 1.23 |
| Deionized | - | 5.63 | 1.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piotrowski, M.; Pawlaczyk, A.; Szynkowska-Jóźwik, M.I.; Maras, P.; Kozicki, M. Radiation-Sensitive Nano-, Micro-, and Macro-Gels and Polymer Capsules for Use in Radiotherapy Dosimetry. Int. J. Mol. Sci. 2025, 26, 6603. https://doi.org/10.3390/ijms26146603
Piotrowski M, Pawlaczyk A, Szynkowska-Jóźwik MI, Maras P, Kozicki M. Radiation-Sensitive Nano-, Micro-, and Macro-Gels and Polymer Capsules for Use in Radiotherapy Dosimetry. International Journal of Molecular Sciences. 2025; 26(14):6603. https://doi.org/10.3390/ijms26146603
Chicago/Turabian StylePiotrowski, Michał, Aleksandra Pawlaczyk, Małgorzata I. Szynkowska-Jóźwik, Piotr Maras, and Marek Kozicki. 2025. "Radiation-Sensitive Nano-, Micro-, and Macro-Gels and Polymer Capsules for Use in Radiotherapy Dosimetry" International Journal of Molecular Sciences 26, no. 14: 6603. https://doi.org/10.3390/ijms26146603
APA StylePiotrowski, M., Pawlaczyk, A., Szynkowska-Jóźwik, M. I., Maras, P., & Kozicki, M. (2025). Radiation-Sensitive Nano-, Micro-, and Macro-Gels and Polymer Capsules for Use in Radiotherapy Dosimetry. International Journal of Molecular Sciences, 26(14), 6603. https://doi.org/10.3390/ijms26146603







