A Puerperal Patient with Leukopenia During Vancomycin Administration: A Case Report and Review of the Literature
Abstract
1. Introduction
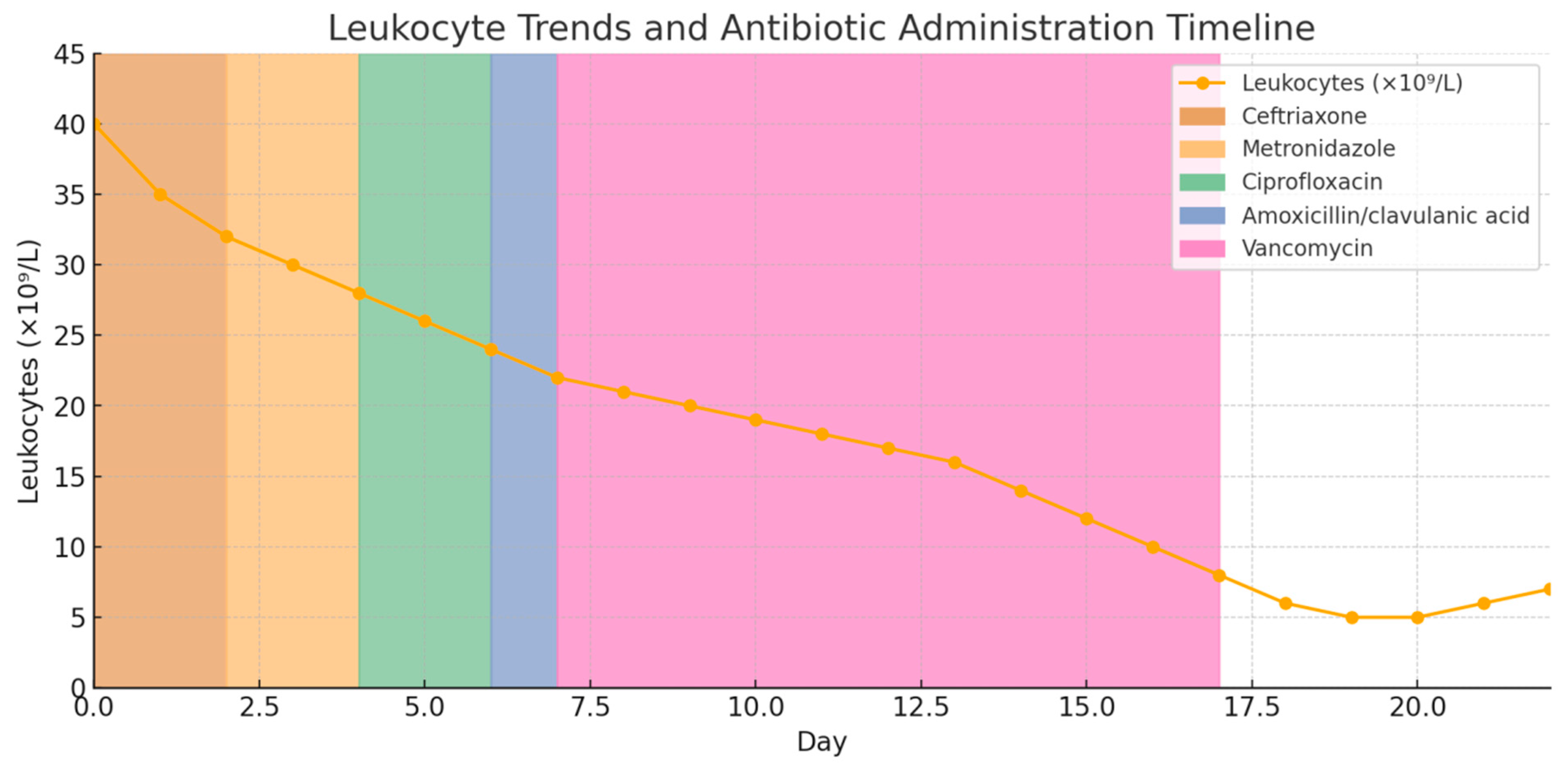
2. Case Presentation
3. Discussion
3.1. Pathophysiological Mechanisms of Antibiotic-Induced Agranulocytosis
3.2. Vancomycin as a Leading Cause of Hematologic Toxicity
3.3. Differential Diagnosis and Consideration of Other Agents
3.4. Monitoring, Management, and Role of G-CSF
4. Clinical Takeaway
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| WBC | White Blood Cell count |
| Neu | Neutrophils |
| Lymph | Lymphocytes |
| Eosin | Eosinophils |
| Baso | Basophils |
| Mono | Monocytes |
| Rbc | Red Blood Cells |
| Hgb | Hemoglobin |
| Plt | Platelets |
| CRP | C-Reactive Protein |
| G-CSF | Granulocyte-Colony Stimulating Factor |
| MRSA | Methicillin-Resistant Staphylococcus aureus |
| CBC | Complete Blood Count |
| IU | International Units |
References
- Cunha, B.A. Antibiotic side effects. Med. Clin. N. Am. 2001, 85, 149–185. [Google Scholar]
- Dickter, J.; Logan, C.; Taplitz, R. Neutropenia and antibiotics: When, what, how and why? Curr. Opin. Infect. Dis. 2023, 36, 218–227. [Google Scholar] [PubMed]
- Dale, D.C. How I diagnose and treat neutropenia. Curr. Opin. Hematol. 2016, 23, 1–4. [Google Scholar] [PubMed]
- Andersohn, F.; Konzen, C.; Garbe, E. Systematic review: Agranulocytosis induced by nonchemotherapy drugs. Ann. Intern. Med. 2007, 146, 657–665. [Google Scholar]
- Schwartzberg, L.S. Neutropenia: Etiology and pathogenesis. Clin. Cornerstone 2006, 8 (Suppl. 5), S5–S11. [Google Scholar] [PubMed]
- di Fonzo, H.; Villegas Gutsch, M.; Castroagudin, A.; Cabrera, M.V.; Mazzei, M.E.; Rueda, D. Agranulocytosis Induced by Vancomycin: Case Report and Literature Review. Am. J. Case Rep. 2018, 19, 1053–1056. [Google Scholar]
- Asai, Y.; Yamamoto, T.; Abe, Y. Comprehensive Analysis of Antibiotic-Induced Agranulocytosis Using the Japanese Adverse Drug Event Report Database. Jpn. J. Antibiot. 2021, 74, 218–226. [Google Scholar]
- Tesfa, D.; Keisu, M.; Palmblad, J. Idiosyncratic drug-induced agranulocytosis: Possible mechanisms and management. Am. J. Hematol. 2009, 84, 428–434. [Google Scholar]
- Cimino, C.; Allos, B.M.; Phillips, E.J. A Review of β-Lactam-Associated Neutropenia and Implications for Cross-reactivity. Ann. Pharmacother. 2021, 55, 1037–1049. [Google Scholar] [CrossRef]
- Sedhai, Y.R.; Lamichhane, A.; Gupta, V. Agranulocytosis. 2023 May 23. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
- Segarra-Newnham, M.; Tagoff, S.S. Probable vancomycin-induced neutropenia. Ann. Pharmacother. 2004, 38, 1855–1859. [Google Scholar]
- Iwazawa, H.; Tanaka, H.; Tatsumichi, T.; Yamaguchi, K.; Takahashi, K.; Suzuki, K.; Motoki, T.; Kanenishi, K.; Kosaka, S. A puerperal patient with agranulocytosis during tazobactam/piperacillin administration: A case report. J. Med. Investig. 2021, 68, 368–371. [Google Scholar] [CrossRef]
- Lam, P.W.; Leis, J.A.; Daneman, N. Antibiotic-induced neutropenia in patients receiving outpatient parenteral antibiotic therapy: A retrospective cohort study. Antimicrob. Agents Chemother. 2023, 67, e0159622. [Google Scholar]
- Holz, J.M. Granulocyte colony-stimulating factor in the treatment of drug-induced agranulocytosis: Experience and recommendations. J. Intern. Med. 2002, 251, 508–514. [Google Scholar]
- Van Tuyl, J.S.; Jones, A.N.; Johnson, P.N. Meropenem-induced neutropenia in a neonate. J. Pediatr. Pharmacol. Ther. 2016, 21, 353–357. [Google Scholar]
- Schattner, A.; Dubin, I. Amoxicillin/clavulanic acid-associated severe neutropenia. Postgrad. Med. J. 2022, 98, 251. [Google Scholar] [PubMed]
- Spetrino, N.; Arazzi, M.; Di Fulvio, G.; Giunta, F.; Grabocka, X.; Longo, M.O.; Micioni, G.; Pezzutto, A.; Silvestri, D.; Tondo, M.; et al. Severe neutropenia during treatment with amoxicillin/clavulanic acid in a patient on regular hemodialysis treatment. G. Ital. Nefrol. 2016, 33, 6. [Google Scholar]
- Villalba, A.; Haq, M.; Zhang, C.; Thakkar, J. Amoxicillin/Clavulanic Acid-Induced Agranulocytosis: A Case Report and Review of the Literature. Case Rep. Hematol. 2019, 2019, 5718716. [Google Scholar]
- Couto, L.; Goulart, A.; Valadão, I.; Garça, M.; Santos, M.B.; Cota, P.; Teixeira, C. Ceftriaxone-induced agranulocytosis. Eur. J. Case Rep. Intern. Med. 2021, 8, 002215. [Google Scholar] [PubMed]
- Aung, Z.Y.; Elmagraby, K.; Nica, A. Neutropenia induced by ceftriaxone and meropenem. Eur. J. Case Rep. Intern. Med. 2024, 11, 004593. [Google Scholar]
- Finegold, S.M. Metronidazole. Ann. Intern. Med. 1980, 93, 585–587. [Google Scholar]
- Bergan, T. Neutropenia associated with metronidazole. Br. Med. J. 1979, 2, 1219. [Google Scholar] [PubMed][Green Version]
- Curtis, B.R. Non-chemotherapy drug-induced neutropenia: Key points to manage the risk. Hematol. Am. Soc. Hematol. Educ. Program 2017, 2017, 187–193. [Google Scholar][Green Version]
- Klastersky, J.; de Naurois, J.; Rolston, K.; Rapoport, B.; Maschmeyer, G.; Aapro, M.; Herrstedt, J.; ESMO Guidelines Committee. Management of febrile neutropaenia: ESMO Clinical Practice Guidelines. Ann. Oncol. 2016, 27, 111–118. [Google Scholar][Green Version]
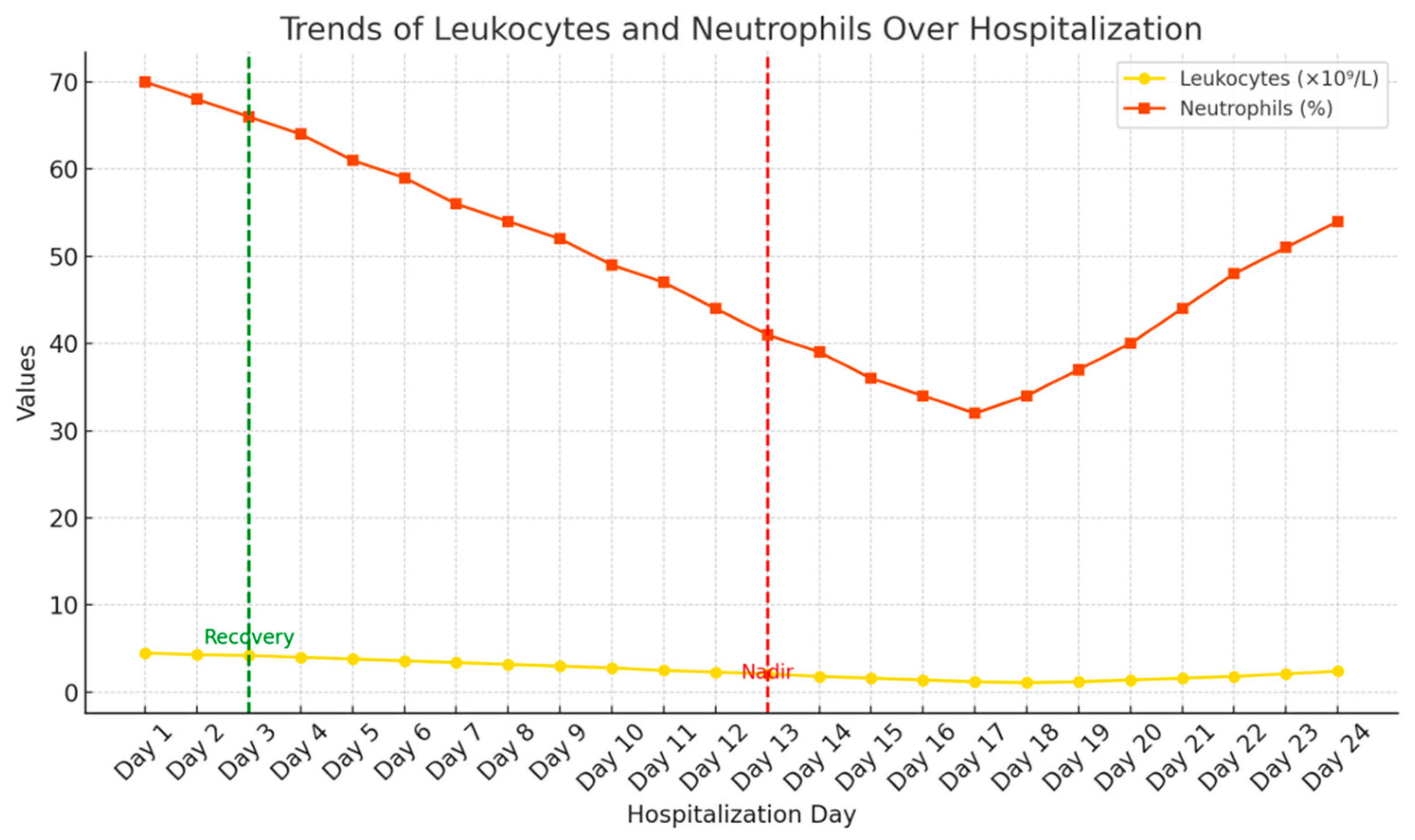

| WBC | 23.1 | 109/L |
| Neu | 90.3 | % |
| Lymph | 5.3 | % |
| Eosin | 0.025 | % |
| Baso | 0.138 | % |
| Mono | 4.22 | % |
| Rbc | 4.27 | 1012/L |
| Hgb | 130.0 | g/L |
| Plt | 166.0 | 109/L |
| Author | Study Type | Antibiotic(s) Involved | Population/Setting | Main Findings |
|---|---|---|---|---|
| di Fonzo H. et al. (2018), [6] | Case Report | Vancomycin | Adult patient | Vancomycin-induced agranulocytosis confirmed via bone marrow exam; recovery post-discontinuation |
| Lam P.W. et al. (2023), [13] | Retrospective Cohort | Vancomycin, Ceftriaxone | Outpatients | Vancomycin linked to highest incidence of neutropenia among IV antibiotics (3.9%) |
| Villalba A. et al. (2019), [18] | Case Report | Amoxicillin–Clavulanate | Adult patient | Agranulocytosis resistant to drug withdrawal; G-CSF needed |
| Couto L. et al. (2021), [19] | Case Report | Ceftriaxone | Adult patient | Neutropenia reversed 48 h after discontinuation |
| Aung Z.Y. et al. (2024), [20] | Case Report | Ceftriaxone, Meropenem | Hospitalized adult | Agranulocytosis resolved after stopping both agents |
| Holz J.M. et al. (2020), [14] | Case Series | Vancomycin, Linezolid, Penicillin G, TMP-SMX | Hospitalized adults | Vancomycin was the most frequently implicated antibiotic (21.7% of cases); median onset of agranulocytosis was 21 days after initiation. G-CSF shortened the duration of neutropenia in most cases. |
| Asai Y. et al. (2022), [7] | Pharmacovigilance Study | Vancomycin | JADER database | Vancomycin had the strongest signal for agranulocytosis among all antibiotics analyzed (72 cases; reporting odds ratio (ROR) 3.54, 95% CI 2.73–4.54). |
| Cimno C. et al. 2020, [9] | Review | β-Lactam-associated neutropenia | Clinical cases | Approximately 10% of patients on prolonged intravenous β-lactam therapy developed neutropenia. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tulic, L.; Ivanovic, K.; Tulic, I.; Vrzic-Petronijevic, S.; Ivanovic, S.; Bratic, D.; Petronijevic, M. A Puerperal Patient with Leukopenia During Vancomycin Administration: A Case Report and Review of the Literature. Int. J. Mol. Sci. 2025, 26, 6584. https://doi.org/10.3390/ijms26146584
Tulic L, Ivanovic K, Tulic I, Vrzic-Petronijevic S, Ivanovic S, Bratic D, Petronijevic M. A Puerperal Patient with Leukopenia During Vancomycin Administration: A Case Report and Review of the Literature. International Journal of Molecular Sciences. 2025; 26(14):6584. https://doi.org/10.3390/ijms26146584
Chicago/Turabian StyleTulic, Lidija, Katarina Ivanovic, Ivan Tulic, Svetlana Vrzic-Petronijevic, Stefan Ivanovic, Danijela Bratic, and Miloš Petronijevic. 2025. "A Puerperal Patient with Leukopenia During Vancomycin Administration: A Case Report and Review of the Literature" International Journal of Molecular Sciences 26, no. 14: 6584. https://doi.org/10.3390/ijms26146584
APA StyleTulic, L., Ivanovic, K., Tulic, I., Vrzic-Petronijevic, S., Ivanovic, S., Bratic, D., & Petronijevic, M. (2025). A Puerperal Patient with Leukopenia During Vancomycin Administration: A Case Report and Review of the Literature. International Journal of Molecular Sciences, 26(14), 6584. https://doi.org/10.3390/ijms26146584






