Glutathione Transferases Omega-1 and -2 Polymorphisms in Cancer: Drivers or Silent Bystanders?
Abstract
1. Introduction
2. The Omega Class of GSTs
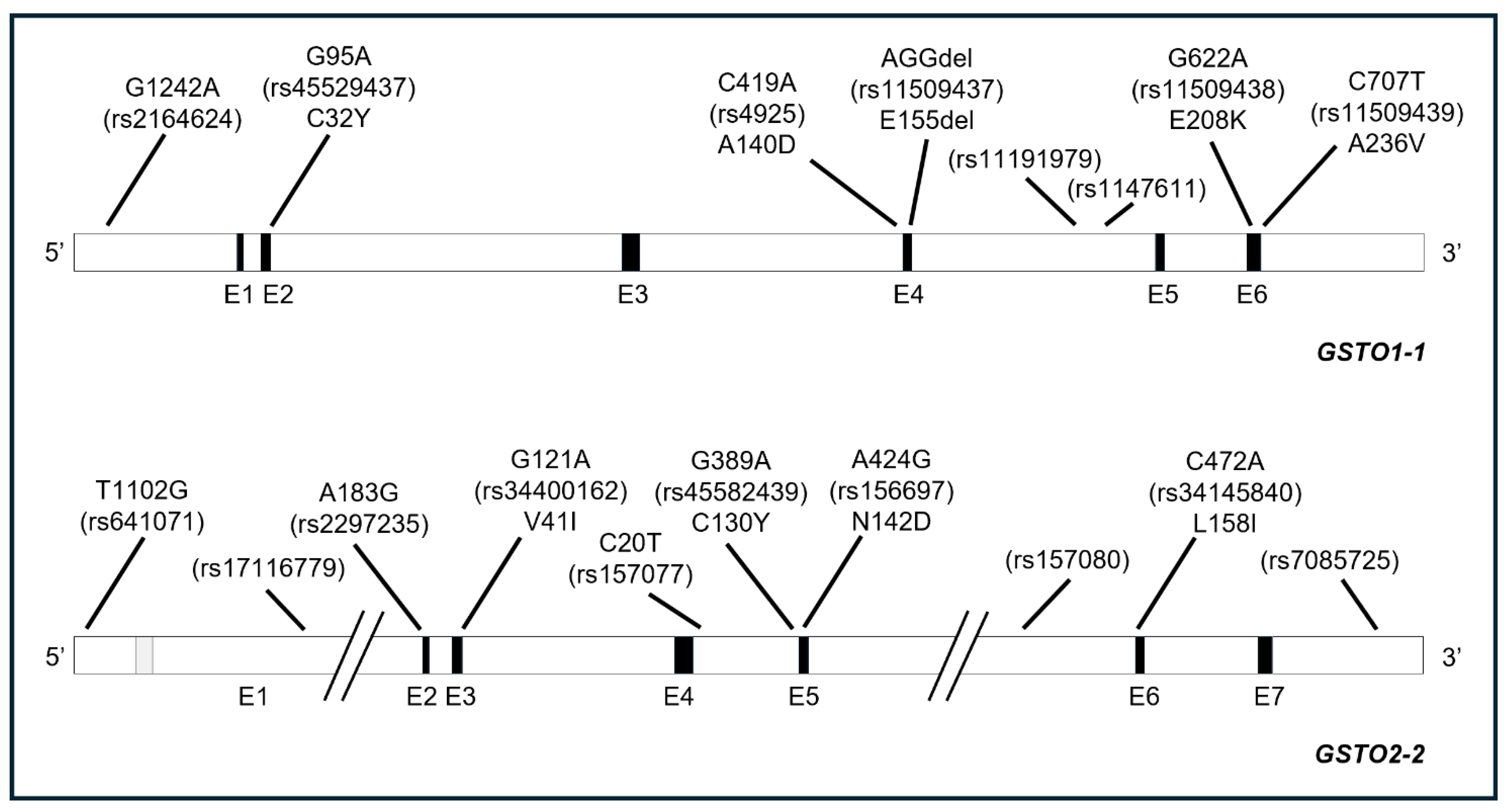
3. GSTO Polymorphisms
3.1. Allele Frequencies
3.2. Effects of GSTOs’ Polymorphisms: Expression and Functions
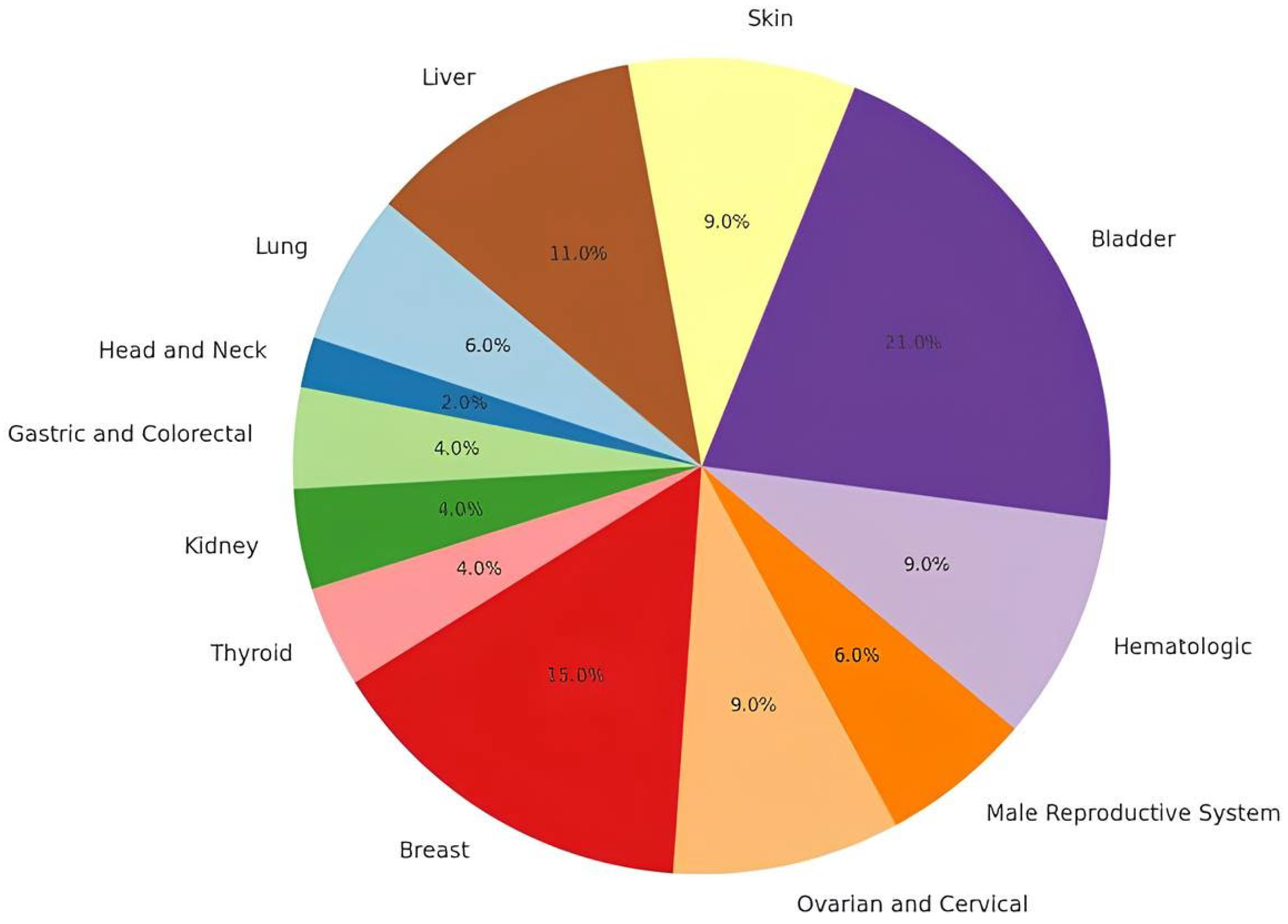
4. GSTOs’ Polymorphisms and Cancer
4.1. Bladder Cancers
4.2. Skin Cancers
4.3. Liver Cancers
4.4. Lung Cancers
4.5. Head and Neck Cancers
4.6. Gastrointestinal Cancers
4.7. Kidney Cancers
4.8. Thyroid Cancer
4.9. Breast Cancer
4.10. Ovarian and Cervical Cancers
4.11. Male Reproductive System Cancers
4.12. Hematologic Cancers
| Type of Cancer | Country | Number of Patients and Control Subjects | Main Findings | Ref. |
|---|---|---|---|---|
| Urothelial bladder carcinoma (UC) | Taiwan | 764 DNA specimens from a long-term follow-up cohort in southwestern Taiwan | Polymorphisms: GSTO1*A140D (C419A; rs4925), GSTO1*E155del (AGG deletion; rs11509437), GSTO1*E208K (G622A; rs11509438), GSTO1*T217N (C650A; rs15032), GSTO2 5′UTR (-183) (A183G; rs2297235), GSTO2*N142D (A424G; rs 156697) Main findings: Increased UC risk in subjects exposed to high arsenic concentrations (cumulative arsenic exposure (CAE) ≥ 20 mg/L*year) and carrying - GSTO1*A140D homozygous variant; - diplotype AGG/AGG of GSTO1*A140D (C419A), GSTO2 5′UTR (-183) (A183G; rs2297235) and GSTO2*N142D (A424G; rs 156697). | [48] |
| Bladder cancer | USA | 219 patients vs. 273 controls | Polymorphisms: GSTO1*A140D (C419A; rs4925), intron variant GSTO-1 (A/C; rs2282326) Main findings: - no significant associations between GSTO-1 polymorphisms and bladder cancer risk overall; - positive association between arsenic exposure and bladder cancer among subjects carrying the homozygous wild-type GSTO1*A140D. | [65] |
| Urothelial bladder carcinoma (UC) | Taiwan | 520 UC cases vs. 520 controls | Polymorphisms: GSTO1*A140D (C419A; rs4925), GSTO2*N142D (A424G; rs156697), GSTO2 polymorphism in 5′UTR gene region (A183G; rs2297235) Main findings: - increased risk of UC for subjects carrying the G/G genotype of GSTO2*A183G; - increased risk of UC for the diplotype GSTO1*C419/GSTO2*A183/GSTO2*G424; - joint effects of GSTO1, GSTO2, and CYP2E1 genotypes and environmental factors (cigarette smoking, alcohol consumption, arsenic, and occupational exposures) on UC risk. | [69] |
| Urothelial bladder carcinoma (UC) | Taiwan | 149 UC cases vs. 251 controls | Polymorphisms: GSTO1*A140D (C419A; rs4925), GSTO1*E208K (G622A; rs11509438), GSTO2*N142D (A424G; rs156697) Main findings: - reduced risk of UC for subjects carrying the homozygous GSTO2*N142D variant; - no association between GSTO1*A140D or GSTO1*E208K and UC risk. | [70] |
| Bladder cancer | USA | 832 patients vs. 1191 controls | Polymorphisms: GSTO2*N142D (A424G; rs156697) and other GSTs (GSTP1, GSTM1, GSTT1, GSTZ1) Main findings: - positive association between GSTO2*N142D variant and bladder cancer risk independently of arsenic exposure. | [71] |
| Muscle invasive bladder cancer | Serbia | 105 patients | Polymorphisms: GSTO1*A140D (C419A; rs4925), GSTO2*N142D (A424G; rs156697), and other GSTs (GSTT1, GSTP1, GSTM1, GSTA1) Main findings: - worse prognosis and shorter survival for homozygous mutant GSTO1 Asp140Asp (*AA) and GSTO2 Asp142Asp (*GG) genotypes; - shorter survival in patients receiving chemotherapy and homozygous mutant for GSTO2 Asp142Asp. | [72] |
| Urothelial carcinoma of the bladder (UCB) | Taiwan | 300 UC patients vs. 233 controls | Polymorphisms: GSTO1*A140D (C419A; rs4925), sulfotransferase 1A1 (SULT1A1)*R213H Main findings: - increased UC risk in subjects with GSTO1 Ala/Ala and SULT1A1 Arg/Arg variants; - increased UC risk in heavy smokers with GSTO1 Ala/Ala and SULT1A1 Arg/Arg variants; - synergistic effect between the GSTO1 Ala/Ala SULT1A1 Arg/Arg genotype on UCB risk. | [73] |
| Transitional cell carcinoma (TCC) of urinary bladder | Serbia | 187 patients vs. 140 controls | Polymorphisms: GSTO1*A140D (C419A; rs4925), GSTO2*N142D (A424G; rs156697) Main findings: - increased TCC risk in subjects with homozygous mutant GSTO2 Asp142Asp genotype (*G/G); - combined effect of wild-type GSTO1 (*C/C or *C/A) genotypes and mutant GSTO2 (*G/G) on TCC risk (haplotype CG); - combined effect of mutant GSTO2 (*G/G) genotype and smoking on TCC risk. | [74] |
| Non-muscle invasive bladder cancer (NMIBC) | China | Discovery cohort 244 patients Validated cohort 86 HCC patients | Polymorphisms: GSTO1*A140D (C419A; rs4925), GSTO2* N142D (A424G; rs156697), GSTP1 (rs1695), ABCB1 (rs3747802, rs3213619) Main findings: - unfavorable recurrence-free survival (RFS), mean survival time (MST), and epirubicin-related toxicity in subjects with at least one GSTO1*A140D variant (AC and AA genotypes); - no correlations in mitomycin-treated subjects; - no effect related to GSTO2* N142D variants. | [75] |
| Non-muscle invasive bladder cancer (NMIBC) | USA | 414 patients | Polymorphisms: A total of 114 SNPs of 21 genes involved in glutathione pathway including GSTO1 rs4925 (C419A), rs11509438 (G622A), GSTO2 rs641071 (-1102 T/G), rs156697 (A424G), and rs3740466 (3′UTR G/A) polymorphisms Main findings: - no significant associations of GSTO polymorphisms and cancer recurrence in patients who underwent to transurethral resection (TUR) alone or in combination with intravesical bacillus Calmette Guérin instillation (BCG) therapy. | [76] |
| Skin lesions | India | 229 cases vs. 199 controls | Polymorphisms: GSTO1*A140D (C419A; rs4925), GSTO1*E208K (G622A; rs11509438), GSTO1*E155del (AGG deletion; rs11509437), GSTO2*N142D (A424G; rs156697), and polymorphisms of purine nucleoside phosphorylase (PNP), arsenic (+3) methyltransferase (As3MT). Main findings: - no association between GSTO1-1 or GSTO2-2 variants and arsenic-induced skin lesions. | [39] |
| Skin lesions | China | 331 patients vs. 519 controls | Polymorphisms: GSTO1 SNPs: rs11191979, rs2164624 (-1242 G/A), rs2282326, rs4925 (C419A); GSTO2 SNPs: rs156697 (A424G), rs157077 (C20T), and rs2297235; other SNPs in PNP. Main findings: - increased risk of arsenic-related skin lesions in carriers of at least one GSTO1*C allele (rs11191979), GSTO1*A allele (rs2164624), and GSTO1*A allele (rs4925); - increased risk of arsenic-related skin lesions in carriers of at least one GSTO2*G allele (rs2297235); - increased risk of arsenic-related skin lesions in carriers of the AG genotype for GSTO2 rs156697 and rs2297235; - increased risk of arsenic-related skin lesions in carriers of haplotype CT between rs4925 and rs11191979 and haplotype GCG among rs156697, rs157077, and rs2297235. | [49] |
| Premalignant skin lesions | Bangladesh | 594 patients vs. 1041 controls | Polymorphisms: GSTO1*A140D (C419A; rs4925), GSTO1*E208K (G622A; rs11509438), GSTO1*E155del (AGG deletion; rs11509437), and methylenetetrahydrofolate reductase (MTHFR rs1801133 and rs1801131) Main findings: - increased risk of skin lesions for subjects carrying the A/A genotype of GSTO1 C419A; - reduced risk of skin lesions for subjects carrying rs11509438 and rs11509437 variants of GSTO1-1; - elevated risk of skin lesions for subjects carrying the AAG/AAG diplotype for the three SNPs of GSTO1 analyzed. | [79] |
| Basal cell skin carcinoma (BCC) | Brazil | 102 patients vs. 124 controls | Polymorphisms: GSTO2*N142D (A424G; rs156697) and variants or deletions of other GSTs (GSTM1, GSTT1, GSTP1) Main findings: - no significant difference between GSTO2-2 polymorphism levels in different types of skin tumors. | [80] |
| Hepatocellular carcinoma (HCC) cholangiocarcinoma colorectal cancer breast cancer | Thailand | 28 cases of HCC, 30 cases of cholangiocarcinoma, 31 cases of colorectal cancer, 30 cases of breast cancer vs. 98 controls | Polymorphisms: GSTO1*A140D (C419A; rs4925), GSTO2*N142D (A424G; rs156697) Main findings: - association of GSTO1*A140D polymorphism with HCC, cholangiocarcinoma, and breast cancer; - no significant association of GSTO1*A140D polymorphism and colorectal cancer; - no significant association of GSTO2*N142D polymorphism with any type of cancer. | [38] |
| Hepatocellular carcinoma (HCC) | China | 214 HCC patients | Polymorphisms: GSTO1: rs2282326; GSTO2: rs17116779, rs156699, rs7085725, rs157077 (C20T); other GSTs (GSTA1, GSTA4, GSTM2, GSTM3, and GSTP1) Main findings: - increased risk of death and a shorter median survival time in subjects carrying at least one variant allele of GSTO2 rs7085725; - increased risk of death in subjects with both GSTO2 rs7085725 and GSTP1 rs4147581 genotypes; - GSTO2 and GSTP1 gene polymorphisms may serve as independent prognostic markers for HCC. | [81] |
| Hepatocellular carcinoma (HCC) | China | 228 patients | Polymorphisms: GSTO2: rs156699, rs157077 (C20T) and rs7085725 Main findings: - significant association between rs157077 variant of GSTO2-2 and OS of transarterial chemoembolization (TACE)-treated HCC patients. | [82] |
| Hepatocellular Carcinoma (HCC) | Egypt | 320 patients vs. 150 healthy controls | Polymorphisms: GSTO1*A140D (C419A; rs4925), GSTO2* N142D (A424G; rs156697) Main findings: - increased risk of hepatitis B virus (HBV) disease progression in subjects with GSTO1*A140 wild-type allele and GSTO2*N142D variant allele. | [83] |
| Cholangiocarcinoma (CCA) | USA | Discovery cohort 370 patients vs. 740 healthy controls Validation cohort 212 patients vs. 424 healthy controls | Polymorphisms: GSTO1*A140D (C419A; rs4925) and SNPs in other inflammation or cancer- associated genes (COX-2, IL6, IL6R, IL6ST, SULF1, VEGFA, WRAP53, NKG2D) Main findings: - no association between allele frequency of GSTO1*A140D variant (and SNPs of the other genes selected) and CCA risk or survival. | [84] |
| Lung cancer | USA | 620 cases of COPD (432 with lung cancer and 188 without) vs. 893 controls | Polymorphisms: Five GSTO1 polymorphisms (rs11191975, rs1147611, rs11509438, rs2164624, rs628480) and six GSTO2 polymorphisms (rs10491045, rs11191990, rs156699, rs157077, rs157080, rs568526) as a part of 470 SNPs in 52 genes involved in glutathione metabolism, DNA repair, and inflammatory response pathways Main findings: - significant association between GSTO2-2 gene and COPD with lung cancer. | [87] |
| Lung cancer | USA | 973 patients with both small cell and non-small cell lung cancer, with platinum-based chemotherapy. | Polymorphisms: Six GSTO1 (including rs4925 and rs2164624) polymorphisms, nine GSTO2 polymorphisms (including rs156697), and other SNPs in glutathione-associated enzymes Main findings: - no association between GSTO1-1 or GSTO2-2 polymorphisms analyzed and lung cancer survival upon cisplatin chemotherapy. | [88] |
| Non-small cell lung cancer (NSCLC) | Turkey | 172 patients vs. 214 healthy controls | Polymorphisms: GSTO1*A140D (C419A; rs4925) Main findings: - no significant association between GSTO1*A140D variant and risk of NSCLC. | [89] |
| Head and neck squamous cell carcinoma (HNSCC) | Thailand | 300 patients vs. 299 controls | Polymorphisms: GSTO1*A140D (C419A; rs4925), GSTO2*N142D (A424G; rs156697) Main findings: - no significant differences in GSTO1 and GSTO2 genotypes between HNSCC and controls; - significant association between wild-type GSTO1*A140 and nodal metastasis and advanced pathological stage; - no significant association between GSTO2*N142D variant and clinico-pathological features. | [91] |
| Gastric cancer | Iran | 67 patients vs. 134 controls | Polymorphisms: GSTO2*N142D (A424G; rs156697) and polymorphisms of other GSTs (GSTM1, GSTT1) Main findings: - no association between GSTO2*N142D variant and gastric cancer risk; - decreased risk of gastric cancer in subjects without history of cancer in their first-degree relatives and with homozygous GSTO2*N142D (D/D) genotype. | [92] |
| Colorectal cancer | Iran | 63 patients vs. 126 healthy controls | Polymorphisms: GSTO2*N142D (A424G; rs 156697) Main findings: - no association between GSTO2*N142D variant and gastric cancer risk; - increased risk of gastric cancer in subjects with history of cancer in their first-degree relatives and with homozygous wild-type GSTO2*N142 (N/N) genotype. | [93] |
| Clear cell renal cell carcinoma (ccRCC) | Serbia | 239 patients vs. 350 controls | Polymorphisms: GSTO1*A140D (C419A; rs4925), GSTO2*N142D (A424G; rs156697), GSTO2 polymorphism in 5′UTR gene region (A183G; rs2297235) Main findings: - increased ccRCC risk in subjects with combined homozygous GSTO1*A140D (D/D) and GSTO2*N142D (D/D) variants; - increased risk of ccRCC in subjects with GSTO1*A (rs4925), GSTO2*G (rs156697), and GSTO2*G (rs2297235) haplotype (H2) compared to haplotype including all three referent alleles (H1); - increased risk of ccRCC in smokers with homozygous GSTO2*N142D (rs156697) variant. | [94] |
| Clear cell renal cell carcinoma (ccRCC) | Serbia | 228 patients | Polymorphisms: GSTO1*A140D (C419A; rs4925), GSTO2*N142D (A424G; rs156697), GSTO2 polymorphism in 5′UTR gene region (A183G; rs2297235). Main findings: - shorter survival in male carriers of GSTO1*C/C wild-type genotype (GSTO1*A140) compared to the carriers of at least one variant allele; - increased risk of overall mortality among male ccRCC patients with GSTO1*C/C wild-type genotype. | [95] |
| Thyroid cancer | Brazil | 145 patients with thyroid nodules (follicular carcinomas, papillary carcinomas, follicular adenoma, multinodular goiters) vs. 173 controls | Polymorphisms: GSTO1*A140D (C419A; rs4925) Main findings: - no association between GSTO1*A140D polymorphism and risk and clinico-pathological features of thyroid nodules. | [96] |
| Thyroid cancer | Brazil | 248 patients with thyroid nodules (benign goiters, follicular adenomas, papillary carcinomas, follicular carcinomas) vs. 277 controls | Polymorphisms: GSTO1*A140D (C419A; rs4925), polymorphisms of other GSTs (GSTM1, GSTT1, GSTP1), CYP1A1, and codon 72 of p53 Main findings: - no association between GSTO1*A140D polymorphism (alone or in combination with other polymorphisms) and clinico-pathological features of thyroid nodules. | [97] |
| Breast cancer | Germany | 1348 patients | Polymorphisms: GSTO1*A140D (C419A; rs4925) and other polymorphisms in oxidative stress-related genes Main findings: - no significant association between GSTO1*A140D variant and overall mortality or possible differential effects by radiotherapy treatment. | [30] |
| Breast cancer | Pakistan | 100 patients vs. 100 healthy controls | Polymorphisms: GSTO2*N142D (A424G; rs156697) and polymorphisms of other GSTs (GSTM1, GSTT1, GSTP1) Main findings: - increased risk of breast cancer in postmenopausal woman homozygous for GSTO2*N142D; - increased risk of breast cancer in premenopausal women with the combined presence of GSTP1 and GSTO2 polymorphisms. | [31] |
| Breast cancer | Denmark | 396 patients vs. 396 matched controls | Polymorphisms: GSTO1*A140D (C419A; rs4925), GSTA1*A. and GSTA1*B alleles. Main findings: - increased risk of estrogen receptor-positive breast cancer for postmenopausal women carrying homozygous GSTO1*A140D variant, as compared with homozygous wild-type; - no clear association between the GSTA1 polymorphism and breast cancer among postmenopausal women. | [98] |
| Breast cancer | Thailand | 101 patients vs. 151 controls | Polymorphisms: GSTO1*A140D (C419A; rs4925), GSTO2*N142D (A424G; rs156697) Main findings: - no association between GSTO1-1 and GSTO2-2 genotypes and the risk of breast cancer; - higher prevalence of homozygous wild-type GSTO1*A140 genotype is significantly correlated with advanced-stage breast cancer. | [99] |
| Breast cancer | Germany | 1021 patients vs. 1015 controls | Polymorphisms: GSTO1*A140D (C419A; rs4925), GSTO1 polymorphism in 5′UTR gene region (G1242A; rs2164624), GSTO2*N142D (A424G; rs156697), GSTO2 polymorphism in 5′UTR gene region (A183G; rs2297235), and polymorphisms of other GSTs (GSTA2, GSTM2, GSTZ) Main findings: - no association between GSTO polymorphisms and breast cancer risk; - no association between GSTO polymorphisms and histopathological tumor characteristics. | [100] |
| Breast cancer | Iran | 181 patients vs. 181 controls | Polymorphisms: GSTO2*N142D (A424G; rs156697), polymorphisms of other GSTs (GSTM1, GSTT1), and XRCC1 Main findings: - no association between GSTO2*N142D polymorphism (allele frequencies and genotypes) and breast cancer risk. | [101] |
| Breast cancer | Iran | 153 patients vs. 150 healthy controls | Polymorphisms: GSTO1*A140D (C419A; rs4925), GSTO2*N142D (A424G; rs156697) Main findings: - increased risk of breast cancer in subjects with A allele of GSTO1*A140D (C419A; rs4925) and G allele of GSTO2*N142D (A424G; rs156697); - increased risk of breast cancer in subjects with CA+AA genotype for GSTO1-1 and GG genotype for GSTO2-2. | [102] |
| Ovarian cancer | Brazil | 69 patients vs. 222 healthy controls | Polymorphisms: GSTO2*N142D (A424G; rs156697), polymorphisms of other GSTs (GSTT1, GSTM1, GSTP1), and codon 72 of p53 Main findings: - no association between GSTO2*N142D variant and the risk of ovarian cancer. | [104] |
| Ovarian cancer | Thailand | 20 patients vs. 41 controls | Polymorphisms: GSTO2*N142D (A424G; rs156697) Main findings: - increased risk of ovarian cancer in subjects with GSTO2*N142D (A424G) polymorphism. | [105] |
| Cervical cancer (CC) | Iran | 50 CC patients vs. 43 patients positive for HPV vs. 43 controls | Polymorphisms: GSTO1*A140D (C419A; rs4925), GSTO2*N142D (A424G; rs156697) Main findings: - reduced risk of human papillomavirus (HPV) 6, 16, 18, and 16/18 infections and CC in subjects with GSTO1*D140 variant (GSTO1*A allele; rs4925), heterozygous GSTO1*A140D (*C/*A genotype), and the combination of heterozygous GSTO1*A140D (140AD) and homozygous wild-type GSTO2*N142 (142NN); no association between GSTO2*N142D polymorphism and HPV infections and cervical cancer. | [107] |
| Ovarian cancer (OC) | Serbia | 110 patients vs. 129 controls | Polymorphisms: GSTO1*A140D (C419A; rs4925), GSTO2*N142D (A424G; rs156697) Main findings: - increased OC risk in homozygous carriers of GSTO2*G (A424G) variant allele; - reduced OC risk in carriers of both GSTO1*A variant allele and GSTO2*A wild-type one (haplotype H4; *A*A). | [106] |
| Testicular germ cell tumor (GCT) | Serbia | 88 patients vs. 96 controls | Polymorphisms: GSTO1*A140D (C419A; rs4925), GSTO2*N142D (A424G; rs156697), GSTO2 polymorphism in 5′UTR gene region (A183G; rs2297235) Main findings: - increased testicular GCT risk in subjects with GSTO1*C/A*C/C genotypes (rs4925; A/D, D/D) and in GSTO2*A/G*G/G genotypes (rs2297235); - increased testicular GCT risk in carriers of combined GSTO2*A/G*G/G (rs156697; N/D, D/D) and GSTO2*A/G*G/G (rs2297235) genotypes. | [34] |
| Prostate cancer | Brazil | 125 patients vs. 100 benign prostatic hyperplasia patients | Polymorphisms: GSTO1*A140D (C419A; rs4925), polymorphisms of other GSTs (GSTT1, GSTM1, GSTP1), and CYP1A1 Main findings: - no significant difference in the frequency of the GSTO1*A140D polymorphism between patients with benign prostatic hyperplasia and those with prostate cancer; - no association of GSTO1*A140D polymorphism with parameters of aggressiveness or response to radiotherapy in prostate cancer patients. | [110] |
| Prostate cancer (PC) | Serbia | 237 patients vs. 236 controls | Polymorphisms: GSTO1*A140D (C419A; rs4925), GSTO2*N142D (A424G; rs156697), and polymorphisms of other GSTs (GSTM1, GSTT1, GSTP1) Main findings: - increased PC risk in subjects with homozygous GSTO1*A/A (rs4925; D/D) and GSTO2*G/G (rs156697; D/D) variant genotypes; - increased PC risk in carriers of both GSTO1*A and GSTO2*G variant alleles (haplotype H2). | [111] |
| Acute lymphoblastic leukemia (ALL) | Thailand | 99 patients vs. 100 controls | Polymorphisms: GSTO1*A140D (C419A; rs4925), GSTO2*N142D (A424G; rs156697) Main findings: - increased ALL risk in with heterozygous GSTO1*A140D variant; - significant association between GSTO2*N142D polymorphism and high-risk group of ALL. | [40] |
| Pre-B acute lymphoblastic leukemia (ALL) | Iran | 100 patients vs. 120 controls | Polymorphisms: GSTO1*A140D (C419A; rs4925), GSTO2*N142D (A424G; rs156697) Main findings: - no association between allele frequency or genotypes of GSTO1*A140D and GSTO2*N142G variants and Pre-B ALL risk; - significant reverse correlation with Pre-B ALL risk, for 140AA/142DD or 140DD/142NN combination genotypes. | [112] |
| B-acute lymphoblastic leukemia (B-ALL) | India | 150 patients vs. 150 controls | Polymorphisms: GSTO1*A140D (C419A; rs4925), GSTO2*N142D (A424G; rs156697), and polymorphisms of other GSTs (GSTM1, GSTT1, GSTP1) Main findings: - no association between GSTO1*A140D (allele or genotype frequencies) and B-ALL risk; - increased cancer risk in subjects with variant G allele (D140) and for heterozygous GSTO2-AG (N/D) and homozygous GSTO2-GG (D/D) variant genotypes; - significant association between GSTO1*C/GSTO2*G haplotype and B-ALL risk; - significantly lower DFS in subjects with GSTO2*GG (D/D) genotype. | [113] |
| Mantle cell lymphoma (MCL) | USA | 89 patients treated with R-HyperCVAD at ten-year follow-up | Polymorphisms: Three GSTO1 polymorphisms (rs1147611, rs4925, rs2164624), three GSTO2 polymorphisms (rs156697, rs157080, rs568526), and other polymorphisms in genes involved in GSH metabolism and DNA damage repair pathways Main findings: - significant associations between three GSTO1 polymorphisms (rs1147611, rs4925, rs2164624) and OS; - significant association between two GSTO2 polymorphisms (rs156697, rs157080) and OS. | [114] |
5. Conclusions
- The physiological levels of GSTOs’ expression in the tissue/organ from which the tumor originates;
- The presence of alternative mechanisms involved in GSTOs-catalyzed reactions (e.g., arsenic metabolism);
- Different GSTOs’ polymorphism frequencies and types among ethnic groups;
- The cancer type, sources of the control, and sample size, i.e., the number of patients recruited, to generate a valuable hypothesis;
- Environmental factors. As stated above, GSTs are classified as low-penetrance genes that contribute to cancer risk when combined with environmental factors (e.g., arsenic, smoking, drugs, etc.);
- The relevance of the specific “mosaic” of functional GSTOs’ polymorphism alterations for a specific tumor; e.g., MMAV and DMAV reductase variations may have consequences in the presence of suitable arsenic concentrations and in some specific tissues; GSTO substrates of glutathionylation/deglutathionylation (and their alterations) may vary in influencing the progression of a specific cancer.
Author Contributions
Funding
Conflicts of Interest
References
- Tew, K.D.; Townsend, D.M. Glutathione-S-transferases as determinants of cell survival and death. Antioxid. Redox Signal. 2012, 17, 1728–1737. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.R.; Reindl, K.M. Glutathione S-Transferases in Cancer. Antioxidants 2021, 10, 701. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Flanagan, J.U.; Jowsey, I.R. Glutathione transferases. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 51–88. [Google Scholar] [CrossRef] [PubMed]
- Mazari, A.M.A.; Zhang, L.; Ye, Z.W.; Zhang, J.; Tew, K.D.; Townsend, D.M. The Multifaceted Role of Glutathione S-Transferases in Health and Disease. Biomolecules 2023, 13, 688. [Google Scholar] [CrossRef]
- Board, P.G.; Coggan, M.; Chelvanayagam, G.; Easteal, S.; Jermiin, L.S.; Schulte, G.K.; Danley, D.E.; Hoth, L.R.; Griffor, M.C.; Kamath, A.V.; et al. Identification, characterization, and crystal structure of the Omega class glutathione transferases. J. Biol. Chem. 2000, 275, 24798–24806. [Google Scholar] [CrossRef]
- Board, P.G.; Menon, D. Glutathione transferases, regulators of cellular metabolism and physiology. Biochim. Biophys. Acta. 2013, 1830, 3267–3288. [Google Scholar] [CrossRef]
- Whitbread, A.K.; Tetlow, N.; Eyre, H.J.; Sutherland, G.R.; Board, P.G. Characterization of the human Omega class glutathione transferase genes and associated polymorphisms. Pharmacogenetics 2003, 13, 131–144. [Google Scholar] [CrossRef]
- Wang, L.; Xu, J.; Ji, C.; Gu, S.; Lv, Y.; Li, S.; Xu, Y.; Xie, Y.; Mao, Y. Cloning, expression and characterization of human glutathione S-transferase Omega 2. Int. J. Mol. Med. 2005, 16, 19–27. [Google Scholar]
- Brock, J.; Board, P.G.; Oakley, A.J. Structural insights into omega-class glutathione transferases: A snapshot of enzyme reduction and identification of a non-catalytic ligandin site. PLoS ONE 2013, 8, e60324. [Google Scholar] [CrossRef]
- Yamamoto, K.; Suzuki, M.; Higashiura, A.; Nakagawa, A. Three-dimensional structure of a Bombyx mori Omega-class glutathione transferase. Biochem. Biophys. Res. Commun. 2013, 438, 588–593. [Google Scholar] [CrossRef]
- Zhou, H.; Brock, J.; Liu, D.; Board, P.G.; Oakley, A.J. Structural insights into the dehydroascorbate reductase activity of human omega-class glutathione transferases. J. Mol. Biol. 2012, 420, 190–203. [Google Scholar] [CrossRef] [PubMed]
- Schmuck, E.M.; Board, P.G.; Whitbread, A.K.; Tetlow, N.; Cavanaugh, J.A.; Blackburn, A.C.; Masoumi, A. Characterization of the monomethylarsonate reductase and dehydroascorbate reductase activities of Omega class glutathione transferase variants: Implications for arsenic metabolism and the age-at-onset of Alzheimer’s and Parkinson’s diseases. Pharmacogenet. Genom. 2005, 15, 493–501. [Google Scholar] [CrossRef]
- Maellaro, E.; Del Bello, B.; Sugherini, L.; Santucci, A.; Comporti, M.; Casini, A.F. Purification and characterization of glutathione-dependent dehydroascorbate reductase from rat liver. Biochem. J. 1994, 301 Pt 2, 471–476. [Google Scholar] [CrossRef]
- Zakharyan, R.A.; Sampayo-Reyes, A.; Healy, S.M.; Tsaprailis, G.; Board, P.G.; Liebler, D.C.; Aposhian, H.V. Human monomethylarsonic acid (MMA(V)) reductase is a member of the glutathione-S-transferase superfamily. Chem. Res. Toxicol. 2001, 14, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- Aposhian, H.V.; Zakharyan, R.A.; Avram, M.D.; Sampayo-Reyes, A.; Wollenberg, M.L. A review of the enzymology of arsenic metabolism and a new potential role of hydrogen peroxide in the detoxication of the trivalent arsenic species. Toxicol. Appl. Pharmacol. 2004, 198, 327–335. [Google Scholar] [CrossRef]
- Whitbread, A.K.; Masoumi, A.; Tetlow, N.; Schmuck, E.; Coggan, M.; Board, P.G. Characterization of the omega class of glutathione transferases. Methods Enzymol. 2005, 401, 78–99. [Google Scholar] [CrossRef]
- Board, P.G.; Anders, M.W. Glutathione transferase omega 1 catalyzes the reduction of S-(phenacyl)glutathiones to acetophenones. Chem. Res. Toxicol. 2007, 20, 149–154. [Google Scholar] [CrossRef]
- Menon, D.; Board, P.G. A role for glutathione transferase Omega 1 (GSTO1-1) in the glutathionylation cycle. J. Biol. Chem. 2013, 288, 25769–25779. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Rossi, R.; Colombo, G.; Giustarini, D.; Milzani, A. Protein S-glutathionylation: A regulatory device from bacteria to humans. Trends Biochem. Sci. 2009, 34, 85–96. [Google Scholar] [CrossRef]
- Pastore, A.; Piemonte, F. S-Glutathionylation signaling in cell biology: Progress and prospects. Eur. J. Pharm. Sci. 2012, 46, 279–292. [Google Scholar] [CrossRef]
- Board, P.G.; Menon, D. Structure, function and disease relevance of Omega-class glutathione transferases. Arch. Toxicol. 2016, 90, 1049–1067. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.M.; Hooftman, A.; Angiari, S.; Tummala, P.; Zaslona, Z.; Runtsch, M.C.; McGettrick, A.F.; Sutton, C.E.; Diskin, C.; Rooke, M.; et al. Glutathione Transferase Omega-1 Regulates NLRP3 Inflammasome Activation through NEK7 Deglutathionylation. Cell Rep. 2019, 29, 151–161.e5. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Choi, J.; Iram, S.; Kim, J. Regulation of Glutathione S-Transferase Omega 1 Mediated by Cysteine Residues Sensing the Redox Environment. Int. J. Mol. Sci. 2024, 25, 5279. [Google Scholar] [CrossRef]
- Piaggi, S.; Raggi, C.; Corti, A.; Pitzalis, E.; Mascherpa, M.C.; Saviozzi, M.; Pompella, A.; Casini, A.F. Glutathione transferase omega 1-1 (GSTO1-1) plays an anti-apoptotic role in cell resistance to cisplatin toxicity. Carcinogenesis 2010, 31, 804–811. [Google Scholar] [CrossRef]
- Dulhunty, A.F.; Hewawasam, R.; Liu, D.; Casarotto, M.G.; Board, P.G. Regulation of the cardiac muscle ryanodine receptor by glutathione transferases. Drug Metab. Rev. 2011, 43, 236–252. [Google Scholar] [CrossRef]
- Mukherjee, B.; Salavaggione, O.E.; Pelleymounter, L.L.; Moon, I.; Eckloff, B.W.; Schaid, D.J.; Wieben, E.D.; Weinshilboum, R.M. Glutathione S-transferase omega 1 and omega 2 pharmacogenomics. Drug Metab. Dispos. 2006, 34, 1237–1246. [Google Scholar] [CrossRef]
- Polimanti, R.; Piacentini, S.; De Angelis, F.; De Stefano, G.F.; Fuciarelli, M. Human GST loci as markers of evolutionary forces: GSTO1*E155del and GSTO1*E208K polymorphisms may be under natural selection induced by environmental arsenic. Dis. Markers 2011, 31, 231–239. [Google Scholar] [CrossRef]
- Wilk, J.B.; Walter, R.E.; Laramie, J.M.; Gottlieb, D.J.; O’Connor, G.T. Framingham Heart Study genome-wide association: Results for pulmonary function measures. BMC Med. Genet. 2007, 8 (Suppl. S1), S8. [Google Scholar] [CrossRef]
- Fu, S.; Wu, J.; Chen, F.; Sun, D.; Fu, S. Polymorphisms of Glutathione S-transferases Omega-1 among ethnic populations in China. BMC Genet. 2008, 9, 29. [Google Scholar] [CrossRef]
- Seibold, P.; Hall, P.; Schoof, N.; Nevanlinna, H.; Heikkinen, T.; Benner, A.; Liu, J.; Schmezer, P.; Popanda, O.; Flesch-Janys, D.; et al. Polymorphisms in oxidative stress-related genes and mortality in breast cancer patients—Potential differential effects by radiotherapy? Breast 2013, 22, 817–823. [Google Scholar] [CrossRef]
- Sohail, A.; Kanwal, N.; Ali, M.; Sadia, S.; Masood, A.I.; Ali, F.; Iqbal, F.; Crickmore, N.; Shaikh, R.S.; Sayyed, A.H. Effects of glutathione-S-transferase polymorphisms on the risk of breast cancer: A population-based case-control study in Pakistan. Environ. Toxicol. Pharmacol. 2013, 35, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Polimanti, R.; Piacentini, S.; Porreca, F.; Fuciarelli, M. Glutathione S-transferase ω class (GSTO) polymorphisms in a sample from Rome (Central Italy). Ann. Hum. Biol. 2010, 37, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.H.; Li, B.Q.; Wu, K.Y.; Yan, H.D.; Gu, M.J.; Yao, X.H.; Dong, H.J.; Zhang, X.; Zhu, J.H. Polymorphisms of Cytochromes P450 and Glutathione S-Transferases Synergistically Modulate Risk for Parkinson’s Disease. Front. Aging Neurosci. 2022, 14, 888942. [Google Scholar] [CrossRef]
- Petrovic, M.; Simic, T.; Djukic, T.; Radic, T.; Savic-Radojevic, A.; Zekovic, M.; Durutovic, O.; Janicic, A.; Milojevic, B.; Kajmakovic, B.; et al. The Polymorphisms in GSTO Genes (GSTO1 rs4925, GSTO2 rs156697, and GSTO2 rs2297235) Affect the Risk for Testicular Germ Cell Tumor Development: A Pilot Study. Life 2023, 13, 1269. [Google Scholar] [CrossRef]
- Kölsch, H.; Linnebank, M.; Lütjohann, D.; Jessen, F.; Wüllner, U.; Harbrecht, U.; Thelen, K.M.; Kreis, M.; Hentschel, F.; Schulz, A.; et al. Polymorphisms in glutathione S-transferase omega-1 and AD, vascular dementia, and stroke. Neurology 2004, 63, 2255–2260. [Google Scholar] [CrossRef]
- Paiva, L.; Marcos, R.; Creus, A.; Coggan, M.; Oakley, A.J.; Board, P.G. Polymorphism of glutathione transferase Omega 1 in a population exposed to a high environmental arsenic burden. Pharmacogenet. Genom. 2008, 18, 1–10. [Google Scholar] [CrossRef]
- Takeshita, H.; Fujihara, J.; Takastuka, H.; Agusa, T.; Yasuda, T.; Kunito, T. Diversity of glutathione s-transferase omega 1 (a140d) and 2 (n142d) gene polymorphisms in worldwide populations. Clin. Exp. Pharmacol. Physiol. 2009, 36, 283–286. [Google Scholar] [CrossRef]
- Marahatta, S.B.; Punyarit, P.; Bhudisawasdi, V.; Paupairoj, A.; Wongkham, S.; Petmitr, S. Polymorphism of glutathione S-transferase omega gene and risk of cancer. Cancer Lett. 2006, 236, 276–281. [Google Scholar] [CrossRef]
- De Chaudhuri, S.; Ghosh, P.; Sarma, N.; Majumdar, P.; Sau, T.J.; Basu, S.; Roychoudhury, S.; Ray, K.; Giri, A.K. Genetic variants associated with arsenic susceptibility: Study of purine nucleoside phosphorylase, arsenic (+3) methyltransferase, and glutathione S-transferase omega genes. Environ. Health Perspect. 2008, 116, 501–505. [Google Scholar] [CrossRef]
- Pongstaporn, W.; Pakakasama, S.; Sanguansin, S.; Hongeng, S.; Petmitr, S. Polymorphism of glutathione S-transferase Omega gene: Association with risk of childhood acute lymphoblastic leukemia. J. Cancer Res. Clin. Oncol. 2009, 135, 673–678. [Google Scholar] [CrossRef]
- Yu, L.; Kalla, K.; Guthrie, E.; Vidrine, A.; Klimecki, W.T. Genetic variation in genes associated with arsenic metabolism: Glutathione S-transferase omega 1-1 and purine nucleoside phosphorylase polymorphisms in European and indigenous Americans. Environ. Health Perspect. 2003, 111, 1421–1427. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Brock, J.; Casarotto, M.G.; Oakley, A.J.; Board, P.G. Novel folding and stability defects cause a deficiency of human glutathione transferase omega 1. J. Biol. Chem. 2011, 286, 4271–4279. [Google Scholar] [CrossRef] [PubMed]
- Schmuck, E.; Cappello, J.; Coggan, M.; Brew, J.; Cavanaugh, J.A.; Blackburn, A.C.; Baker, R.T.; Eyre, H.J.; Sutherland, G.R.; Board, P.G. Deletion of Glu155 causes a deficiency of glutathione transferase Omega 1-1 but does not alter sensitivity to arsenic trioxide and other cytotoxic drugs. Int. J. Biochem. Cell Biol. 2008, 40, 2553–2559. [Google Scholar] [CrossRef] [PubMed]
- Tanaka-Kagawa, T.; Jinno, H.; Hasegawa, T.; Makino, Y.; Seko, Y.; Hanioka, N.; Ando, M. Functional characterization of two variant human GSTO 1-1s (Ala140Asp and Thr217Asn). Biochem. Biophys. Res. Commun. 2003, 301, 516–520. [Google Scholar] [CrossRef]
- Allen, M.; Zou, F.; Chai, H.S.; Younkin, C.S.; Miles, R.; Nair, A.A.; Crook, J.E.; Pankratz, V.S.; Carrasquillo, M.M.; Rowley, C.N.; et al. Glutathione S-transferase omega genes in Alzheimer and Parkinson disease risk, age-at-diagnosis and brain gene expression: An association study with mechanistic implications. Mol. Neurodegener. 2012, 7, 13. [Google Scholar] [CrossRef]
- Xu, Y.T.; Wang, J.; Yin, R.; Qiu, M.T.; Xu, L.; Wang, J.; Xu, L. Genetic polymorphisms in Glutathione S-transferase Omega (GSTO) and cancer risk: A meta-analysis of 20 studies. Sci. Rep. 2014, 4, 6578. [Google Scholar] [CrossRef]
- Rodrigues, E.G.; Kile, M.; Hoffman, E.; Quamruzzaman, Q.; Rahman, M.; Mahiuddin, G.; Hsueh, Y.; Christiani, D.C. GSTO and AS3MT genetic polymorphisms and differences in urinary arsenic concentrations among residents in Bangladesh. Biomarkers 2012, 17, 240–247. [Google Scholar] [CrossRef]
- Hsu, L.I.; Chen, W.P.; Yang, T.Y.; Chen, Y.H.; Lo, W.C.; Wang, Y.H.; Liao, Y.T.; Hsueh, Y.M.; Chiou, H.Y.; Wu, M.M.; et al. Genetic polymorphisms in glutathione S-transferase (GST) superfamily and risk of arsenic-induced urothelial carcinoma in residents of southwestern Taiwan. J. Biomed. Sci. 2011, 18, 51. [Google Scholar] [CrossRef]
- Luo, L.; Li, Y.; Gao, Y.; Zhao, L.; Feng, H.; Wei, W.; Qiu, C.; He, Q.; Zhang, Y.; Fu, S.; et al. Association between arsenic metabolism gene polymorphisms and arsenic-induced skin lesions in individuals exposed to high-dose inorganic arsenic in northwest China. Sci. Rep. 2018, 8, 413. [Google Scholar] [CrossRef]
- Kalinina, E. Glutathione-Dependent Pathways in Cancer Cells. Int. J. Mol. Sci. 2024, 25, 8423. [Google Scholar] [CrossRef]
- Townsend, D.; Tew, K. Cancer drugs, genetic variation and the glutathione-S-transferase gene family. Am. J. Pharmacogenomics 2003, 3, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Ni, R.Z.; Xiao, M.B.; Guo, J.G.; Zhou, J.W. Comparative proteomic analysis of differentially expressed proteins in human pancreatic cancer tissue. Hepatobiliary Pancreat. Dis. Int. 2009, 8, 193–200. [Google Scholar] [PubMed]
- Piaggi, S.; Marchi, S.; Ciancia, E.; Debortoli, N.; Lazzarotti, A.; Saviozzi, M.; Raggi, C.; Fierabracci, V.; Visvikis, A.; Bisgaard, H.C.; et al. Nuclear translocation of glutathione transferase omega is a progression marker in Barrett’s esophagus. Oncol. Rep. 2009, 21, 283–287. [Google Scholar] [PubMed][Green Version]
- Li, Y.; Zhang, Q.; Peng, B.; Shao, Q.; Qian, W.; Zhang, J.Y. Identification of glutathione S-transferase omega 1 (GSTO1) protein as a novel tumor-associated antigen and its autoantibody in human esophageal squamous cell carcinoma. Tumour Biol. 2014, 35, 10871–10877. [Google Scholar] [CrossRef]
- Lombardi, S.; Fuoco, I.; di Fluri, G.; Costa, F.; Ricchiuti, A.; Biondi, G.; Nardini, V.; Scarpato, R. Genomic instability and cellular stress in organ biopsies and peripheral blood lymphocytes from patients with colorectal cancer and predisposing pathologies. Oncotarget 2015, 6, 14852–14864. [Google Scholar] [CrossRef]
- Yan, X.D.; Pan, L.Y.; Yuan, Y.; Lang, J.H.; Mao, N. Identification of platinum-resistance associated proteins through proteomic analysis of human ovarian cancer cells and their platinum-resistant sublines. J. Proteome Res. 2007, 6, 772–780. [Google Scholar] [CrossRef]
- Paul, S.; Jakhar, R.; Bhardwaj, M.; Kang, S.C. Glutathione-S-transferase omega 1 (GSTO1-1) acts as mediator of signaling pathways involved in aflatoxin B1-induced apoptosis-autophagy crosstalk in macrophages. Free Radic. Biol. Med. 2015, 89, 1218–1230. [Google Scholar] [CrossRef]
- Djukic, T.; Simic, T.; Pljesa-Ercegovac, M.; Matic, M.; Suvakov, S.; Coric, V.; Dragicevic, D.; Savic-Radojevic, A. Upregulated glutathione transferase omega-1 correlates with progression of urinary bladder carcinoma. Redox Rep. 2017, 22, 486–492. [Google Scholar] [CrossRef]
- Saisawang, C.; Wongsantichon, J.; Robinson, R.C.; Ketterman, A.J. Glutathione transferase Omega 1-1 (GSTO1-1) modulates Akt and MEK1/2 signaling in human neuroblastoma cell SH-SY5Y. Proteins 2019, 87, 588–595. [Google Scholar] [CrossRef]
- Steinmaus, C.; Ferreccio, C.; Yuan, Y.; Acevedo, J.; González, F.; Perez, L.; Cortés, S.; Balmes, J.R.; Liaw, J.; Smith, A.H. Elevated lung cancer in younger adults and low concentrations of arsenic in water. Am. J. Epidemiol. 2014, 180, 1082–1087. [Google Scholar] [CrossRef]
- Lan, C.C.; Yu, H.S.; Ko, Y.C. Chronic arsenic exposure and its adverse health effects in Taiwan: A paradigm for management of a global environmental problem. Kaohsiung J. Med. Sci. 2011, 27, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Rachamalla, M.; Chinthada, J.; Kushwaha, S.; Putnala, S.K.; Sahu, C.; Jena, G.; Niyogi, S. Contemporary Comprehensive Review on Arsenic-Induced Male Reproductive Toxicity and Mechanisms of Phytonutrient Intervention. Toxics 2022, 10, 744. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Jasmine, F.; Kibriya, M.G.; Liu, M.; Cheng, X.; Parvez, F.; Islam, T.; Ahmed, A.; Rakibuz-Zaman, M.; Jiang, J.; et al. Interaction between arsenic exposure from drinking water and genetic polymorphisms on cardiovascular disease in Bangladesh: A prospective case-cohort study. Environ. Health Perspect. 2015, 123, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Sinha, D.; Prasad, P. Health effects inflicted by chronic low-level arsenic contamination in groundwater: A global public health challenge. J. Appl. Toxicol. 2020, 40, 87–131. [Google Scholar] [CrossRef]
- Beebe-Dimmer, J.L.; Iyer, P.T.; Nriagu, J.O.; Keele, G.R.; Mehta, S.; Meliker, J.R.; Lange, E.M.; Schwartz, A.G.; Zuhlke, K.A.; Schottenfeld, D.; et al. Genetic variation in glutathione S-transferase omega-1, arsenic methyltransferase and methylene-tetrahydrofolate reductase, arsenic exposure and bladder cancer: A case-control study. Environ. Health 2012, 11, 43. [Google Scholar] [CrossRef]
- Liao, P.J.; Hsu, K.H.; Chiou, H.Y.; Chen, C.J.; Lee, C.H. Joint effects of genomic markers and urinary methylation capacity associated with inorganic arsenic metabolism on the occurrence of cancers among residents in arseniasis-endemic areas: A cohort subset with average fifteen-year follow-up. Biomed. J. 2021, 44 (Suppl. S2), S218–S225. [Google Scholar] [CrossRef]
- Marnell, L.L.; Garcia-Vargas, G.G.; Chowdhury, U.K.; Zakharyan, R.A.; Walsh, B.; Avram, M.D.; Kopplin, M.J.; Cebrián, M.E.; Silbergeld, E.K.; Aposhian, H.V. Polymorphisms in the human monomethylarsonic acid (MMA V) reductase/hGSTO1 gene and changes in urinary arsenic profiles. Chem. Res. Toxicol. 2003, 16, 1507–1513. [Google Scholar] [CrossRef]
- Chowdhury, U.K.; Zakharyan, R.A.; Hernandez, A.; Avram, M.D.; Kopplin, M.J.; Aposhian, H.V. Glutathione-S-transferase-omega [MMA(V) reductase] knockout mice: Enzyme and arsenic species concentrations in tissues after arsenate administration. Toxicol. Appl. Pharmacol. 2006, 216, 446–457. [Google Scholar] [CrossRef]
- Wang, Y.H.; Yeh, S.D.; Shen, K.H.; Shen, C.H.; Juang, G.D.; Hsu, L.I.; Chiou, H.Y.; Chen, C.J. A significantly joint effect between arsenic and occupational exposures and risk genotypes/diplotypes of CYP2E1, GSTO1 and GSTO2 on risk of urothelial carcinoma. Toxicol. Appl. Pharmacol. 2009, 241, 111–118. [Google Scholar] [CrossRef]
- Chung, C.J.; Pu, Y.S.; Su, C.T.; Huang, C.Y.; Hsueh, Y.M. Gene polymorphisms of glutathione S-transferase omega 1 and 2, urinary arsenic methylation profile and urothelial carcinoma. Sci. Total Environ. 2011, 409, 465–470. [Google Scholar] [CrossRef]
- Lesseur, C.; Gilbert-Diamond, D.; Andrew, A.S.; Ekstrom, R.M.; Li, Z.; Kelsey, K.T.; Marsit, C.J.; Karagas, M.R. A case-control study of polymorphisms in xenobiotic and arsenic metabolism genes and arsenic-related bladder cancer in New Hampshire. Toxicol. Lett. 2012, 210, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Djukic, T.I.; Savic-Radojevic, A.R.; Pekmezovic, T.D.; Matic, M.G.; Pljesa-Ercegovac, M.S.; Coric, V.M.; Radic, T.M.; Suvakov, S.R.; Krivic, B.N.; Dragicevic, D.P.; et al. Glutathione S-transferase T1, O1 and O2 polymorphisms are associated with survival in muscle invasive bladder cancer patients. PLoS ONE 2013, 8, e74724. [Google Scholar] [CrossRef]
- Tung, M.C.; Wang, Y.H.; Yeh, S.D.; Wu, C.C.; Chen, K.C.; Huang, Z.M.; Huang, M.T.; Chiou, H.Y. Combined effects of GSTO1 and SULT1A1 polymorphisms and cigarette smoking on urothelial carcinoma risk in a Taiwanese population. J. Formos. Med. Assoc. 2014, 113, 640–647. [Google Scholar] [CrossRef]
- Djukic, T.; Simic, T.; Radic, T.; Matic, M.; Pljesa-Ercegovac, M.; Suvakov, S.; Coric, V.; Pekmezovic, T.; Novakovic, I.; Dragicevic, D.; et al. GSTO1*C/GSTO2*G haplotype is associated with risk of transitional cell carcinoma of urinary bladder. Int. Urol. Nephrol. 2015, 47, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Yang, X.; Cheng, Y.; Liu, X.; Li, X.; Zhao, R.; Qin, C.; Lu, Q.; Yin, C. GSTP1 and GSTO1 single nucleotide polymorphisms and the response of bladder cancer patients to intravesical chemotherapy. Sci. Rep. 2015, 5, 14000. [Google Scholar] [CrossRef]
- Ke, H.L.; Lin, J.; Ye, Y.; Wu, W.J.; Lin, H.H.; Wei, H.; Huang, M.; Chang, D.W.; Dinney, C.P.; Wu, X. Genetic Variations in Glutathione Pathway Genes Predict Cancer Recurrence in Patients Treated with Transurethral Resection and Bacillus Calmette-Guerin Instillation for Non-muscle Invasive Bladder Cancer. Ann. Surg. Oncol. 2015, 22, 4104–4110. [Google Scholar] [CrossRef]
- Jahrreiss, V.; Pradere, B.; Laukhtina, E.; Mori, K.; Shariat, S.F. Catalog of exogenous risk factors for bladder carcinogenesis. Curr. Opin. Urol. 2020, 30, 449–456. [Google Scholar] [CrossRef]
- Centeno, J.A.; Mullick, F.G.; Martinez, L.; Page, N.P.; Gibb, H.; Longfellow, D.; Thompson, C.; Ladich, E.R. Pathology related to chronic arsenic exposure. Environ. Health Perspect. 2002, 110 (Suppl. S5), 883–886. [Google Scholar] [CrossRef]
- Ahsan, H.; Chen, Y.; Kibriya, M.G.; Slavkovich, V.; Parvez, F.; Jasmine, F.; Gamble, M.V.; Graziano, J.H. Arsenic metabolism, genetic susceptibility, and risk of premalignant skin lesions in Bangladesh. Cancer Epidemiol. Biomarkers Prev. 2007, 16, 1270–1278. [Google Scholar] [CrossRef]
- Leite, J.L.; Morari, E.C.; Granja, F.; Campos, G.M.; Guilhen, A.C.; Ward, L.S. Influence of the glutathione s-transferase gene polymorphisms on the susceptibility to basal cell skin carcinoma. Rev. Med. Chil. 2007, 135, 301–306. [Google Scholar] [CrossRef]
- Qu, K.; Liu, S.S.; Wang, Z.X.; Huang, Z.C.; Liu, S.N.; Chang, H.L.; Xu, X.S.; Lin, T.; Dong, Y.F.; Liu, C. Polymorphisms of glutathione S-transferase genes and survival of resected hepatocellular carcinoma patients. World J. Gastroenterol. 2015, 21, 4310–4322. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Qu, K.; Huang, Z.; Xu, X.; Zhang, J.; Zhang, L.; Liu, S.; Chang, H.; Lin, T.; Liu, Y.; et al. Glutathione S-transferase O2 gene rs157077 polymorphism predicts response to transarterial chemoembolization in hepatocellular carcinoma. Tumour Biol. 2015, 36, 6463–6469. [Google Scholar] [CrossRef] [PubMed]
- Shaban, N.Z.; Salem, H.H.; Elsadany, M.A.; Ali, B.A.; Hassona, E.M.; Mogahed, F.A. Distribution of Glutathione S-Transferase Omega Gene Polymorphism with Different Stages of HBV Infection Including Hepatocellular Carcinoma in the Egyptian Population. Asian Pac. J. Cancer Prev. 2016, 17, 2145–2150. [Google Scholar] [CrossRef] [PubMed]
- Chaiteerakij, R.; Juran, B.D.; Aboelsoud, M.M.; Harmsen, W.S.; Moser, C.D.; Giama, N.H.; Allotey, L.K.; Mettler, T.A.; Baichoo, E.; Zhang, X.; et al. Association between variants in inflammation and cancer-associated genes and risk and survival of cholangiocarcinoma. Cancer Med. 2015, 4, 1599–1602. [Google Scholar] [CrossRef]
- de Jong, K.; Boezen, H.M.; Hacken, N.H.; Postma, D.S.; Vonk, J.M. LifeLines cohort study. GST-omega genes interact with environmental tobacco smoke on adult level of lung function. Respir. Res. 2013, 14, 83. [Google Scholar] [CrossRef]
- Yanbaeva, D.G.; Wouters, E.F.; Dentener, M.A.; Spruit, M.A.; Reynaert, N.L. Association of glutathione-S-transferase omega haplotypes with susceptibility to chronic obstructive pulmonary disease. Free Radic. Res. 2009, 43, 738–743. [Google Scholar] [CrossRef]
- de Andrade, M.; Li, Y.; Marks, R.S.; Deschamps, C.; Scanlon, P.D.; Olswold, C.L.; Jiang, R.; Swensen, S.J.; Sun, Z.; Cunningham, J.M.; et al. Genetic variants associated with the risk of chronic obstructive pulmonary disease with and without lung cancer. Cancer Prev. Res. 2012, 5, 365–373. [Google Scholar] [CrossRef]
- Moyer, A.M.; Sun, Z.; Batzler, A.J.; Li, L.; Schaid, D.J.; Yang, P.; Weinshilboum, R.M. Glutathione pathway genetic polymorphisms and lung cancer survival after platinum-based chemotherapy. Cancer Epidemiol. Biomarkers Prev. 2010, 19, 811–821. [Google Scholar] [CrossRef]
- Ada, T.G.; Ada, A.O.; Kunak, S.C.; Alpar, S.; Gulhan, M.; Iscan, M. Association between glutathione S-transferase omega 1 A140D polymorphism in the Turkish population and susceptibility to non-small cell lung cancer. Arh. Hig. Rada Toksikol. 2013, 64, 61–67. [Google Scholar] [CrossRef]
- Hiyama, T.; Yoshihara, M.; Tanaka, S.; Chayama, K. Genetic polymorphisms and head and neck cancer risk (Review). Int. J. Oncol. 2008, 32, 945–973. [Google Scholar][Green Version]
- Sanguansin, S.; Petmitr, S.; O-Charoenrat, P.; Pongstaporn, W. Association of glutathione S-transferase omega gene polymorphisms with progression of head and neck cancer. Mol. Biol. Rep. 2012, 39, 10915–10920. [Google Scholar] [CrossRef] [PubMed]
- Masoudi, M.; Saadat, I.; Omidvari, S.; Saadat, M. Genetic polymorphisms of GSTO2, GSTM1, and GSTT1 and risk of gastric cancer. Mol. Biol. Rep. 2009, 36, 781–784. [Google Scholar] [CrossRef] [PubMed]
- Masoudi, M.; Saadat, I.; Omidvari, S.; Saadat, M. Association between N142D genetic polymorphism of GSTO2 and susceptibility to colorectal cancer. Mol. Biol. Rep. 2011, 38, 4309–4313. [Google Scholar] [CrossRef]
- Radic, T.M.; Coric, V.M.; Pljesa-Ercegovac, M.S.; Basta-Jovanovic, G.M.; Radojevic-Skodric, S.M.; Dragicevic, D.P.; Matic, M.G.; Bogdanovic, L.M.; Dzamic, Z.M.; Simic, T.P.; et al. Concomitance of Polymorphisms in Glutathione Transferase Omega Genes Is Associated with Risk of Clear Cell Renal Cell Carcinoma. Tohoku J. Exp. Med. 2018, 246, 35–44. [Google Scholar] [CrossRef]
- Radic, T.; Coric, V.; Bukumiric, Z.; Pljesa-Ercegovac, M.; Djukic, T.; Avramovic, N.; Matic, M.; Mihailovic, S.; Dragicevic, D.; Dzamic, Z.; et al. GSTO1*CC Genotype (rs4925) Predicts Shorter Survival in Clear Cell Renal Cell Carcinoma Male Patients. Cancers 2019, 11, 2038. [Google Scholar] [CrossRef] [PubMed]
- Granja, F.; Morari, E.C.; Assumpção, L.V.; Ward, L.S. GSTO polymorphism analysis in thyroid nodules suggest that GSTO1 variants do not influence the risk for malignancy. Eur. J. Cancer Prev. 2005, 14, 277–280. [Google Scholar] [CrossRef]
- Bufalo, N.E.; Leite, J.L.; Guilhen, A.C.; Morari, E.C.; Granja, F.; Assumpcao, L.V.; Ward, L.S. Smoking and susceptibility to thyroid cancer: An inverse association with CYP1A1 allelic variants. Endocr. Relat. Cancer 2006, 13, 1185–1193. [Google Scholar] [CrossRef]
- Olsen, A.; Autrup, H.; Sørensen, M.; Overvad, K.; Tjønneland, A. Polymorphisms of glutathione S-transferase A1 and O1 and breast cancer among postmenopausal Danish women. Eur. J. Cancer Prev. 2008, 17, 225–229. [Google Scholar] [CrossRef]
- Chariyalertsak, S.; Purisa, W.; Sangrajrang, S. Role of glutathione S-transferase omega gene polymorphisms in breast-cancer risk. Tumori 2009, 95, 739–743. [Google Scholar] [CrossRef]
- Andonova, I.E.; Justenhoven, C.; Winter, S.; Hamann, U.; Baisch, C.; Rabstein, S.; Spickenheuer, A.; Harth, V.; Pesch, B.; Brüning, T.; et al. No evidence for glutathione S-transferases GSTA2, GSTM2, GSTO1, GSTO2, and GSTZ1 in breast cancer risk. Breast Cancer Res. Treat. 2010, 121, 497–502. [Google Scholar] [CrossRef]
- Masoudi, M.; Saadat, I.; Omidvari, S.; Saadat, M. Additive effects of genetic variations of xenobiotic detoxification enzymes and DNA repair gene XRCC1 on the susceptibility to breast cancer. Breast Cancer Res. Treat. 2010, 120, 263–265. [Google Scholar] [CrossRef] [PubMed]
- Sharif, M.R.; Sharif, A.; Kheirkhah, D.; Taghavi Ardakan, M.; Soltani, N. Association of GSTO1 A140D and GSTO2 N142D Gene Variations with Breast Cancer Risk. Asian Pac. J. Cancer Prev. 2017, 18, 1723–1727. [Google Scholar] [CrossRef] [PubMed]
- Rothman, N.; Wacholder, S.; Caporaso, N.E.; Garcia-Closas, M.; Buetow, K.; Fraumeni, J.F., Jr. The use of common genetic polymorphisms to enhance the epidemiologic study of environmental carcinogens. Biochim. Biophys. Acta 2001, 1471, C1–C10. [Google Scholar] [CrossRef] [PubMed]
- Morari, E.C.; Lima, A.B.; Bufalo, N.E.; Leite, J.L.; Granja, F.; Ward, L.S. Role of glutathione-S-transferase and codon 72 of P53 genotypes in epithelial ovarian cancer patients. J. Cancer Res. Clin. Oncol. 2006, 132, 521–528. [Google Scholar] [CrossRef]
- Pongstaporn, W.; Rochanawutanon, M.; Wilailak, S.; Linasamita, V.; Weerakiat, S.; Petmitr, S. Genetic alterations in chromosome 10q24.3 and glutathione S-transferase omega 2 gene polymorphism in ovarian cancer. J. Exp. Clin. Cancer Res. 2006, 25, 107–114. [Google Scholar]
- Simic, P.; Coric, V.; Pljesa, I.; Savic-Radojevic, A.; Zecevic, N.; Kocic, J.; Simic, T.; Pazin, V.; Pljesa-Ercegovac, M. The Role of Glutathione Transferase Omega-Class Variant Alleles in Individual Susceptibility to Ovarian Cancer. Int. J. Mol. Sci. 2024, 25, 4986. [Google Scholar] [CrossRef]
- Zamani, S.; Sohrabi, A.; Rahnamaye-Farzami, M.; Hosseini, S.M. Glutathione S-transferase omega gene polymorphism as a biomarker for human papilloma virus and cervical cancer in Iranian women. J. Turk. Ger. Gynecol. Assoc. 2018, 19, 193–200. [Google Scholar] [CrossRef]
- Berenguer, C.V.; Pereira, F.; Câmara, J.S.; Pereira, J.A.M. Underlying Features of Prostate Cancer-Statistics, Risk Factors, and Emerging Methods for Its Diagnosis. Curr. Oncol. 2023, 30, 2300–2321. [Google Scholar] [CrossRef]
- Wilson, K.M.; Mucci, L.A. Diet and Lifestyle in Prostate Cancer. Adv. Exp. Med. Biol. 2019, 1210, 1–27. [Google Scholar] [CrossRef]
- Lima, M.M., Jr.; Oliveira, M.N.; Granja, F.; Trindade, A.C.; De Castro Santos, L.E.; Ward, L.S. Lack of association of GSTT1, GSTM1, GSTO1, GSTP1 and CYP1A1 polymorphisms for susceptibility and outcome in Brazilian prostate cancer patients. Folia Biol. 2008, 54, 102–108. [Google Scholar]
- Santric, V.; Dragicevic, D.; Matic, M.; Djokic, M.; Pljesa-Ercegovac, M.; Radic, T.; Suvakov, S.; Nikitovic, M.; Stankovic, V.; Milojevic, B.; et al. Polymorphisms in Genes Encoding Glutathione Transferase Pi and Glutathione Transferase Omega Influence Prostate Cancer Risk and Prognosis. Front. Mol. Biosci. 2021, 8, 620690. [Google Scholar] [CrossRef]
- Rezazadeh, D.; Moradi, M.T.; Kazemi, A.; Mansouri, K. Childhood Pre-B acute lymphoblastic leukemia and glutathione S-transferase omega 1 and 2 polymorphisms. Int. J. Lab. Hematol. 2015, 37, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Baba, S.M.; Pandith, A.A.; Shah, Z.A.; Geelani, S.A.; Bhat, J.R.; Gul, A.; Guru, S.A.; El-Serehy, H.A.; Koul, A.M.; Mansoor, S. GSTT1null and rs156697 Polymorphism in GSTO2 Influence the Risk and Therapeutic Outcome of B-Acute Lymphoblastic Leukemia Patients. Front. Oncol. 2021, 11, 714421. [Google Scholar] [CrossRef]
- Thompson, M.A.; Wei, C.; Moyer, A.M.; Cunningham, J.M.; Wang, L.; Fayad, L.; Feng, L.; Rodriguez, M.A.; Cabanillas, F.; Kantarjian, H.M.; et al. Single Nucleotide Polymorphisms (SNPs) in Genes for Glutathione-Related Metabolism, Cyclin D1, and DNA Repair As Predictive Biomarkers in Mantle Cell Lymphoma Patients Treated with R-HyperCVAD with Ten Year Clinical Follow-up. Blood 2011, 118, 3650. [Google Scholar] [CrossRef]

| Enzymatic Activity | GSTO1-1 | GSTO2-2 |
|---|---|---|
| DHA reductase | Yes [12,13] | Yes [8,12] |
| Inorganic arsenate reductase | Yes [15] | ----- |
| MMAV reductase | Yes [12,14] | Yes [12] |
| DMAV reductase | Yes [12,14] | Yes [8,12] |
| S-(Phenacyl)glutathione reductase | Yes [17] | No [17] |
| Glutaredoxin (Grx)-like thioltransferase reactions | Yes [5,16] | Yes [6,12] |
| Deglutathionylation | Yes [6] | No [6] |
| Glutathionylation | Yes [18] | Yes [18] |
| Classical glutathione transferase | Yes [5] | Yes [8]; No [12] |
| Enzymatic Activity | GSTO1-1 A140D | GSTO1-1 E155del | GSTO1-1 E208K | GSTO2-2 N142D |
|---|---|---|---|---|
| DHA reductase | NC [7,12] | ↑↑ [12] | NC [12,18] | NC [12] |
| MMAV reductase | NC [7,12] | ↑↑ [12] | NC [7] ↑ [12] | NC [7,16] |
| DMAV reductase | NC [18] | ↑↑ [12] | ↑↑ [12] | NC [12] |
| S-(phenacyl)glutathione reductase | NC [18] | NC [17] | NC [17] | ----- |
| Glutaredoxin (Grx)-like thioltransferase reactions | NC [7,12] ↓ [44] | ↑↑ [7,12] | ↑ [12] NC [18] | NC [12,16] |
| Deglutathionylation | ↓ [18] | ----- | ↓ [18] | ----- |
| Glutathionylation | ↑↑ [18] | ----- | ----- | ----- |
| Classical glutathione transferase | NC [18,44] | ↑↑ [7] | ----- | ----- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belcastro, E.; Paties Montagner, G.; Pompella, A.; Piaggi, S.; Corti, A. Glutathione Transferases Omega-1 and -2 Polymorphisms in Cancer: Drivers or Silent Bystanders? Int. J. Mol. Sci. 2025, 26, 6586. https://doi.org/10.3390/ijms26146586
Belcastro E, Paties Montagner G, Pompella A, Piaggi S, Corti A. Glutathione Transferases Omega-1 and -2 Polymorphisms in Cancer: Drivers or Silent Bystanders? International Journal of Molecular Sciences. 2025; 26(14):6586. https://doi.org/10.3390/ijms26146586
Chicago/Turabian StyleBelcastro, Eugenia, Giulia Paties Montagner, Alfonso Pompella, Simona Piaggi, and Alessandro Corti. 2025. "Glutathione Transferases Omega-1 and -2 Polymorphisms in Cancer: Drivers or Silent Bystanders?" International Journal of Molecular Sciences 26, no. 14: 6586. https://doi.org/10.3390/ijms26146586
APA StyleBelcastro, E., Paties Montagner, G., Pompella, A., Piaggi, S., & Corti, A. (2025). Glutathione Transferases Omega-1 and -2 Polymorphisms in Cancer: Drivers or Silent Bystanders? International Journal of Molecular Sciences, 26(14), 6586. https://doi.org/10.3390/ijms26146586







